Abstract
New 2H-1,4-benzoxazine derivatives were synthesized and tested for their agonist properties on the ATP-sensitive K+ channels (KATP) of native rat skeletal muscle fibres by using the patch-clamp technique. The novel modifications involved the introduction at position 2 of the benzoxazine ring of alkyl substituents such as methyl (–CH3), ethyl (–C2H5) or propyl (–C3H7) groups, while maintaining pharmacophore groups critical for conferring agonist properties.
The effects of these molecules were compared with those of cromakalim in the presence or absence of internal ATP (10−4 M). In the presence of internal ATP, all the compounds increased the macropatch KATP currents. The order of potency of the molecules as agonists was −C3H7 (DE50=1.63 × 10−8 M) >−C2H5 (DE50=1.11 × 10−7 M)>–CH3 (DE50=2.81 × 10−7 M)>cromak-slim (DE50= 1.42 × 10−5 M). Bell-shaped dose–response curves were observed for these compounds and cromakalim indicating a downturn in response when a certain dose was exceeded.
In contrast, in the absence of internal ATP, all molecules including cromakalim inhibited the KATP currents. The order of increasing potency as antagonists was cromakalim (IC50=1.15 × 10−8 M)⩾–CH3 (IC50=2.6 × 10−8 M)>–C2H5 (IC50=4.4 × 10−8 M)>–C3H7 (IC50=1.68 × 10−7 M) derivatives.
These results suggest that the newly synthesized molecules and cromakalim act on muscle KATP channel by binding on two receptor sites that have opposite actions. Alternatively, a more simple explanation is to consider the existence of a single site for potassium channel openers regulated by ATP which favours the transduction of the channel opening. The alkyl chains at position 2 of the 2H-1,4-benzoxazine nucleus is pivotal in determining the potency of benzoxazine derivatives as agonists or antagonists.
Keywords: KCO; 2H-1,4-benzoxazine; cromakalim; KATP channel; skeletal muscle; patch clamp
Introduction
The K+ channel openers (KCOs) are a structurally diverse group of drugs showing a broad spectrum of potentially therapeutic applications including asthma, urinary incontinence, hypertension, angina, hypoglycaemia, neuromuscular disorders and some forms of epilepsy (Longman & Hamilton, 1992; Schwanstecher et al., 1998). First and second generation KCOs have been synthesised and tested for their capability to open the ATP-sensitive K+ channel (KATP) of different tissues leading to hyperpolarisation of the cells and reduction of the influx of Ca2+ ions through voltage-dependent Ca2+ channels (Lawson, 2000).
Pharmacological investigations have shown that cromakalim, a first-generation KCO, can be effective in the treatment of neuromuscular disorders. This molecule is able to repolarise the muscle fibers of hypokalemic periodic paralyses (hypoPP) patients as well as of K+-depleted rats, an animal model of hypoPP, and in some myotonic patients is also able to suppress ‘in vitro' the abnormal hyperexcitability of the fibers (Quasthoff et al., 1990; Tricarico et al., 1998, 1999). However, the use of first generation KCOs in skeletal muscle disorders is limited by their lack of tissue selectivity that in turn is related to their side effects such as hypotension, hypertricosis and headache (Andersson, 1992). Knowledge of the tissue-selective expression of various sulphonylurea receptor (SURs) subunits and pore forming subunits (kirs) of the KATP channel complexes has made it possible to investigate tissue-selective KCOs. However, to date molecules targeting the skeletal muscle KATP channels are not known.
In order to search for molecules more potent than cromakalim in activating the muscular KATP channel and to better investigate their mechanism of action, a series of new 2H-1,4-benzoxazine derivatives have been synthesised. The novel structural modifications that we performed in the 2H-1,4-benzoxazine nucleus involved the replacement of one hydrogen atom at position 2 on the benzoxazine ring with different alkyl substituents such as methyl (–CH3) ethyl (–C2H5) or propyl (–C3H7) groups. This gave us the opportunity to evaluate the effects of the lipophilicity on muscular KATP channel activity.
The 2H-1,4-benzoxazine derivatives are known to be structural analogues of benzopyrans, the difference being the replacement of the carbon atom at position 4 of the benzopyran ring with a nitrogen atom. Structure–activity relations performed on isolated organs have shown that the introduction of the nitro or amino group in positions 6 and 7 of the 2H-1,4-benzoxazine nucleus, respectively, or the synthesis of tricyclic derivatives by condensing an oxadiazole ring to the same part of the nucleus give rise to molecules more potent than cromakalim or levcromakalim in relaxing the arterial smooth muscle or in reducing the arterial mean blood pressure (Matsumoto et al., 1996, 1999, 2000; Caliendo et al., 1998). In most cases, the vasodilating effects of these compounds were antagonised ‘in vitro' by glibenclamide, the well known KATP channel blocker, suggesting that the KATP channel is the main target of the action of these molecules in smooth muscle cells. Furthermore, 2H-1,4-benzoxazine derivatives can be considered cyclic analogues of the anilide tertiary carbinols (Grant et al., 1994; Matsumoto et al., 1996, 1999; Caliendo et al., 1998). These latter open chain KCOs are known for their relaxant effects on the bladder and urethra smooth muscle (Grant et al., 1994). However, the mechanism of action of these KCOs appears to be complex showing stimulatory and inhibitory responses (Jow & Numann, 1999; Teramoto et al., 2001). Similarly pinacidil, an open chain KCO derivative, activates or inhibits the KATP channels in skeletal muscle depending on the level of the KATP channel activity (Hehl & Neumcke, 1994). In addition, inhibitory responses of diverse K+ channels have been observed with structural analogues of cromakalim, such as the chromanols that inhibit KCNQ1 channels, reducing the IKs currents in heart cells by a stereospecific interaction with the pore-forming subunit (Seebohm et al., 2001).
