Abstract
Since most inflammatory mediators that stimulate granulocyte responsiveness also delay apoptosis, it is often assumed that activation and longevity are causally related. Using isolated human peripheral blood neutrophils and eosinophils, we examined this association by exploiting the proinflammatory lipid mediators, the leukotrienes (LTs), and investigated granulocyte function and apoptosis.
LTB4 induced elevation of intracellular free Ca2+ concentration ([Ca2+]i), cell polarisation and retardation of neutrophil apoptosis, although the antiapoptotic effect occurred only at concentrations ⩾300 nM. LTB4-induced activation was attenuated by CP-105,696, a BLT1-specific antagonist suggesting classical LTB4 receptor BLT1 involvement.
Despite demonstrating the presence of the neutrophil intracellular LTB4 receptor peroxisome-proliferator activator receptor-α (PPARα) in neutrophils, the selective PPARα agonist WY-14,643 did not mimic LTB4-induced prosurvival effects.
LTB4-induced survival, however, also appeared to be mediated by BLT1 since CP-105,696 inhibited the LTB4-mediated antiapoptotic effect. Furthermore, based on studies with CP-105,696 and 5-lipoxygenase inhibitors, lipopolysaccharide (LPS)-, granulocyte–macrophage colony-stimulating factor (GM-CSF)-, dexamethasone- and dibutyryl-cAMP (db-cAMP)-induced delay of neutrophil apoptosis did not involve autocrine production of LTB4.
Although LTB4 and LTD4 induced human eosinophil [Ca2+]i elevation and polarization, these LTs did not influence eosinophil apoptosis. Furthermore, LTB4- and LTD4-induced eosinophil activation was attenuated by CP-105,696 and the Cys-LT1 receptor antagonist montelukast, respectively, highlighting specific receptor dependency.
Thus, mediator-triggered granulocyte activation and antiapoptotic pathways are distinct events that can be differentially regulated.
Keywords: Leukotrienes, neutrophils, eosinophils, apoptosis, calcium
Introduction
Granulocytes are key effector cells of the immune system, where neutrophils provide first-line protection against bacterial and fungal invasion and eosinophils play an important role in mediating antiparasitic defences. Over-recruitment, inappropriate activation or dysregulated clearance of these cells likely contribute to the pathogenesis and propagation of a wide variety of inflammatory disorders. While neutrophil products can cause tissue damage in inflammatory diseases such as chronic obstructive pulmonary disease and rheumatoid arthritis, inappropriate release of eosinophil products is associated with allergic disorders such as allergic rhinitis and asthma (Rossi & Haslett, 1998; Giembycz & Lindsay, 1999; Gompertz & Stockley, 2000). Apoptosis or programmed cell death is a critical process regulating the lifespan of inflammatory cells and the resolution phase of inflammation (Haslett, 1999; Ward et al., 1999b; Lawrence et al., 2002). Apoptosis is associated with the maintenance of plasma membrane integrity, downregulation of functional responsiveness (Whyte et al., 1993b), and triggers apoptotic cell recognition and engulfment by phagocytic cells (Savill et al., 1989; Stern et al., 1992; Walsh et al., 1999). Remarkably, the phagocytic clearance of these cells fails to excite an inflammatory response (Meagher et al., 1992); indeed, ingestion of apoptotic granulocytes in vitro induces an anti-inflammatory cytokine and mediator profile in the phagocyte (Stern et al., 1996; Fadok et al., 1998; Liu et al., 1999). Thus, this process provides an efficient and noninflammatory mechanism for the clearance of potentially toxic and unneeded cells from the inflamed site.
Neutrophils and eosinophils are derived from a common myeloid precursor, and when activated, exhibit similar responses (e.g., cell polarisation, chemotaxis, adhesion, degranulation and superoxide anion generation). Comparative studies of functional responses in these cells have contributed significantly to our understanding of granulocyte behaviour, including apoptosis. Of interest, despite their close haematopoietic origins, apoptosis of eosinophil and neutrophil granulocytes can be regulated in a selective manner. For example, glucocorticoids induce eosinophil apoptosis but retard this process in the neutrophil (Meagher et al., 1996). Many of the proinflammatory mediators present at an inflamed site not only influence granulocyte responsiveness but also delay the rate of constitutive apoptosis. For example, lipopolysaccharide (LPS) and granulocyte/macrophage colony-stimulating factor (GM-CSF) have been shown to upregulate neutrophil function by priming for enhanced functional responsiveness to secretagogue agonists and also prolong cell longevity by delaying apoptosis (Colotta et al., 1992; Lee et al., 1993). A similar dual effect has been observed in eosinophils stimulated with IL-5 and GM-CSF with both cytokines enhancing eosinophil function and delaying apoptosis (Stern et al., 1992; Walsh et al., 1996). Thus, it is often perceived that a close and inevitable relation exists between granulocyte activation status and longevity; indeed, a large family of inflammatory mediators promote granulocyte function by initially inducing cell priming and subsequently by delaying the onset of constitutive apoptosis (Mecklenburgh et al., 1999). In this study, we investigated this association by exploiting the archetypal proinflammatory lipid mediators, the leukotrienes (LTs).
The LTs are produced by the oxygenation of deacylated membrane-bound arachidonic acid through a reaction catalysed by 5-lipoxygenase (5-LO) and 5-lipoxygenase-activating protein (FLAP) to yield 5-hydroperoxyeisosatetraenoic acid (5-HPETE), which is converted to leukotriene A4 (LTA4), an unstable epoxide intermediate. In neutrophils, and other cells that contain LTB4 synthase (e.g., alveolar macrophages), LTA4 is converted to LTB4 (Samuelsson et al., 1987). Indeed, neutrophils when activated by a variety of stimuli (e.g., the calcium ionophore, A23187 and receptor-directed stimuli such as platelet-activating factor (PAF), formyl-leucyl-phenylalanine (fMLP) and GM-CSF) are capable of synthesising and releasing LTB4 (Lin et al., 1982; Samuelsson et al., 1987; Dahinden et al., 1988; Rossi et al., 1993). LTB4 has also been shown to be a powerful activator of neutrophil responsiveness causing cell polarisation, chemotaxis, adhesion, degranulation and reactive oxygen species generation (Ford-Hutchinson et al., 1980; Rossi et al., 1993). In addition, LTB4 has been shown to influence neutrophil longevity by delaying apoptosis (Hebert et al., 1996). LTB4 is believed to elicit its effects by interacting with distinct seven transmembrane G-protein-coupled receptors, the classical BLT1 receptor (Yokomizo et al., 1997) and the recently identified BLT2 receptor (Kamohara et al., 2000; Serhan & Prescott, 2000; Yokomizo et al., 2000). The other receptor type that binds LTB4 that has received much recent attention is the peroxisome-proliferator activator receptor-α (PPARα) (Devchand et al., 1996). This receptor is a member of the PPAR group of transducer proteins belonging to the steroid/thyroid/retinoid receptor family, which regulate the expression of target genes by binding to PPAR response elements. PPARα is primarily expressed in tissues that have high fatty acid catabolism, including the liver and the immune system (Devchand et al., 1996).
In eosinophils, mast cells and other cells that contain LTC4 synthase, LTA4 is converted mainly to LTC4, through conjugation with reduced glutathione. LTC4 is exported from the cytosol to the extracellular environment and sequentially cleaved to form LTD4 and the less potent LTE4. These cysteinyl LTs are now well established as mediators of immediate hypersensitivity reactions, and are potent agonists of bronchoconstriction and vascular permeability and play a role in the persistent tissue eosinophilia that is characteristic of asthma (Leff, 2000). Cysteinyl LTs may influence various stages of the eosinophil life cycle including bone marrow differentiation, adhesion and margination; however, the effects of the cysteinyl LTs on the activation status and functional longevity of these cells are ill defined. In this study, we have examined the relation between granulocyte activation and apoptosis by comparing the effects of the LTs on neutrophil and eosinophil function and lifespan in vitro.
