Abstract
The mode of Ca2+ channel blocking by gabapentin [1-(aminomethyl)cyclohexane acetic acid] was compared to those of other Ca2+ channel blockers, and the potential role of Ca2+ channel antagonists in providing protection against hypoxic injury was subsequently investigated in rat cerebrocortical slices.
mRNA for the α2δ subunits of Ca2+ channels was found in rat cerebral cortex.
Nitric oxide (NO) synthesis estimated from cGMP formation was enhanced by KCl stimulation, which was mediated primarily by the activation of N- and P/Q-type Ca2+ channels. Gabapentin blocked both types of Ca2+ channels, and preferentially reversed the response to 30 mM K+ stimulation compared with 50 mM K+ stimulation. In contrast, verapamil preferentially inhibited the response to depolarization by the higher concentration (50 mM) of K+.
Gabapentin inhibited KCl-induced elevation of intracellular Ca2+ in primary neuronal culture.
Hypoxic injury was induced in cerebrocortical slices by oxygen deprivation in the absence (severe injury) or presence of 3 mM glucose (mild injury). Gabapentin preferentially inhibited mild injury, while verapamil suppressed only severe injury. ω-Conotoxin GVIA (ω-CTX) and ω-agatoxin IVA (ω-Aga) were effective in both models.
NO synthesis was enhanced in a manner dependent on the severity of hypoxic insults. Gabapentin reversed the NO synthesis induced by mild insults, while verapamil inhibited that elicited by severe insults. ω-CTX and ω-Aga were effective in both the cases.
Therefore, the data suggest that gabapentin and verapamil cause activity-dependent Ca2+ channel blocking by different mechanisms, which are associated with their cerebroprotective actions and are dependent on the severity of hypoxic insults.
Keywords: Gabapentin, hypoxic injury, lactate dehydrogenase, cGMP, nitric oxide, voltage-gated Ca2+ channels, rat cerebrocortical slices, ω-agatoxin IVA, ω-conotoxin GVIA, verapamil
Introduction
Gabapentin is currently used as a therapeutic agent for refractory epilepsy (Ramsay, 1994), and also for a wide-range of neuropathic pain conditions including diabetic neuropathy (Backonja et al., 1998), postherpetic neuralgia (Rowbotham et al., 1998), migraine, and pain associated with cancer and multiple sclerosis (Di Trapani et al., 2000; Laird & Gidal, 2000; Nicholson, 2000). The broad spectrum of pharmacological activity of gabapentin has led a number of investigators to determine its modes of actions, although the precise mechanisms are yet unknown.
Gabapentin has been shown to bind the α2δ subunit of voltage-gated Ca2+ channels thereby inhibiting Ca2+ influx (Gee et al., 1996). Voltage-gated Ca2+ channels have been purified and cloned from various biological tissues, and are broadly classified into high- and low-voltage-gated channels. The high-voltage-gated Ca2+ channels can be subdivided into L-, P/Q-, and N-type depending on their biophysical characteristics. Ca2+ channels are heteromeric complexes consisting of a functional α1 subunit and auxiliary α2δ, β, and γ subunits (for a review, see Hofmann et al., 1999). The α1 subunit accounts for the ion channel-containing voltage sensor and binding sites for various drugs and toxins. Ca2+ flux through the α1 subunit is modulated by interactions with α2δ, β, and γ subunits. At present, three isoforms of α2δ subunits have been cloned from rabbit, human, and mouse Ca2+ channels (α2δ-1, α2δ-2, and α2δ-3) (Ellis et al., 1988; Klugbauer et al., 1999).
Several lines of evidence have suggested that excessive Ca2+ influx through voltage-gated Ca2+ channels represents the etiology of ischemic brain damage (Buchan et al., 1994; Lysko et al., 1994; Lynch et al., 1995), although the precise cellular mechanisms underlying cerebral injury after hypoxia or cerebral ischemia remain to be elucidated. A number of Ca2+ channel blockers, such as the N-type Ca2+ channel blocker, SNX-111 (Buchan et al., 1994), and the P/Q-type Ca2+ channel blocker, ω-agatoxin IVA (ω-Aga) (Asakura et al., 1997) have been reported to protect neurons against focal as well as global cerebral ischemia. We have also demonstrated that the hypoxic injury induced by a transient exposure to hypoxia/glucose deprivation is reversed by N- and P/Q-type but not L-type Ca2+ channel blockers in rat cerebrocortical slices (Oka et al., 2000b). In addition, the synthesis of nitric oxide (NO) is remarkably enhanced during exposure to hypoxia/glucose deprivation, which is also reduced by an N-type Ca2+ channel blocker, ω-conotoxin GVIA (ω-CTX) (Regan et al., 1991), or a P/Q-type Ca2+ channel blocker, ω-Aga (Mintz et al., 1992). Several NO synthase inhibitors such as L-NG-nitroarginine methyleter (L-NAME) and L-NG-monomethylarginine (L-NMMA) also inhibit hypoxic injury (Oka et al., 2000b). Taken together, the enhancement of NO synthase activity following the activation of N-type or P/Q-type Ca2+ channels may contribute to hypoxic injury in rat cerebrocortical slices.
To determine whether gabapentin inhibits the activity of N- and P/Q-type Ca2+ channels, we examined its effects on KCl-evoked NO synthesis in rat cerebrocortical slices and compared it to representative Ca2+ channel blockers, such as verapamil, ω-CTX, and ω-Aga. The effects of gabapentin and these Ca2+ channel blockers on hypoxic injury induced in rat cerebrocortical slices were subsequently compared relative to their Ca2+ channel-blocking properties.
Methods
Animals
Male 10 to 14 week-old Sprague–Dawley rats and pregnant ddY strain mice were obtained from Shimizu Laboratory Supplies, Co., Kyoto, Japan. Rats were housed in groups of 5–6, and mice were individually bred in a plastic cage in a room maintained at 21–25°C, 45–65% humidity, and a 12 h light/dark cycle (lights automatically on at 8:00 a.m.). Food and water were provided ad libitum. All animal procedures were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committee of Kyoto Pharmaceutical University.