In the present work, macropatch KATP currents were recorded using a patch-clamp technique in the presence of cromakalim or in the presence of newly synthesised 2H-1,4-benzoxazine derivatives. The potency of the 2H-1,4-benzoxazine derivatives relative to cromakalim was evaluated by constructing dose–response curves for the compounds under investigation in the presence of internal ATP or in the absence of the nucleotide.
Methods
Muscle preparations and single fibre isolation
The flexor digitorum brevis (FDB) muscles were dissected from male Wistar rats under urethane anaesthesia (1.2 g kg−1). After dissection, the animals were rapidly killed with an overdose of urethane according to the ‘Guide for Care and Use of Laboratory Animals' prepared by the National Academy of Sciences. Single muscle fibers were prepared by enzymatic dissociation (Tricarico et al., 1998).
Drugs and solutions
The normal Ringer solution contained 145 × 10−3 M NaCl, 5 × 10−3 M KCl, 1 × 10−3 M MgCl2, 0.5 × 10−3 M CaCl2, 5 × 10−3 M glucose and 1 × 10−2 M 3-(N-morpholino)propanesulphonic acid (MOPS), pH=7.2. The patch pipette solution contained 150 × 10−3 M KCl, 2 × 10−3 M CaCl2 and 1 × 10−2 M MOPS, pH=7.2. The bath solution contained 150 × 10−3 M KCl, 5 × 10−3 M EGTA and 1 × 10−2 M MOPS, pH=7.2. Stock solution of ATPK2 (5 × 10−3 M) was prepared by dissolving the chemical in the bath solution. Stock solutions of cromakalim (7.6 × 10−1 M) and of the benzoxazine derivatives (7.6 × 10−1 M) were prepared by dissolving the drugs in dimethylsulphoxide (DMSO). Microliter amounts of the stock solutions were then added to the bath solution as needed to obtain concentrations of cromakalim ranging between 10−12 and 8 × 10−4 M, and of the benzoxazine derivatives between 10−12 and 10−4 M (DMSO 1.3 × 10−8–0.05%). Higher concentrations could not be tested because of the low solubility of the substances in the bath solution. The drugs were tested in the presence or absence of an internal 10−4 M concentration of ATPK2.
DMSO applied at 0.05% concentration to the excised patches in the presence of a 10−4 M concentration of ATPK2 did not increase the KATP channel activity (solvent control).
We prefer to use ATPK2 instead of MgATP to avoid the effects of second messengers such as PIP2 or related molecules and pumps such as the 3Na+/2 K+ ATP-ase, which are known to induce activation of the KATP channel in excised patches in the presence of MgATP (Kabakov, 1998; Song & Ashcroft, 2001). Furthermore, it is known that cromakalim and other KCOs are able to stimulate the native skeletal muscle KATP channels even in the absence of internal Mg2+ ions (Forestier et al., 1996).
Synthesis of the new benzoxazine derivatives
The starting compounds were (±)-2-alkyl-2-(4-chloro-phenoxy) acetic acids, the preparation of which has been previously reported (Bettoni et al., 1987, 1992). These acids were treated with 90% HNO3 at 0°C for 2–3 h to give the 2-nitro benzene derivatives which were reduced and cycled to the corresponding benzoxazinones with iron and HCl (6 N) in refluxing 1,4-dioxane for 2–3 h. The condensation of the benzoxazinones with 3-aminopyridine, in the presence of TiCl4 and anisole, was carried out in refluxing dry toluene for 6–12 h and provided the desired compounds in 10–40% yields (Fryer et al., 1969). The physical properties of the final drugs are as follows:
(R/S)-6-chloro-2-methyl-3-(pyridin-3-yl-amino)-2H-1,4-benzoxazine maleate salt. m.p. 148–150°C. 1H-NMR (300 MHz, DMSO-d6): δ 1.32 (d, 3H, CH3); 4.92 (q, 1H, CH); 6.23 (s, 2H, CH=CH); 6.87 (d, 1H, benzenic proton); 6.96 (dd, 1H, benzenic proton); 7.10 (d, 1H, benzenic proton); 7.43 (q, 1H, pyridinic proton); 8.28 (d, 1H, pyridinic proton); 8.36 (d, 1H, pyridinic proton); 8.96 (s, 1H, pyridinic proton); 9.80 (bb, 2H, NH+COOH, D2O exchanged). GC–MS, m/z: 273 (M+, 100); 275 (M++2, 32).
(R/S)-6-chloro-2-ethyl-3-(pyridin-3-yl-amino)-2H-1,4-benzoxazine maleate salt. m.p. 149–151°C. 1H-NMR (300 MHz, DMSO-d6): δ 0.95 (t, 3H, CH3); 1.63 (m, 2H, CH2); 3.00–5.00 (bb, 2H, NH+COOH, D2O exchanged); 4.68 (t, 1H, CH); 6.22 (s, 2H, CH=CH); 6.89 (d, 1H, benzenic proton); 6.95 (dd, 1H, benzenic proton); 7.09 (d, 1H, benzenic proton); 7.43 (q, 1H, pyridinic proton); 8.27 (d, 1H, pyridinic proton); 8.37 (d, 1H, pyridinic proton); 8.97 (s, 1H, pyridinic proton). GC–MS, m/z: 287 (M+, 100); 289 (M++2, 33).
