Abstract
Patch clamp recordings were made from periaqueductal grey (PAG) neurons in vitro to investigate the cellular actions of opioids in wild-type C57B16/J mice and mutant mice lacking the first exon of the μ-opioid (MOP) receptor.
In wild-type mice, the κ-(KOP) agonist U-69593 (300 nM) and the mixed μ/δ-opioid agonist met-enkephalin (10 μM), but not the δ-(DOP) agonist deltorphin (300 nM), reduced the amplitude of evoked GABAA-mediated inhibitory postsynaptic currents (IPSCs). Met-enkephalin and U-69593 also reduced the rate of spontaneous miniature IPSCs, but had no effect on their amplitude and kinetics. In μ-receptor-deleted mice, only U-69593 (300 nM) reduced the amplitude of evoked IPSCs.
In wild-type mice, the MOP agonist DAMGO (3 μM) produced an outward current in 76% of the neurons. Deltorphin and U-69593 produced outward currents in 24 and 32% of the neurons, respectively. In μ-receptor-deleted mice, deltorphin and U-69593 produced similar outward currents in 32 and 27% of the neurons, respectively, while DAMGO was without effect. All neurons in both the wild-type and μ-receptor-deleted mice responded with similar outward currents to either the GABAB receptor agonist baclofen (10 μM), or the opioid-like receptor ORL1 (NOP) agonist nociceptin (300 nM).
The DAMGO-, deltorphin-, U-69593-, baclofen- and nociceptin-induced currents displayed inward rectification and reversed polarity at −109 to −116 mV.
These findings indicate that μ-, δ- and κ-opioid receptor activation has complex pre- and postsynaptic actions within the mouse PAG. This differs to the rat PAG where only μ-opioid receptor actions have been observed.
Keywords: Opioid, receptor, analgesia, potassium current, synaptic transmission, mouse, knockout
Introduction
The midbrain periaqueductal grey (PAG) plays a pivotal role in the integration of an animal's response to threat, stress and pain (Bandler et al., 1994). The PAG is an important central site of the analgesic actions of opioids, forming part of a descending antinociceptive pathway which projects via the rostral ventromedial medulla (RVM) to modulate nociceptive transmission at the level of the spinal cord (Fields et al., 1991). Functional and electrophysiological studies in the rat have led to the hypothesis that μ-opioids activate this antinociceptive pathway by reducing inhibitory GABAergic influences (disinhibition) on descending projection neurons within the PAG and RVM.
At the cellular level, μ-opioid disinhibition in the rat PAG and RVM is thought to occur by direct inhibition of GABAergic interneurons and presynaptic inhibition of transmitter release from GABAergic terminals onto projection neurons. Anatomical studies have established the presence of μ-, δ- and κ-opioid receptors in the rat PAG (Kalyuzhny et al., 1996; Mansour et al., 1996) and mouse (Depaoli et al., 1994; Jenab et al., 1995; Kitchen et al., 1997). In rat PAG, μ-opioid receptor activation inhibits a subpopulation of PAG neurons, by directly increasing an inwardly rectifying potassium conductance (Chieng et al., 1994a; Osborne et al., 1996) and by inhibiting voltage-dependent calcium channels (Kim et al., 1997; Connor et al., 1998). In addition, μ-opioids inhibit GABAergic synaptic transmission via a presynaptic mechanism (Chieng et al., 1994b; Vaughan et al., 1997). δ- and κ-opioid receptor-mediated actions have not been demonstrated at the cellular level in the rat PAG.
The introduction of targeted gene mutagenesis has facilitated the pharmacological analysis of opioid-mediated analgesia (Kieffer et al., 2002). While there are a number of anatomical and behavioural studies on opioid analgesic systems using mutant mouse strains lacking genes for specific opioid receptors, studies at the cellular level in the mouse are lacking. Recently, it has been demonstrated that μ-, but not δ- and κ-opioid inhibit voltage-dependent calcium channels in mouse PAG neurons and that μ-opioid inhibition is absent in mutant mice lacking the first exon of MOR-1 (Connor et al., 1999). In the present study we have examined the actions of μ-, δ- and κ-opioid receptor activation on GABAergic synaptic transmission and a potassium conductance in PAG neurons from wild-type C57B16/J mice and mutant mice lacking the first exon of MOR-1.
