Abstract
The mechanism underlying the enhancement by superoxide dismutase (SOD) of endothelium-dependent relaxation was investigated in rabbit mesenteric resistance arteries.
SOD (200 U ml−1) increased the production of H2O2 in smooth muscle cells (as indicated by the use of an H2O2-sensitive fluorescent dye).
Neither SOD nor catalase (400 U ml−1) modified either the resting membrane potential or the hyperpolarization induced by acetylcholine (ACh, 1 μM) in smooth muscle cells.
In arteries constricted with noradrenaline, the endothelium-dependent relaxation induced by ACh (0.01–1 μM) was enhanced by SOD (200 U ml−1) (P<0.01). This action of SOD was inhibited by L-NG-nitroarginine (nitric oxide (NO)-synthase inhibitor) but not by either charybdotoxin+apamin (Ca2+-activated-K+-channel blockers) or diclofenac (cyclooxygenase inhibitor).
Neither ascorbate (50 μM) nor tiron (0.3 mM), superoxide scavengers, had any effect on the ACh-induced relaxation, but each attenuated the enhancing effect of SOD on the ACh-induced relaxation. Similarly, catalase (400 U ml−1) inhibited the effect of SOD without changing the ACh-induced relaxation.
In endothelium-denuded strips constricted with noradrenaline, SOD enhanced the relaxation induced by the NO donor 1-hydroxy-2-oxo-3-(N-methyl-3-aminopropyl)-3-methyl-1-triazene (NOC-7) (P<0.05). Ascorbate and catalase each attenuated this effect of SOD.
H2O2 (1 μM) enhanced the relaxation on the noradrenaline contraction induced by NOC-7 and that induced by 8-bromo-cGMP, a membrane-permeable analogue of guanosine 3′,5′ cyclic monophosphate (cGMP).
SOD had no effect on cGMP production, whether measured in endothelium-intact strips following an application of ACh (0.1 μM) or in endothelium-denuded strips following an application of NOC-7 (0.1 μM).
It is suggested that in rabbit mesenteric resistance arteries, SOD increases the ACh-induced, endothelium-dependent relaxation by enhancing the action of NO in the smooth muscle via its H2O2-producing action (rather than via a superoxide-scavenging action).
Keywords: Nitric oxide, superoxide dismutase, endothelium, vascular smooth muscle, resistance artery, reactive oxygen, hydrogen peroxide
Introduction
Vascular endothelial cells release vasorelaxing factors (such as nitric oxide (NO), prostacyclin and endothelium-derived hyperpolarizing factor (EDHF)) and these play an important role in the regulation of vascular tone (Moncada et al., 1991; Kuriyama et al., 1998). It has been found that cells located in the vascular wall (such as endothelial cells, smooth muscle cells and fibroblasts) are able to produce superoxide, which binds to and inactivates NO, thus causing a downregulation of the function of endothelium-derived NO (Daemen et al., 1991; Griendling et al., 1994; Harrison, 1997).
It is known that both the extracellular and intracellular isoforms of Cu/Zn-superoxide dismutase (SOD) are present in these cells and play a vital role in protecting the NO produced in endothelial cells (Mügge et al., 1991; Omar et al., 1991; Abrahamsson et al., 1992; Mian & Martin, 1995). In addition, the superoxide scavengers ascorbate and tiron (4,5-dihydroxy-1,3-benzene-disulphonic acid) are known to be highly effective at such scavenging and to protect NO from destruction under conditions of oxidant stress (Som et al., 1983; Gotoh & Niki, 1992; Dudgeon et al., 1998; MacKenzie et al., 1999). Thus, it has been speculated that SOD, ascorbate and tiron may all enhance the endothelium-dependent, NO-mediated relaxation in various types of vascular preparations as a result of their scavenging of superoxide.
The H2O2 that is produced by SOD via the dismutation of superoxide has various actions in vascular tissues: under resting conditions it produces a contraction in rat and rabbit aorta (Bharadwaj & Prasad, 1995; Yang et al., 1998), rat mesenteric artery (Gao & Lee, 2001) and rabbit pulmonary artery (Sheehan et al., 1993), while in preconstricted vessels it evokes relaxation in the canine middle cerebral and porcine coronary arteries (Barlow et al., 2000; Iida & Katusic, 2000) and cat cerebral arterioles (Wei et al., 1996). The relaxations induced by H2O2 have variously been reported to be endothelium-dependent (Yang et al., 1999), endothelium-independent (Barlow & White, 1998; Iesaki et al., 1999) or both (Rubanyi & Vanhoutte, 1986; Zembowicz et al., 1993). Recently, it was found that in endothelial-NO-synthase-knockout mice, the H2O2 produced by SOD in endothelial cells hyperpolarizes the smooth muscle cell membrane and produces a relaxation in small mesenteric arteries (Matoba et al., 2000). In preliminary experiments, we found that in rabbit mesenteric resistance arteries, exogenously applied Cu/Zn-SOD, but not ascorbate, enhanced ACh-induced endothelium-dependent relaxation. Furthermore, catalase, an enzyme that acts as a dismutase on H2O2 (forming water and oxygen), significantly inhibited the above effect of SOD. Taken together, these results suggest that H2O2 may play a role in the enhancement of endothelium-dependent relaxation by SOD in resistance arteries, although the mechanism underlying this action of the SOD has not yet been fully clarified.
We investigated the above mechanism in rabbit mesenteric resistance arteries, since the effects on the electrical and mechanical properties of these arteries seen in response to endothelial stimulation or nitrate compounds have been well characterized (Kuriyama et al., 1998; Yamashita et al., 1999). In the present experiments, we first tried to identify the endothelium-dependent relaxing factors responsible for the action of Cu/Zn-SOD. Second, to explore the possibility that superoxide scavenging might be responsible for the effect of SOD, we examined the effect of SOD on endothelium-dependent relaxation in the presence of ascorbate or catalase. Third, we used the H2O2-sensitive fluorescent dye CM-H2DCF (5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein) to investigate whether extracellularly applied Cu/Zn-SOD can indeed increase the cellular concentration of H2O2 in smooth muscle cells. Fourth, the amount of guanosine 3′,5′ cyclic monophosphate (cGMP) produced in response to SOD was investigated (a) in the absence or presence of ACh (in endothelium-intact strips) and (b) in the absence or presence of the NO donor NOC-7 (1-hydroxy-2-oxo-3-(N-methyl-3-aminopropyl)-3-methyl-1-triazene) (Hrabie et al., 1993) (in endothelium-denuded strips). Finally, the effect of SOD on the relaxation induced by the membrane-permeable cGMP analogue 8-bromo-cGMP was examined in endothelium-denuded strips.
Methods
Tissue preparation
Male Japan white albino rabbits (supplied by Kitayama Labes, Ina, Japan), weighing 1.9–2.5 kg, were anaesthetized by injection of pentobarbitone sodium (40 mg kg−1, given intravenously) and then killed by exsanguination. The protocols used conformed with guidelines on the conduct of animal experiments issued by Nagoya City University Medical School and were approved by the Committee on the Ethics of Animal Experiments in Nagoya City University Medical School. The third and fourth branches of the mesenteric artery distributing to the region of the ileum (diameter, approximately 0.10–0.13 mm) were excised immediately, then cleaned by removal of connective tissue in Krebs solution under a binocular microscope at room temperature. After each artery had been cut open parallel to its long axis using small scissors, circularly cut strips were carefully prepared so as not to damage the endothelium, as described previously (Yamashita et al., 1999). In some strips, the endothelium was carefully removed by gentle rubbing of the internal surface of the vessel using small pieces of razor blade. Satisfactory ablation of the endothelium was pharmacologically verified by testing for the absence of a relaxing effect when ACh (3 μM) was applied during a noradrenaline-induced contraction, as described previously (Itoh et al., 1992; Yamashita et al., 1999).
Recording of mechanical responses
Circularly cut strips (0.2–0.3 mm long, 0.07–0.10 mm wide, 0.02–0.03 mm thick) were prepared for tension measurement. A strip of mesenteric artery was placed in a chamber with a capacity of 0.3 ml and superfused with warmed (36–37°C) Krebs solution at a flow rate of about 2 ml min−1. Both ends of the preparation were fixed using fine silk threads to allow isometric tension to be recorded using a strain-gauge transducer (AE801; SensoNor a.s., Horten, Norway). A resting tension of 2–3 mg was applied so as to obtain a maximum contraction to 128 mM K+. Each preparation was allowed to equilibrate for 1–2 h before the start of the experiment. Unless otherwise stated, the concentration of noradrenaline used was 10 μM and propranolol (nonselective β-adrenoceptor antagonist, 3 μM) was coapplied with the noradrenaline to prevent it activating β-adrenoceptors. Noradrenaline produced a phasic, followed by a tonic contraction in endothelium-intact and -denuded strips. When the noradrenaline-induced tonic contraction was normalized with respect to the maximum amplitude of the contraction induced by 128 mM K+, the values obtained were 0.826±0.164 in endothelium-intact strips and 1.242±0.188 in endothelium-denuded strips. These values were significantly different from each other (n=15, P<0.0001).
