Abstract
Fluorescence techniques offer a way to circumvent several problems associated with many radioligand binding and functional assays and the need for large numbers of cells. Fluorescent ligands also offer the advantage of allowing real time direct visualisation of ligand – receptors interactions. A fluorescent analogue of CGP 12177 (BODIPY-TMR-CGP) has thus been evaluated as a β2-adrenoceptor ligand in CHO-K1 cells expressing the human β2-adrenoceptor.
Studies of 3H-cAMP accumulation showed that BODIPY-TMR-CGP stimulated an increase in cAMP accumulation and cyclic AMP response element (CRE)-mediated gene transcription with an EC50 of 21–28 nM. Both of these responses were antagonised by the selective β2-adrenoceptor antagonist ICI 118551.
Binding studies with 3H-CGP 12177, and functional studies of CRE-regulated gene transcription showed that the BODIPY-TMR-CGP interaction with the human β2-adrenoceptor is of very long duration.
Visualisation of the binding of BODIPY-TMR-CGP to single living mammalian cells was clearly demonstrated by confocal microscopy and showed that this ligand was able to selectively label cell surface β2-adrenoceptors in living CHO-K1 cells transfected with the human β2-adrenoceptor with an apparent KD of 27 nM. Studies with cells expressing a β2-adrenoceptor–green fluorescent protein (GFP) fusion protein provided further strong evidence that BODIPY-TMR-CGP was binding to the β2-adrenoceptor.
BODIPY-TMR-CGP is therefore a long-acting fluorescent β2-adrenoceptor agonist that can be used to label β2-adrenoceptors in the plasma membrane of living cells.
Keywords: BODIPY-TMR-CGP, β2-adrenoceptor, fluorescent ligands, reporter-genes, single-cell imaging, long-acting agonist
Introduction
Fluorescent techniques offer a way to circumvent several of the problems associated with radioligand binding assays and the associated need for large numbers of cells with relatively high levels of receptor expression (McGrath et al., 1996). Fluorescent ligands have the advantage of allowing direct visualisation of the receptors using microscopy and hence the actual location of receptors within single living cells can be followed over time. Furthermore, agonists that retain their agonist efficacy after tagging with a fluorophore offer an opportunity to study the dynamics of the receptor activation process (Harikumar et al., 2002). The aim of this study was to characterise a fluorescent ligand for the study of β2-adrenoceptors.
CGP 12177 was initially described as a high-affinity nonselective antagonist at β1- and β2-adrenoceptors (Lang & Lemmer, 1985; Thede-Reynolds et al., 1986; Haddad et al., 1987; Steinkraus et al., 1990). More recently, however, CGP 12177 has been shown to have partial agonist activity at β1- (Pak & Fishman, 1996; Konkar et al., 2000; Lowe et al., 2002), β2- (Pak & Fishman, 1996; Baker et al., 2002) and β3-adrenoceptors (Mohell & Dicker, 1989; Muzzin et al., 1992; D'Allaire et al., 1995). Unlike many β-adrenoceptor ligands, the high hydrophilicity of CGP 12177 means that it primarily binds to cell surface β-adrenoceptors (Staehelin et al., 1983; Hertel et al., 1983a,1983b). As a result of this unique property, 3H-CGP 12177 has been frequently used to measure the levels of cell surface β-adrenoceptors and to study β-adrenoceptor internalisation (Wilkinson & Wilkinson, 1985; Lacasa et al., 1986; Kallay et al., 1991; Fabbri et al., 2001).
As CGP 12177 almost exclusively labels cell surface receptors, it is an excellent candidate for the generation of fluorescent probes to study β-adrenoceptors. Several different fluorescent bordifluoropyrromethane (BODIPY) derivatives of CGP 12177 have been synthesized (Heithier et al., 1994). One variant of BODIPY-CGP 12177 has been evaluated in (sf9) insect cells expressing the human β2-adrenoceptor at a high level (Heithier et al., 1994). Another BODIPY analogue of CGP 12177 has also been used, with limited success, to visualise β1-adrenoceptors on the surface of primary rat cerebral cortical astrocytes (Thorlin et al., 2000).
In view of our recent observation that CGP 12177 is a potent stimulant of gene transcription at the human β2-adrenoceptor (Baker et al., 2002), we have investigated the agonist properties of another fluorescent analogue, BODIPY-TMR-CGP. Here we report that BODIPY-TMR-CGP (Figure 1) is a long-acting agonist at the human β2-adrenoceptor expressed in CHO-K1 cells, when measured at the level of both cAMP accumulation and cAMP response element (CRE)-mediated reporter gene transcription. We also show that this compound can be used to visualise its real-time binding to receptors at the single-cell level in living mammalian cells. A preliminary account of part of this work has been presented to the British Pharmacological Society (Baker et al., 2001).
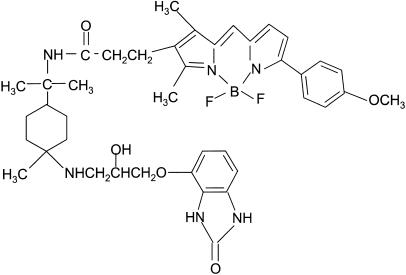
Figure 1.
Structure of BODIPY-TMR-CGP.
Methods
Materials
Cell culture reagents were from Sigma Chemicals (Poole, Dorset, U.K.) except Fetal calf serum which was from PAA Laboratories (Teddington, Middlesex, U.K.). 3H-adenine, 3H-CGP 12177 and 14C-cAMP were obtained from Amersham International (Buckinghamshire, U.K.); ICI 118551, CGP 12177 from Tocris Cookson (Avonmounth, Bristol, U.K.) and BODIPY-TMR-CGP (BODIPY-TMR-(±)-CGP 12177) from Molecular Probes (Eugene, OR, U.S.A.). All other reagents were supplied by Sigma Chemicals (Poole, Dorset, U.K.).