Methods
Wild-type and knockout mice homozygous for a deletion of exon 1 of MOR-1 were used, as described previously (Connor et al., 1999; Schuller et al., 1999). Briefly, the targeting vector used to produce the gene-targeted mice lacking exon 1 was constructed in two steps. First, a 6.5 kb HindIII/XhoI fragment corresponding to part of intron 1 of MOR-1 was cloned into the HindIII and XhoI restriction sites, located between the pMC1neo sequence and the HSV-TK gene of the plasmid knockout vector. Then a 2.5 kb NotI/BamHI fragment, located 5′ of exon 1 of MOR-1, was cloned into the NotI and BamHI sites 3′ of the Neo gene. Successful targeting events were detected by Southern blot, which was also used to identify embryonic stem cells in which one allele of MOR-1 had been replaced by the mutant allele during homologous recombination. Heterozygous mice were produced following germ-line transmission of microinjected embryonic stem cells, and homozygous MOR-1 knockout mice derived from heterozygote/heterozygote mating were viable and fertile. Knockout mice used in the present study were littermates derived from matings of homozygous MOR-1 knockout mice. Wild-type mice were of the parental C57B16/J strain.
Male and female mice (15–40 days old) were anaesthetised with halothane, decapitated and coronal midbrain slices containing PAG were cut (220 μM) in ice-cold artificial cerebrospinal fluid (ACSF). The slices were maintained at 34°C in a submerged chamber containing ACSF equilibrated with 95% O2 and 5% CO2. The slices were then individually transferred to a chamber and superfused continuously (2 ml min−1) with ACSF (32°C) of composition: (mM): NaCl 126, KCl 2.5, NaH2PO4 1.4, MgCl2 1.2, CaCl2 2.4, glucose 11 and NaHCO3 25. PAG neurons were visualised using infrared Nomarski optics on an upright microscope (Olympus BX50).
For experiments on synaptic currents, whole-cell voltage clamp recordings (holding potential −70 mV) were made using an Axopatch 200B (Axon Instruments, Foster City, U.S.A.). A Cs+-based internal solution was used which contained (mM): CsCl 140, EGTA 10, HEPES 5, CaCl2 2 and MgATP 2 (pH 7.3, osmolarity 270–290 mosmol l−1). For experiments on postsynaptic K+ currents, either perforated patch voltage-clamp recordings with an internal solution comprising (mM): K-acetate, 120; HEPES, 40; EGTA, 10; MgCl2, 5; Pluronic F-127, 0.25 mg ml−1; amphotericin B, 0.12 mg ml−1, or whole-cell patch-clamp recordings with an internal solution comprising (mM): K-gluconate, 95; KCl, 30; NaCl, 15; MgCl2, 2; HEPES, 10; EGTA, 11; MgATP, 2; NaGTP 0.25 were used (holding potential −62 mV). Series resistance (<15 MΩ for whole cell and <50 MΩ for perforated patch) was compensated by 80% and continuously monitored during experiments. Postsynaptic opioid responses of neurons obtained using perforated and whole-cell patch-clamp recordings were similar, and these results were pooled. Liquid junction potentials of –12 mV for K-acetate and K-gluconate-based internal solutions and –4 mV for CsCl-based internal solutions were corrected.
Postsynaptic K+ current recordings were filtered (500 Hz low-pass filter) and sampled (1 kHz) for off-line analysis (Axograph 4, Axon Instruments). Inhibitory postsynaptic currents (IPSCs) were filtered (1 kHz low-pass filter) and sampled (5 kHz) for on-line and later off-line analysis (Axograph 4). Electrically evoked IPSCs were elicited in neurons via bipolar tungsten, or theta-glass stimulating electrodes placed 50–200 μm from the recording electrode (rate 0.05–0.1 Hz, stimuli: 2–70 V, 20–400 μs) in the presence of 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX; 3–5 μM). Spontaneous miniature IPSCs were obtained in neurons in the presence of tetrodotoxin (TTX) (0.3 μM) and CNQX (3–5 μM). Miniature IPSCs above a preset threshold (4–5 standard deviations above baseline noise) were automatically detected by a sliding template algorithm (Axograph 4), then manually checked offline. Miniature IPSCs were counted in 5 s epochs every 6 s to construct plots of event rate versus time.