Isolation of mRNA from rat cerebral cortex and RT–PCR analysis
Total RNA was isolated from rat cerebral cortex using Sepasol RNA I reagent (Nacalai Tesque, Kyoto, Japan) according to the manufacturer's instructions. Subsequently, poly(A)+ RNA was isolated using Oligotex-dT30 (TaKaRa Biochemicals, Ohtsu, Japan) and was subjected to RT–PCR. PCR was performed with primers on the basis of published sequences for rat α2δ isoforms. The primers specific for α2δ-1 were 5′-AAC AGA TCT AAA GCC CTG GTG CGC C-3′ (forward primer) and 5′-ACC CAT GGA GAA GCT GGA TAA TAT CG-3′ (reverse primer), which correspond to nucleotide positions 504–901 of cDNA [accession no. U73483]. The primers specific for α2δ-2 were 5′-ATT GAC GGT GTG ATG CGG ATT TTT G-3′ (forward primer) and 5′-GAC ATC GTA CAG GTC AAT CTT CTT G-3′ (reverse primer), which correspond to nucleotide positions 693–1280 of cDNA [accession no. AF247139]. The primers specific for α2δ-3 were 5′-GTG GGG AGA TAA AAT CCA TCG CTG-3′ (forward primer) and 5′-GCT CTT TAA CTG GGA CAT CTG TGC-3′ (reverse primer), which correspond to nucleotide positions 149–1525 of cDNA [accession no. NM_009785]. The primers specific for glyceraldehydes-3-phosphate dehydrogenase (GAPDH) were 5′-CCA TCA CCA TCT TCC AGG AG-3′ (forward primer) and 5′-CCT GCT TCA CCA CCT TCT TG-3′ (reverse primer), which correspond to nucleotide positions 283–858 of cDNA [accession no. X02231]. Semiquantitative PCR was performed according to the following conditions: 94°C for 45 s, 58°C for 45 s, 72°C for 90 s, repeated for 20–27 cycles. For each primer set, an increasing number of PCR cycles with otherwise fixed conditions was performed to determine the optimal number of cycles to be used. The numbers of cycles were 23 for all three α2δ subunits. For every PCR reaction, GAPDH was used as internal control. PCR product was loaded on a 1.0% agarose gel and stained with ethidium bromide. The stained PCR bands were photographed and captured into digital image analyzer. The density of bands from PCR images was analyzed by Scion Image for Windows (version 3.0). Results are expressed as the ratio of the optical density of the band of the PCR product of interest to that of GAPDH.
Measurement of KCl-evoked NO synthesis in slices of the rat cerebral cortex
NO synthesis was estimated from cGMP production in slices of the rat cerebral cortex, as described previously (Oka et al., 2000c;2001). Briefly, rats were decapitated under ether anesthesia, and their brains were quickly removed. After that, the rat cerebral cortex was dissected on ice and 500-μm thick serial coronal sections were prepared using a McIlwain tissue chopper (Mickle Laboratory Engineering Co., Gomshall, U.K.). Then, the chopped tissue was transferred to a glass dish containing ice-cold Krebs–Ringer bicarbonate (KRB) buffer pH 7.4 (NaCl 118 mM, KCl 4.7 mM, CaCl2 2.54 mM, MgCl2 1.2 mM, KH2PO4 1.2 mM, NaHCO3 25 mM, and glucose 11.5 mM), and each slice was gently isolated using forceps. A piece of each slice was transferred to a 12-well plastic dish containing 2 ml KRB buffer and preincubated at 37°C for 1 h under continuous bubbling with a gas mixture of 95% O2/5% CO2. After preincubation, the slice was incubated with 30 or 50 mM KCl for 20 min in the presence of 1 mM 3-isobutyl-1-methylxanthine (IBMX), and the reaction was terminated by the addition of perchloric acid (final concentration was 0.2 M). Each slice was homogenized, and the homogenate was centrifuged at 10,000 × g for 15 min at 4°C. The resultant supernatant was neutralized with 10% K2CO3, then centrifuged at 10,000 × g for 15 min at 4°C. The cGMP content in the supernatant was determined using the Amersham cGMP enzyme immunoassay kit. The tissue pellet was dissolved in 1 ml of 1 M NaOH and the protein content was determined by the method of Bradford (1976) using bovine serum albumin as the standard.
Primary neuronal culture
Primary neuronal cultures were prepared from the cerebral cortex of fetal mice, as described previously (Oka et al., 1999;2000c). Briefly, 15-day-old fetal mice were decapitated under ether anesthesia, and their brains were quickly removed. After the cerebral cortex was dissected, the meninges were carefully removed in a Ca2+-free Puck's solution (pH 7.4). Tissues were minced and washed with Ca2+-free Puck's solution, followed by treatment with 0.1% trypsin dissolved in Ca2+-free Puck's solution at 37°C for 5 min under the stream of gas mixture of 95% O2/5% CO2. The trypsin digestion was terminated by the addition of ice-cold Dulbecco's modified Eagle's medium (DMEM) supplemented with 30 mM glucose, 10 mM HEPES, carbenicillin (0.1 mg ml−1), streptomycin (0.1 mg ml−1), and 20% foetal bovine serum, and tissues were triturated with a Pasteur pipette. The dispersed cells were collected after centrifugation at 900 × g at 4°C for 2 min. The resultant pellet was then resuspended in DMEM followed by trituration, then the cell suspension was passed through a nylon sieve (mesh size 60 μm). A portion (1.5 ml) of the cell suspension containing 1.45 × 106 cells ml−1 was inoculated on a 35-mm glass-bottom dish (MatTek, Ashland, MA, U.S.A.). The medium was replaced by freshly prepared DMEM containing 15% fetal bovine serum and the cells were successively incubated for 2 days. On the third day, cells were exposed to 20 μM cytosine-β-D-arabinofuranoside dissolved in DMEM containing 15% fetal bovine serum for 24 h to prevent the growth of non-neuronal cells. The culture medium was then replaced by freshly prepared DMEM supplemented with 15% fetal bovine serum.