Methods
Neutrophil and eosinophil isolation
Neutrophils and eosinophils were isolated from the peripheral venous blood of healthy adult donors by dextran sedimentation followed by centrifugation through discontinuous PBS-Percoll gradients (Rossi et al., 1998; Ward et al., 1999a). Only neutrophil preparations with a purity of >98% were used. Eosinophils were separated from contaminating neutrophils using an immunomagnetic separation step with sheep anti-mouse IgG-Dynabeads (Dynabeads M-450, Dynal, Merseyside, U.K.) coated with the murine antineutrophil antibody 3G8 (anti-CD16; a gift from Dr J. Unkeless, Mount Sinai Medical School, New York, U.S.A.). Cells were mixed with washed 3G8-coated Dynabeads at a bead : neutrophil ratio of 3 : 1 on a rotary mixer at 4°C for 20 min, and the beads removed magnetically by two 3 min stationary magnetic contacts (Dynal Magnetic Particle Concentrator, MPC-1) to yield an eosinophil population of >98% purity. After harvesting and purification, cells were washed twice in PBS (without divalent cations) and once in PBS containing MgCl2 and CaCl2 before resuspending in appropriate buffer.
Culture of granulocytes
Neutrophils (5 × 106 cells ml−1) and eosinophils (2.5 × 106 cells ml−1) were resuspended in Iscove's MDM (Life Technologies, Paisley, U.K.) containing 50 U ml−1 penicillin and 50 U ml−1 streptomycin and supplemented with 10% autologous serum (or 0.1% BSA) and cultured in flat-bottomed 96-well Falcon flexiwell plates (Becton-Dickinson, U.K.) at 37°C in a humidified 5% CO2 atmosphere for the time periods indicated. The cells were cultured in the absence or presence of the reagents of interest as described in the figure legends and unless otherwise stated, all experiments were done at least three times with each treatment performed in triplicate.
Morphological assessment of granulocyte apoptosis
After culture, cells were gently resuspended and 100 μl harvested from each well, cytocentrifuged in a Shandon Cytospin II (Shandon, U.K.) and the resulting slide preparations air dried, fixed in methanol and stained with Diff-Quik™. Cell morphology was examined by × 100 objective oil immersion light microscopy to determine the proportion of cells with highly characteristic apoptotic morphology (Savill et al., 1989; Stern et al., 1992; Ward et al., 1999a). For each condition examined, slides were prepared from triplicate incubations and after coding, a total of at least 500 granulocytes were counted over a minimum of five high power fields with the observer blinded to the assay conditions. The data were expressed as the mean per cent apoptosis±s.e.m. Cell viability was determined in parallel by the ability of granulocytes to exclude the vital dye trypan blue assessed by light microscopy and, on occasion, by cellular incorporation of propidium iodide as assessed by flow cytometry (Ward et al., 1999a). Under all conditions and treatments used, there was no loss of cell membrane integrity and cell loss was minimal.
Annexin V binding
A separate and independent assessment of apoptosis was performed by flow cytometry using FITC-labelled recombinant human annexin V that binds to phosphatidylserine exposed on the surface of apoptotic cells. Stock annexin V (Bender MedSystems, Vienna, Austria) was diluted 1 : 200 with binding buffer and then added (25 μl) to 75 μl of the recovered cell samples. Following a 10 min incubation at 4°C, these samples were fixed by the addition of 100 μl of 3% paraformaldehyde before analysis using an EPICS Profile II (Coulter Electronics, Luton, U.K.) or a FACSCalibur (Becton Dickinson, Oxford, U.K.) (Ward et al., 1999a). The fluorescence from a minimum of 5000 cells was recorded.
Determination of cytosolic free calcium ion levels ([Ca2+]i)
Freshly isolated granulocytes were washed (3 ×) in HBSS (Ca2+ and Mg2+ free) before being resuspended at 107 ml−1 in HBSS (Ca2+ and Mg2+ free) and incubated with fura-2/AM (final concentration 2 μM) for 30 min at 37°C (O'Flaherty & Rossi, 1993). The cells were then washed (2 ×) and left in HBSS (Ca2+ and Mg2+ free) for a further 10 min for optimal de-esterification of the fura-2-acetoxymethyl ester, before finally resuspending the granulocytes at 2 × 106 cells ml−1 in HBSS (containing Ca2+ and Mg2+). Changes in fluorescence following agonist addition were determined using a Perkin-Elmer LS50B fluorimeter, with dual wavelength excitation (340 and 380 nm) and emission at 510 nm. The fluorimeter has a fitted thermostated cuvette compartment and stirring attachment to ensure complete mixing of reagents at 37°C. Calibration of [Ca2+]i was performed as described (O'Flaherty & Rossi, 1993).
Granulocyte shape change assay
Freshly isolated granulocytes (180 μl, 2 × 106 cells ml−1 in PBS containing CaCl2 and MgCl2) were equilibrated at 37°C in a shaking water bath for 15 min in the presence of buffer alone or the antagonist of interest. Agonists were then added to the required concentration and incubations continued for a further 15 min before the addition of 200 μl of 2.5% glutaraldehyde. Samples were analysed for shape change by flow cytometry (Coulter EPICS Profile II; Coulter Electronics, Luton, U.K.) (Kitchen et al., 1996). Percentage shape change was calculated from the mean forward light scatter of each sample by gating on the nonshape-changed population.
Western blotting for PPARα
Cell samples (5 × 106 cells ml−1) were lysed (Ward et al., 1999a,2002; Fujihara et al., 2002) and the whole cell lysates were run on a 9% SDS gel and after transfer to nitrocellulose membranes (Amersham Pharmacia Biotech U.K. Ltd), blocked by 5% milk protein before an overnight incubation with the polyclonal PPARα antibody, N/19 (SantaCruz Biotechnology Inc., Santa Cruz, CA, U.S.A.) diluted 1 : 500. After washing, blots were incubated with HRP-conjugated antibiotin antibody diluted at 1 : 2500 and developed using standard ECL reagents.
Materials
Further specific materials were obtained as follows: LTB4, LTC4, LTD4, and WY-14,643 (Biomol, Affinity Research Products, Mamhead, U.K.) and GM-CSF, IL-5 (R&D Systems Europe Ltd, Oxon, U.K.) and MK-886 (Calbiochem Ltd, Beeston, Nottingham, U.K.). CP-105,696 was a gift from Henry J. Showell, Pfizer Inc., Connecticut, U.S.A. Montelukast (1-(((1(R)-(3-(2-(7-chloro-2-quinolinyl)-(E)-ethenyl)phenyl)(3-2-(1-hydroxy-1-methylethyl)phenyl)propyl)thio)methyl) cyclopropane) acetic acid sodium salt) was a gift from Dr Richard Tomiak, Merck, Sharp and Dohme Ltd. All other reagents were obtained from Sigma Chemical Co., Poole, U.K. and were of the highest purity.
Statistical analysis
The results are expressed as means±s.e.m. of (n) the number of independent experiments each using cells isolated from separate donors with each treatment performed in triplicate, unless otherwise stated. Statistical analysis was performed by ANOVA with comparisons between groups made using the Newman–Kuels procedure. Differences were considered significant when P<0.05.