Stock solutions of all drugs were diluted to working concentrations using ACSF immediately before use and applied by superfusion. D-Phe-Cys-Tyr-D-Trp-Arg-Pen-Thr-NH2 (CTAP), Tyr-D-Ala-Gly-N-Me-Phe-Gly-ol enkephalin (DAMGO), deltorphin II, methionine-enkephalin (met-enkephalin) and nociceptin/orphaninFQ (Phe-Gly-Gly-Phe-Thr-Gly-Ala-Arg-Lys-Ser-Ala-Arg-Lys-Leu-Ala-Asn-Gln) were obtained from Auspep (Parkville, Victoria, Australia); baclofen, bicuculline methiodide, DPDPE ([d-Pen2,5]-enkephalin), picrotoxin, strychnine hydrochloride and U-69593 were obtained from Sigma (Sydney, Australia); CGP-55845, CNQX, ICI-174,864 and nor-binaltorphimine dihydrochloride (nor-BNI) were from Tocris Cookson (Bristol, U.K.); TTX from Alomone (Jerusalem, Israel); H-Tyr-Ticψ [CH2NH]-Phe-Phe-OH (TIPP[ψ]) was provided by Professor P. Schiller (Clinical Research Institute of Montreal, Montreal, Canada). All pooled data are expressed as means±s.e.m. and all statistical comparisons were made using Student's t-test (unpaired two-tailed test with unequal variance), or χ2 tests for differences among proportions. Results from male and female mice were similar and were pooled for analysis.
Results
μ- and κ-Opioid receptor activation presynaptically inhibits GABAergic synaptic transmission in wild-type mice
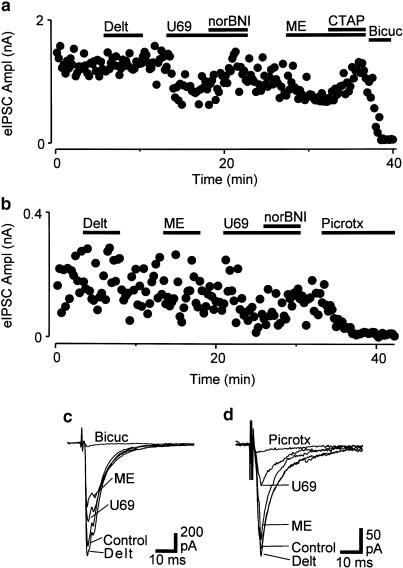
When PAG neurons from wild-type mice were voltage clamped to a membrane potential of −70 mV, in the presence of non-NMDA glutamate (CNQX, 3–5 μM) and glycine (strychnine, 3 μM) receptor antagonists, local electrical stimulation evoked IPSCs (amplitude 1390±310 pA, n=19) which were abolished by the GABAA receptor antagonist bicuculline (30 μM) or picrotoxin (100 μM) (Figure 1a).
Figure 1.
μ- and κ-opioids inhibit evoked GABAergic synaptic currents in mouse PAG. Effect of opioids on electrically evoked IPSCs in PAG neurons from (a,c) wild-type mice and (b,d) mice with a μ-receptor deletion. Traces in (a,b) depict the time course of the amplitude of evoked IPSCs in PAG neurons during application of U-69593 (U69, 300 nM), nor-BNI (300 nM), deltorphin II (Delt, 300 nM), met-enkephalin (ME, 10 μM), CTAP (1 μM), then either bicuculline (Bicuc, 30 μM) or picrotoxin (Pic, 100 μM). (c,d) show averaged raw traces of evoked IPSCs from neurons depicted in (a) and (b), respectively. (a–d) are taken from two neurons held at –70 mV in the presence of CNQX (3 μM) and strychnine (3 μM).
Superfusion of the μ/δ-opioid receptor agonist met-enkephalin (10 μM) produced a reversible reduction in the amplitude of evoked IPSCs in all neurons tested (mean inhibition=55±5%, n=11, Figure 1a). The met-enkephalin-induced inhibition was abolished by addition of the μ-opioid receptor antagonist CTAP in all neurons tested (1 μM, n=5). Superfusion of the κ-opioid receptor agonist U-69593 (300 nM) reduced the amplitude of evoked IPSCs in all neurons tested (mean inhibition=49±6%, n=11, Figure 1a). The U-69593-mediated inhibition was abolished by addition of the κ-opioid receptor antagonist nor-BNI (300 nM, n=7). In contrast, superfusion of the δ-opioid receptor agonist deltorphin II (300 nM) had no effect on the amplitude of evoked IPSCs (mean inhibition=1±3%, n=9, Figure 1a).
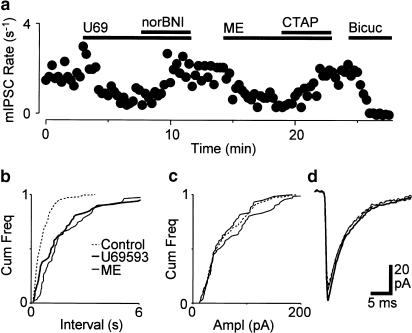
Spontaneous miniature IPSCs were readily observed in the presence of CNQX (3–5 μM), strychnine (3 μM) and TTX (0.3 μM). This concentration of TTX abolished evoked postsynaptic currents (n=4). In wild-type mice, superfusion of met-enkephalin (10 μM) reduced the rate of miniature IPSCs, but had no effect on their amplitude distributions or kinetics (Figure 2, n=14). On average, the mean miniature IPSC rate was reduced by 58±3% and the mean amplitude was reduced by −1±6% during superfusion of met-enkephalin. In addition, superfusion of U-69593 (300 nM) reduced the rate of miniature IPSCs, but had no effect on their amplitude distributions or kinetics in all neurons tested (Figure 2, n=6). On average, the mean miniature IPSC rate was reduced by 35±6% during superfusion of U-69593, whereas the mean amplitude was reduced by 2±8%.
