Abstract
Urotensin-II (U-II) is among the most potent mammalian vasoconstrictors identified and may play a role in the aetiology of essential hypertension. Currently, only one mouse U-II receptor (UT) gene has been cloned. It is postulated that this protein is solely responsible for mediating U-II-induced vasoconstriction.
This hypothesis has been investigated in the present study, which assessed basal haemodynamics and vascular reactivity to hU-II in wild-type (UT(+/+)) and UT receptor knockout (UT(−/−)) mice.
Basal left ventricular end-diastolic and end-systolic volumes/pressures, stroke volumes, mean arterial blood pressures, heart rates, cardiac outputs and ejection fractions in UT(+/+) mice and in UT(−/−) mice were similar.
Relative to UT(+/+) mouse isolated thoracic aorta, where hU-II was a potent spasmogen (pEC50=8.26±0.08) that evoked relatively little vasoconstriction (17±2% 60 mM KCl), vessels isolated from UT(−/−) mice did not respond to hU-II. However, in contrast, the superior mesenteric artery isolated from both the genotypes did not contract in the presence of hU-II. Reactivity to unrelated vasoconstrictors (phenylephrine, endothelin-1, KCl) and endothelium-dependent/independent vasodilator agents (carbachol, sodium nitroprusside) was similar in the aorta and superior mesenteric arteries isolated from both the genotypes.
The present study is the first to directly link hU-II-induced vasoconstriction with the UT receptor. Deletion of the UT receptor gene results in loss of hU-II contractile action with no ‘nonspecific' alterations in vascular reactivity. However, as might be predicted based on the limited contractile efficacy recorded in vitro, the contribution that hU-II and its receptor make to basal systemic haemodynamics appears to be negligible in this species.
Keywords: UT receptor, knockout mouse, GPR14, SENR, urotensin-II, endothelium, endothelin-1
Introduction
Urotensin-II (U-II) is a cyclic peptide (Pearson et al., 1980) originally isolated from the goby urophysis (Bern & Lederis, 1969), a highly vascularized endocrine organ located at the caudal end of teleost fish spinal cords. Isoforms of this peptide are found not only in fish (Bern et al., 1985; Conlon et al., 1996), but also in amphibians (Conlon et al., 1992) and mammals (Coulouarn et al., 1998; 1999). In addition to altering metabolic (Sheridan & Bern 1986; Kelsall & Balment 1998; Silvestre et al., 2001), CNS (Gartlon et al., 2001; Filipeanu et al., 2002; Lu et al., 2002; Pelletier et al., 2002), osmoregulatory (Shenouda et al., 2002) and pulmonary function (Hay et al., 2000), U-II exhibits profound effects upon the cardiovascular system (Douglas & Ohlstein, 2000a; Maguire & Davenport, 2002). Indeed, human urotensin-II (hU-II) has been characterized as the most potent mammalian vasoconstrictor identified (Ames et al., 1999; Douglas et al., 2000). Recently, elevated U-II expression has been reported in both hypertensive (Matsushita et al., 2001) and heart failure patients (Douglas et al., 2002; Richards et al., 2002) suggesting that U-II isopeptides may play a role in regulating mammalian cardiovascular homeostasis. Similarly, U-II levels are higher in patients on dialysis (Totsune et al., 2001), with renal tubular abnormality (Matsushita et al., 2001) and portal hypertension (Heller et al., 2002), suggesting that U-II may also participate in the (patho)physiology of renal and liver disease.
The G-protein-coupled receptor originally termed GPR14/SENR (Marchese et al., 1995; Tal et al., 1995), and recently renamed the urotensin-II receptor (UT) by IUPHAR (see Douglas & Ohlstein, 2000b), has been identified as a receptor for U-II (Ames et al., 1999; Liu et al., 1999). Human, rat, mouse and monkey UT receptors have been cloned (Marchese et al., 1995; Tal et al., 1995; Ames et al., 1999; Elshourbagy et al., 2002). The amino-acid sequence identity of the monkey UT receptor is 97 and 77% identical to the human and rat sequence, while the mouse UT receptor is 76 and 93% identical to the human and rat sequence, respectively. However, it is unclear as to whether these sequences represent receptor homologues or sequence paralogues. Data to support the existence of UT receptor subtypes are ambiguous at best. Using multi-tissue Northern analysis, Liu et al. (1999) observed different UT receptor transcript sizes in human tissues. Functional data suggesting the existence of UT receptor subtypes have also been reported. Coy et al. (2000) demonstrated that although the somatostatin analogue (Cpa-c[D-Cys-Pal-D-Trp-Lys-Val-Cys]-Cpa-amide) was able to inhibit U-II-induced phasic contraction in the rat isolated aorta, the peptidic moiety was unable to block tonic contraction. Further, Camarda et al. (2002) demonstrated that [Orn8]U-II acts as a full agonist at HEK293 cells expressing recombinant rat UT receptor, but is a competitive antagonist in rat isolated aortae. Although these observations could be interpreted as consistent with the existence of UT receptor subtypes, such evidence is far from definitive.
The UT receptor is clearly expressed in vascular tissue (Ames et al., 1999; Maguire et al., 2000), and it is possible therefore that U-II exerts its effects on vascular smooth muscle tone via an interaction with UT. However, a direct link between U-II, the UT receptor and vasoconstriction has yet to be established (Ames et al., 1999; Douglas & Ohlstein, 2001a). In this study we have used homologous recombination in embryonic stem (ES) cells to generate mice lacking the UT receptor coding region to determine the effect(s) of this receptor on vascular reactivity both in vivo and in vitro.
Methods
All procedures were performed in accredited facilities in accordance with institutional guidelines (Animal Care and Use Committee, GlaxoSmithKline), the requirements of the United Kingdom Animals (Scientific Procedures) Act (1986) and the Guide for the Care and Use of Laboratory Animals (DHSS #NIH 85-23).
Targeting the UT gene and generation of mutant mice
Gene targeting was performed in murine E14.1 ES cells, replacing the single coding exon of the UT receptor locus with a positive selection cassette containing the neomycin phosphotransferase gene (Neo) driven by the phosphoglycerate kinase I (PGK) promoter. 5′- and 3′-homology arms, both of ∼4.0 kb, were cloned from a 129SVJ mouse genomic bacterial artificial chromosome (BAC) library and placed on either side of the positive selection cassette. Homologous recombination in neomycin-resistant ES cells was confirmed by Southern blot of BamHI-digested genomic DNA using an ∼800 bp BamHI/SmaI restriction fragment as the 5′ external probe (which detects 6.5 and 6.0 kb bands at the wild-type and targeted locus, respectively). Approximately one in 80 G418-resistant clones had undergone homologous recombination. Homologous recombination at the 3′ end was confirmed in these ES cell clones by Southern blot of HindIII-digested genomic DNA using a ∼700 bp XmnI/HindIII restriction fragment as the 3′-external probe (which detects 5.5 and 5.0 kb bands at the wild-type and targeted locus, respectively). Three targeted clones were injected into C57B16/J-derived blastocysts. Male chimeras were crossed with C57B16/J females to give N1F0 offspring, which were subsequently intercrossed to generate N1F1 offspring. In addition, N1F0 offspring were successively backcrossed to C57B16/J females to generate N5F0 mice. These were intercrossed to create an N5F1 population.