Endothelium-dependent relaxation was induced by ACh (0.01–1 μM) during the contraction induced by 10 μM noradrenaline. Each preparation was first contracted with noradrenaline+propranolol. Then, after a steady-state contraction had been attained (at 6 min after the noradrenaline application), ACh (0.01–1 μM) was cumulatively applied from low to high concentration (for 2 min at each concentration) during the ongoing noradrenaline-induced contraction. At 1 min after removal of the final concentration of ACh, noradrenaline+propranolol was washed out and the tension of the artery returned to the resting level. This protocol was repeated as required at 30-min intervals.
When the effect of SOD (200 U ml−1), ascorbate (50 μM), tiron (0.3 mM) or catalase (400 U ml−1) on the ACh (0.03–0.3 μM)-induced relaxation was to be examined, one of the blockers was applied after a 3-min application of noradrenaline, with ACh being subsequently applied in the presence of noradrenaline+blocker. When the effect to be examined was that of ascorbate, tiron or catalase on the action of SOD on the ACh-induced relaxation, the effect of one of these agents on the ACh-induced relaxation was first recorded, and then the effect of SOD on the ACh-induced response was examined in its presence.
To examine the possible roles played by NO, endothelium-derived hyperpolarizing factor (EDHF) and prostaglandins (PGs) in the action of SOD in endothelium-intact strips, such strips were treated with L-NG-nitroarginine (L-NOARG, 0.1 mM) for 60 min, with diclofenac (3 μM) for 60 min or with charybdotoxin (CTx, 0.1 μM)+apamin (0.1 μM) in diclofenac-treated strips for 3 min. The ACh-induced relaxation was then observed in the presence of agent and the effect of SOD (200 U ml−1) on the ACh-induced relaxation was also observed in the presence of agent.
The effect of SOD (200 U ml−1) on the relaxation induced by NOC-7 (0.1–100 nM) was examined in endothelium-denuded strips. The experimental protocol was essentially the same as that used for examining the effect of SOD on ACh-induced relaxation in endothelium-intact strips, unless otherwise noted. The action of ascorbate (50 μM) or catalase (400 U ml−1) on the effect of SOD on the NOC-7-induced relaxation was examined as follows. After recording the control NOC-7 response, the NOC-7-induced relaxation was examined in the presence of ascorbate or catalase. Next, the effect of SOD (200 U ml−1) on the NOC-7 response was examined in the presence of ascorbate or catalase. In strips in which the effect of ascorbate+SOD was examined, the effect of SOD alone was finally examined on the NOC-7-induced relaxation following a 30-min washout of ascorbate+SOD. When the effect of the hydroxyl-radical scavenger dimethylthiourea was to be examined on the action of SOD on the NOC-7-induced relaxation, dimethylthiourea (1 mM) was pretreated for 30 min after recording the control NOC-7-induced relaxation; then, the effect of SOD (200 U ml−1) was examined in the presence of dimethylthiourea.
The effect of H2O2 on the NOC-7-induced relaxation was examined in endothelium-denuded strips. Noradrenaline (10 μM) was applied and, after 6 min, NOC-7 (1–100 nM) was cumulatively applied (for 2 min at each concentration) in the presence of noradrenaline. After a 30-min interval, the strip was again contracted with noradrenaline and then NOC-7 was applied cumulatively in the presence of noradrenaline+H2O2. H2O2 (1 μM) was applied for 3 min after a 3-min application of noradrenaline and NOC-7 was applied for 2 min at each concentration. When the effect of H2O2 (1 μM) on the 8-bromo-cGMP-induced relaxation was to be examined in endothelium-denuded strips, 8-bromo-cGMP (3–300 μM) was cumulatively applied (for 3 min at each concentration) in the presence of noradrenaline. In other respects, the protocol was the same as that used to examine the effect of H2O2 on the NOC-7-induced relaxation.
Recording of membrane potential
The membrane potential of smooth muscle cells in endothelium-intact strips was measured using conventional microelectrode techniques, as described previously (Yamashita et al., 1999). To this end, an endothelium-intact strip was pinned to the bottom of a chamber of 0.7 ml volume and superfused with warmed (36–37°C) Krebs solution at a flow rate of about 2 ml min−1. Glass microelectrodes were made from borosilicate glass tubing (o.d.=1.2 mm with a glass filament inside; Hilgenberg, Malsfeld, Germany) and then filled with 1 M KCl. Their resistances were 80–180 MΩ and each electrode was inserted into the smooth muscle cell from the luminal side. Membrane potentials recorded using an Axoclamp-2B amplifier (Axon Instruments, Foster, CA, U.S.A.) were displayed on a cathode-ray oscilloscope (model VC-6020; Hitachi, Tokyo, Japan) and the data were stored at an acquisition rate of 100 Hz using an Axoscope 7.0/Digidata 1200 data-acquisition system (Axon Instruments) on an IBM/AT-compatible PC.
ACh (1 μM) was first applied for 2 min followed by a 30-min washout (to record the control ACh response). To examine the role of PGs and EDHF on the ACh-induced hyperpolarization, diclofenac (3 μM) or CTx (0.1 μM)+apamin (0.1 μM) was pretreated and ACh was again applied, this time in the presence of diclofenac or CTx+apamin. When the effect of SOD or catalase on the ACh-induced hyperpolarization was to be tested, SOD (200 U ml−1) or catalase (400 U ml−1) was pretreated for 5 min after recording the control ACh (1 μM) response and ACh was again applied, this time in the presence of SOD. Each series was performed on a separate cell.
Measurement of H2O2 production
An endothelium-denuded strip of rabbit mesenteric artery was placed in a chamber with a capacity of 1 ml, each end of the preparation being fixed using a small tungsten wire (diameter 0.02 mm). The change in the intracellular concentration of H2O2 ([H2O2]i) within the smooth muscle cells was estimated from the increase in the fluorescence intensity of CM-H2DCF (Molecular Probes, OR, U.S.A.). The strip was loaded with CM-H2DCF by a single application of membrane-permeable CM-H2DCF diacetate (CM-H2DCF-DA, 20 μM) for 2 h at 37°C and then transferred to a fluorescence microscope (ECLIPSE TE300; Nikon, Tokyo, Japan) equipped with a CCD camera (C6790; Hamamatsu Photonics, Hamamatsu, Japan). The focus was adjusted to reveal individual smooth muscle cells and the experiment was started after a 30-min perfusion with Krebs solution (at a flow rate of about 1 ml min−1) under dark conditions. The strip of artery was illuminated with light of 470 nm (with a half-width of 20 nm) for 36 ms every 30 s (to minimize photobleaching of the dye) and emitted light of 535 nm (with a half-width of 20 nm) was collected. The fluorescence intensities were first recorded for 15 min (to obtain the untreated control value), and then SOD (200 U ml−1) or MnCl2 (30 μM) was applied for 15 min followed by a 10-min wash out. Finally, H2O2 (10 μM) was applied for 15 min (to ensure that the fluorescence did increase in response to H2O2). The ratios of the fluorescence values (F/F0) obtained from four to five smooth muscle cells were averaged to estimate the change in [H2O2]i, the values F0 and F representing the fluorescence intensities obtained just before and 15 min after application of a given stimulant, respectively. The data analysis was performed using commercial software (AquaCosmos; Hamamatsu Photonics, Hamamatsu, Japan).
Assay for cGMP
After strips had been suspended (without applying tension) for 2 h in Krebs solution at 37°C, either ACh (0.1 μM) in the case of endothelium-intact strips or NOC-7 (0.1 μM) in the case of endothelium-denuded strips was added for 5 min in the absence or presence of SOD (200 U ml−1), the reaction being halted by soaking the strips in ice-cold 8% trichloroacetic acid. Strips were homogenized in a solution containing trichloroacetic acid in a glass homogenizer (Suzuki et al., 1991). The homogenate was then centrifuged and the pellet used for the protein assay. The supernatant fraction was treated with water-saturated ether three times and assayed for cGMP using an enzyme-immunoassay kit purchased from Amersham Pharmacia Biotech (Tokyo, Japan). For the assay protocol, we followed the manual supplied by Amersham Pharmacia Biotech.