Figure 2.
μ- and κ-opioids presynaptically inhibit GABAergic synaptic transmission in mouse PAG. (a) Time course of miniature IPSC rate during superfusion of U-69593 (U69, 300 nM), nor-BNI (300 nM), met-enkephalin (ME, 10 μM), CTAP (1 μM) and bicuculline (Bicuc, 30 μM). Cumulative distribution plots of the (b) inter-event interval and (c) amplitude of miniature IPSCs before, then during U-69593 and met-enkephalin (number of events=213, 71, 61 for 120 s epochs, respectively). (d) Averaged raw traces of miniature IPSCs as for (b,c). (a–d), are taken from one neuron held at –70 mV in the presence of CNQX (3 μM), strychnine (3 μM) and TTX (300 nM).
μ-, δ- and κ-Opioid receptor activation produces an outward current in subpopulations of PAG neurons from wild-type mice
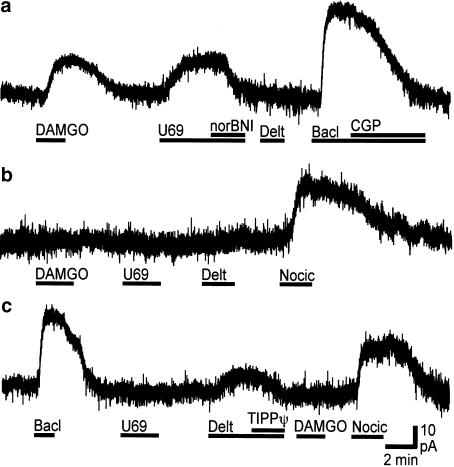
When PAG neurons from wild-type mice were voltage clamped to a membrane potential of –60 mV superfusion of the μ-opioid receptor agonist DAMGO (3 μM) produced an outward current in the majority of neurons tested (Figure 3). The DAMGO induced current was abolished by addition of CTAP (1 μM, n=5). Deltorphin II (300 nM) or DPDPE (300 nM) produced an outward current in a subpopulation of neurons (Figure 3), which was abolished by addition of the δ-opioid receptor antagonists ICI-174864 (300 nM, n=5), or TIPP[ψ] (100 nM, n=3). U-69593 (300 nM–1 μM) produced an outward current in a subpopulation of neurons (Figure 3) which was abolished by addition of nor-BNI (300 nM, n=3). Met-enkephalin (10 μM) produced outward currents in the majority of neurons tested (Figure 4a).
Figure 3.
μ-, δ- and κ-Opioids produce an outward current in subpopulations of mouse PAG neurons. Current traces of (a) μ- and κ-opioid responsive, (b) opioid nonresponsive and (c) δ-opioid responsive PAG neurons from wild-type mice during application of DAMGO (3 μM), U-69593 (U69, 300 nM), nor-BNI (300 nM), deltorphin (Delt, 300 nM), TIPP[ψ] (100 nM), nociceptin (Nocic, 300 nM), baclofen (Bacl, 10 μM) and CGP-55845 (CGP, 1 μM). (a–c) represent whole-cell patch-clamp recordings from three different neurons held at –60 mV.
Figure 4.
The proportion of μ-opioid, but not δ-, κ-opioid, ORL1 and GABAB responding neurons differs between wild-type and μ-receptor deleted mice. (a) Bar chart of the percentage of neurons from wild-type (μ (+/+), open bar) and μ-receptor deleted mice (μ (−/−), filled bar) in which DAMGO (3 μM), deltorphin (Delt, 300 nM), U-69593 (U69, 300 nM), met-enkephalin (ME, 10 μM), nociceptin (Nocic, 300 nM) and baclofen (Bacl, 10 μM) produced an outward current. (b) Bar chart of the magnitude of the current (mean±s.e.m.) produced in responsive neurons from wild-type and μ-receptor-deleted mice. Layout of (b) is same as for (a).
