Abstract
Angiopoietin-1 (Ang1) is an angiogenic growth factor that binds to the Tie2 receptor on vascular endothelium, promoting blood vessel maturation and integrity. In the present study, we have investigated whether Ang1 also possesses anti-inflammatory properties by determining its effects on endothelial barrier function, neutrophil (PMN) adherence to endothelial cells (EC) and production of the PMN chemotactic factor interleukin-8 (IL-8).
Pretreatment of endothelial monolayers with Ang1 attenuated the permeability increase induced by thrombin in both lung microvascular cells and a human endothelial cell line. Similarly, Ang1 prevented the permeability-inducing effects of platelet-activating factor, bradykinin and histamine.
Pretreatment of EC with Ang1 also reduced the adherence of PMN to EC stimulated by thrombin. In contrast to its ability to counteract the increase in monolayer permeability brought about by various inflammatory agents, Ang1 did not affect the ability of histamine, PAF, or tumor necrosis factor-α to stimulate PMN adherence to EC.
In addition to its ability to inhibit PMN adherence, Ang1 diminished IL-8 production from EC challenged with thrombin in a concentration-dependent manner.
When EC were preincubated with the specific Rho kinase (ROCK) inhibitor Y-27632, we observed a reduction in PMN adherence in response to thrombin, as well as a decrease in thrombin-stimulated IL-8 production. Coincubation of monolayers with Y-27632 and Ang1 did not further attenuate the above-mentioned responses. However, Ang-1 failed to inhibit the activation of RhoA in response to thrombin, suggesting that inhibition of EC adhesiveness for PMN and IL-8 production by Ang1 does not result from reduced ROCK activation.
We conclude that Ang1 can counteract several aspects of the inflammatory response, including endothelial permeability, PMN adherence to EC as well as inhibition of IL-8 production by EC.
Keywords: Tie2, angiopoietin-1, neutrophils, endothelium, angiogenesis, IL-8, thrombin, permeability, inflammation, ROCK
Introduction
Angiopoietins are a novel family of growth factors that bind to the endothelial receptor tyrosine kinase Tie2 and regulate several aspects of the angiogenic process (Yancopoulos et al., 2000). The importance of the Ang/Tie2 pathway in normal development of the cardiovascular system is underscored by the observation that transgenic animals with targeted disruption of the locus for Ang1 or its cognate receptor exhibit embryonic lethality because of cardiovascular defects (Sato et al., 1995; Davis et al., 1996). Unlike most angiogenic growth factors angiopoietin-1 (Ang1) is not an endothelial cell mitogen; it does, however, stimulate endothelial cell (EC) migration, it promotes sprouting of EC and facilitates the assembly of EC into more complex networks in in vitro models (Koblizek et al., 1998; Witzenbichler et al., 1998; Hayes et al., 1999). In addition, Ang1 is a survival factor for EC mediating blood vessel maturation and stabilization (Papapetropoulos et al., 2000; Jones et al., 2001).
Recent studies have revealed a new aspect of Ang1 biology, namely its ability to restrict the permeability increase in response to edema-promoting agents. Indeed, transgenic overexpression (Thurston et al., 1999) or adenovirus-mediated gene transfer (Thurston et al., 2000) of Ang1 protects blood vessels against plasma leackage in mice challenged with serotonin, platelet-activating factor (PAF) or vascular endothelial growth factor (VEGF). Further in vitro studies have demonstrated a direct inhibitory effect of Ang1 on endothelial monolayer permeability increased by VEGF or thrombin (Gamble et al., 2000). An anti-inflammatory role for Ang1 was also suggested by experiments showing that Ang1 reduces VEGF-stimulated leukocyte adherence to EC (Kim et al., 2001), as well as polymorphonuclear neutrophil (PMN) migration across endothelial monolayers stimulated with TNF-α (Gamble et al., 2000).
Inflammation is a normal immune response associated with injury and infection (Nathan, 2002). Although a healing response, chronic or uncontrolled inflammation plays an important pathogenetic role in many diseases (Nathan, 2002). The endothelium, normally a tight barrier between the blood and the underlying tissues, reacts to inflammatory stimuli by exhibiting increased leakage to macromolecules, as well as increased binding and migration of leukocytes. The aim of the present study was to determine whether the endothelial-specific ligand Ang1 is able to prevent the actions of common inflammatory mediators by reducing endothelial permeability, PMN adherence and cytokine production by EC.
Methods
Materials
Two forms of Ang1 were used in our study: a recombinant, chimeric version of Ang1 was used in most experiments (Papapetropoulos et al., 1999) and was provided by Regeneron Pharmaceuticals. Native angiopoietin 1 fused with a 6xHis tag at its carboxy terminus was purchased from R&D Systems (Minneapolis, MN, U.S.A.). Bovine lung microvascular endothelial cells (BLMVEC) were from VEC Technologies Inc. (Rensselaer, NY, U.S.A.). Tissue culture plates were obtained from Greiner GmbH. Y-27632, endotoxin-free BSA and thrombin were purchased from Calbiochem-Novabiochem GmbH (Bad Soden, Germany). Human TNF-α was obtained from Chemicon International Inc (Temecula, CA, U.S.A.). The IL-8 ELISA kit was purchased from Diaclone Research (Besançon, France). Media, serum, antibiotics and cell culture supplements were obtained from Life Technologies, Inc. (Paisley, U.K.). The RhoA antibody was purchased form Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). The Ficoll–Hypaque and the glutathione sepharose beads were obtained from Amersham Biosciences (Vienna, Austria); the Transwell inserts were from Corning (Corning, NY, U.S.A.); fibronectin was obtained from Roche Diagnostics GmbH (Mannheim, Germany). The protein determination dye was purchased from Bio-Rad (Hercules, CA, U.S.A.). Most other biochemical reagents including bradykinin, PAF, ABTS, hydrogen peroxide and horseradish peroxidase were purchased from Sigma (St Louis, MO, U.S.A.).
