Abstract
Phorbol ester decreases muscle tension in the rat myometrium, and the effect is more potent in late-pregnant myometrium than in nonpregnant myometrium. In the present study, we have examined the contribution of protein kinase C (PKC) isoforms to the phorbol ester-induced inhibition of tension in rat uterine smooth muscle.
Thymeleatoxin (THX), a selective activator of conventional-type PKC (cPKC), and 12-deoxyphorbol 13-isobutyrate (DPB), an activator of pan PKC, inhibited the tension induced by high K+, and inhibitions were significantly increased in pregnant myometrium compared to nonpregnant myometrium. The inhibition by DPB and THX of high K+-induced tension was significantly attenuated when PKC was downregulated by long-term pretreatment with THX and inhibited by Go6976, a cPKC inhibitor.
Of the cPKCs, PKCα is predominantly expressed in the rat myometrium, as detected by Western blot analysis. The expression of PKCα gradually increases from the beginning of gestation, reaching a maximum at day 21 of pregnancy. Treatment with DPB induced PKCα to translocate from the cytosol to the membrane in the pregnant myometrium. PKCɛ and PKCζ, other dominant PKC isoforms in the rat myometrium, decrease during gestation, reaching a minimum in late pregnancy.
These results suggest that cPKC may be at least partly involved in the PKC-mediated inhibition of muscle tension in the rat myometrium.
Keywords: PKCα, rat myometrial tension, pregnancy, translocation, relaxation
Introduction
Protein kinase C (PKC) is a major signalling mediator activated by external stimuli, including hormones, neurotransmitters, and growth factors. These signals induce the hydrolysis of a membrane phospholipid, phosphatidylinositol (PI), via PI-specific phospholipase C (PLC), generating inositol 1,4,5-triphosphate and 1,2-diacylglycerol (DAG). DAG in turn increases the activity of PKC. Activation of PKC evokes a tension that is independent of the increment in intracellular Ca2+ levels ([Ca2+]i) and myosin light chain (MLC) phosphorylation in vascular smooth muscle (Sato et al., 1992). PKC plays an important role in the regulation of uterine smooth muscle tension (Savineau & Mironneau, 1990; Karibe et al., 1991; Phillippe, 1994; Ruzycky & Ameredes, 1996). We have previously reported that phorbol ester, a potent PKC activator, inhibits increases in [Ca2+]i and tension elicited by high-level K+ and uterotonic agonists in rat uterine smooth muscle (Kim et al., 1996). The phorbol ester-induced inhibition was observed similarly, but more potently in pregnant myometrium than in nonpregnant myometrium. Moreover, the elevation of hormonal levels as gestation advances and the physical stretch induced by the fetus can activate PKC in the myometrium because of increased production of DAG, a physiological activator of PKC (Wray, 1993; Ruzycky & Kulick, 1996).
PKC consists of a family of 12 isoforms distinguishable by their cofactor dependency during activation, which play different roles in transmembrane signal transduction (Nishizuka, 1995; Webb et al., 2000). Individual isoforms have distinct biological functions and show different tissue specificity (Kim et al., 1999; Maruyama et al., 1999). The distribution of the isoforms may also be altered following cell activation (Ali & Sarna, 2002). At least seven PKC isoforms are present in the uterine smooth muscle of humans and animal species (Ruzycky & Kulick, 1996; Breuiller-Fouche et al., 1998; Migliaccio et al., 1998; Tertrin-Clary et al., 1999). Since individual isoforms of PKC have different roles and undergo different distributional changes with the same stimulation, it can be assumed that the activation of PKC is closely related to the elevation or diminution of tension, and that differences in PKC activity in different gestational stages involve differences in isoform expression and/or activation.
Although it has been suggested that individual PKC isoforms are involved in proliferation or tension in uterine smooth muscle (Breuiller-Fouche et al., 1998; Tertrin-Clary et al., 1999; Eude et al., 2002), the role of individual PKC isoforms in myometrial tension has not been determined. In the present study, we examined the role of PKC isoforms in the inhibitory regulation of myometrial tension at different gestational stages.
Methods
Animal and tension preparations
Female Wistar rats (200–250 g) were used for this study. Vaginal smears were taken, and proestrus rats were mated with male rats overnight. The day upon which sperms were observed in the vaginal lavage was defined as day 0 of gestation. The normal length of gestation in the rat colony was 21 days. The uteri of pregnant rats were removed at days 7, 14, 19, or 21 of gestation. Myometria, isolated from rats in estrus, were used as the nonpregnant control myometria. Rats were stunned and bled, and a strip of uterine muscle (1–2 mm wide and 7–8 mm long) was isolated from the middle of each horn in the longitudinal direction. In experiments wherein conventional-type PKC (cPKC) was downregulated, strips from day 21 pregnant rats were placed in Dulbecco's modified Eagle's medium (DMEM) containing 100 mg ml−1 streptomycin and 100 U ml−1 penicillin without or with 10−6 M thymeleatoxin (THX) for 6 h at 37°C in a humidity-controlled CO2 incubator.
