Abstract
Kinin B2 receptor antagonists or tissue kallikrein (t-KK) inhibitors prevent oedema formation and associated sequelae in caerulein-induced pancreatitis in the rat. We have now further investigated the mechanism of kinin generation in the pancreas.
Kinins were elevated in the pancreatic tissue already before oedema formation became manifest. Peak values (421±59 pmol g−1 dry wt) were reached at 45 min and remained elevated for at least 2 h; a second increase was observed at 24 h. Pretreatment with the B2 receptor antagonist icatibant abolished kinin formation, while post-treatment was ineffective.
Total kininogen levels were very low in the pancreas of controls, but increased 75-fold during acute pancreatitis. This increase was absent in rats that were pretreated with icatibant.
During pancreatitis, t-KK-like and plasma kallikrein (p-KK)-like activity in the pancreas, as well as trypsinogen activation peptide (TAP) increased significantly. Icatibant pretreatment further augmented t-KK about 100-fold, while p-KK was significantly attenuated; TAP levels remained unaffected.
Endogenous protease inhibitors (α1-antitrypsin, α2-macroglobulin) were low in normal tissues, but increased 45- and four-fold, respectively, during pancreatitis. This increase was abolished when oedema formation was prevented by icatibant.
In summary, oedema formation is initiated by t-KK; the ensuing plasma protein extravasation supplies further kininogen and active p-KK to the tissue. Concomitantly, endogenous protease inhibitors in the oedema fluid inhibit up to 99% of active t-KK. Our data thus suggest a complex interaction between kinin action and kinin generation involving positive and negative feedback actions of the inflammatory oedema.
Keywords: Kallikrein-kinin system, pancreatitis, inflammation, kinins, bradykinin, kininogen, kallikrein, α1-anti-trypsin, α2-macroglobulin
Introduction
Acute pancreatitis is a severe disease that is associated with premature enzyme activation within the pancreatic tissue, intense symptoms of pain and a pronounced oedema formation, which, in a lesser proportion of patients, can progress towards necrosis. While only about 15–20% of cases exhibit this progression to the severe form of necrotizing pancreatitis (Büchler, 1991), the overall mortality is still about 20% (Amurawaye & Brown, 1991). Current intensive treatment regimens primarily aim at relieving pain, reducing stimulation of the exocrine function of the pancreas and maintaining homeostasis. Treatments aiming at interfering with specific inflammatory mechanisms as yet have, however, not resulted in a breakthrough in clinical therapy. During the past decades, the numbers of diagnosed cases and hospital admissions have increased noticeably in Western countries (Eland et al., 2000), so that a better understanding of the pathophysiological mechanisms is of paramount importance.
The pathogenesis of acute pancreatitis is still unclear, but includes biliary, alcoholic, hereditary and idiopathic aetiologies (Karne & Gorelick, 1999). Investigations on pathophysiological mechanisms in acute pancreatitis are mostly carried out in vivo using separate experimental models for the interstitial-oedematous and haemorrhagic-necrotizing forms of the disease, respectively (see Lerch & Adler, 1994). Exocrine hyperstimulation of the pancreas using the cholecystokinin analogue, caerulein (Lampel & Kern, 1977) is the standard experimental model for the more frequent, oedematous, form of acute pancreatitis because it closely mimics clinical cases with respect to histological, ultrastructural, biochemical and haemodynamic findings (Watanabe et al., 1984; Steer & Meldolesi, 1987; Willemer et al., 1990).
In previous investigations on the role of the kinins in caerulein-induced pancreatitis, we have found that the oedema formation in this model is almost entirely due to the release of endogenous kinins which exert their action via activation of kinin B2 receptors (Griesbacher & Lembeck, 1992). Blockade of kinin action with peptide or nonpeptide B2 receptor antagonists not only prevents oedema formation but also prevents further consequences such as hypotension, hypovolaemia, haemoconcentration and accumulation of activated digestive enzymes within the pancreatic tissue (Griesbacher et al., 1993,1995). In a most recent study (Griesbacher et al., 2002), we have found that the same effects can be obtained by selectively inhibiting tissue kallikrein (t-KK), while inhibition of plasma-type kallikrein (p-KK) remained without effect. In the present investigation, we have aimed at obtaining a closer insight into the mechanism of kinin generation during caerulein-induced acute pancreatitis by determining the time-course of the generation of immunoreactive kinins, as well as tissue levels of kininogens, and activities of tissue- and plasma-type kallikrein and endogenous protease inhibitors in the pancreatic tissue. Using these data, we also want to propose to replace the conventional, linear concept of the mechanism of action of the kallikrein–kinin system in inflammation by a more complex concept involving feedback interactions between kinin action and kinin generation.
Methods
Experimental procedure
Female Sprague–Dawley rats (200–250 g body weight; Institute for Laboratory Animal Science and Genetics, University of Vienna, Austria) were anaesthetized with a combination of pentobarbitone sodium (40 mg kg−1, i.p.) and phenobarbitone sodium (20 mg kg−1, i.p.). A jugular vein was cannulated for the i.v. infusion of caerulein (4 nmol kg−1 h−1); control animals were infused with phosphate-buffered saline (8 ml kg−1 h−1) instead. The i.v. infusions were carried out for periods of 10, 45 or 120 min. In further experimental groups, rats were killed by i.p. injection of an overdose of pentobarbitone sodium 6 or 24 h after the beginning of a 2 h infusion of caerulein or saline. In order to prevent the breakdown of kinins by kininase II (identical to angiotensin-converting enzyme) in the pancreatic tissue samples taken for the measurements of immunoreactive kinins (see below), captopril (50 μmol kg−1) was injected i.p.; in order to assure comparable experimental conditions in the entire study, captopril was also administered to the rats in the remaining experiments. The pretreatment with captopril does not interfere with the formation of the inflammatory oedema as the water content of the pancreas in rats with acute pancreatitis following captopril pretreatment (13.2±1.7 g g−1 dry wt; n=5) was similar to that observed in rats without the kininase II inhibitor (13.8±0.4 g g−1 dry wt; n=5). As in previous studies (Griesbacher & Lembeck, 1992; Griesbacher et al., 1993,1995), icatibant (100 nmol kg−1); or its vehicle (154 mM NaCl solution; 1 ml kg−1); was injected s.c. at −20 min. In experiments involving measurements at 6 or 24 h, the injections of icatibant (or NaCl solution) were repeated three times at 2 h intervals at 50 nmol kg−1 (0.5 ml kg−1) since half-life of icatibant in rats is approximately 2 h (Wirth, Aventis AG, personal communication). In these animals, anaesthesia was kept up for about 6–8 h, after which animals were allowed to recover.
All animal experiments followed the Principles of Laboratory Animal Care (NIH) and the Austrian Law on Experiments in Living Animals. Permission for the experiments was granted by the Commission for Animal Experiments of the Austrian Ministry of Science.
Measurements
In order to determine factors released into or activated in the extracellular space of the pancreatic tissue, portions of about 300–500 mg of the gland were excised and weighed. The samples were carefully divided into smaller pieces and placed immediately into 2 ml cold (4°C) 154 mM NaCl solution. For tissue samples intended for the later measurements of immunoreactive kinins, the NaCl solution was supplemented with aprotinin (104 U ml−1), soybean trypsin inhibitor (0.8 mg ml−1), hexadimethrine bromide (polybrene; 4 mg ml−1) 1,10-phenanthroline (10 mg ml−1) and EDTA (20 mg ml−1) (Shimamoto & Iimura, 1987) to prevent degradation of kinins. The samples were then centrifuged at 2 × 105 m s−2 for 25 min, the supernatant was lyophilized in a Christ Beta 2-16 freeze dryer (Rieger Industriesysteme, Vienna, Austria) and stored at −80°C until assayed. For the determination of water content, the tissue was dried overnight in a vacuum centrifuge. The difference between wet and dry weight was taken as fluid content of the samples; water content of tissues is given as fluid weight per dry weight of tissues.