The selective agonists DAMGO, deltorphin II (300 nM) and U-69593 produced outward currents in 78% (n=21/27), 24% (n=9/38) and 32% (n=12/38) of neurons, respectively (Figure 4a). The response to at least two of these agonists was examined simultaneously in some neurons (Figure 3). In these neurons, a similar proportion of DAMGO responsive (20%, n=3/15) and DAMGO nonresponsive neurons (17%, n=1/6) responded to deltorphin (P>0.05, χ2=0.03). In addition, a similar proportion of DAMGO-responsive (35%, n=6/17) and DAMGO-nonresponsive neurons (17%, n=1/6) responded to U-69593 (P>0.05, χ2=0.73).
The GABAB receptor agonist baclofen (10 μM, n=44) and/or the ORL1 receptor agonist nociceptin (300 nM, n=19) produced an outward current in all neurons tested, whether or not they previously responded to μ-, δ- or κ-opioid agonists (Figure 3 and Figure 4). The baclofen-induced current was abolished by addition of the GABAB receptor antagonist CGP-55845 (1 μM, n=7).
μ-, δ- and κ-Opioid receptor activation opens an inwardly rectifying potassium current in PAG neurons from wild-type mice
In wild-type mice, the met-enkephalin-, DAMGO-, deltorphin-,U-69593, baclofen- and nociceptin-induced currents reversed polarity at membrane potentials of −116±3 mV (n=37), −109±3 mV (n=7), −113±6 mV (n=5), −111±9 mV (n=5), −111±3 mV (n=18) and −113±3 mV (n=6), respectively. The met-enkephalin-induced current showed inward rectification as the slope conductance was greater when measured between −110 and −130 mV (0.43±0.06 nS) than between −60 and −90 mV (0.30±0.04 nS). When measured over the same potentials, the DAMGO-induced current had slope conductances of 0.37±0.04 and 0.11±0.02 nS, the deltorphin induced current had slope conductances of 0.54±0.04 and 0.12±0.03 nS, the U-69593 induced current had slope conductances of 0.35±0.02 and 0.13±0.03 nS, the baclofen-induced current had slope conductances of 0.57±0.03 and 0.41±0.03 nS, and, the nociceptin induced current had slope conductances of 1.19±0.04 and 0.48±0.03 nS.
μ-Receptor-deleted mice
In PAG neurons from mice with a μ-receptor deletion, local electrical stimulation in the presence of CNQX (3–5 μM) and strychnine (3 μM) evoked IPSCs (amplitude 1130±330 pA, n=15), which were abolished by bicuculline (30 μM) or picrotoxin (100 μM) (Figure 1b). In these mice, met-enkephalin (10 μM) and deltorphin II (300 nM) had no effect on the amplitude of evoked IPSCs (Figure 1b). The mean inhibition produced by met-enkephalin and deltorphin was 3±6% (n=7) and 2±6% (n=6), respectively. In contrast, U-69593 (300 nM) reduced the amplitude of evoked IPSCs by an average of 45±9% (Figure 1b, n=8). The inhibition produced by U-69593 was reversed by nor-BNI (300 nM, n=5). The magnitude of the U-69593-induced inhibition was similar in wild-type and μ-receptor-deleted mice (P>0.05, t-test).
In PAG neurons from μ-receptor-deleted mice, DAMGO (3 μM) produced no change in membrane current (n=6). In contrast, deltorphin-II (300 nM), U-69593 (300 nM) and met-enkephalin (10 μM) produced outward currents in 32% (n=6/19), 27% (n=6/22) and 39% (n=9/23) of the neurons, respectively (Figure 4a). Baclofen (10 μM, n=43) and nociceptin (300 nM, n=18) produced an outward current in all the neurons tested (Figure 4a). The proportion of deltorphin (χ2=0.49, P>0.05) and U-69593 (χ2=0.06, P>0.05), but not met-enkephalin (χ2=8.73, P<0.005) responding neurons was similar in wild-type and μ-receptor-deleted mice. In responding neurons, the magnitude of the currents produced by met-enkephalin, deltorphin, U-69593, nociceptin and baclofen were similar in wild-type and μ-receptor-deleted mice (Figure 4b, P>0.05, t-test).
Discussion
In the present study, it was demonstrated that opioids produce a complex spectrum of cellular actions mediated by μ-, δ- and κ-opioid receptors in the mouse PAG. These observations differ from prior studies in the rat, where only μ-opioid receptor-mediated actions have been observed at the cellular level.