Solutions
The ionic composition of the Krebs solution was as follows (mM): Na+, 137.4; K+, 5.9; Mg2+, 1.2; Ca2+, 2.6; HCO3−, 15.5; H2PO4−, 1.2; Cl−, 134; glucose, 11.5. High-K+ solution (128 mM) was prepared by replacing sodium chloride with potassium chloride isosmotically. All the solutions used in the present experiments contained guanethidine (5 μM, to prevent effects due to release of sympathetic transmitters). The solutions were bubbled with 95% oxygen and 5% carbon dioxide and the pH was adjusted to 7.3–7.4 using NaOH and HCl.
Drugs
The drugs used in the present experiments were as follows: noradrenaline, diclofenac sodium salt and 8-bromo-guanosine 3′,5′ cyclic monophosphate (8-bromo-cGMP; Sigma Chemical Co. St Louis, MO, U.S.A.), L-NG-nitroarginine (L-NOARG), charybdotoxin (CTx) and apamin (Peptide Institute Inc., Osaka, Japan), SOD (Cu/Zn-type, from bovine erythrocytes), L-(+)-ascorbic acid sodium salt and catalase (from bovine liver) (Wako Pure Chemical Ind., Osaka, Japan), tiron (4,5-dihydroxy-1,3-benzene-disulphonic acid) and NOC-7 (3-(2-hydroxy-1-methylethyl-2-nitrosohydrazino)-N-methyl-1-propanamine) (Dojindo, Kumamoto, Japan), ACh-HCl (Daiichi Pharmaceutical, Tokyo, Japan) and guanethidine (Tokyo Kasei, Tokyo, Japan). NOC-7 was dissolved in 0.1 M NaOH and CM-H2DCF-DA in dimethyl sulphoxide (as a 10 mM stock solution). All other drugs were dissolved in ultra-pure Milli-Q water (Japan Millipore Corp., Tokyo, Japan).
Statistical analysis
The effects of ACh, NOC-7 and 8-bromo-cGMP were each expressed as a relative tension, the tension level just before application of a given agent being normalized as a relative tension of 1.0. The EC50 value (the concentration producing 50% of the maximum effect) for the relaxant action of ACh was obtained by fitting the data points for each strip by a nonlinear least-squares method using software (Kaleida Graph; Synergy Software, PA, U.S.A.) written for the Macintosh Computer (Apple Co. Ltd). All results are expressed as the mean±s.e.m. The n values represent the number of strips (approximately equal to the number of animals, unless otherwise stated). A two-way repeated-measures ANOVA (followed by Scheffé's F test for post hoc analysis) or a Student's paired or unpaired t-test with an F test were used for statistical analysis. The level of significance was set at P<0.05.
Results
Effect of ACh on membrane potential
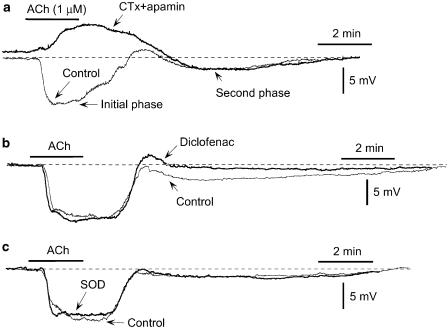
The resting membrane potential of the smooth muscle cells in our endothelium-intact strips was –55.1±0.3 mV (22 cells from eight strips), and ACh (1 μM) produced an initial, followed by a second phase of hyperpolarization. CTx (0.1 μM)+apamin (0.1 μM) depolarized the membrane (P<0.01, n=5) and blocked the ACh-induced initial-phase hyperpolarization (P<0.01; instead, there was depolarization by 3.2±0.7 mV) without changing the second phase (P>0.05; Table 1, Figure 1a). Although diclofenac did not modify the resting membrane potential (n=6, P>0.5), it significantly inhibited the ACh-induced second-phase hyperpolarization (P<0.01) without changing the initial phase (P>0.1; Table 1, Figure 1b). By contrast, SOD (200 U ml−1) affected neither the resting membrane potential (n=6, P>0.2) nor the initial- or second-phase hyperpolarization (P>0.1; Table 1, Figure 1c).
Table 1.
Effects of charybdotoxin (CTx)+apamin, diclofenac, SOD, catalase and H2O2 on resting membrane potential (RMP) and ACh-induced hyperpolarization
| ACh-induced hyperpolarization (mV) | |||||||
|---|---|---|---|---|---|---|---|
| RMP (mV) | Initial phase | Second phase | |||||
| n | Before | After | Before | After | Before | After | |
| CTx (0.1 μM)+apamin (0.1 μM) | 5 | −56.0±0.2 | −54.2±0.4** | 6.9±0.5 | 0.0±0.0** | 2.2±0.1 | 2.8±0.2 |
| Diclofenac (3 μM) | 6 | −55.2±0.9 | −54.9±0.7 | 6.8±0.5 | 8.2±0.9 | 1.9±0.3 | 0.6±0.1** |
| SOD (200 U ml−1) | 6 | −55.2±0.5 | −55.7±0.3 | 6.9±1.5 | 6.6±1.2 | 1.5±0.1 | 1.5±0.2 |
| Catalase (400 U ml−1) | 6 | −54.8±0.6 | −54.1±0.7 | 9.7±1.0 | 9.2±1.0 | 3.4±0.7 | 3.7±0.9 |
| H2O2 (1 μM) | 5 | −53.8±0.4 | −54.4±1.4 | 7.3±1.4 | 8.5±1.8 | 1.2±0.1 | 1.8±0.3 |
The maximum amplitudes of the initial- and second-phase hyperpolarizations induced by ACh (1 μM) were measured. Data are expressed as mean±s.e.m.
P<0.01 vs before application of agent(s) (Student's paired t-test). n indicates number of strips.
Figure 1.
Effects of charybdotoxin (CTx)+apamin, diclofenac and SOD on ACh-induced hyperpolarization in smooth muscle cells of the rabbit mesenteric resistance artery. (a) Effect of ACh on membrane potential in the absence (‘control') and presence of CTx (0.1 μM)+apamin (0.1 μM). ACh produced an initial, followed by a second-phase hyperpolarization. ACh (1 μM) was applied for 2 min as indicated by the bar. (b) Effect of diclofenac (3 μM) on ACh-induced hyperpolarization. (c) Effect of SOD (200 U ml−1) on ACh-induced response. Broken lines indicate original resting membrane potential level in each cell (in a given panel, recordings were made from one and the same cell). Traces in (a)–(c) were obtained from different strips. The data were representative of five to six experiments and the results were reproducible.
It was recently suggested that an increase in the H2O2 concentration in endothelial cells may be responsible for ACh-induced hyperpolarization in the smooth muscle cells of small mesenteric arteries in endothelial-NO-synthase-knockout mice (Matoba et al., 2000). To test whether this holds true for the present tissue, we examined the effect of catalase on the ACh (1 μM)-induced hyperpolarization. In fact, catalase (400 U ml−1) altered neither the resting membrane potential (n=6, P>0.5) nor the initial- or second-phase hyperpolarization (P>0.5; Table 1). Similarly, H2O2 (1 μM) modified neither the resting membrane potential (n=5, P>0.1) nor the initial- or second-phase hyperpolarization (P>0.1; Table 1).
Effect of SOD on ACh-induced, endothelium-dependent relaxation
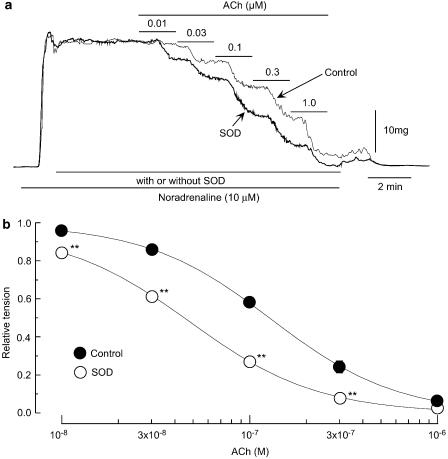
In rabbit mesenteric arteries constricted with noradrenaline, ACh evokes endothelium-dependent relaxation (Yamashita et al., 1999). In the present experiments, the EC50 value for this effect of ACh was 194±58 nM (n=8). SOD (200 U ml−1) did not significantly modify the contraction induced by 10 μM noradrenaline (26.5±2.5 vs 25.8±5.1 mg, n=8, P>0.1) but it enhanced the ACh-induced relaxation with a significant shift of the relation to the left (EC50 39±6 nM, P<0.01; Figure 2). A recovery from the effect of SOD did not occur even after a 60-min washout (data not shown).