Cell culture
BLMVEC were cultured in MCDB-131 medium containing 10% FCS, 150 μg ml−1 bovine cerebellum extract, 100 μg ml−1 heparin, 100 μg ml−1 streptomycin, and 200 U ml−1 penicillin. The EA.hy926 endothelial cell line, derived from immortalized human umbilical vein endothelial cells (HUVEC) (Edgell et al., 1983) was grown in DMEM containing 10% FCS, 2 mM glutamine, HAT (hypoxanthine 0.1 mM, aminopterin 0.4 μM, thymidine 16 μM), and antibiotics. For PMN adherence experiments and IL-8 production, the cells were seeded in 24-well plates and used 3–4 days later, at confluence.
Permeability experiments
The cells were seeded at a density of 0.15 × 106 on 0.3 cm2 porous filters coated with 1 μg human fibronectin (for EA.hy 926 cells) or left uncoated (for BLMVEC). The permeability experiments were performed on day 3 after seeding. For experiments with BLMVEC, the apical side of monolayers was exposed to 250 ng ml−1 Ang1 or its solvent (TBS with 0.05% CHAPS, pH 7.5; final dilution 1 : 530) for 2 h in 225 μl DMEM without phenol red and containing 1% endotoxin-free bovine serum albumin (BSA), before the addition of inflammatory stimuli for another 30 or 60 min. The basolateral side was incubated with 1 ml DMEM alone. At the end of the experiment, the passage of BSA into the basolateral compartment was quantified with the Bio-Rad protein assay kit. For experiments with EA.hy926 cells, the apical side of monolayers was incubated with 250 ng ml−1 of Ang1 or its solvent for 1 h in 225 μl DMEM without phenol red and with 1% BSA, before the addition of inflammatory stimuli for 45 min. The basolateral side was incubated with 1 ml DMEM containing 1% BSA. A volume of 5 μg ml−1 horseradish peroxidase (HRP) were then added to the apical compartment for the last 30 min of the experiment. The passage of HRP into the basolateral compartment was quantified by colorimetry. For this, the basolateral supernatant was collected and 100 μl of 1 M citrate pH 4.2 was added. A volume of 100 μl of sample were transferred to a 96-well microtiter plate and 100 μl of substrate (2 mM2,2′-azino-bis[3-ethylbenzthiazoline 6-sulfonic acid] diammonium (ABTS) and 0.06% H2O2 in 100 mM citrate buffer, pH 4.2) were added. The colorimetric reaction was stopped by adding 30 μl of 5.5% sodium dodecyl sulfate (0.5% final) and read at 405 nm. Standards were made with serial dilutions of the same stock of HRP, and processed in the same way as described above. The assay was linear in the range of 2–75 ng ml−1 HRP.
PMN adherence to EC
PMN from citrated blood collected from healthy donors were isolated by dextran sedimentation followed by density gradient centrifugation in Ficoll–Hypaque. Contaminating red blood cells were lysed by hypotonic shock with cold water for 40 s, PMN were washed twice and resuspended at a concentration of 107 ml−1 in DMEM with 0.1% BSA. EA.hy926 cells were gently rinsed in HBSS containing Ca2+ and Mg2+ (HBSS+) prewarmed to 37°C, before incubation with Ang1 or vehicle for 1 h, in the presence or absence of 10 μM Y-27632. The cells were then challenged for 30 min with various inflammatory stimuli, before adding 106 PMN well−1 for another 30 min. In some experiments, PMN were added only after all the agents incubated with the EC were washed away. Nonadherent PMN were then rinsed three times with warm HBSS+. Adherent PMN were quantified by coloration of a substrate of the PMN-specific azurophil granule marker, myeloperoxidase (MPO), as previously described (Pizurki et al., 2000). Briefly, adherent PMN were lysed with 0.5 ml ice-cold 1% Triton X-100. The remaining MPO trapped within PMN-derived DNA was dissolved by adding 10 μl of 10 mg ml−1 DNase (200 μg ml−1 final). After 15 min of shaking on ice, 50 μl of 1 M citrate pH 4.2 were added. The activity of MPO was performed as described for permeability experiments. Standards were made with serial dilutions of the initial stock of PMN, and processed in the same way as described above. The assay was linear in the range of 4–600 × 103 PMN ml−1.
IL-8 production by endothelial monolayers
Confluent EA.hy 926 cells were incubated 8 h with culture medium containing 0.1% BSA, before being treated with Ang1 or solvent for 1 h, in the presence or absence of 10 μM Y 27632, and then challenged with thrombin which was added for 16 h. IL-8 in the supernatant was measured using an ELISA kit.