Results
Effect of LTB4 on human neutrophil apoptosis
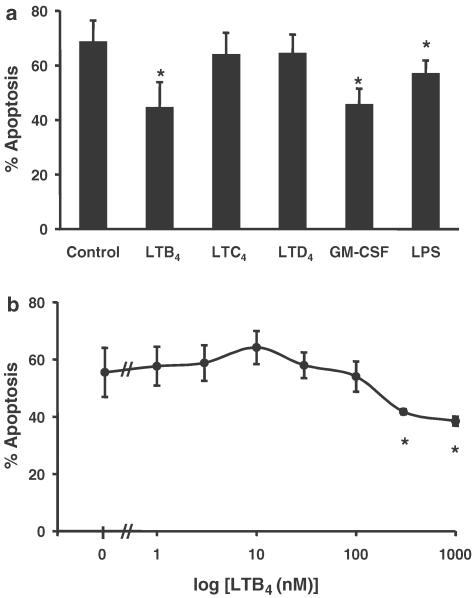
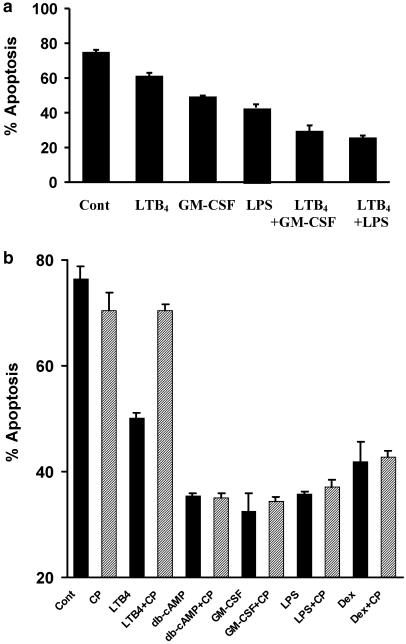
Initially, we demonstrated that incubation of human neutrophils for 20 h with LTB4 (1 μM) delayed the rate of constitutive apoptosis as assessed by morphological criteria (Figure 1a). In contrast, the cysteinyl LTs LTC4 (1 μM) and LTD4 (1 μM) had no effect on the constitutive rate of apoptosis in these cells (Figure 1a). The extent of the retardation of neutrophil apoptosis observed with LTB4 was similar to that observed with human recombinant GM-CSF (50 U ml−1) and LPS (1 μg ml−1) (Figure 1a). Assessment of cell recovery (haemocytometer counts) and viability (trypan blue exclusion) demonstrated that the inflammatory mediators investigated had no effect on either of these parameters as compared to vehicle-treated controls (data not shown). Experiments performed to examine the concentration dependency of LTB4-mediated neutrophil survival revealed that this response was only observed with relatively high concentrations of LTB4 (⩾300 nM; Figure 1b). Accordingly, an LTB4 concentration of 1 μM was selected for use in all further apoptosis studies.
Figure 1.
Effect of LTs on human neutrophil apoptosis. Neutrophils (5 × 106 cells ml−1) were cultured in serum-supplemented Iscove's MDM alone (control) or in identical medium containing stimuli (final concentration). (a) LTB4 (1 μM), LTC4 (1 μM), LTD4 (1 μM), GM-CSF (50 U ml−1) or LPS (1 μg ml−1) and (b) LTB4 (1 nM–1 μM). Cells were harvested following 20 h in culture and assessed for morphological features of apoptosis. Data represent the means±s.e.m. of three separate experiments, each performed in triplicate, where * represents P<0.05 compared to control values.
Effect of LTB4 on neutrophil [Ca2+]i levels and cell polarisation
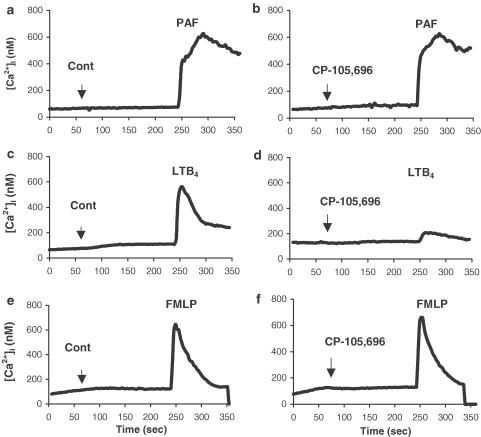
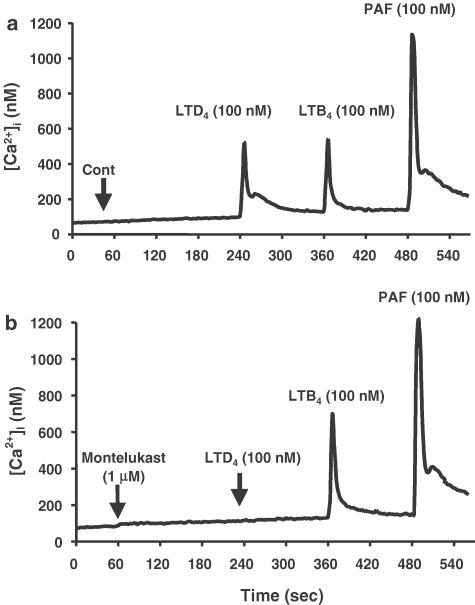
Signalling mechanisms such as changes in [Ca2+]i are fundamental in regulating neutrophil activation and may play a key role regulating granulocyte apoptosis (Whyte et al., 1993a). We therefore examined the effects of LTB4 on [Ca2+]i in human neutrophils and compared the responses to those induced by other agonists. [Ca2+]i levels in freshly isolated neutrophils were measured using the Ca2+-sensitive dye fura-2 following the addition of PAF (100 nM), LTB4 (100 nM) or fMLP (100 nM) as indicated in Figure 2. The resting [Ca2+]i levels recorded in these experiments (<100 nM) were consistent with those reported in previous studies in neutrophils (O'Flaherty & Rossi, 1993; Whyte et al., 1993a). Addition of PAF, LTB4 or fMLP induced a rapid rise in [Ca2+]i to a peak of approximately 600 nM, before returning to control values (Figure 2). To investigate the mechanism of LTB4-mediated elevation of [Ca2+]i, neutrophils were preincubated in the presence or absence of the specific BLT1 receptor antagonist CP-105,696 ((+)-1-(3S,4R)-[3-(4-phenyl-benzyl)-4-hydroxy-chroman-7-yl]-cyclopentane carboxylic acid; Showell et al., 1995), prior to the addition of LTB4, PAF or fMLP (100 nM). CP-105,696 (1 μM) had no effect on either the basal levels of [Ca2+]i, or on PAF- or fMLP-induced responses, but abrogated LTB4-mediated elevation of [Ca2+]i (Figure 2) indicating that this response is a specific, BLT1 receptor-mediated event. Moreover, the data suggest that fMLP- and PAF-induced elevation of [Ca2+]i is not mediated by the autocrinic production of LTB4.
Figure 2.
Measurement of cytosolic calcium levels in freshly isolated human neutrophils in response to agonist stimulation. Cytosolic calcium measurements (nM) in fura-2-loaded, neutrophils (2 × 106 cells ml−1) over 350 s. Buffer alone (control, a,c,e) or CP-105,696 (1 μM; b,d,f) was added to the cell suspension followed by the sequential addition of PAF (100 nM; a, b), LTB4 (100 nM; c, d), or fMLP (100 nM; e, f), after 245 s. The data show traces obtained from a typical experiment performed at least three times.