Measurement of intracellular Ca2+ concentration ([Ca2+]i) in primary neuronal culture
The [Ca2+]i was determined as described previously (Oka et al., 1999;2000c). Briefly, on the fifth day of in vitro culture, cells were preloaded with 10 μM fura-2/AM in 10 mM HEPES–NaOH buffer pH 7.05: KCl 5.4 mM, NaCl 130 mM, CaCl2 2.5 mM, and glucose 5.5 mM, at 37°C for 30 min, followed by further incubation for 30 min to hydrolyze acetoxymethyl ester. The fura-2 loaded cells were imaged on an inverted microscope (Olympus IMT-2, Tokyo, Japan), using a × 40, 0.6 numerical aperture immersion objective (Olympus) and a charge-coupled device camera (C-2400-08, Hamamatsu Photonics, Shizuoka) fitted with a 500 TV line. A 75-W xenon arc lamp was used to provide fluorescence excitation. Ratio images were obtained by acquiring pairs of images at alternating excitation wavelengths (340/380 nm) and filtering the emission at 510 nm (OSP-3, Olympus). Images were recorded every 5 s with a video camera (Hamamatsu Photonics) equipped with an Argus-50/CA system (Hamamatsu Photonics), which controlled the image acquisition and display. The fluorescence images of fura-2 loaded neuron were corrected by subtracting the background images measured in a field lacking neuronal cells. The images at individual wavelengths were averaged over 32 frames. Cells were depolarized with 50 mM KCl for 30 s (during 120–150 s after start of [Ca2+]i monitoring) in the absence or presence of gabapentin (300 μM and 1 mM). The basal [Ca2+]i was determined by averaging the F340/380 ratio during 60 s before KCl stimulation, while the maximal [Ca2+]i value was measured after KCl stimulation. The effect of gabapentin on KCl-stimulated rise in [Ca2+]i was evaluated by comparing the percentage of respective basal values. [Ca2+]i was monitored in 26–41 neurons in one experiment, and data were obtained from three to four experiments.
Hypoxia/reoxygenation-induced tissue injury in slices of the rat cerebral cortex
Hypoxic tissue injury was induced in rat cerebrocortical slices, as described previously (Oka et al., 2000c). Briefly after immersing the whole brain in ice-cold 5% glucose solution for 10 min, the cerebral cortex was dissected on ice and 500-μm-thick serial coronal sections were prepared as described above. A piece of each slice was transferred to a 12-well plate containing 2 ml KRB buffer and preincubated at 37°C for 1 h under continuous bubbling with a gas mixture of 95% O2/5% CO2. After preincubation, the slice was incubated in KRB buffer supplemented with 95% N2/5% CO2 in the absence of glucose (severe injury model) or in the presence of 3 mM glucose (mild injury model) at 37°C for 45 min under continuous bubbling with 95% N2/5% CO2, followed by reoxygenation for 5 h in normal KRB buffer. Tissue injury was assessed by the leakage of lactate dehydrogenase (LDH) into the incubation medium. A 50-μl portion of the incubation medium was taken at 5 h after the reoxygenation, and LDH activity in the medium was determined using an enzymatic assay kit (Wako Pure Chemical, Osaka, Japan). In the normoxic group, slices were incubated for 5 h and 45 min in KRB under continuous bubbling with 95% O2/5% CO2. At the end of the experiments, brain slices were dissolved in 1 ml of 1 M NaOH and protein content was determined as described above.
Measurement of NO synthesis during exposure of rat cerebrocortical slices to hypoxia
NO synthesis was estimated from cGMP production in the incubation medium after addition of 0.5 mM GTP, 1 mM IBMX, and the soluble fraction of rat cerebellum as the source of guanylate cyclase to the incubation medium, according to the method described previously (Oka et al., 2000b,2000c). The cerebral cortex and cerebellum were dissected on ice and serial coronal sections of the cerebral cortex were prepared as described above. The cerebellum was homogenized with 10-volumes of ice-cold buffer-A (Tris 50 mM, pH 7.4, EDTA 1 mM, dithiothreitol 1 mM, and PMSF 0.2 mM) using a Polytron homogenizer (PT 3000, Kinematica AG, Littau, Switzerland). After centrifugation at 30,000 × g for 30 min, the supernatant fraction was used as a source of guanylate cyclase. A cerebrocortical slice was transferred to a 24-well plastic plate containing 2 ml KRB buffer, and preincubated at 37°C for 1 h under continuous bubbling with 95% O2/5% CO2. After preincubation, the incubation medium was discarded and the slice was further incubated in 2 ml of oxygen-deprived medium in the absence of glucose (severe injury model) or in the presence of 3 mM glucose (mild injury model) for 45 min. During the incubation period, 0.5 mM GTP, 1 mM IBMX, and a 20-μl aliquot of the supernatant fraction of rat cerebellum (containing 50–80 μg protein) were included in the incubation medium. The cGMP produced in the incubation medium was measured by using the Amersham cGMP enzyme immunoassay kit.
Analysis of data
Data were analyzed using SAS (SAS/STAT, Ver. 6, fourth edition, 1990, SAS Institute Ins., Cary, NC, U.S.A.). Unless otherwise indicated, data were analyzed by one-way analysis of variance, followed by Dunnett's test. The IC50 values were calculated from the least-squares linear regression method.
Drugs and solution
The following chemicals and drugs were obtained from commercial sources: gabapentin, nifedipine, and IBMX (Sigma-RBI, St Louis, MO, U.S.A.), ω-CTX and ω-Aga (Peptide Institute, Osaka, Japan). Fura-2/AM was purchased from Dojindo (Osaka, Japan), and dissolved in dimethyl sulfoxide (10 mM stock). Other chemicals were all of guaranteed grade.
