Abstract
This study examines the involvement of kinins in neutrophil migration into rat subcutaneous air pouches triggered by lipopolysaccharide (LPS), as well as the putative roles played by kinin B1 and B2 receptors, tumour necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β) and selectins in this response.
LPS (5 ng to 10 μg cavity−1) injected into the 6-day-old pouch induced a dose- and time-dependent neutrophil migration which peaked between 4 and 6 h, and was maximal following the dose of 100 ng cavity−1 (saline: 0.46±0.1; LPS: 43±3.70 × 106 cells cavity−1 at 6 h).
Bradykinin (BK) (600 nmol) injected into the pouch of saline-treated rats induced only modest neutrophil migration (0.73±0.16 × 106 cells cavity−1). A more robust response to BK (3.2±0.25 × 106 cells cavity−1) was seen in animals pretreated with captopril, but this was still smaller than the responses to IL-1β or TNF-α (15 pmol: 23±2.2 × 106 and 75 pmol: 29.5±2 × 106 cells cavity−1, respectively). Nevertheless, the B1 agonist des-Arg9-BK (600 nmol) failed to induce neutrophil migration.
HOE-140 (1 and 2 mg kg−1), a B2 receptor antagonist, reduced LPS-induced neutrophil migration. HOE-140 also reduced the neutrophil migration induced by BK, but had no effect on the migration promoted by IL-1β or TNF-α. des-Arg9-[Leu8]-BK, B1 receptor antagonist was ineffective in changing neutrophil migration caused by any of these stimuli.
Neutrophil migration induced by LPS or BK was reduced by interleukin-1 receptor antagonist (IL-1ra) (1 mg kg−1), sheep anti-rat TNF serum (anti-TNF serum) (0.3 ml cavity−1), and the nonspecific selectin inhibitor fucoidin (10 mg kg−1).
TNF-α levels in the pouch fluid were increased by LPS or BK injection, peaking at 0.5–1 h and gradually declining thereafter up to 6 h. IL-1β levels increased steadily throughout the 6 h period. HOE-140 markedly inhibited the rise in IL-1β and TNF-α levels in pouch fluid triggered by both stimuli.
These results indicate that BK participates importantly in selectin-dependent neutrophil migration into the air pouch triggered by LPS in the rat, by stimulating B2 receptors coupled to synthesis/release of TNF-α and IL-1β.
Keywords: Air pouch, LPS, kinins, B1 and B2 kinin receptors, IL-1β, TNF-α, selectins, neutrophil migration
Introduction
Kinins mediate their biological actions via stimulation of two types of membrane G protein-coupled receptors, named B1 and B2 (Regoli & Barabé, 1980). The B2 receptors displaying high affinity for the agonists bradykinin (BK) and Lys-BK are constitutively expressed on the membranes of many cells in both the periphery and in the central nervous system, and can be blocked, potently, selectively and competitively by several antagonists including HOE-140 and NPC 17731 (Hall, 1992 for review). On the other hand, B1 receptors are usually absent or largely underexpressed in most tissues in normal conditions, with rare exceptions, but their expression can be induced or increased by treatment with lipopolysaccharide (LPS) or cytokines, or following adrenalectomy (Campos et al., 1996;1998; Pesquero et al., 1996; Cabrini et al., 2001). B1 receptors exhibit high affinity for the agonists des-Arg9-BK (DABK) and des-Arg10-Lys-BK, which are metabolites of BK and Lys-BK, respectively, and can be selectively antagonised by the B1 receptor antagonists, des-Arg9-[Leu8]-BK (DALBK) and des-Arg10-[Leu8]-Lys-BK. Most of the physiological or pathological effects of kinins result from their ability to stimulate neuropeptide release from sensory nerve fibres (Geppetti, 1993; Ahluwalia & Perretti, 1996), synthesis of cytokines (Tiffany & Burch, 1989; Pang & Knox, 1998), prostaglandins, leucotrienes (Sakamoto et al., 1994) and nitric oxide (Volpe et al., 1996).
Kinins are responsible for many signs and symptoms of the inflammatory response including pain/hyperalgesia, vasodilatation, contraction of venular endothelial cells promoting the extravasation of plasma proteins and fluids (Calixto et al., 2000 for review). These peptides can also affect leucocyte migration. For example, injection of BK into the pleural cavity of naive mice (Saleh et al., 1997) or of antigen into that of previously sensitised rats (Bandeira-Melo et al., 1999) triggers local neutrophil accumulation amenable to inhibition by B2 receptor antagonists. In mice, DABK evokes B1 receptor-mediated neutrophil accumulation in the pleural cavity of naive animals (Vianna & Calixto, 1998), as well as trafficking of these cells across mesenteric postcapillary venules following priming of the peritoneal cavity with interleukin-1 beta (IL-1β) (McLean et al., 2000).
Another interesting model to study inflammation is that of the air pouch (Edwards et al., 1981), an experimentally formed cavity induced by injection of sterile air into the dorsum of the animal. After 6 days, the air pouch shows a well-defined lining structure constituted largely of fibroblasts, about 10–40% of esterase positive macrophage-like cells and a few mast cells, which enables the subcutaneous administration of substances that induce or modulate local inflammatory responses, and is readily accessible for sample collection and evaluation of cellular content. Injections of carrageenan and kaolin into the air pouch induce marked and parallel increases in both the levels of BK in the pouch fluid and in vascular permeability of the cavity's subcutaneous borders (Barlas et al., 1985; Kumakura et al., 1987;1988a; Tsurufuji & Kumakura, 1989). Injection of IL-1β into the mouse air pouch induces neutrophil accumulation largely mediated by B1 (but not B2) receptors, as well as unveils significant chemotactic activity of the B1 agonist DABK towards these cells (Ahluwalia & Perretti, 1996). Nonetheless, no evidence has been provided so far as to the role(s) played by kinins and their receptors on the neutrophil accumulation evoked by an exogenous inflammatory stimulus in the air pouch, such as LPS.