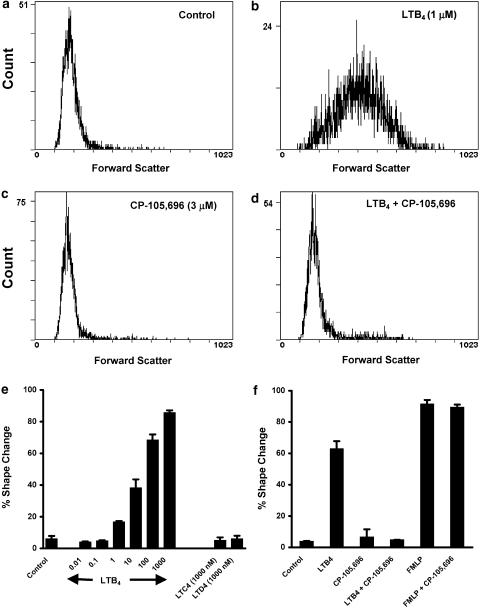
We next examined the effect of a range of LTB4 concentrations on cell polarisation. Incubation of freshly isolated neutrophils with LTB4 for 10 min induced a dramatic polarisation response as assessed by changes in forward scatter by flow cytometric analysis (compare Figure 3a and b). This response exhibited a concentration-dependency and was sensitive to much lower LTB4 concentrations of 1 nM and above (Figure 3e). The cysteinyl LTs LTC4 and LTD4 failed to elicit a response at 1 μM, a concentration that was maximal for LTB4-induced shape change (Figure 3e). Preincubation of neutrophils (15 min at 37°C) with the BLT1 receptor antagonist CP-105,696 (3 μM) had no effect on basal shape change (compare Figure 3a and c) but abrogated the maximal (1 μM) LTB4-induced shape change (compare Figure 3b and d) without influencing fMLP-induced polarisation (Figure 3f). Taken together, these data indicate that the rapid activation responses to LTB4 observed in human neutrophils are BLT1 receptor-mediated and support the use of CP-105,696 as a potent and specific BLT1 receptor antagonist.
Figure 3.
Effect of LTB4 on shape change in human neutrophils. Neutrophils (2 × 106 cells ml−1 in 180 μl PBS with CaCl2 and MgCl2) were equilibrated for 15 min at 37°C, then preincubated with 10 μl CP-105,696 (3 μM) or vehicle prior to stimulation for 10 min with 10 μl LTB4 (1 μM), or vehicle. (a) represents control, (b) LTB4, (c) CP-105,696 and (d) LTB4 plus CP-105,696. (e) Shows the concentration–response for LTB4-induced shape change where neutrophils (2 × 106 cells ml−1 in 190 μl PBS with CaCl2 and MgCl2) were equilibrated for 15 min at 37°C, then incubated with 10 μl buffer alone (control), LTB4 (0.01 nM–1 μM), LTC4 (1 μM) or LTD4 (1 μM) for 10 min. (f) Shows the specificity of CP-105,696 for BLT1-mediated shape change where neutrophils (2 × 106 cells ml−1 in 180 μl PBS with CaCl2 and MgCl2) were equilibrated for 15 min at 37°C, then incubated with 10 μl buffer alone (control), or CP-105,696 (3 μM) prior to stimulation for 10 min with 10 μl LTB4 (1 μM), fMLP (100 nM), or buffer alone. All reactions were terminated by addition of 200 μl 2.5% glutaraldehyde. (a–d) Show representative flow cytometry (EPICS Profile II), x-axis: mean forward light scatter (FS); y-axis: relative cell number. (e) and (f) show per cent shape change as analysed by flow cytometry as detailed in Methods. Data represent means±s.e.m. of one representative experiment of three, each performed in triplicate.
Effect of a BLT1 receptor antagonist on LTB4-mediated survival of human neutrophils
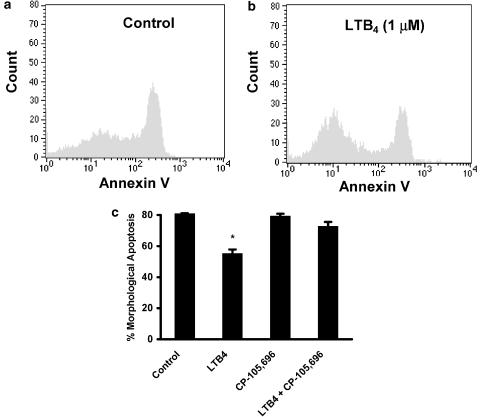
As a separate assessment of apoptosis, we show that incubation of neutrophils with LTB4 (1 μM) for 20 h delayed apoptosis as assessed by annexin V binding (Figure 4a and b). Initial preliminary experiments showed that the presence of autologous serum interfered with the ability of CP-105,696 to inhibit LTB4-mediated retardation of neutrophil apoptosis (data not shown). We therefore cultured our cells in the presence of 0.1% BSA since delayed apoptosis induced by LTB4 is not dependent on the presence of serum and neutrophils cultured for 20 h in the absence of any protein would invariably undergo secondary necrosis. Thus, to explore the receptor-dependency of LTB4-mediated delay of neutrophil apoptosis, cells were cocultured in Iscove's containing 0.1% BSA with the BLT1 receptor antagonist CP-105,696 at a concentration shown in this study to completely antagonise maximal LTB4-stimulated [Ca2+]i elevation and shape change (Figure 2 and Figure 3c). LTB4-delayed neutrophil apoptosis was now almost totally inhibited by CP-105,696 (Figure 4c).
Figure 4.
Effect of BLT1 receptor antagonist CP-105,696 on LTB4-mediated retardation of human neutrophil apoptosis. Neutrophils (5 × 106 cells ml−1) were cultured in serum-supplemented Iscove's MDM alone (control, a) or in the presence of LTB4 (1 μM, b). Neutrophils were resuspended following 20 h in culture and incubated with FITC-labelled recombinant human Annexin V to determine phosphatidylserine expression. The cells were then fixed and analysed using an EPICS Profile II. Mean fluorescence values are shown for a minimum of 5000 cells for each condition. (c) Neutrophils (5 × 106 cells ml−1) were preincubated (37°C, 15 min) in serum-supplemented Iscove's MDM containing BSA (0.1%) and treated with LTB4 (1 μM) with or without CP-105,696 (3 μM). Samples were harvested to assess for morphological features of apoptosis; data represent the means±s.e.m. of three independent experiments performed in triplicate, where * represents P<0.05 compared to control values.
Effect of a BLT1 receptor antagonist and a FLAP inhibitor on GM-CSF-, LPS-, dexamethasone- and db-cAMP-mediated survival of human neutrophils
It has been suggested that the delay of neutrophil apoptosis by GM-CSF, LPS and dexamethasone is mediated by autocrine generation of LTB4 (Lee et al., 1999). To pursue this hypothesis in more detail, we examined: (i) whether the survival effects of LTB4 and GM-CSF/LPS were additive; (ii) the effect of FLAP and 5-LO inhibitors on GM-CSF and LPS-mediated survival and (iii) whether the effects of these inflammatory mediators, dexamethasone and the cell-permeable analogue of cAMP, db-cAMP was modified by CP-105,696 at a concentration shown to block LTB4-mediated shape change, [Ca2+]i transients and survival. Coculture experiments demonstrated that the antiapoptotic effects of LTB4 and GM-CSF, and LTB4 and LPS were in fact additive (Figure 5a), perhaps suggesting that these agents act via discrete rather than common signalling pathways. This latter observation however requires further investigation. Moreover, preincubation of neutrophils (30 min, 37°C) with the specific FLAP inhibitor (MK-886; 1 μM), or the BLT1 antagonist CP-105,696 (1 and 3 μM), had no influence on basal apoptosis per se and did not inhibit GM-CSF-, LPS-, dexamethasone- or db-cAMP-mediated suppression of apoptosis (Table 1, Figure 5b). Taken together, these data demonstrate that GM-CSF and LPS are unlikely to mediate directly their survival signal in neutrophils via the generation and autocrine action of LTB4.
Figure 5.
(a) Effect of coculture with GM-CSF and LPS on LTB4-mediated retardation of human neutrophil apoptosis. Neutrophils (5 × 106 cells ml−1) were incubated in serum-supplemented Iscove's MDM alone or in identical medium containing LTB4 (1 μM), GM-CSF (50 U ml−1), LPS (1 μg ml−1), LTB4 (1 μM) plus GM-CSF (50 U ml−1) or LTB4 (1 μM) plus LPS (1 μg ml−1). Neutrophils were harvested following 20 h in culture and assessed for morphological features of apoptosis. Data represent the means±s.e.m. of one representative experiment of three, each performed in triplicate. (b) Effect of CP-105,696 on LTB4-, db-cAMP-, GM-CSF-, LPS- and dexamethasone-mediated retardation of human neutrophil apoptosis. Neutrophils (5 × 106 cells ml−1) were pre-incubated (37°C, 15 min) in BSA (0.1%)-supplemented Iscove's MDM alone (control; black bars) or in identical medium containing CP-105,696 at final concentrations of 3 μM (hatched bars) prior to cell culture in the medium alone (control), LTB4 (1 μM), db-cAMP (0.2 mM), GM-CSF (50 U ml−1), LPS (1 μg ml−1) or dexamethasone (1 μM). Neutrophils were harvested following 20 h in culture and assessed for morphological features of apoptosis. Data represent the means±s.e.m. of triplicate samples from an individual experiment from at least 3 separate experiments.