Results
Detection of mRNA for the α2δ-1, -2, and -3 subunits of voltage-gated Ca2+ channels in rat cerebral cortical membranes by RT–PCR
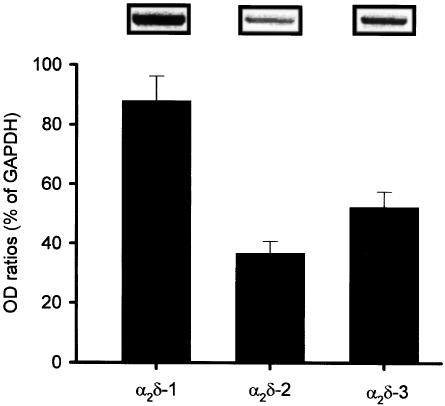
The mRNA for the α2δ-1, -2, and -3 subunits of voltage-gated Ca2+ channels in rat cerebral cortex was determined by a semiquantitative RT–PCR. In preliminary experiments, we verified that the PCR was completed during the exponential phase of amplification and that the amplification was linear at least up to 25 cycles (data not shown). Under these conditions (23 cycles), the rank order of their mRNA expression level was as follows: α2δ-1>α2δ-3>α2δ-2 (Figure 1).
Figure 1.
mRNA expression of α2δ-1, α2δ-2, and α2δ-3 in rat cerebral cortex. The mRNA (0.5 μg) was subjected to RT–PCR using primer pairs specific for α2δ-1, α2δ-2, and α2δ-3. Expected size of PCR products is 398, 588, and 1377 bp for α2δ-1, α2δ-2, and α2δ-3, respectively. The relative expression level was determined by normalizing the optimal density (OD) of α2δ subunits with that of GAPDH. Each column represents the mean±s.e.m. of n=3 independent experiments.
Comparative effects of gabapentin and verapamil on the KCl-evoked NO synthesis in rat cerebral cortex slices
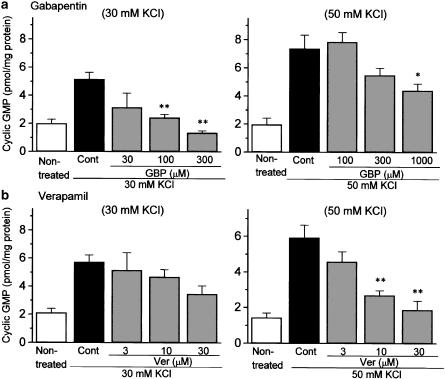
As shown in Figure 2, the stimulation of slices with 30 or 50 mM KCl produced a marked increase in NO synthesis, as estimated from cGMP formation, although the increase was greater for 50 mM KCl. Gabapentin (30–300 μM) reversed 30 mM KCl-evoked NO synthesis in a concentration-dependent manner (Figure 2a, left), whereas the agent showed far less marked inhibition of 50 mM KCl-evoked NO synthesis. Significant inhibition was observed only at the highest concentration tested (1000 μM) (Figure 2a, right). In contrast, high concentrations of verapamil (3–30 μM) concentration-dependently inhibited the 50 mM KCl-induced NO synthesis, but had no significant influence on the 30 mM KCl-evoked response (Figure 2b).
Figure 2.
Comparative effects of gabapentin and verapamil on KCl-evoked NO synthesis in rat cerebral cortex slices. NO synthesis was estimated from cGMP formation. After preincubation for 1 h, slices were incubated with 30 or 50 mM KCl for 20 min in the presence of 1 mM IBMX. Gabapentin (GBP: 30–1000 μM) or verapamil (Ver: 3–30 μM) was included with KCl stimulation. Each column represents the mean±s.e.m. of n=5 experiments. *P<0.05, **P<0.01 as compared with control group (Dunnett's test).
Effect of gabapentin on KCl-evoked NO synthesis mediated by P/Q- and N-type Ca2+ channels in rat cerebral cortex slices
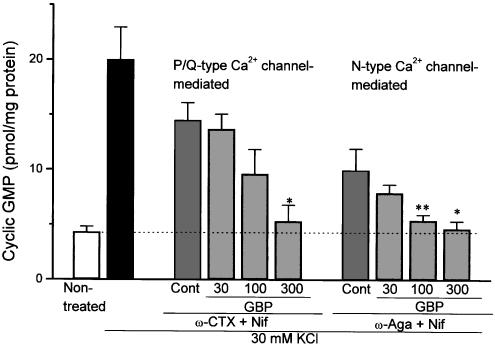
We have already found that KCl-evoked NO synthesis in rat cerebral cortex slices was inhibited almost completely by the combination of ω-CTX (5 μM) and ω-Aga (0.2 μM), but attenuated only slightly by nifedipine, suggesting that the response is mediated predominantly through activation of N- and P/Q-type Ca2+ channels (Oka et al., 2001). To determine the effect of gabapentin on each Ca2+ channel subclass, we examined its effects on KCl-evoked NO synthesis in the presence of subclass-selective Ca2+ channel blockers. The KCl-evoked response determined in the presence of ω-CTX and nifedipine appeared to be mediated by a P/Q-type Ca2+ channel, while that measured in the presence of ω-Aga and nifedipine was likely because of the activation of an N-type Ca2+ channel. As shown in Figure 3, gabapentin (30–300 μM) inhibited the P/Q-type Ca2+ channel at an IC50 value of 98 μM (51–208 μM, 95% confidence limit), and the N-type Ca2+ channel at an IC50 value of 66 μM (25–114 μM, 95% confidence limit) in rat cerebrocortical slices.
Figure 3.
Comparative effects of gabapentin on KCl-evoked NO synthesis mediated by P/Q- and N-type Ca2+ channels in rat cerebral cortex slices. The KCl-evoked NO synthesis was measured in the presence of either ω-conotoxin GVIA (ω-CTX: 5 μM)+nifedipine (Nif: 1 μM) or ω-agatoxin IVA (ω-Aga: 0.2 μM)+nifedipine (1 μM). Gabapentin (30–300 μM) was added to the incubation medium together with 1 mM IBMX and the respective Ca2+ channel blockers. Each column represents the mean±s.e.m. of n=5 experiments. *P<0.05, **P<0.01 as compared with ω-CTX+Nif-treated or ω-Aga+Nif-treated control group.