Cell culture
Chinese hamster ovary cells stably expressing both the human β2-adrenoceptor (CHO-β2) and a reporter gene, secreted placental alkaline phosphatase (SPAP), under the transcriptional control of a six CRE promoters (McDonnell et al., 1998) were grown at 37°C in Dulbecco's modified Eagle's medium/Nutrient mix F12 (DMEM/F12) containing 10% fetal calf serum (FCS) and 2 mM L-glutamine in a humidified 5% CO2 : 95% air atmosphere. A CHO-K1 cells line expressing a C-terminal green fluorescent protein (GFP) tagged human β2-adrenoceptor was also used (Baker et al., 2002). CHO-K1 cells (not transfected), grown in identical conditions, were used as controls as appropriate.
Cyclic AMP accumulation and CRE-mediated gene transcription (SPAP)
3H-cyclic AMP accumulation was measured in 3H-adenine-labelled CHO-β2 cells as described previously (Baker et al., 2002). Briefly, prelabelled cells were incubated at 37°C for 15 min with IBMX (isobutylmethylxanthine, 100 μM) and antagonists before agonists were added for a further 10 min. The reaction was then terminated by the addition of 50 μl concentrated HCl and 3H-cyclic AMP separated by Dowex-alumina chromatography.
CRE-mediated reporter gene production (secreted placental alkaline phosphatase, SPAP) was measured as previously reported (Baker et al., 2002). Briefly, cells were incubated with antagonists for 30 min prior to incubation with agonists for a further 5 h at 37°C. Secreted SPAP activity was quantified by following the colour change caused by the hydrolysis of p-nitrophenol phosphate (PNPP) (Baker et al., 2002).
3H-CGP 12177 whole-cell binding
Cells were grown to confluence in 96-well plates. The media were removed and replaced with 100 μl DMEM/F12 or DMEM/F12 containing competing drugs. 3H-CGP 12177 in 100 μl was then added immediately, or when stated, and the plates incubated at 37°C in 5% CO2 for 1–3 h as stated. Nonspecific binding was determined using 100 nM ICI 118551. The media and drugs were then removed, each well washed with 2 × 200 μl phosphate-buffered saline and 200 μl Microscint 20 (Packard Bioscience, Pangbourne, Berks, U.K.) added to each well. Radioactivity was then determined using a Topcount. Protein was determined by the method of Lowry et al. (1951).
Confocal microscopy
Confocal microscopy was performed using a Zeiss LSM510 laser scanning microscope with a Zeiss 40 × 1.3NA oil immersion lens. Cells were grown in eight-well borosilicate chambered-coverglass plates (Nalgene Nunc International, Fisher Scientific, Loughborough, U.K.) in 400 μl DMEM-F12 media containing 10% FCS and 2 mM glutamine. Prior to experimentation, the media were replaced with 400 μl Hepes-buffered saline (HBS). BODIPY-TMR-CGP (in HBS) was added for 10 min (unless otherwise stated) at room temperature and the cells washed with HBS before imaging (1024 × 1024 pixels; averaging at 16 frames). Competing ligands were added for 30 min (unless otherwise stated) prior to addition of BODIPY-TMR-CGP for a further 10 min in either the continued presence of the competing ligand or following wash out of the ligands. The binding of BODIPY-TMR-CGP to cells was monitored using single-line excitation at 543 nm (emission 560 nm long pass filter). Where CHO-β2-GFP expressing cells were imaged with BODIPY-TMR-CGP, 543 nm and argon 488 nm lasers were used with multi-tracking. This allowed the sample to be illuminated with one laser at a time in order to avoid any bleed through of the 488 nm laser causing excitation of the BODIPY-TMR-CGP. For images taken at 37°C, the cells were grown on a glass coverslips that were placed in a specially designed holder. The stage and microscope objective were then maintained at 37°C throughout the experiment.
Approximately 140 cells were viewed in each image. On each experimental day, a control well (50 nM BODIPY-TMR-CGP) was imaged. The maximal image brightness was then set to a value greater than that achieved with the control well, and the confocal settings (laser intensity, digital values for image brightness, background and contrast) were then kept constant for the rest of that experimental day. Quantitative analysis of the effects of different drugs on the binding of BODIPY-TMR-CGP in a given experiment was performed by measuring the average pixel intensities of each image captured. The Zeiss software provides the frequency of pixels recorded at each of 256 grey-scale intensities for each image taken. The total image intensity (arbitrary units; calculated as the sum of the product of frequency × grey-scale intensity for each grey-scale intensity value between 0 and 255) was then calculated from this frequency distribution. The average pixel intensity was then calculated from this value. For any given experimental condition a minimum of three different cell preparations and drug dilutions were used. In the text, ‘n' refers to the number of different complete experiments performed.
Data analysis
Agonist and antagonist concentration – response curves were fitted to a four-parameter logistic equation through computer-assisted nonlinear regression using the programme Prism 2 as previously described (Hopkinson et al., 2000). Antagonist dissociation constants were assessed at fixed antagonist concentrations (assuming competitive antagonism) by observing the shift in the agonist concentration–response curve using the equation:
 |
where DR (dose-ratio) is the ratio of the concentrations of agonist required to produce an identical response in the presence and absence of antagonist, [A] is the concentration of antagonist and KD is the antagonist dissociation constant (Arunlakshana & Schild, 1959). Where appropriate partial agonist dissociation constants were estimated according to the method of Stephenson (1956). Relative agonist efficacy was estimated in terms of the maximal response (cyclic AMP or SPAP) compared with the maximal response to isoprenaline measured in the same experiment.
BMAX and KD values for the specific binding of 3H-CGP 12177 were determined from a fit of the saturation data according to the equation: specific binding=BMAX × A/(A+KD). Ki values of competing drugs were determined from the concentration required to inhibit specific binding by 50% (IC50) using the expression Ki=IC50/(1+A/KD) where A is the concentration of 3H-CGP 12177 and KD is its dissociation constant.