RhoA activity assays
The GST-C21 construct codes for the 90 amino acids of the Rho effector protein rhotekin that carry the sequence responsible for Rho binding fused with glutathione S-transferase (GST) (Sander et al., 1999). E. coli cells were transformed with GST-C21 and grown at 30°C. Expression of recombinant protein was induced by adding 0.1 mM isopropylthiogalactoside for 2 h. Cells were harvested, resuspended in lysis buffer (10 mM Tris pH 8.0, 0.2 mM Na2S2O5, 2 mM MgCl2, 2 mM DTT, 20% sucrose, 10% glycerol) and sonicated. Cell lysates were centrifuged at 4°C for 30 min at 12,000 × g and the supernatant was incubated with glutathione-coupled Sepharose 4B beads for 30 min at 4°C. The beads were washed three times in lysis buffer and resuspended in the GST-fishing buffer (10% glycerol, 50 mM Tris pH 7.4, 100 mM NaCl, 1% NP-40 and 2 mM MgCl2). Binding assays were performed as described (Sander et al., 1999). Briefly, confluent EA.hy 926 cells were rinsed twice with HBSS+ and preincubated for 1 h with Ang1 or solvent in culture medium containing 0.1% BSA. Thrombin (0.01 U ml−1) was then added for 5 or 10 min and the cells were lysed. The cells were washed with ice-cold PBS once and lysed with GST-fishing buffer. Lysates were then centrifuged at 12,000 × g for 20 min at 4°C and the supernatant was incubated with glutathione-coupled sepharose beads for 30 min at 4°. The beads containing active RhoA bound to GST-rhotekin were washed and subjected to SDS–PAGE followed by Western blotting with a RhoA antibody.
Data analysis and statistics
Data are expressed as means±s.e.m. of the number of indicated observations. Statistical significance was tested using ANOVA followed by an appropriate post hoc test (the LSD test was used for all comparisons with the exception of Figure 4b, where the post-hoc test used was the Dunnett's). Statistical significance was set at P<0.05.
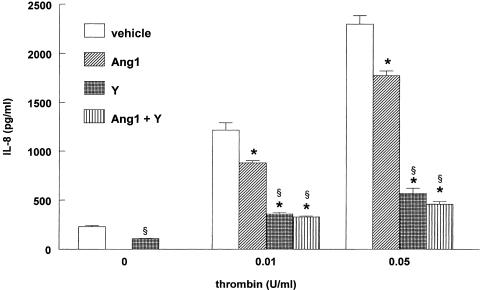
Figure 4.
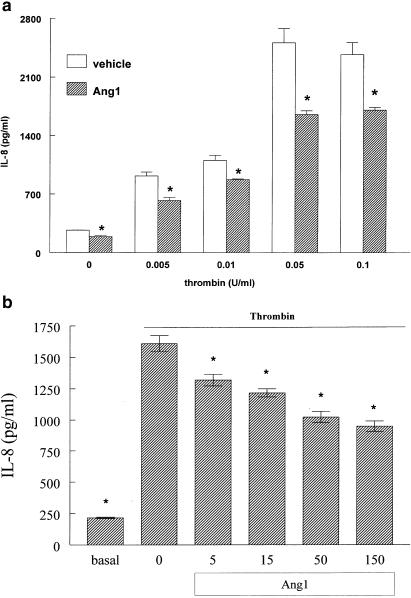
Ang1 decreases IL-8 production stimulated by thrombin. (a) Confluent EA.hy 926 cells were preincubated with 250 ng ml−1 Ang1 or solvent for 1 h, before being exposed to different concentrations of thrombin for 16 h. Values are means±s.e.m., n=3; *P<0.05 from vehicle. (b) EA.hy 926 cells were preincubated with the indicated concentration of native Ang1 or solvent for 1 h, before being exposed to 0.05 U ml−1 thrombin for 16 h. Values are means±s.e.m., n=5; *P<0.05 from vehicle.
Results
Ang1 prevents the increase in endothelial permeability induced by inflammatory agents
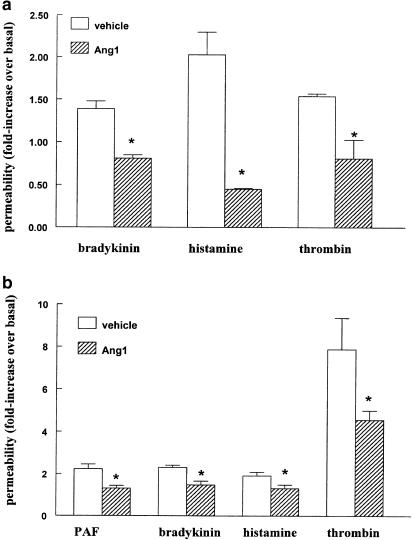
We determined whether Ang1 counteracts the permeability increasing effect of various inflammatory agents, such as thrombin, histamine and bradykinin. Initial experiments were performed with primary cultures of bovine lung microvascular endothelial cells (BLMVEC). Figure 1a shows that the increase in transendothelial permeability stimulated by these edemagenic agents was prevented by pretreatment with Ang1. As BLMVEC rapidly lose over passages in culture their ability to form tight monolayers that restrict the passage of macromolecules, we used a HUVEC-derived cell line (EA.hy926) in all of our subsequent experiments. EA.hy926 cells are known to display similar characteristics as their parental cells with respect to the response to inflammatory mediators in permeability studies (Rabiet et al., 1996), as well as leucocyte adherence assays (Brown et al., 1993). They also express the endothelial-specific Tie2 receptor (Maisonpierre et al., 1997). Permeability studies with EA.hy926 cells were done by measuring the passage of HRP instead of BSA, as BSA had to be present on both the apical and basolateral side of the monolayers for the duration of the experiment to maintain endothelial barrier function. Figure 1b shows that Ang1 displayed similar anti-permeability properties on EA.hy926 monolayers, consistent with our observations in BLMVEC. The ∼two-fold increase in permeability induced by histamine or bradykinin, as well as the 11-fold increase in response to thrombin, were significantly inhibited by Ang1. Interestingly, Ang1 was also capable of antagonizing the effect exerted by PAF, another classical edemagenic agent.
Figure 1.