Genotyping of study populations
N1F1 and N5F1 study populations were genotyped by polymerase chain reaction (PCR) and Southern blot of genomic DNA isolated from the hearts of animals used in the studies. Hearts of wild-type (UT(+/+)) and UT receptor knockout (UT(−/−)) mice were cut into small pieces (∼1 mm) and placed into polypropylene tubes. Extraction buffer (2 ml; 10 mM Tris-HCl pH 8.0, 50 mM NaCl, 100 mM ethylenediaminetetraacetic acid (EDTA), 0.5% SDS, 20 μg ml−1 RNase, 100 μg ml−1 Proteinase K) was added and samples were incubated at 50–55°C for 4 h until completely lysed. The mixture was then extracted two times each with phenol, phenol/chloroform and chloroform (Maniatis et al., 1989). Genomic DNA was precipitated by adding 2.5 volumes of cold ethanol, washed with 70% v v−1 ethanol (−20°C) and dissolved in 200 μl of (10 mM Tris-HCl, 1 mM EDTA TE, pH 8.0). Once dissolved, the purity and concentration of the DNA were measured by spectrophotometry (absorbency at 260 and 280 nm wavelength).
PCR amplification was performed in 50 μl aliquots (50 mM KCl, 10 mM Tris-HCl [pH 8.3], 17 mM MgCl2, 200 nM 2′-deoxynucleotide 5′-triphosphates (dNTPs), 10% v v−1 dimethylsulphoxide, 1.25 U Taq DNA polymerase; Perkin-Elmer, Norwalk, CT, U.S.A.) using 200 ng of genomic DNA as the template and PCR primers specific to the neomycin resistance gene present at the targeted locus (5′-TGA ACA AGA TGG ATT GCA CGC AGG TTC TCC GGC-3′ and 5′-GCC AAG CTC TTC AGC AAT ATC ACG GGT AGC-3′, yielding an ∼700 bp product) and mouse UT gene-specific primers (5′-CTG GCT GAC CTG CTG TAT CTG CT-3′ and 5′-CAG GGT CAC ACA AAG CAC TCT CA-3′, yielding an ∼900 bp product). A 500 bp mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) amplicon was used as the internal control (5′-TGG CCA AGG TCA TCC ATG AC-3′ and 5′-GTC CAC CAC CCT GTT GCT GTA G-3′, yielding an ∼500 bp product). Amplification was performed for 30 cycles at 60°C annealing for 30 s, 72°C extension for 90 s and 94°C denaturing for 30 s. Amplification of a 500 bp (GAPDH)/700 bp doublet alone corresponded to a UT(−/−) genotype and a 500 bp (GAPDH)/900 bp doublet alone corresponded to a UT(+/+) genotype.
Genomic DNA (20 μg) was digested with BamHI and run on an agarose gel (1%). The agarose gel was then treated with denaturing solution (0.5 M NaOH, 1.5 M NaCl) for 45 min, followed by neutralization with 0.5 M Tris-HCl (pH 7.5), 1.5 M NaCl for 30–40 min. The DNA was transferred to a nylon membrane (GeneScreen Plus, NEN Life Science Products, MA, U.S.A.) and probed with cDNA corresponding to the full-length mouse UT receptor open reading frame (ORF). cDNA fragments were labelled with [α-32P] 2′-deoxycytidine 5′-triphosphate (dCTP) using standard random primed methods (T7 QuickPrime; Pharmacia Biotech, Piscataway, NJ, U.S.A.). Membranes were prehybridized for 2 h at 42°C and incubated overnight at 42°C with 1 × 109 cpm μg−1 denatured radiolabelled probe in standard buffer (50% deionized formamide, 6 × sodium chloride, sodium citrate (SSC), 5 × Denhardt's reagent, 0.5% sodium dodecyl sulphate (SDS), 100 μg ml−1 denatured, fragmented salmon sperm DNA). Membranes were washed under conditions of low stringency (three 15 min washes in 1 × SSC, 0.1% SDS at 28°C) followed by a high stringency wash in 0.1 × SSC/0.1% SDS for 30 min at 55°C. Hybridization signals were detected by conventional X-ray autoradiography (Hyper film, Amersham Life Science, U.K.) and phosphor imaging (Storm 860, Molecular Dynamics, Sunnyvale, CA, U.S.A.).
Haemodynamics and echocardiography
Male wild-type (UT(+/+)) and homozygous UT receptor knockout (UT(−/−)) mice, anaesthetized with 1.5% isoflurane, underwent transthoracic echocardiographic determination of left ventricular end-diastolic volume (EDV), end-systolic volume (ESV), stroke volume (SV), cardiac output (CO) and ejection fraction (EF). Further to this, mice were reanaesthetized the following day for haemodynamic evaluation where a fluid-filled catheter was inserted into the left carotid artery for the measurement of mean arterial blood pressure (MAP) and heart rate (HR). The catheter was then advanced into the left ventricle (LV) to obtain measurements of left ventricular end- systolic (LVESP) and end-diastolic pressure (LVEDP). At the end of the study, selected organs (right and left kidney, heart, right and left ventricle and lungs) were isolated and wet weights were measured.
Preparation and utilization of mouse isolated aortae and mesenteric arteries
Male (4 months; 27 g) wild-type (UT(+/+)) and UT receptor knockout (UT(−/−)) mice were anaesthetized with inhaled isoflurane (5% in O2) and killed by cervical dislocation. Proximal descending thoracic aortae were isolated and cleaned of adherent tissue. Vessels approximately 3 mm in length were suspended in 10 ml organ baths containing Krebs of the following composition (mM): NaCl, 112.0; KCl, 4.7; KH2PO4, 1.2; MgSO4, 1.2; CaCl2, 2.5; NaHCO3, 25.0; dextrose, 11.0. Krebs was maintained at 37±1°C and aerated with 95% O2 : 5% CO2 (pH 7.4). For contraction studies, vessels were denuded of endothelium by rubbing with a fine forceps and indomethacin (10 μM) was added to the buffer. Changes in isometric force were measured under 0.5g optimal resting tension using FT03 force–displacement transducers (Grass Instruments, Quincy, MA, U.S.A.) coupled to Model 7D polygraphs.