In the case of the measurement of binding of BODIPY-TMR-CGP to intact cells using confocal microscopy, the image intensity values obtained at each concentration of fluorescent ligand were fitted to the following equation:
where BMAX is the maximal fluorescent intensity, B is the concentration of BODIPY-TMR-CGP, M is the slope of the nonspecific binding component and C is the intercept with the Y-axis (reflecting the background image intensity).
For competition experiments, Ki values of competing drugs were determined from the concentration required to inhibit the specific fluorescent binding of BODIPY-TMR-CGP by 50% (IC50) using the expression Ki=IC50/(1+A/KD), where A is the concentration of BODIPY-TMR-CGP and KD is its dissociation constant.
All data are presented as mean±s.e.m. The ‘n' in the text refers to the number of separate experiments.
Results
Functional responses of BODIPY-TMR-CGP
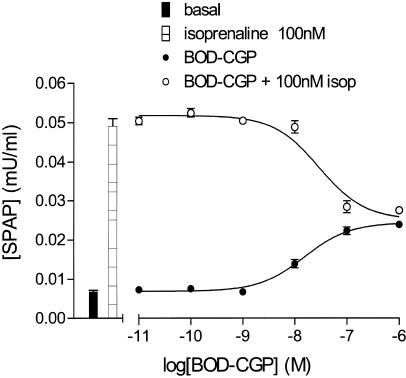
BODIPY-TMR-CGP appeared as a partial agonist in this system stimulating an increase in CRE-mediated SPAP production from CHO-β2 cells in a concentration-dependent manner (EC50=20.6 nM, maximal response 45% of that obtained with isoprenaline; Figure 2a; Table 1 ). This is similar to the data obtained previously with the parent compound CGP 12177 (Baker et al., 2002; Table 1). This BODIPY-TMR-CGP-induced response was antagonised by ICI 118551 (apparent KD=1.5 nM; Table 1; Figure 2b). This value was similar to that obtained when isoprenaline was used as agonist in the same series of experiments (ICI 118551 KD=1.32± 0.29 nM; n=6) and that reported previously (0.9 nM; Baker et al., 2002). Preincubation with a fixed concentration of BODIPY-TMR-CGP antagonised the isoprenaline concentration – response curve in a manner consistent with its partial agonist actions and gave an apparent KD value of 3.9 nM (Figure 2c; Table 1). Unexpectedly, this was an order of magnitude lower than the EC50 obtained for BODIPY-TMR-CGP-stimulated SPAP production (P<0.05; unpaired t-test). However, when increasing concentrations of BODIPY-TMR-CGP were added simultaneously with a fixed concentration of isoprenaline (100 nM), the response to isoprenaline was gradually reduced down to a maximum similar to that obtainable with BODIPY-TMR-CGP alone (Figure 3). The IC50 value for this inhibitory effect was 30.3±5.1 nM (n=4), which was similar to the EC50 value for BODIPY-TMR-CGP (Table 1) suggesting that both drugs are competing at the same site.
Figure 2.
(a) CRE-mediated SPAP production in response to isoprenaline and BODIPY-TMR-CGP obtained from CHO-β2 cells. Data points are mean±s.e.m. (triplicate determinations) from a single experiment and are representative of 15 separate experiments. Bar represents the SPAP production from unstimulated cells. (b) SPAP production induced by BODIPY-TMR-CGP in the presence and absence of 30 nM ICI 118551. Points represent mean±s.e.m. of triplicate determinations. The bars show basal SPAP production and that in response to 30 nM ICI 118551 and this single experiment is representative of nine separate determinations. (c) SPAP production induced by isoprenaline in the presence and absence of 100 nM BODIPY-TMR-CGP. Bars represent basal response and SPAP production in the presence of 100 nM BODIPY-TMR-CGP. Data points are mean±s.e.m. (triplicate determinations) and this single experiment is representative of six separate determinations.
Table 1.
Concentration–response parameters for BODIPY-TMR-CGP and CGP 12177-stimulated cAMP accumulation and gene transcription and interactions with isoprenaline and ICI 118551
| *CGP 12177 | BODIPY-TMR-CGP | n | |
|---|---|---|---|
| cAMP accumulation | |||
| EC50 | 1.26 nM | 28.15±3.98 nM | 3 |
| EMAX (% max isoprenaline) | 6.50% | 3.50±0.08% | 3 |
| KD (antagonism of isoprenaline) | 0.17 nM | 2.46±0.70 nM | 4 |
| KD ICI 118551 | 1.45 nM | 2.20±0.99 nM | 3 |
| CRE-mediated gene transcription (SPAP) | |||
| EC50 | 0.22 nM | 20.60±2.14 nM | 44 |
| EMAX (% max isoprenaline) | 59.8% | 45.2±1.8% | 44 |
| KD (antagonism of isoprenaline) | 1.35 nM | 3.86±0.84 nM | 12 |
| KD ICI 118551 | 0.31 nM | 1.50±0.38 nM | 9 |
Data taken from Baker et al. (2002).
CGP 12177=parent compound. Values (EC50 and EMAX) are mean±s.e.m. for cAMP accumulation or CRE-mediated gene transcription from n separate experiments. EMAX values are expressed as a percentage of the maximal (10 μM) isoprenaline response measured in the same experiment. KD values were estimated from the rightward shift of the concentration – response curve for isoprenaline obtained in the presence of a fixed concentration of BODIPY-TMR-CGP or CGP 12177 (Figure 2c). The KD values for ICI 118551 were obtained from parallel shifts of concentration–response curves (Figure 2b) as described under Methods.
Figure 3.