Ang1 prevents permeability increases across endothelial monolayers. (a) BLMVEC monolayers were exposed to 250 ng ml−1 Ang1 or solvent for 2 h before adding 1 μM bradykinin for 1 h, 100 μM histamine for 30 min, or 1 U ml–1 thrombin for 1 h. Values are means+s.e.m., n=3; *P<0.05 from vehicle. The passage of BSA under baseline conditions was of 31.5±5 μg monolayer−1 (30 min)–1. (b) EA.hy 926 monolayers were exposed to 250 ng ml−1 Ang1 or solvent for 1 h before adding 10 nM PAF, 1 μM bradykinin, 100 μM histamine or 0.01 U ml−1 thrombin for 45 min. Values are means±s.e.m., n=6–12; *P<0.05 from vehicle. The passage of HRP under baseline conditions was 16.4±1.7 ng HRP monolayer−1 (30 min)−1.
Ang1 decreases PMN adherence stimulated by thrombin
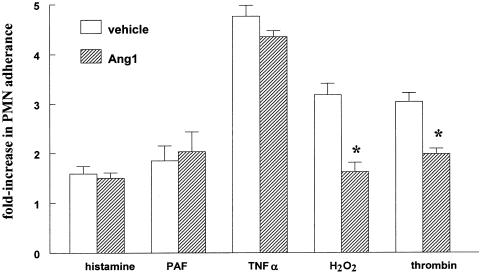
We next determined the effect of Ang1 on PMN adherence to EA.hy 926 cells. Figure 2 shows that pretreatment of EC with Ang1 resulted in a significant inhibition in the number of PMN adhering to EC stimulated by thrombin. As PAR-1 activation on EC is known to promote the formation of reactive oxygen species (ROS) that contribute to thrombin signaling, we also determined the ability of Ang1 to reduce H2O2- stimulated PMN adherence. Similar to what was observed with thrombin, Ang1 inhibited the effect of H2O2 on PMN adherence. In contrast, Ang1 failed to decrease PMN adherence to EC in response to histamine, PAF or TNF-α (Figure 2).
Figure 2.
Ang1 decreases neutrophil adherence stimulated by thrombin or H2O2. Confluent EA.hy 926 cells were preincubated with 250 ng ml−1 Ang1 or solvent for 1 h before treatment with 50 nM PAF for an additional hour, 1 ng ml−1 hTNFα for 4 h 30 min, 0.005 U ml−1 thrombin for 1 h, 0.5 mM H2O2 for 1 h or 10 μM histamine for 1 h. PMN were then added for 30 min. Values are means±s.e.m., n=3–4; *P<0.05 from vehicle.
Role of Rho kinase (ROCK) in thrombin-stimulated PMN adherence
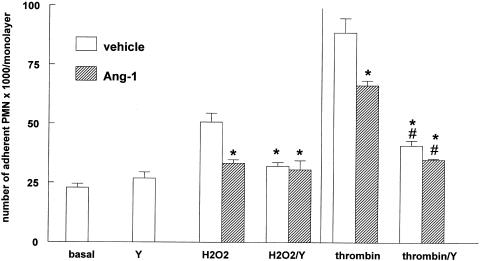
To determine whether the endothelial Rho/ROCK signaling pathway is involved in PMN adherence increased by thrombin or H2O2, experiments were conducted in the presence of Y-27632, a pharmacological inhibitor specific for ROCK. Preincubation for 1 h with 10 μM Y-27632 had no effect on baseline PMN adherence, but strongly reduced the adherence stimulated by either thrombin or H2O2 (Figure 3). This indicates that the effects of thrombin and H2O2 are mediated, at least in part, by activation of ROCK. Although Y-27632 exerted a strong inhibition of thrombin-mediated PMN adherence, it did not decrease it to baseline levels. Interestingly, in cells pretreated with Y-27632 Ang1 did not further attenuate PMN adherence. It was recently shown that engagement of β2 integrins on human PMN induces activation of RhoA in the PMN (Dib et al., 2001). Using the above-mentioned experimental conditions, it was not possible to determine whether PMN adherence decreased by the ROCK inhibitor Y-27632 resulted from an effect on the EC and/or the PMN. Therefore, experiments were repeated in such a way that only the EC were exposed to the inhibitor, that is by washing the inhibitor away after incubation with the EC and before adding the PMN. The results were similar to those obtained when both EC and PMN were incubated with Y-27632 (data not shown), demonstrating that PMN adherence prevented by Y-27632 results from an effect on the EC, not the PMN.
Figure 3.
Inhibition of Rho kinase attenuates neutrophil adherence. Confluent EA.hy 926 cells were preincubated with 250 ng ml−1 Ang1 or solvent for 1 h, with or without 10 μM Y-27632 (Y), before treatment with 0.5 mM H2O2 or 0.005 U ml–1 thrombin for 30 min. PMN were then added for another 30 min. Values are means±s.e.m., n=4; *P<0.05 from H2O2 or thrombin, #P<0.05 from basal. Y-27632 inhibited the effect of thrombin and H2O2 by 73 and 67%, respectively.
Ang1 decreases IL-8 production induced by thrombin
PMN adherence can be stimulated by thrombin's action on EC either acutely, through mobilization or changes in structure of adhesion molecules that occur within minutes (Toothill et al., 1990; Sugama et al., 1992) or in a more delayed fashion that involves transcription of cytokines and adhesion molecules that occurs after several hours (Kaplanski et al., 1997). To determine whether Ang1 can also counteract the chronic effects of thrombin, IL-8 production (a major PMN chemoattractant) was assayed in the presence or absence of Ang1. Figure 4a shows that Ang1 significantly decreased IL-8 production by EA.hy926 cells, in baseline as well as thrombin-induced conditions. All of the concentrations of thrombin tested were affected by Ang1, with a 28–45% inhibition being observed. The form of Ang1 used in our experiments up to this point was a chimeric form of Ang1 that is easier to produce and purify (Maisonpierre et al., 1997). In order to determine whether the endogenous ligand of the Tie2 receptor has the same biological effects on IL-8 release, cells were pretreated with various concentrations of native Ang1 and thrombin-induced IL-8 production was determined. Similar to what was observed with chimeric Ang1, native Ang-1 reduced IL-8 production (Figure 4b).