Immunoreactive kinins in the pancreas were determined by radioimmunoassay using antibodies raised in rabbits after coupling of bradykinin to bovine serum albumin with 1-ethyl-3-(3-dimethyl-aminopropyl)carbodiimide (Goodfriend et al., 1964). The detection limit was 50 pg bradykinin with [3H] bradykinin as tracer. The antibodies recognized kinin-like peptides that show agonist activity at B2 receptors, including kallidin (Lys-bradykinin) and rat T-kinin (lle-Ser-bradykinin) (see Table 1). The peptide B2 receptor antagonist icatibant (D-Arg-[Hyp3, Thi5, D-Tic7, Oic8]-bradykinin), as well as peptide agonist or antagonist ligands for the B1 receptor, or kinin degradation fragments were not recognized. Kinin concentrations in the tissue are expressed as picomoles of bradykinin-like immunoreactivity per gram dry weight of tissue.
Table 1.
Crossreactivity of the antibodies used for measurement of immunoreactive kinins
| B2 receptor agonists | % | |||||||||||
| Bradykinin | Arg | Pro | Pro | Gly | Phe | Ser | Pro | Phe | Arg | 100.0 | ||
| Kallidin (Lys-bradykinin) | Lys | Arg | Pro | Pro | Gly | Phe | Ser | Pro | Phe | Arg | 66.7 | |
| Met-Lys-bradykinin | Met | Lys | Arg | Pro | Pro | Gly | Phe | Ser | Pro | Phe | Arg | 58.8 |
| T-kinin (lle-Ser-bradykinin) | lle | Ser | Arg | Pro | Pro | Gly | Phe | Ser | Pro | Phe | Arg | 100.0 |
| [Thr6]-bradykinin | Arg | Pro | Pro | Gly | Phe | Thr | Pro | Phe | Arg | 100.0 | ||
| [Lys1]-bradykinin | Lys | Pro | Pro | Gly | Phe | Ser | Pro | Phe | Arg | 91.0 | ||
| Tyr-bradykinin | Tyr | Arg | Pro | Pro | Gly | Phe | Ser | Pro | Phe | Arg | 83.3 | |
| B2 receptor antagonist | ||||||||||||
| Icatibant | D-Arq | Arg | Pro | Hyp | Gly | Thi | Ser | D-Tic | Oic | Arg | <0.1 | |
| B1 receptor agonist | ||||||||||||
| Des-Arg9-bradykinin | Arg | Pro | Pro | Gly | Phe | Ser | Pro | Phe | — | 1.1 | ||
| B1 receptor antagonist | ||||||||||||
| Des-Arg9-[Leu8]-bradykinin | Arg | Pro | Pro | Gly | Phe | Ser | Pro | Leu | — | 0.1 | ||
| Other analogues | ||||||||||||
| D-Arg-bradykinin | D-Arg | Arg | Pro | Pro | Gly | Phe | Ser | Pro | Phe | Arg | <0.1 | |
| [Tyr8]bradykinin | Arg | Pro | Pro | Gly | Phe | Ser | Pro | Tyr | Arg | 1.3 | ||
| [D-Phe7]bradykinin | Arg | Pro | Pro | Gly | Phe | Ser | D-Phe | Phe | Arg | <0.1 | ||
| Bradykinin fragments | ||||||||||||
| Bradykinin (1–7) | Arg | Pro | Pro | Gly | Phe | Ser | Pro | — | — | <0.1 | ||
| Bradykinin (1–6) | Arg | Pro | Pro | Gly | Phe | Ser | — | — | — | <0.1 | ||
| Bradykinin (1–5) | Arg | Pro | Pro | Gly | Phe | — | — | — | — | <0.1 | ||
| Bradykinin (2–9) | — | Pro | Pro | Gly | Phe | Ser | Pro | Phe | Arg | <0.1 | ||
| Bradykinin (2–7) | — | Pro | Pro | Gly | Phe | Ser | Pro | — | — | <0.1 | ||
Recognition of bradykinin is set as 100%. Amino-acid replacements in the bradykinin sequence are shown underlined.
Kininogen levels in the pancreas at 2 h were quantified as total trypsin-induced kinin generation after acid inactivation of trypsin inhibitors and kinin-metabolizing kininases (Uchida & Katori, 1986). Briefly, 20 μl of the samples obtained by ultracentrifugation were acidified with 180 μl 0.03 M HCl and incubated at 37°C for 15 min. After addition of 10 μl 0.5 M NaOH and 30 μl 0.3 M Tris/HCl (pH 7.8), the samples were incubated at 37°C for 30 min with 10 μl of a 2 mg ml−1 solution of trypsin in 2.5 mM HCl. After addition of 1 ml ethanol and incubation at 70°C for 10 min, the samples were lyophilized and stored at −80°C until assayed by radioimmunoassay for kinins.
For the measurements of kallikrein activities in the pancreas at 2 h, tissue samples of about 500 mg were homogenized and diluted up to 1 : 128 in Tris buffer. Activities of t-KK and plasma kallikrein (P-KK) were determined photometrically using the chromogenic substrates S-2266 (D-Val-Leu-Arg-p-nitroanilide) and S-2302 (D-Pro-Phe-Arg-p-nitroanilide), respectively, as described by Amundsen et al. (1979) and Ito & Statland (1981). All measurements were carried out in duplicates. Purified porcine kallikrein preparations were used to control the performance of the system.
Trypsinogen activation peptide (TAP; Asp-Asp-Asp-Asp-Lys) at the amino terminus of vertebrate trypsinogens is cleaved from trypsinogen during its activation to trypsin and thus is used as a marker for the generation of trypsin during acute pancreatitis (Nakae et al., 1995). TAP was measured using a specific enzyme immunoassay (Biotrin, Dublin, Ireland). Samples of the pancreatic extracts, obtained after a 2 h infusion of caerulein, were serially diluted up to 1 : 512 in assay diluent and measured in duplicates. TAP values are given as picomoles per gram dry weight of tissue.
The activities in the pancreatic tissue of α1-antitrypsin (α1-AT) and α2-macroglobulin (α2-M) were measured at 2 h by commercially available chromogenic substrate tests (Unitest α1-AT assay, Unitest α2-M assay; Unicorn Diagnostics, London, U.K., supplied by CoaChrom Diagnostica, Vienna, Austria). The activity of α1-AT was calculated using a standard plasma containing a known amount (0.95 U ml−1) of α1-AT. The standard plasma used for the calculation of α2-M activities in the pancreatic tissue samples contained 1.06 U ml−1 of α2-M.