Table 1.
Effect of the FLAP inhibitor MK-886 on LTB4, GM-CSF and LPS-mediated retardation of human neutrophil apoptosis
| Treatment | Control | MK-866 |
|---|---|---|
| Control | 72.5±5.0 | 72.0±5.8 |
| LTB4 | 59.1±3.3 | 58.9±3.7 |
| GM-CSF | 50.9±2.7 | 51.8±3.7 |
| LPS | 51.1±9.2 | 49.3±8.0 |
Human neutrophils (5 × 106 cells ml−1) were preincubated (37°C, 30 min) in serum supplemented Iscove's MDM alone or in identical medium containing MK-886 (1 μM) prior to cell culture in the medium alone (control), LTB4 (1 μM), GM-CSF (50 U ml−1) or LPS (1 μg ml−1). Neutrophils were harvested following 20 h in culture and assessed for morphological features of apoptosis (expressed as percentage of apoptotic cells). Data represent the means±s.e.m. of three separate experiments, each performed in triplicate.
Role of PPARα in LTB4-mediated survival of human neutrophils
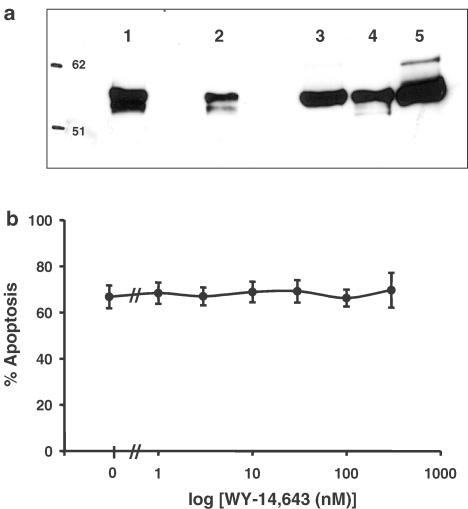
It is now well recognised that LTB4 is a potent activating ligand for PPARα (Devchand et al., 1996). In these studies, we have detected PPARα (molecular weight 55 kDa) in the cytosol of freshly isolated human neutrophils and eosinophils as well as monocytes and monocyte-derived macrophages by Western blotting (Figure 6a). To determine whether the LTB4-stimulated survival signal in human neutrophils is mediated via this receptor, we exploited the synthetic PPAR-activating ligand pirinixic acid (WY-14643), which has been shown to be selective for PPARα (Yu et al., 1995). Culture of neutrophils for 20 h with a wide range of concentrations of WY-14643 (1–300 μM) had no influence on the rate of constitutive apoptosis in these cells (Figure 6b). Therefore, although PPARα is present in human neutrophils, stimulation of this receptor does not mimic LTB4-mediated retardation of neutrophil death suggesting that the survival effect of LTB4 may not be mediated via activation of this receptor.
Figure 6.
Detection of PPARα in freshly isolated human leukocytes (a). Leukocyte cytosolic samples (5–10 × 106 cells in 100 μl of lysis buffer as detailed in the Methods section) were run on a 9% acrylamide gel and probed for human PPARα. Lanes represent: (1) fresh neutrophils (10 × 106), (2) fresh neutrophils (5 × 106), (3) fresh eosinophils (10 × 106), (4) monocytes (10 × 106), (5) monocyte-derived macrophages (10 × 106). Effect of WY-14,643, a synthetic PPARα-activating ligand, on human neutrophil apoptosis (b). Human neutrophils (5 × 106 cells ml−1) were incubated in serum-supplemented Iscove's MDM alone (control) or in identical medium containing a range of concentrations of WY-14643 (1–300 μM). Cells were harvested following 20 h in culture and assessed for morphological features of apoptosis. Data represent the means±s.e.m. of three separate experiments, each performed in triplicate.
Effects of LTs on eosinophil [Ca2+]i levels
The effects of LTs on [Ca2+]i in freshly isolated human eosinophils were measured using fura-2 in a similar set of experiments as described for neutrophils. The rise in eosinophil [Ca2+]i in response to LTB4 (100 nM; Figure 7a), was abrogated by preincubation with the BLT1 receptor antagonist CP-105,696 (data not shown). While LTC4 had no effect on [Ca2+]i (Figure 7a), incubation with LTD4 induced a rapid and transient rise in [Ca2+]i (Figure 7a and b). Moreover, LTD4-mediated flux in [Ca2+]i was blocked by preincubation with the Cys-LT1 receptor antagonist montelukast (1 μM) (Figure 7b), which had no influence on resting [Ca2+]i or on LTB4 or PAF-mediated responses (Figure 7b). Interestingly, there was no evidence of heterologous desensitisation with regard to the [Ca2+]i responses induced by LTB4, LTD4 and PAF (see Figure 7 and data not shown). This supports the existence of a specific Cys-LT1-receptor mediated LTD4-induced flux in [Ca2+]i in human eosinophils.
Figure 7.
Effect of the Cys-LT1 receptor antagonist montelukast on agonist-induced calcium fluxes in human eosinophils. Cytosolic calcium measurements (nM) in fura-2-loaded eosinophils (2 × 106 cells ml−1 in HBSS without phenol red, with CaCl2 and MgCl2) over 540 s. Buffer alone (control, a) or montelukast (1 μM, b) was added to the cell suspension after 60 s, followed by the sequential addition of LTD4 (100 nM), LTB4 (100 nM) and PAF (100 nM) after 245, 365 and 485 s, respectively. The data show traces obtained from a typical experiment performed at least three times.
Figure 8.
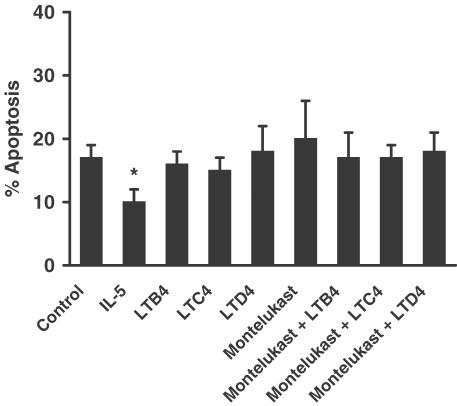
Effect of LTs (LTB4, LTC4, LTD4) and montelukast on human eosinophil apoptosis at 20 h in vitro. Eosinophils (2.5 × 106 cells ml−1) were cultured in serum-supplemented Iscove's MDM alone (control) or in identical medium containing: final concentration LTB4 (1 μM), LTC4 (1 μM), LTD4 (1 μM), montelukast (10 μM) or IL-5 (50 ng ml−1). Coculture conditions were also prepared with LTB4, LTC4 LTD4 (1 μM) in the presence of montelukast (10 μM). Eosinophils were harvested following 20 h in culture and assessed for morphological features of apoptosis. Data represent the means±s.e.m. of three independent experiments, each performed in triplicate, where * represents P<0.05 compared to control values.