(R/S)-6-chloro-2-propyl-3-(pyridin-3-yl-amino)-2H-1,4-benzoxazine (free base). m.p. 180–200°C. 1H-NMR (300 MHz, DMSO-d6): δ 0.86 (t, 3H, CH3); 1.30–1.70 (m, 4H, CH2–CH2); 4.66 (dd, 1H, CH); 6.69 (d, 1H, benzenic proton); 6.78 (dd, 1H, benzenic proton); 6.98 (d, 1H, benzenic proton); 7.22 (q, 1H, pyridinic proton); 8.15 (d, 1H, pyridinic proton); 8.36 (d, 1H, pyridinic proton); 8.81 (s, 1H, pyridinic proton); 9.30 (bb, 1H, NH, D2O exchanged). GC–MS, m/z: 301 (M+, 68); 303 (M++2, 24); 259 (100).
Microanalyses of all the final molecules were within ±0.4% of theoretical values. For pharmacological experiments, these molecules were used as racemate and free bases.
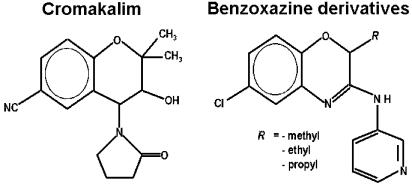
Molecular modelling studies have shown that the minimal factors required to confer KCO properties to benzopyran and other bicyclic compounds is the presence of four common regions; two representing areas of lipophilic interaction such as the aromatic ring condensed with the pyran ring and the alkyl substituent at position 2 of the benzopyran ring, and the others having hydrogen bonding forming capacity represented by the oxygen atom of the amide group at position 4 and by the electron-withdrawing group at position 6 of the benzopyran nucleus (Koga et al., 1993). According to this pharmacophore model, our molecules showed at least two lipophilic areas represented by the aromatic ring condensed with the oxazine nucleus and by an alkyl substituent at position 2 of the benzoxazine ring. The region with hydrogen bonding forming capacity is represented by the pyridylamino group at position 3, while at position 6 of the same nucleus an electron-withdrawing chlorine atom is present. The novel structural modifications in the 2H-1,4-benzoxazine nucleus involved the replacement of one hydrogen atom at position 2 of the benzoxazine ring with different alkyl substituents such as –CH3, –C2H5 or –C3H7 groups (Figure 1).
Figure 1.
Chemical structures of cromakalim and of the newly synthesised benzoxazine derivatives. The 2H-1,4-benzoxazine derivatives are structural analogues of benzopyrans, the difference being the replacement of the carbon atom at position 4 with a nitrogen atom. The new molecules contain different alkyl substituents of variable length such as the methyl, ethyl and propyl at position 2 of the benzoxazine nucleus. All the synthesized molecules have a pyridylamine group with hydrogen bond forming capacity at position 3 and an electron-withdrawing group such as the chlorine atom at position 6 of the benzoxazine nucleus. The introduction of different alkyl substituents at position 2 of the benzoxazine ring gives the opportunity to evaluate the influence of the increase of lipophilicity and/or of alkyl chain length in this part of the molecule.
Patch-clamp experiments
Experiments were performed in inside-out configurations using the standard patch-clamp technique. Recordings of channel currents were performed during voltage steps of 6 s going from 0 mV of holding potential to −60 mV immediately after excision, at 20°C, in the presence of 150 × 10−3 M KCl on both sides of the membrane in the absence (controls) or in the presence of 10−4 M ATP in the bath solution. The macropatch currents were recorded at 1 kHz sampling rates (filter=0.2 kHz) using an Axopatch-1D amplifier equipped with a CV-4 headstage (Axon Instruments, Foster City, CA, U.S.A.). Pipettes having an average tip opening area of 6.1±0.8 μm2 (macropatches=211) were used to measure the currents sustained by multiple KATP channels and their pharmacological properties.
The currents flowing through the macropatches excised from different fibres were digitally averaged, and were calculated by subtracting the base-line level of the currents from the open channel level. The base-line level for the KATP current was measured in the presence of ATP (5 × 10−3 M). Macropatches containing voltage-dependent K+ channels or inward rectifier K+ channels were excluded from the analysis. Current amplitude was measured using the Clampfit program (Axon Instruments, Foster City, CA, U.S.A.). No correction for liquid junction potential was made, estimated to be <1.9 mV in our experimental conditions.
No more than two different concentrations of the drugs were applied to the same excised macropatch. Washout periods followed the first and the second applications of the drug solutions. Patches showing rundown or that did not fully recover during washout after the drug solution applications were excluded from the analysis.
logP, logD and pKa calculation
The relation between the lipophilicity and the biological activity of the 2H-1,4-benzoxazine derivatives tested was evaluated by calculating the log P and log D at pH 7, defined as logarithm of the partition coefficient and logarithm of the distribution coefficient at a particular pH, respectively. The degree of ionisation of our molecules was evaluated by calculating the pKa defined as the negative logarithm of the dissociation constant. In our molecules the potential basic centers are represented by the pyridine nitrogen atom and the endocyclic nitrogen atom of the amidine function. Theoretical log P, log D and pKa values were calculated by using ACD software V. 6.0 (Advanced Chemistry Development Inc., Toronto, Ontario, Canada M5H 3V9).
Statistics
The data are expressed as mean±standard error unless otherwise specified. The concentration–response relation of the KATP currents constructed in the presence of internal ATP fits the product of two equations describing the interaction of a ligand with two sites mediating opposite effects, the stimulatory effect or the inhibitory effect (Rovati & Nicosia, 1994), while the concentration–response relations of the KATP currents versus drug concentrations constructed in the absence of ATP are well fitted by one inhibitory term.