SPAP production induced by BODIPY-TMR-CGP in the presence and absence of 100 nM isoprenaline. Bars represent basal response and SPAP production in the presence of 100 nM isoprenaline. Data points are mean±s.e.m. (triplicate determinations) and this single experiment is representative of four separate experiments.
At the level of cAMP, BODIPY-TMR-CGP caused a much smaller β2-adrenoceptor-mediated increase in 3H-cAMP accumulation (EC50=28.1 nM, Table 1), a similar finding to its parent compound. Preincubation with BODIPY-TMR-CGP was also able to antagonise the isoprenaline-induced cAMP accumulation, causing a parallel shift of the isoprenaline concentration–response curve to yield an apparent KD value for BODIPY-TMR-CGP of 2.5 nM (Table 1). Thus again, there was a 10-fold difference between the EC50 and KD values for BODIPY-TMR-CGP.
3H-CGP 12177 whole-cell binding
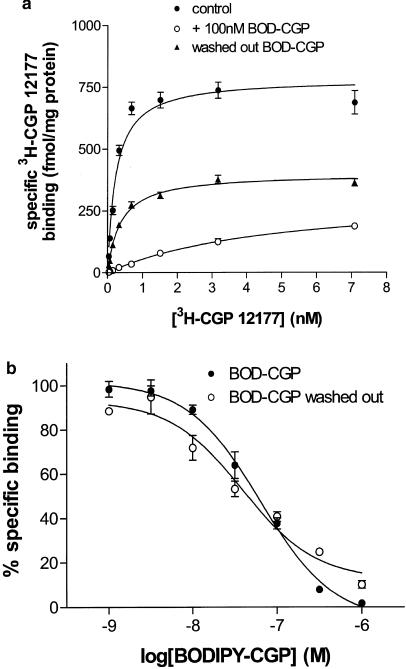
The specific binding of 3H-CGP 12177 to intact CHO-β2 cells was best described by a single site with a KD value of 0.20± 0.02nM (n=4) and a Bmax of 696.1±75.7fmol mg protein−1 (n=4, Figure 4a). This binding was inhibited by ICI 118551 with a Ki of 0.54±0.02 nM (n=4), consistent with the values obtained in both functional studies above. BODIPY-TMR-CGP was also able to inhibit specific binding of 3H-CGP 12177 yielding an apparent Ki of 15.84±2.97 nM (n=4; Figure 4b). This was consistent with the EC50 values obtained for BODIPY-TMR-CGP in both cAMP accumulation and gene transcription responses, but very different from the KD value estimated from shifts of isoprenaline concentration–response curves for these functional studies (Table 1). To determine whether a true equilibrium was obtained between 3H-CGP 12177 and BODIPY-TMR-CGP, the kinetics of BODIPY-TMR-CGP displacement were examined. The saturation curve for 3H-CGP 12177 in the presence of 100 nM BODIPY-TMR-CGP did not reach the same Bmax as the control curve, indicative of a noncompetitive interaction (Figure 4a). Furthermore, following an hour preincubation with 100 nM BODIPY-TMR-CGP, and extensive washing over a period of an hour, before the addition of the 3H-CGP 12177 for a further 2 h, the Bmax for 3H-CGP 12177 binding (360.2± 37.3 fmol mg protein−1; n=4) was still substantially reduced (52.5±5.2% of control; Figure 4a). This was despite the fact that the KD for 3H-CGP 12177 was similar to the control situation. This indicates that BODIPY-TMR-CGP had effectively removed a significant proportion of the receptor population, probably as a consequence of very slow dissociation of BODIPY-TMR-CGP from the receptor. In keeping with this observation, a similar apparent KD for BODIPY-TMR-CGP was obtained from the displacement of 3H-CGP 12177 even when the BODIPY-TMR-CGP was extensively washed out over the period of an hour before the addition of the radioligand (Ki=9.09±1.66 nM, n=4; Figure 4b).
Figure 4.
(a) Specific binding of 3H-CGP 12177 to CHO-β2 cells in control conditions (absence of BODIPY-TMR-CGP; 2 h incubation, filled circles), in the presence of 100 nM BODIPY-TMR-CGP (BOD-CGP, open circles) added 1 h before 3H-CGP 12177 (2 h subsequent incubation in the presence of both ligands), and following wash out of the BODIPY-TMR-CGP before addition of 3H-CGP 12177 (triangles). In this latter case, BODIPY-TMR-CGP was incubated for 1 h, then each well washed twice with 200 μl phosphate-buffered saline. The cells were then incubated in media for a further hour in order to give time for any BODIPY-TMR-CGP to dissociate from the receptor. The cells were then washed twice again before the 3H-CGP 12177 was added for a 2 h incubation. Nonspecific binding was determined in the presence of 100 nM ICI 118551. Data points represent mean±s.e.m. for triplicate values and this single experiment is representative of four separate experiments. Curves were fitted by nonlinear regression as described under Methods. Fitted parameters in this experiment for BMAX and KD were: (filled circles) 784.7 fmol mg protein−1, 0.23 nM; (open circles) 315.9 fmol mg protein−1, 4.8 nM; (triangles) 313.1 fmol mg protein−1, 0.36 nM. (b) Inhibition of the specific binding of 0.3 nM 3H-CGP 12177 by BODIPY-TMR-CGP (BOD-CGP). Closed symbols show BODIPY-TMR-CGP and 3H-CGP 12177 after 2 h incubation. Open symbols are where the BODIPY-TMR-CGP was washed out as described in (a) before incubation with radioligand. Data points represent mean±s.e.m. for triplicate determinations in a single experiment and is representative of four experiments.