In light of such considerations, the present study investigates the possible role(s) played by kinins and both their receptor types in neutrophil migration induced by LPS in the 6-day-old air pouch model in the rat. In addition, the contribution of the proinflammatory cytokines, tumour necrosis factor alpha (TNF-α) and IL-1β, as well as of cell adhesion selectin molecules in this response was also assessed.
Methods
Animals
The experiments were conducted on male Wistar rats weighing 180–200 g, housed at 24±1°C under a 12 : 12 h light–dark cycle (lights on at 07:00 h), with free access to food and tap water and carried out between 13:00 and 17:00 h. Animal housing conditions and all experimental procedures were previously approved by the Ethics Committee on Use of Animals in Research of the University of São Paulo, and were carried out at this institution in accordance with the European Community laws governing the use of experimental animals.
Induction of air pouches and determination of stimulus dose
Subcutaneous air pouches were formed according to the method described by Edwards et al. (1981). Under ether anaesthesia and after shaving the dorsum of each animal, 20 ml of sterile air was injected into the subcutaneous tissue of the dorsal midline, caudal to the scapulae. On day 3, pouches were reinflated with 10 ml of sterile air to maintain patency of the cavity. On day 6, LPS (5, 10 or 100 ng, or 1, 5 or 10 μg), BK (60, 200 and 600 nmol), DABK (600 nmol), rat recombinant IL-1β and TNF-α (1.5–150 pmol), all diluted in 1 ml of sterile saline (0.9% NaCl) were injected into the pouches. Control animals were treated identically with vehicle alone.
Cell collection, total and differential cell counts
The animals were anaesthetised with ether and killed by exsanguination 2–24 h after injection of LPS in the time-course study, or at 6 h in the remaining experiments. Pouches were washed with 10 ml of PBS, pH 7.4 (composition, mmol l−1: NaCl 136.9, KCI 2.7, Na2HPO4 9, KH2PO4 1.47) containing 5 U ml−1 of heparin and 5 ml samples were withdrawn for cell counts. Total and differential cell counts were performed as described by Souza & Ferreira (1985).
Experimental protocols
Selective bradykinin B1 (DALBK) or B2 (HOE-140) receptor antagonists were injected subcutaneously (s.c., at a site distant from the air pouch) at doses of 0.5 up to 2 mg kg−1, 30 min before the intrapouch injection of one of the following stimuli: LPS (100 ng), BK (600 nmol), IL-1β (15 pmol), TNF-α (75 pmol), or the corresponding vehicle. The IL-1 receptor antagonist (interleukin-1 receptor antagonist (IL-1ra), 1 mg kg−1) was initially coinjected into the pouches together with LPS or BK, followed 1 h later by a second injection of the compound alone. Undiluted sheep anti-rat TNF serum (anti-TNF, 0.3 ml cavity−1) was coadministered with either LPS or BK. Fucoidin was injected intravenously at a dose of 10 mg kg−1, 15 min prior to LPS or BK. To minimise kinin degradation, 5 mg kg−1 of captopril, a kininase II inhibitor (Corrêa & Calixto, 1993; Damas et al., 1996) was injected s.c. 1 h before BK or DABK. Aiming to examine the role of endogenous kinins in the neutrophil migration to LPS, a set of animals was pre treated with captopril before this stimulus. Cell collection for total and differential cell counts was usually carried out 6 h after injection of the inflammatory stimuli, except for the experiments evaluating the time-course for neutrophil migration.
Quantitative determination of IL-1β and TNF-α concentrations
The pouch fluid for IL-1β and TNF-α determination was collected at 0.5, 1, 3 and 6 h after LPS or BK injection in animals pretreated (30 min) with HOE-140 (1 mg kg−1) or saline. The cytokine concentrations in each sample (those obtained from LPS-injected animals were always diluted two-fold) were determined by the quantitative sandwich enzyme immunoassay technique (ELISA) using the Quantikine® M kits for rats (R&D Systems, U.S.A.) following the manufacturer's instructions. The limits of detection for IL-1β and TNF-α were 62.5 and 12.5 pg ml−1, respectively.
Drugs
The drugs used were: bacterial lipopolysaccharide (LPS, E. coli serotype 0111:B4, Lot 2630), bradykinin (BK), des-Arg9-BK (DABK), des-Arg9-[Leu8]-BK (DALBK), fucoidin and captopril (all from Sigma Chemicals Co., St Louis, MO, U.S.A.). HOE-140 (D-Arg°-Hyp3-Thi5-DTic7-Oic8-BK) was kindly supplied by Hoechst Marion Roussel, São Paulo, Brazil. Rat recombinant IL-1β, rat recombinant TNF-α, human recombinant interleukin-1 receptor antagonist (IL-1ra) and sheep anti-rat TNF serum (anti-TNF) were kindly provided by Dr S. Poole, NIBSC, Hertfordshire, U.K.
Statistical analysis
Values are reported as means±standard error of the means (s.e. mean) of the number of neutrophils or concentrations of IL-1β and TNF-α present in the air pouches after injection of the stimuli. Statistical significance was assessed by unpaired Student's t-test for cytokine assays and analysis of variance (ANOVA) followed by the Scheffé's test for multiple comparisons for neutrophil migration. The level of significance was set at P<0.05.