Figure 2.
Effect of SOD on ACh-induced relaxation in endothelium-intact strips. (a) Effect of SOD (200 U ml−1) on the ACh-induced relaxation on the contraction induced by noradrenaline (10 μM). ‘Control': in the absence of SOD, ‘SOD': in the presence of SOD. Agents were applied as indicated by the bars. (b) Summary of the effect of SOD on ACh-induced relaxation. Data are shown as means and s.e.m. **P<0.01 vs control (two-way repeated-measures ANOVA and Scheffé's F test).
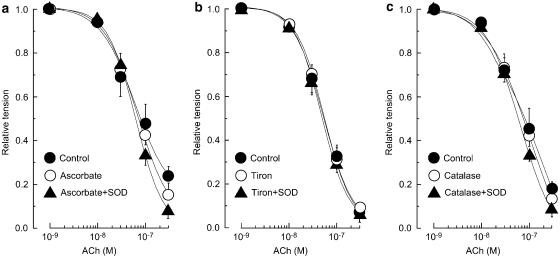
Ascorbate (50 μM) modified neither the noradrenaline-induced contraction (23.6±4.1 vs 23.4±3.8 mg, n=4, P>0.9) nor the ACh-induced relaxation in endothelium-intact strips (P>0.1; Figure 3a). In the presence of ascorbate, SOD did not significantly modify the ACh-induced relaxation (n=4, P>0.5; Figure 3a). The effect of ascorbate+SOD was significantly different from that of SOD alone (P<0.05; Figure 3a vs Figure 2b). Similarly, neither tiron (0.3 mM, n=4) nor catalase (400 U ml−1, n=4) modified either the noradrenaline-induced contraction (P>0.1 in each case) or the ACh-induced relaxation (P>0.5) but each of these agents blocked the enhancing effect of SOD on the ACh-induced relaxation (P>0.5; Figure 3b and c). The effects of tiron+SOD and catalase+SOD were significantly weaker than that of SOD alone (P<0.05 in each case; Figure 3b or c vs Figure 2b).
Figure 3.
Effects of ascorbate, tiron and catalase on ACh-induced relaxation in the absence or presence of SOD (200 U ml−1) in endothelium-intact strips. (a) Effect of ascorbate (50 μM) on ACh-induced relaxation. ‘Control': in the absence of agents; ‘Ascorbate': in the presence of ascorbate; ‘Ascorbate+SOD': the action of SOD in the presence of ascorbate. (b) Effect of tiron (0.3 mM) on ACh-induced relaxation. ‘Control': in the absence of agents; ‘Tiron': in the presence of tiron; ‘Tiron+SOD': the action of SOD in the presence of tiron. (c) Effect of catalase (400 U ml−1) on ACh-induced relaxation. ‘Control': in the absence of agents; ‘Catalase': in the presence of catalase; ‘Catalase+SOD': the action of SOD in the presence of catalase. Data are shown as means and s.e.m.
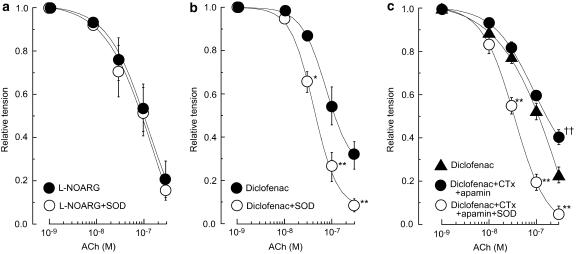
In endothelium-intact strips, L-NOARG (0.1 mM) significantly enhanced the noradrenaline (10 μM)-induced contraction (19.2±1.5 and 35.8±2.6 mg before and after application of L-NOARG, respectively, n=4, P<0.01) and attenuated the ACh-induced relaxation (P<0.01) with a significant rightward shift in the EC50 value (8.3±3.5 vs 59.3±16.8 nM, n=4, P<0.05). In the presence of L-NOARG, SOD had no effect on either the noradrenaline-induced contraction (n=4, P>0.1) or the ACh-induced relaxation (n=4, P>0.1) (not shown). The concentration of noradrenaline used in tests on L-NOARG-treated strips was reduced to 0.5 μM (18.2±1.5 mg, n=4) so that the amplitude of the contraction induced by noradrenaline was about the same as that observed before L-NOARG application. Under these conditions, SOD had no effect on the ACh-induced relaxation (n=4, P>0.5; Figure 4a).
Figure 4.
Effects of L-NG-nitroarginine (L-NOARG), CTx+apamin and diclofenac on the SOD (200 U ml−1)-induced enhancement of the ACh-induced relaxation in endothelium-intact strips. Effect of SOD on the ACh relaxation on the ongoing contraction induced by (a) 0.5 μM noradrenaline in L-NOARG (0.1 mM)-treated strips, (b) 10 μM noradrenaline in strips treated with diclofenac (3 μM), and (c) 10 μM noradrenaline in the presence of CTx (0.1 μM)+apamin (0.1 μM) in strips treated with diclofenac (3 μM). Data are shown as means and s.e.m. *P<0.05, **P<0.01 vs corresponding response in the absence of SOD (two-way repeated-measures ANOVA and Scheffé's F test). †† P<0.01 vs ‘Diclofenac'.
In endothelium-intact strips, SOD, when applied in the presence of diclofenac (3 μM), did not significantly modify the noradrenaline-induced contraction (23.8±3.8 vs 21.8±5.2 mg, n=4, P>0.2) but it did enhance the ACh-induced relaxation (P<0.02; Figure 4b). CTx (0.1 μM)+apamin (0.1 μM) significantly enhanced the contraction induced by noradrenaline (22.8±2.1 vs 26.1±2.2 mg, n=4, P<0.01) and inhibited the relaxation induced by ACh (P<0.05) in the presence of diclofenac. In the presence of diclofenac+CTx+apamin, SOD did not modify the noradrenaline-induced contraction (25.2±2.1 mg, n=4, P>0.3) but it did enhance the ACh-induced relaxation (P<0.01; Figure 4c).
Effects of SOD and MnCl2 on NOC-7-induced relaxation in endothelium-denuded strips
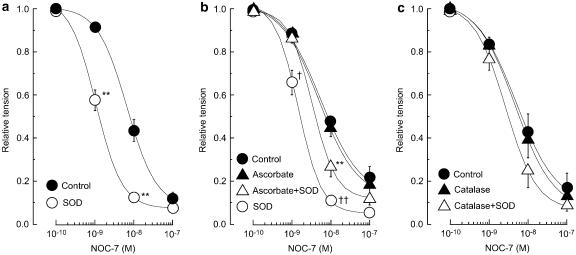
In endothelium-denuded strips constricted with noradrenaline (10 μM), NOC-7 (1–100 nM) evoked concentration-dependent relaxation. Under these conditions, SOD did not significantly modify the noradrenaline-induced contraction (55.1±8.1 vs 45.8±4.5 mg, n=4, P>0.2) but it did enhance the relaxation induced by NOC-7 (P<0.01; Figure 5a). This enhancing effect of SOD on the NOC-7-induced relaxation was also observed (and was similar in magnitude) in endothelium-denuded strips treated with 3 μM diclofenac (n=4, P<0.01; Table 2). The hydroxyl-radical scavenger dimethylthiourea (1 mM) had no effect on the relaxation induced by NOC-7 (1–100 nM), and SOD (200 U ml−1) significantly enhanced the NOC-7-induced relaxation in the presence of dimethylthiourea (n=6, P<0.01; Table 2).
Figure 5.
Effects of SOD, ascorbate and catalase on the NOC-7-induced relaxation on the ongoing noradrenaline (10 μM)-contraction in endothelium-denuded strips. (a) Effect of SOD (200 U ml−1) on NOC-7-induced relaxation. ‘Control': in the absence of agent; ‘SOD': the action of SOD. (b) Effect of ascorbate (50 μM) on NOC-7-induced relaxation in the absence and presence of SOD (200 U ml−1). ‘Control': in the absence of agents; ‘Ascorbate': in the presence of ascorbate; ‘Ascorbate+SOD': the action of SOD in the presence of ascorbate; ‘SOD': the action of SOD after a 60-min removal of ascorbate. (c) Effect of catalase (400 U ml−1) on NOC-7-induced relaxation in the absence and presence of SOD (200 U ml−1). ‘Control': in the absence of agents; ‘Catalase': in the presence of catalase; ‘Catalase+SOD': the action of SOD in the presence of catalase. Data are shown as means and s.e.m. **P<0.01 vs control (two-way repeated-measures ANOVA and Scheffé's F test). † P<0.05, †† P<0.01 vs ‘Ascorbate+SOD'.