Measurement of tension
Each strip was attached to a holder under a resting tension of 10 mN. After equilibration for 20 min in a physiological salt solution (PSS), strips were repeatedly exposed to a 40 mM K+ solution until responses became stable. PSS contained (in mM): NaCl 136.9, KCl 5.4, CaCl2 1.5, MgCl2 1.0, NaHCO3 23.8, glucose 5.5, and ethylenediaminetetraacetic acid (EDTA) 0.01. The high concentration of K+ was prepared by replacing NaCl with equimolar KCl. These solutions were saturated with a mixture of 95% O2 and 5% CO2 at 37°C and pH 7.4. Muscle tension was recorded isometrically with a force–displacement transducer (FT03, Grass, RI, U.S.A.) connected to a polygraph system (RPS7C8, Grass).
PKC immunoblotting
Strips were isolated as described for the tension measurement experiments, and were snap-frozen in liquid N2 after treatment with various stimulants for different times. The samples were then homogenized in a sample buffer containing 200 mM Tris-HCl (pH 7.4), 300 mM sucrose, 10 mM ethylene glycol-bis (β-amino ethyl ether) tetraacetic acid (EGTA), 5 mM EDTA, 0.3% 2-mercaptoethanol, 0.3 mM phenylmethylsulfonyl fluoride, and 5 mM dithiothreitol. The homogenate was centrifuged at 100,000 × g for 60 min at 4°C (TL-100, Beckman, Fullerton, CA, U.S.A.) and the cytosolic fraction was removed as the supernatant. The resulting pellet was resuspended in a homogenizing buffer containing 1.0% Triton X-100 for 30 min and centrifuged at 100,000 × g for 60 min. The supernatant was assumed to be the membrane fraction. Protein concentrations were determined using a protein assay kit (BioRad, Hercules, CA, U.S.A.), utilizing a colorimetric assay for protein based on the Bradford dye-binding procedure (Bradford, 1976). Protein homogenates were diluted 1 : 1 (vol : vol) with sodium dodecyl sulfate (SDS) sample buffer containing 40 mM Tris-HCl (pH 6.8), 8 mM EGTA, 4% 2-mercaptoethanol, 40% glycerol, 0.01% bromophenol blue, and 4% SDS, and then boiled for 5 min.
Equal amounts (10–30 μg lane−1) of protein were separated in each lane of a 10% SDS–polyacrylamide gel. Electrophoretically separated proteins were transferred to a nitrocellulose membrane (Amersham Pharmacia Biotech, Buckinghamshire, U.K.). Membranes were incubated for 30 min in phosphate-buffered saline including 0.05% Tween 20 (PBST) containing 5% nonfat dried milk, and then incubated with individual PKC isoform-specific antibodies diluted 1 : 1000–5000 overnight at 4°C. Following incubation with horseradish peroxidase-conjugated anti-rabbit IgG (1 : 1000) for 60 min, the blots were developed using the enhanced chemiluminescence (ECL) detection system (Amersham Pharmacia Biotech). In some experiments, a rat brain cytosolic fraction was also run in parallel as a positive control for the detection of PKC isoforms. Quantitative analysis of antibody-specific bands was performed with an image analyser (BioRad).
Materials
Polyclonal anti-PKCα antibody was purchased from Upstate Biotech (Lake Placid, NY, U.S.A.). Polyclonal anti-PKCɛ and anti-PKCζ antibodies were purchased from Santa Cruz Biotech (Santa Cruz, CA, U.S.A.). Polyclonal anti-PKCβI anti-PKCβII, and anti-PKCγ antibodies, phenylmethylsulfonyl fluoride, Triton-X 100, dithiothreitol, and 2-mercaptoethanol were purchased from Sigma (St Louis, MO, U.S.A.). 12-Deoxyphorbol 13-isobutyrate (DPB) was purchased from Funakoshi (Tokyo, Japan). THX and Go6976 were purchased from Calbiochem (La Jolla, CA, U.S.A.). Dimethyl sulfoxide (DMSO) was used to dissolve and dilute DPB, THX, and Go6976. The final concentration of DMSO in the tissue baths was not greater than 0.1%.
Data analysis
The results of experiment are expressed as means±s.e.m. Unpaired Student's t-test was used to compare the data, and values were considered to be significantly different at P<0.05.