Results
Neutrophil migration into subcutaneous air pouches induced by LPS, BK, DABK, IL-1β and TNF-α
In a preliminary set of experiments, we assessed the dose–response curve for the neutrophil-recruiting effect of LPS, injected into the pouches in doses ranging between 5 ng and 10 μg cavity−1. At the doses of 5 ng and 100 ng, LPS enhanced neutrophil counts 6 h after injection from 0.46±0.10 (vehicle control, n=5) to 21.6±1.9 (n=8) and 43±3.7 × 106 cells cavity−1 (n=9), respectively. Doses of LPS in excess of 100 ng cavity−1 failed to cause any additional increases in neutrophil influx (1 μg: 37±3.1, n=7; 5 μg: 45.6±4.2, n=5; 10 μg: 37.2±3.7 × 106 cells cavity−1, n=9). Therefore, the dose of 100 ng was selected for all the subsequent experiments in which LPS was used.
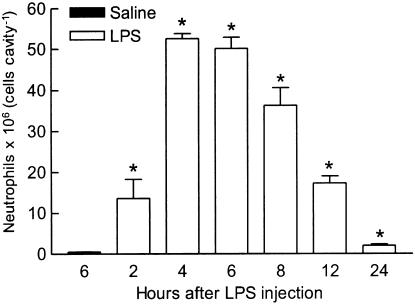
Figure 1 shows that the intrapouch injection of 100 ng of LPS induced a significant and time-dependent neutrophil migration, which started at 2 h, reached a maximum between 4 and 6 h, and gradually subsided thereafter, to attain near control levels by 24 h. Thus, cell collection was performed 6 h after injection of the stimuli in all further experiments. In sharp contrast, the number of neutrophils in pouches injected with saline was minimal at all times studied (near 0.5 × 106 cells cavity−1), as illustrated in Figure 1 and others.
Figure 1.
Time-course of neutrophil migration induced by LPS, 100 ng cavity−1, injected in a volume of 1 ml into the rat air pouch. Control animals received the same volume of sterile saline (only value for 6 h is shown in figure). The bars represent the mean±s.e. mean number of neutrophils (6–9 animals) present in pouch fluid collected at the times indicated. *Significantly different from control animals, P<0.05, Scheffé's test.
In untreated animals (i.e. rats that were not treated with captopril), 600 nmol of BK produced only a modest neutrophil migration (0.73±0.16 × 106 cells cavity−1, n=9). In captopril-treated animals, however, this effect of BK was markedly potentiated, so that doses of 60, 200 and 600 nmol cavity−1 of the peptide stimulated dose–dependent neutrophil accumulation to values of 0.78±0.2, n=6; 1.35±0.16, n=9 and 3.2±0.25 × 106 cells cavity−1, n=9, respectively. The highest dose of BK (600 nmol cavity−1) was chosen for all further experiments. Doses of BK lower than 60 nmol cavity−1 induced negligible neutrophil migration, even in captopril-treated rats (for instance, 10 nmol: 0.2±0.1 × 106 cells cavity−1, n=9). As seen with BK, captopril pretreatment also potentiated the neutrophil migration induced by 100 ng cavity−1 of LPS (saline: 34.4±1.7, n=6; captopril: 41.6±1.6 × 106 cells cavity−1, n=6, 6 h, P<0.05). Conversely, DABK at doses of 200 and 600 nmol failed to induce significant neutrophil migration in captopril-treated animals (0.5±0.1, n=7, and 0.3±0.05 × 106 cells cavity−1, n=9, respectively).
Dose–response curves for induction of neutrophil migration were also established for IL-1β and TNF-α in the pouches of rats not treated with captopril. As IL-1β yielded a bell-shaped curve (1.5 pmol: 15±2.4, n=5; 15 pmol: 24±2.2, n=7; 45 pmol: 22.7±1.7, n=9; 150 pmol: 11.2±1.2 × 106 cells cavity−1, n=7), the maximally effective dose of 15 pmol was selected for the subsequent experiments in which this cytokine was employed. The curve for TNF-α-induced neutrophil migration was monophasic (1.5 pmol: 6.7±1.1, n=5; 15 pmol: 24±2.5, n=9; 75 pmol: 29.5±2 × 106 cells cavity−1, n=7). Although no clear-cut maximal effect was detected, we chose the dose of 75 pmol of TNF-α for subsequent experiments with this cytokine.
Effect of B1 and B2 kinin receptor antagonists on LPS, BK, TNF-α and IL-1β-induced neutrophil migration to subcutaneous air pouches
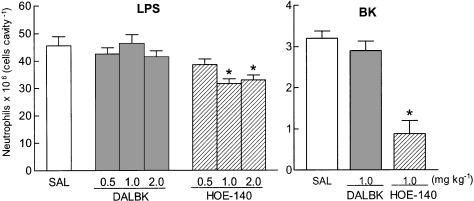
Figure 2 (left panel) shows that subcutaneous injection of the B2-selective receptor antagonist, HOE-140, 30 min beforehand, reduced LPS-induced neutrophil migration significantly at either 1 or 2 mg kg−1 (but not at 0.5 mg kg−1). Neutrophil migration was inhibited by 36% relative to vehicle-pre-treated LPS-treated controls following 1 mg kg−1 of HOE-140, and the higher dose did not cause any additional inhibitory effect. This B2-selective receptor antagonist (at 1 mg kg−1, s.c.) also markedly reduced (by 70%) the neutrophil migration induced by BK (600 nmol, Figure 2, right panel). In sharp contrast, identical treatment with the B1-selective receptor antagonist, DALBK (0.5–2 mg kg−1 s.c., 30 min beforehand), did not significantly change the pattern of neutrophil influx induced either by LPS (Figure 2, left panel) or BK (Figure 2, right panel). The antagonistic action of DALBK was confirmed by a parallel positive control experiment in which fever induced by intracerebroventricular (i.c.v.) injection of 10 nmol of BK was significantly reduced (from 0.9±0.1, n=5 to 0.4±0.02°C, n=6, 3 h after BK injection) in rats treated intravenously (i.v.) with DALBK (1 mg kg−1, 30 min beforehand).