Substances
Caerulein (Sigma Chem. Co., St. Louis, MO, U.S.A.) was dissolved in phosphate-buffered saline (composition in mM: NaCl 136.9, KCl 2.7, KH2PO4 1.5, Na2HPO4 7.7). All salts were of analytical grade and were obtained from Merck (Darmstadt, Germany). Icatibant (D-Arg-[Hyp3, Thi5, D-Tic7, Oic8]-bradykinin) was a generous gift of Aventis AG (Frankfurt am Main, Germany). Further substances were: pentobarbitone sodium (Nembutal; Sanofi Santé Animale, Libourne, France), phenobarbitone sodium (Apoka, Vienna), S-2266 and S-2302 (CoaChrom Diagnostica, Vienna, Austria), aprotinin (Trasylol; Bayer, Leverkusen, Germany), soy bean trypsin inhibitor, hexadimethrine bromide, phenanthroline, EDTA, trypsin (diphenylcarbamyl chloride-treated) from bovine pancreas (Sigma, St. Louis, MO, U.S.A.) and kallikrein from porcine pancreas (Calbiochem, San Diego, CA, U.S.A.).
Statistical evaluation
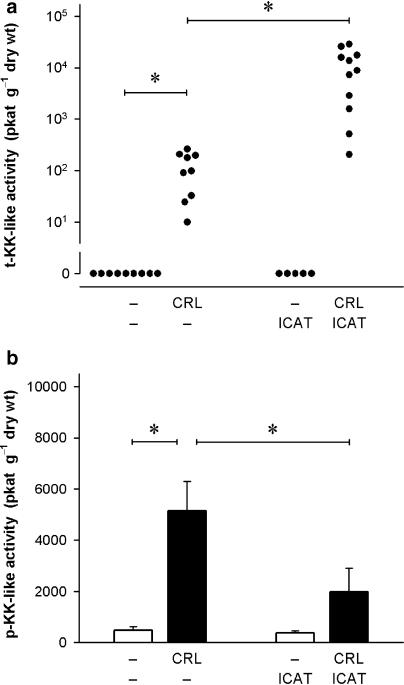
Since the variances of data obtained in caerulein-induced pancreatitis differed greatly from those obtained in control animals without pancreatitis, and data frequently showed significant deviations from normal distributions, comparisons between different treatment groups were made using nonparametric analysis of variance and multiple nonparametric comparisons for independent data (Zar, 1984). Probability values of P<0.05 were considered significant. All values presented are arithmetical means with s.e.mean, except t-KK-like activities in the pancreatic tissue which are shown as individual values on a logarithmic ordinate (see Figure 2a) in order to allow the illustration of differences of values obtained in caerulein-induced pancreatitis from baseline values obtained in control animals.
Figure 2.
Effect of B2 kinin receptor blockade on kallikrein-like activities in the pancreatic tissue during caerulein (CRL)-induced pancreatitis. Activities of (a) tissue-type kallikrein (t-KK) and (b) plasma-type kallikrein (p-KK) were determined after a 2 h infusion of CRL using the chromogenic substrates S-2266 and S-2302, respectively. CRL (4 nmol kg−1 h−1) was infused i.v. for 120 min; control rats received phosphate-buffered saline instead. The B2 antagonist icatibant (ICAT; 100 nmol kg−1) was administered s.c. 20 min before the experiment. Significance of difference as indicated by the horizontal bars: *P<0.05. Values are given as individual values (a) or means+s.e.mean (b); n=5–11.
Results
Oedema formation and immunoreactive kinins in the pancreas
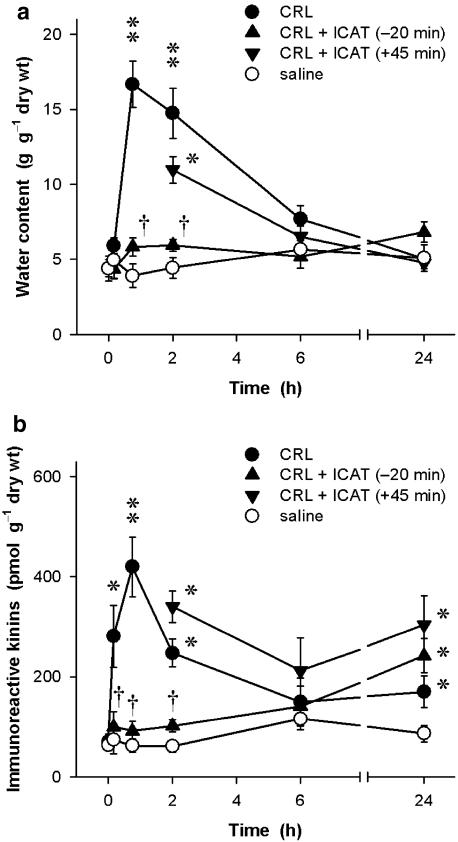
During the acute phase of acute interstitial-oedematous pancreatitis induced by a 2 h infusion of caerulein at a dose used for exocrine hyperstimulation (4 nmol kg−1 h−1), vascular permeability in the pancreatic tissue increased rapidly. The oedema reached maximum values already at 45 min (Figure 1a). Water content of the pancreatic tissue at this time point was three to four-fold higher than that of saline-infused control animals (P<0.01). Already 4 h after the end of a 2 h infusion of caerulein, the pancreatic oedema was largely resolved as the pancreatic water content had returned to values that were not significantly different from that of control tissues. At the same time, however, histological evaluation of the tissue revealed that the cell vacuolization, which is typical for the acinar cell damage, still persisted and also could be found on the day following the experiment (data not shown). Blockade of kinin B2 receptors by pretreatment of the animals with icatibant (100 nmol kg−1, s.c.; repeated up to three times at 2 h intervals with doses of 50 nmol kg−1) completely abolished oedema formation (Figure 1a). Conversely, icatibant had no effect on oedema formation when the antagonist treatment was started 45 min after the beginning of the caerulein infusion, that is, at a time when oedema formation had already reached its peak values.
Figure 1.
Time course of (a) the inflammatory oedema (water content in g g−1 dry wt) and (b) levels of immunoreactive kinins (pmol bradykinin-like immunoreactivity g−1 dry wt) during caerulein-induced acute pancreatitis in anaesthetized rats. Caerulein (CRL; 4 nmol kg−1 h−1 for 2 h) or phosphate-buffered saline (8 ml kg−1 h−1) was infused via a jugular vein. Icatibant (ICAT; 100 nmol kg−1), or 154 mM NaCl (1 ml kg−1), was given s.c. either as a pretreatment 20 min prior to CRL or as treatment 45 min after the start of the experiment. For measurements at 6 and 24 h, the administration of icatibant (50 nmol kg−1) or vehicle (0.5 ml kg−1) was repeated three times at 2 h intervals. Significance of difference from saline controls: *P<0.05, **P<0.01; significance of difference from CRL alone: †P<0.05. Means±s.e.mean; n=5–10.
Immunoreactive kinin levels determined in the same tissue samples also increased rapidly in response to the caerulein infusion (Figure 1b). A significant (P<0.05) increase in immunoreactive kinins was already found after a 10 min infusion of caerulein, that is, at a time when oedema formation had not yet commenced. Maximum kinin concentrations of 421±59 pmol bradykinin-like immunoreactivity per gram dry weight (n=8) were reached at 45 min which represents an about six-fold increase over basal values obtained in rats that had not received caerulein (69±16 pmol g−1 dry wt, n=6; P<0.01). Similar to the time-course of oedema formation, kinin levels had returned to values of control animals by 6 h. However, 24 h after induction of pancreatitis, a second increase in immunoreactive kinins was noted in the tissue. At this time point, kinin levels (171±31 pmol g−1 dry wt, n=8) were about twice as high as those of saline-infused controls (86±17 pmol g−1 dry wt, n=7; P<0.05).