Demonstration of long duration of action in functional studies – CRE-mediated gene transcription
In order to confirm that this long duration of action was also seen in functional studies, the agonist (isoprenaline or BODIPY-TMR-CGP) was added to the CHO-β2 cells and incubated for 10 min at 37°C. The agonist and media were removed from the wells, the cells washed with 1 ml fresh serum-free media, and a further fresh 1 ml serum-free media added to each well. This process should have reduced the free ligand concentration by 10,000 fold. The plates were then incubated for 4 h 50 min at 37°C and the CRE-SPAP gene transcription as described previously (Baker et al., 2002). Under these conditions, by removing isoprenaline after the first 10 min of the 5 h incubation, reporter gene transcription was not seen at original concentrations below 1 μM (n=4). However, the response to BODIPY-TMR-CGP was little altered after wash out of the ligand and the response indices (EC50 29.82±4.25 nM, EMAX=86.6±6.1% of that achieved when BODIPY-TMR-CGP response was not washed out at 10 min, n=4) were similar to when BODIPY-TMR-CGP remained present for the full 5 h. This confirms that whereas isoprenaline can be washed out of the system and thus limit the gene transcription response, BODIPY-TMR-CGP remained bound to the receptor.
Lack of β-agonist-mediated 3H-cAMP accumulation and gene transcription responses in native CHO-K1 cells
There was no increase in either the 3H-cAMP accumulation or CRE-mediated gene transcription in response to isoprenaline or BODIPY-TMR-CGP in untransfected CHO-K1 cells or those transfected with the CRE-SPAP reporter and not the receptor (n=3). There was also no specific binding of 3H-CGP 12177 to untransfected CHO-K1 cells again confirming the absence of any other β-adrenergic receptors in these cells (n=4; data not shown). This confirms that the cAMP and SPAP responses to β2-agonists described above are dependent in this cell line upon the presence of the human β2-adrenoceptor.
Confocal microscopy
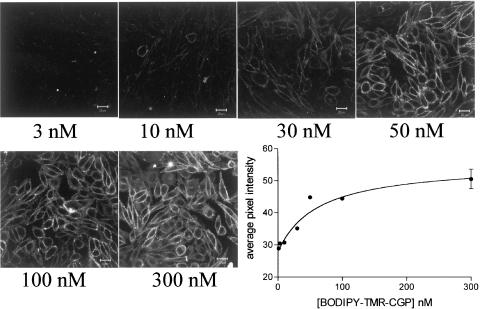
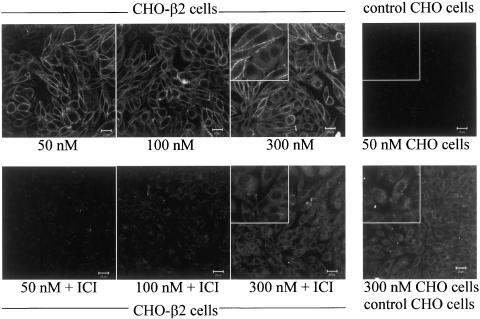
Following 10 min exposure, BODIPY-TMR-CGP (50 nM) was able to label the plasma membrane of CHO-β2 cells (Figure 5; n=83 wells). Concentrations of 30 nM and above were necessary to give clear membrane labelling with BODIPY-TMR-CGP (Figure 5; n=5 complete experiments). This membrane labelling was clearly seen irrespective of whether the cells were imaged in the presence, or following wash out, of the ligands (cf. Figures 5, 6, 7 and 8). Quantification of the image fluorescence by average pixel intensity gave KD values for BODIPY-TMR-CGP from the saturation curves very similar to those obtained from the other measurements above (KD=27.6±6.4 nM, n=4, Figure 5). Other experiments (data not shown) indicated that an incubation period of 7.5 min was necessary for clear binding to occur at 20°C, and that binding remained unchanged without evidence of internalisation at this temperature for at least 60 min (n=3).
Figure 5.
Confocal images and quantification of the binding of increasing concentrations (3–300 nM) of BODIPY-TMR-CGP (10 min exposure) to CHO-β2 cells. Data are from a single experiment and are representative of four separate experiments. The final panel shows the quantification of these data. Average pixel intensities were calculated from the grey-scale images as described under Methods. Values represent the mean of up to four separate images obtained in a single experiment. Similar data were obtained in three other experiments. Cells were imaged following washout of all ligands.
Figure 6.
Confocal images showing the effect of increasing concentrations of 30 min preincubation with (a) ICI 118551 or (b) CGP 12177 on the binding of 50 nM BODIPY-TMR-CGP to CHO-β2 cells (10 min) in the continued presence of ICI 118551 or CGP 12177. Images shown are from a single experiment. The final panel shows the quantification of these data. Average pixel intensities were calculated from the grey-scale images as described under Methods. Values represent the mean of up to four separate images obtained in a single experiment. Similar data were obtained in (a) three and (b) two other experiments. Cells were imaged following washout of all ligands.
Figure 7.
Confocal images showing the effects of 50–300 nM concentrations of BODIPY-TMR-CGP (10 min exposure) in CHO-β2 cells and control CHO-K1 cells. In CHO-β2 cells experiments were performed with and without 30 min pre-incubation with 100 nM ICI 118551. Inset images show a close up of a group of cells (from the centre of each image) at four times the magnification of the rest of the image. Images are from a single experiment that was representative of 155 cells in 10 different experiments. Cells were imaged following washout of all ligands.
Figure 8.
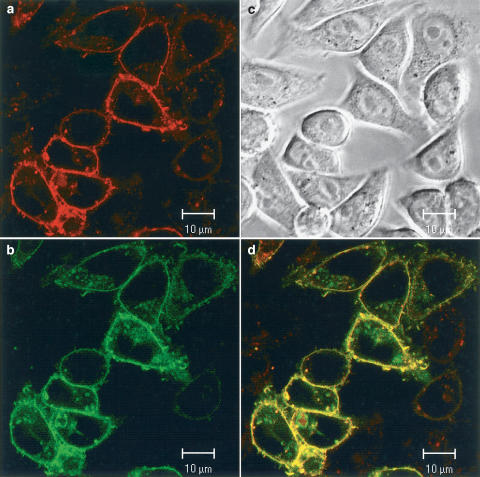
Binding of BODIPY-TMR-CGP to CHO-β2-GFP cells. In (b) the fluorescence monitored in the green GFP channel (488 nm) is shown. (a) Shows the binding of 50 nM BODIPY-TMR-CGP to the same cells as in (b) in the red channel (543 nm). (d) Shows an overlay of the two images (a and b) with colocalisation shown by the yellow pixels. (c) Shows the phase contrast image of these cells. In this case, the BODIPY-TMR-CGP has not been washed out before imaging.