Results
Effects of PKC activator on myometrial tension
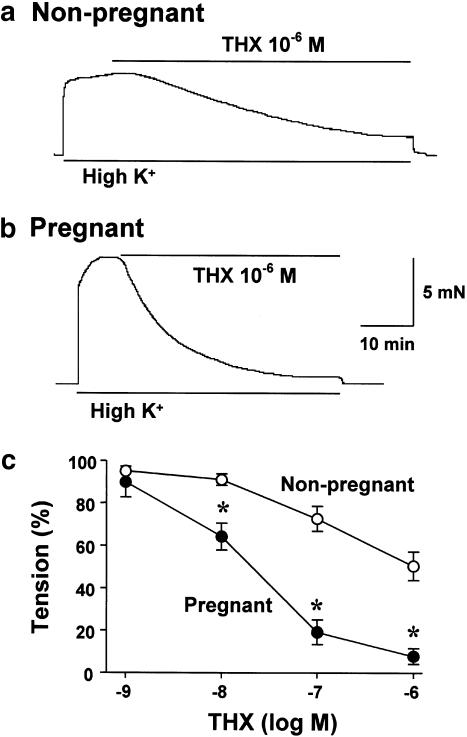
Isolated myometria from nonpregnant and day 21 pregnant rats were contracted with high K+ solution and then exposed to DPB and THX, activators of pan PKC and cPKC, respectively. DPB inhibited the tension induced by 40 mM K+ in a concentration-dependent manner, and inhibitions were potent in the myometrium from day 21 pregnant rat compared with that in the myometrium from nonpregnant rat (data not shown), which is consistent with our previous report (Kim et al., 1996). THX inhibited the tension induced by 40 mM K+ in myometria from nonpregnant and day 21 pregnant rats (Figure 1a, b). Although similar patterns of the inhibition by THX were obtained in both myometria, the concentration–response curves were significantly potent in day 21 pregnant myometrium than in nonpregnant myometrium (Figure 1c). In the quiescent preparation, neither DPB (10−6 M) nor THX (10−6 M) evoked any changes in tension (data not shown).
Figure 1.
Effects of thymeleatoxin (THX) on high K+-induced tension in the myometria from nonpregnant and pregnant rats. Myometrial strips from nonpregnant and day 21 pregnant rats were stimulated repeatedly with 40 mM K+. After the response to high K+ was determined, THX were applied. Typical recordings of THX (10−6 M)-induced inhibition of 40 mM K+-induced contraction in nonpregnant (a) and pregnant (b) myometria. (c) Concentration–response courses of the inhibition induced by THX. Each point represents the mean±s.e.m. of four to five experiments. * Denotes significant difference from the results in nonpregnant myometrium (P<0.05).
Changes in PKC isoforms during gestation
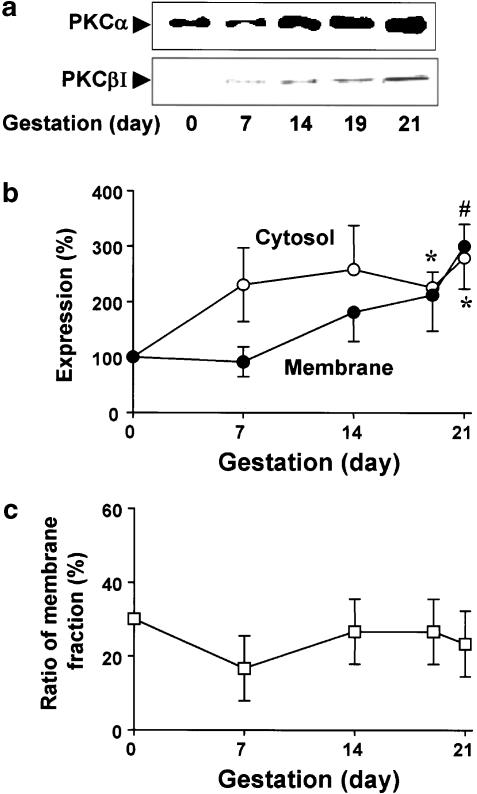
Results from the mechanical study suggest that cPKC may induce the inhibition of muscle tension in the rat myometrium. Since the contractility of the myometrium changed during gestation, it can be assumed that the expression of cPKC isoforms may be altered by pregnancy. Therefore, we attempted to detect the expression of cPKC isoforms in the myometria of nonpregnant rats and at each stage of gestation, using Western blot analysis with polyclonal antibodies. Figure 2a shows a typical immunoblotting pattern using a polyclonal antibody directed against PKCα to probe membrane extracts from nonpregnant myometrium and myometrium at each stage of pregnancy. In the absence of any stimulus, PKCα was clearly found in the nonpregnant myometrium and in the myometrium at all stages of pregnancy. PCKα in the cytosolic fraction represented 71.5±9.0 (n=3) of the total amount of this isoform in the nonpregnant myometrium. The expression of the PKCα isoform in the cytosolic fraction increased with advancing gestation, reaching a maximum at day 21 of pregnancy (279±56.0% of nonpregnant myometrium, n=3) (Figure 2b). In the membrane fraction, the expression of the PKCα isoform also increased with the advance of gestation (300±40.0% of nonpregnant myometrium, n=3) (Figure 2b). Figure 2c shows the ratio of membrane fraction in the quiescent state relative to total expression of PKCα during gestation. The ratio of membrane fractions was not significantly changed during gestation. However, immunodetection of PKCβI was faint (Figure 2a), and PKCβII and PKCγ were not detectable under these conditions. These results show that of the cPKCs, PKCα is predominantly expressed in the rat myometrium and significantly increased with advancing gestation.