When icatibant was given as a pretreatment 20 min prior to induction of acute pancreatitis with caerulein, the generation of kinins in the pancreatic tissue (Figure 1b) also was almost completely abolished (P<0.05). Immunoreactive kinin values were not different from those of control animals without pancreatitis throughout the period of icatibant treatment (up to 6 h). This effect of icatibant was absent when the antagonist was administered 45 min after the beginning of the induction of pancreatitis, that is, when the B2 antagonist could not have an effect on oedema formation any more. In these animals, immunoreactive kinins determined in the pancreatic tissue at 2 and 6 h were not different from those of caerulein-infused animals without treatment with the antagonist. When kinin levels in the tissue were determined 24 h after the induction of pancreatitis, icatibant had no inhibitory effect on these values regardless of whether the antagonist was given before the onset, or after the complete formation of the acute inflammatory oedema. However, it must be noted that no antagonist was administered later than 6 h after the start of the 120 min caerulein infusion. In control animals without pancreatitis, icatibant had no effect on basal kinin levels in the pancreas and also had no effect on the water content of the tissue (not shown in Figure 1).
Kallikrein activities in the pancreas
The activities of kallikreins, the specific kinin-generating enzymes, were determined using the synthetic chromogenic tripeptide derivatives S-2266 and S-2302, which are substrates for the two major types of kallikrein, t-KK and p-KK, respectively. No t-KK-like activity was found in the pancreatic tissue of control animals. When acute pancreatitis was induced by a 2 h infusion of caerulein, significant levels of t-KK-like activity, ranging from 11 up to 263 pkat g−1 dry wt (n=12), were determined in the tissue (Figure 2a). Control tissues contained low levels of p-KK-like activity (482±134 pkat g−1 dry wt, n=6), which was increased about 10-fold during acute pancreatitis (5143±1153 pkat g−1 dry wt, n=6; P<0.05; Figure 2b). It should be noted that tissue samples used for the determination of t-KK had been stored frozen for a longer period than the samples used for the measurement of p-KK-like activity, so that a direct comparison between t-KK and p-KK-like activities should not be made. In a recent investigation (Griesbacher et al., 2002) basal t-KK-like activities in pancreas samples that had been stored frozen for less than 1 week were indeed measurable (10–350 pkat g−1 dry wt), but still lower than basal p-KK-like activities in the pancreatic tissue (200–1100 pkat g−1 dry wt).
Prevention of oedema formation by pretreatment with icatibant further augmented t-KK-like activity in the pancreas significantly (P<0.05) about 100-fold (11,200±3035 pkat g−1dry wt, n=11). In contrast, the p-KK-like activity in the pan-creatic tissue was significantly attenuated (1979±931 pkat g−1dry wt, n=6; P<0.05) by the B2 receptor antagonist. In control rats without induction of pancreatitis, the basal activities of both t-KK and p-KK remained unchanged by the pretreatment with icatibant.
Trypsinogen activation
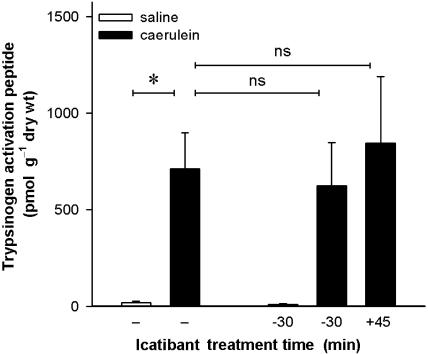
Activation of trypsinogen into trypsin was determined by measurement of the aequimolar activation by-product, TAP (Figure 3). The pancreatic tissue of control animals without pancreatitis only showed very low TAP concentrations (21±7 pmol g−1 dry wt, n=6). After induction of pancreatitis by a 2 h infusion of caerulein, these values were increased about 35-fold to 711±188 pmol g−1 dry wt (n=10; P<0.05). Blockade of kinin B2 receptors with icatibant did not have any effect on trypsinogen activation at this time point, regardless of whether the antagonist was given as a pretreatment 20 min before caerulein or 45 min after the start of the caerulein infusion. Icatibant also did not affect the low basal values of trypsinogen activation in rats without induction of pancreatitis.
Figure 3.
Effect of the B2 receptor antagonist icatibant on activation of trypsin during acute caerulein-induced pancreatitis. Trypsin activation was quantified by measuring the by-product of trypsinogen activation, trypsinogen activation peptide, at the end of a 2 h infusion of caerulein (4 nmol kg−1 h−1) or its vehicle (phosphate-buffered saline; 1 ml kg−1 h−1). The B2 antagonist icatibant (100 nmol kg−1) or its vehicle (154 mM NaCl solution, 1 ml kg−1) was injected s.c. either 20 min before or 45 min after the start of the i.v. infusion of caerulein or saline. Significance of difference as indicated by the horizontal bars: *P<0.05. Means+s.e.means; n=5–10.
At 24 h after the induction of pancreatitis, the tissue concentrations of TAP returned to values identical to those obtained in control animals (1.0–34 pmol g−1 dry wt). These values were also not affected by the treatment with icatibant (data not shown in Figure 3).
Kininogen concentrations in the pancreas
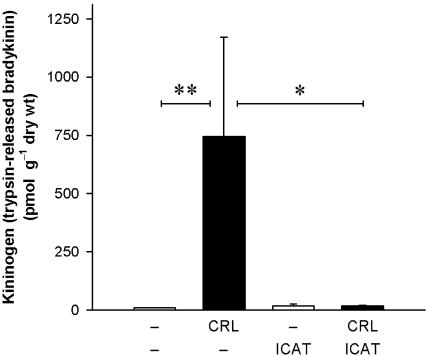
Kininogen values in the pancreas (Figure 4) were determined as total kinin release after trypsin digestion of kininogens after acid treatment for the destruction of endogenous kininogenase inhibitors and kinin-metabolizing enzymes. Kinins measured by radioimmunoassay thus provide a measure for total kininogens in the samples assayed. Basal kininogen levels in the pancreatic tissue were low and amounted to 9.9±0.5 pmol g−1 dry wt (n=6). During acute pancreatitis, total kininogen levels increased markedly (745±426 pmol g−1dry wt, n=7; P<0.01), but were highly variable between individual animals. Prevention of oedema formation by pretreatment with the B2 receptor antagonist icatibant completely abolished the caerulein-induced increases in total kininogens in the pancreas (17±3 pmol g−1 dry wt, n=6; P<0.05). Basal kininogen levels in animals without induction of pancreatitis were not affected by icatibant.
Figure 4.
Effect of the B2 receptor antagonist icatibant on kininogen levels in the pancreatic tissue during caerulein-induced pancreatitis. Kininogen was quantified as the amount of immuno-reactive kinins after trypsin-treatment of tissue extracts. All tissue samples were taken at the end of a 2 h i.v. infusion of caerulein (CRL; 4 nmol kg−1 h−1) or its vehicle, phosphate-buffered saline (8 ml kg−1 h−1). Icatibant (100 nmol kg−1) was given s.c. 20 min prior to the start of the experiment; control animals were injected with an appropriate volume (1 ml kg−1) of a 154 mM NaCl solution. Significance of difference as indicated by the horizontal bars: *P<0.05, **P<0.01. Values are means+s.e.mean; n=4–7.