Preincubation with increasing concentrations of either ICI 118551 or CGP 12177 was able to inhibit the binding of 50 nM BODIPY-TMR-CGP in CHO-β2 cells (Figure 6a; n=5 and Figure 6b; n=3). The Ki values obtained from average pixel intensities for the inhibition of BODIPY-TMR-CGP (50 nM) binding by ICI 118551 (2.38±0.48 nM, n=4, Figure 6a) and CGP 12177 (0.42±0.22 nM, n=3, Figure 6b) were also similar to those obtained for these compounds from both functional studies and 3H-CGP 12177 binding. Preincubation with isoprenaline (IC50∼0.1 μM, n=3, data not shown) was also able to inhibit the binding of BODIPY-TMR-CGP to CHO-β2 cells although the substantial isoprenaline-induced receptor internalisation that occurs with concentrations of 1 μM and above complicates interpretation of these data (Baker et al., 2002).
The nonspecific binding properties of BODIPY-TMR-CGP were also examined. BODIPY-TMR-CGP (50 nM) did not cause membrane labelling in control untransfected CHO-K1 cells (Figure 7; n=13); however, once exposed to 300 nM BODIPY-TMR-CGP a diffuse intracellular fluorescence was seen (Figure 7, n=4). CHO-β2 cells exposed to higher concentrations of BODIPY-TMR-CGP showed two distinct patterns of fluorescent labelling; membrane labelling and a diffuse cytoplasmic fluorescence (Figure 7, n=6). ICI 118551 (100 nM) was able to inhibit the binding of 50–300 nM BODIPY-TMR-CGP to the plasma membrane of cells, but did not affect its uptake into the cytoplasm (Figure 7, n=4). The parent fluorophore alone, BODIPY-TMR (50–300 nM), caused a similar cytoplasmic fluorescence to that of the high concentration BODIPY-TMR-CGP in both CHO-K1 cells and CHO-β2 cells (n=4, data not shown). This suggests that the diffuse intracellular fluorescence seen at higher concentrations of BODIPY-TMR-CGP is probably a consequence of a nonspecific, non-β2-adrenoceptor-mediated process due to fluorophore moiety.
In order to further confirm that BODIPY-TMR-CGP was labelling the β2-adrenoceptor, we have investigated the extent to which it binds to a GFP-tagged β2-adrenoceptor (Figure 8). The GFP-tagged β2-adrenoceptor (green) was localised to the plasma membrane in CHO-β2-GFP cells (Figure 8b) and was able to bind the red BODIPY-TMR-CGP (Figure 8a). When the two images are superimposed (yellow), the binding of BODIPY-TMR-CGP (red) can be seen to localise well with the β2-GFP fluorescence (Figure 8d, n=155 cells in 10 wells). It is clear that the cells with greater β2-GFP expression were able to bind more of the red BODIPY-TMR-CGP. Furthermore, these data show that there has been no receptor internalisation induced by BODIPY-TMR-CGP. Experiments performed at 37°C also showed no internalisation of the β2-GFP-tagged receptor following 60 min incubation with BODIPY-TMR-CGP (data not shown).
Discussion
Although CGP 12177 has traditionally been considered to be a high-affinity β1- and β2-adrenoceptor antagonist (Lang & Lemmer, 1985; Thede-Reynolds et al., 1986; Haddad et al., 1987; Steinkraus et al., 1990), it has also been shown to be a partial agonist in CHO-K1 cells transfected with the human β2-adrenoceptor (Pak & Fishman, 1996; Baker et al., 2002). In this study, we have shown that the fluorescent analogue BODIPY-TMR-CGP is also a partial agonist of both cAMP accumulation and CRE-mediated gene transcription. Although, the EC50 (circa 30 nM) of this fluorescent compound is 100-fold less than the EC50 for the parent compound (0.3 nM; Baker et al., 2002), the agonist efficacy is very similar. For example, BODIPY-TMR-CGP was able to produce a maximal stimulation of gene transcription which represented 45.2% of that obtained with the full agonist isoprenaline (cf. 59.8% with CGP 12177; Baker et al., 2002, see Table 1). Thus, although the addition of the relatively large fluorophore to the smaller CGP 12177 moiety has reduced the binding affinity of BODIPY-TMR-CGP, the functional ability of the molecule to stimulate agonist responses is largely maintained. Maximal cyclic AMP responses to BODIPY-TMR-CGP were lower (relative to isoprenaline) than those for CRE-mediated gene transcription, but this probably reflects the amplification of the signalling cascades leading from cAMP to the final reporter gene response (Rees et al., 1999; Hill et al., 2001). This amplification is also of similar magnitude to that seen with the parent compound (Baker et al., 2002).
The agonist effect of BODIPY-TMR-CGP was potently antagonised by the selective β2-adrenoeptor antagonist ICI 118551. No responses were seen to BODIPY-TMR-CGP in cells transfected with the CRE-SPAP reporter, but not the receptor. This confirms that the BODIPY-TMR-CGP effects were occurring entirely via the transfected β2-adrenoceptor.