Figure 2.
Effect of DALBK and HOE-140 on neutrophil migration induced by injection of LPS (left panel) or BK (right panel) into the rat air pouch. DALBK and HOE-140 were injected subcutaneously (s.c.), at doses indicated, 30 min before LPS (100 ng cavity−1) or BK (600 nmol cavity−1). Control animals received s.c. injection of sterile saline (SAL). Captopril (5 mg kg−1, s.c.) was given 1 h before BK as stimulus in the pouch. The bars represent the mean±s.e. mean number of neutrophils (6–9 animals) detected in pouch fluid collected 6 h after LPS or BK. *Significantly different from saline-treated animals (SAL), P<0.05, Scheffé's test.
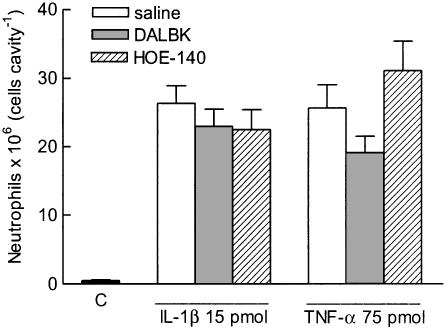
Figure 3 shows that prior treatment with either DALBK or with HOE-140 (each at 1 mg kg−1 s.c., 30 min beforehand) did not significantly modify neutrophil migration induced by IL-1β (15 pmol) or TNF-α (75 pmol).
Figure 3.
Effect of DALBK or HOE-140 on neutrophil migration induced by IL-1β or TNF-α into the rat air pouch. DALBK or HOE-140, each at 1 mg kg−1, was injected subcutaneously (s.c.) 30 min before IL-1β or TNF-α. The two control groups received either sterile saline alone into the pouch (C) or were pretreated s.c. with sterile saline instead of B1 or B2 receptor antagonist. The bars represent the means±s.e. mean number of neutrophils (6–9 animals) detected in pouch fluid collected 6 h after IL-1β or TNF-α. All values of cytokine-treated groups differed from C, P<0.05, Scheffé's test.
Effect of IL-1ra, anti-TNF serum and fucoidin on neutrophil migration induced by LPS and BK
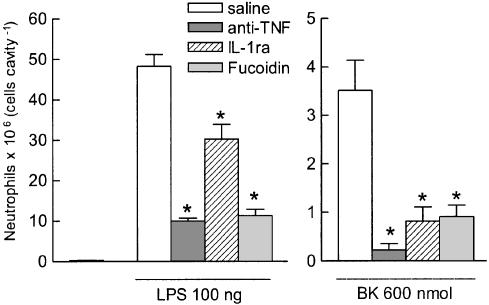
The neutrophil migration induced by LPS or by BK was considerably reduced (by 37 and 77%, respectively) by coadministration of IL-1ra (1 mg kg−1) together with either stimulus, followed by another injection of the same dose 1 h later (Figure 4, left and right panels, respectively). Coinjection of anti-TNF (0.3 ml cavity−1) was considerably more effective than IL-1ra in reducing neutrophil migration triggered by these stimuli, having reduced the effect of LPS by 80% and almost abolished (93% reduction) that caused by BK (Figure 4, left and right panels). Fucoidin (10 mg kg−1, i.v. 15 min beforehand) was also effective in reducing the neutrophil migration caused by either LPS (79% reduction, right panel) or BK (74% reduction, left panel).
Figure 4.
Effect of IL-1 receptor antagonist (IL-1ra), sheep anti-rat TNF serum (anti-TNF) or fucoidin on neutrophil migration induced by LPS (left panel) or BK (right panel) into the rat air pouch. IL-1ra (1 mg kg−1) or anti-TNF (0.3 ml cavity−1) was coinjected with the stimuli into the air pouches. An additional and similar dose of IL-1ra was also given 1 h later in that particular group. Fucoidin (10 mg kg−1, i.v.) was injected 15 min before the stimuli. Control animals received sterile saline instead of IL-1ra, anti-TNF or fucoidin. Animals that received BK as a stimulus were pretreated with captopril (5 mg kg−1, s.c., 1 h beforehand). Bars represent the mean±s.e. mean number of neutrophils (6–9 animals) detected in pouch fluid collected 6 h after LPS or BK. *Significantly different from saline treated animals, P<0.05, Scheffé's test.
Effect of HOE-140 on the levels of IL-1β and TNF-α in pouches stimulated with either LPS or BK
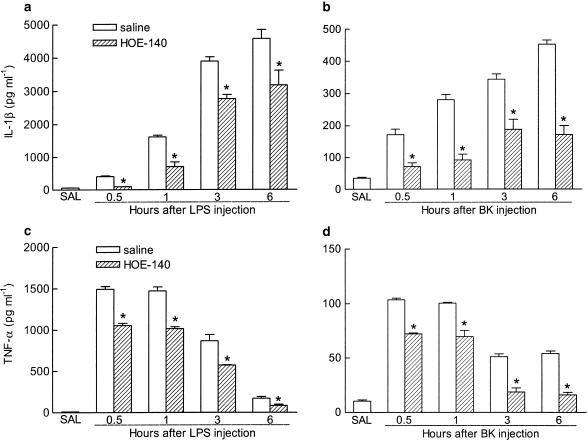
Another series of experiments was carried out to correlate neutrophil migration induced by either LPS or BK with the levels of IL-1β and TNF-α in the pouch fluid of rats pretreated with HOE-140 (1 mg kg−1, s.c.) or vehicle. To this effect, pouch fluids were collected 6 h after saline injection or 0.5, 1, 3 and 6 h after intrapouch injection of either LPS (100 ng) or BK (600 nmol). In saline-injected pouches, the levels of TNF-α and IL-1β were close to the detection limit (Figure 5). At all times studied, LPS induced a more prominent (between 10- and 15-fold greater) increase of TNF-α and IL-1β levels in the pouch fluid (Figure 5, panels a and c, respectively) than BK (Figure 5, panels b and d, respectively). For both stimuli, the levels of IL-1β rose steadily throughout the observation period reaching the maximum up to 6 h after administration. Conversely, TNF-α levels in pouch fluid increased very rapidly after either LPS or BK, reaching peak values at 0.5–1 h after injection, which decreased progressively thereafter. The pretreatment of rats with HOE-140 markedly reduced the levels of both IL-1β and TNF-α in the pouch fluid in response to LPS or BK at all time points examined (Figure 5).