Table 2.
Effects of SOD (200 U ml−1) on the NOC-7-induced relaxation on the contraction induced by noradrenaline in endothelium-denuded strips in the absence and presence of diclofenac or dimethylthiourea
| NOC-7-induced relaxation | |||||||||||
| Noradrenaline-induced contraction (mg) | EC50 (nM) | Emax (%) | |||||||||
| n | Before agent | After agent | Before agent | After agent | Before agent | After agent | |||||
| Control | SOD (−) | SOD (+) | Control | SOD (−) | SOD (+) | Control | SOD (−) | SOD (+) | |||
| Diclofenac (3 μM) | 4 | 52.8±5.2 | 51.9±6.2 | 47.8±3.2 | 26.3±10.7 | 38.0±11.5 | 1.3±0.2* | 66.7±1.3 | 70.0±0.8 | 95.4±0.4** | |
| Dimethylthiourea (1 mM) | 6 | 49.6±6.2 | 48.9±5.8 | 44.9±4.8 | 11.2±2.3 | 18.1±6.1 | 1.4±0.3** | 86.5±1.5 | 89.9±1.4 | 96.3±0.5** | |
Emax represents the maximum relaxation induced by 0.1 μM NOC-7 during the ongoing contraction induced by noradrenaline (10 μM). Control: in the absence of SOD and agents; SOD (−): in the presence of agent but without SOD; SOD (+): in the presence of agent plus SOD (200 U ml−1). Data are expressed as mean±s.e.m.
*P<0.05 or **P<0.01 vs SOD (−) in the presence of agent (Student's paired t-test). n indicates number of strips.
Ascorbate (50 μM) affected neither the noradrenaline-induced contraction (n=4, P>0.5) nor the NOC-7-induced relaxation (P>0.3) in endothelium-denuded strips. In the presence of ascorbate, SOD did not modify the noradrenaline-induced contraction (P>0.3) but it did enhance the NOC-7-induced relaxation (P<0.001; Figure 5b). However, the effect of SOD seen in the presence of ascorbate was significantly smaller than that seen after a 60-min washout of ascorbate (i.e. in the absence of ascorbate, P<0.02). In endothelium-denuded strips, catalase (400 U ml−1) altered neither the noradrenaline-induced contraction (n=4, P>0.5) nor the NOC-7-induced relaxation (P>0.5). In the presence of catalase, SOD affected neither the noradrenaline-induced contraction (n=4, P>0.3) nor the NOC-7-induced relaxation (P>0.1; Figure 5c). Under our conditions, an enhancing effect of SOD on the NOC-7-induced relaxation was not obtained following a 60-min washout of catalase, suggesting that the effect of catalase was not completely washable (at least, not by a 60-min washout).
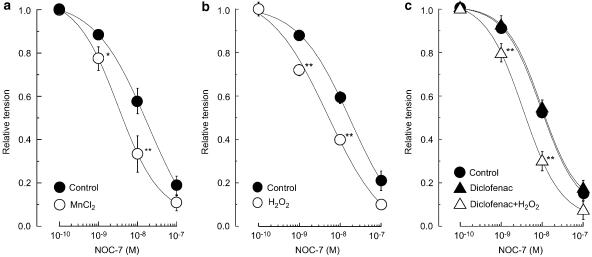
In endothelium-denuded strips, MnCl2 (30 μM) did not modify the contraction induced by 10 μM noradrenaline (37.8±5.2 and 36.3±6.5 mg in the absence and presence of MnCl2, respectively, n=5, P>0.5) but it did enhance the relaxation induced by NOC-7 (1–100 nM; P<0.05; Figure 6a). The enhancing effect of MnCl2 persisted even after a 60-min washout.
Figure 6.
Effects of MnCl2 and H2O2 (in the absence or presence of diclofenac) on the relaxation induced by NOC-7 during the contraction induced by 10 μM noradrenaline in endothelium-denuded strips. (a) Effect of MnCl2 (30 μM) on the NOC-7-induced relaxation. ‘Control': in the absence of MnCl2; ‘MnCl2': in the presence of MnCl2. (b) Effect of H2O2 (1 μM) on the NOC-7-induced relaxation. ‘Control': in the absence of H2O2; ‘H2O2': in the presence of H2O2. (c) Effect of H2O2 (1 μM) on the NOC-7-induced relaxation in the absence and presence of diclofenac. ‘Control': in the absence of diclofenac and H2O2; ‘Diclofenac': in the presence of diclofenac alone. ‘Diclofenac+H2O2': in the presence of both diclofenac and H2O2. Data are shown as means and s.e.m. *P<0.05, **P<0.01 vs control (two-way repeated-measures ANOVA and Scheffé's F test).
Effects of H2O2 on relaxations induced by NOC-7 and 8-bromo-cGMP in endothelium-denuded strips
In endothelium-denuded strips, H2O2 (1 μM) had no effect on the noradrenaline-induced contraction (39.6±2.4 and 38.4±3.8 mg in the absence and presence of H2O2, respectively, n=4, P>0.5) but it enhanced the NOC-7-induced relaxation (P<0.001; Figure 6b). The enhancing effect of H2O2 persisted even after a 60-min washout.
Catalase (400 U ml−1) abolished the action of H2O2 (1 μM) when it was coapplied with the H2O2. However, catalase did not significantly modify the action of H2O2 when it was applied after the enhancing effect of H2O2 had developed (n=4, P>0.05, not shown). In the presence of SOD (200 U ml−1), H2O2 (1 μM) did not significantly modify the NOC-7-induced relaxation (n=5, P>0.1 vs SOD alone). Moreover, SOD (200 U ml−1) had no significant effect on the NOC-7-induced relaxation in the presence of 1 μM H2O2 (n=5, P>0.5).
Diclofenac (3 μM) affected neither the noradrenaline-induced contraction (35.8±4.8 vs 36.3±5.2 mg, n=4, P>0.5) nor the NOC-7-induced relaxation (P>0.5; Figure 6c). In the presence of diclofenac, H2O2 (1 μM) did not modify the noradrenaline-induced contraction (n=4, P>0.5) but it did enhance the NOC-7-induced relaxation (P<0.001; Figure 6c).
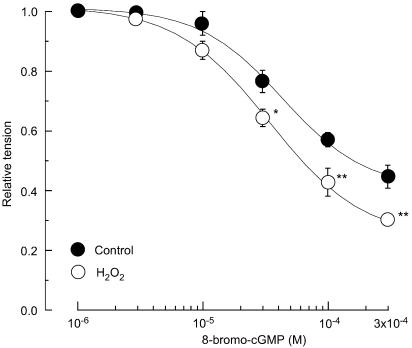
The membrane-permeable, phosphodiesterase-resistant cGMP analogue 8-bromo-cGMP produced a relaxation on the contraction induced by 10 μM noradrenaline (Figure 7). H2O2 (1 μM) significantly enhanced the 8-bromo-cGMP-induced relaxation (n=4, P<0.001).
Figure 7.
Effect of H2O2 on relaxation induced by 8-bromo-cGMP in endothelium-denuded strips. Effect of H2O2 (1 μM) on the 8-bromo-cGMP-induced relaxation obtained during a contraction induced by 10 μM noradrenaline. ‘Control': in the absence of H2O2; ‘H2O2': in the presence of H2O2. Data are shown as means and s.e.m. *P<0.05, **P<0.01 vs control (two-way repeated-measures ANOVA and Scheffé's F test).
Effects of SOD and MnCl2 on H2O2 production in smooth muscle cells
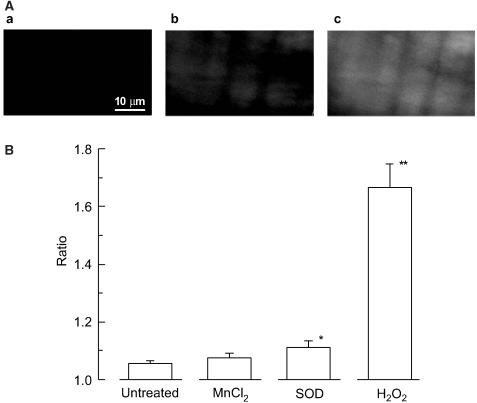
To examine whether extracellularly applied SOD increases the cellular concentration of H2O2, its effect on change in the fluorescence intensities of the H2O2-sensitive dye CM-H2DCF was studied in the smooth muscle cell. SOD (200 U ml−1) significantly increased the CM-H2DCF fluorescence ratio in smooth muscle cells (n=4, P<0.05; Figure 8). This effect of SOD was abolished when it was coapplied with catalase (400 U ml−1). MnCl2 (30 μM) tended to increase the ratio, but not significantly (n=4, P=0.07). H2O2 (1 μM) increased the ratio to a similar extent as SOD (1.18±0.02, n=4, P<0.05), while at 10 μM it increased the ratio to a value more than six times that seen with SOD (n=8, P<0.01; Figure 8).