Figure 2.
Immunoblotting analysis of cPKCs from nonpregnant and pregnant myometria. Uterine fractions were prepared as described in ‘Methods'. (a) Upper and lower panels show representative expression of PKCα in the membrane fraction and PKCβI in the cytosolic fraction, respectively. (b) Statistical results in the cytosolic and membrane fractions were obtained from three independent experiments. The level of PKCα in nonpregnant myometrium was defined as 100%. (c) Quantification of the gestation-induced changes in the membrane distribution of PKCα. The ratio of membrane fractions was expressed as a percentage of the total expression refers to the sum of immunoreactivity in the cytosolic and membrane fractions. *, # Denote significant differences from the results of the cytosolic and the membrane fractions in nonpregnant myometrium, respectively (P<0.05).
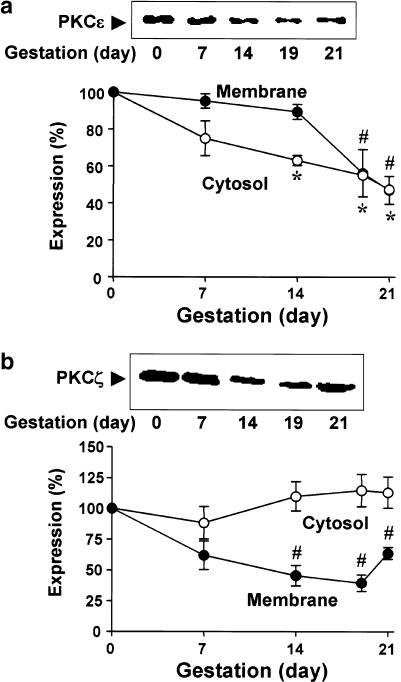
In this study, we have identified the expression of PKCɛ and PKCζ, which are representative isoforms of the novel and atypical types of PKC, respectively, in the myometrium of nonpregnant rats and at each stage of gestation. PKCɛ was more expressed in the membrane fraction (69.4±10.6%) compared to the cytosolic fraction (30.6±10.6%, n=4) from nonpregnant myometrium. As shown in Figure 3a, the expression of PKCɛ in the cytosolic fraction decreased with advancing gestation, reaching a minimum at day 21 of pregnancy (47±2.1% of nonpregnant myometrium, n=3). In the membrane fraction, the expression of the PKCɛ isoform also decreased with gestation and reached a minimum in the myometrium on day 21 of pregnancy (47±7.4% of nonpregnant myometrium, n = 3).
Figure 3.
Immunoblotting analysis of PKC isoforms from nonpregnant and pregnant myometria. The expression of PKCɛ (a) and PKCζ (b) were determined in the myometria from nonpregnant and pregnant rats. The statistical results for the cytosolic and membrane fractions were obtained from three independent experiments. The levels of PKC isoforms in nonpregnant myometrium were defined as 100%. *, # Denote significant differences from the results of the cytosolic and the membrane fractions in nonpregnant myometrium, respectively (P<0.05).
PKCζ was also detected in the myometrial extracts of all groups (Figure 3b). In the nonpregnant myometrium, PKCζ was more expressed in the cytosolic fraction (66.1±8.9%) compared to the membrane fraction (34.9±8.9%, n=4). Levels of PKCζ in the cytosolic fractions did not change significantly during gestation. On the other hand, PKCζ in the membrane fraction decreased significantly during gestation and reached a minimum at day 19 of pregnancy at 39±11.4% (n=3) of the level in the nonpregnant myometrium.
Effects of cPKC inhibition on the relaxation
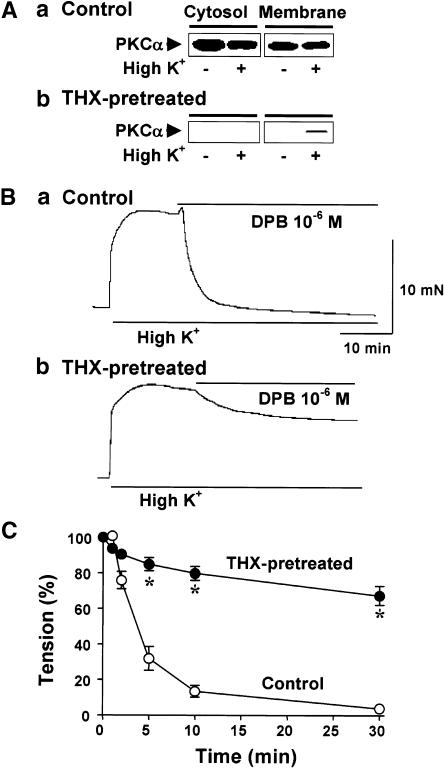
The effects of DPB and THX on myometrial tension in muscle strips with downregulated cPKC and cPKC inhibitor were examined. Myometrial strips from day 21 pregnant rats were incubated without or with THX (10−6 M) for 6 h, and the expression of PKCα and tension were measured. The expression of PKCα was significantly reduced in muscle strips preincubated with THX (Figure 4A). In control muscle strips incubated in DMEM containing 0.1% DMSO, muscle tension induced by high K+ solution was inhibited by treatment with DPB (10−6 M), similarly to the response in the freshly isolated strips. The relaxation induced by DPB was significantly attenuated in THX-pretreated muscle strips (Figure 4B and C).