Figure 5.
Effect of HOE-140 on the levels of IL-1β and TNF-α in the fluid from rat air pouch injected with either LPS or BK. HOE-140 (1 mg kg−1) was injected subcutaneously (s.c.) 30 min before LPS (100 ng cavity−1, panels a and c) or BK (600 nmol cavity−1, panels b and d). The appropriate control groups received sterile saline either s.c. (instead of HOE-140) or into the pouch (SAL; instead of LPS or BK), and the fluid was collected 6 h later. Animals that received BK as the stimulus were pretreated with captopril (5 mg kg−1, s.c., 1 h beforehand). The bars represent the means±s.e. mean of duplicates for each animal (n=4). *Significantly different from saline treated animals, P<0.05, Student's t-test.
Discussion
Confirming and extending previous studies (Graham et al., 1965; Pasquale et al., 1991; Ahluwalia & Perretti, 1996; Saleh et al., 1997; Vianna & Calixto, 1998; Lo et al., 1999; Campos et al., 2002), the results obtained in the present study show that endogenous kinins exert an important role in the neutrophil migration to inflammatory sites.
Although not as effective as LPS, IL-1β or TNF-α, BK induced dose-dependent neutrophil migration into the air pouch of rats previously treated with captopril. However, in animals that were not treated with captopril, 600 nmol of BK triggered a modest neutrophil migration. Since BK is quickly metabolised in vivo (Erdös, 1990; Kumakura et al., 1988b) and neutrophil migration is such a slowly developing and long-lasting phenomenon, it is possible that the ability of a single bolus injection of BK to recruit neutrophils into the air pouch is far more limited than that displayed by endogenous BK generated over a broader time period in response to an inflammatory stimulus. Supporting this hypothesis, HOE-140, a potent and long-acting B2 receptor antagonist (Wirth et al., 1991), reduced the neutrophil migration induced by LPS in captopril-untreated animals and, importantly, also inhibited substantially that caused by BK in captopril-treated animals. Moreover, captopril potentiated the ability of LPS to trigger neutrophil migration, further strengthening the role of endogenous kinins in bringing about this effect of LPS. By inhibiting kininase II, captopril also blocks the generation of angiotensin II. It is, however, unlikely that this action underlies its ability to potentiate LPS-induced neutrophil migration as angiotensin II has been found to stimulate, rather than suppress, neutrophil migration in vivo and in vitro (Elferink & Koster, 1997; Piqueras et al., 2000).
Unlike HOE-140, the selective and competitive B1 receptor antagonist, DALBK, failed to alter neutrophil counts in the fluid of pouches stimulated with LPS or BK. This apparent lack of involvement of B1 receptor-mediated mechanisms in LPS-induced neutrophil migration is further substantiated by our finding that DABK, a specific B1 receptor agonist, failed to induce neutrophil accumulation in the air pouch. DABK is also ineffective in promoting neutrophil influx into the naive mouse air pouch (Ahluwalia & Perretti, 1996), and migration of human neutrophils in vitro, even though these later cells where modestly responsive to BK in a HOE-140-inhibitable fashion (Böckmann & Paegelow, 2000). On the other hand, we also found that both kinin B2 and B1 receptor antagonists were completely ineffective in changing neutrophil influx caused by IL-1β or TNF-α into the rat air pouch, at least up to 6 h after cytokine injection. The lack of effect of DALBK against IL-1β-induced cell migration in the rat air pouch is at variance with the significant inhibitory effect of this B1 receptor antagonist against neutrophil accumulation triggered by injection of this cytokine into the mouse air pouch (Ahluwalia & Perretti, 1996). This difference between species appears to be restricted to this particular model, as DABK induces significant neutrophil migration when injected into the pleural cavity of both rats (saline: 0.24±0.08; DABK: 2.3±0.3 × 106 cells cavity−1, n=7 for each group; unplublished results) and mice (Vianna & Calixto, 1998). Moreover, we have reported that subcutaneous IL-1β rapidly enhances the expression of B1 mRNA in the rat paw (Campos et al., 2002), an effect that was fully dependent on neutrophil migration, but which fully subsided within 6 h after injection. Finally, the efficacy of the DALBK sample used in the current study to block B1 receptors was confirmed by the finding that, given i.c.v. to rats, this antagonist markedly reduced the fever induced by BK (data described in Results, manuscript in preparation). At present, it is unclear why B1 receptors are implicated in neutrophil migration into the rat pleural cavity and paw skin, but not into the air pouch. Perhaps the macrophage-like cells lining the 6-day-old air pouch are indeed macrophages still at an earlier stage of differentiation/maturation than those resident in pleural lining or skin, and thus differ in their pattern of receptor expression and responsiveness to cytokines and other inflammatory stimuli (for a review see Böckmann & Paegelow, 2000).