The Halpern–Mulvany wire myograph (Model 610M; Danish Myo Technology, Denmark) was used for measurement of isometric force development of endothelium-intact superior mesenteric arteries (optimal resting tension of 0.5g) and data were recorded using a Grass 7400 direct thermal recorder.
Following a 60 min equilibration period, vessels were treated with standard concentrations of KCl (60 mM) and phenylephrine (1 μM) to which subsequent agonist-induced responses were normalized. Once the contractile response to phenylephrine had plateaued, carbachol (10 μM) was added to the vessels in order to evaluate endothelial integrity.
Cumulative concentration–response curves to phenylephrine (0.1 nM–10 μM), angiotensin-II (0.1 nM–10 μM), endothelin-1 (0.1 nM–1 μM) and hU-II (0.01 nM–3 μM) were obtained for each vessel by adding the spasmogen to the tissue bath at half-log increments. During relaxation studies, vessels were preconstricted with an EC80 concentration of phenylephrine and contractile tone was reversed by adding cumulative amounts of carbachol (1 nM–30 μM) or sodium nitroprusside (SNP; 0.1 nM–1 μM). Each response was allowed to plateau before the addition of subsequent agonist concentrations. Vessels were allowed to recover for at least 30 min between subsequent agonist–response curves, and were not exposed to subsequent agonists after treatment with either endothelin-1 or hU-II.
Statistical and data analysis
All values are expressed as mean±s.e.m. and n represents the total number of animals from which the vessels were isolated. Statistical comparisons were made using an unpaired, two-tailed t-test or Fisher's exact tests and differences were considered significant when P⩽0.05. Concentration–response curves were fitted to a logistic equation as previously described (Douglas et al., 1995):
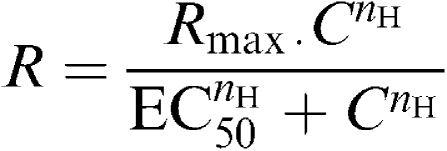 |
where R is the contraction response; C, the concentration of agonist; EC50, the concentration of agonist required to produce a half-maximal response; nH, the Hill coefficient; Rmax, the maximal contractile or relaxant response.
Drugs and reagents
hU-II was custom synthesized by California Peptide Research Inc. (Napa, CA, U.S.A.). Angiotensin-II, carbachol, endothelin-1, phenylephrine and SNP were from Sigma (St Louis, MO, U.S.A.). All other reagents used were of analytical grade. All drug solutions were made fresh on the day of experimentation and stored in a light-tight container on ice.
Results
Targeting of the UT gene and generation of mutant mice
Targeted deletion of the mouse UT receptor gene was successfully achieved using standard homologous recombination in ES cells. Specifically, the single coding exon of the UT receptor was replaced with the positive selection cassette, thus removing all coding sequence (Figure 1a). Chimeric mice derived from the targeted 129Ola ES cell lines were crossed with C57B1/6J females to generate N1F0 heterozygotes, which were intercrossed giving rise to overtly healthy N1F1 homozygous mutant offspring in the expected Mendelian ratio. Successful targeting of the locus and germline transmission was confirmed by Southern analysis (Figure 1b). The N5F1 study population was generated by successive backcrossing of male heterozygotes to C57B1/6J females to generate N5F0 heterozygotes, which were then intercrossed.
Figure 1.
Disruption of the UT receptor gene by homologous recombination. (a) Targeting strategy for the UT receptor locus. (i) Structure of the UT receptor genomic locus. The single coding exon is indicated as urotensin-II receptor open reading frame (‘UT ORF'). The position of the ∼800 bp BamHI/SmaI (B/S; see below) and XmaI/HindIII (X/H; see below) restriction fragment probes used to identify homologous recombination events at the 5′ and 3′ ends, respectively, are also indicated, (ii) Structure of the targeting construct. The 5′ and 3′ arms of homology are ∼4kb SmaI – PstI (S-P; see below) and ∼4 kb XmnI (X, see below) restriction fragments, respectively. The PstI site (P; see below) is 158 bp upstream of the UT initiation codon and the 5′ XmnI site (X; see below) is 40 bp downstream of the termination codon. The PGK-Neo selection cassette is indicated. (iii) Structure of the targeted locus. Homologous recombination replaces the single coding exon with a PGK-Neo selection cassette. Restriction sites: B, BamHII; S, SmaI; P, PstI; H, HindIII; X, XmnI. (b) Southern blot analysis of N1F1 offspring tail DNA (wild-type, UT(+/+); heterozygote, UT(+/−); homozygous UT receptor knockout, UT(−/−)), digested with BamHI and probed with the BamHI/SmaI restriction fragment. The 6.5 kb wild-type locus specific band and 6.0 kb targeted locus specific band are indicated.
PCR and Southern blot analysis of heart genomic DNA was used to identify wild-type mice and mice homozygous for the UT targeted mutation (Figure 2). Wild-type mice show the UT-specific PCR product of 900 bp and lack the 700 bp neomycin resistance gene product. Conversely, mice homozygous for the UT targeted mutation show the neomycin resistance gene product of 700 bp and lack the 900 bp UT-specific PCR product (Figure 2a). This PCR genotyping was confirmed by Southern blot analysis of BamHI-digested genomic DNA probed with a UT gene-specific restriction fragment where, in contrast to UT(+/+) mice, the 6.5 kb BamHI restriction fragment containing the UT receptor gene was absent in UT(−/−) mice (Figure 2b).
Figure 2.
UT receptor gene analysis of wild-type (UT(+/+)) and UT receptor knockout (UT(−/−)) mice as determined by (a) PCR and (b) Southern blot. PCR analysis indicates that the 900 bp UT receptor gene is replaced with the neomycin resistance gene product (Neo) of 700 bp in UT(−/−). The lack of UT receptor gene in UT(−/−) is confirmed by Southern blot analysis of BamHI-digested heart genomic DNA probed with probed radiolabelled with cDNA corresponding to the full-length mouse UT receptor ORF.