Activation of μ- and κ-opioid receptors inhibited GABAergic synaptic transmission in mouse PAG by a presynaptic mechanism. The selective μ- and κ-opioid agonists DAMGO and U-69593 reduced the amplitude of evoked IPSCs, while the δ-opioid agonist deltorphin was without effect. Indicative of a presynaptic site of action, DAMGO and U-69593 reduced the rate of spontaneous miniature IPSCs without any effect on their amplitude distributions or kinetics. The present study is the first to demonstrate κ-opioid inhibition of GABAergic synaptic transmission in the nervous system. Previous studies in the rat PAG have detected only μ-opioid inhibition of GABAergic and glutamatergic synaptic transmission (Chieng et al., 1994b; Vaughan et al., 1997). κ-Opioid presynaptic inhibition of glutamatergic, but not GABAergic synaptic transmission has been reported in other brain regions (Pinnock, 1992; Wagner et al., 1992; Ackley et al., 2001; Hjelmstad et al., 2001).
Activation of μ-, δ- and κ-opioid receptors directly inhibited subpopulations of mouse PAG neurons by increasing an inwardly rectifying K-conductance. The DAMGO-, deltorphin- and U-69593 induced currents reversed at a potential similar to that predicted by the Nernst equation for a K+-conductance and were associated with an increase in conductance which was greater at more negative potentials. These observations differ to those of previous studies which have reported only μ-opioid postsynaptic modulation of K-conductance in the rat PAG (Chieng et al., 1994a), and of voltage-dependent Ca-conductances in the mouse and rat PAG (Connor et al., 1998, 1999). However, δ- and/or κ-opioid-mediated increases in K-conductance have been observed in the spinal cord and other brain regions (Grudt et al., 1993; Tanaka et al., 1994; Pan et al., 1997; Svoboda et al., 1999).
In the present study, DAMGO had pre- and postsynaptic actions in wild type, but not in μ-receptor deleted mice. In addition, the proportion of δ-, κ-opioid and nociceptin-responding neurons and the magnitude of their responses was similar in wild-type and μ-receptor-deleted mice. These observations provide a functional correlate of previous autoradiographic studies which have demonstrated that μ-opioid receptors are absent, but the levels of δ-, κ-opioid and ORL1 receptors are not altered in mice with a μ-receptor deletion (Matthes et al., 1996; Kitchen et al., 1997; Sora et al., 1997; Loh et al., 1998; Schuller et al., 1999; Chen et al., 2000; Slowe et al., 2001). Met-enkephalin had postsynaptic actions which persisted in μ-receptor deleted mice that were likely to be mediated by δ-receptors, although a role for other purported μ-opioid receptor splice variants cannot be excluded (Schuller et al., 1999).
What are the consequences of the observed cellular actions of opioids in the mouse PAG? The disinhibition hypothesis states that μ-opioids produce analgesia in the rat by reducing the inhibitory influence of GABAergic interneurons on PAG projection neurons which project via the RVM to the spinal cord (Fields et al., 1991). μ-opioid disinhibition is likely to be mediated presynaptic inhibition of transmitter release from GABAergic terminals and by postsynaptic inhibition of presumptive interneurons in rat PAG (Chieng et al., 1994a,1994b; Osborne et al., 1996; Vaughan et al., 1997). The present observations in the mouse are partly similar to prior studies in the rat where μ-opioid receptor mediated pre- and postsynaptic disinhibitory actions have been observed. Firstly, it was found that μ-opioids presynaptically inhibited GABAergic synaptic transmission in the mouse PAG. Secondly, it was found that μ-opioids postsynaptically inhibited the majority of neurons in the mouse PAG. While the majority of identified projection neurons in the rat ventrolateral PAG do not respond to μ-opioids, most unidentified neurons are μ-opioid responsive (Chieng et al., 1994a; Osborne et al., 1996; Vaughan et al., 2000). It might be hypothesised that the μ-opioid responsive and nonresponsive neurons are likely to be interneurons and projection neurons, respectively, although the opioid responses of PAG projection neurons in the mouse remain to be examined. These findings suggest that μ-opioids are likely to produce similar cellular effects on descending analgesic pathways from the PAG in both species.
Unlike prior studies in the rat, δ- and κ-opioid, receptor-mediated effects were observed in the mouse PAG. Firstly, it was found that κ-opioids presynaptically inhibited GABAergic synaptic transmission in the mouse PAG. Secondly, both μ-opioid responsive and nonresponsive neurons were inhibited by δ- and κ-opioids in the mouse PAG. These observations suggest that, like μ-opioids, κ-opioids can produce analgesia via presynaptic disinhibitory mechanisms in the mouse PAG. However, δ- and κ-opioids have postsynaptic actions which might either produce analgesia, or oppose μ-opioid-induced analgesia. Overall, the cellular actions of δ- and κ-opioids only partly overlap those of μ-opioids and are likely to be more complex than a generalised disinhibition of descending analgesic pathways.