Figure 8.
Effects of MnCl2, SOD and H2O2 on the fluorescence obtained with the H2O2-sensitive dye CM-H2DCF in smooth muscle cells of endothelium-denuded strips. (A) fluorescent images: a, image obtained just before application of SOD; b, at 15 min after application of SOD (200 U ml−1); c, at 15 min after application of H2O2 (10 μM). Similar results were obtained in three other preparations. (B) Summary of the effects of MnCl2 (30 μM), SOD (200 U ml−1) and H2O2 (10 μM) on the fluorescence. To obtain the fluorescence ratio, the fluorescence value was normalized with respect to that recorded just before the application of agent. Data are shown as means and s.e.m. *P<0.05, **P<0.01 vs untreated (Student's unpaired t-test).
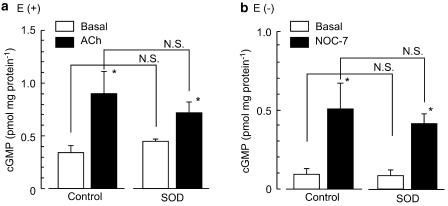
Effects of SOD on cGMP production
Under basal conditions, the concentration of cGMP was 0.33±0.07 pmol mg protein−1 in endothelium-intact strips (n=4) and 0.09±0.03 pmol mg protein−1 in endothelium-denuded strips (n=4, P<0.05). ACh (0.1 μM) and NOC-7 (0.1 μM) significantly increased the concentration of cGMP in endothelium-intact strips and endothelium-denuded strips, respectively (n=4, P<0.05 in each case). SOD (200 U ml−1) had no effect on the concentration of cGMP whether applied in the absence or presence of ACh in endothelium-intact strips (n=4, P>0.1 in each case) or in the absence or presence of NOC-7 in endothelium-denuded strips (n=4, P>0.5 in each case) (Figure 9). Likewise, neither tiron (0.3 mM) nor ascorbate (50 μM) significantly modified the concentration of cGMP whether applied in the presence of ACh in endothelium-intact strips (0.85±0.20 pmol mg protein−1 and 0.76± 0.16 pmol mg protein−1, n=4, P>0.5 in each case) or in the presence of NOC-7 in endothelium-denuded strips (0.47±0.13 pmol mg protein−1 and 0.42±0.08 pmol mg protein−1, n=4, P>0.5).
Figure 9.
Effects of SOD on the production of cGMP. Effects of SOD (200 U ml−1) on cGMP production (a) in the absence or presence of ACh (0.1 μM) in endothelium-intact (E(+)) strips and (b) in the absence or presence of NOC-7 (0.1 μM) in endothelium-denuded (E(−)) strips. Data are shown as means and s.e.m. *P<0.05 vs basal (Student's unpaired t-test). N.S. not significantly different.
Discussion
In the present experiments, SOD enhanced the ACh-induced, endothelium-dependent relaxation obtained during a noradrenaline-induced contraction. Furthermore, the SOD-induced enhancement of the ACh-induced relaxation was inhibited by L-NOARG (an NO-synthase inhibitor) but not by diclofenac (a cyclooxygenase inhibitor) whether applied in the absence or presence of CTx (an inhibitor of intermediate-conductance KCa channels)+apamin (an inhibitor of small-conductance KCa channels). Since an inhibitory action of L-NOARG on the effect of SOD was also observed when the concentration of noradrenaline was reduced so that the noradrenaline-induced contractions obtained before and after the application of L-NOARG were amplitude-matched, it is suggested that this action of L-NOARG is not secondary to its enhancement of the noradrenaline-induced contraction. Moreover, SOD enhanced the relaxation induced by NOC-7 (an NO donor) in endothelium-denuded strips. These results indicate that SOD enhances the ACh-induced endothelium-dependent relaxation through an increase in the action of endothelium-derived NO.
Endothelial cells and smooth muscle cells each generate superoxide and this reacts rapidly with NO, leading to a loss of the latter's vasodilator activity (Gryglewski et al., 1986; Rubanyi & Vanhoutte, 1986; Griendling et al., 1994; Harrison, 1997). It has been suggested that SOD plays a vital role in protecting endothelium-derived NO from destruction by endogenously generated superoxide (Mügge et al., 1991; Omar et al., 1991; Abrahamsson et al., 1992; Mian & Martin, 1995), and that by so doing, SOD enhances endothelium-dependent NO-mediated relaxation (Ohlstein & Nichols, 1989; Kasten et al., 1994; Mian & Martin, 1995). Ascorbate is another effective scavenger of superoxide (Som et al., 1983; Gotoh & Niki, 1992). In rat aorta, this agent produces a relaxation on the phenylephrine-induced contraction in the presence of endothelium that is abolished by endothelial denudation or treatment with an inhibitor of NO synthase (Dudgeon et al., 1998). This suggests that ascorbate, like SOD, protects basal NO against superoxide.
Notwithstanding the above similarity between ascorbate and SOD, we found in the present experiments that the superoxide scavenger ascorbate did not modify the NO-mediated relaxations induced by ACh (in endothelium-intact strips) and NOC-7 (in endothelium-denuded strips). The present results are, in part, consistent with previous findings in rat aorta showing that ascorbate (>30 μM), despite having the ability to protect NO from destruction by superoxide, has no effect on the relaxation induced by ACh in strips with intact endothelium (Dudgeon et al., 1998). Surprisingly, we found that ascorbate inhibited the effects of SOD on the NO-mediated relaxations induced by ACh (in endothelium-intact strips) and NOC-7 (in endothelium-denuded strips). The inhibition by a superoxide scavenger of the SOD-induced enhancement of endothelium-derived-NO-mediated relaxation was confirmed by the use of another superoxide scavenger tiron (MacKenzie et al., 1999). Furthermore, catalase (which converts H2O2 to water and oxygen), but not the hydroxyl-radical scavenger dimethylthiourea, inhibited the enhancing effect of SOD on these NO-mediated relaxations (without changing the NO-mediated relaxations obtained in the absence of SOD). Moreover, we found that SOD, but not tiron (0.3 mM; Mok et al., 1998), increased the intracellular concentration of H2O2 in smooth muscle cells (assessed from the increase in the fluorescence of the H2O2-sensitive dye CM-H2DCF), although the efficacy with which this type of SOD (the wild type) enters the cells remains unknown. In addition, MnCl2 (30 μM), a chemically distinct SOD mimetic that produces H2O2 (MacKenzie & Martin, 1998), tended to increase the CM-H2DCF signal in the smooth muscle cells and it enhanced the NOC-7-induced relaxation in endothelium-denuded strips. Taken together, these results suggest that H2O2 plays an essential role in the SOD-induced enhancement of NO-mediated relaxation in rabbit mesenteric resistance arteries. In other words, SOD may enhance NO-induced relaxation not via its superoxide-scavenging action but via its H2O2-producing action. This hypothesis is supported by our finding that SOD had no effect on the amount of cGMP produced (a) in the absence or presence of ACh (in endothelium-intact strips) or (b) in the absence or presence of NOC-7 (in endothelium-denuded strips). Since it is known that NO activates soluble guanylyl cyclase and increases the cellular concentration of cGMP, thus producing a smooth-muscle relaxation (Moncada et al., 1991; Kuriyama et al., 1998), it would be expected that SOD would increase the cellular concentration of cGMP if this enzyme enhanced NO-mediated relaxation by scavenging superoxide. Hence, the scavenging action of SOD against superoxide is unlikely to explain the SOD-induced enhancement of NO-mediated relaxation. Importantly, we found that in endothelium-denuded strips, a low concentration (1 μM) of H2O2 enhanced the NOC-7-induced relaxation. The above evidence suggests that in rabbit mesenteric resistance arteries, SOD converts superoxide into H2O2 and thereby enhances NO-mediated relaxation. Furthermore, we also found that H2O2 (1 μM) significantly enhanced the relaxation induced by the membrane-permeable, phosphodiesterase-resistant cGMP analogue 8-bromo-cGMP in endothelium-denuded strips. Taken together, all this suggests that H2O2 enhances the activation of cGMP-dependent protein kinase by this messenger, thus enhancing the NO-mediated smooth muscle relaxation.