Role of ROCK in thrombin-stimulated IL-8 production
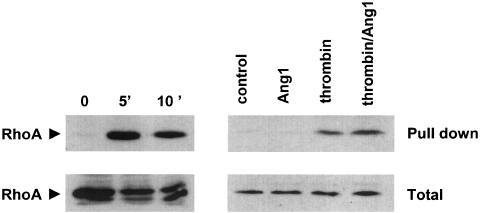
We then investigated whether the Rho/ROCK pathway plays a role in thrombin-induced IL-8 production. Figure 5 demonstrates that Y-27632 strongly decreased both basal and thrombin-induced IL-8 release by EA.hy 926 cells. Moreover, Ang1 did not exert an additive effect to that observed with Y-27632. Although Y-27632 exerted a strong inhibition on IL-8 production in cells challenged with thrombin, it did not decrease it to baseline levels, either in the presence or in the absence of Ang1. Overall, the data obtained with IL-8 are very similar to those obtained with PMN adherence (compare Figure 3 and Figure 5) and suggest that Ang1 might act by inhibiting ROCK activity. To directly test this possibility we determined the extent of RhoA activation, a small GTPase that stimulates ROCK activity, in cells treated with thrombin in the presence and absence of Ang-1. Cells treated with thrombin for 5 or 10 min displayed increased binding of the GTP-bound (active) form of RhoA to rhotekin as compared to control untreated cells. However, pretreatment of EA.hy 926 cells with Ang-1 failed to decrease the thrombin-induced activation of RhoA (Figure 6).
Figure 5.
Inhibition of Rho kinase attenuates IL-8 production. Confluent EA.hy 926 cells were preincubated with 250 ng ml−1 Ang1 or solvent for 1 h, in the presence or absence of 10 μM Y-27632 (Y), before being exposed to different concentrations of thrombin for 16 h. Values are means±s.e.m., n=3; *P<0.05 from corresponding vehicle, §P<0.05 from basal. Y-27632 inhibited the response to 0.01 and 0.05 U ml−1 thrombin by 87 and 84%, respectively.
Figure 6.
Ang1 does not affect thrombin-induced RhoA activation. Confluent EA.hy 926 cells were preincubated either with 0.01 U ml−1 thrombin for 5 or 10 min (left) or with 250 ng ml−1 Ang1 or solvent for 1 h, before stimulation with 0.01 U ml−1 thrombin for 5 min (right). Top (pull down): cell lysates (1 mg reaction−1) were incubated with GST-rhotekin and processed as described in the methods section. Membranes were incubated with an antibody for RhoA. Bottom (total): before performing the GST pull down, 50 μg of each sample was analyzed by Western blot with the RhoA antibody to demonstrate the presence of equal amounts of RhoA in all samples. Blots shown are from a representative experiment performed three times with identical results.
Discussion
In the present study, we have demonstrated that angiopoietin-1 inhibits various biological responses associated with inflammation, namely EC permeability, PMN adherence to the EC and cytokine production from activated EC. Initially, we showed that pretreatment of cells with Ang1 reduces the increase in permeability brought about by the inflammatory agents thrombin, bradykinin and histamine. Our data are in agreement with studies reporting an inhibitory effect of Ang1 in mustard oil- and VEGF-induced permeability in the mouse skin (Thurston et al., 2000), as well as with the antipermeability effect of Ang1 in HUVEC monolayers in vitro (Gamble et al., 2000); moreover, our data extend the antipermeability actions of Ang1 to a new vascular bed, namely the lung vasculature. Endothelial barrier dysfunction after exposure to inflammatory mediators results from both actinomyosin-driven cell contraction and loss of cell–cell junctional integrity. Although Ang1 was shown to reduce baseline phosphorylation of the intercellular junction proteins PECAM-1 and VE-cadherin (Gamble et al., 2000), it is still not known whether it also inhibits phosphorylation of junctional proteins in cells challenged with inflammatory agents. Collectively, our data indicate that Ang1 reduces the permeability-increasing effects of different inflammatory mediators possibly by suppressing a common pathway utilized by these edemagenic agents.
In addition to endothelial leakage of macromolecules, increased recruitment of leucocytes is observed at sites of inflammation. Thrombin promotes increased binding of leucocytes to the endothelium through both a rapid mobilization of P-selectin and change of ICAM-1 conformation at the EC surface (Toothill et al., 1990; Sugama et al., 1992), as well as a delayed, transcriptionally regulated, induction of ICAM-1 and E-selectin (Kaplanski et al., 1997; Rahman et al., 1999). Pretreatment of cells with Ang1 reduced the acute adherence of PMN in response to thrombin. In the present study, we have not determined whether Ang1 also affects the delayed response to thrombin on EC adhesion molecules. However, Kim et al. (2001) recently reported that Ang1 reduces VEGF-stimulated leukocyte adherence to EC by reducing the synthesis of ICAM-1, VCAM-1 and E-selectin mRNA, demonstrating that Ang1 can also suppress the upregulation of these molecules at the transcriptional level. However, unlike what was observed with VEGF, Ang1 reduced only basal, but not TNF-α-induced E-selectin expression (Gamble et al., 2000). In contrast to the decrease in permeability exerted by Ang1 in response to a variety of inflammatory mediators, PMN adherence to EC was prevented only when the cells were exposed to thrombin, and not in response to histamine, PAF or TNF-α. Thrombin is known to promote PAF release rapidly from EC and part of the effect of thrombin on PMN adherence is attributable to PAF (Toothill et al., 1990). Since in our experiments Ang1 did not inhibit PAF-stimulated PMN adherence Ang1 must inhibit the PAF-independent component of the response to thrombin. A previous study by Gamble et al. (2000), demonstrated that Ang1 reduces PMN transmigration in response to TNF-α without inhibiting TNF-α-induced E-selectin expression. These observations, taken together with our finding that Ang1 does not reduce PMN adherence to EC activated by TNF-α support the hypothesis that Ang1 affects TNFα-induced transjunctional diapedesis per se without influencing the prerequisite processes of tethering and firm adhesion to EC.