Basal haemodynamics, echocardiography and body weight
Targeted deletion of the UT receptor did not alter basal systemic haemodynamics or major organ weights. Basal left ventricular EDV, ESV, SV, CO and EF determined by transthoracic echocardiography in the wild-type (UT(+/+)) mice were similar to those values determined in UT receptor knockout (UT(−/−)) mice (Table 1 ). Inaddition, mean arterial blood pressure, heart rate and left ventricular systolic and end-diastolic pressure were similar in both the genotypes (Table 1). In accord with these haemodynamic data, body weights and organ-to-body-weight ratios were similar in UT(+/+) and UT(−/−) mice (Table 2 ).
Table 1.
Basal echocardiographic and haemodynamic measurements in wild-type (UT(+/+)) and UT receptor knockout (UT(−/−)) mice
| Genotype | MBP (mmHg) | Heart rate (min−1) | LVESP (mmHg) | LVEDP (mmHg) | EDV (ml) | ESV (ml) | SV (ml) | CO (mlmin−1) | EF (%) |
| UT(+/+) | 78±3 | 383±22 | 93±7 | 2.5±0.8 | 0.091±0.002 | 0.057±0.004 | 0.034±0.018 | 14.0±0.9 | 38±2 |
| n | 7 | 7 | 5 | 5 | 15 | 15 | 15 | 15 | 15 |
| UT(−/−) | 79±3 | 369±27 | 87±4 | 1.0±0.3 | 0.086±0.003 | 0.052±0.002 | 0.035±0.002 | 14.2±1.0 | 40±2 |
| n | 8 | 8 | 8 | 8 | 16 | 16 | 16 | 16 | 16 |
All values are expressed as mean±s.e.m. Statistical comparisons were made using unpaired two-tailed t-tests. No statistical differences were identified between the UT(+/+) and UT(−/−) mice.
Table 2.
Organ-to-body-weight ratios in wild-type (UT(+/+)) and UT receptor knockout (UT(−/−)) mice
| Genotype | Body weight (g) | Left kidney(10−3) | Right kidney(10−3) | Heart(10−3) | Left ventricle(10−3) | Right ventricle(10−3) | Lung(10−3) |
| UT(+/+) (n=8) | 27.5±0.9 | 6.1±0.2 | 6.5±0.2 | 4.2±0.2 | 3.2±0.1 | 0.8±0.1 | 4.9±0.2 |
| UT(−/−) (n=8) | 27.1±0.6 | 6.2±0.1 | 6.3±0.1 | 4.3±0.1 | 3.3±0.1 | 0.8±0.1 | 5.0±0.1 |
All values are expressed as mean±s.e.m. Kidney:, heart: and lung: to-body-weight ratios are expressed as mgg−1. Statistical comparisons were made using unpaired two-tailed t-tests. No statistical differences were identified between the UT(+/+) and UT(−/−) mice.
Effects of vasoactive agents in mouse-isolated arteries
Relative to the responses in UT(+/+) mice, where hU-II was a potent (EC50 of ∼5 nM) and low-efficacy (Rmax<20% response to 60 mM KCl) vasoconstrictor, aortae isolated from UT(−/−) mice did not respond to hU-II (Table 3, Figure 3 and Figure 4). However, this observation was not accompanied by any general aberration in vascular reactivity, since responses to unrelated vasoactive agents were unaltered. Thoracic aortae isolated from UT(+/+) and UT(−/−) mice responded to cumulative concentrations of KCl, phenylephrine and endothelin-1 with similar potencies and efficacies (Table 3, Figure 4). Similarly, loss of the UT receptor did not alter the responses to the endothelium-dependent and -independent vasodilators, carbachol and SNP (Table 3, Figure 5).
Table 3.
Responses to various vasoactive compounds in thoracic aortae and superior mesenteric artery isolated from wild-type (UT(+/+)) and UT receptor knockout (UT(−/−)) mice
| Thoracic aorta | −log[EC50] | Rmax (% normalized) | Responders/n | |||
| Vasoactive agent | UT(+/+) | UT(−/−) | UT(+/+) | UT(−/−) | UT(+/+) | UT(−/−) |
| Urotensin-II | 8.35±0.07 | – | 17±2 | 0* | 5/7 | 0/8* |
| Phenylephrine | 7.33±0.07 | 7.39±0.04 | 115±6 | 117±8 | 6/6 | 6/6 |
| Endothelin-1 | 8.51±0.06 | 8.39±0.19 | 15±3 | 16±2 | 9/9 | 8/10 |
| Angiotensin-II | – | – | 0 | 0 | 0/4 | 0/4 |
| KCl | 1.64±0.03 | 1.58±0.03 | 132±8 | 136±7 | 6/6 | 6/6 |
| Carbachol | 6.33±0.17 | 6.08±0.25 | 65±5 | 67±6 | 6/6 | 5/6 |
| Sodium nitroprusside | 8.87±0.09 | 8.85±0.10 | 100±0 | 100±0 | 6/6 | 6/6 |
| Mesenteric artery | −log[EC50] | Rmax (% normalized) | Responders/n | |||
| Vasoactive agent | UT(+/+) | UT(−/−) | UT(+/+) | UT(−/−) | UT(+/+) | UT(−/−) |
| Urotensin-II | – | – | 0 | 0 | 0/6 | 0/6 |
| Phenylephrine | 5.83±0.10 | 5.68±0.13 | 148±8 | 134±14 | 6/6 | 6/6 |
| Endothelin-1 | 8.66±0.32 | 8.84±0.21 | 10±4 | 10±3 | 4/4 | 5/5 |
| Angiotensin-II | – | – | 0 | 0 | 0/4 | 0/4 |
| KCl | 1.42±0.04 | 1.42±0.04 | 244±44 | 384±145 | 5/5 | 5/5 |
| Carbachol | 6.69±0.16 | 6.41±0.13 | 100±0 | 100±0 | 6/6 | 6/6 |
| Sodium nitroprusside | 8.66±0.32 | 8.84±0.21 | 100±0 | 100±0 | 6/6 | 6/6 |
All values are expressed as mean±s.e.m. Statistical comparisons were made using unpaired two-tailed t-tests or Fisher's exact tests:
P<0.01 compared to values obtained in UT(+/+) mice. Responses to KCl are normalized to 1 μM phenylephrine and responses to phenylephrine, endothelin-1, angiotensin-II and urotensin-II are normalized to 60 mM KCl. Responses to carbachol and sodium nitroprusside are expressed as per cent reversal of 1 μM phenylephrine-induced tone.
Figure 3.
Representative experimental traces illustrating the contractile responses to hU-II in the aortae isolated from wild-type (UT(+/+)) and UT receptor knockout (UT(−/−)) mice. In contrast to (a) UT(+/+) mice (where hU-II is a potent spasmogen evoking relatively little vasoconstriction), (b) aortae isolated from UT(−/−) do not respond to hU-II. Vessels were precontracted with 60 mM KCl. Following washing (W), endothelial cell integrity was evaluated by adding 1 μM phenylephrine (PE) and observing the response to 10 μM carbachol (CCh). The vertical and horizontal scale bars represent tension (0.5 and 0.25g) and time (10 min), respectively.