Figure 4.
Effects of cPKC down-regulation on PKCα expression and 12- deoxyphorbol 13-isobutyrate-induced relaxation in pregnant myometrium. Myometrial strips from day 21 pregnant rats were pretreated with thymeleatoxin (10−6 M, THX) for 6 h to downregulate cPKC (THX-pretreated in A and B), or with 0.1% DMSO for control experiments (control in A and B). (A) Expression of PKCα in control and THX-pretreated pregnant myometria. (B and C) Inhibitory effects of 12-deoxyphorbol 13-isobutyrate (DPB) on 40 mM K+-induced tension in control and THX-pretreated pregnant myometria (n=6–8). *Denotes significant difference from the results of control (P<0.05).
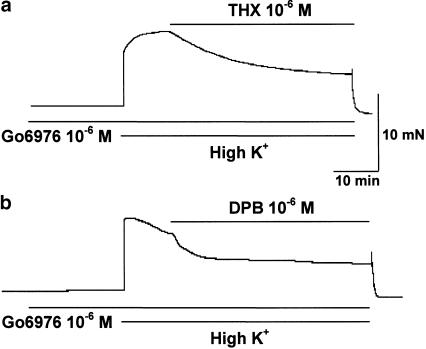
In the intact myometrium from day 21 pregnant rats, pretreatment with Go6976 (10−6 M), an inhibitor of cPKC, for 20 min attenuated the inhibition by THX (10−6 M) of the tension induced by 40 mM K+ (43.1±8.3% of K+-induced contraction, n=5, Figure 5a). Go6976 (10−6 M) also diminished the inhibition by DPB (10−6 M) of 40 mM K+-induced contraction in the myometrium from day 21 pregnant rats (58.7±5.8% of K+-induced contraction, n=5, Figure 5b). In the quiescent preparation, Go6976 (10−6 M) did not evoke any changes in tension (Figure 5a and b).
Figure 5.
Effects of Go6976 on thymeleatoxin- and 12-deoxyphorbol 13-isobutyrate-induced relaxation in pregnant myometrium. Myometrial strips from day 21 pregnant rats pretreated with Go6976 (10−6 M) for 20 min were contracted with 40 mM K+, and then exposed to thymeleatoxin (10−6 M, THX) and 12-deoxyphorbol 13-isobutyrate (10−6 M, DPB), respectively. Results are typical recordings obtained from five independent experiments.
Changes in subcellular distribution of PKCα by treatment with phorbol ester
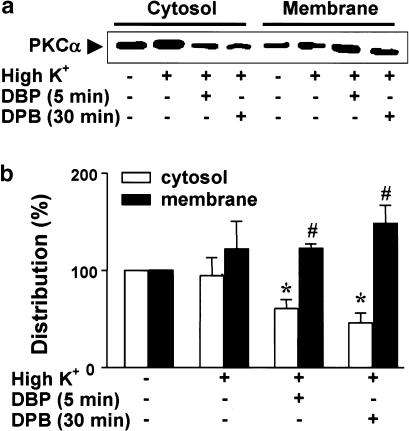
It has been reported that PKC undergoes changes in subcellular distribution with extracellular stimuli (Haller et al., 1990). To estimate whether PKCα in the myometrium is activated by phorbol ester, we examined the translocation of the enzyme after treatment of the tissue with DPB. As shown in Figure 6, the subcellular distribution of PKCα did not change after treatment with 40 mM K+. When the strips were treated with DPB (10−6 M), PKCα levels in the membrane fraction increased significantly, and decreased significantly in the cytosolic fraction (Figure 6b).
Figure 6.
Translocation of PKCα induced by treatment with 12-deoxyphorbol 13-isobutyrate in pregnant myometrium. (a) Myometrial strips from day 21 pregnant rats were stimulated with 40 mM K+ and 10−6 M 12-deoxyphorbol 13-isobutyrate (10−6 M, DPB), for different times (5 and 30 min). (b) Statistical results for the cytosolic and membrane fractions were obtained from three independent experiments. The level of PKCα in the quiescent state was defined as 100%. *, # Denote significant differences from the results of the cytosol and the membrane in the quiescent states, respectively (P<0.05).