Endogenous serine protease inhibitors
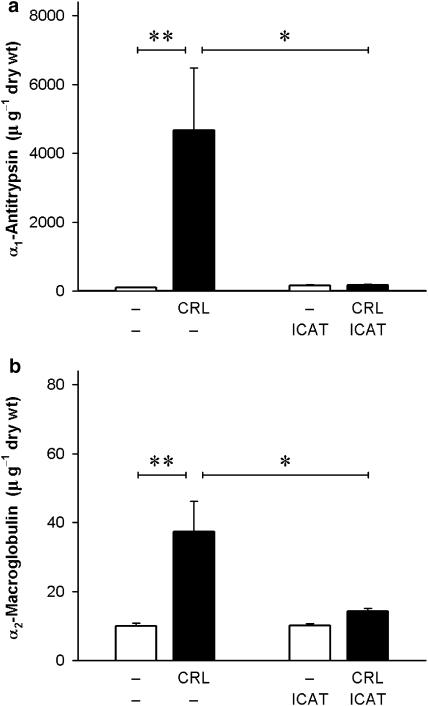
α1-AT and α2-M were determined in the pancreatic tissue (Figure 5) as examples of endogenous serine protease inhibitors, which are capable of inhibiting the activity of kinin-generating enzymes. Basal levels in control animals without pancreatitis were similar for both inhibitors (9.9±0.5 U g−1 dry wt, n=5, for α1-AT, and 10.1±0.7 U g−1dry wt, n=5, for α2-M). During caerulein-induced pancreatitis, both α1-AT and α2-M increased significantly. However, this increase was much more pronounced for α1-AT (Figure 5a), which increased more than 45-fold (4675±1802 U g−1 dry wt, n=6; P<0.01), as compared to α2-M (Figure 5b), which was increased only about four-fold (37.3±8.8 U g−1 dry wt, n=6; P<0.01). These increases in the tissue levels of α1-AT and α2-M activities were completely absent in rats following pretreatment with icatibant (P<0.05). Basal values of both α1-AT and α2-M in control animals without pancreatitis remained unaffected by the B2 receptor antagonist.
Figure 5.
Activities of (a) α1-antitrypsin (α1-AT) and (b) α2-macroglobulin (α2-M) in the pancreatic tissue in acute caerulein-induced pancreatitis in the rat. Caerulein (CRL; 4 nmol kg−1 h−1 for 2 h) was infused via a jugular vein; control animals received phosphate-buffered saline (1 ml kg−1 h−1). The B2 receptor antagonist icatibant (ICAT; 100 nmol kg−1), or its vehicle (154 mM NaCl solution, 1 ml kg−1), was injected s.c. 20 min prior to the experiment. Tissue samples were taken immediately at the end of the 2 h infusion. Significance of difference as indicated by the horizontal bars: *P<0.05, **P<0.01. Columns represent mean values, vertical lines give s.e.mean; n=4–7.
Discussion
The endogenous kinins, bradykinin (Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg) and kallidin (Lys-bradykinin), have potent vasodilator and permeability-enhancing properties. Furthermore, kinins are probably the most potent endogenous pain-inducing mediators (Juan & Lembeck, 1974). Kinin actions are mediated by the constitutive kinin B2 receptor, or, especially after tissue damage, by the inducible B1 receptor (Regoli et al., 1993). As a result of their actions, the release of kinins has been implicated in the pathophysiology of tissue injury and inflammation (Marceau et al., 1983). Kinins are released from larger precursor peptides, high molecular weight kininogen (HK) and low molecular weight kininogen (LK) by specific enzymes, the kallikreins. In addition, cellular proteases, which are not selective for kininogens, may also contribute to kinin formation, especially after cell injury (Proud & Kaplan, 1988). As the better part of experimental studies on the role of the kallikrein–kinin system in vivo are carried out in rats, a particularity of the kallikrein–kinin system in this species has to be borne in mind. In rats, a third kinin, lle-Ser-bradykinin (T-kinin), can be released from its precursor, T-kininogen, by trypsin and certain other proteases or by a specific T-kininogenase but not by t-KK or p-KK (Greenbaum & Okamoto, 1988).
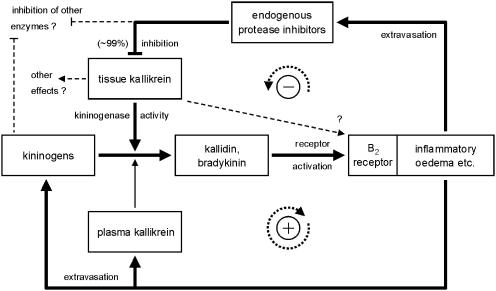
In the present investigation, we have aimed at more closely investigating the components of the kallikrein–kinin system (kinins, kallikreins, kininogens) in order to get a better insight into the mechanism of kinin formation during this inflammatory disease (Figure 6).
Figure 6.
Proposed mechanism of the interaction of kinin action and kinin generation during acute caerulein-induced pancreatitis in the rat. Kinins are generated by activated t-KK from kininogens, and subsequently activate B2 receptors to increase vascular permeability. Extravasation of plasma proteins is required for further influx of kininogens, kinin generation and oedema formation, thus forming a positive feedback loop. Active p-KK also is brought into the tissue with the oedema and may contribute to kinin generation in parallel to t-KK once the positive feedback has been initiated. A suggested direct activation of B2 receptors by t-KK could, if at all, only contribute to the very initial increases in vascular permeability. Up to 99% of the activity of t-KK is inhibited by endogenous protease inhibitors supplied to the tissue by the inflammatory oedema, forming a potent negative feedback mechanism.
Immunoreactive kinins in the pancreas
Kinins were measured by radioimmunoassay in order to investigate the time-course of kinin generation during acute pancreatitis. The antibodies that were used in the present study exclusively recognized kinin-like peptides with agonist activity on the kinin B2 receptor (compare Table 1). The somewhat lower cross-reactivity of the antibodies with kallidin (about 67%) could lead to slight underestimates if kallidins were the predominant kinin present in the samples assayed. However, kallidin (Lys-bradykinin) is absent in the rat, since the sequences of rat HK or LK contain an arginine instead of a lysine residue preceding the bradykinin sequence (Kato et al., 1985), while Arg-bradykinin does not seem to be released (Girolami et al., 1986; Hagiwara et al., 1995). Rat T-kinin (lle-Ser-bradykinin) is equally well recognized as bradykinin, whereas icatibant or bradykinin metabolites do not crossreact with the antibodies.
Although peak values of immunoreactive kinins (see Figure 1) were reached at the same time as the inflammatory oedema, that is, at about 45 min, kinins were already significantly elevated at a time when an increase in vascular permeability was not yet observed, that is, at 10 min, suggesting that kinin generation is initiated in the tissue itself. Kinin levels returned to values not different to those of control animals by 6 h (4 h after the end of the caerulein infusion), which correlated with the almost complete resolution of oedema formation at that time point. A second interesting observation was that at 24 h, a second, significant, increase in kinin levels could be observed. Even rats that had received a treatment with icatibant during the acute stage of the inflammation exhibited this second phase of kinin generation in the pancreas. For practical reasons, the treatment of the experimental animals with icatibant had been stopped 6 h after the induction of pancreatitis. Although icatibant has a duration of action in vivo, which in rats may extend to several hours after a single injection (Lembeck et al., 1991; Wirth et al., 1991), the antagonist must be expected to be fully metabolized or cleared well before the last time point of observation, that is, at 24 h. It is interesting to note that at this time point no increased water content of the tissue could be observed despite the elevation in tissue kinin levels. This should certainly be addressed in future investigations.