Acknowledgments
Supported by the National Health & Medical Research Council of Australia (Grant #153844), NIH (Grants #RO1DA12926–01, #DA-09040) and The Medical Foundation of The University of Sydney. We thank Dr P. Schiller for his kind gift of TIPP[ψ].
Abbreviations
- ACSF
artificial cerebrospinal fluid
- IPSC
inhibitory postsynaptic current
- PAG
periaqueductal grey
- RVM
rostral ventromedial medulla
References
- ACKLEY M.A., HURLEY R.W., VIRNICH D.E., HAMMOND D.L. A cellular mechanism for the antinociceptive effect of a kappa opioid receptor agonist. Pain. 2001;91:377–388. doi: 10.1016/S0304-3959(00)00464-4. [DOI] [PubMed] [Google Scholar]
- BANDLER R., SHIPLEY M.T. Columnar organization in the midbrain periaqueductal gray – modules for emotional expression. Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- CHEN H.C., SEYBOLD V.S., LOH H.H. An autoradiographic study in μ-opioid receptor knockout mice. Mol. Brain Res. 2000;76:170–172. doi: 10.1016/s0169-328x(99)00346-0. [DOI] [PubMed] [Google Scholar]
- CHIENG B., CHRISTIE M.J. Hyperpolarization by opioids acting on μ-receptors of a sub-population of rat periaqueductal gray neurones in vitro. Br. J. Pharmacol. 1994a;113:121–128. doi: 10.1111/j.1476-5381.1994.tb16183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIENG B., CHRISTIE M.J. Inhibition by opioids acting on μ-receptors of GABAergic and glutamatergic postsynaptic potentials in single rat periaqueductal gray neurones in vitro. Br. J. Pharmacol. 1994b;113:303–309. doi: 10.1111/j.1476-5381.1994.tb16209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNOR M., CHRISTIE M.J. Modulation of calcium channel currents of acutely dissociated rat periaqueductal grey neurons. J. Physiol. 1998;509:47–58. doi: 10.1111/j.1469-7793.1998.047bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNOR M., SCHULLER A., PINTAR J.E., CHRISTIE M.J. μ-opioid receptor modulation of calcium channel current in periaqueductal grey neurons from C57B16/J mice and mutant mice lacking MOR-1. Br. J. Pharmacol. 1999;126:1553–1558. doi: 10.1038/sj.bjp.0702457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEPAOLI A.M., HURLEY K.M., YASADA K., REISINE T., BELL G. Distribution of κ-opioid receptor messenger RNA in adult mouse brain – an in situ hybridization histochemistry study. Mol. Cell. Neurosci. 1994;5:327–335. doi: 10.1006/mcne.1994.1039. [DOI] [PubMed] [Google Scholar]
- FIELDS H.L., HEINRICHER M.M., MASON P. Neurotransmitters in nociceptive modulatory circuits. Annu. Rev. Neurosci. 1991;14:219–245. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- GRUDT T.J., WILLIAMS J.T. κ-Opioid receptors also increase potassium conductance. Proc. Natl. Acad. Sci. USA. 1993;90:11429–11432. doi: 10.1073/pnas.90.23.11429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HJELMSTAD G.O., FIELDS H.L. Kappa opioid receptor inhibition of glutamatergic transmission in the nucleus accumbens shell. J. Neurophysiol. 2001;85:1153–1158. doi: 10.1152/jn.2001.85.3.1153. [DOI] [PubMed] [Google Scholar]
- JENAB S., KEST B., FRANKLIN S.O., INTURRISI C.E. Quantitative distribution of the delta opioid receptor mRNA in the mouse and rat CNS. Life Sci. 1995;56:2343–2355. doi: 10.1016/0024-3205(95)00228-x. [DOI] [PubMed] [Google Scholar]
- KALYUZHNY A.E., ARVIDSSON U., WU W., WESSENDORF M.W. Mu-opioid and delta-opioid receptors are expressed in brainstem antinociceptive circuits–studies using immuno-cytochemistry and retrograde tract-tracing. J. Neurosci. 1996;16:6490–6503. doi: 10.1523/JNEUROSCI.16-20-06490.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIEFFER B.L., GAVERIAUX-RUFF C. Exploring the opioid system by gene knockout. Prog. Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- KIM C.J., RHEE J.S., AKAIKE N. Modulation of high-voltage activated Ca2+ channels in the rat periaqueductal gray neurons by mu-type opioid agonist. J. Neurophysiol. 1997;77:1418–1424. doi: 10.1152/jn.1997.77.3.1418. [DOI] [PubMed] [Google Scholar]