Discussion
In the present study, we have focused on the possible role of cPKC isoforms in the inhibitory regulation of tension by PKC activation in rat uterine smooth muscle. Our data indicate that nonpregnant and pregnant myometria express PKCα and βI, and that the events of gestation are associated with changes in the expression of these PKC isoforms. Furthermore, activated PKCα showed a distinct change in subcellular distribution under stimulation with phorbol ester, which is consistent with the findings of earlier studies (Patel et al., 1996; Taggart et al., 1999; Eude et al., 2000; Giardina et al., 2001). Furthermore, in the mechanical study, both phorbol ester and a cPKC activator THX (Barman, 2001) inhibited myometrial tension, and inhibitions were increased in pregnant myometrium compared with that in nonpregnant myometrium. An important observation presented herein is that inhibitions of phorbol ester and THX were markedly modified by gestation, which closely matched the increased pattern in the expression of cPKC. Furthermore, the relaxation induced by phorbol ester was significantly attenuated by long-term treatment with the activator THX, which significantly downregulates the expression of cPKC (Kang et al., 2001). In addition, inhibitions induced by phorbol ester and THX were strongly attenuated by Go6976, a selective inhibitor of cPKC specific to PKCα and β (Martiny-Baron et al., 1993; Giardina et al., 2001). It has been reported that THX is involved in the regulation of vascular tension (Barman, 2001), and that PKCα does not contribute to an improvement in myometrial tension (Eude et al., 2000). These results suggest that phorbol ester-induced relaxation in the rat myometrium may be mediated, at least in part, by the activation of cPKC.
Since the tension of the myometrium simply depends on the increment of [Ca2+]i rather than on Ca2+-independent mechanisms such as Ca2+-sensitization (Szal et al., 1994; Kim et al., 1996), the decrease in tension because of PKC activation may result from a decrease in [Ca2+]i, possibly through the activation of a membrane Ca2+ pump (Kim et al., 1996). An alternative explanation is that PKC activation decreases [Ca2+]i by inhibiting the L-type Ca2+ channel (Galizzi et al., 1987; Haymes et al., 1992). In several smooth muscle systems, PKC activation has been reported to elicit a dual effect: the elevation and the diminution of mechanical activity (Savineau & Mironneau, 1990; Mitsui & Karaki, 1993). In the present study, the activation of PKC resulted in a relaxation of the myometrium, as has been reported elsewhere (Phillippe, 1994). The different roles of the PKC isoforms in the regulation of tension in smooth muscles may be a factor in the diversity of smooth muscle function.
In the present study, the amount of PKCɛ, a novel-type isoform, decreased in both the cytosolic and membrane fractions during gestation, and these levels reached a minimum at day 21 of pregnancy in the rat. The PKCɛ isoform plays a particular role in Ca2+-independent tension in vascular smooth muscle (Khalil et al., 1992). Walsh et al. (1996) suggested that activation of PKCɛ induces the phosphorylation of calponin to alleviate the inhibition of crossbridge cycling. PKCζ, an atypical-type isoform, on the other hand, is involved in cell growth and hypertrophy (Liou & Morgan, 1994) and gestational hypertrophy in rat myometrium (Ruzycky & Kulick, 1996; Eude et al., 2000). However, because the expression of PKCζ in the rat myometrium did not change with gestation, it can be concluded that this isoform is not involved in myometrial contractility.
In summary, cPKC undergoes dramatic changes in expression during gestational events and is induced to translocate by stimulation with phorbol ester. An activator of cPKC-inhibited myometrial tension, and long-term treatment with this activator and the selective inhibition of cPKC attenuated phorbol ester-induced relaxation. Therefore, we suggest for the first time that cPKC is associated with the relaxation of rat uterine smooth muscle upon PKC activation.
Acknowledgments
This study was supported by Konkuk University Research Fund in 2000 and the Human Science Foundation in Japan.