Surprisingly, blockade of kinin actions using the kinin B2 receptor antagonist icatibant also completely prevented the increases in immunoreactive kinins in the pancreas (see Figure 1b). The underlying reason for this observation must have occurred during the very early stages of the acute inflammation, as treatment with icatibant at 45 min, that is, at a time when the oedema formation had been complete, remained without consequence for tissue kinin levels. Kinin B2 receptor antagonists do not interfere with the induction of pancreatitis itself, as such compounds do not inhibit acinar cell damage (Lerch et al., 1995). However, a direct effect of icatibant on the activity of kallikrein, or changes in kininogen supply to the pancreatic tissue by prevention of the kinin-mediated oedema could serve as potential explanations.
Kallikrein activities in the pancreatic tissue
Kallikreins are the specific kininogenases cleaving kinins from their precursors, the kininogens. The B2 receptor antagonist icatibant is a peptide kinin analogue (D-Arg-[Hyp3, Thi5, D-Tic7, Oic8]-bradykinin; Lembeck et al., 1991), so that it is conceivable that it can interfere with the action of kallikrein. Indeed, a direct, inhibitory, effect of icatibant (Hoe-140) on the activity of rat t-KK has been demonstrated in vitro (Damas et al., 1995). During acute pancreatitis, both t-KK-like and p-KK-like activities are increased in the pancreatic tissue (see Figure 2). The increase in t-KK-like activity is most likely due to the release from acinar cells, where t-KK is localized physiologically (Bendayan & Ørstavik, 1982). Conversely, the increase in p-KK-like activity in the tissue can be attributed to an influx from the blood plasma because this increase was found to be absent after prevention of oedema formation. Since both types of kallikrein are strongly increased during the acute inflammation, the fact that all kinin-mediated effects in the caerulein model can be prevented by inhibition of t-KK but not of p-KK (Griesbacher et al., 2002) needs some further consideration. The most likely explanation is that an action of t-KK is required for the initial increases in vascular permeability, while p-KK only contributes to kinin release in parallel to t-KK once p-KK has extravasated into the tissue. An inhibition of p-KK hence will not have an effect on oedema formation if t-KK activity in the tissue is high enough to account for kinin generation on its own. In contrast, an inhibitor of t-KK will be effective since it not only inhibits t-KK directly, but also prevents the influx of p-KK and therefore eliminates, or at least significantly reduces, the action of both types of kallikrein in the pancreatic tissue (compare Figure 5 in Griesbacher et al., 2002).
Trypsin is another protease that could potentially contribute to kinin release. TAP is frequently measured to estimate trypsin activation. In the present investigation, elevated tissue levels of TAP were indeed observed (see Figure 3). However, based on the results with selective KK inhibitors, we have recently concluded that trypsin is not likely to contribute to kinin generation in caerulein-induced pancreatitis (Griesbacher et al., 2002). Furthermore, trypsin activation is not dependent on oedema formation.
However, a rather puzzling finding was that pretreatment with icatibant was associated with an about 100-fold increase in t-KK-like activity in the pancreatic tissue during the acute inflammation. A direct effect of icatibant on t-KK, therefore, could be excluded as the reason why blockade of B2 receptors almost completely prevents the generation of kinins in the pancreas.
Kininogen in the pancreas
Kininogen levels in the pancreatic tissue are almost undetectable under basal conditions, but increase up to more than 100-fold during acute pancreatitis (see Figure 4). The source of the increased levels of kininogen in the pancreas most likely is influx from the plasma rather than synthesis within the tissue, as production of kininogen following an inflammatory stimulus has only been demonstrated in the liver, but not in other organs of the rat (Chao et al., 1988). Initial, minimal, increases in vascular permeability will supply kininogen to the tissue which then is available for the generation of kinins and will further increase vascular permeability including a further influx of kininogens. Kinin-mediated plasma protein extravasation supplying kininogen to the tissue for further kinin generation thus constitutes an essential positive feedback loop which is required for the build-up of the inflammatory oedema.
Pretreatment with the kinin B2 receptor antagonist icatibant completely abolished the accumulation of kininogen in the pancreas during acute pancreatitis (see Figure 4), which shows that prevention of oedema formation and plasma protein extravasation also precludes the influx of kininogens from the plasma into the pancreatic tissue. This also can explain why under these conditions, despite the substantial further augmentation of t-KK activity, tissue levels of immunoreactive kinins do not increase but are comparable to control baseline levels (Figure 1b). Two other, hypothetical, explanations are highly unlikely. Firstly, a complete consumption of kininogen by the highly increased kallikrein activity would require the presence of kininogen before cleavage can take place. In addition, increased kininogen consumption should result in increased, rather than decreased, kinin levels in the tissue. Secondly, a direct, inhibitory, effect of icatibant on kininogen synthesis in the liver has not been reported to date; in fact, the B2 receptor antagonist might even increase (T-)kininogen synthesis during inflammation (Raymond et al., 1996). Based on the present results, the general concept that ‘kinin generation is primarily determined by the activity and availability of kallikrein since the level of kininogen is not a rate-limiting factor' (e.g. Chao & Chao, 1995) should be reconsidered. At least in acute pancreatitis in vivo, kinin generation in the pancreatic tissue is in fact absolutely dependent upon the availability of kininogens in the tissue, which by itself requires the increase in vascular permeability for plasma proteins.
An interesting investigation by Hecquet et al. (2000), using cultured cells stably transfected with the human B2 receptor, has suggested that kallikreins and other serine proteases can also activate B2 receptors directly, that is, independent of kinin generation from kininogens. However, a direct activation of B2 receptors by kallikrein could serve as a possibility only for an initial increase in vascular permeability, that is, when kininogens in the tissue are absent or very low. The formation of an overt oedema still depends on the influx of kininogen into the tissue with the subsequent release of kinins (see above).
Activity of endogenous protease inhibitors in the pancreas
When measuring kallikrein activity, it must be taken into account that endogenous inhibitors may limit the activity of the enzyme. In plasma, a number of such endogenous protease inhibitors are present (Fritz et al., 1979) which could gain access to the pancreatic tissue with the inflammatory plasma protein extravasation. As examples of such inhibitors, we have measured the activity of α1-AT and α2-M (see Figure 5). While α1-AT potently inhibits t-KK (Hörl et al., 1982), α2-M also inhibits p-KK (Harpel, 1970). Indeed, we could demonstrate that both inhibitors significantly increase in the pancreatic tissue during caerulein-induced pancreatitis. Hence, it must be expected that kallikrein activity during acute oedematous pancreatitis is the combined result of an activation of the enzyme and a concomitant inhibition by endogenous protease inhibitors. The prevention of the oedema formation completely abolished the influx of α1-AT, α2-M, and very likely also that of other endogenous inhibitors, which can explain the further increase in t-KK activity in the pancreas of icatibant-pretreated animals. Based on the fact that in the absence of oedema formation t-KK activity increases by about 100-fold, it can be estimated that the endogenous protease inhibitors supplied by the inflammatory oedema inhibit up to 99% of t-KK, and certainly will have a similarly potent inhibitory effect on other enzymes activated in the tissue during acute pancreatitis. The absence of such inhibitory components after prevention of oedema formation could well serve as an explanation why pretreatment with icatibant has been reported to be associated with the occurrence of vascular damage in the later stages of caerulein-induced acute pancreatitis (Weidenbach et al., 1995). With respect to proteinase inhibition, it is noteworthy that kininogens also function as inhibitors of cysteine proteinases such as cathepsins (Müller-Esterl et al., 1986) of which some may contribute significantly to pathological trypsinogen activation in the early course of pancreatitis (Lerch et al., 2000). Such an inhibitory function may even be more pronounced after the kinin sequence has been cleaved out of the kininogen molecule (Higashiyama et al., 1986).