Our results allow us to postulate that BK might be positioned upstream to the cascade of cytokines involved in leucocyte migration into LPS-inflamed pouches. In fact, IL-1ra significantly reduced neutrophil migration in response to both LPS and BK, while anti-TNF reduced the response induced by LPS, and almost abolished that induced by BK. These findings are also strengthened by previous studies showing that cytokines exert a critical role in inflammatory responses to both LPS and BK (Ferreira et al., 1993a,1993b). Likewise, our demonstration that HOE-140 significantly decreased the levels of either IL-1β or TNF-α in LPS- and BK-treated pouch fluid further highlights the importance of B2 receptor activation in the release of cytokines and chemotactic substances by a variety of cell types (Koyama et al., 1998;2000; Sato et al., 1996;2000). It has been reported that LPS is undetectable in plasma up to 8 h after its injection into the rat air pouch, but is present in significant amounts at least up to 2 h after its intraperitoneal injection (Cartmell et al., 2001). Thus, it seems to pertinent to speculate that, most likely, LPS stimulates kinin generation from within the air pouch itself.
The 10- to 15-fold greater capability of LPS in increasing TNF-α and IL-1β levels in the pouch fluid, as compared to BK, may be ascribed to more efficient coupling of the former stimulus to mechanisms that amplify the cascade of mediators involved in cell migration. This view is supported by the greater number of neutrophils mobilised by LPS, in comparison to BK. On the other hand, the prompt increase in TNF-α levels (peak at 0.5 h) and the slow increase in those of IL-1β (peak at 3 h) in the pouch fluid following LPS have also been shown previously by Miller et al. (1997).
Another important result of the present study was that fucoidin, a non-selective selectin inhibitor, reduced substantially the neutrophil migration induced by either BK or LPS. TNF-α and IL-1β can both induce the expression of cell adhesion molecules (Panés et al., 1999for review). Selectins in particular are critical for the rolling and adhesion of circulating neutrophils on endothelial cells of vessels in the vicinity of inflammatory sites (Mansson et al., 2000; Campos et al., 2002). Thus, it is likely that BK, acting through B2 receptors subtype, promotes synthesis of IL-1β and TNF-α, which, in turn, activate the selectins.
In conclusion, the present study indicates that BK, acting on B2 (but not B1) receptors, is critically involved in the neutrophil migration induced by LPS, when assessed in the 6-day-old rat air pouch. This B2 receptor-mediated action of endogenous BK seems to rely on the induction of synthesis and/or release of TNF-α and IL-1β, and selectin activation.
Acknowledgments
We gratefully acknowledge the kind gift of rat recombinant IL-1β and TNF-α, human recombinant interleukin-1 receptor antagonist (IL-1ra) and sheep anti-rat TNF serum (anti-TNF) from Professor S. Poole (National Institute of Biological and Standards and Control, Hertfordshire, U.K.) and the gift of HOE-140 from Hoechst Marrion Roussel, São Paulo, Brazil. Thanks are also due to Mrs M.C.C. Melo, Mrs J.A. Vercesi, Mr C.A.A. Silva and Mr R.C. Penatti for technical assistance. We thank the helpful comments and the linguistic revision of the manuscript by Professor Giles A. Rae (Department of Pharmacology, Centre of Biological Sciences, UFSC, Florianópolis, SC, Brazil). This work was supported by a grant from FAPESP (97/09837-6) and by CNPq. D.R. Santos is a PhD student receiving a fellowship from FAPESP.
Abbreviations
- anti-TNF
sheep anti-rat TNF serum
- BK
bradykinin
- DABK
des-Arg9-BK
- DALBK
Des-Arg9-[Leu8]-BK
- i.c.v.
intracerebroventricular
- IL-1β
interleukin-1 beta
- IL-1ra
interleukin-1 receptor antagonist
- i.v.
intravenously
- LPS
lipopolysaccharide
- PBS
phosphate-buffered saline
- s.c.
subcutaneously
- TNF-α
tumour necrosis factor alpha
References
- AHLUWALIA A., PERRETTI M. Involvement of bradykinin B1 receptors in the polymorphonuclear leukocyte accumulation induced by IL-1βin vivo in the mouse. J. Immunol. 1996;156:269–274. [PubMed] [Google Scholar]
- BANDEIRA-MELO C., CALHEIROS A.S., SILVA P.M., CORDEIRO R.S., TEIXEIRA M.M., MARTINS M.A. Suppressive effect of distinct bradykinin B2 receptor antagonists on allergen-evoked exudation and leukocyte infiltration in sensitized rats. Br. J. Pharmacol. 1999;127:315–320. doi: 10.1038/sj.bjp.0702536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLAS A., SUGIO K., GREENBAUM L.M. Release of T-kinin and bradykinin in carrageenin induced inflammation in the rat. FEBS Lett. 1985;190:268–270. doi: 10.1016/0014-5793(85)81297-7. [DOI] [PubMed] [Google Scholar]
- BÖCKMANN S., PAEGELOW I. Kinins and kinin receptors: importance for the activation of leukocytes. J. Leukoc. Biol. 2000;68:587–592. [PubMed] [Google Scholar]