The stimulatory component can be described by the term
while the inhibitory component can be described by the term
For equation (1), Idrug+ATP is the KATP currents measured in the presence of the molecules under study and in the presence of internal ATP (10−4 M); Amax, is the per cent maximal activation of the KATP currents produced by the molecules under study, and it is calculated in respect to the current levels measured in the presence of ATP (10−4 M) alone. For equation (2), Idrug, is the KATP currents measured in the presence of the molecules under study but in the absence of ATP (controls); Imax, is the per cent maximal inhibition of the KATP currents produced by the molecules under study, and it is calculated in respect to the maximal current levels measured in the absence of ATP; DE50 is the concentration of the drug needed to enhance the current by 50%, calculated in respect to the maximal activation produced by the drugs in the presence of internal ATP; IC50 is the concentration of the drug needed to reduce the current by 50%, calculated in respect to the maximal inhibition produced by the drugs in the absence of internal ATP; [Drug] is the concentration of the drug tested; n is the slope factor of the curves calculated in the presence (equation (1)) or absence (equation (2)) of ATP. The algorithms of the fitting procedures used are based on a Marquardt least-squares fitting routine.
Results
Effects of 2H-1,4-benzoxazine derivatives and cromakalim on muscle KATP channels in the presence of internal ATP
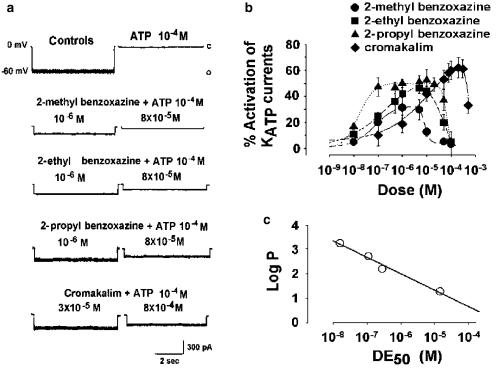
The effects of increasing concentrations of the newly synthesised 2H-1,4-benzoxazine derivatives or cromakalim on muscle KATP currents of excised macropatches recorded in the presence of 10−4 M ATP in the bath were investigated. We found that all the new compounds increased the macropatch KATP currents with different degrees of potency depending on the length of the alkyl chain at position 2 of the 2H-1,4-benzoxazine ring (Figures 1; 2a, b).
Figure 2.
Effects of K+ channel openers on KATP channels of rat skeletal muscle fibres in the presence of internal ATP. (a) Digital average of KATP current recorded in the excised inside-out macropatches, at −60 mV (V m), with high cytosolic KCl solutions on both sides of the membrane, in the absence (controls) (n=31 macropatches), in the presence of 10−4 M concentration of ATP (n=31 macropatches), in the presence of 10−4 M ATP+the 2H-1,4-benzoxazine derivatives having the methyl (n=6 macropatches), ethyl (n=5 macropatches) or propyl (n=7 macropatches) groups at position 2 of the benzoxazine ring or in the presence of 10−4 M ATP+cromakalim (n=4 macropatches). C and O in the traces indicate closed and open channel levels, respectively, (b) Concentration–response relation of the 2H-1,4-benzoxazine derivatives having the–CH3, –C2H5 or –C3H7 groups at position 2 of the benzoxazine ring, and of cromakalim on the KATP currents inhibited by a 10−4 M concentration of internal ATP. All the compounds under investigation including cromakalim produced a concentration-dependent increase of the KATP current at the lower doses, in contrast inhibiting it at the highest doses, (c) Log P plot versus the DE50 of the 2H-1,4-benzoxazine derivatives and cromakalim. An inverse relation was observed between the log P, as index of lipophilicity, and DE50 of the compounds under investigation showing a coefficient of correlation of −0.97.
In the range of concentrations from 10−8 to 10−6 M, the 2–CH3, 2–C2H5 and 2–C3H7,-2H-1,4-benzoxazine derivatives induced a stimulation of the KATP currents. The 2–C3H7-2H-1,4-benzoxazine derivative increased the current concentration-dependently and was the most potent molecule as KATP channel agonist compared to the other structurally related analogues (Figure 2a, b).
However, the constructed concentration–response curves of the compounds showed a dualistic behaviour; in fact, bell-shaped concentration–response curves were observed for these compounds exhibiting a downturn in response when a certain concentration was exceeded (Figure 2a, b). Cromakalim also induced a significant stimulation of the current in the range of concentrations from 10−7 to 3 × 10−4 M showing a downturn in response at higher concentrations (Figure 2a, b). On the basis of these findings, a function describing the interaction of a ligand with two sites mediating opposite effects, one stimulatory and the other inhibitory, was used to fit the experimental data. The calculation of the fitted parameters of equation (1) showed that the order of potency of the molecules as agonists expressed as DE50 was –C3H7>–C2H5>–CH3>cromakalim (Table 1).
Table 1.