Effect of LTs on eosinophil apoptosis
In these studies, we sought to determine the potential role of LTB4 and the cysteinyl LTs (LTC4 and LTD4) in modulating eosinophil apoptosis. In initial experiments, we examined the effects of LTB4, LTC4, and LTD4 (1 μM) on eosinophil longevity at 20 h, when the constitutive rate of apoptosis in these cells was still relatively very low (17.0±1.2%; Figure 8). At this time point, we were unable to detect any significant effects of the LTs on eosinophil apoptosis. The cys-LT1 receptor antagonist montelukast (10 μM) was also unable to modulate the constitutive rate of eosinophil apoptosis, either on its own, or following coculture with the LTs (Figure 8). The responsiveness of these cells to stimuli that have been previously reported to modulate apoptosis was confirmed by the delay of apoptosis by IL-5 (50 ng ml−1) (Stern et al., 1992). In experiments where cells were harvested following longer periods in culture, where the rates of constitutive apoptosis recorded were much higher (40h : 30.3±1.9%; 60 h: 70.7±1.2%), we were still unable to detect any influence of LTB4, LTC4, LTD4 or montelukast on eosinophil apoptosis (data not shown). Similar results were also obtained at these time points using LTs at 10 and 300 nM, and montelukast at 3 μM (data not shown). Thus, in these studies, neither LTB4 nor the cysteinyl LTs, LTC4 and LTD4 had any influence on the rate of eosinophil apoptosis at the various concentrations and time points investigated.
Discussion
In this study, we have examined the effects of the proinflammatory lipid mediators, the LTs, on granulocyte activation and survival. LTB4 is a potent stimulus of rapid activation events in neutrophils (i.e., [Ca2+]i fluxes and shape change). For example, LTB4-stimulated cell polarisation occurred at concentrations as low as 1 nM, with an EC50 of ≅10 nM. In addition, LTB4-induced [Ca2+]i elevation and shape change were attenuated by the specific BLT1 receptor antagonist CP-105,696 whereas these responses induced by PAF and fMLP appear to be mediated by non-LTB4 receptor-dependent mechanisms.
To investigate LTB4-mediated survival of human neutrophils, we examined the potential role of specific receptor-dependent mechanisms. Our initial studies using CP-105,696 also suggested that LTB4-induced retardation of this response appears to be mediated via the BLT1 receptor. This conclusion is supported by our studies investigating the now recognised LTB4 receptor PPARα. For this, we initially probed for the presence of the receptor by Western blotting analysis and show for the first time that PPARα (molecular weight 55 kDa) is present in whole-cell lysates of freshly isolated human neutrophils and eosinophils, as well as monocytes and monocyte-derived macrophages. Despite the presence of PPARα protein in freshly isolated neutrophils, culture of these cells for 20 h with a wide range of concentrations of the synthetic and selective PPARα-activating ligand pirinixic acid (WY-14643) (1–300 μM) had no influence on the rate of constitutive apoptosis in these cells. The higher concentrations of WY-14643 used in our study are by far in excess of that used (10 μM) to enhance interferon-γ plus TNF-α-induced apoptosis in isolated human differentiated macrophages (Chinetti et al., 1998). Of particular relevance to our studies is the recent characterisation and cloning of a novel cell surface-bound G-protein-coupled receptor for LTB4, designated BLT2 (Yokomizo et al., 2000; Kamohara et al., 2000). This receptor binds LTB4 with a Kd value of 23 nM compared with 1.1 nM for BLT1, but still efficiently transduces intracellular signalling. In contrast to BLT1, which is expressed predominantly in leucocytes, BLT2 is expressed more ubiquitously with a large amount of BLT2 mRNA detected in human spleen as well as in peripheral blood leucocytes (Kamohara et al., 2000; Yokomizo et al., 2000). Yokomizo et al. (2000) also revealed that several BLT1 antagonists failed to inhibit LTB4 binding to BLT2 demonstrating that BLT2 is pharmacologically distinct and may mediate cellular functions in tissues other than leucocytes. Further work is required to investigate the potential role of this receptor in LTB4-induced neutrophil function and, in particular, apoptosis. This is especially important given that there is evidence suggesting that LTB4 may mediate retardation of neutrophil apoptosis via cell surface receptor signalling events distinct from those involved in neutrophil degranulation and respiratory burst (Hebert et al., 1996). Taken together, however, our results suggest that the BLT1, and not the PPARα receptor, is the likely receptor responsible for the retardation of neutrophil apoptosis induced by LTB4.
We have also demonstrated, using the specific FLAP antagonist MK-886, that GM-CSF- and LPS-mediated survival in neutrophils does not appear to be mediated via the generation and autocrine effects of LTB4. These data conflict with a recent report (Lee et al., 1999) that proposed that the delay of neutrophil apoptosis observed with GM-CSF, LPS and dexamethasone could be attributed to the generation of LTB4. These latter observations are supported by a recent report showing that BLT1 receptor expression is upregulated by dexamethasone and that this effect may be one of the mechanisms through which glucocorticoids can prolong neutrophil survival. Indeed, dexamethasone-induced survival may be mediated by LTB4 as determined by use of an LTB4 receptor antagonist (Stankova et al., 2002). Difference between these studies and our observations may be because of differences in isolation procedures, activation/priming status of the cells, culture conditions or reagents and inhibitors used. Our studies however indicate that, whatever the precise mechanism utilised by LTB4 to mediate its suppression of neutrophil apoptosis, it is unlikely that dexamethasone, LPS, GM-CSF or db-cAMP delay apoptosis by this mechanism since blockade of LTB4 action by CP-105,696 and/or LTB4 synthesis by the FLAP inhibitor do not abrogate the effects of these agents.
It has been assumed previously that a close relation exists between neutrophil activation and survival in that agents which serve to prime neutrophils for enhanced functional responsiveness to secretagogue signals or directly activate these cells also increase functional longevity by delaying the onset of constitutive apoptosis. We have shown that while LTD4, and to a lesser extent LTB4, were potent stimuli for elevation of [Ca2+]i in freshly isolated human eosinophils, neither LTB4, nor the cysteinyl LTs LTC4 and LTD4 had any influence on the basal rate of apoptosis. Although it has been reported that cysteinyl LTs can delay apoptosis of eosinophils isolated from asthmatic patients (Lee et al., 2000), our data suggest that the LTs, despite elevating [Ca2+]i, do not prolong survival of eosinophils isolated from normal or mildly atopic individuals. Furthermore, analyses of the documented effects of various agonists on granulocyte activation reveals that there may be no unifying hypothesis to correlate these two events, that is that the mechanisms leading to granulocyte activation do not appear to be critical for delaying granulocyte apoptosis. This is supported by the observation that TNFα, a potent neutrophil priming agent, induces neutrophil apoptosis at early time points (<8 h) while delaying this process at later times (<20 h) (Murray et al., 1997). Moreover, while nitric oxide (NO) donors induce neutrophil apoptosis (Blaylock et al., 1998; Fortenberry et al., 1998; Ward et al., 2000; Taylor et al., 2001), these reagents also have the capacity to downregulate other functions such as shape change and chemotaxis (Ward et al., 2000). Interestingly, the ultimate biological effect of NO on apoptosis and function may depend on the source and concentration of NO, and the cell under investigation (Taylor et al., 2003). In addition, neutrophils exposed to agents that elevate cAMP are rendered less responsive to activation but exhibit a greater longevity (Rossi et al., 1995; Martin et al., 2001). This is further supported by our demonstration that preincubation of neutrophils with PAF (1 μM) prior to stimulation with fMLP (100 nM), a protocol that results in a dramatic upregulation of fMLP-induced superoxide anion release (Ward et al., 2000), has no effect on neutrophil apoptosis following 6 and 20 h in culture (data not shown). These data offer the possibility that more rational and selective anti-inflammatory therapies may be designed and further underline the importance of elucidating and understanding the mechanisms of activation and signalling in response to local LT generation and other inflammatory agents present at sites of inflammation.
Acknowledgments
This work was supported by The National Asthma Campaign (01/042), The Medical Research Council Program Grant (G9016491), The Wellcome Trust (035662) and by Merck Sharp and Dohme. JM and CW are joint first authors.