Functional studies show that the EC50 value for BODIPY-TMR-CGP for both cAMP accumulation and CRE-SPAP reporter gene production is similar to the KD value obtained from inhibition of 3H-CGP-12177 binding (circa 15–25 nM) as would be predicted from classical receptor theory (Kenakin & Beek, 1984). The ability of increasing concentrations of BODIPY-TMR-CGP to antagonise the SPAP responses elicited by a fixed concentration of isoprenaline were also consistent with a KD value of 30 nM. However, the KD values determined for both cAMP and SPAP responses from the ability of a fixed concentration of BODIPY-CGP-TMR to shift the concentration–responses curves of isoprenaline to higher agonist concentrations suggested a much higher affinity. In these calculations, the methods of Aranlakshana & Schild (1959) and Stephenson (1956) assume that the antagonist is fully competitive and that a full equilibrium has been reached. The above discrepancy may therefore be because of the kinetic properties of the fluorescent ligand.
To investigate this anomaly further, binding experiments were conducted in CHO-β2 cells that were pretreated with BODIPY-TMR-CGP and this fluorescent compound washed out before the radioligand was added. The ability of BODIPY-TMR- CGP to inhibit 3H-CGP 12177 was virtually unchanged in this situation and the maximal saturation possible by the radioligand was markedly reduced. This suggests that the binding of BODIPY-TMR-CGP has an extremely slow dissociation from the receptor. This is further suggested by the gene transcription assay where the ligands have been washed out after an initial 10 min incubation. Where isoprenaline was present for only the first 10 min of the 5 h incubation period, gene transcription response was severely limited suggesting that a 10 min isoprenaline incubation is not sufficient to initiate gene transcription. However, the response to BODIPY-TMR-CGP was essentially unchanged suggesting that the drug remained bound to the receptor despite attempts to washout the drug.
An explanation for the apparent higher affinity of BODIPY-TMR-CGP in both functional studies is therefore that within the time course of both assays, BODIPY-TMR-CGP is effectively ‘irreversible' and thus acts to ‘remove' receptors from the system. The isoprenaline concentration response in the presence of BODIPY-TMR-CGP is therefore shifted further to the right than if true equilibrium was achieved, that is, BODIPY-TMR-CGP has effectively reduced the spare receptor reserve for the full agonist isoprenaline. This mode of long duration of action is very different from that of other long-acting β2-agonists, for example, salmeterol. The long-acting property of salmeterol is because of its irreversible anchoring to transmembrane region 4 of the receptor, the active head group is short acting and is thus dislodged by competing ligands in a usual competitive manner (Coleman et al., 1996; Green et al., 1996). BODIPY-TMR-CGP, on the other hand, appears to have a long duration of action within the active site itself and consequently is not readily removed by other competing ligands.
In order to investigate the potential use of BODIPY-TMR-CGP to image β2-adrenoceptors in living cells, we used confocal microscopy approaches with the CHO-β2 cells. Localised plasma membrane labelling was clearly seen with BODIPY-TMR-CGP at concentrations of 30 nM and above. Similar images were obtained whether taken in the continued presence of the fluorescent ligand or following wash out of this long-acting molecule. The concentration required to label cell surface β2-adrenoceptors was consistent with the EC50 values and binding studies above. The fluorescent binding of BODIPY-TMR-CGP (50 nM) was selective for the β2-adrenoceptor as it did not occur in untransfected cells, and was inhibited by concentrations of ICI 118551 close to the KD obtained in the functional assays described above. In this respect, BODIPY-TMR-CGP appears to have retained the useful hydrophilic cell surface binding properties of CGP 12177 and will be a potentially useful fluorescent probe for cell surface β2-adrenoceptors. BODIPY-TMR-CGP binding was also inhibited by both CGP 12177 and isoprenaline giving KD values similar to those achieved in previous radioligand binding studies (Baker et al., 2002) suggesting that BODIPY-TMR-CGP is acting at the same site on the receptor as the other ligands.
However, when higher concentrations of BODIPY-TMR-CGP (300 nM) were used, a widespread cytoplasmic fluorescence was seen in addition to the membrane fluorescence and this was not inhibited by ICI 118551. This intracellular fluorescence occurred in both the CHO-β2 cells and untransfected CHO-K1 cells. When used alone, the native BODIPY-TMR fluorophore (50–300 nM) also caused a similar diffuse cytoplasmic labelling in both CHO-β2 and CHO-K1 cells. This suggests that this cytoplasmic uptake of high concentration (300 nM) BODIPY-TMR-CGP and the native BODIPY-TMR alone is not a β2-adrenoceptor-mediated process and is occurring by another route. It is clear, however, from the present studies that concentrations of BODIPY-TMR-CGP up to 100 nM allow selective receptor labelling while minimising the nonreceptor-mediated cytoplasmic uptake.
In summary, we conclude BODIPY-TMR-CGP is a long-acting fluorescent β2-adrenoceptor ligand that can be clearly used to label β2-adrenoceptors in the plasma membrane of living mammalian cells. Functional studies of both cAMP accumulation and gene transcription reveal that this ligand is a partial agonist at human β2-adrenoceptors. These partial agonist properties, together with its long duration of action, should make this molecule an important tool to study both receptor activation at the single receptor level, and to probe for cell surface β2-adrenoceptors in single native human cells in health and disease.
Acknowledgments
JGB is a Wellcome Trust Clinical Training Fellow. The work was funded by Wellcome Trust Grants 057199 and 060949.