Effect of gabapentin on KCl-induced elevation of [Ca2+]i in primary neuronal culture
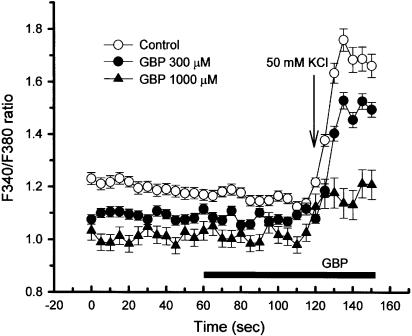
To confirm that the inhibitory action of gabapentin on KCl-evoked NO synthesis is because of the blockade of Ca2+ entry, we examined its effects on the KCl-induced elevation of [Ca2+]i, which was estimated by the ratio of fluorescence intensities at excitation wavelengths of 340 and 380 nm (F340/F380 ratio), in primary neuronal culture of mouse cerebral cortex. Gabapentin inhibited the elevation of F340/F380 ratio evoked by 50 mM KCl in a concentration-dependent manner (Figure 4). Significant inhibitory effects by gabapentin were observed at 1000 μM.
Figure 4.
KCl-induced elevation of intracellular free Ca2+ concentration ([Ca2+]i) and its reversal by gabapentin in primary neuronal culture of mouse cerebral cortex. On the fifth day of culture, cells were preloaded with fura-2/AM at 37°C for 30 min, followed by further incubation for 30 min to hydrolyze the ester. [Ca2+]i was measured from the ratio of fluorescence intensities at excitation wavelengths of 340 and 380 nm using an Olympus fluorescence microscope and an Argus-50/CA system. Cells were exposed to 50 mM KCl for 30 s (during 120–150 s). Gabapentin (300 or 1000 μM) was added 60 s before the end of KCl stimulation, as indicated by a solid bar. [Ca2+]i was monitored every 5 s.
Effect of gabapentin and various types of Ca2+ channel blockers on mild and severe hypoxic injury in rat cerebral cortex slices
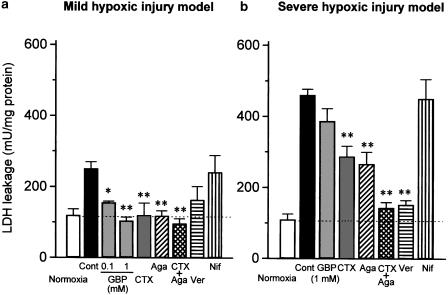
Two modes of hypoxic injury were induced in rat cerebrocortical slices by transient exposure to either hypoxia/glucose deprivation (severe injury model) or hypoxia/3 mM glucose (mild injury model) for 45 min, followed by further incubation in normal KRB buffer supplemented with 95% O2/5% CO2 for 5 h. As shown in Figure 5a, the LDH leakage in the mild hypoxic injury model was 2.5-fold higher than that in normoxic slices, while the value was more marked (4.6-fold) in the severe hypoxic injury model. Gabapentin at 100 and 1000 μM markedly attenuated the mild hypoxic injury (Figure 5a), whereas it did not affect, even at 1000 μM, the severe hypoxic injury model (Figure 5b). In contrast to the action of gabapentin, verapamil (30 μM) preferentially inhibited the LDH leakage in the severe hypoxic injury model without significantly affecting mild hypoxic injury. On the other hand, ω-CTX (5 μM) and ω-Aga (0.2 μM) significantly inhibited both mild hypoxic injury and severe hypoxic injury, although the effects were more marked for the mild hypoxic injury model. Neither mild hypoxic injury nor severe hypoxic injury was attenuated by the L-type Ca2+ channel blocker nifedipine.
Figure 5.
Comparative effects of various Ca2+ channel blockers in the mild and severe hypoxic injury models using rat cerebrocortical slices. After preincubation for 1 h, slices were exposed to hypoxia for 45 min in the presence of 3 mM glucose (a: mild injury model) or the absence of glucose (b: severe injury model), followed by reoxygenation for 5 h. Gabapentin (100 or 1000 μM), ω-CTX (5 μM), ω-Aga (0.2 μM), nifedipine (Nif: 1 μM), or verapamil (Ver: 30 μM) were included during hypoxia and reoxygenation periods. Tissue injury was determined by LDH concentrations. Each column represents the mean±s.e.m. of n=5 experiments. *P<0.05, **P<0.01 as compared with control group (Dunnett's test).
Effects of gabapentin and verapamil on NO synthesis induced in rat cerebrocortical slices by mild and severe hypoxic insults
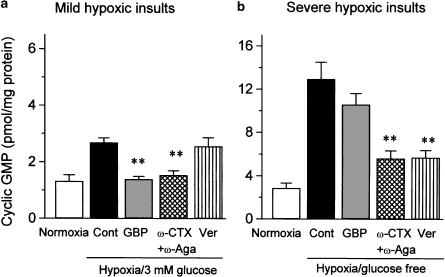
NO synthesis, estimated from cGMP production in the extracellular fluids, was enhanced during the mild and severe hypoxic insults, although the extent was much greater in slices exposed to severe hypoxic insults (Figure 6) (452.8±54.5% in severe model and 203.0±12.2% in mild model, n=5). As observed for cerebroprotection, gabapentin (1000 μM) preferentially reduced NO synthesis induced by mild hypoxic insults, while verapamil (30 μM) reversed NO synthesis evoked by severe but not mild hypoxic insults (Figure 6). The combination of ω-CTX and ω-Aga significantly attenuated NO synthesis caused by mild as well as severe hypoxic insults.
Figure 6.
Comparative effects of various Ca2+ channel blockers on NO synthesis induced in rat cerebrocortical slices by mild (a) or severe (b) hypoxic insults. NO synthesis was estimated from cGMP formation in the extracellular fluids after addition of 0.5 mM GTP and 1 mM IBMX. After preincubation for 1 h, slices were exposed to hypoxia for 45 min in the presence of 3 mM glucose (a) or in the absence of glucose (b). Gabapentin (1000 μM), ω-CTX (5 μM), ω-Aga (0.2 μM), or verapamil (Ver: 30 μM) were included during exposure to hypoxic insults. Each column represents the mean±s.e.m. of n=5 experiments. **P<0.01 as compared with control group (Dunnett's test).