Conclusions
In summary, our data support a more complex view of the mode of operation of the kallikrein–kinin system in an acute inflammatory disease. While the system has traditionally been described basically in a linear fashion, it is now evident that it involves potent positive as well as negative feedback functions. During the acute stage of caerulein-induced pancreatitis, kinins are generated by t-KK, and subsequently activate kinin B2 receptors to increase vascular permeability. A recently suggested direct activation of B2 receptors by KK could, if at all, only play a role in the initial induction of increased vascular permeability, that is, when no, or very little, kininogen is present in the tissue. The substrates for kinin generation, the kininogens, are, for the most part, supplied to the tissue by the kinin-mediated influx of plasma proteins. The inflammatory oedema only then builds up along this positive feedback loop. Kallikrein of the plasma type also gains access to the tissue in active form, thus further augmenting the kinin-generating potential in the pancreas. In contrast to this proinflammatory role of oedema formation, endogenous protease inhibitors in the oedema fluid inhibit up to 99% of the activated t-KK and conceivably may have similar effects on other proteases that become activated in the tissue during acute inflam-mation. Our data, therefore, suggest that oedema formation, in addition to its proinflammatory role, must be regarded also as a mechanism of considerable relevance for protection from tissue damage. This protective role of inflammatory oedema formation has not been addressed with appropriate interest in the past and should be studied in much more detail in future investigations.
Acknowledgments
The present investigation was supported by the Jubilee Funds of the Austrian National Bank (ÖNB grant 6507).
Abbreviations
- α1-AT
α1-antitrypsin
- α2-M
α2-macroglobulin
- D-Tic
D-(1,2,3,4-tetrahydroisoquinolin-3-yl-carbonyl)
- HK
high molecular weight kininogen
- Hyp
trans-(4-hydroxy)-L-proline
- LK
low molecular weight kininogen
- Oic
L-((3aS,7aS)-octahydroindol-2-yl-carbonyl)
- p-KK
plasma kallikrein
- TAP
trypsinogen activation peptide
- Thi
L-(3-(2-thienyl)alanyl)
- t-KK
tissue kallikrein
References
- AMUNDSEN E., PUTTER J., FRIBERGER P., KNOS M., LARSBRATEN M., CLAESON G. Methods for the determination of glandular kallikreins by means of a chromogenic tripeptide substrate. Adv. Exp. Med. Biol. 1979;120A:83–95. doi: 10.1007/978-1-4757-0926-1_9. [DOI] [PubMed] [Google Scholar]
- AMURAWAYE E.O., BROWN R.A. Acute pancreatitis – 30 years' experience at a teaching hospital. Can J. Surg. 1991;34:137–143. [PubMed] [Google Scholar]
- BENDAYAN M., ØRSTAVIK T.B. Immunocytochemical localization of kallikrein in the rat exocrine pancreas. J. Histochem. Cytochem. 1982;30:58–66. doi: 10.1177/30.1.6915073. [DOI] [PubMed] [Google Scholar]
- BÜCHLER M. Objectification of the severity of acute pancreatitis. Hepatogastroenterology. 1991;38:101–108. [PubMed] [Google Scholar]
- CHAO J., CHAO L. Biochemistry, regulation and potential function of kallistatin. Biol. Chem. Hoppe Seyler. 1995;376:705–713. [PubMed] [Google Scholar]
- CHAO J., SWAIN C., CHAO S., XIONG W., CHAO L. Tissue distribution and kininogen gene expression after acute-phase inflammation. Biochim. Biophys. Acta. 1988;964:329–339. doi: 10.1016/0304-4165(88)90033-5. [DOI] [PubMed] [Google Scholar]
- DAMAS J., BOURDON V., SOUZA PINTO J.-C. The myostimulating effect of tissue kallikrein on rat uterus. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;351:535–541. doi: 10.1007/BF00171046. [DOI] [PubMed] [Google Scholar]
- DRAY A., PERKINS M. Bradykinin and inflammatory pain. Trends Neurosci. 1993;16:99–104. doi: 10.1016/0166-2236(93)90133-7. [DOI] [PubMed] [Google Scholar]
- ELAND I.A., STURKENBOOM M.J., WILSON J.H., STRICKER B.H. Incidence and mortality of acute pancreatitis between 1985 and 1995. Scand. J. Gastroenterol. 2000;35:1110–1116. doi: 10.1080/003655200451261. [DOI] [PubMed] [Google Scholar]
- FRITZ H., FINK E., TRUSCHEIT E. Kallikrein inhibitors. Fed. Proc. 1979;38:2753–2759. [PubMed] [Google Scholar]
- GIROLAMI J.P., ALHENC-GELAS F., DOS REIS M.L., BASCANDS J.L, SUC J.M., CORVOL P., MENARD J. Hydrolysis of rat high molecular weight kininogen by purified rat urinary kallikrein: identification of bradykinin as the kinin formed. Adv. Exp. Med. Biol. 1986;198A:137–145. doi: 10.1007/978-1-4684-5143-6_19. [DOI] [PubMed] [Google Scholar]
- GOODFRIEND T.L., LEVINE L., FASMAN G.D. Antibodies to bradykinin and angiotensin: a use of carbodiimides in immunology. Science. 1964;144:1344–1346. doi: 10.1126/science.144.3624.1344. [DOI] [PubMed] [Google Scholar]
- GREENBAUM L.M., OKAMOTO H. T-kinin and T-kininogen. Methods Enzymol. 1988;163:272–282. doi: 10.1016/0076-6879(88)63026-6. [DOI] [PubMed] [Google Scholar]
- GRIESBACHER T., KOLBITSCH C., TIRAN B., LEMBECK F. Effects of the bradykinin antagonist, icatibant (Hoe 140), on pancreas and liver functions during and after experimental pancreatitis in rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;352:557–564. doi: 10.1007/BF00169391. [DOI] [PubMed] [Google Scholar]
- GRIESBACHER T., LEMBECK F. Effects of the bradykinin antagonist, HOE 140, in experimental acute pancreatitis. Br. J. Pharmacol. 1992;107:356–360. doi: 10.1111/j.1476-5381.1992.tb12751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIESBACHER T., RAINER I., TIRAN B., EVANS D.M. Involvement of tissue kallikrein but not plasma kallikrein in the development of symptoms mediated by endogenous kinins in acute pancreatitis in rats. Br. J. Pharmacol. 2002;137:692–700. doi: 10.1038/sj.bjp.0704910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIESBACHER T., TIRAN B., LEMBECK F. Pathological events in experimental acute pancreatitis prevented by the bradykinin antagonist, Hoe 140. Br. J. Pharmacol. 1993;108:405–411. doi: 10.1111/j.1476-5381.1993.tb12817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA Y., KOJIMA M., KURAISHI T., HAYASHI I., MIYATA T., OH-ISHI S. Identification of rat urinary bradykinin as bradykinin. Life Sci. 1995;57:997–1002. doi: 10.1016/0024-3205(95)02035-h. [DOI] [PubMed] [Google Scholar]