Figure 4.
Loss of the UT receptor does not alter the responses to vasoconstrictors other than hU-II in the mouse isolated thoracic aorta. Log concentration-response curves to (a) hU-II, (b) endothelin-1, (c) phenylephrine and (d) KCl in aortae isolated from wild-type (UT(−/+) and UT receptor knockout (UT(−/−)) mice. Values are mean and vertical bars represent the s.e.m. Curves were derived by fitting experimental data to a logistic equation (Douglas et al., 1995).
Figure 5.
Loss of the UT receptor does not alter the responses to vasodilators in the mouse isolated aorta. Log concentration-response curves to (a) carbachol and (b) SNP in aortae isolated from wild-type (UT(+/+)) and UT receptor knockout (UT(−/−)) mice. Vessels were preconstricted with an EC80 concentration of phenylephrine and contractile tone was reversed by adding cumulative amounts of carbachol or SNP. Values are mean and vertical bars represent the s.e.m. Curves were derived by fitting experimental data to a logistic equation (Douglas et al., 1995).
As in the aorta, responses to endothelin-1, phenylephrine, angiotensin-II, KCl, carbachol and SNP were similar in the superior mesenteric arteries isolated from both the genotypes. However, in contrast to the response to hU-II observed in the aorta (vasoconstriction in UT(+/+) mice), the superior mesenteric artery isolated from both the UT(−/−) and UT(+/+) mice did not contract in the presence of hU-II (Table 3).
Discussion
U-II, among the most potent peptidic mammalian vasoconstrictors identified, is purported to elicit a contractile response via an interaction with its G-protein-coupled receptor, UT (Douglas & Ohlstein, 2000a, 2000b; Maguire & Davenport, 2002). Interestingly, and in contrast to the majority of knockout studies investigating vasoactive peptide systems (where receptor subtypes exist typically), the hU-II/UT system is somewhat atypical inasmuch as no receptor subtypes have been identified to date. As in other mammalian species, one mouse UT receptor gene has been cloned. Therefore, it is assumed that this specific protein mediates U-II-induced vasoconstriction. However, since this has not been previously investigated, the present study represents the first to provide a direct link between the UT receptor and vasoactivity, an association achieved via the generation of UT-receptor-deficient (knockout) mice.
In order to determine the effects of the UT receptor on cardiovascular homeostasis, basal haemodynamic measurements were made in wild-type mice (UT(+/+)) and UT receptor knockout mice (UT(−/−)). Basal echocardiographic and haemodynamic measurements in UT(−/−) mice were similar to those determined in UT(+/+) mice, suggesting that loss of the mouse UT receptor does not uncover an overt cardiovascular phenotype in vivo. This observation is not surprising since systemic administration of hU-II does not effect basal haemodynamics in this species (R. Willette, unpublished observation).
hU-II induces potent vasoconstriction in blood vessels isolated from the rat, rabbit, dog, pig, monkey (Douglas et al., 2000; Saetrum Opgaard et al., 2000; Camarda et al., 2002) and man (MacLean et al., 2000; Maguire et al., 2000; Paysant et al., 2001; Russell et al., 2001; Camarda et al., 2002). Vasoconstriction appears to be dependent on the species and anatomical origin of the vessel, that is, hU-II-induced contraction is restricted to isolated thoracic aortae in the rat and the spasmogen is a universal arterial vasoconstrictor in the cynomolgus monkey (Douglas et al., 2000). Previous studies have demonstrated that vertebrate U-II isopeptides (fish, amphibian and mammalian) exhibit similar functional potencies across species (Gardiner et al., 2001 [haemodynamic responses in the rat]; Russell et al., 2001 [contractile activity in isolated trabeculae]). Indeed, competition binding studies reveal that both mouse and human U-II isopeptides exhibit similarly high affinities (2.5 and 4.0 nM Kis, respectively) for mouse UT receptor (Elshourbagy et al., 2002). Together, these data demonstrate that hU-II is an agonist at the mouse UT receptor and that the relatively poor contractile activity described herein is not likely to reflect the use of a nonmurine (i.e. human) isoform of U-II in mouse isolated tissue. Although Douglas et al. (2000) reported no contractile response to hU-II in the mouse-isolated aorta, the ability of hU-II to contract various arteries isolated from wild-type (UT(+/+)) mice was evaluated. As in the rat and dog, where vasoconstriction has only been observed in vessels proximal to the aortic arch (Douglas et al., 2000), the present study reported that hU-II contracted UT(+/+) mouse isolated aortae but failed to elicit a contractile response in the superior mesenteric arteries. However, in contrast to the response previously observed in the rat aorta, hU-II induced relatively little vasoconstriction in the UT(+/+) mouse aorta (Rmax was only 17±2% response to 60 mM KCl). The fact that the present investigation was able to elicit a contractile response in wild-type UT(+/+) mice, albeit small in size, contrasts a previous report (Douglas et al., 2000) where hU-II was purported to lack contractile activity in mouse aortae. This apparent disparity likely reflects difficulties encountered when trying to record such small responses (i.e. <20% response to 60 mM KCl, likely because of low UT receptor expression/spare receptor reserve in this tissue; Douglas, 2003).
In addition to being a potent vasoconstrictor in vessels isolated from several species, hU-II has been reported to induce vasodilation in rat (Bottrill et al., 2000) and human (Stirrat et al., 2001) isolated resistance arteries. Consistent with the effects in rat-isolated resistance arteries, Gibson et al. (1986), Gardiner et al. (2001) and Lin et al. (2003) have all reported vasodilation to be the predominant cardiovascular response when hU-II is given systemically as a bolus dose in the rat (infusions, however, are associated with pressor responses in the rat; Bennett et al., 2002). Although i.v. administration of hU-II does not induce a systemic hypotensive response in the mouse as it does in the rat (R. Willette, unpublished observation), it remains to be determined if a vasodilator response can be elicited with hU-II in mouse isolated arteries (and, if so, whether such a phenotype is lost as a function of UT deletion UT(−/−) mouse).