Abbreviations
- BLT
LTB4 receptor
- [Ca2+]I
intracellular free Ca2+ concentration
- Cys-LT
cysteinyl leukotriene
- db-cAMP
dibutyryl-cAMP
- fMLP
formyl-leucyl-phenylalanine
- GM-CSF
granulocyte/macrophage colony-stimulating factor
- LPS
lipopolysaccharide
- PAF
platelet-activating factor
- PPAR
peroxisome-proliferator activator receptor
References
- BLAYLOCK M.G., CUTHBERTSON B.H., GALLEY H.F., FERGUSON N.R., WEBSTER N.R. The effect of nitric oxide and peroxynitrite on apoptosis in human polymorphonuclear leukocytes. Free Radic. Biol. Med. 1998;25:748–752. doi: 10.1016/s0891-5849(98)00108-7. [DOI] [PubMed] [Google Scholar]
- CHINETTI G., GRIGLIO S., ANTONUCCI M., TORRA I.P., DELERIVE P., MAJD Z., FRUCHART J.C., CHAPMAN J., NAJIB J., STAELS B. Activation of proliferator-activated receptors α and γ induces apoptosis of human monocyte-derived macrophages. J. Biol. Chem. 1998;273:25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- COLOTTA F., RE F., POLENTARUTTI N., SOZZANI S., MANTOVANI A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- DAHINDEN C.A., ZINGG J., MALY F.E., DE WECK A.L. Leukotriene production in human neutrophils primed by recombinant human granulocyte/macrophage colony-stimulating factor and stimulated with the complement component C5A and FMLP as second signals. J. Exp. Med. 1988;167:1281–1295. doi: 10.1084/jem.167.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVCHAND P.R., KELLER H., PETERS J.M., VAZQUEZ M., GONZALEZ F.J., WAHLI W. The PPARα–leukotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- FADOK V.A., BRATTON D.L., KONOWAL A., FREED P.W., WESTCOTT J.Y., HENSON P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORD-HUTCHINSON A.W., BRAY M.A., DOIG M.V., SHIPLEY M.E., SMITH M.J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980;286:264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- FORTENBERRY J.D., OWENS M.L., BROWN M.R., ATKINSON D., BROWN L.A. Exogenous nitric oxide enhances neutrophil cell death and DNA fragmentation. Am. J. Respir. Cell. Mol. Biol. 1998;18:421–428. doi: 10.1165/ajrcmb.18.3.2875. [DOI] [PubMed] [Google Scholar]
- FUJIHARA S., WARD C., DRANSFIELD I., HAY R.T., UINGS I.J., HAYES B., FARROW S.N., HASLETT C., ROSSI A.G. Inhibition of nuclear factor-κB activation un-masks the ability of TNF-α to induce human eosinophil apoptosis. Eur. J. Immunol. 2002;32:457–466. doi: 10.1002/1521-4141(200202)32:2<457::AID-IMMU457>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- GIEMBYCZ M.A., LINDSAY M.A. Pharmacology of the eosinophil. Pharmacol. Rev. 1999;51:213–340. [PubMed] [Google Scholar]
- GOMPERTZ S., STOCKLEY R.A. Inflammation–role of the neutrophil and the eosinophil. Semin. Respir. Infect. 2000;15:14–23. doi: 10.1053/srin.2000.0150014. [DOI] [PubMed] [Google Scholar]
- HASLETT C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am. J. Respir. Crit. Care Med. 1999;160:S5–S11. doi: 10.1164/ajrccm.160.supplement_1.4. [DOI] [PubMed] [Google Scholar]
- HEBERT M-J., TAKANO T., HOLTHÖFER H., BRADY H.R. Sequential morphologic events during apoptosis of human neutrophils. Modulation by lipoxygenase-derived eicosanoids. J. Immunol. 1996;157:3105–3115. [PubMed] [Google Scholar]
- KAMOHARA M., TAKASAKI J., MATSUMOTO M., SAITO T., OHISHI T., ISHII H., FURUICHI K. Molecular cloning and characterization of another leukotriene B4 receptor. J. Biol. Chem. 2000;275:27000–27004. doi: 10.1074/jbc.C000382200. [DOI] [PubMed] [Google Scholar]
- KITCHEN E., ROSSI A.G., CONDLIFFE A.M., HASLETT C., CHILVERS E.R. Demonstration of reversible priming of human neutrophils using platelet-activating factor. Blood. 1996;88:4330–4337. [PubMed] [Google Scholar]
- LAWRENCE T., WILLOUGHBY D.A., GILROY D.W. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunol. 2002;2:787–795. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- LEE A., WHYTE M.K.B., HASLETT C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J. Leukoc. Biol. 1993;54:283–288. [PubMed] [Google Scholar]
- LEE E., LINDO T., JACKSON N., MENG-CHOONG L., REYNOLDS P., HILL A., HASWELL M., JACKSON S., KILFEATHER S. Reversal of human neutrophil survival by leukotriene B4 receptor blockade and 5-lipoxygenase and 5-lipoxygenase activating protein inhibitors. Am. J. Respir. Crit. Care Med. 1999;160:2079–2085. doi: 10.1164/ajrccm.160.6.9903136. [DOI] [PubMed] [Google Scholar]
- LEE E., ROBERTSON T., SMITH J., KILFEATHER S. Leukotriene receptor antagonists and synthesis inhibitors reverse survival in eosinophils of asthmatic individuals. Am. J. Respir. Crit. Care Med. 2000;161:1881–1886. doi: 10.1164/ajrccm.161.6.9907054. [DOI] [PubMed] [Google Scholar]
- LEFF A.R. Role of leukotrienes in bronchial hyperresponsiveness and cellular responses in airways. Am. J. Respir. Crit. Care Med. 2000;161:S125–S132. doi: 10.1164/ajrccm.161.supplement_1.ltta-25. [DOI] [PubMed] [Google Scholar]
- LIN A.H., MORTON D.R., GORMAN R.R. Acetyl glyceryl ether phosphorylcholine stimulates leukotriene B4 synthesis in human polymorphonuclear leukocytes. J. Clin. Invest. 1982;70:1058–1065. doi: 10.1172/JCI110693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Y., COUSIN J.M., HUGHES J., VAN DAMME J., SECKL J.R., HASLETT C., DRANSFIELD I., SAVILL J., ROSSI A.G. Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J. Immunol. 1999;162:3639–3646. [PubMed] [Google Scholar]
- MARTIN M.C., DRANSFIELD I., HASLETT C., ROSSI A.G. Cyclic AMP regulation of neutrophil apoptosis occurs via a novel protein kinase A-independent signaling pathway. J. Biol. Chem. 2001;276:45041–45050. doi: 10.1074/jbc.M105197200. [DOI] [PubMed] [Google Scholar]
- MEAGHER L.C., COUSIN J.M., SECKL J.R., HASLETT C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J. Immunol. 1996;156:4422–4428. [PubMed] [Google Scholar]
- MEAGHER L.C., SAVILL J.S., BAKER A., FULLER R.W., HASLETT C. Phagocytosis of apoptotic neutrophils does not induce macrophage release of thromboxane B2. J. Leukoc. Biol. 1992;52:269–273. [PubMed] [Google Scholar]
- MECKLENBURGH K., MURRAY J., BRAZIL T., WARD C., ROSSI A.G., CHILVERS E.R. Role of neutrophil apoptosis in the resolution of pulmonary inflammation. Monaldi Arch. Chest Dis. 1999;54:345–349. [PubMed] [Google Scholar]
- MURRAY J., BARBARA J.A.J., DUNKLEY S.A., LOPEZ A.F., VAN OSTADE X., CONDLIFFE A.M., DRANSFIELD I., HASLETT C., CHILVERS E.R. Regulation of neutrophil apoptosis by tumor necrosis factor-α: requirement for TNFR55 and TNFR75 for induction of apoptosis in vitro. Blood. 1997;90:2772–2783. [PubMed] [Google Scholar]
