Abstract
We have investigated the effects of loperamide on intracellular Ca2+ stores and membrane K+ channels in insulin-secreting hamster insulinoma (HIT-T15) cells.
In cell-attached patch-clamp mode, loperamide (3–250 μM) activated large single-channel currents. The loperamide-activated currents were tentatively identified as Ca2+-activated K+ channel (KCa) currents based on their single-channel conductance (145 pS), apparent reversal potential, and insensitivity to tolbutamide. Smaller single-channel currents with a conductance (32 pS) indicative of adenosine triphosphate-sensitive K+ channels (KATP channels) were also recorded, but were insensitive to loperamide.
Surprisingly, the loperamide-activated currents persisted in the absence of extracellular Ca2+. Yet under these conditions, we still measured loperamide-induced Ca2+ increases. These effects are dose dependent. Loperamide had no effects in the inside-out patch configuration, suggesting that loperamide does not directly activate the channels with large conductance, but does so secondarily to release of Ca2+ from intracellular stores.
Carbachol (100 μM), an agonist of muscarinic receptors, which mediates IP3-dependent intracellular Ca2+ release, enhanced the effects of loperamide on KCa channels.
Both the putative KCa currents and Ca2+ signals induced by loperamide (with ‘0' [Ca2+]o) were abolished when the intracellular Ca2+ stores had been emptied by pretreating the cells with either carbachol or thapsigargin, an endoplasmic reticulum Ca2+-ATPase inhibitor that blocks reuptake of calcium.
These data indicate that loperamide in insulin-secreting β-cells evokes intracellular Ca2+ release from IP3-gated stores and activates membrane currents that appear to be carried by KCa, rather than KATP channels.
Keywords: Ca2+-activated K+ channel, stimulus–secretion coupling, endoplasmic reticulum, ion channels, patch clamp, charybdotoxin, IP3-gated stores, thimerosal
Introduction
In this paper, we examine the effect of loperamide on single-channel K+ currents and intracellular Ca2+ signals in insulin-secreting hamster insulinoma (HIT-T15) cells.
Loperamide is a widely used antidiarrheal opioid receptor agonist, but at higher concentrations it is thought also to modulate the activity of ionic channels either directly or as a result of altered Ca2+ signaling. Thus, colonic mucosal secretion may be blocked by loperamide by a mechanism involving Cl− channels, Ca2+-activated K+ (KCa) channels, and the calmodulin system (Diener et al., 1988). In hippocampal neurons, loperamide at micromolar concentrations blocks a broad spectrum of high-voltage-activated Ca2+ channels, and, less effectively, NMDA-activated currents (Church et al., 1994). Furthermore, in a wide variety of cell types, loperamide has been found to increase Ca2+ influx, but only after targeting IP3-sensitive intracellular Ca2+ stores with the likely consequence of mobilizing store-operated Ca2+ (SOC) channels (Daly et al., 1995; Harper et al., 1997; Daly & Harper, 2000). To clarify the effects of loperamide on ionic channels and Ca2+ signaling, we studied single-channel currents and intracellular Ca2+ transients in individual clonal pancreatic β cells (HIT-T15 cells).
Pancreatic β cells respond to intermediate glucose concentrations (∼8 mM) with a reduction in K+ permeability leading to membrane depolarization followed by volleys of Ca2+-dependent action potentials that elevate the cytoplasmic Ca2+ concentration ([Ca2+]i) and trigger the release of insulin (Atwater et al., 1978; Wollheim & Sharp, 1981; Henquin & Meissner, 1984; Prentki & Matschinsky, 1987). At least three types of K+ channels play a role in β cells. First, an ATP-sensitive channel is blocked by intracellular ATP (Cook & Hales, 1984) or by glucose metabolism (Ashcroft et al., 1984; Misler et al., 1986) and has been linked to the depolarization of the membrane from −70 mV (in the absence of glucose) to −60 mV (in the presence of glucose), which is the threshold for activation of Ca2+-current-mediated action potentials. Second, a delayed rectifier K+ channel is rapidly activated during each action potential spike (Rorsman & Trube, 1986; Zunkler et al., 1988). Third, different K+ channels (KCa) with high conductance, activated by [Ca2+]i and membrane depolarization, are conspicuously present in pancreatic β cells from rodents and insulin-secreting cell lines (Atwater et al., 1979; Marty & Neher, 1982; Atwater et al., 1983; Cook et al., 1984; Eddlestone et al., 1989; Tabcharani & Misler, 1989; Ämmälä et al., 1991; Bordin et al., 1995; Göpel et al., 1999). However, the specific roles of each of the subtypes of β cell KCa channels have not been determined.
The role of intracellular Ca2+ stores in stimulus-secretion coupling is also uncertain. In addition to directly affecting exocytosis, sequestration and release of Ca2+ could affect membrane potential via Ca2+-activated channels or store-operated channels. Such mechanisms likely underlie the enhancement of glucose-induced electrical activity and insulin secretion by muscarinic agonists (Bertram et al., 1995; Bordin et al., 1995), which stimulate IP3-dependent mobilization of stored Ca2+. Glucose causes an initial uptake of Ca2+ into the endoplasmic reticulum (Roe et al., 1994; Chow et al., 1995), possibly followed by voltage- or Ca2+-dependent release of the ion (Roe et al., 1993; Worley et al., 1994). These observations suggest that intracellular Ca2+ stores and the ionic currents coupled to them may also be involved in glucose-induced electrical signaling in the pancreatic β cell.
The primary purpose of this study was to examine the effects of loperamide on HIT cells, which are known to possess ionic channels and Ca2+ stores of the types implicated in the mode of action of the drug. Contrary to what might be expected from the literature, we found that loperamide appeared to activate the large conductance KCa channel, yet did not require extracellular Ca2+. With this insight, we proceeded to demonstrate that loperamide mobilized Ca2+ from intracellular stores, and that it therefore may serve as a means to explore the regulatory role of intra- and extracellular Ca2+ in the control of insulin secretion in pancreatic β cells.
Methods
Cell preparation
Experiments were performed on pancreatic β cells from a cell line derived from HIT-T15 cells. The stock was purchased from American Type Culture Collection (Manassas, VA, U.S.A.) and maintained in F-12 K medium supplemented with 10% dialyzed horse serum, 2.5% fetal bovine serum, 100 U ml−1 penicillin, and 0.05 mg ml−1 streptomycin in a 5% CO2 atmosphere at 37°C. The medium was changed every 3 days and the cells were subcultured once a week. The passage range of the HIT cells used was 59–70.
Cells were plated on 35-mm dishes and maintained in culture for 2–3 days. Before experiments, the culture medium was replaced with extracellular Krebs-Ringer (KR) solution containing (mM): 140 NaCl, 4 KCl, 2.6 CaCl2, 1 MgCl2, 2.8 or 5.6 glucose, and 10 HEPES at pH 7.4. In many of the experiments, we used a ‘Ca2+-free' extracellular KR solution, which contained no added Ca2+, but included 5 mM of the Ca2+-chelator EGTA to bind trace contaminants. The glucose concentration was 2.8 or 5.6 mM in different experiments, which rendered most cells quiescent.
Loperamide, carbachol, tobutamide, thapsigargin, and thimerosal were from Sigma Chemical Co. (St Louis, MO, U.S.A.). Charybdotoxin was from RBI, which is now owned by Sigma. F-12 Nutrient Mixture medium, fetal bovine serum, dialyzed horse serum, trypsin-EDTA (0.05% trypsin and 0.53 mM EDTA), and penicillin-streptomycin (10,000 U ml−1 penicillin G sodium and 10,000 μg/ml streptomycin sulfate) were from GIBCO/BRL, (Rockville, MD, U.S.A.) and Biofluids Inc. (Rockville, MD, U.S.A.).