Fitting parameters of the concentration–response curves of cromakalim and 2H-1,4-benzoxazine derivatives versus the skeletal muscle KATP currents in the presence of absence of internal ATP
| In the presence of ATP (10−4 M) | In the absence of ATP | |||||
|---|---|---|---|---|---|---|
| Compounds | DE50 (M) | n | % Maximal activation | IC50m (M) | n | % Maximal inhibition |
| Cromakalim | 1.42 × 10−5 | 1.1 | 63 | 1.15 × 10−8 | 0.89 | 65 |
| Benzoxazine derivatives R=–CH3 | 2.81 × 10−7 | 1.2 | 35 | 2.6 × 10−8 | 0.9 | 61 |
| R=–C2H5 | 1.11 × 10−7 | 1.1 | 45 | 4.4 × 10−8 | 0.8 | 48 |
| R=–C3H7 | 1.63 × 10−8 | 1.3 | 54 | 1.68 × 10−7 | 0.9 | 41 |
The parameters reported in the table were calculated using the fitting routine as described in the Methods. The 2H-1,4-benzoxazine derivatives had a methyl (–CH3), ethyl (–C2H5) or propyl (–C3H7) group at position 2 of the nucleus. DE50 is the concentration of the drug needed to enhance the current by 50%, calculated in respect to the maximal activation produced by the drugs in the presence of internal ATP; the per cent maximal activation of the KATP currents produced by the molecules under study is calculated in respect to the current levels measured in the presence of ATP (10−4 M) alone as reported in equation (1) of the Methods; IC50 is the concentration of the drug needed to reduce the current by 50%, calculated in respect to the maximal inhibition produced by the drugs in the absence of internal ATP; the per cent maximal inhibition of the KATP currents produced by the molecules under study is calculated in respect to the maximal current levels measured in the absence of ATP as reported in equation (2) of the Methods. n is the slope factor calculated in the presence or absence of ATP as reported in equations (1) and (2) (see Methods), respectively.
To evaluate the role of the lipophilicity and the degree of ionisation of the 2H-1,4-benzoxazine derivatives in determining the activation of the KATP channel in our system, the log P, log D at pH 7 and pKa values were calculated. No difference was observed between the calculated log P and log D values for the 2H-1,4-benzoxazine derivatives under study. Furthermore, the calculation of the pKa gave values of 4.91±0.11 for the protonated form of the pyridine nitrogen and 3.13±0.4 for the protonated form of the endocyclic nitrogen atom of the amidine function, respectively. These values were identical for all the 2H-1,4-benzoxazine derivatives. These findings indicated that our molecules are expected to be poorly protonated at the pH of 7.2 used in our experiments and in consequence of the fact that the lipophilicity plays a major role in determining the pharmacological activity. In fact, an inverse correlation was observed between the log P, which is an index of lipophilicity, and the DE50 of the 2H-1,4-benzoxazine derivatives (Figure 2c; Table 1). Cromakalim had the lowest value of log P among the benzoxazine series, and lower than that experimentally measured (Adlar et al., 1995).
Differences in the maximal efficacy as agonists were observed within the 2H-1,4-benzoxazine series and cromakalim (Table 1).
Effects of 2H-1,4-benzoxazine derivatives and cromakalim on muscle KATP channels in the absence of internal ATP
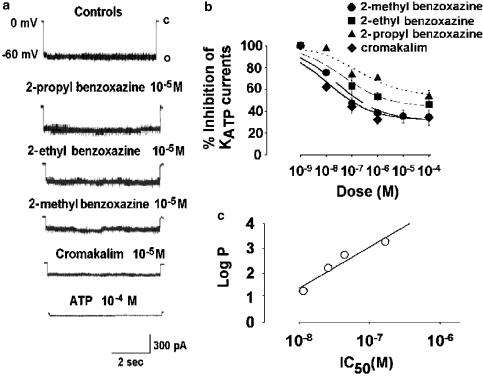
In the absence of internal ATP, the 2H-1,4-benzoxazine molecules under investigation and cromakalim all inhibited the KATP currents (Figure 3a, b).
Figure 3.
Effects of K+ channel openers on KATP channels of rat skeletal muscle fibres in the absence of internal ATP. (a) Digital average of KATP current recorded in the excised inside-out macropatches, at −60 mV (V m), with high cytosolic KCl solutions on both sides of the membrane, in the absence (controls) (n=26 macropatches), or in the presence of the 2H-1,4-benzoxazine derivatives having the methyl (n=5 macropatches), ethyl (n=4 macropatches) or propyl (n=6 macropatches) groups at position 2 on the benzoxazine ring or in the presence of cromakalim (n=7 macropatches). C and O in the traces indicate closed and open channel levels, respectively. (b) Concentration–response relations of the 2H-1,4-benzoxazine derivatives having the –CH3, –C2H5 or –C3H7 groups at position 2 of the benzoxazine ring, and of cromakalim on the KATP currents in the absence of ATP. All the compounds under investigation produced a concentration-dependent inhibition of the KATP current, (c) Log P plot versus the IC50 of the 2H-1,4-benzoxazine derivatives and cromakalim. The calculated coefficient of correlation between the log P and IC50 of the compounds under investigation was 0.8.
In the range of concentrations from 10−10 to 10−4 M, the 2H-1,4-benzoxazine derivatives and cromakalim reduced the KATP currents concentration-dependently, reaching a plateau around 10−5 M concentration (Figure 3a, b). A function describing the interaction of a ligand with one inhibitory component mediating the inhibitory response was used to fit the experimental data for all the compounds under investigation. The calculation of the fitted parameters of equation (2) showed that the order of potency of the molecules as antagonists in the absence of internal ATP, expressed as IC50, was cromakalim⩾–CH3>–C2H5>–C3H7 derivatives (Table 1).
A direct correlation was observed between the IC50 and the log P values of the 2–C3H7, 2–C2H5 and 2–CH3-2H-1,4-benzoxazine derivatives (Figure 3c; Table 1).
Differences in the maximal efficacy as antagonists were observed within the 2H-1,4-benzoxazine derivatives and cromakalim (Table 1).