Once the contractile responses to hU-II were observed in tissues isolated from UT(+/+) mice, they were compared to those in UT(−/−) mice. Relative to UT(+/+) mouse isolated thoracic aorta where hU-II was a potent spasmogen, vessels isolated from UT(−/−) mice did not respond to hU-II. Thus, loss of the UT receptor results in the loss of U-II-induced contractile action. However, loss of the UT receptor gene did not alter the contractile responses of KCl, phenylephrine, endothelin-1 or angiotensin-II in the isolated aorta. Similarly, responses to the endothelium-dependent vasodilator carbachol or SNP, an endothelium-independent vasodilator, were not altered by the deletion of the UT receptor. Together, these data suggest that loss of the UT receptor results in the selective loss of hU-II contractile activity, that is, the lack of response to hU-II in UT(−/−) mice was not accompanied by any alteration in reactivity to unrelated spasmogens. However, even though the contractile efficacy of hU-II was low in mouse isolated aorta, it is pertinent to note that neither endothelin-1 nor angiotensin-II was able to elicit contractile responses equivalent to those seen with KCl or phenylephrine in this vessel. Although the aorta is a conduit vessel, since both angiotensin-II and endothelin-1 play a significant (patho)physiological role in the control of haemodynamic function/regional vascular resistance in man, one must recognize the potential shortcomings of using this species for studying the effects of vasoactivc neurohormones.
In summary, the present study includes the first description of the generation of UT receptor knockout mice and constitutes the first direct link between the UT receptor and U-II-induced vasoconstriction. Deletion of the UT gene results in a loss of U-II contractile action within the mouse vasculature (without causing any ‘nonspecific' alterations in vascular reactivity to other spasmogens). However, the contribution that U-II and its receptor make to the regulation/maintenance of basal systemic haemodynamics appears to be negligible in this species. Although loss of the UT receptor does not alter basal haemodynamics in the mouse, further experiments are required to assess the role of UT in the mouse under conditions of stress/pathology (e.g. salt-sensitive hypertension in bradykinin B2 receptor knockout mice; Alfie et al., 1996). Nevertheless, these mice may represent a useful tool for delineating other (patho)physiological roles for U-II outside the vasculature such as regulating endocrine/metabolic, CNS and pulmonary function. For example, U-II has been implicated in diabetes/obesity (U-II inhibits insulin release in the perfused rat pancreas; Silvestre et al., 2001) and atherosclerosis (increased U-II binding in coronary artery atherosclerotic lesions; Maguire & Davenport, 2002). Since nonpeptide UT receptor antagonists are not currently commercially available, breeding UT receptor knockout mice onto a genetic background of diabetes, obesity and/or atherosclerosis (db/db, ob/ob and ApoE/LDLR deficient mice, etc.) may be an alternative/supplemental method for determining the influenceof U-II and its receptor on the aetiology of cardiorenal disease.
Acknowledgments
David J. Behm and Stephen M. Harrison contributed equally to this paper. We thank Barbara Abrams, Wendy Crowell and Renee Hernandez for their technical assistance and comments during the preparation of this paper. We also thank GSK Laboratory Animal Sciences for animal husbandry and GSK Discovery Genetics for PCR genotyping.
Abbreviations
- BAC
bacterial artificial chromosome
- CO
cardiac output
- dCTP
2′-deoxycytidine 5′-triphosphate
- dNTP
2′-deoxynucleotide 5′-triphosphate
- EDTA
ethylenediaminetetraacetic acid
- EDV
end-diastolic volume
- EF
ejection fraction
- ES
embryonic stem cells
- ESV
end-systolic volume
- GADPH
glyceraldehyde-3-phosphate dehydrogenase
- HR
heart rate
- LVEDP
left ventricular end-diastolic pressure
- LVESP
left ventricular end-systolic pressure
- MAP
mean arterial blood pressure
- Neo
neomycin phosphotransferase-encoding gene
- ORF
open reading frame
- PCR
polymerase chain reaction
- PGK
phosphoglycerate kinase I promoter
- SDS
sodium dodecyl sulphate
- SNP
sodium nitroprusside
- SSC
150 mM NaCl, 15 mM sodium citrate, pH 7.0
- SV
stroke volume
- TE
10 mM Tris-HCl, 1 mM EDTA
- hU-II
human urotensin-II
- UT
urotensin-II receptor
- UT(−/−)
homozygous UT receptor knockout mice
- UT(+/−)
heterozygous UT receptor knockout mice
- UT(+/+)
wild-type mice
References
- ALFIE M.E., YANG X.P., HESS F., CARRETERO O.A. Salt-sensitive hypertension in bradykinin B2 receptor knockout mice. Biochem. Biophys. Res. Commun. 1996;224:625–630. doi: 10.1006/bbrc.1996.1076. [DOI] [PubMed] [Google Scholar]
- AMES R.S., SARAU H.M., CHAMBERS J.K., WILLETTE R.N., AIYAR N.V., ROMANIC A.M., LOUDEN C.S., FOLEY J.J., SAUERMELCH C.F., COATNEY R.W., AO Z., DISA J., HOLMES S.D., STADEL J.M., MARTIN J.D., LIU W.S., GLOVER G.I., WILSON S., MCNULTY D.E., ELLIS C.E., ELSHOURBAGY N.A., SHABON U., TRILL J.J., HAY D.W.P., OHLSTEIN E.H., BERGSMA D.J., DOUGLAS S.A. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- BENNETT T., MARCH J.E., KEMP P.A., GARDINER S.M. Regional haemodynamic responses to infusion of human urotensin II (hUII) in conscious Sprague–Dawley rats. Br. J. Pharmacol. 2002;135:200. [Google Scholar]
- BERN H.A., PEARSON D., LARSON B.A., NISHIOKA R.S. Neurohormones from fish tails: the caudal neurosecretory system. I. ‘Urophysiology' and the caudal neurosecretory system of fishes. Rec. Prog. Horm. Res. 1985;41:533–552. doi: 10.1016/b978-0-12-571141-8.50016-0. [DOI] [PubMed] [Google Scholar]