Recording of single-channel and whole-cell membrane currents
Patch-clamp experiments were conducted at room temperature (20–25°C). For single-channel recording, we used mainly the cell-attached configuration. In a few experiments, we used excised patches in the inside-out configuration (Figure 5). Electrodes were made from microhematocrit capillary glass (Blue-Tip) using a BB-CH-PC microcomputer-controlled multistep puller (Mecanex, Geneva, Switzerland). Electrodes were coated to near the tip with Sylgard (Dow Corning, Midland, MI, U.S.A.). For cell-attached measurements, pipettes were filled with a solution of the following composition (mM): 140 KCl, 1 MgCl2, 1 CaCl2, and 5 Na-HEPES and had tip resistance from 8 to 10 MΩ. Recordings started after a high resistance seal (>5 GΩ) was formed between the pipette tip and the cell membrane. Single-channel currents were recorded using an EPC-7 patch-clamp amplifier (List Electronics, Darmstadt-Eberstadt, Germany), and stored continuously in digital form on VCR tapes using a pulse code modulator (Sony PCM 501-ES). Single-channel current records were later analyzed using commercially available software packages (pCLAMP, IPROC and LPROC, Axon Instrument, Foster City, CA, U.S.A.).
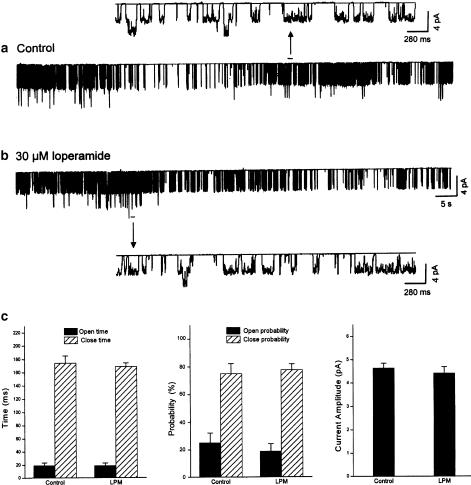
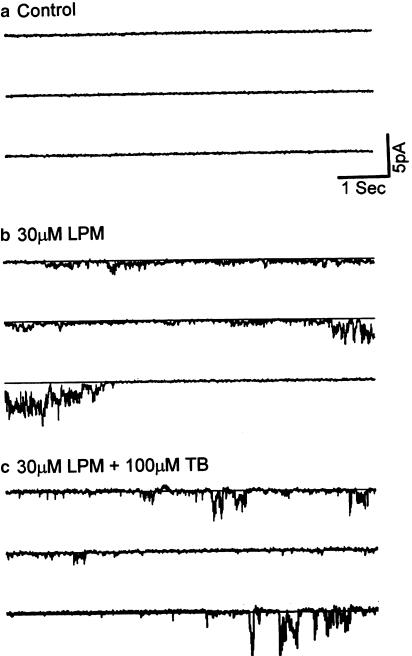
Figure 5.
Loperamide has no direct effect on the large single-channel currents measured in excised patches from HIT cells. Recording from an inside-out patch configuration before (panel a) and after (panel b) addition of 30 μM loperamide to the solution superfusing the cytoplasmic side of the cell membrane. Insets show details on an expanded time scale. The graphs in panel c illustrate the mean open time (left), open probability (middle), or amplitude (right) of the single-channel current before (control) and after (LPM) addition of loperamide. Vertical bars show s.e.m., n=3. (Holding potential=0 mV; all solutions were Ca2+ free and contained 5 mM EGTA; records are representative of five similar experiments conducted in different cells.)
Ca2+-imaging
Two-dimensional confocal Ca2+ imaging was used to estimate the intracellular Ca2+ concentration in multiple nondialyzed cells loaded with 5 μM Fluo-3 AM for 20–60 min at 37°C. The experiments were conducted following previously published methods (Cleemann et al., 1998). In Ca2+-imaging experiments, rapid switching (<50 ms) between different external solutions was accomplished using an electronically controlled multibarrelled perfusion system (Cleemann & Morad, 1991).
Static images of Ca2+-dependent fluorescence (Figures 3, 8) show a change in the average fluorescence intensity (ΔF) calculated from several frames recorded before and during exposure to loperamide. Changes in the intracellular Ca2+ concentration were estimated pseudoratiometrically (F/F0) by measuring the time course of fluorescence (F) relative to the initial fluorescence prior to interventions (F0) (Wang et al., 2000). Considering the affinity of Fluo-3 (Kd≅350 nM) and the approximate resting [Ca2+]i (50–100 nM), it is estimated that measured fluorescence values (F/F0<5) do not produce saturation. Bleaching was assessed by measuring for extended periods under control conditions and was minimized by using signal averaging in combination with a low intensity of the scanning excitation beam.
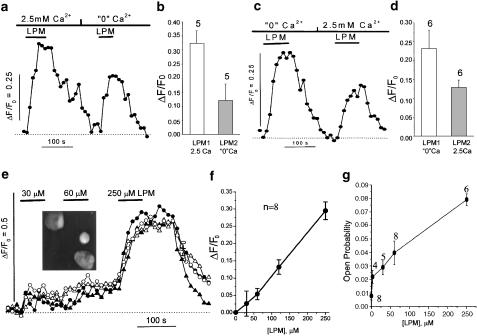
Figure 3.
The loperamide-induced increase in intracellular Ca2+ is independent of extracellular Ca2+. Panels (a, c, and e) show the change in the intracellular Ca2+ concentration measured with Fluo-3 in representative cells. The increase in the intensity of Ca2+-induced fluorescence (ΔF) was normalized relative to the fluorescence (F0) measured at the beginning of the recordings. The additions of loperamide and the change of extracellular Ca2+ concentration are indicated by bars over the traces. The upper panels show comparisons of rise in intracellular Ca2+ evoked by 250 μM loperamide when the extracellular Ca2+ concentration was first 2.5 and then 0 mM (panels a, b; n=5), or vice versa (panels c, d, n=6). Panel f shows the relation between the increase in intracellular Ca2+ and the concentration of loperamide based on experiments with successive increases in the loperamide concentration (panel e). The solvent for loperamide (up to 0.8% ethanol) produced no detectable change in the Ca2+ signal. The inset in Panel (e) shows a confocal image of Fluo-3 loaded HIT cells, often clustered 2 or 3 together. Brightly fluorescent Ca2+-overloaded cells, and nonresponsive cells were excluded from numerical analysis. Panel g shows the dose-response curve for the open probability of the single-channel current. The threshold of detection was set at ∼0.5 pA, so that smaller single-channel currents were also included in the analysis giving rise to an open probability of 0.008±0.003 in the absence of the drug.
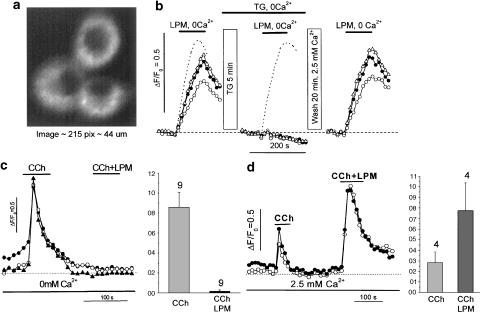
Figure 8.