Discussion
In the present study, NO synthesis, as estimated from cGMP formation, was used as an index of Ca2+ channel activity, since NO synthase is readily activated by the elevation of intracellular Ca2+. Along with other investigators, we have already shown that production of KCl-evoked cGMP is reversed by NO synthase inhibitors L-NAME and L-NMMA, an NO scavenger hemoglobin, and calmodulin inhibitors including W-7. These results indicate that the increase in cGMP content is mediated exclusively by the stimulation of guanylate cyclase by NO generated by Ca2+-dependent constitutive-type NO synthase. Moreover, the KCl-evoked NO synthesis in rat cerebrocortical slices is reversed by ω-CTX and ω-Aga, while nifedipine slightly (by 16%) but significantly inhibited the KCl response (Oka et al., 2001). These results indicate that the KCl-evoked NO synthesis is mediated mostly through activation of N- and P/Q-type Ca2+ channels.
In the present study, gabapentin preferentially inhibited the response induced by a low concentration of KCl (30 mM) but only weakly affected the response to a high concentration (50 mM) of KCl. It is unlikely that the inhibition of KCl-evoked NO synthesis by gabapentin results from activities other than Ca2+ channel blockage, such as the direct inhibition of NO synthase, because gabapentin did attenuate the elevation of [Ca2+]i induced in primary neuronal culture by KCl (Figure 4). To further determine which subclasses of Ca2+ channels are blocked by gabapentin, the effect of this compound on KCl-evoked NO synthesis was examined in the presence of subclass-selective Ca2+ channel blockers. Since KCl-evoked NO synthesis is mediated almost exclusively by N-, P/Q-, and L-type Ca2+ channels, as described above, the NO synthesis measured in the presence of ω-CTX and nifedipine is likely associated with P/Q-type Ca2+ channels, while that observed in the presence of ω-Aga and nifedipine seems to be mediated through activation of N-type Ca2+ channels. These results revealed that gabapentin blocked N-type as well as P/Q-type Ca2+ channels to nearly the same extent.
Recently, several studies have shown that gabapentin blocks Ca2+ channels. For example, it inhibits the KCl-evoked release of [3H] noradrenaline from human neocortical slices in an ω-Aga-sensitive manner (Fink et al., 2002), and blocks N-, L-, and P/Q-type Ca2+ currents in dorsal root ganglion neurons, as demonstrated by a whole-cell patch clamp technique (Sutton et al., 2002). The gabapentin IC50 values (66–98 μM) for inhibiting KCl-evoked responses obtained in the present experiment were comparable to those reported by other investigators, who reported 48 μM for inhibiting KCl-evoked [3H] noradrenaline release (Fink et al., 2000) and 14 μM for inhibiting KCl-induced elevation of [Ca2+]i in rat neocortical synaptosomes (Meder & Dooley, 2000).
Ng et al. (2001) have reported that the inhibitory effect of gabapentin on Ca2+ channels is mediated by stimulation of GABAB receptors. To confirm this possibility, we examined the effect of gabapentin on forskolin-stimulated cAMP formation in rat cerebrocortical slices, because GABAB receptors are coupled with the inhibitory trimeric G protein Gi to suppress adenylate cyclase activity (Wojcik & Holo-painen, 1992). In our experiment, however, the higher concentration of gabapentin (1000 μM) failed to inhibit forskolin-stimulated cAMP formation, although the GABAB receptor agonist baclofen (30 μM) markedly inhibited cAMP formation, that is, the cAMP formation in the slices in groups treated with forskolin (10 μM), forskolin+baclofen (30 μM), forskolin+gabapentin (1 mM) were 603±41, 216±18 and 582±38% of basal, respectively (mean±s.e.m. of n=3 experiments), thereby ruling out the possibility that gabapentin blocks Ca2+ channels via GABAB receptor stimulation.
In contrast to the action of gabapentin, verapamil was more effective in suppressing NO synthesis under higher depolarized conditions – 50 mM KCl compared with 30 mM KCl. We could not fully explain the difference in the mode of Ca2+ channel blockade between gabapentin and verapamil. The putative mechanism of Ca2+ channel function modulation by gabapentin is binding to the α2δ-subunit of the Ca2+ channel complex (Gee et al., 1996). The α1 subunit constitutes the ion pore and has Ca2+ channel function on its own. Other subunits including α2δ subunits are thought to only modulate the Ca2+ influx through the α1 subunit. Therefore, it is probable that the Ca2+ channel-modulatory action of gabapentin through association with α2δ subunits is not so efficacious as to inhibit the response to intense depolarizing stimuli. Moreover, the α2δ subunits is localized at the extracellular site of membranes, while the voltage sensor is supposedly located in the transmembrane region of α1 subunit. This may explain why the Ca2+ channel-blocking action of gabapentin is less marked as the depolarizing stimuli were intensified. In contrast to the present findings, a few investigators have reported that gabapentin inhibit voltage dependently the Ca2+ currents in cultured rat dorsal root ganglions (Alden & Garcia, 2001; Sutton et al., 2002). At present, we do not know the precise reason for the difference in the mode of Ca2+ channel-blocking action of gabapentin between their results and our data. The difference in the Ca2+ channel subclasses involving the predominant Ca2+ responses between dorsal root ganglion neurons and cerebrocortical neurons may cause such a difference.
On the other hand, the binding site of verapamil is considered to be within the sixth transmembrane segment (S6) of domain IV of the α1-subunit of Ca2+ channels (Hering et al., 1996). Verapamil is reported to block multiple types of Ca2+ channels including L-, N-, and P/Q-type Ca2+ channels in a voltage-dependent manner (Cai et al., 1997). This may be because of the higher affinity of this compound for the activated or inactivated state of Ca2+ channels.