Activation of endothelial vascular cells by thrombin triggers the release of ROS (Holland et al., 1998). Oxidative stress causes EC dysfunction leading to increases in both endothelial permeability and adherence for PMN, as well as PMN-dependent endothelial injury (Lum & Roebuck, 2001). Given the important role played by ROS in vascular inflammation, we determined whether Ang1 affects H2O2-mediated increases in EC adherence for PMN. Our data provide evidence that Ang1 reduces a ROS-mediated inflammatory response, suggesting that Ang1 has the potential to prevent oxidative stress-induced damage.
In order to investigate the signaling pathways mediating thrombin-induced adherence of PMN that might be targets for Ang1, we exposed EC to the ROCK inhibitor Y-27632. ROCK, has been implicated in the activation of adhesion molecules by inflammatory mediators (Wojciak-Stothard et al., 1999; Takeuchi et al., 2000). Moreover, ROCK has been shown to mediate the endothelial reorganization of actin cytoskeleton that is necessary for leukocyte adherence and migration across cell junctions (Adamson et al., 1999; Wojciak-Stothard et al., 1999). Our data with the pharmacological inhibitor Y-27632 shows that the endothelial ROCK plays a predominant role in both thrombin and H2O2-mediated PMN adherence. As the inhibitory effect of Ang1 and Y-27632 on PMN adherence was not additive, we interpreted this finding as evidence that Ang1 decreases the responses to thrombin by interfering with the activation of ROCK. To directly test this hypothesis, we measured thrombin-induced activation of ROCK by monitoring RhoA activity. RhoA is a major activator of ROCK in many cells, including EC and has also been shown to mediate thrombin-induced permeability (Essler et al., 1998; Van Nieuw Amerongen et al., 2000). In agreement with observations in human umbilical vein EC (Van Nieuw Amerongen et al., 2000), treatment of EA.hy 926 with thrombin increased the conversion of RhoA to its GTP-bound active form. However, pretreatment of cells with Ang1 did not reduce RhoA activation, suggesting that Ang1 does not inhibit ROCK activation by affecting this upstream activator of ROCK. Alternatively, Ang1 may inhibit other signaling molecules required for ROCK activation or might interfere with the activation of molecules downstream of ROCK that are required for thrombin's action. The finding that Y-27632 attenuates thrombin-induced PMN adherence to a greater extent than Ang1 suggests that Ang1 inhibits only some of these pathways.
The last anti-inflammatory property of Ang1 we describe herein is its ability to reduce thrombin-increased production of the PMN chemotactic cytokine IL-8. Thus, Ang1 has the potential to exert an inhibitory effect on both acute and chronic thrombin-induced responses, by preventing rapid increases in PMN adherence via direct inhibition of EC adhesion molecule mobilization, as well as delayed PMN recruitment, via downregulation of IL-8 production. We also show that thrombin-mediated IL-8 production occurs, at least in part, through the Rho/ROCK pathway, a fact that is reported for the first time. Activation of Rho/ROCK was previously shown to modulate gene transcription in EC. It mediates thrombin-induced suppression of endothelial nitric oxide synthase expression (Eto et al., 2001), as well as TNF-α-induced activity of nuclear factor-κB (NF-κB) (Perona et al., 1997).
In conclusion, this study demonstrates that Ang1 displays important anti-inflammatory properties in vitro, such as reinforcement of endothelial barrier function, attenuation of endothelial adhesiveness for PMNs and inhibition of proinflammatory cytokine production by activated EC. Our observations on the inhibitory effects of Ang1 in activated EC are in line with the current hypothesis that Ang1 has a protective and stabilizing role in the vasculature, directing EC towards a quiescent, anti-inflammatory, as well as anticoagulant phenotype (Wong et al., 1997; Papapetropoulos et al., 1999; Yancopoulos et al., 2000; Kim et al., 2002). Ang1 may, thus, be used as a therapeutic agent in inflammatory diseases; this possibility is further supported by recent findings that Ang1 exhibits anti-inflammatory properties in the retinal vasculature of diabetic rats by reducing leucocyte adherence and EC injury (Joussen et al., 2002).
Acknowledgments
We are indebted to Regeneron Pharmaceuticals, Inc. (Tarrytown, NY, U.S.A.) for providing the recombinant Ang1, to Dr Cora-Jean Edgell for the generous gift of EA.hy 926 cells and to Dr R.C. Roovers for providing the GST-C21 construct. We also thank Ms Athanasia Hatzianastasiou for excellent technical assistance and Dr Grammatiki Hatzidimitriou for providing blood from the Blood Donation Center in Evangelismos Hospital. This study was supported by grants from the Thorax Foundation and from the Greek Secretariat of Research and Technology.