Thapsigargin (panels a, b) and carbachol (panels c, d) abolished the loperamide-induced rise in intracellular Ca2+. Panel a: confocal image of three HIT cell clusters producing the records in panel b. Panel b shows changes in intracellular Ca2+ signals produced by loperamide (250 μM) under control conditions (left), following 5 min exposure to thapsigargin (2 μM) in Ca2+-free solution (middle), and after 20 min washout and reequilibration with standard KR solution (2.5 mM Ca2+; right). The traces in a dashed line are from a control experiment with the same timing and exposure to Ca2+-free solution, but without thapsigargin. The lower panes show Ca2+-transients evoked first by carbachol alone (CCh, 100 μM) and then together with 250 μM loperamide (CCh+LPM) in experiments with Ca2+-free solution (panel c) and standard KR solution with 2.5 mM Ca2+ (panel d). The bar graphs show average signals.
Results
Loperamide activates a high-conductance channel and enhances intracelluar Ca2+ even in the absence of extracellular Ca2+
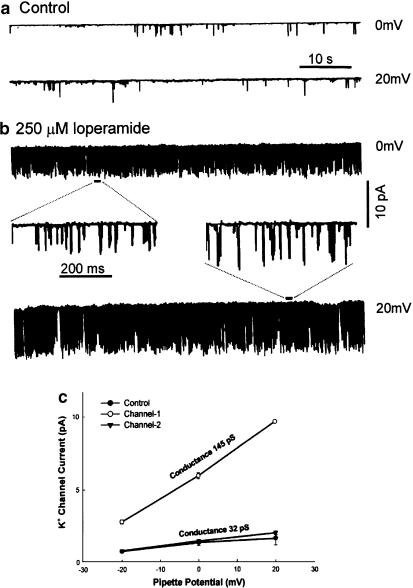
Figure 1 shows traces from a cell-attached patch recording from a HIT cell. Loperamide (250 μM) greatly increased the frequency of single-channel openings at both 0 and +20 mV. The superfusing KR solution contained 2.5 mM Ca2+ throughout the experiment. Loperamide decreased the mean closed time from 819 to 29 ms so that an expanded time scale had to be used (insets) to resolve individual openings after the addition of the drug. The measured unitary currents were of small amplitude (<2 pA) under control conditions (panel a) while numerous single-channel currents of larger amplitude (2–10 pA) were dominant (panel b) after switching to the solution with loperamide. Analysis of the records obtained at different holding potentials (−20 to +20 mV) suggests that loperamide induced openings of a high-conductance channel of 145 pS (Figure 1c, channel 1) without affecting the unitary properties of the low-conductance channel of 32 pS (Figure 1c, channel 2) seen before the addition of the drug (Figure 1c, control). The large single-channel currents were rarely observed under control conditions. They appeared with increasing frequency but constant amplitude when the superfusion control solution was exchanged with one containing loperamide over a period of ∼1 min. The patch pipette contained a KCl-based solution, so that it is plausible that the measured inward currents were carried mainly by K+. Notice that we aimed to preserve physiological Ca2+ fluxes and therefore did not take any measures, such as KCl depolarization, to stabilize the potential of the cell membrane outside the patch. It is possible therefore that the unclamped membrane potential of the cell, and thereby the potential difference across the patch, may have changed somewhat after activation of single-channel activity by loperamide. The recordings shown in Figure 1 suggest that loperamide activates a large conductance channel in HIT cells.
Figure 1.
Loperamide activates channels with high conductance in HIT cells. The tracings show single-channel currents recorded at different holding potentials (0, +20 mV) from a cell-attached patch of a HIT cell under control conditions (panel a) and in the presence of 250 μM loperamide (panel b). Loperamide caused a large increase in the open probability of the channels so that recording with an expanded time-scale was required to resolve single-channel openings (insets of panel b). Panel c illustrates the single-channel slope conductances of two channels (145 and 32 pS) determined from recordings at −20, 0, and 20 mV. The superfusing solutions used in this experiment all contained 2.5 mM Ca2+ and 2.8 mM glucose.
The following experiments were carried out to test the hypothesis that the high-conductance channel activated by loperamide is a KCa channel, while the low-conductance channel is an ATP-dependent K+ channel that does not respond to loperamide. The activation of a Ca2+-activated K+ current might depend on the influx of Ca2+ through Ca2+ channels in the cell membrane or on the release of Ca2+ from intracellular Ca2+ stores. To distinguish these possibilities we carried out experiments in which loperamide was applied in a Ca2+-free solution to which 5 mM EGTA had been added to bind trace contaminants (Figure 2).
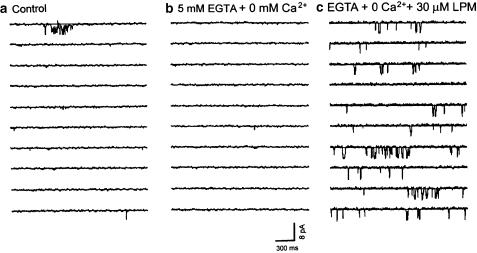
Figure 2.
Activation of large single-channel currents by loperamide in the absence of extracellular Ca2+. Single-channel currents recorded in the cell-attached configuration from a HIT cell superfused with KR solution containing 2.5 mM Ca2+ (panel a), 3 min after changing to a Ca2+-free solution to which 5 mM EGTA had been added (panel b), and 5 min after changing to a Ca2+-free solution which, in addition to 5 mM EGTA, also contained 30 μM loperamide (panel c). The glucose concentration was 5.6 mM throughout the experiment.
In the normal KR solution with 2.5 mM CaCl2, only a few openings of the high-conductance channel were observed (panel a). Removing extracellular Ca2+ and adding 5 mM EGTA to the superfusion solution (Ca2+-free solution) abolished all channel activity (Figure 2b). Yet under these conditions, exposure to 30 μM loperamide still produced the characteristic large single-channel currents (Figure 2c), indicating that extracellular Ca2+ is not required to activate the loperamide-sensitive current.
Using 2-D confocal Ca2+ imaging of multiple cultured HIT cells, we explored whether loperamide might mobilize Ca2+ from an intracellular store or whether its effect was independent of Ca2+ signaling. Figure 3 illustrates changes in the intracellular Ca2+ activity measured pseudoratiometrically with fluo-3 (Cleemann et al., 1998). In the presence of 2.5 mM Ca2+, rapid exposure to loperamide (250 μM) caused a substantial rise in intracellular Ca2+. The Ca2+ signal reached a steady level in ∼30 s and returned to the basal level 2–5 min after removal of loperamide. When loperamide was applied again after first switching to a Ca2+-free solution and adding the drug 1 min later, it still produced an increase in intracellular Ca2+, although the signal was generally of somewhat reduced amplitude (Figure 3a and b). To examine the reason for this decline we reversed the sequence so that loperamide was applied first in the absence and then in the presence of 2.5 mM Ca2+ (Figure 3c and d). The results were nearly identical suggesting that the decreasing response observed in most cells during repeated exposure to loperamide was not related to absence or presence of extracellular Ca2+. The more significant finding is therefore that loperamide, independent of extracellular Ca2+, may activate a Ca2+-dependent K+ current by releasing Ca2+ from intracellular stores.