At present, the mechanism underlying the H2O2-induced facilitation of the cGMP-dependent pathway involved in NO-mediated relaxation remains unknown. It has been suggested that cGMP produces vascular smooth muscle relaxation through an increase in myosin-light-chain dephosphorylation (Lee et al., 1997; Sauzeau et al., 2000). However, cGMP produces a smooth muscle relaxation without myosin-light-chain dephosphorylation in swine carotid arteries (Rembold et al., 2000). Thus, the mechanism underlying cGMP-induced smooth muscle relaxation remains controversial. H2O2 activates both protein kinase C and tyrosine kinase in vascular smooth muscle and endothelial cells (Jin & Rhoades, 1997; Lum & Roebuck, 2001), but the involvement of these kinases in the facilitation of cyclic-GMP-mediated relaxation by H2O2 seems unlikely since it has been suggested that they contribute to the H2O2-induced enhancement (rather than inhibition) of vascular smooth muscle contraction (Jin & Rhoades, 1997; Lum & Roebuck, 2001). The mechanism underlying the hydrogen-peroxide-induced augmentation of cGMP-dependent relaxation will need to be clarified in future work.
We are uncertain as to why the superoxide-scavenging effect of SOD (which serves to protect NO) did not play a significant role in the SOD-induced enhancement of NO-mediated relaxation in the present experiments. Likewise, we are unclear as to why scavenging of superoxide (assessed from the action of ascorbate and tiron) did not significantly enhance NO-mediated relaxation in rabbit mesenteric resistance arteries. Possibly, the amount of superoxide produced in this particular artery may not be enough to modify NO-induced relaxation to a significant extent under our experimental conditions. Alternatively, since in the present experiments we pretreated with the antioxidants for a very short time (3 min), the results may represent only the extracellular actions of the antioxidants against superoxide, not the intracellular ones. These points remain to be clarified. It has been suggested that ascorbate is unlikely to act purely as an antioxidant because the rate constant for the interaction of superoxide with ascorbate is about five orders of magnitude lower than that for the interaction of superoxide with SOD (Griendling & Alexander, 1997). In our preliminary experiments, we found that the inhibitory action of ascorbate on the effect of SOD on ACh-induced endothelium-dependent relaxation was obtained only when ascorbate was applied as a pretreatment (i.e. before application of SOD, as in the present experiments). Therefore, as suggested by Griendling & Alexander (1997), ascorbate may act somewhere in the pathway responsible for superoxide production and thus reduce its production, leading to a reduced generation of H2O2 and thereby attenuating the SOD-induced enhancement of NO-mediated relaxation.
In rabbit mesenteric resistance arteries, ACh-stimulated endothelial cells release NO, prostacyclin and endothelium-derived hyperpolarizing factor (EDHF) (Kuriyama et al., 1998; Yamashita et al., 1999) and NO does not contribute to hyperpolarization (Murphy & Brayden, 1995; Kuriyama et al., 1998). Thus, it has been suggested that ACh produces a hyperpolarization through an action mediated by EDHF and endothelium-derived prostaglandins (Murphy & Brayden, 1995; Parkington et al., 1995; Nishiyama et al., 1998; Yamashita et al., 1999). By contrast, in mesenteric arteries in endothelial-NO-synthase-knockout mice it has recently been suggested that ACh produces a hyperpolarization via an action mediated by H2O2 synthesized within the endothelial cells (Matoba et al., 2000). Furthermore, those authors found that catalase (1250 U ml−1) attenuated both the hyperpolarization and the relaxation induced by ACh, suggesting that H2O2 is a possible candidate for EDHF. In the present experiments, however, neither SOD (200 U ml−1) nor catalase (400 U ml−1) modified the resting membrane potential or the ACh-induced hyperpolarization in smooth muscle cells in rabbit mesenteric arteries, although admittedly the concentration of catalase used in the present experiments was lower than that used by Matoba et al. (2000) in the mouse. Furthermore, H2O2 (1 μM) modified neither the resting membrane potential nor the ACh-induced hyperpolarization in the smooth muscle cells of the rabbit mesenteric resistance artery. These results suggest that at this low concentration, H2O2 cannot be EDHF in this particular resistance artery.
In conclusion, in rabbit mesenteric resistance arteries, SOD enhances ACh-induced endothelium-dependent relaxation through an increase in the action of endothelium-derived NO on the smooth muscle. Since H2O2 facilitates the cGMP-dependent pathway that is involved in the NO-induced relaxation of vascular smooth muscle, it is suggested that SOD produces H2O2 and that this contributes to the SOD-induced enhancement of NO-mediated relaxation in these arteries.
Acknowledgments
We thank Drs R.J. Timms and F. Edwards for critical readings of the manuscript. We also thank Mr Toshio Kamiya for his cooperation in this study. This work was partly supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan.
Abbreviations
- CM-H2DCF
5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein
- CTx
charybdotoxin
- EDHF
endothelium-derived hyperpolarizing factor
- L-NOARG
L-NG-nitroarginine
- NOC-7
1-hydroxy-2-oxo-3-(N-methyl-3-aminopropyl)-3-methyl-1-triazene
- SOD
superoxide dismutase
- tiron
4,5-dihydroxy-1,3-benzene-disulphonic acid
References
- ABRAHAMSSON T., BRANDT U., MARKLUND S.L., SJOQVIST P.O. Vascular bound recombinant extracellular superoxide dismutase type C protects against the detrimental effects of superoxide radicals on endothelium-dependent arterial relaxation. Circ. Res. 1992;70:264–271. doi: 10.1161/01.res.70.2.264. [DOI] [PubMed] [Google Scholar]
- BARLOW R.S., ABDALLA M.E., RICHARD E.W. H2O2 opens BKCa channels via the PLA2-arachidonic acid signaling cascade in coronary artery smooth muscle. Am. J. Physiol. 2000;279:H475–H483. doi: 10.1152/ajpheart.2000.279.2.H475. [DOI] [PubMed] [Google Scholar]
- BARLOW R.S., WHITE R.E. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am. J. Physiol. 1998;275:H1283–H1289. doi: 10.1152/ajpheart.1998.275.4.H1283. [DOI] [PubMed] [Google Scholar]
- BHARADWAJ L., PRASAD K. Mediation of H2O2-induced vascular relaxation by endothelium-derived relaxing factor. Mol. Cell. Biochem. 1995;149–150:267–270. doi: 10.1007/BF01076587. [DOI] [PubMed] [Google Scholar]
- DAEMEN M.J., LOMBARDI D.M., BOSMAN F.T., SCHWARTZ S.M. Angiotensin II induces smooth muscle cell proliferation in the normal and injured rat arterial wall. Circ. Res. 1991;68:450–456. doi: 10.1161/01.res.68.2.450. [DOI] [PubMed] [Google Scholar]
- DUDGEON S., BENSON D.P., MACKENZIE A., PAISLEY-ZYSZKIEWICZ K., MARTIN W. Recovery by ascorbate of impaired nitric oxide-dependent relaxation resulting from oxidant stress in rat aorta. Br. J. Pharmacol. 1998;125:782–786. doi: 10.1038/sj.bjp.0702120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAO Y.J., LEE R.M.K.W. Hydrogen peroxide induces a greater contraction in mesenteric arteries of spontaneously hypertensive rats through thromboxane A2 production. Br. J. Pharmacol. 2001;134:1639–1646. doi: 10.1038/sj.bjp.0704420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTOH N., NIKI E. Rates of interactions of superoxide with vitamin E, vitamin C and related compounds as measured by chemiluminescence. Biochim. Biophys. Acta. 1992;1115:201–207. doi: 10.1016/0304-4165(92)90054-x. [DOI] [PubMed] [Google Scholar]
- GRIENDLING K.K., ALEXANDER R.W. Oxidative stress and cardiovascular disease. Circulation. 1997;96:3264–3265. [PubMed] [Google Scholar]