Abbreviations
- Ang1
angiopoietin-1
- BLMVEC
bovine lung microvascular endothelial cells
- EC
endothelial cells
- HRP
horseradish peroxidase
- ICAM-1
intercellular adhesion molecule-1
- IL-8
interleukin-8
- MPO
myeloperoxidase
- PMN
neutrophil
- PAF
platelet-activating factor
- ROS
reactive oxygen species
- ROCK
Rho kinase
- TNFα
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
References
- ADAMSON P., ETIENNE S., COURAUD P.O., CALDER V., GREENWOOD J. Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM-1 via a rho-dependent pathway. J. Immunol. 1999;162:2964–2973. [PubMed] [Google Scholar]
- BROWN K., VORA A., BIGGERSTAFF J., EDGELL C., OIKLE S., MAZURE G., TAUB N., MEAGER A., HILL T., WATSON, C Application of an immortalized human endothelial cell line to the leukocyte:endothelial assay. J. Immunol. Methods. 1993;163:13–22. doi: 10.1016/0022-1759(93)90234-x. [DOI] [PubMed] [Google Scholar]
- DAVIS S., ALDRICH T.H., JONES P.F., ACHESON A., COMPTON D.L., JAIN V., RYAN T.E., BRUNO J., RADZIEJEWSKI C., MAISONPIERRE P.C., YANCOPOULOS G.D. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- DIB K., MELANDER F., ANDERSSON T. Role of p190RhoGAP in beta 2 integrin regulation of RhoA in human neutrophils. J. Immunol. 2001;166:6311–6322. doi: 10.4049/jimmunol.166.10.6311. [DOI] [PubMed] [Google Scholar]
- EDGELL C.J., MCDONALD C.C., GRAHAM J.B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. U.S.A. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESSLER M., AMANO M., KRUSE H.-J., KAIBUCHI K., WEBER P.C., AEPFELBACHER M. Thrombin inactivates myosin light chain phosphatase via rho and its target rho kinase in human endothelial cells. J. Biol. Chem. 1998;273:21867–21874. doi: 10.1074/jbc.273.34.21867. [DOI] [PubMed] [Google Scholar]
- ETO M., BARANDIER C., RATHGEB L., KOZAI T., JOCH H., YANG Z., LUSCHER T.F. Thrombin suppresses endothelial nitric oxide synthase and upregulates endothelin-converting enzyme-1 expression by distinct pathways: role of Rho/ROCK and mitogen-activated protein kinase. Circ. Res. 2001;89:583–590. doi: 10.1161/hh1901.097084. [DOI] [PubMed] [Google Scholar]
- GAMBLE J., DREW J., TRESIZE L., UNDERWOOD A., PARSONS M., KASMINKAS L., RUDGE J., YANCOPOULOS G., VADAS M. Angiopoietin-1 is an anti-permeability and anti-inflammatory agent in vitro and targets cells junctions. Circ. Res. 2000;87:603–607. doi: 10.1161/01.res.87.7.603. [DOI] [PubMed] [Google Scholar]
- HAYES A.J., HUANG W.Q., MALLAH J., YANG D., LIPPMAN M.E., LI L.Y. Angiopoietin-1 and its receptor Tie-2 participate in the regulation of capillary-like tubule formation and survival of endothelial cells. Microvasc. Res. 1999;58:224–237. doi: 10.1006/mvre.1999.2179. [DOI] [PubMed] [Google Scholar]
- HOLLAND J.A., MEYER J.W., CHANG M.M., O'DONNELL R.W., JOHNSON D.K., ZIEGLER L.M. Thrombin stimulated reactive oxygen species production in cultured human endothelial cells. Endothelium. 1998;6:113–121. doi: 10.3109/10623329809072198. [DOI] [PubMed] [Google Scholar]
- JONES N., ILJIN K., DUMONT D., ALITALO K. Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat. Rev. 2001;2:1–11. doi: 10.1038/35067005. [DOI] [PubMed] [Google Scholar]
- JOUSSEN A.M., POULAKI V., TSUJIKAWA A., QIN W., QAUM T., XU Q., MOROMIZATO Y., BURSELL S.E., WIEGAND S.J., RUDGE J., IOFFE E., YANCOPOULOS G.D., ADAMIS A.P. Suppression of diabetic retinopathy with angiopoietin-1. Am. J. Pathol. 2002;160:1683–1693. doi: 10.1016/S0002-9440(10)61115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLANSKI G., FABRIGOULE M., BOULAY V., DINARELLO C.A., BONGRAND P., KAPLANSKI S., FARNARIER C. Thrombin induces endothelial type II activation in vitro: IL-1 and TNF-alpha-independent IL-8 secretion and E-selectin expression. J. Immunol. 1997;158:5435–5441. [PubMed] [Google Scholar]
- KIM I., MOON S.O., PARK S.K., CHAE S.W., KOH G.Y. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circ. Res. 2001;89:477–479. doi: 10.1161/hh1801.097034. [DOI] [PubMed] [Google Scholar]
- KIM I., OH J.L., RYU Y.S., SO J.N., SESSA W.C., WALSH K., KOH G.Y. Angiopoietin-1 negatively regulates expression and activity of tissue factor in endothelial cells. FASEB J. 2002;16:126–128. doi: 10.1096/fj.01-0556fje. [DOI] [PubMed] [Google Scholar]
- KOBLIZEK T.I., WEISS C., YANCOPOULOS G.D., DEUTSCH U., RISAU W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr. Biol. 1998;8:529–532. doi: 10.1016/s0960-9822(98)70205-2. [DOI] [PubMed] [Google Scholar]