Dose dependence of the loperamide effects
The dose dependence of loperamide was tested by quantifying both single-channel and intracellular Ca2+ measurements. Successive applications of increasing concentrations of loperamide (30, 60, and 250 μM) evoked Ca2+ transients of increased amplitude that settled at a steady level within 30–60 s after each solution change (Figure 3e). The effect of loperamide on intracellular Ca2+ was significant at 30 μM, and increased nearly linearly with increasing concentration up to 250 μM (Figure 3f). In single-channel recordings, the different concentrations of loperamide (3, 30, 60, and 250 μM in the Ca2+-free external solution) caused a similar increase in the open probability (Figure 3g). These results indicate that the effects of loperamide on both Ca2+ transients and open probability increased nearly linearly up to 250 μM.
Tolbutamide does not suppress the current activated by loperamide
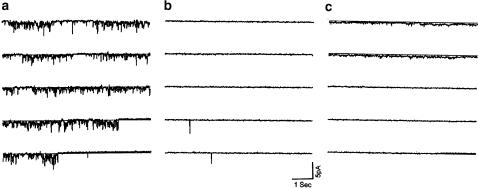
The experiment illustrated in Figure 4 shows that the current activated by loperamide is not blocked by tolbutamide, a cell-permeant specific blocker of KATP channels. No single-channel activity was observed in normal KR solution with 2.5 mM CaCl2 and 0 mV holding potential (Figure 4a). Switching to 30 μM loperamide in Ca2+-free KR solution elicited prominent single-channel openings. In the presence of loperamide, tolbutamide (100 μM in Ca2+-free KR solution) appeared not to reduce the channel activity (Figure 4c). This observation was confirmed by quantitative off-line analysis in which the detection threshold was set low (0.25 pA) to include both the large- and small-conductance channels. With loperamide alone (cf. Figure 4b), 1185 events in n=3 records yielded a mean open time of 14.4±4.5 ms (s.d.), open probability 10±10% (s.d.), and mean amplitude 1.1±0.1 pA (s.d.). Analysis of matched records obtained after addition of tolbutamide (cf. Figure 4c) produced 830 events with mean open time 14.0±1.2 ms (s.d.), open probability 7±5% (s.d.), and mean amplitude 1.6±0.1 pA (s.d.). The similarity of single-channel data in the absence and presence of tolbutamide suggests that the KATP channel does not contribute significantly to the loperamide-activated current.
Figure 4.
The single-channel currents activated by loperamide are insensitive to tolbutamide. Single-channel currents were measured in the cell-attached configuration under control conditions with 2.5 mM Ca2+ (panel a) after 3 min exposure to 30 μM loperamide (LPM) in ‘0' Ca2+ solution (panel b), and after 100 μM of the specific KATP channel blocker tolbutamide had been added to the loperamide-containing, but Ca2+-free solution (panel c). The records are representative of three similar experiments conducted in different cells. The glucose concentration was 5.6 mM.
Interestingly, we found that when charybdotoxin (ChTX), a specific antagonist of the ‘maxi' KCa channel, was added to the bath solution, loperamide subsequently did not activate noticeable single-channel currents (data not shown). However, the implications of this finding for the identification of the loperamide-activated channel are not clear, since even a large concentration of the highly basic peptide ChTX (100 nM) may not gain access to the cell-attached membrane patch unless perfused through the pipette.
Experiments of the type illustrated in Figure 4 were performed with the drugs (loperamide, tolbutamide, and charybdotoxin) added not only to ‘Ca2+-free' KR solution, but also to KR solution with the normal 2.5 mM Ca2+. Although the experiments with normal Ca2+ concentration were not chosen for illustration, they gave rise to loperamide-induced currents similar to those seen in Ca2+-free solution. Thus, it is neither the Ca2+-free solution (Figure 2b), nor loperamide in combination with ‘Ca2+-free' solution that activates the large-conductance current.
Loperamide has no effects in an inside-out patch configuration
To investigate whether loperamide directly affects the large conductance channel, we measured the single-channel currents from cultured HIT cells in the inside-out patch configuration. The internal membrane of the cell was exposed to the KR solution containing 0 mM CaCl2 and 5 mM EGTA. The holding potential was 0 mV. Under these conditions, numerous channel openings appeared (Figure 5a). Mean open time was 18.5±3 ms (n=3), close time 176.1±10 ms (n=3), open probability 25±6% (n=3), close probability 75±8% (n=3), and current amplitude 4.6±0.1 pA (n=3) (Figure 5c). Applying loperamide (30 μM) to the bath solution (internal side of cell membrane) did not increase the channel activity (Figure 5b). Mean open time was 18.2±3 ms (n=3), close time 170±6 ms (n=2), open probability 20±4% (n=3), close probability 78±4% (n=3), and current amplitude 4.4±0.2 pA (n=3) (Figure 5c). Compared to control, loperamide had no significant effect on channel activity when applied to excised patches (Figure 5c).
Carbachol enhanced the loperamide-stimulated KCa channel activity
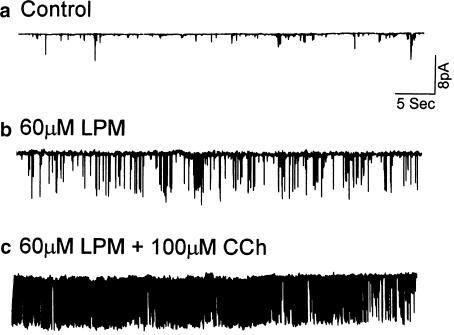
Carbachol is a muscarinic receptor agonist, which causes release of Ca2+ from IP3-sensitive intracellular Ca2+ stores. It stimulates KCa channel activity in human submandibular gland (HSG) cells (Liu et al., 1998). The calcium-mobilizing effects of loperamide motivated comparison with muscarinic agonists. Figure 6 shows the activity of the large conductance channel in a cell-attached recording from a single cultured HIT cell. In normal KR solution containing 2.5 mM Ca2+, the channel was almost always closed (Figure 6a). Application of loperamide (60 μM) activated the large single-channel currents (Figure 6b) and the addition of carbachol (100 μM) to the loperamide-containing, but Ca2+-free, solution increased the activity without changing the amplitude of the single-channel current (Figure 6c). This enhancement of the loperamide effect by carbachol is consistent with the idea that the intracellular Ca2+ stores are the source of the Ca2+ that activates the high-conductance channel.
Figure 6.
Carbachol increased the open probability of the loperamide-stimulated channel in HIT cells. Recordings under control conditions with 2.5 mM Ca2+ (panel a), when the cell was exposed to 60 μM loperamide in Ca2+-free solution (panel b) and when 100 μM carbachol was also added to the loperamide-containing but Ca2+-free solution (panel c). (Recording in the cell-attached configuration; holding potential=0 mV; glucose concentration was 2.8 mM; records are representative of three similar experiments conducted in different cells.)
The loperamide effects were abolished by thapsigargin and carbachol
The effect of loperamide on single-channel currents and [Ca2+]i was also examined in experiments in which thapsigargin was used to empty intracellular Ca2+ stores. Thapsigargin inhibits the Ca2+-ATPase of the endoplasmic reticulum and thereby the reuptake required to maintain the Ca2+ stores over a period of minutes. Figure 7a shows the activity of the large-conductance channel recorded immediately after incubation with thapsigargin (1 μM, 10 min) in Ca2+-free bath solution. Notice that numerous channel openings were observed when thapsigargin was first added suggesting an increase in [Ca2+]i as thapsigargin blocked the Ca-ATPase (Figure 7a) that is required to maintain the stores by counteracting their small continuous leak of Ca2+. The channel activity disappeared over a period of several minutes and after 20 min the patch was essentially quiet (Figure 7b). Under these conditions, the application of 30 μM loperamide in Ca2+-free extracellular solution failed to stimulate large single-channel currents (Figure 7c). These results indicate that loperamide is ineffective after thapsigargin-sensitive intracellular Ca2+ stores have been emptied.