Several lines of evidence have shown that Ca2+ channels, particularly N- and P/Q-type subclasses, are implicated in the pathogenesis of ischemic or hypoxic brain damage. We have recently shown that the enhanced formation of NO is involved in the hypoxia/glucose deprivation-induced injury, in which several NO synthase inhibitors, such as L-NAME and L-NMMA, and an NO scavenger, sodium 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (carboxy-PTIO), effectively inhibit hypoxic injury (Oka et al., 2000a). We also showed that the NO synthesis estimated from the cGMP formation in the extracellular fluids was remarkably enhanced during hypoxic insults (Oka et al., 2000b,2000c;2002). In the present study, the NO synthesis was enhanced by exposing the rat cerebrocortical slices to hypoxia/glucose deprivation or low glucose concentration. Interestingly, the extent of the increase in cGMP formation was proportional to that of LDH leakage. Gabapentin preferentially inhibited the enhancement of NO synthesis as well as LDH leakage induced by mild hypoxic insults, whereas verapamil reversed the changes in these biochemical markers induced by severe but not mild hypoxic insults. Therefore, the cerebroprotective actions of gabapentin and verapamil observed in the present study may be due at least in part to the inhibition of NO synthesis via Ca2+ channel blockade.
Plasma or brain concentrations of gabapentin achieved during monotherapy for epilepsy or neuropathy range between 10 and 100 μM (Fink et al., 2002). In the present study, gabapentin at 100 μM was effective in protecting brain tissues against mild hypoxic insults. Therefore, it is likely that the cerebroprotective action of gabapentin observed in the present study is physiologically relevant.
In conclusion, mRNAs for several types of α2δ subunits of Ca2+ channels were expressed in the rat cerebral cortex. Gabapentin, a ligand for α2δ subunits, inhibited NO synthesis evoked by 30 mM but had a weak inhibitory effect on the response to 50 mM KCl, in which it reversed almost equally the responses mediated by P/Q- and N-type Ca2+ channels. In primary neuronal culture, gabapentin reversed the KCl-induced elevation of [Ca2+]i. In contrast, verapamil inhibited more effectively the response to 50 mM KCl than that to 30 mM KCl. The exposure of rat cerebrocortical slices to hypoxic insults caused an enhancement of NO synthesis, the extent of which was related to that of the LDH leakage. As observed in the reduction in KCl-evoked NO synthesis, gabapentin inhibited NO synthesis and LDH leakage induced by only mild hypoxic insults, while verapamil attenuated only the tissue responses to severe hypoxic insults.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (C) (13672404) from the Ministry of Education, Science, Sports and Culture of Japan.
Abbreviations
- ω-Aga
ω-agatoxin IVA
- [Ca2+]i
intracellular Ca2+
- ω-CTX
ω-conotoxin GVIA
- KRB
Krebs–Ringer bicarbonate solution
- NO
nitric oxide
- LDH
lactate dehydrogenase
References
- ASAKURA K., MATSUO Y., KANEMASA T., NINOMIA M. P/Q-type Ca2+ channel blocker ω-agatoxin IVA protects against brain injury after focal ischemia in rats. Brain Res. 1997;776:140–145. doi: 10.1016/s0006-8993(97)00975-x. [DOI] [PubMed] [Google Scholar]
- ALDEN K.J., GARCIA J. Differential effect of gabapentin on neuronal and muscle calcium currents. J. Pharmacol. Exp. Ther. 2001;297:727–735. [PubMed] [Google Scholar]
- BACKONJA M., BEYDOUN A., EDWARDS K.R., SCHWARTZ S.L., FONSECA V., HES M., LAMOREAUX L., GAROFALO E. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280:1831–1836. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- BRADFORD M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BUCHAN A.M., GERTLER S.Z., LI H., XUE D., HUANG Z.-O., CHAUNDRY K.E., BARNES K., LESIUK H.J. A selective N-type Ca2+-channel blocker prevents CA1 injury 24 h following severe forebrain ischemia and reduces infarction following focal ischemia. J. Cerebr. Blood Flow Metab. 1994;14:903–910. doi: 10.1038/jcbfm.1994.121. [DOI] [PubMed] [Google Scholar]
- CAI D., MULLE J.G., YUE D.T. Inhibition of recombinant Ca2+ channels by benzothiazepines and phenylalkylamines: class-specific pharmacology and underlying molecular determinants. Mol. Pharmacol. 1997;51:872–881. [PubMed] [Google Scholar]
- DI TRAPANI G., MEI D., MARRA C., MAZZA S., CAPUANO A. Gabapentin in the prophylaxis of migraine: a double-blind randomized placebo-controlled study. Clin. Ter. 2000;151:145–148. [PubMed] [Google Scholar]
- ELLIS S.B., WILLIAMS M.E., WAYS N.R., BRENNER R., SHARP A.H., LEUNG A.T., CAMPBELL K.P., MCKENNA E., KOCH W.J., HUI A., SCHWARTZ A., HARPOLD M.M. Sequence and expression of mRNAs encoding the α1 and α2 subunits of a DHP-sensitive calcium channel. Science. 1988;241:1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- FINK K., DOOLEY D.J., MEDER W.P., SUMAN-CHAUHAN N., DUFFY S., CLUSMANN H., GOTHERT M. Inhibition of neuronal Ca2+ influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42:229–236. doi: 10.1016/s0028-3908(01)00172-1. [DOI] [PubMed] [Google Scholar]