- CABRINI D.A., CAMPOS M.M., TRATSK K.S., MERINO V.F., SILVA J.A., Jr, SOUZA G.E.P., AVELLAR M.C., PESQUERO J.B., CALIXTO J.B. Molecular and pharmacological evidence for modulation of kinin B(1) receptor expression by endogenous glucocorticoids hormones in rats. Br. J. Pharmacol. 2001;132:567–577. doi: 10.1038/sj.bjp.0703846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALIXTO J.B., CABRINI D.A., FERREIRA J., CAMPOS M.M. Kinins in pain and inflammation. Pain. 2000;87:1–5. doi: 10.1016/S0304-3959(00)00335-3. [DOI] [PubMed] [Google Scholar]
- CAMPOS M.M., SOUZA G.E.P., CALIXTO J.B. Upregulation of B1 receptor expression mediating des-Arg9-BK-induced rat paw oedema by systemic treatment with bacterial endotoxin. Br. J. Pharmacol. 1996;117:793–798. doi: 10.1111/j.1476-5381.1996.tb15262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPOS M.M., SOUZA G.E.P., CALIXTO J.B. Modulation of kinin B1 but not B2 receptor-mediated rat paw edema by IL-1β and TNFα. Peptides. 1998;19:1269–1276. doi: 10.1016/s0196-9781(98)00087-4. [DOI] [PubMed] [Google Scholar]
- CAMPOS M.M., SOUZA G.E.P., RICCI N.D., PESQUERO J.L., TEIXEIRA M.M., CALIXTO J.B. The role of migrating leukocytes in IL-1β-induced up-regulation of kinin B1 receptors in rats. Br. J. Pharmacol. 2002;135:1107–1114. doi: 10.1038/sj.bjp.0704488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARTMELL T., LUHESHI G.N., HOPKINS S.J., ROTHWELL N.R., POOLE S. Role of endogenous interleukin-1 receptor antagonist in regulating fever induced by localised inflammation in the rat. J. Physiol. 2001;531:171–180. doi: 10.1111/j.1469-7793.2001.0171j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORRÊA C.R., CALIXTO J.B. Evidence for participation of B1 and B2 kinin receptors in formalin-induced nociceptive response in the mouse. Br. J. Pharmacol. 1993;110:193–198. doi: 10.1111/j.1476-5381.1993.tb13791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAMAS J., LIEGEOIS J.F., SIMMONS W.H. Potentiation of the pro-inflammatory effects of bradykinin by inhibition of angiotensin-converting enzyme and aminopeptidase P in rat paws. Naunyn Schmiedebergs Arch. Pharmacol. 1996;354:670–676. doi: 10.1007/BF00170844. [DOI] [PubMed] [Google Scholar]
- EDWARDS J.C.W., SEDGWICK A.D., WILLOUGHBY D.A. The formation of a structure with the features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system. J. Pathol. 1981;134:147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- ELFERINK J.G., KOSTER B.M. The stimulation of human neutrophil migration by angiotensin II: its dependence on Ca2+ and the involvement of cyclic GMP. Br. J. Pharmacol. 1997;121:643–648. doi: 10.1038/sj.bjp.0701167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERDÖS E.G. Some old and some new ideas on kinin metabolism. J. Cardiovasc. Pharmacol. 1990;15:S20–S24. [PubMed] [Google Scholar]
- FERREIRA S.H., LORENZETTI B.B., CUNHA F.Q., POOLE S. Bradykinin release of TNF-α plays a key role in the development of inflammatory hyperalgesia. Agents Actions. 1993a;38:C7–C9. doi: 10.1007/BF01991120. [DOI] [PubMed] [Google Scholar]
- FERREIRA S.H., LORENZETTI B.B., POOLE S. Bradykinin initiates cytokine-mediated inflammatory hyperalgesia. Br. J. Pharmacol. 1993b;110:1227–1231. doi: 10.1111/j.1476-5381.1993.tb13946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEPPETTI P. Sensory neuropeptide release by bradykinin: mechanisms and pathophysiological implications. Regul. Pept. 1993;47:1–23. doi: 10.1016/0167-0115(93)90268-d. [DOI] [PubMed] [Google Scholar]
- GRAHAM R.C., EBERT R.H., RATNOFF O.D., MOSES J.M. Pathogenesis of inflammation: II. In vivo observation of the inflammatory effects of activated Hageman factor and bradykinin. J. Exp. Med. 1965;121:807–823. doi: 10.1084/jem.121.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL J.M. Bradykinin receptors: pharmacological properties and biological roles. Pharmacol Ther. 1992;56:131–190. doi: 10.1016/0163-7258(92)90016-s. [DOI] [PubMed] [Google Scholar]
- KOYAMA S., SATO E., NOMURA H., KUBO K., MIURA M., YAMASHITA T., NAGAI S., IZUMI T. Bradykinin stimulates type II alveolar cells to release neutrophil and monocyte chemotactic activity and inflammatory cytokines. Am. J. Pathol. 1998;153:1885–1893. doi: 10.1016/S0002-9440(10)65702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOYAMA S., SATO E., NUMANAMI H., KUBO K., NAGAI S., IZUMI T. Bradykinin stimulates lung fibroblasts to release neutrophil and monocyte chemotactic activity. Am. J. Respir. Cell Mol. Biol. 2000;22:75–84. doi: 10.1165/ajrcmb.22.1.3752. [DOI] [PubMed] [Google Scholar]
- KUMAKURA S., KAMO I., TSURUFUJI S. Role of bradykinin in the vascular permeability response induced by carrageenin in rats. Br. J. Pharmacol. 1988a;93:739–746. doi: 10.1111/j.1476-5381.1988.tb11457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUMAKURA S., KAMO I., TSURUFUJI S. Nature of kininase activity in the exudate in kaolin-induced inflammation of the air pouch type in rats. Eur. J. Pharmacol. 1988b;149:267–275. doi: 10.1016/0014-2999(88)90657-7. [DOI] [PubMed] [Google Scholar]
- KUMAKURA S., TSURUFUJI S., KUROOKA S., SUNAHARA N. Role of bradykinin in the vascular permeability response induced with kaolin in rats. J. Pharmacol. Exp. Ther. 1987;243:1067–1073. [PubMed] [Google Scholar]