Figure 7.
Loperamide shows no stimulatory effects at the single-channel level after pretreatment with thapsigargin. panel a: Single-channel currents recorded immediately after incubation with 1.0 μM thapsigargin in Ca2+-free solution. Panel b: Recording after continuous exposure to thapsigargin for 20 min. Panel c: after 3 min exposure to 30 μM loperamide in Ca2+-free solution. (Recording in the cell-attached configuration; holding potential 0 mV; glucose concentration was 5.6 mM; this record is representative of three similar experiments conducted in different cells.)
Figure 8 shows intracellular Ca2+ measurements from cultured HIT-T15 cells loaded with 5 μM Fluo-3AM (Figure 8a). Exposure to 250 μM loperamide in Ca2+-free perfusion solution caused a marked increase in [Ca2+]i (Figure 8b, left traces; ΔF/F0=0.5). After 5 min application of thapsigargin (2 μM in Ca2+-free solution) to empty intracellular stores, loperamide failed to produce an increase in intracellular Ca2+ (Figure 8b, middle traces). After washout of thapsigargin with KR solution containing 2.5 mM Ca2+ for 20 min, loperamide recovered the ability to raise intracellular Ca2+ (Figure 8b, right traces).
To ascertain if 5 min incubation with zero Ca2+ would prevent the agonist from subsequently eliciting a response, whether or not thapsigargin was present, the same experiment was carried out with the cells incubated in Ca2+-free KR solution for 5 min without thapsigargin. The second application of loperamide now gave nearly the same rise in [Ca2+]i (Figure 8b middle, dot line trace) as control (Figure 8b left, dotted line trace), indicating that 5 min in Ca2+-free solution does not deplete the stores in the absence of thapsigargin.
To further compare the effects of loperamide and carbachol, we also tested their combined effects on [Ca2+]i (Figure 8c and d). In the Ca2+-free extracellular solution, carbachol (100 μM) caused a rapid increase of [Ca2+]i. The following application of carbachol plus loperamide (250 μM) caused no detectable elevation of [Ca2+]i (Figure 8c), indicating that 100 μM carbachol had effectively emptied the loperamide-sensitive Ca2+ store. In the presence of 2.5 mM extracellular Ca2+, this emptying apparently did not take place since the addition of loperamide to the second application of carbachol, now produced an elevation of [Ca2+]i (Figure 8d).
The results in Figures 7 and 8 indicate that both the activation of large-conductance channels and the rise in [Ca2+]i normally seen with loperamide are blocked when intracellular Ca2+ stores have been depleted by prior application of thapsigargin or carbachol.
Since carbachol is known to target IP3-sensitive stores, we tested the effects of thimerosal to sensitize the IP3 receptors to ambient levels of IP3 (Mihai et al., 1999). Rapid application of 100 μM thimerosal caused a significant rise in [Ca2+]i that continued to climb as long as the drug was present (ΔF/F0=0.2–0.4 after 1 min) and was cumulative during repeated exposures, but did not decline noticeably during washout periods (2–5 min; data not shown). This sustained effect of thimerosal was clearly different from the rapidly reversible effects of loperamide, but it was difficult to evaluate the possible synergistic effects of the two drugs. Nevertheless, the experiments with thimerosal support the presence of an IP3-releasable pool of intracellular Ca2+.
Discussion and conclusions
Loperamide is an agonist of opioid receptors (Awouters et al., 1983; Ooms et al., 1984; Burleigh, 1988) and is usually used as an antidiarrheal agent. In addition loperamide has nonopioid effects such as inhibition of calmodulin (Zavecz et al., 1982; Diener et al., 1988) and calcium channels (Reynolds et al., 1984; Burleigh, 1988; Church et al., 1994; Daly et al., 1995). Loperamide has also been shown to enhance Ca2+ influx in various types of cells when SOC channels had been previous activated (Daly et al., 1995; Harper et al., 1997; Daly & Harper, 2000). Here we report for the first time the effect of loperamide on KCa channel activity in insulin-secreting cells and the mechanism underlying the effect.
Does loperamide stimulate charybdotoxin-sensitive KCa channel activity?
In our study loperamide stimulated openings of a large-conductance (145 pS) K+ channel and had no effects on a small conductance (32 pS) K+ channel (Figure 1). The conductance of the latter channel is consistent with that of the KATP channel, which is formed by complexes of an inward rectifying K+ channel (Kir6.2) and a sulfonylurea receptor (SUR1) (Ashcroft et al., 1984; Cook & Hales, 1984; Rorsman & Trube, 1985; Ashcroft et al., 1988; Inagaki et al., 1995). KATP channels play an important role in glucose and sulfonylurea-induced depolarization of the β cell, and may also be involved in driving membrane potential oscillations (bursts) at intermediate glucose concentrations (7–10 mM; Ding et al., 1996; Detimary et al., 1998; He et al., 1998). However, our results with the KATP channel agonist tolbutamide, which had no effect on the K+ channel activated by loperamide (Figure 4), show that loperamide acts on a different K+ channel. The conductance of the K+ channel activated by loperamide (145 pS) is similar to that of the charybdotoxin-sensitive ‘maxi' KCa channel which has been identified in both rodent pancreatic β cells and insulin-secreting cell lines (Cook et al., 1984; Li et al., 1999). Surprisingly, loperamide-induced activation of the 145 pS K+ channel did not require the presence of Ca2+ in the superfusing KR solution (Figure 2). (In such experiments we did not also remove Ca2+ from the pipette solution, but we estimate that the minute amounts of Ca2+ that might have entered through the cell-attached patch would be rapidly distributed on the timescale of the loperamide response and therefore insignificant compared to the Ca2+ that might enter though the rest of the cell membrane, when it was present in the bath.) Our further studies showed that loperamide-induced activation of the channel is most likely Ca2+ dependent, with activation of the channel occurring subsequent to release of stored Ca2+ (Figure 3). Thus, loperamide had no direct effect on K+ channel activity when applied to the intracellular side of inside-out membrane patches (Figure 5), but did produce increases in intracellular Ca2+, even when applied in a Ca2+-free medium (Figure 3). The dose dependences of single-channel open probability and intracellular Ca2+ rise were nearly linear up to 250 μM loperamide (Figure 3e, f, and g). The effects were barely detectable at concentrations below 3 μM, indicating that they are not mediated by opioid receptors, which have IC50 values in the submicromolar range (Giagnoni et al., 1983). At 250 μM the effects of loperamide showed no sign of saturation and developed more slowly than the effects of carbchol (100 μM; Figure 8c and d), suggesting that the Ca2+ release mechanism had not been fully activated.
While this evidence is consistent with activation of the large conductance or ‘maxi' Ca2+-activated K+ channel, KCa, we cannot entirely exclude the contribution of other ionic channels, for example, a Ca2+-activated Cl− channel or nonselective cation channel, to the current activated by loperamide. A more definitive identification of the KCa channel might involve ascertaining block by charybdotoxin, verifying that the reversal potential is governed by the K+ concentrations, and performing simultaneous measurements of loperamide-induced current and [Ca2+]i.