- BOTTRILL F.E., DOUGLAS S.A., HILEY C.R., WHITE R. Human urotensin-II is an endothelium-dependent vasodilator in rat small arteries. Br. J. Pharmacol. 2000;130:1865–1870. doi: 10.1038/sj.bjp.0703513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMARDA V., RIZZI A., CALO G., GENDRON G., PERRON S.I., KOSTENIS E., ZAMBONI P., MASCOLI F., REGOLI D. Effects of human urotensin II in isolated vessels of various species; comparison with other vasoactive agents. Naunyn-Schmiedeberg's Arch. Pharmacol. 2002;365:141–149. doi: 10.1007/s00210-001-0503-0. [DOI] [PubMed] [Google Scholar]
- CONLON J.M., O'HARTE F., SMITH D.D., TONON M.C., VAUDRY H. Isolation and primary structure of urotensin II from the brain of a tetrapod, the frog Rana ridibunda. Biochem. Biophys. Res. Commun. 1992;188:578–583. doi: 10.1016/0006-291x(92)91095-8. [DOI] [PubMed] [Google Scholar]
- CONLON J.M., YANO K., WAUGH D., HAZON N. Distribution and molecular forms of urotensin II and its role in cardiovascular regulation in vertebrates. J. Expl. Zool. 1996;275:226–238. [PubMed] [Google Scholar]
- COULOUARN Y., JEGOU S., TOSTIVINT H., VAUDRY H., LIHRMANN I. Cloning, sequence analysis and tissue distribution of the mouse and rat urotensin II precursors. FEBS Lett. 1999;457:28–32. doi: 10.1016/s0014-5793(99)01003-0. [DOI] [PubMed] [Google Scholar]
- COULOUARN Y., LIHRMANN I., JEGOU S., ANOUAR Y., TOSTIVINT H., BEAUVILLAIN J.C., CONLON J.M., BERN H.A., VAUDRY H. Cloning of the cDNA encoding the urotensin II precursor in frog and human reveals intense expression of the urotensin II gene in motoneurons of the spinal cord. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15803–15808. doi: 10.1073/pnas.95.26.15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COY D.H., ROSSOWSKI W.J., CHENG B.L., HOCART S.J., TAYLOR J.E. Novel urotensin-II (UII) antagonists point to multiple receptor involvement in UII bioactivity. Reg. Pep. 2000;94:48. [Google Scholar]
- DOUGLAS S.A.Human urotensin-II as a novel cardiovascular target: ‘heart' of the matter or simply a fishy ‘tail' Curr. Opin. Pharmacol. 2003(In press) [DOI] [PubMed]
- DOUGLAS S.A., BECK G.R., ELLIOTT J.D., OHLSTEIN E.H. Pharmacological evidence for the presence of three distinct functional endothelin receptor subtypes in the rabbit lateral saphenous vein. Br. J. Pharmacol. 1995;114:1529–1540. doi: 10.1111/j.1476-5381.1995.tb14936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS S.A., OHLSTEIN E.H. Human urotensin-II, the most potent mammalian vasoconstrictor identified to date, as a therapeutic target for the management of cardiovascular disease. Trends Cardiovasc. Med. 2000a;10:229–237. doi: 10.1016/s1050-1738(00)00069-4. [DOI] [PubMed] [Google Scholar]
- DOUGLAS S.A., OHLSTEIN E.H.Urotensin receptors The IUPHAR Compendium of Receptor Characterization and Classification 2000bLondon: IUPHAR Media; 365–372.ed. Girdlestone, D. pp [Google Scholar]
- DOUGLAS S.A., SULPIZIO A.C., PIERCY V., SARAU H.M., AMES R.S., AIYAR N.V., OHLSTEIN E.H., WILLETTE R.N. Differential vasoconstrictor activity of human urotensin-II in vascular tissue isolated from the rat, mouse, dog, pig, marmoset and cynomolgus monkey. Br. J. Pharmacol. 2000;131:1262–1274. doi: 10.1038/sj.bjp.0703690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS S.A., TAYARA L., OHLSTEIN E.H., HALAWA N., GIAID A. Congestive heart failure and expression of myocardial urotensin II. Lancet. 2002;359:1990–1997. doi: 10.1016/S0140-6736(02)08831-1. [DOI] [PubMed] [Google Scholar]
- ELSHOURBAGY N.A., DOUGLAS S.A., SHABON U., HARRISON S., DUDDY G., SECHLER J.L., AO Z., MALEEFF B.E., NASELSKY D., DISA J., AIYAR N.V. Molecular and pharmacological characterization of genes encoding urotensin-II peptides and their cognate G-protein-coupled receptors from the mouse and monkey. Br. J. Pharmacol. 2002;136:9–22. doi: 10.1038/sj.bjp.0704671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILIPEANU C.M., BRAILOIU E., LE DUN S., DUN N.J. Urotensin-II regulates intracellular calcium in dissociated rat spinal cord neurons. J. Neurochem. 2002;83:879–884. doi: 10.1046/j.1471-4159.2002.01196.x. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., MARCH J.E., KEMP P.A., DAVENPORT A.P., BENNETT T. Depressor and regionally-selective vasodilator effects of human and rat urotensin II in conscious rats. Br. J. Pharmacol. 2001;132:1625–1629. doi: 10.1038/sj.bjp.0704051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARTLON J., PARKER F., HARRISON D.C., DOUGLAS S.A., ASHMEADE T.E., RILEY G.J., HUGHES Z.A., TAYLOR S.G., MUNTON R.P., HAGAN J.J., HUNTER J.A., JONES D.N. Central effects of urotensin-II following ICV administration in rats. Psychopharmacology. 2001;155:426–433. doi: 10.1007/s002130100715. [DOI] [PubMed] [Google Scholar]
- GIBSON A., WALLACE P., BERN H.A. Cardiovascular effects of urotensin II in anesthetized and pithed rats. Gen. Comp. Endocrinol. 1986;64:435–439. doi: 10.1016/0016-6480(86)90080-8. [DOI] [PubMed] [Google Scholar]
- HAY D.W., LUTTMANN M.A., DOUGLAS S.A. Human urotensin-II is a potent spasmogen of primate airway smooth muscle. Br. J. Pharmacol. 2000;131:10–12. doi: 10.1038/sj.bjp.0703533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLER J., SCHEPKE M., NEEF M., WOITAS R., RABE C., SAUERBRUCH T. Increases urotensin II plasma levels in patients with cirrhosis and portal hypertension. J. Hepatol. 2002;37:767–772. doi: 10.1016/s0168-8278(02)00295-7. [DOI] [PubMed] [Google Scholar]
- KELSALL C.J., BALMENT R.J. Native urotensins influence cortisol secretion and plasma cortisol concentration in the euryhaline flounder, Platichthys flesus. Gen. Comp Endocrinol. 1998;112:210–219. doi: 10.1006/gcen.1998.7166. [DOI] [PubMed] [Google Scholar]