- FINK K., MEDER W., DOOLEY D.J., GOTHERT M. Inhibition of neuronal Ca2+ influx by gabapentin and subsequent reduction of neurotransmitter release from rat neocortical slices. Br. J. Pharmacol. 2000;130:900–906. doi: 10.1038/sj.bjp.0703380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEE N.S., BROWN J.P., DISSANAYAKE V.U., OFFORD J., THURLOW R., WOODRUFF G.N. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the α2δ subunit of a calcium channel. J. Biol. Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- HERING S., ACZÉL S., GRABNER M., DÖRING F., BERJUKOW S., MITTERDORFER J., SINNEGGER M.J., STRIESSNIG J., DEGTIAR V.E., WANG Z., GLOSSMANN H. Transfer of high sensitivity for benzothiazepines from L-type to class A (BI) calcium channels. J. Biol. Chem. 1996;271:24471–24475. doi: 10.1074/jbc.271.40.24471. [DOI] [PubMed] [Google Scholar]
- HOFMANN F., LACINOVA L., KLUGBAUER N. Voltage-dependent calcium channels: from structure to function. Rev. Physiol. Biochem. Pharmacol. 1999;139:33–87. doi: 10.1007/BFb0033648. [DOI] [PubMed] [Google Scholar]
- KLUGBAUER N., LACINOVA L., MARAIS E., HOBOM M., HOFMANN F. Molecular diversity of the calcium channel α2δ subunit. J. Neurosci. 1999;19:684–691. doi: 10.1523/JNEUROSCI.19-02-00684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAIRD M.A., GIDAL B.E. Use of gabapentin in the treatment of neuropathic pain. Ann. Pharmacother. 2000;34:802–807. doi: 10.1345/aph.19303. [DOI] [PubMed] [Google Scholar]
- LYNCH J.J., YU S.P., CANZONIERO L.M.T., SENSI S.L., CHOI D.W. Sodium channel blockers reduce oxygen-glucose deprivation-induced cortical neuronal injury when combined with glutamate receptor antagonists. J. Pharmacol. Exp. Ther. 1995;273:554–560. [PubMed] [Google Scholar]
- LYSKO P.G., WEBB C.L., YUE T.-L., GU J.-L., FEUERSTEIN G. Neuroprotective effect of tetrodotoxin as a Na+ channel modulator and glutamate release inhibitor in cultured rat cerebellar neurons and in gerbil global brain ischemia. Stroke. 1994;25:2476–2482. doi: 10.1161/01.str.25.12.2476. [DOI] [PubMed] [Google Scholar]
- MEDER W.P., DOOLEY D.J. Modulation, of K+-induced synaptosomal calcium influx by gabapentin. Brain Res. 2000;875:157–159. doi: 10.1016/s0006-8993(00)02610-x. [DOI] [PubMed] [Google Scholar]
- MINTZ I.M., ADAMS M.E., BEAN B.P. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992;9:85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- NG G.Y., BERTRAND S., SULLIVAN R., ETHIER N., WANG J., YERGEY J., BELLEY M., TRIMBLE L., BATEMAN K., ALDER L., SMITH A., MCKERNAN R., METTERS K., O'NEILL G.P., LACAILLE J.C., HEBERT T.E. Gamma-aminobutyric acid type B receptors with specific heterodimer composition and postsynaptic actions in hippocampal neurons are targets of anticonvulsant gabapentin action. Mol. Pharmacol. 2001;59:144–152. [PubMed] [Google Scholar]
- NICHOLSON B. Gabapentin use in neuropathic pain syndromes. Acta Neurol. Scand. 2000;101:359–371. doi: 10.1034/j.1600-0404.2000.0006a.x. [DOI] [PubMed] [Google Scholar]
- OKA M., HAYASHI S., ITOH Y. Electrophysiological and neurochemical evidence for state-dependent Ca2+ channel blockade by a novel neuroprotective agent NS-7. Pharmacol. Toxicol. 2001;89:123–128. doi: 10.1034/j.1600-0773.2001.d01-145.x. [DOI] [PubMed] [Google Scholar]
- OKA M., HIROUCHI M., ITOH Y., UKAI Y. Involvement of peroxynitrite and hydroxyradical generated from nitric oxide in hypoxia/reoxygenation injury in rat cerebrocortical slices. Neuropharmacology. 2000a;39:1319–1330. doi: 10.1016/s0028-3908(99)00197-5. [DOI] [PubMed] [Google Scholar]
- OKA M., ITOH Y., FUJITA T. Halothane attenuates the cerebroprotective action of several Na+ and Ca2+ channel blockers via reversal of their ion channel blockade. Eur. J. Pharm. 2002;452:175–181. doi: 10.1016/s0014-2999(02)02298-7. [DOI] [PubMed] [Google Scholar]
- OKA M., ITOH Y., UKAI Y. Involvement of Na+ and Ca2+ channel activation and resultant nitric oxide synthesis in glutamate-mediated hypoxic injury in rat cerebrocortical slices. Life Sci. 2000b;67:2331–2343. doi: 10.1016/s0024-3205(00)00814-6. [DOI] [PubMed] [Google Scholar]
- OKA M., ITOH Y., UKAI Y. Preferential inhibition by a novel Na+/Ca2+ channel blocker NS-7 of severe to mild ischemic injury in rat cerebrocortical slices: a possible involvement of a highly voltage-dependent blockade of Ca2+ channel. J. Pharmacol. Exp. Ther. 2000c;293:522–529. [PubMed] [Google Scholar]
- OKA M., ITOH Y., UKAI Y., KIMURA K. Blockade by NS-7, a neuroprotective compound, of both L-type and P/Q-type Ca2+ channels involving depolarization-stimulated nitric oxide synthase activity in primary neuronal culture. J. Neurochem. 1999;72:1315–1322. doi: 10.1046/j.1471-4159.1999.0721315.x. [DOI] [PubMed] [Google Scholar]
- RAMSAY R.E. Clinical efficacy and safety of gabapentin. Neurology. 1994;44:S23–S30. [PubMed] [Google Scholar]
- REGAN L.J., SAH D.W.Y., BEAN B.P. Ca2+ channels in rat central and peripheral neurons: high-threshold current resistant to dihydropyridine blockers and ω-conotoxin. Neuron. 1991;6:269–280. doi: 10.1016/0896-6273(91)90362-4. [DOI] [PubMed] [Google Scholar]
- ROWBOTHAM M., HARDEN N., STACEY B., BERNSTEIN P., MAGNUS-MILLER L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280:1837–1842. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- SUTTON K.G., MARTIN D.J., PINNOCK R.D., LEE K., SCOTT R.H. Gabapentin inhibits high-threshold calcium channel currents in cultured rat dorsal root ganglion neurons. Br. J. Pharmacol. 2002;135:257–265. doi: 10.1038/sj.bjp.0704439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOJCIK W.J., HOLOPAINEN I. Role of central GABAB receptors in physiology and pathology. Neuropsychopharmacology. 1992;6:201–213. [PubMed] [Google Scholar]