Activation of the KCa current by loperamide vs the rise in intracellular Ca2+
The release of Ca2+ by loperamide is closely correlated with the activation of the KCa current as indicated by the dose–response curves (Figure 3f and g), and the finding that the timing and amplitude of this current consistently reflects [Ca2+]i: (a) The responses of KCa current and [Ca2+]i to carbachol are stronger (and faster) than those to loperamide (Figure 6 vs Figure 8c and d), (b) thapsigargin gives rise to relatively modest transient responses of both KCa current (Figure 7a and b) and [Ca2+]i (not shown), and (c) loperamide does not activate KCa when the releasable Ca2+ stores have been depleted by thapsigargin (Figure 7c vs 8b) or charbachol (Figure 7c vs 8c). Furthermore, 30 μM loperamide, which generally produced a significant response (Figure 3g), did not activate KCa current when applied (in Ca2+-free solution) to inside-out patches. Of course, such experiments are always subject to the criticism that some essential component (e.g. ATP/ADP) may be missing from the cytosolic aspect of the membrane after excising the patch. Yet, on balance the evidence indicates that loperamide does not directly modulate the KCa channel, but activates it by raising [Ca2+]i as observed also with excised patches (Findlay et al., 1985).
Loperamide releases Ca2+ from a thapsigargin-sensitive store
In order to characterize the nature of the loperamide-sensitive intracellular Ca2+ store, we performed experiments in the presence of thapsigargin. Thapsigargin causes depletion of Ca2+ from the endoplasmic reticulum by inhibiting the sarco-endoplasmic reticulum Ca2+ ATPase, blocking uptake of the ion into the store (Thastrup et al., 1989). Following application of thapsigargin, loperamide (in Ca2+-free solution) was no longer able to activate KCa channels (Figure 7) or increase intracellular Ca2+ (Figure 8). In this context, we notice that the patch-clamp experiments performed without Ca2+ in the bathing solution were not compromised by the Ca2+ remaining in the patch pipette, since in that case the depletion of intracellular Ca2+ stores by thapsigargin would not have abolished the activation of current by loperamide. We conclude that loperamide releases Ca2+ from thapsigargin-sensitive intracellular stores in insulin-secreting cells. Interestingly, depletion of thapsigargin-sensitive Ca2+ stores has been associated with activation of store-operated currents in both β cells and insulin-secreting cell lines (Bode & Goke, 1994; Worley et al., 1994; Bertram et al., 1995; Liu & Gylfe, 1997; Miura et al., 1997; Roe et al., 1998), and loperamide has been reported to be a positive modulator of such channels (Harper et al., 1997). However, the K+ channel activated by loperamide in this study is unlikely to be a store-operated channel per se, since application of thapsigargin caused only a transient opening of the channel (Figure 7a and b), most likely resulting from the transient increase in intracellular Ca2+ produced by the SERCA inhibitor (Figure 8). Furthermore, loperamide did not enhance the activity of the channels when applied after thapsigargin, as has been reported for SOC channels (Harper et al., 1997).
IP3-gated Ca2+ stores are targeted by both loperamide and muscarinic agonists
Direct measurements of ER Ca2+ dynamics in rodent β cells have shown that IP3 acts on a subset of the thapsigargin-sensitive Ca2+ store (Tengholm et al., 1999;2000; Maechler et al., 1999). We therefore targeted IP3-gated Ca2+ stores using carbachol and thimerosal.
Carbachol enhanced the stimulatory effect of loperamide on KCa channels (Figure 6). Without extracellular Ca2+, carbachol rapidly and completely emptied IP3-sensitive stores and abolished the loperamide effect (Figure 8c). On the other hand, with normal extracellular Ca2+ (2.5 mM) the Ca2+ stores remained intact, and loperamide enhanced the carbachol-induced Ca2+ release (Figure 8d). These results suggest that loperamide in HIT cells (i) may mobilize Ca2+ stores similar to those that respond to muscarinic receptor agonists, (acetyl choline and carbachol), in mouse (Nenquin et al., 1984) and rat (Mathias et al., 1985; Morgan et al., 1985) pancreatic islets, (ii) are insensitive to mitochondrial poisons (Gylfe & Hellman, 1986), and (iii) produce Ca2+ efflux that correlates with the rise in the islet IP3 concentration (Morgan et al., 1985). Using thimerosal to sensitize IP3 receptors to basal IP3 levels (Mihai et al., 1999), we observed a rise in Ca2+ that confirmed the presence of IP3-gated stores, but did not explore whether thimerosal enhanced the effects of loperamide.
Our results indicate that loperamide caused release of Ca2+ from intracellular stores that can be emptied by thapsigargin and carbachol and therefore may be located in the endoplasmic reticulum, maintained by SERCA, and gated by IP3.
In summary, we have found that loperamide releases Ca2+ from a thapsigargin-sensitive intracellular store. The augmentation of the intracellular Ca2+ produced by this action of loperamide is sufficient to activate an ion channel with biophysical properties similar or identical to those of the ‘maxi' KCa channel. Loperamide may therefore serve as a useful tool for further studies of the coupling between intracellular Ca2+ stores and membrane potential in physiological regulation of insulin secretion.
Acknowledgments
We thank Dr A. Sherman, for his helpful comments. Some of the experimentation was performed at the Laboratory of Cell Biochemistry and Biology, NIDDK, National Institutes of Health, Bethesda, MD 20892, U.S.A. It was supported in part by a grant from the American Diabetes Association (to L. Cleemann).
Abbreviations
- [Ca2+]i
intracellular Ca2+ concentration
- KATP channel
adenosine triphosphate-sensitive K+ channel
- KCa channel
Ca2+-activated K+ channel
- SOC channel
store-operated Ca2+ channel
References
- ÄMMÄLÄ C., LARSSON O., BERGGREN P.-O., BOKVIST K., JUNTTI-BERGGREN L., KINDMARK H., RORSMAN P. Inositol trisphosphate-dependent periodic activation of a Ca2+-activated K+ conductance in glucose-stimulated pancreatic β-cells. Nature. 1991;353:849–852. doi: 10.1038/353849a0. [DOI] [PubMed] [Google Scholar]
- ASHCROFT F.M., ASHCROFT S.J.H., HARRISON D.E. Properties of single potassium channels modulated by glucose in rat pancreatic β-cells. J. Physiol. 1988;400:501–527. doi: 10.1113/jphysiol.1988.sp017134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHCROFT F.M., HARRISON D.E., ASHCROFT S.J.H. Glucose induces closure of single potassium channels in isolated rat pancreatic β-cells. Nature. 1984;312:446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- ATWATER I., DAWSON C.M., RIBALET B., ROJAS E. Potassium permeability activated by intracellular calcium ion concentration in the pancreatic beta-cell. J. Physiol. 1979;288:575–588. [PMC free article] [PubMed] [Google Scholar]