- LO E.J, GREEN P.G., MIAO F.J., RELCHLING D.B., LEVINE J.D. Bradykinin-induced neurogenic migration of neutrophils into the rat knee joint. Neuroreport. 1999;10:3821–3824. doi: 10.1097/00001756-199912160-00018. [DOI] [PubMed] [Google Scholar]
- MANSSON P., ZHANG X.W., JEPPSSON B., JOHNELL O., THORLACIUS H. Critical role of P-selectin-dependent rolling in tumor necrosis factorα-induced leukocyte adhesion and extravascular recruitment in vivo. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000;362:190–196. doi: 10.1007/s002100000268. [DOI] [PubMed] [Google Scholar]
- MCLEAN P.G., AHLUWALIA A., PERRETTI M. Association between kinin B1 receptor expression and leukocyte trafficking across mouse mesentereric postcapillary venules. J. Exp. Med. 2000;192:367–380. doi: 10.1084/jem.192.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER A.J., LUHESHI G.N., ROTHWELL N.J., HOPKINS S. Local cytokine induction by LPS in the rat air pouch and its relationship to the febrile response. Am. J. Physiol. 1997;272:R857–R861. doi: 10.1152/ajpregu.1997.272.3.R857. [DOI] [PubMed] [Google Scholar]
- PANÉS J., PERRY M., GRANGER D.N. Leukocyte-endothelial cell adhesion: avenues for therapeutic intervention. Br. J. Pharmacol. 1999;126:537–550. doi: 10.1038/sj.bjp.0702328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANG L., KNOX A.J. Bradykinin stimulates IL-8 production in cultures of human airway smooth muscle cells: role of cyclooxygenase products. J. Immunol. 1998;161:2509–2515. [PubMed] [Google Scholar]
- PASQUALE C.P., MARTINS M.A., BOZZA P.T., SILVA P.M.R., NETO H.C.C.F., PIRES A.L.A., CORDEIRO R.S.B. Bradykinin induces eosinophil accumulation in the rat pleural cavity. Int. Arch. Allergy Appl. Immunol. 1991;95:244–247. doi: 10.1159/000235436. [DOI] [PubMed] [Google Scholar]
- PESQUERO J.B., PESQUERO J.L., OLIVEIRA S.M., ROSCHER A.A., METZGER R., GANTEN D., BADER M. Molecular cloning and functional characterisation of a mouse bradykinin B1 receptor gene. Biochem. Byophys. Res. Commun. 1996;220:219–225. doi: 10.1006/bbrc.1996.0384. [DOI] [PubMed] [Google Scholar]
- PIQUERAS L., KUBES P., ALVAREZ A., O'CONNOR E., ISSEKUTZ A.C., ESPLUGUES J.V., SANZ M.J. Angiotensin II induces leukocyte-endothelial cell interactions in vivo via AT1 and AT2 receptor-mediated P-selectin upregulation. Circulation. 2000;102:2118–2123. doi: 10.1161/01.cir.102.17.2118. [DOI] [PubMed] [Google Scholar]
- REGOLI D., BARABÉ J. Pharmacology of bradykinin and related kinins. Pharmacol. Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- SAKAMOTO W., KAGA M., HANDA H., GOTOH K., SUZUKI S., FUJIE K., INOUE N., NISHIHIRA J. Bradykinin and Met-T-kinin-Leu stimulated PGE2 production by rat macrophage and fibroblast. Braz. J. Med. Biol. Res. 1994;27:1923–1927. [PubMed] [Google Scholar]
- SALEH T.S.F., CALIXTO J.B., MEDEIROS Y.S. Pro-inflammatory effects induced by bradykinin in a murine model of pleurisy. Eur. J. Pharmacol. 1997;331:43–52. doi: 10.1016/s0014-2999(97)01005-4. [DOI] [PubMed] [Google Scholar]
- SATO E., KOYAMA S., NOMURA H., KUBO K., SEKIGUCHI M. Bradykinin stimulates alveolar macrophages to release neutrophil, monocyte, and eosinophil chemotactic activity. J. Immunol. 1996;157:3122–3129. [PubMed] [Google Scholar]
- SATO E., NELSON D.K., KOYAMA S., HOYT J.C., ROBBINS R.A. Bradykinin stimulates eotaxin production by a human lung fibroblast cell line. J. Allergy Clin. Immunol. 2000;106:117–123. doi: 10.1067/mai.2000.107400. [DOI] [PubMed] [Google Scholar]
- SOUZA G.E.P., FERREIRA S.H. Blockade by antimacrophage serum of the migration of PMN neutrophils into the inflamed peritoneal cavity. Agents Actions. 1985;17:97–103. doi: 10.1007/BF01966691. [DOI] [PubMed] [Google Scholar]
- TIFFANY C.W., BURCH R.M. Bradykinin stimulates tumor necrosis factor and interleukin-1 release from macrophages. FEBS Lett. 1989;247:189–192. doi: 10.1016/0014-5793(89)81331-6. [DOI] [PubMed] [Google Scholar]
- TSURUFUJI S., KUMAKURA S. Role of bradykinin in exudative reactions of inflammation in rats. Dermatologica. 1989;179:64–67. doi: 10.1159/000248452. [DOI] [PubMed] [Google Scholar]
- VIANNA R.M.J., CALIXTO J.B. Characterization of the mechanisms underlying the inflammatory response induced by des-Arg9-BK in mouse pleurisy. Br. J. Pharmacol. 1998;123:281–291. doi: 10.1038/sj.bjp.0701590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLPE A.R., GIARDINA B., PREZIOSI P., CARMIGNANI M. Biosynthesis of endothelium-derived nitric oxide by bradykinin as endogenous precursor. Immunopharmacology. 1996;33:287–290. doi: 10.1016/0162-3109(96)00043-4. [DOI] [PubMed] [Google Scholar]
- WIRTH K.J., HOCK F.J., ALBUS U., LINS W., ALPERMANN H.G., ANAGNOSTOPOULOS H., HENKE ST., BREIPOHL G., KÖNIG W., KNOLLE J., SCHÖLKENS B.A. HOE 140 a new potent and long acting bradykinin-antagonist: in vivo studies. Br. J. Pharmacol. 1991;102:774–777. doi: 10.1111/j.1476-5381.1991.tb12249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]