- O'FLAHERTY J.T., ROSSI A.G. 5-Hydroxyicosatetraenoate stimulates neutrophils by a stereospecific, G protein-linked mechanism. J. Biol. Chem. 1993;268:14708–14714. [PubMed] [Google Scholar]
- ROSSI A.G., COUSIN J.M., DRANSFIELD I., LAWSON M.F., CHILVERS E.R., HASLETT C. Agents that elevate cAMP inhibit human neutrophil apoptosis. Biochem. Biophys. Res. Commun. 1995;217:892–899. doi: 10.1006/bbrc.1995.2855. [DOI] [PubMed] [Google Scholar]
- ROSSI A.G., HASLETT C.Inflammation, cell injury and apoptosis Lung Biology in Health and Disease 1998New York: Marcel-Dekker; 9–24.ed. Lenfant, C. pp [Google Scholar]
- ROSSI A.G., HASLETT C., HIRANI N., GREENING A.P., RAHMAN I., METZ C.N., BUCALA R., DONNELLY S.C. Human circulating eosinophils secrete macrophage migration inhibitory factor (MIF). Potential role in asthma. J. Clin. Invest. 1998;101:2869–2974. doi: 10.1172/JCI1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSSI A.G., MACINTYRE D.E., JONES C.J.P., MCMILLAN R.M. Stimulation of human polymorphonuclear leukocytes by leukotriene B4 and platelet-activating factor: an ultrastructural and pharmacological study. J. Leukoc. Biol. 1993;53:117–125. doi: 10.1002/jlb.53.2.117. [DOI] [PubMed] [Google Scholar]
- SAMUELSSON B., DAHLEN S.E., LINDGREN J.A., ROUZER C.A., SERHAN C.N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- SAVILL J.S., WYLLIE A.H., HENSON J.E., WALPORT M.J., HENSON P.M., HASLETT C. Macrophage phagocytosis of ageing neutrophils in inflammation. Programmed cell death in the neutrophil leads to recognition by macrophages. J. Clin. Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERHAN C.N., PRESCOTT S.M. The scent of a phagocyte: advances on leukotriene B4 receptors. J. Exp. Med. 2000;192:F5. doi: 10.1084/jem.192.3.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOWELL H.J., PETTIPHER E.R., CHENG J.B., BRESLOW R., CONKLYN M.J., FARRELL C.A., HINGORANI G.P., SALTER E.D., HACKMAN B.C., WIMBERLY D.J., DOHERTY N.S., MELVIN L.S., REITER L.A., BIGGERS M.S., KOCK K. The in vitro and in vivo pharmacologic activity of the potent and selective leukotriene B4 receptor antagonist CP-105696. J. Pharmacol. Exp. Ther. 1995;273:176–184. [PubMed] [Google Scholar]
- STANKOVA J., TURCOTTE S., HARRIS J., ROLA-PLESZCZYNSKI M. Modulation of leukotriene B4 receptor-1 expression by dexamethasone: potential mechanism for enhanced neutrophil survival. J. Immunol. 2002;168:3570–3576. doi: 10.4049/jimmunol.168.7.3570. [DOI] [PubMed] [Google Scholar]
- STERN M., MEAGHER L., SAVILL J., HASLETT C. Apoptosis in human eosinophils. Programmed cell death in the eosinophil leads to phagocytosis by macrophages and is modulated by IL-5. J. Immunol. 1992;148:3543–3549. [PubMed] [Google Scholar]
- STERN M., SAVILL J., HASLETT C. Human mono-cyte-derived macrophage phagocytosis of senescent eosinophils undergoing apoptosis. Mediation by αvβ3/CD36/thrombospondin recognition mechanism and lack of phlogistic response. Am. J. Pathol. 1996;149:911–921. [Google Scholar]
- TAYLOR E.L., MEGSON I.L., HASLETT C., ROSSI A.G. Dissociation of DNA fragmentation from other hallmarks of apoptosis in nitric oxide-treated neutrophils: differences between individual nitric oxide donor drugs. Biochem. Biophys. Res. Commun. 2001;289:1229–1236. doi: 10.1006/bbrc.2001.6122. [DOI] [PubMed] [Google Scholar]
- TAYLOR E.L., MEGSON I.L., HASLETT C., ROSSI A.G. Nitric oxide; a key regulator of inflammatory cell apoptosis. Cell Death Differ. 2003;10:418–430. doi: 10.1038/sj.cdd.4401152. [DOI] [PubMed] [Google Scholar]
- WALSH G.M., SEXTON D.W., BLAYLOCK M.G., CONVERY C.M. Resting and cytokine-stimulated human small airway epithelial cells recognize and engulf apoptotic eosinophils. Blood. 1999;94:2827–2835. [PubMed] [Google Scholar]
- WALSH G.M., WILLIAMSON M.L., SYMON F.A., WILLARS G.B., WARDLAW A.J. Ligation of CD69 induces apoptosis and cell death in human eosinophils cultured with granulocyte-macrophage colony-stimulating factor. Blood. 1996;87:2815–2821. [PubMed] [Google Scholar]
- WARD C., CHILVERS E.R., LAWSON M.F., PRYDE J.G., FUJIHARA S., FARROW S.N., HASLETT C., ROSSI A.G. NF-κB activation is a critical regulator of human granulocyte apoptosis in vitro. J. Biol. Chem. 1999a;274:4309–4319. doi: 10.1074/jbc.274.7.4309. [DOI] [PubMed] [Google Scholar]
- WARD C., DRANSFIELD I., CHILVERS E.R., HASLETT C., ROSSI A.G. Pharmacological manipulation of granulocyte apoptosis: potential therapeutic targets. Trends Pharmacol. Sci. 1999b;20:503–509. doi: 10.1016/s0165-6147(99)01391-7. [DOI] [PubMed] [Google Scholar]
- WARD C., DRANSFIELD I., MURRAY J., FARROW S.N., HASLETT C., ROSSI A.G. Prostaglandin D2 and its metabolites induce caspase-dependent granulocyte apoptosis that is mediated via inhibition of IκBα degradation using a peroxisome proliferator-activated receptor-γ-independent mechanism. J. Immunol. 2002;168:6232–6243. doi: 10.4049/jimmunol.168.12.6232. [DOI] [PubMed] [Google Scholar]
- WARD C., WONG T.H., MURRAY J., RAHMAN I., HASLETT C., CHILVERS E.R., ROSSI A.G. Induction of human neutrophil apoptosis by nitric oxide donors: evidence for a caspase-dependent, cyclic-GMP-independent, mechanism. Biochem. Pharmacol. 2000;59:305–314. doi: 10.1016/s0006-2952(99)00329-9. [DOI] [PubMed] [Google Scholar]
- WHYTE M.K.B., MEAGHER L.C., HARDWICK S., SAVILL J.S., HASLETT C. Transient elevations of cytosolic free calcium retard subsequent apoptosis in neutrophils in vitro. J. Clin. Invest. 1993a;92:446–455. doi: 10.1172/JCI116587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHYTE M.K.B., MEAGHER L.C., MACDERMOT J., HASLETT C. Impairment of function in aging neutrophils is associated with apoptosis. J. Immunol. 1993b;150:5123–5134. [PubMed] [Google Scholar]
- YOKOMIZO T., IZUMI T., CHANG K., TAKUWA Y., SHIMIZU T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- YOKOMIZO T., KATO K., TERAWAKI K., IZUMI T., SHIMIZU T. A second leukotriene B4 receptor, BLT2: a new therapeutic target in inflammation and immunological disorders. J. Exp. Med. 2000;192:421–432. doi: 10.1084/jem.192.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YU K., BAYONA W., KALLEN C.B., HARDING H.P., RAVERA C.P., MCMAHON G., BROWN M., LAZAR M.A. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J. Biol. Chem. 1995;270:23975–23983. doi: 10.1074/jbc.270.41.23975. [DOI] [PubMed] [Google Scholar]