- ATWATER I., RIBALET B., ROJAS E. Cyclic changes in potential and resistance of the beta-cell membrane induced by glucose in islets of Langerhans from mouse. J. Physiol. 1978;278:117–139. doi: 10.1113/jphysiol.1978.sp012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATWATER I., ROSARIO L.M., ROJAS E. Properties of the Ca-activated K+ channel in pancreatic beta-cells. Cell Calcium. 1983;4:451–461. doi: 10.1016/0143-4160(83)90021-0. [DOI] [PubMed] [Google Scholar]
- AWOUTERS F., NIEMEGEERS J.E., JANSSEN P.A.J. Pharmacology of antidiarrheal drugs. Annu. Rev. Pharmacol. Toxicol. 1983;23:279–301. doi: 10.1146/annurev.pa.23.040183.001431. [DOI] [PubMed] [Google Scholar]
- BERTRAM R., SMOLEN P., SHERMAN A., MEARS D., ATWATER I., MARTIN F., SORIA B. A role for calcium release-activated current (CRAC) in cholinergic modulation of electrical activity in pancreatic beta-cells. Biophys. J. 1995;68:2323–2332. doi: 10.1016/S0006-3495(95)80414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BODE H.P., GOKE B. Protein kinase C activates capacitative calcium entry in the insulin secreting cell line RINm5F. FEBS Lett. 1994;339:307–311. doi: 10.1016/0014-5793(94)80436-2. [DOI] [PubMed] [Google Scholar]
- BORDIN S., BOSCHERO A.C., CARNEIRO E.M., ATWATER I. Ionic mechanisms involved in the regulation of insulin secretion by muscarinic agonists. J. Membr. Biol. 1995;148:177–184. doi: 10.1007/BF00207273. [DOI] [PubMed] [Google Scholar]
- BURLEIGH D.E. Opioid and non-opioid actions of loperamide on cholinergic nerve function in human isolated colon. Eur. J. Pharmacol. 1988;152:39–46. doi: 10.1016/0014-2999(88)90833-3. [DOI] [PubMed] [Google Scholar]
- CHOW R.H., LUND P.E., LOSER S., PANTEN U., GYLFE E. Coincidence of early glucose-induced depolarization with lowering of cytoplasmic Ca2+ in mouse pancreatic beta-cells. J. Physiol. 1995;485:607–617. doi: 10.1113/jphysiol.1995.sp020756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHURCH J., FLCTCHER E.J., ABDEL-HAMID K., MACDONALD J.F. Loperamide blocks high-voltage-activated calcium channels and N-methyl-D-aspartate-evoked responses in rat and mouse cultured hippocampal pyramidal. Mol. Pharmacol. 1994;45:747–757. [PubMed] [Google Scholar]
- CLEEMANN L., MORAD M. Role of Ca2+ channel in cardiac excitation–contraction coupling in the rat: evidence from Ca2+ transients and contraction. J. Physiol. 1991;432:283–312. doi: 10.1113/jphysiol.1991.sp018385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLEEMANN L., WANG W., MORAD M. Two-dimensional confocal images of organization, density, and gating of focal Ca2+ release sites in rat cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 1998;95:10984–10989. doi: 10.1073/pnas.95.18.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOK D.L., HALES C.N. Intracellular ATP directly block K+ channels in pancreatic B-cells. Nature. 1984;311:271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- COOK D.L., IKEUCHI M., FUJIMOTO W.Y. Lowering of pHi inhibits Ca2+- activated K+ channels in pancreatic β-cells. Nature (London) 1984;311:269–271. doi: 10.1038/311269a0. [DOI] [PubMed] [Google Scholar]
- DALY J.W., HARPER J. Loperamide: novel effects on capacitative calcium influx. Cell Mol. Life Sci. 2000;57:149–157. doi: 10.1007/s000180050504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALY J.W., LUEDERS J., PADGETT W.L., SHIN Y., GUSOVSKY F. Maitotoxin-elicited calcium influx in cultured cells. Effect of calcium-channel blockers. Biochem. Pharmacol. 1995;50:1187–1197. doi: 10.1016/0006-2952(95)00257-z. [DOI] [PubMed] [Google Scholar]
- DETIMARY P., GILON P., HENQUIN J.C. Interplay between cytoplasmic Ca2+ and the ATP/ADP ratio: a feedback control mechanism in mouse pancreatic islets. Biochem. J. 1998;333:269–274. doi: 10.1042/bj3330269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIENER M., KNOBLOCH S.F., RUMMEL W. Action of loperamide on neuronally mediated and Ca2+- or cAMP-mediated secretion in rat codon. Eur. J. Pharmacol. 1988;152:217–225. doi: 10.1016/0014-2999(88)90716-9. [DOI] [PubMed] [Google Scholar]
- DING W.G., HE L.P., OMATSU-KANBE M., KITASATO H. A possible role of the ATP-sensitive potassium ion channel in determining the duration of spike-bursts in mouse pancreatic beta-cells. Biochim. Biophys. Acta. 1996;1279:219–226. doi: 10.1016/0005-2736(95)00249-9. [DOI] [PubMed] [Google Scholar]
- EDDLESTONE G.T., RIBALET B., CIANI S. Comparative study of K channel behavior in β cell lines with different secretory responses to glucose. J. Membr. Biol. 1989;109:123–134. doi: 10.1007/BF01870851. [DOI] [PubMed] [Google Scholar]
- FINDLAY I., DUNNE M.J., PETERSEN O.H. High-conductance K+ channel in pancreatic islet cells can be activated and inactivated by internal calcium. J. Membr. Biol. 1985;83:169–175. doi: 10.1007/BF01868748. [DOI] [PubMed] [Google Scholar]
- GIAGNONI G., CASIRAGHI L., SENINI R., REVEL L., PAROLARO D., SALA M., GORI E. Loperamide: evidence of interaction with mu and delta opioid receptors. Life Sci. 1983;33:315–318. doi: 10.1016/0024-3205(83)90506-4. [DOI] [PubMed] [Google Scholar]
- GÖPEL S.O., KANNO T., BARG S., ELIASSON L., GALVANOVSKIS J., RENSTRÖM E., RORSMAN P. Activation of Ca2+-dependent K+ channels contributes to rhythmic firing of action potentials in mouse pancreatic β cells. J. Gen. Physiol. 1999;114:759–770. doi: 10.1085/jgp.114.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GYLFE E., HELLMAN B. Glucose-stimulated sequestration of Ca2+ in clonal insulin-releasing cells. Evidence for an opposing effect of muscarinic-receptor activation. Biochem. J. 1986;233:865–870. doi: 10.1042/bj2330865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARPER J.L., SHIN Y., DALY J.W. Loperamide: a positive modulator for store-operated calcium channels. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14912–14917. doi: 10.1073/pnas.94.26.14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HE L.P., MEARS D., ATWATER I., KITASATO H. Glucagon induces suppression of ATP-sensitive K+ channel activity through a Ca2+/calmodulin-dependent pathway in mouse pancreatic β-cells. J. Membr. Biol. 1998;166:237–244. doi: 10.1007/s002329900465. [DOI] [PubMed] [Google Scholar]
- HENQUIN J.C., MEISSNER H.P. Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in pancreatic β-cells. Experientia. 1984;40:1043–1052. doi: 10.1007/BF01971450. [DOI] [PubMed] [Google Scholar]