Discussion
We provided evidence that molecules belonging to the class of the 2H-1,4-benzoxazine derivatives, in the presence of internal ATP, lead to significant stimulation of the muscle native KATP channels with a different degree of potency depending on the alkyl chains at position 2 of the 2H-1,4-benzoxazine nucleus. This phenomenon is consistent with the hypothesis that the increase in lipophilicity is a critical factor in determining the potency of these molecules as muscle KATP channel agonists. This is supported by the inverse correlation existing between the calculated log P values, as lipophilicity index, and the DE50 of the molecules as KATP channel agonists.
However, to evaluate the possible influence of the different degree of ionisation of the 2H-1,4-benzoxazine derivatives on the KATP channel activity, the log D values for these molecules at pH 7.0 were calculated. The fact that the calculated log D values were identical to those of log P indicates that the 2H-1,4-benzoxazine does not affect the KATP channel derivatives in their protonated forms. This is certainly because of their weak basic property as demonstrated by the findings that all the 2H-1,4-benzoxazine derivatives showed quite low calculated pKa values which were also identical for all the 2H-1,4-benzoxazine derivatives under study. This means that there is no difference in their ionisation state which can be explained with the little influence that the different alkyl groups at position 2 of the benzoxazine ring exert on the basicity of our molecules. The main basic centre is in fact represented by the nitrogen atom of the pyridine ring located at a long distance from the alkyl groups.
These considerations all together suggest that the increase in the lipophilicity favours the access of the molecule to the stimulatory site through the membrane or can permit a stronger interaction of the molecule with a hydrophobic area of the binding site. The candidate hydrophobic area where our molecules can bind is the TMDII region of the SUR (SUR2A) subunit of muscle KATP channel complex, with TMD 17 playing a prominent role in the binding and affinity of KCO to SUR (D'hahan et al., 1999; Uhde et al., 1999; Ashcroft & Gribble, 2000; Moreau et al., 2000). The hydrophobic characteristic of the TMD II region is consistent with the lipophilic properties of the benzopyran molecules and also of the benzoxazine derivatives (D'hahan et al., 1999; Babenko et al., 2000).
Another point of interest is the fact that the 2H-1,4-benzoxazine derivatives and cromakalim in the presence of internal ATP showed bell-shaped concentration–response curves stimulating the KATP channels at lower concentrations but inhibiting the channels at higher concentrations. Furthermore, in the absence of internal ATP, all KCOs tested inhibited KATP channel currents.
At least two mechanisms could explain these phenomena. Firstly, these molecules could interact with two sites mediating opposite effects, one site being a stimulatory site available for drug binding in the presence of ATP, the other being the inhibitory site which is unmasked in the absence of nucleotide. This hypothesis is supported by the fact that our molecules in the presence of ATP caused a full inhibition of channel currents at the highest concentration tested. In fact, the general idea is that the full inhibition of channels or receptors by agonists at high concentrations is evidence for the existence of two different sites mediating opposite actions (Rovati & Nicosia, 1994).
Secondly, bell-shaped concentration–response curves with reduced maximal efficacy could be the result of a partial agonist action of the molecules on a single binding site (Rovati & Nicosia, 1994). In this case, the dualistic effects of our KCO and cromakalim could be the result of ATP-regulated KCO binding, so that ATP would favour the transduction of channel opening, whereas in the absence of the nucleotide channel closure is favoured. Competition experiments performed in skeletal muscle using [3H] P1075 have shown that the binding of KCO including cromakalim to the membrane fraction is supported by nucleotides. In most cases, a 1 : 1 stoichiometry was found suggesting the existence of a single ATP-regulated site for KCO (Dickinson et al., 1997). Similar results have been obtained for the vascular SUR2B and pancreatic SUR1 subunits expressed in cell lines in which nucleotides support binding of various KCOs (Schwanstecher et al., 1998).
Although binding studies support the existence of a single site for KCOs whose binding is ATP-regulated, we believe that the existence of two different KCO sites mediating opposite effects on KATP channel still cannot be excluded. In fact, it is well known that the binding of a molecule to a receptor site is also affected by the quaternary structure of the channel in the native membrane which is conserved in patch-clamp experiments but is lost in binding experiments. This is the case of KCOs binding to the KATP channel which is an octameric association of SUR and kir subunits, in which the affinity measured by binding experiments as well as the stoichiometry of the reaction do not match those measured by patch-clamp experiments (Schwanstecher et al., 1998). In our experiments, the weak correlation observed between the log P and the potency of the 2–CH3, 2–C2H5 or 2–C3H7-2H-1,4-benzoxazine derivatives as antagonists (IC50) evaluated in the absence of ATP suggests that the lipophilicity is not pivotal in determining the inhibitory effects of KCO. This predicts that sites different from the agonist sites for KCO may be located at the interface between the hydrophilic/hydrophobic area. Furthermore, the contribution of kir6.2, the pore-forming subunit of the KATP channel complex, to the inhibitory effects of the KCO observed in our experiments cannot be excluded (D'hahan et al., 1999; Ashcroft & Gribble, 2000).
The fact that pinacidil activates or inhibits the KATP channel in skeletal muscle depending on the level of KATP channel activity, or that the anilide tertiary carbinol derivative ZD6169 shows dualistic actions on the smooth muscle and ventricular KATP channels suggests that the phenomenon that we observed is not a unique property of the cyclic KCOs but can also be extended to the open chain KCOs (Hehl & Neumcke, 1994; Teramoto et al., 2001).
The dualistic mode of action of the KCOs resembles that observed for some physiological modulators of KATP channels. In fact, ADP at lower concentrations induces stimulation of the skeletal muscle KATP channel, while at higher concentrations it inhibits the channels (Allard & Lazdunski, 1992). This phenomenon has been explained by the high affinity interaction of ADP with the second nucleotide binding fold of the SUR subunit, and with the low affinity interaction with the ATP inhibitory site located on the kir6.2 subunit of the channel.