- LUM H., ROEBUCK K.A. Oxidant stress and endothelial cell dysfunction. Am. J. Physiol. Cell Physiol. 2001;280:C719–C741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- MAISONPIERRE P.C., SURI C., JONES P.F., BARTUNKOVA S., WIEGAND S.J., RADZIEJEWSKI C., COMPTON D., MCCLAIN J., ALDRICH T.H., PAPADOPOULOS N., DALY T.J., DAVIS S., SATO T.N., YANCOPOULOS G.D. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- NATHAN C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- PAPAPETROPOULOS A., FULTON D., MAHBOUBI K., KALB R.G., O_CONNOR D.S., LI F., ALTIERI D.C., SESSA W.C. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J. Biol. Chem. 2000;275:9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- PAPAPETROPOULOS A., GARCIA_CARDENA G., DENGLER T.J., MAISONPIERRE P.C., YANCOPOULOS G.D., SESSA W.C. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab. Invest. 1999;79:213–223. [PubMed] [Google Scholar]
- PERONA R., MONTANER S., SANIGER L., SANCHEZ-PEREZ I., BRAVO R., LACAL J.C. Activation of the nuclear factor-kappaB by Rho, CDC42, and Rac-1 proteins. Genes Dev. 1997;11:, 463–475. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- PIZURKI L., MORRIS M.A., CHANSON M., SOLOMON M., PAVIRANI A., BOUCHARDY I., SUTER S. Cystic fibrosis transmembrane conductance regulator does not affect neutrophil migration across cystic fibrosis airway epithelial monolayers. Am. J. Pathol. 2000;156:1407–1416. doi: 10.1016/S0002-9440(10)65009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABIET M., PLANTIER J., RIVAL Y., GENOUX Y., LAMPUGNANI M., DEJANA E. Thrombin-induced increase in endothelial permeability is associated with changes in cell-to-cell junction organization. Arterioscler Thromb. Vasc. Biol. 1996;16:488–496. doi: 10.1161/01.atv.16.3.488. [DOI] [PubMed] [Google Scholar]
- RAHMAN A., ANWAR K.N., TRUE A.L., MALIK A.B. Thrombin-induced p65 homodimer binding to downstream NF-kappa B site of the promoter mediates endothelial ICAM-1 expression and neutrophil adhesion. J. Immunol. 1999;162:5466–5476. [PubMed] [Google Scholar]
- SANDER E., TEN KLOOSTER J., VAN DELFT S., VAN DER KAMMEN R., COLLARD J. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migration behavior. J. Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO T.N., TOZAWA Y., DEUTSCH U., WOLBURG-BUCHHOLZ K., FUJIWARA Y., GENDRON-MAGUIRE M., GRIDLEY T., WOLBURG H., RISAU W., QIN Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- SUGAMA Y., TIRUPPATHI C., OFFAKIDEVI K., ANDERSEN T.T., FENTON II J.W., MALIK A.B. Thrombin-induced expression of endothelial P-selectin and intercellular adhesion molecule-1: a mechanism for stabilizing neutrophil adhesion. J. Cell Biol. 1992;119:935–944. doi: 10.1083/jcb.119.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI S., KAWASHIMA S., RIKITAKE Y., UEYAMA T., INOUE N., HIRATA K., YOKOYAMA M. Cerivastatin suppresses lipopolysaccharide-induced ICAM-1 expression through inhibition of Rho GTPase in BAEC. Biochem. Biophys. Res. Commun. 2000;269:97–102. doi: 10.1006/bbrc.2000.2238. [DOI] [PubMed] [Google Scholar]
- THURSTON G., RUDGE J.S., IOFFE E., ZHOU H., ROSS L., CROLL S.D., GLAZER N., HOLASH J., MCDONALD D.M., YANCOPOULOS G.D. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat. Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- THURSTON G., SURI C., SMITH K., MCCLAIN J., SATO T.N., YANCOPOULOS G.D., MCDONALD D.M. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- TOOTHILL V.J., VAN MOURIK J.A., NIEWENHUIS H.K., METZELAAR M.J., PEARSON J.D. Characterization of the enhanced adhesion of neutrophil leukocytes to thrombin-stimulated endothelial cells. J. Immunol. 1990;145:283–291. [PubMed] [Google Scholar]
- VAN NIEUW AMERONGEN G.P., VAN DELFT S., VERMEER M.A., COLLARD J.G., VAN HINSBERGH V.W. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ. Res. 2000;87:335–340. doi: 10.1161/01.res.87.4.335. [DOI] [PubMed] [Google Scholar]
- WITZENBICHLER B., MAISONPIERRE P.C., JONES P., YANCOPOULOS G.D., ISNER J.M. Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie2. J. Biol. Chem. 1998;273:18514–18521. doi: 10.1074/jbc.273.29.18514. [DOI] [PubMed] [Google Scholar]
- WOJCIAK-STOTHARD B., WILLIAMS L., RIDLEY A.J. Monocyte adhesion and spreading on human endothelial cells is dependent on rho-regulated receptor clustering. J. Cell Biol. 1999;145:1293–1307. doi: 10.1083/jcb.145.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WONG A., HAROON Z., WERNER S., DEWHIRST M., GREENBERG C., PETERS K.G. Tie2 expression ad phosphorylation in angiogenic and quiescent adult tissues. Circ. Res. 1997;81:567–574. doi: 10.1161/01.res.81.4.567. [DOI] [PubMed] [Google Scholar]
- YANCOPOULOS G.D., DAVIS S., GALE N.W., RUDGE J.S., WIEGAND S.J., HOLASH J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]