- INAGAKI N., GONOI T., CLEMENT J.P., IV, NAMBA N., INAZAWA J., GONZALEZ G., AGUILAR-BRYAN L., SEINO S., BRYAN J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- LI Z.W., DING J.P., KALYANARAMAN V., LINGLE C.J. RINm5f cells express inactivating BK channels whereas HIT cells express noninactivating BK channels. J. Neurophysiol. 1999;81:611–624. doi: 10.1152/jn.1999.81.2.611. [DOI] [PubMed] [Google Scholar]
- LIU X., ROJAS E., AMBUDKAR I.S. Regulation of KCa current by store-operated Ca2+ influx depends on internal Ca2+ release in HSG cells. Am. J. Physiol. 1998;275:C571–C580. doi: 10.1152/ajpcell.1998.275.2.C571. [DOI] [PubMed] [Google Scholar]
- LIU Y.J., GYLFE E. Store-operated Ca2+ entry in insulin-releasing pancreatic beta-cells. Cell Calcium. 1997;22:277–286. doi: 10.1016/s0143-4160(97)90066-x. [DOI] [PubMed] [Google Scholar]
- MAECHLER P., KENNEDY E.D., SEBO E., VALEVA A., POZZAN T., WOLLHEIM C.B. Secretagogues modulate the calcium concentration in the endoplasmic reticulum of insulin-secreting cells. Studies in aequorin-expressing intact and permeabilized ins-1 cells. J. Biol. Chem. 1999;274:12583–12592. doi: 10.1074/jbc.274.18.12583. [DOI] [PubMed] [Google Scholar]
- MARTY A., NEHER E. Ionic channels in cultured rat pancreatic beta cells. J. Physiol. 1982;326:36P–37P. [Google Scholar]
- MATHIAS P.C., CARPINELLI A.R., BILLAUDEL B., GARCIA-MORALES P., VALVERDE I., MALAISSE W.J. Cholinergic stimulation of ion fluxes in pancreatic islets. Biochem. Pharmacol. 1985;34:3451–3457. doi: 10.1016/0006-2952(85)90717-8. [DOI] [PubMed] [Google Scholar]
- MIHAI R., LAI T., SCHOFIELD G., FARNDON J.R. Thimerosal increases the responsiveness of the calcium receptor in human parathyroid and rMTC6-23 cells. Cell Calcium. 1999;26:95–101. doi: 10.1054/ceca.1999.0055. [DOI] [PubMed] [Google Scholar]
- MISLER S., FALKE L.C., GILLIS K., MCDANIEL M.L. A metabolite-regulated potassium channel in rat pancreatic B cells. Proc. Natl. Acad. Sci. U.S.A. 1986;83:7119–7123. doi: 10.1073/pnas.83.18.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIURA Y., HENQUIN J.C., GILON P. Emptying of intracellular Ca2+ stores stimulates Ca2+ entry in mouse pancreatic beta-cells by both direct and indirect mechanisms. J. Physiol. 1997;503:387–398. doi: 10.1111/j.1469-7793.1997.387bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN N.G., RUMFORD G.M., MONTAGUE W. Studies on the mechanism by which melittin stimulates insulin secretion from isolated rat islets of Langerhans. Biochim. Biophys. Acta. 1985;845:526–532. doi: 10.1016/0167-4889(85)90221-6. [DOI] [PubMed] [Google Scholar]
- NENQUIN M., AWOUTERS P., MATHOT F., HENQUIN J.C. Distinct effects of acetylcholine and glucose on 45calcium and 86rubidium efflux from mouse pancreatic islets. FEBS Lett. 1984;176:457–461. doi: 10.1016/0014-5793(84)81218-1. [DOI] [PubMed] [Google Scholar]
- OOMS L.A.A., DEGRYSE A., JANSSEN P.A.J. Mechanisms of action of loperamide. Scand. J. Gastroenterol. Suppl. 1984;96:145–155. [PubMed] [Google Scholar]
- PRENTKI M., MATSCHINSKY F.M. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol. Rev. 1987;67:1185–1248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- REYNOLDS I.J., GOULD R.J., SNYDER S.H. Loperamide: blockade of calcium channels as a mechanism for antidiarrheal effects. J. Pharmacol. Exp. Therap. 1984;31:628–632. [PubMed] [Google Scholar]
- ROE M.W., LANCASTER M.E., MERTZ R.J., WORLEY J.F., III, DUKES I.D. Voltage-dependent intracellular calcium release from mouse islets stimulated by glucose. J. Biol. Chem. 1993;268:9953–9956. [PubMed] [Google Scholar]
- ROE M.W., PHILIPSON L.H., FRANGAKIS C.J., KUZNETSOV A., MERTZ R.J., LANCASTER M.E., SPENCER B., WORLEY J.F, III, DUKES I.D. Defective glucose-dependent endoplasmic reticulum Ca2+ sequestration in diabetic mouse islets of Langerhans. J. Biol. Chem. 1994;269:18279–18282. [PubMed] [Google Scholar]
- ROE M.W., WORLEY J.F., III, OIAN F., TAMARINA N., MITTA A.A., DRALYUK F., BLAIR N.T., MERTZ R.J., PHILIPSON L.H., DUKES I.D. Characterization of a Ca2+ release-activated nonselective cation current regulating membrane potential and [Ca2+]i oscillations in transgenically derived beta-cells. J. Biol. Chem. 1998;273:10402–10410. doi: 10.1074/jbc.273.17.10402. [DOI] [PubMed] [Google Scholar]
- RORSMAN P., TRUBE G. Calcium and delayed potassium currents in mouse pancreatic beta-cells under voltage-clamp conditions. J. Physiol. 1986;374:531–550. doi: 10.1113/jphysiol.1986.sp016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RORSMAN P., TRUBE G. Glucose-dependent K+-channels in pancreatic β-cells are regulated by intracellular ATP. Pflügers Arch. 1985;405:305–309. doi: 10.1007/BF00595682. [DOI] [PubMed] [Google Scholar]
- TABCHARANI J.A., MISLER S. Ca2+- activated K+ channels in rat pancreatic islet β cells: permeation, gating and blockade by cations. Biochim. Biophys. Acta. 1989;982:62–72. doi: 10.1016/0005-2736(89)90174-0. [DOI] [PubMed] [Google Scholar]
- TENGHOLM A., HELLMAN B., GYLFE E. Mobilization of Ca2+ stores in individual pancreatic beta-cells permeabilized or not with digitonin or alpha-toxin. Cell Calcium. 2000;27:43–51. doi: 10.1054/ceca.1999.0087. [DOI] [PubMed] [Google Scholar]
- TENGHOLM A., HELLMAN B., GYLFE E. Glucose regulation of free Ca2+ in the endoplasmic reticulum of mouse pancreatic beta cells. J. Biol. Chem. 1999;274:36883–36890. doi: 10.1074/jbc.274.52.36883. [DOI] [PubMed] [Google Scholar]
- THASTRUP O., DAWSON A.P., SCHARFF O., FODER B., CULLEN P.J., DROBAK B.K., BYERRUM P.J., CHRISTENSEN S.B., HANLEY M.R. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions. 1989;43:187–193. doi: 10.1007/BF01986687. [DOI] [PubMed] [Google Scholar]
- WANG W., CLEEMANN L., JONES L.R., MORAD M. Modulation of focal and global Ca2+ release in calsequestrin-overexpressing mouse cardiomyocytes. J. Physiol. 2000;524:399–414. doi: 10.1111/j.1469-7793.2000.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLHEIM C.B., SHARP G.W.G. Regulation of insulin release by calcium. Physiol. Rev. 1981;61:914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]
- WORLEY J.F., III, MCINTYRE M.S., SPENCER B., DUKES I.D. Depletion of intracellular Ca2+ stores activates a maitotoxin-sensitive nonselective cationic current in beta-cells. J. Biol. Chem. 1994;269:32055–32058. [PubMed] [Google Scholar]
- ZAVECZ J.H., JACKSON T.E., LIMP G.L., YELLIN T.O. Relationship between anti-diarrheal activity and binding to calmodulin. Eur. J. Pharmacol. 1982;78:375–377. doi: 10.1016/0014-2999(82)90042-5. [DOI] [PubMed] [Google Scholar]
- ZUNKLER B.J., TRUBE G., OHNO-SHOSAKU T. Forskolin-induced block of delayed rectifying K+ channels in pancreatic beta-cells is not mediated by cAMP. Pflugers Arch. 1988;411:613–619. doi: 10.1007/BF00580856. [DOI] [PubMed] [Google Scholar]