- GRIENDLING K.K., MINIERI C.A., OLLERENSHAW J.D., ALEXANDER R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- GRYGLEWSKI R.J., PALMER R.M., MONCADA S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- HARRISON D.G.Endothelial function and oxidant stress Clin. Cardiol. 199720II-11-17 [PubMed] [Google Scholar]
- HRABIE J.A., KLOSE J.R., WINK D.A., KEEFER L.K. New nitric oxide-releasing zwitterions derived from polyamines. J. Org. Chem. 1993;58:1472–1476. [Google Scholar]
- IESAKI T., GUPTE S.A., KAMINSKI P.M., WOLIN M.S. Inhibition of guanylate cyclase stimulation by NO and bovine arterial relaxation to peroxynitrite and H2O2. Am. J. Physiol. 1999;277:H978–H985. doi: 10.1152/ajpheart.1999.277.3.H978. [DOI] [PubMed] [Google Scholar]
- IIDA Y., KATUSIC Z.S. Mechanisms of cerebral arterial relaxations to hydrogen peroxide. Stroke. 2000;31:2224–2230. doi: 10.1161/01.str.31.9.2224. [DOI] [PubMed] [Google Scholar]
- ITOH T., SEKI N., SUZUKI S., ITO S., KAJIKURI J., KURIYAMA H. Membrane hyperpolarization inhibits agonist-induced synthesis of inositol 1,4,5-trisphosphate in rabbit mesenteric artery. J. Physiol. 1992;451:307–328. doi: 10.1113/jphysiol.1992.sp019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIN N., RHOADES R.A. Activation of tyrosine kinases in H2O2-induced contraction in pulmonary artery. Am. J. Physiol. 1997;272:H2686–H2692. doi: 10.1152/ajpheart.1997.272.6.H2686. [DOI] [PubMed] [Google Scholar]
- KASTEN T.P., SETTLE S.L., MISKO T.P., CURRIE M.G., NICKOLS G.A. Manganese potentiation of nitric oxide-mediated vascular relaxation. Eur. J. Pharmacol. 1994;253:35–43. doi: 10.1016/0014-2999(94)90754-4. [DOI] [PubMed] [Google Scholar]
- KURIYAMA H., KITAMURA K., ITOH T., INOUE R. Physiological features of visceral smooth muscle cells, with special reference to receptors and ion channels. Physiol. Rev. 1998;78:811–920. doi: 10.1152/physrev.1998.78.3.811. [DOI] [PubMed] [Google Scholar]
- LEE M.R., LI L., KITAZAWA T. Cyclic GMP causes Ca2+ desensitization in vascular smooth muscle by activating the myosin light chain phosphatase. J. Biol. Chem. 1997;272:5063–5068. doi: 10.1074/jbc.272.8.5063. [DOI] [PubMed] [Google Scholar]
- LUM H., ROEBUCK K.A. Oxidant stress and endothelial cell dysfunction. Am. J. Physiol. 2001;280:C719–C741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- MACKENZIE A., FILIPPINI S., MARTIN W. Effects of superoxide dismutase mimetics on the activity of nitric oxide in rat aorta. Br. J. Pharmacol. 1999;127:1159–1164. doi: 10.1038/sj.bjp.0702670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKENZIE A., MARTIN W. Loss of endothelium-derived nitric oxide in rabbit aorta by oxidant stress: restoration by superoxide dismutase mimetics. Br. J. Pharmacol. 1998;124:719–728. doi: 10.1038/sj.bjp.0701899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATOBA T., SHIMOKAWA H., NAKASHIMA M., HIRAKAWA Y., MUKAI Y., HIRANO K., KANAIDE H., TAKESHITA A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J. Clin. Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIAN K.B., MARTIN W. Differential sensitivity of basal and acetylcholine-stimulated activity of nitric oxide to destruction by superoxide anion in rat aorta. Br. J. Pharmacol. 1995;115:993–1000. doi: 10.1111/j.1476-5381.1995.tb15909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOK J.S.L., PAISLEY K., MARTIN W. Inhibition of nitrergic neurotransmission in the bovine retractor penis muscle by an oxidant stress: effects of superoxide dismutase mimetics. Br. J. Pharmacol. 1998;124:111–118. doi: 10.1038/sj.bjp.0701809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M., HIGGS E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- MÜGGE A., ELWELL J.H., PETERSON T.E., HARRISON D.G. Release of intact endothelium-derived relaxing factor depends on endothelial superoxide dismutase activity. Am. J. Physiol. 1991;260:C219–C225. doi: 10.1152/ajpcell.1991.260.2.C219. [DOI] [PubMed] [Google Scholar]
- MURPHY M.E., BRAYDEN J.E. Apamin-sensitive K+ channels mediate an endothelium-dependent hyperpolarization in rabbit mesenteric arteries. J. Physiol. 1995;489:723–734. doi: 10.1113/jphysiol.1995.sp021086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIYAMA M., HASHITANI H., FUKUTA H., YAMAMOTO Y., SUZUKI H. Potassium channels activated in the endothelium-dependent hyperpolarization in guinea-pig coronary artery. J. Physiol. 1998;510:455–465. doi: 10.1111/j.1469-7793.1998.455bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHLSTEIN E.H., NICHOLS A.J. Rabbit polymorphonuclear neutrophils elicit endothelium-dependent contraction in vascular smooth muscle. Circ. Res. 1989;65:917–924. doi: 10.1161/01.res.65.4.917. [DOI] [PubMed] [Google Scholar]
- OMAR H.A., CHERRY P.D., MORTELLITI M.P., BURKE-WOLIN T., WOLIN M.S. Inhibition of coronary artery superoxide dismutase attenuates endothelium-dependent and -independent nitrovasodilator relaxation. Circ. Res. 1991;69:601–608. doi: 10.1161/01.res.69.3.601. [DOI] [PubMed] [Google Scholar]
- PARKINGTON H.C., TONTA M.A., COLEMAN H.A., TARE M. Role of membrane potential in endothelium-dependent relaxation of guinea-pig coronary arterial smooth muscle. J. Physiol. 1995;484:469–480. doi: 10.1113/jphysiol.1995.sp020679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REMBOLD C.M., FOSTER D.B., STRAUSS J.D., WINGARD C.J., VAN EYK J.E. cGMP-mediated phosphorylation of heat shock protein 20 may cause smooth muscle relaxation without myosin light chain dephosphorylation in swine carotid artery. J. Physiol. 2000;524:865–878. doi: 10.1111/j.1469-7793.2000.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBANYI G.M., VANHOUTTE P.M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am. J. Physiol. 1986;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- SAUZEAU V., LE JEUNE H., CARIO-TOUMANIANTZ C., SMOLENSKI A., LOHMANN S.M., BERTOGLIO J., CHARDIN P., PACAUD P., LOIRAND G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J. Biol. Chem. 2000;275:21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- SHEEHAN D.W., GIESE E.C., GUGINO S.F., RUSSELL J.A. Characterization and mechanisms of H2O2-induced contractions of pulmonary arteries. Am. J. Physiol. 1993;264:H1542–H1547. doi: 10.1152/ajpheart.1993.264.5.H1542. [DOI] [PubMed] [Google Scholar]
- SOM S., RAHA C., CHATTERJEE I.B. Ascorbic acid: a scavenger of superoxide radical. Acta. Vitaminol. Enzymol. 1983;5:243–250. [PubMed] [Google Scholar]
- SUZUKI S., KAJIKURI J., SUZUKI A., ITOH T. Effects of endothelin-1 on endothelial cells in the porcine coronary artery. Circ. Res. 1991;69:1361–1368. doi: 10.1161/01.res.69.5.1361. [DOI] [PubMed] [Google Scholar]
- WEI E.P., KONTOS H.A., BECKMAN J.S. Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am. J. Physiol. 1996;271:H1262–H1266. doi: 10.1152/ajpheart.1996.271.3.H1262. [DOI] [PubMed] [Google Scholar]
- YAMASHITA A., KAJIKURI J., OHASHI M., KANMURA Y., ITOH T. Inhibitory effects of propofol on acetylcholine-induced, endothelium-dependent relaxation and prostacyclin synthesis in rabbit mesenteric resistance arteries. Anesthesiology. 1999;91:1080–1089. doi: 10.1097/00000542-199910000-00029. [DOI] [PubMed] [Google Scholar]
- YANG Z.-W., ZHANG A., ALTURA B.T., ALTURA B.M. Hydrogen peroxide-induced endothelium-dependent relaxation of rat aorta. Involvement of Ca2+ and other cellular metabolites. Gen. Pharmacol. 1999;33:325–336. doi: 10.1016/s0306-3623(99)00019-1. [DOI] [PubMed] [Google Scholar]
- YANG Z.-W., ZHENG T., ZHANG A., ALTURA B.T., ALTURA B.M. Mechanisms of hydrogen peroxide-induced contraction of rat aorta. Eur. J. Pharmacol. 1998;344:169–181. doi: 10.1016/s0014-2999(97)01576-8. [DOI] [PubMed] [Google Scholar]
- ZEMBOWICZ A., HATCHETT R.J., JAKUBOWSKI A.M., GRYGLEWSKI R.J. Involvement of nitric oxide in the endothelium-dependent relaxation induced by hydrogen peroxide in the rabbit aorta. Br. J. Pharmacol. 1993;110:151–158. doi: 10.1111/j.1476-5381.1993.tb13785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]