- HARPEL P.C. Human plasma alpha2-macroglobulin. An inhibitor of plasma kallikrein. J. Exp. Med. 1970;132:329–352. doi: 10.1084/jem.132.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HECQUET C., TAN F., MARCIC B.M., ERDÖS E.G. Human bradykinin B2 receptor is activated by kallikrein and other serine proteases. Mol. Pharmacol. 2000;58:828–836. doi: 10.1124/mol.58.4.828. [DOI] [PubMed] [Google Scholar]
- HIGASHIYAMA S., OHKUBO I., ISHIGURO H., KUNIMATSU M., SAWAKI K., SASAKI M. Human high molecular weight kininogen as a thiol proteinase inhibitor: presence of the entire inhibition capacity in the native form of heavy chain. Biochemistry. 1986;25:1669–1675. doi: 10.1021/bi00355a034. [DOI] [PubMed] [Google Scholar]
- HÖRL W.H., SCHÄFER R.M., HEIDLAND A. Role of urinary alpha1-antitrypsin in Padutin® (kallikrein) inactivation. Eur. J. Clin. Pharmacol. 1982;22:541–544. doi: 10.1007/BF00609628. [DOI] [PubMed] [Google Scholar]
- ITO R., STATLAND B.E. Centrifugal analysis for plasma kallikrein activity, with use of the chromogenic substrate S-2302. Clin. Chem. 1981;27:586–593. [PubMed] [Google Scholar]
- JUAN H., LEMBECK F. Action of peptides and other algesic agents on paravascular pain receptors of the isolated perfused rabbit ear. Naunyn-Schmiedeberg's Arch. Pharmacol. 1974;283:151–164. doi: 10.1007/BF00501142. [DOI] [PubMed] [Google Scholar]
- KARNE S., GORELICK F.S. Etiopathogenesis of acute pancreatitis. Surg. Clin. North Am. 1999;79:699–710. doi: 10.1016/s0039-6109(05)70036-0. [DOI] [PubMed] [Google Scholar]
- KATO H., ENJYOJI K., MIYATA T., HAYASHI I., OH-ISHI S., IWANAGA S. Demonstration of arginyl-bradykinin moiety in rat HMW kininogen: direct evidence for liberation of bradykinin by rat glandular kallikreins. Biochem. Biophys. Res. Commun. 1985;28:289–295. doi: 10.1016/s0006-291x(85)80157-1. [DOI] [PubMed] [Google Scholar]
- LAMPEL M., KERN H.F. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch. [A] Pathol. Anat. Histol. 1977;373:97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]
- LEMBECK F., GRIESBACHER T., ECKHARDT M., HENKE S., BREIPOHL G., KNOLLE J. New, long-acting, potent bradykinin antagonists. Br. J. Pharmacol. 1991;102:297–304. doi: 10.1111/j.1476-5381.1991.tb12169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LERCH M.M., ADLER G. Experimental animal models of acute pancreatitis. Int. J. Pancreatol. 1994;15:159–170. [PubMed] [Google Scholar]
- LERCH M.M., HALANGK W., KRÜGER B. The role of cysteine proteases in intracellular pancreatic serine protease activation. Adv. Exp. Med. Biol. 2000;477:403–411. doi: 10.1007/0-306-46826-3_41. [DOI] [PubMed] [Google Scholar]
- LERCH M.M., WEIDENBACH H., GRESS T., ADLER G. Effect of kinin inhibition in experimental acute pancreatitis. Am. J. Physiol. 1995;269:G490–G499. doi: 10.1152/ajpgi.1995.269.4.G490. [DOI] [PubMed] [Google Scholar]
- MARCEAU F., LUSSIER A., REGOLI D., GIRAUD G.P. Pharmacology of kinins: their relevance to tissue injury and inflammation. Gen. Pharmacol. 1983;14:209–229. doi: 10.1016/0306-3623(83)90001-0. [DOI] [PubMed] [Google Scholar]
- MÜLLER-ESTERL W., IWANAGA S., NAKANISHI S. Kininogens revisited. Trends Biochem. Sci. 1986;11:336–339. [Google Scholar]
- NAKAE Y., NARUSE S., KITAGAWA M., HIRAO S., YAMAMOTO R., HAYAKAWA T. Activation of trypsinogen in experimental models of acute pancreatitis in rats. Pancreas. 1995;10:306–313. doi: 10.1097/00006676-199504000-00014. [DOI] [PubMed] [Google Scholar]
- PROUD D., KAPLAN A.P. Kinin formation: mechanisms and role in inflammatory disorders. Ann. Rev. Immunol. 1988;6:49–83. doi: 10.1146/annurev.iy.06.040188.000405. [DOI] [PubMed] [Google Scholar]
- RAYMOND P., DÉCARIE A., LANTIN F., RAUT R., MORAIS R., COUTURE R., ADAM A. A role for B1 and B2 kinin receptors in the modulation of T-kininogen during the acute phase response of inflammation. Peptides. 1996;17:1163–1170. doi: 10.1016/s0196-9781(96)00174-x. [DOI] [PubMed] [Google Scholar]
- REGOLI D., JUKIC D., GOBEIL F., RHALEB N.-E. Receptors for bradykinin and related kinins: a critical review. Can. J. Physiol. Pharmacol. 1993;71:556–567. doi: 10.1139/y93-079. [DOI] [PubMed] [Google Scholar]
- SHIMAMOTO K., IIMURA O. Measurement of circulating kinins, their changes by inhibition of kininase II and their possible blood pressure lowering effect. Agents Actions Suppl. 1987;22:297–307. doi: 10.1007/978-3-0348-9299-5_31. [DOI] [PubMed] [Google Scholar]
- STEER M.L., MELDOLESI J. The cell biology of experimental pancreatitis. N. Engl. J. Med. 1987;316:144–150. doi: 10.1056/NEJM198701153160306. [DOI] [PubMed] [Google Scholar]
- UCHIDA Y., KATORI M. Independent consumption of high and low molecular weight kininogens in vivo. Adv. Exp. Med. Biol. 1986;198A:113–118. doi: 10.1007/978-1-4684-5143-6_16. [DOI] [PubMed] [Google Scholar]
- WATANABE O., BACCINO P.M., STEER M.L., MELDOLESI J. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am. J. Physiol. 1984;246:G457–G467. doi: 10.1152/ajpgi.1984.246.4.G457. [DOI] [PubMed] [Google Scholar]
- WEIDENBACH H., LERCH M.M., GRESS T.M., PFAFF D., TURI S., ADLER G. Vasoactive mediators and the progression from oedematous to necrotizing experimental acute pancreatitis. Gut. 1995;37:434–440. doi: 10.1136/gut.37.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLEMER S., BIALEK R., ADLER G. Localization of lysosomal and digestive enzymes in cytoplasmic vacuoles in caerulein-pancreatitis. Histochemistry. 1990;94:161–170. doi: 10.1007/BF02440183. [DOI] [PubMed] [Google Scholar]
- WIRTH K., HOCK F.J., ALBUS U., LINZ W., ALPERMANN H.G., ANAGNOSTOPOULOS H., HENKE S., BREIPOHL G., KÖNIG W., KNOLLE J., SCHÖLKENS B.A. Hoe 140 a new potent and long acting bradykinin-antagonist: in vivo studies. Br. J. Pharmacol. 1991;102:774–777. doi: 10.1111/j.1476-5381.1991.tb12249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAR J.H. Biostatistical Analysis 1984Englewood Cliffs, NJ: Prentice-Hall; 2nd edn [Google Scholar]