Currently, we are searching for agonists on native skeletal muscle KATP channels showing stimulatory effects without the inhibitory component. This will be useful in selecting molecules able to restore fully the abnormally reduced skeletal muscle KATP conductance in hypoPP (Tricarico et al., 1998, 1999). An enantioselective synthesis of the optical isomers of the tested benzoxazine derivatives is also in progress to evaluate the influence of the absolute configuration on the biological activity of these drugs.
Acknowledgments
This work was supported by Italian–Telethon grant no. 1208. We thank Dr Sophie Talon for support during the experiments and Dr Giuseppe Carbonara for helpful hints for the structural characterisation of the benzoxazine derivatives.
Abbreviations
- –CH3
methyl
- –C2H5
ethyl
- –C3H7
propyl
- DMSO
dimethylsulphoxide
- FDB
flexor digitorum brevis
- KATP
ATP-sensitive K+ channel
- KCOs
potassium channel openers
- Kir6.2
inward rectifier K+ channel 6.2 subunit
- log D
logarithm of the distribution coefficient at a particular pH
- log P
logarithm of the partition coefficient
- pKa
negative logarithm of the dissociation constant
- SUR2A
sulphonylureas receptor 2A subunit
References
- ADLAR M., OKAFO G., MEENAN E., CAMILLERI P.Rapid estimation of octanol–water partition coefficients using deoxycholate micelles in capillary electrophoresis J. Chem. Soc. Chem. Commun. 19952241–2243.No. 21
- ALLARD B., LAZDUNSKI M. Nucleotides diphosphates activate the ATP-sensitive potassium channels in mouse skeletal muscle. Pflugers Arch. 1992;422:185–192. doi: 10.1007/BF00370419. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.E. Clinical pharmacology of potassium channel openers. Pharmacol. Toxicol. 1992;70:244–254. doi: 10.1111/j.1600-0773.1992.tb00466.x. [DOI] [PubMed] [Google Scholar]
- ASHCROFT F.M., GRIBBLE F.M. New windows on the mechanism of action of KATP channel openers. TiPS. 2000;21:439–445. doi: 10.1016/s0165-6147(00)01563-7. [DOI] [PubMed] [Google Scholar]
- BABENKO A.P., GONZALES G., BRYAN J. Pharmaco-topology of sulfonylurea receptors. J. Biol. Chem. 2000;275:717–720. doi: 10.1074/jbc.275.2.717. [DOI] [PubMed] [Google Scholar]
- BETTONI G., FERRORELLI S., LOIODICE F., TANGARI N., TORTORELLA V., GASPARRINI F., MISITI D., VILLANI C. Chiral α-substituted α-aryloxy acetic acids: synthesis, absolute configuration, chemical resolution and direct separation by HPLC. Chirality. 1992;4:193–203. [Google Scholar]
- BETTONI G., LOIODICE F., TORTORELLA V., CONTE-CAMERINO D., MAMBRINI M., FERRANINI E., BRYANT S.H. Stereospecificity of the chloride ion channel: the action of chiral clofibric acid analogues. J. Med. Chem. 1987;30:1267–1270. doi: 10.1021/jm00391a002. [DOI] [PubMed] [Google Scholar]
- CALIENDO G., GRIECO P., PERISSUTTI E., SANTAGATA V., SANTINI A., ALBRIZIO S., FATTORUSSO C., PINTO A., SORRENTINO R. Synthesis, biological activity and conformational study of 1,4-benzoxazine derivatives as potassium channel modulators. Eur. J. Med. Chem. 1998;33:957–967. [Google Scholar]
- D'HAHAN N., JACQUET H., MOREAU C., CATTY P., VIVAUDOU M. A transmembrane domain of the sulfonylurea receptor mediates activation of ATP-sensitive K+ channels by K+ channel openers. Mol. Pharmacol. 1999;56:308–315. doi: 10.1124/mol.56.2.308. [DOI] [PubMed] [Google Scholar]
- DICKINSON K.E.J., BRYSON C.C., COHEN R.B., ROGERS L., GREEN D.W., ATWAL K.S. Nucleotide regulation and characteristics of potassium channel opener binding to skeletal muscle membranes. Mol. Pharmacol. 1997;52:473–481. doi: 10.1124/mol.52.3.473. [DOI] [PubMed] [Google Scholar]
- FORESTIER C., PIERRARD J., VIVAUDOU M. Mechanism of action of K channel openers on skeletal muscle KATP channels. Interactions with nucleotides and protons. J. Gen. Physiol. 1996;107:489–502. doi: 10.1085/jgp.107.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRYER R.I., EARLEY J.V., FIELD G.F., ZALLY W., STERNBACH L.H. A synthesis of amidines from cyclic amides. J. Org. Chem. 1969;34:1143–1145. [Google Scholar]
- GRANT T.L., OHNMACHT C.J., HOWE B.B. Anilide tertiary carbinols: a novel series of K+ channel openers. TiPS. 1994;15:402. doi: 10.1016/0165-6147(94)90082-5. [DOI] [PubMed] [Google Scholar]
- HEHL S., NEUMCKE B. Diverse effects of pinacidil on KATP channels in mouse skeletal muscle in the presence of different nucleotides. Cardiovasc. Res. 1994;28:841–846. doi: 10.1093/cvr/28.6.841. [DOI] [PubMed] [Google Scholar]