- KITCHEN I., SLOWE S.J., MATTHES H.W.D., KIEFFER B. Quantitative autoradiographic mapping of mu-delta- and kappa-opioid receptors in knockout mice lacking the mu-opioid receptor gene. Brain Res. 1997;778:73–88. doi: 10.1016/s0006-8993(97)00988-8. [DOI] [PubMed] [Google Scholar]
- LOH H.H., LIU H.C., CAVALLI A., YANG W.L., CHEN Y.F., WEI L.N. Mu opioid receptor knockout in mice – effects on ligand-induced analgesia and morphine lethality. Mol. Brain Res. 1998;54:321–326. doi: 10.1016/s0169-328x(97)00353-7. [DOI] [PubMed] [Google Scholar]
- MANSOUR A., BURKE S., PAVLIC R.J., AKIL H., WATSON S.J. Immunohistochemical localization of the cloned kappa(1)receptor in the rat cns and pituitary. Neuroscience. 1996;71:671–690. doi: 10.1016/0306-4522(95)00464-5. [DOI] [PubMed] [Google Scholar]
- MATTHES H.W.D., MALDONADO R., SIMONIN F., VALVERDE O., SLOWE S., KITCHEN I., BEFORT K., DIERICH A., LEMEUR M., DOLLE P., TZAVARA E., HANOUNE J., ROQUES B.P., KIEFFER B.L. Loss of morphine-induced analgesiareward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- OSBORNE P.B., VAUGHAN C.W., WILSON H.I., CHRISTIE M.J. Opioid inhibition of rat periaqueductal grey neurones with identified projections to rostral ventromedial medulla in vitro. J. Physiol. 1996;490:383–389. doi: 10.1113/jphysiol.1996.sp021152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAN Z.Z., TERSHNER S.A., FIELDS H.L. Cellular mechanism for anti-analgesic action of agonists of the kappa-opioid receptor. Nature. 1997;389:382–385. doi: 10.1038/38730. [DOI] [PubMed] [Google Scholar]
- PINNOCK R.D. Activation of kappa-opioid receptors depresses electrically evoked excitatory postsynaptic potentials on 5-HT-sensitive neurones in the rat dorsal raphe nucleus in vitro. Brain Res. 1992;583:237–246. doi: 10.1016/s0006-8993(10)80029-0. [DOI] [PubMed] [Google Scholar]
- SCHULLER A.G.P., KING M.A., ZHANG J.W., BOLAN E., PAN Y.X., MORGAN D.J., CHANG A., CZICK M.E., UNTERWALD E.M., PASTERNAK G.W., PINTAR J.E. Retention of heroin and morphine-6 beta-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat. Neurosci. 1999;2:151–156. doi: 10.1038/5706. [DOI] [PubMed] [Google Scholar]
- SLOWE S.J., CLARKE S., LENA I., GOODY R.J., LATTANZI R., NEGRI L., SIMONIN F., MATTHES H.W.D., FILLIOL D., KIEFFER B.L., KITCHEN I. Autoradiographic mapping of the opioid receptor-like 1 (ORL1) receptor in the brains of mu-delta- or kappa-opioid receptor knockout mice. Neuroscience. 2001;106:469–480. doi: 10.1016/s0306-4522(01)00308-6. [DOI] [PubMed] [Google Scholar]
- SORA I., FUNADA M., UHL G.R. The μ-opioid receptor is necessary for [DPEN2D-PEN5]enkephalin-induced analgesia. Eur. J. Pharmacol. 1997;324:R1–R2. doi: 10.1016/s0014-2999(97)10016-4. [DOI] [PubMed] [Google Scholar]
- SVOBODA K.R., ADAMS C.E., LUPICA C.R. Opioid receptor subtype expression defines morphologically distinct classes of hippocampal interneurons. J. Neurosci. 1999;19:85–95. doi: 10.1523/JNEUROSCI.19-01-00085.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANAKA E., NORTH R.A. Opioid actions on rat anterior cingulate cortex neurons in vitro. J. Neurosci. 1994;14:1106–1113. doi: 10.1523/JNEUROSCI.14-03-01106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAUGHAN C.W., CHRISTIE M.J. Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. J. Physiol. 1997;498:463–472. doi: 10.1113/jphysiol.1997.sp021872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAUGHAN C.W., CONNOR M., BAGLEY E.E., CHRISTIE M.J. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol. Pharmacol. 2000;57:288–295. [PubMed] [Google Scholar]
- WAGNER J.J., CAUDLE R.M., CHAVKIN C. Kappa-opioids decrease excitatory transmission in the dentate gyrus of the guinea pig hippocampus. J. Neurosci. 1992;12:132–141. doi: 10.1523/JNEUROSCI.12-01-00132.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]