Abbreviations
- [Ca2+]i
intracellular Ca2+ level
- cPKC
conventional-type PKC
- DAG
1,2-diacylglycerol
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethyl sulfoxide
- DPB
12-deoxyphorbol 13-isobutyrate
- ECL
enhanced chemiluminescence
- EDTA
ethylenediaminetetraacetic acid
- EGTA
ethylene glycol-bis (β-amino ethyl ether) tetraacetic acid
- MLC
myosin light chain
- PBST
phosphate-buffered saline including 0.05% Tween 20
- PI
phosphatidylinositol
- PKC
protein kinase C
- PLC
phospholipase C
- PSS
physiological salt solution
- SDS
sodium dodecyl sulfate
- THX
thymeleatoxin
References
- ALI I., SARNA S.K. Selective modulation of PKC isozymes by inflammation in canine colonic circular muscle cells. Gastroenterology. 2002;122:483–494. doi: 10.1053/gast.2002.31215. [DOI] [PubMed] [Google Scholar]
- BARMAN S.A. Effect of protein kinase C inhibition on hypoxic pulmonary vasoconstriction. Am. J. Physiol. 2001;280:L888–L895. doi: 10.1152/ajplung.2001.280.5.L888. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BREUILLER-FOUCHE M., TERTRIN-CLARY C., HELUY V., FOURNIER T., FERRE F. Role of protein kinase C in endothelin-1-induced contraction of human myometrium. Biol. Reprod. 1998;59:153–159. doi: 10.1095/biolreprod59.1.153. [DOI] [PubMed] [Google Scholar]
- EUDE I., DALLOT E., FERRE F., BREUILLER-FOUCHE M. Protein kinase C alpha is required for endothelin-1-induced proliferation of human myometrial cells. Biol. Reprod. 2002;66:44–49. doi: 10.1095/biolreprod66.1.44. [DOI] [PubMed] [Google Scholar]
- EUDE I., PARIS B., CABROL D., FERRE F., BREUILLER-FOUCHE M. Selective protein kinase C isoforms are involved in endothelin-1-induced human uterine contraction at the end of pregnancy. Biol. Reprod. 2000;63:1567–1573. doi: 10.1095/biolreprod63.5.1567. [DOI] [PubMed] [Google Scholar]
- GALIZZI J.P., QAR J., FOSSET M., VAN RENTERGHEM C., LAZDUNSKI M. Regulation of calcium channels in aortic muscle cells by protein kinase C activators (diacylglycerol and phorbol esters) and by peptides (vasopressin and bombesin) that stimulate phosphoinositide breakdown. J. Biol. Chem. 1987;262:6947–6950. [PubMed] [Google Scholar]
- GIARDINA J.B., TANNER D.J., KHALIL R.A. Oxidized-LDL enhances coronary vasoconstriction by increasing the activity of protein kinase C isoforms alpha and epsilon. Hypertension. 2001;37:561–568. doi: 10.1161/01.hyp.37.2.561. [DOI] [PubMed] [Google Scholar]
- HALLER H., SMALLWOOD J.I., RASMUSSEN H. Protein kinase C translocation in intact vascular smooth muscle strips. Biochem. J. 1990;270:375–381. doi: 10.1042/bj2700375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYMES A.A., KWAN Y.W., ARENA J.P., KASS R.S., HINKLE P.M. Activation of protein kinase C reduces L-type calcium channel activity of GH3 pituitary cells. Am. J. Physiol. 1992;262:C1211–C1219. doi: 10.1152/ajpcell.1992.262.5.C1211. [DOI] [PubMed] [Google Scholar]
- KANG J., YANG M., JOU I., JOE E. Identification of protein kinase C isoforms involved in interferon-gamma-induced expression of inducible nitric oxide synthase in murine BV2 microglia. Neurosci. Lett. 2001;299:205–208. doi: 10.1016/s0304-3940(01)01515-4. [DOI] [PubMed] [Google Scholar]
- KARIBE H., OISHI K., UCHIDA M.K. Involvement of protein kinase C in Ca2+-independent contraction of rat uterine smooth muscle. Biochem. Biophys. Res. Commun. 1991;179:487–494. doi: 10.1016/0006-291x(91)91397-u. [DOI] [PubMed] [Google Scholar]
- KHALIL R.A., LAJOIE C., RESNICK M.S., MORGAN K.G. Ca2+-independent isoforms of protein kinase C differentially translocate in smooth muscle. Am. J. Physiol. 1992;263:C714–C719. doi: 10.1152/ajpcell.1992.263.3.C714. [DOI] [PubMed] [Google Scholar]
- KIM B.K., OZAKI H., HORI M., KARAKI H. Increased inhibitory effect of phorbol ester on cytosolic Ca2+ level and contraction in rat myometrium after gestation. Jpn. J. Pharmacol. 1996;72:111–118. doi: 10.1254/jjp.72.111. [DOI] [PubMed] [Google Scholar]
- KIM T.T., SAUNDERS T., BIEBER E., PHILLIPPE M. Tissue-specific protein kinase C isoform expression in rat uterine tissue. J. Soc. Gynecol. Invest. 1999;6:293–300. doi: 10.1016/s1071-5576(99)00035-0. [DOI] [PubMed] [Google Scholar]