- KABAKOV A. Activation of KATP channels by Na/K pump in isolated cardiac myocytes and giant membrane patches. Biophys. J. 1998;75:2858–2867. doi: 10.1016/S0006-3495(98)77728-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOGA H., OHTA M., SATO H., ISHIZAWA T., NABATA H. Design of potent K+ channel openers by pharmacophore model. Bioorg. Med. Chem. Lett. 1993;3:625–631. [Google Scholar]
- JOW B., NUMANN R. The effects of ZD6169 on the ATP-dependent K+ current (IKATP) in isolated cat ventricular myocytes. Eur. J. Pharmacol. 1999;383:197–202. doi: 10.1016/s0014-2999(99)00630-5. [DOI] [PubMed] [Google Scholar]
- LAWSON K. Potassium channel openers as potential therapeutic weapons in ion channel disease. Kidney Int. 2000;57:838–845. doi: 10.1046/j.1523-1755.2000.00923.x. [DOI] [PubMed] [Google Scholar]
- LONGMAN S.D., HAMILTON T.C. Potassium channel activator drugs: mechanism of action, pharmacological properties, and therapeutic potential. Med. Res., Rev. 1992;12:73–148. doi: 10.1002/med.2610120202. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO Y., TSUZUKI R., MATSUHISA A., MASUDA N., YAMAGIWA Y., YANAGISAWA I., SHIBANUMA T., NOHIRA H. Novel potassium channel activators. II1). Synthesis and pharmacological evaluation of 3,4-dihydro-2H-1,4-benzoxazine derivatives: modification of the aromatic part. Chem. Pharm. Bull. 1999;47:971–979. doi: 10.1248/cpb.47.971. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO Y., TSUZUKI R., MATSUHISA A., TAKANAMA K., YODEN T., UCHIDA W., ASANO M., FUJITA S., YANAGISAWA I., FUJIKURA T. Novel potassium channel activators: synthesis and structureactivity relationship studies of 3,4-dihydro-2H-1, 4-benzoxazine derivatives. Chem. Pharm. Bull. 1996;44:103–114. doi: 10.1248/cpb.44.103. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO Y., UCHIDA A., NAKAHARA H., YANAGISAWA I., SHIBANUMA T., NOHIRA H. Novel potassium channel activators. III. Synthesis and pharmacological evaluation of 3,4-dihydro-2H-1,4-benzoxazine derivatives: modification at the position 2. Chem. Pharm. Bull. 2000;48:428–432. doi: 10.1248/cpb.48.428. [DOI] [PubMed] [Google Scholar]
- MOREAU C., JACQUET H., PROST Al., D'HAHAN N., VIVAUDOU M. The molecular basis of the specificity of action of KATP channel openers. EMBO J. 2000;19:6644–6651. doi: 10.1093/emboj/19.24.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUASTHOFF S., SPULER A., SPITTELMEISTER W., LEHMANN-HORN F., GRAFE P. K+ channel openers suppress myotonic activity of human skeletal muscle in vitro. Eur. J. Pharmacol. 1990;186:125–128. doi: 10.1016/0014-2999(90)94068-9. [DOI] [PubMed] [Google Scholar]
- ROVATI E.G., NICOSIA S. Lower efficacy: interaction with an inhibitory receptor or partial agonism. TiPS. 1994;15:141–144. doi: 10.1016/0165-6147(94)90073-6. [DOI] [PubMed] [Google Scholar]
- SCHWANSTECHER M., SIEVERDING C., DORSCHNER H., GROSS I., AGUILAR-BRYAN L., SCHWANSTECHER C., BRYAN J. Potassium channel openers require ATP to bind to and act through sulfonylurea receptors. EMBO J. 1998;17:5529. doi: 10.1093/emboj/17.19.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEEBOHM G., LERCHE C., PUSCH M., STEINMEYER K., BRUGGEMANN A., BUSCH A.E. A kinetic study on the stereospecific inhibition of KCNQ1 and Iks by the chromanol 293B. Br. J. Pharmacol. 2001;134:1647–1654. doi: 10.1038/sj.bjp.0704421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONG D.K., ASHCROFT F.M. ATP-modulation of K(subATP) channel ATP-sensitivity varies with the type of SUR subunit. J. Biol. Chem. 2001;276:7143–7149. doi: 10.1074/jbc.M009959200. [DOI] [PubMed] [Google Scholar]
- TERAMOTO N., YUNOKI T., TAKANO M., YONEMITSU Y., MASAKI I., SUEISHI K., BRADING A.F., ITO Y. Dual action of ZD6169, a novel K(+) channel opener, on ATP-sensitive K(+) channels in pig urethral myocytes. Br. J. Pharmacol. 2001;133:154–164. doi: 10.1038/sj.bjp.0704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRICARICO D., PIERNO S., MALLAMACI R., SIRO BRIGIANI G., CAPRIULO R., SANTORO G., CONTE CAMERINO D. The biophysical and pharmacological characteristics of skeletal muscle KATP channels are modified in K+ depleted rats, an animal model of hypokalemic periodic paralysis. Mol. Pharmacol. 1998;54:197–206. doi: 10.1124/mol.54.1.197. [DOI] [PubMed] [Google Scholar]
- TRICARICO D., SERVIDEI S., TONALI P., JURKAT-ROTT K., CONTE CAMERINO D. Impairment of skeletal muscle adenosine triphosphate-sensitive K+ channels in patients with hypokalemic periodic paralysis. J. Clin. Invest. 1999;103:675–682. doi: 10.1172/JCI4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UHDE I., TOMAN A., GROSS I., SCHWANSTECHER C., SCHWANSTECHER M. Identification of the potassium channel opener site on sulfonylurea receptors. J. Biol. Chem. 1999;274:28079–28082. doi: 10.1074/jbc.274.40.28079. [DOI] [PubMed] [Google Scholar]