- LIN Y., TSUCHIHASHI T., MATSUMURA K., ABE I., IIDA M. Central cardiovascular action of urotensin II in conscious rats. J. Hypertens. 2003;21:159–165. doi: 10.1097/00004872-200301000-00026. [DOI] [PubMed] [Google Scholar]
- LIU Q., PONG S.S., ZENG Z., ZHANG Q., HOWARD A.D., WILLIAMS D.L., DAVIDOFF M., WANG R., AUSTIN C.P., MCDONALD T.P., BAI C., GEORGE S.R., EVENS J.F., CASKEY C.T. Identification of urotensin II as the endogenous ligand for the orphan G-protein-coupled receptor GPR14. Biochem. Biophys. Res. Commun. 1999;266:174–178. doi: 10.1006/bbrc.1999.1796. [DOI] [PubMed] [Google Scholar]
- LU Y., ZOU C, HUANG D., TANG C. Cardiovascular effects of urotensin II in different brain areas. Peptides. 2002;23:1631. doi: 10.1016/s0196-9781(02)00104-3. [DOI] [PubMed] [Google Scholar]
- MACLEAN M.R., ALEXANDER D., STIRRAT A., GALLAGHER M., DOUGLAS S.A., OHLSTEIN E.H., MORECROFT I., POLLAND K. Contractile responses to human urotensin-II in rat and human pulmonary arteries: effect of endothelial factors and chronic hypoxia in the rat. Br. J. Pharmacol. 2000;130:201–204. doi: 10.1038/sj.bjp.0703314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGUIRE J.J., DAVENPORT A.P. Is urotensin-II the new endothelin. Br. J. Pharmacol. 2002;137:579–588. doi: 10.1038/sj.bjp.0704924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGUIRE J.J., KUC R.E., DAVENPORT A.P. Orphan-receptor ligand human urotensin-II: receptor localization in human tissues and comparison of vasoconstrictor responses with endothelin-1. Br. J. Pharmacol. 2000;131:441–446. doi: 10.1038/sj.bjp.0703601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANIATIS T., FRITSCH E.F., SAMBROOK J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- MARCHESE A., HEIBER M., NGUYEN T., HENG H.H., SALDIVIA V.R., CHENG R., MURPHY P.M., TSUI L.C., SHI X., GREGOR P., GEORGE S.R., O'DOWD B.F., DOCHERTY J.M. Cloning and chromosomal mapping of three novel genes, GPR9, GPR10, and GPR14, encoding receptors related to interleukin 8, neuropeptide Y, and somatostatin receptors. Genomics. 1995;29:335–344. doi: 10.1006/geno.1995.9996. [DOI] [PubMed] [Google Scholar]
- MATSUSHITA M., SHICHIRI M., IMAI T., IWASHINA M., TANAKA H., TAKASU N., HIRATA Y. Co-expression of urotensin II and its receptor (GPR14) in human cardiovascular and renal tissues. J. Hypertens. 2001;19:2185–2190. doi: 10.1097/00004872-200112000-00011. [DOI] [PubMed] [Google Scholar]
- PAYSANT J., RUPIN A., SIMONET S., FABIANI J.N., VERBEUREN T.J. Comparison of the contractile responses of human coronary bypass grafts and monkey arteries to human urotensin-II. Fundam. Clin. Pharmacol. 2001;15:227–231. doi: 10.1046/j.1472-8206.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- PEARSON D., SHIVELY J.E., CLARK B.R., GESCHWIND I.I., BARKLEY M., NISHIOKA R.S., BERN H.A. Urotensin II; a somatostatin-like peptide in the caudal neurosecretory system of fishes. Proc. Natl. Acad. Sci. U.S.A. 1980;77:5021–5024. doi: 10.1073/pnas.77.8.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PELLETIER G., LIHRMANN I., VAUDRY H. Role of androgens in the regulation of urotensin II precursor mRNA expression in the rat brainstem and spinal cord. Neuroscience. 2002;115:525–532. doi: 10.1016/s0306-4522(02)00413-x. [DOI] [PubMed] [Google Scholar]
- RICHARDS A.M., NICHOLLS M.G., LAINCHBURY J.G., FISHER S., YANDLE T.G. Plasma urotensin II in heart failure. Lancet. 2002;360:545–546. doi: 10.1016/s0140-6736(02)09709-x. [DOI] [PubMed] [Google Scholar]
- RUSSELL F.D., MOLENAAR P., O'BRIEN D.M. Cardiostimulant effects of urotensin-II in human heart in vitro. Br. J. Pharmacol. 2001;132:5–9. doi: 10.1038/sj.bjp.0703811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAETRUM OPGAARD O., NOTHACKER H., EHLERT F.J., KRAUSE D.N. Human urotensin II mediates vasoconstriction via an increase in inositol phosphates. Eur. J. Pharmacol. 2000;406:265–271. doi: 10.1016/s0014-2999(00)00672-5. [DOI] [PubMed] [Google Scholar]
- SHENOUDA A., DOUGLAS S.A., OHLSTEIN E.H., GIAID A. Localization of urotensin-II immunoreactivity in normal human kidneys and renal carcinoma. J. Histochem. Cytochem. 2002;50:885–889. doi: 10.1177/002215540205000702. [DOI] [PubMed] [Google Scholar]
- SHERIDAN M.A., BERN H.A. Both somatostatin and the caudal neuropeptide, urotensin II, stimulate lipid mobilization from coho salmon liver incubated in vitro. Reg. Peptides. 1986;14:333–344. doi: 10.1016/0167-0115(86)90175-8. [DOI] [PubMed] [Google Scholar]
- SILVESTRE R.A., RODRIQUES-GALLARDO J., EGIDO E.M., MARCO J. Inhibition of insulin release by urotensin II–a study on the perfused rat pancreas. Horm. Metab. Res. 2001;33:379–381. doi: 10.1055/s-2001-15414. [DOI] [PubMed] [Google Scholar]
- STIRRAT A., GALLAGHER M., DOUGLAS S.A., OHLSTEIN E.H., BERRY C., KIRK A., RICHARDSON M., MACLEAN M.R. Potent vasodilator responses to human urotensin-II in human pulmonary and abdominal resistance arteries. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H925–H928. doi: 10.1152/ajpheart.2001.280.2.H925. [DOI] [PubMed] [Google Scholar]
- TAL M., AMMAR D.A., KARPUJ M., KRIZHANOVSKY V., NAIM M., THOMPSON D.A. A novel putative neuropeptide receptor expressed in neural tissue, including sensory epithelia. Biochem. Biophys. Res. Commun. 1995;209:752–759. doi: 10.1006/bbrc.1995.1563. [DOI] [PubMed] [Google Scholar]
- TOTSUNE K., TAKAHASHI K., ARIHARA Z., SONE M., SATOH F., ITO S., KIMURA Y., SASANO H., MURAKAMI O. Role of urotensin II in patients on dialysis. Lancet. 2001;358:774–775. doi: 10.1016/S0140-6736(01)06002-0. [DOI] [PubMed] [Google Scholar]