- LIOU Y.M., MORGAN K.G. Redistribution of protein kinase C isoforms in association with vascular hypertrophy of rat aorta. Am. J. Physiol. 1994;267:C980–C989. doi: 10.1152/ajpcell.1994.267.4.C980. [DOI] [PubMed] [Google Scholar]
- MARTINY-BARON G., KAZANIETZ M.G., MISCHAK H., BLUMBERG P.M., KOCHS G., HUG H., MARME D., SCHACHTELE C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J. Biol. Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- MARUYAMA Y., SAKAI Y., NOBE K., MOMOSE K. Subcellular distribution of protein kinase C isoforms in gastric antrum smooth muscle of STZ-induced diabetic rats. Life Sci. 1999;64:1933–1940. doi: 10.1016/s0024-3205(99)00138-1. [DOI] [PubMed] [Google Scholar]
- MIGLIACCIO S., WASHBURN T.F., FILLO S., RIVERA H., TETI A., KORACH K.S., WETSEL W.C. Modulation of estrogen receptor levels in mouse uterus by protein kinase C isoenzymes. Endocrinology. 1998;139:4598–4606. doi: 10.1210/endo.139.11.6300. [DOI] [PubMed] [Google Scholar]
- MITSUI M., KARAKI H. Contractile and relaxant effects of phorbol ester in the intestinal smooth muscle of guinea-pig taenia caeci. Br. J. Pharmacol. 1993;109:229–233. doi: 10.1111/j.1476-5381.1993.tb13558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIZUKA Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- PATEL V., BROWN C., BOARDER M.R. Protein kinase C isoforms in bovine aortic endothelial cells: role in regulation of P2Y- and P2U-purinoceptor stimulated prostacyclin release. Br. J. Pharmacol. 1996;118:123–130. doi: 10.1111/j.1476-5381.1996.tb15374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPPE M. Protein kinase C, an inhibitor of oxytocin-stimulated phasic myometrial contractions. Biol. Reprod. 1994;50:855–859. doi: 10.1095/biolreprod50.4.855. [DOI] [PubMed] [Google Scholar]
- RUZYCKY A.L., AMEREDES B.T. Oxytocin-mediated recruitment of slowly cycling cross bridges and isometric force in rat myometrium. Am. J. Physiol. 1996;270:E203–E208. doi: 10.1152/ajpendo.1996.270.2.E203. [DOI] [PubMed] [Google Scholar]
- RUZYCKY A.L., KULICK A. Estrogen increases the expression of uterine protein kinase C isozymes in a tissue specific manner. Eur. J. Pharmacol. 1996;313:257–263. doi: 10.1016/0014-2999(96)00525-0. [DOI] [PubMed] [Google Scholar]
- SATO K., HORI M., OZAKI H., TAKANO-OHMURO H., TSUCHIYA T., SUGI H., KARAKI H. Myosin phosphorylation-independent contraction induced by phorbol ester in vascular smooth muscle. J. Pharmacol. Exp. Ther. 1992;261:497–505. [PubMed] [Google Scholar]
- SAVINEAU J.P., MIRONNEAU J. An analysis of the action of phorbol 12, 13-dibutyrate on mechanical activity in rat uterine smooth muscle. J. Pharmacol. Exp. Ther. 1990;255:133–139. [PubMed] [Google Scholar]
- SZAL S.E., REPKE J.T., SEELY E.W., GRAVES S.W., PARKER C.A., MORGAN K.G. [Ca2+]i signaling in pregnant human myometrium. Am. J. Physiol. 1994;267:E77–E87. doi: 10.1152/ajpendo.1994.267.1.E77. [DOI] [PubMed] [Google Scholar]
- TAGGART M.J., LEE Y.H., MORGAN K.G. Cellular redistribution of PKCalpha, rhoA, and ROKalpha following smooth muscle agonist stimulation. Exp. Cell Res. 1999;251:92–101. doi: 10.1006/excr.1999.4565. [DOI] [PubMed] [Google Scholar]
- TERTRIN-CLARY C., EUDE I., FOURNIER T., PARIS B., BREUILLER-FOUCHE M., FERRE F. Contribution of protein kinase C to ET-1-induced proliferation in human myometrial cells. Am. J. Physiol. 1999;276:E503–E511. doi: 10.1152/ajpendo.1999.276.3.E503. [DOI] [PubMed] [Google Scholar]
- WALSH M.P., HOROWITZ A., CLEMENT-CHOMIENNE O., ANDREA J.E., ALLEN B.G., MORGAN K.G. Protein kinase C mediation of Ca2+-independent contractions of vascular smooth muscle. Biochem. Cell Biol. 1996;74:485–502. doi: 10.1139/o96-053. [DOI] [PubMed] [Google Scholar]
- WEBB B.L., HIRST S.J., GIEMBYCZ M.A. Protein kinase C isoenzymes: a review of their structure, regulation and role in regulating airways smooth muscle tone and mitogenesis. Br. J. Pharmacol. 2000;130:1433–1452. doi: 10.1038/sj.bjp.0703452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRAY S. Uterine contraction and physiological mechanisms of modulation. Am. J. Physiol. 1993;264:C1–C18. doi: 10.1152/ajpcell.1993.264.1.C1. [DOI] [PubMed] [Google Scholar]