Abbreviations
- BODIPY-TMR
bordifluoropyrromethane-tetramethylrhodamine
- BODIPY-TMR-CGP (or BOD-CGP)
bordifluoropyrromethane-tetramethylrhodamine-(±)CGP 12177
- CRE
cyclic AMP response element
- GFP
green fluorescent protein
- SPAP
secreted placental alkaline phosphatase
References
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. Chemother. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER J.G., HALL I.P., HILL S.J.Pharmacology and direct visualization of the fluorescent β2-adrenoceptor ligand BODIPY-CGP 12177 in CHO cells transfected with the human β2-adrenoceptor Br. J. Pharmacol. 200113450p [Google Scholar]
- BAKER J.G., HALL I.P., HILL S.J. Agonist and Antagonist effects of CGP 12177 on β2-adrenoceptor-stimulated gene transcription in CHO cells transfected with the human β2-adrenoceptor. Br. J. Pharmacol. 2002;137:400–408. doi: 10.1038/sj.bjp.0704855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLEMAN R.A., JOHNSON M., NIALS A.T., VARDEY C.J. Exosites: their current status, and their relevance to the duration of action of long-acting beta 2-adrenoceptor agonists. Trends Pharmacol. Sci. 1996;17:324–330. [PubMed] [Google Scholar]
- D'ALLAIRE F., ATGIE C., MAURIEGE P., SIMARD P.M., BUKOWIECKI L.J. Characterization of beta 1- and beta 3-adrenoceptors in intact brown adipocytes of the rat. Br. J. Pharmacol. 1995;114:275–282. doi: 10.1111/j.1476-5381.1995.tb13223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FABBRI E., SELVA C., MOON T.W., CAPUZZO A. Characterization of 3H-CGP-12177 binding to beta-adrenergic receptors in intact eel hepatocytes. Gen. Comp. Endocrinol. 2001;121:223–231. doi: 10.1006/gcen.2000.7591. [DOI] [PubMed] [Google Scholar]
- GREEN S.A., SPASOFF A.P., COLEMAN R.A., JOHNSON M., LIGGETT S.B. Sustained activation of a G protein-coupled receptor via “anchored” agonist binding. Molecular localization of the salmeterol exosite within the β2-adrenergic receptor. J. Biol. Chem. 1996;271:24029–24035. doi: 10.1074/jbc.271.39.24029. [DOI] [PubMed] [Google Scholar]
- HADDAD C., WILKINSON M., ROEDER L.M., TILDON J.T., ARMOUR J.A. Binding of the hydrophilic beta-adrenergic antagonist 3H-CGP-12177 to cardiac tissue slices: characterization and ontogenetic studies in dogs. Can. J. Physiol. Pharmacol. 1987;65:1928–1933. doi: 10.1139/y87-300. [DOI] [PubMed] [Google Scholar]
- HARIKUMAR K.G., PINON D.I., WESSELS W.S., PRENDERGAST F.G., MILLER L.J. Environment and mobility of a series of fluorescent reporters at the amino terminus of structurally related peptide agonists and antagonists bound to the cholecystokinin receptor. J. Biol. Chem. 2002;277:18552–18560. doi: 10.1074/jbc.M201164200. [DOI] [PubMed] [Google Scholar]
- HEITHIER H., HALLMANN D., BOEGE F., REILANDER H., DEES C., JAEGGI K.A., ARNDT-JOVIN D., JOVIN T.M., HELMREICH E.J.M. Synthesis and properties of fluorescent β-adrenoceptor ligands. Biochemistry. 1994;33:9126–9134. doi: 10.1021/bi00197a015. [DOI] [PubMed] [Google Scholar]
- HERTEL C., MULLER P., PORTENIER M., STAEHELIN M. Determination of the desensitization of beta-adrenergic receptors by 3H-CGP-12177. Biochem. J. 1983a;216:669–674. doi: 10.1042/bj2160669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERTEL C., STAEHELIN M., PERKINS J.P. Evidence for intravesicular beta-adrenergic receptors in membrane fractions from desensitized cells: binding of the hydrophilic ligand CGP-12177 only in the presence of alamethicin. J. Cyclic Nucleotide Protein Phosphor. Res. 1983b;9:119–128. [PubMed] [Google Scholar]
- HILL, S J., BAKER J.G., REES S. Reporter gene systems for the study of G-protein-coupled receptors. Curr. Opin. Pharmacol. 2001;1:526–532. doi: 10.1016/s1471-4892(01)00091-1. [DOI] [PubMed] [Google Scholar]
- HOPKINSON H.E., LATIF M.L., HILL S.J. Non-competitive antagonism of β2-agonist-mediated cyclic AMP accumulation by ICI 118551 in BC3H1 cells endogenously expressing constitutively active β2-adrenoceptors. Br. J. Pharmacol. 2000;131:124–130. doi: 10.1038/sj.bjp.0703535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALLAY Z., SMISTEROVA J., SOLTES L., MARKO V. Evaluation of 3H-CGP-12177 and 3H-DHA binding to beta 2-adrenoceptors of rat reticulocytes by means of affinity spectra. J. Recept. Res. 1991;11:909–917. doi: 10.3109/10799899109064687. [DOI] [PubMed] [Google Scholar]
- KENAKIN T.P., BEEK D. Relative efficacy of prenalterol and pirbuterol for beta-1 adrenoceptors: measurement of agonist affinity by alteration of receptor number. J. Pharmacol. Exp. Ther. 1984;229:340–345. [PubMed] [Google Scholar]
- KONKAR A.A., ZHENGXIAN Z., GRANNEMAN J.G. Aryloxypropanolamine and catecholamine ligand interactions with the β1-adrenergic receptor: evidence for interaction with distinct conformations of β1-adrenergic receptors. J. Pharmacol. Exp. Ther. 2000;294:923–932. [PubMed] [Google Scholar]
- LACASA D., MAURIEGE P., LAFONTAN M., BERLAN M., GIUDICELLI Y. A reliable assay for beta-adrenoceptors in intact isolated human fat cells with a hydrophilic radioligand 3H-CGP 12177. J. Lipid Res. 1986;27:368–376. [PubMed] [Google Scholar]
- LANG P.H., LEMMER B. Evidence for two specific affinity states of 3H-antagonist binding to cardiac beta-adrenoceptors and influence of Gpp(NP)p. J. Cyclic Nucleotide Protein Phosphor. Res. 1985;10:341–360. [PubMed] [Google Scholar]
- LOWE M.D., LYNHAM J.A., GRACE A.A., KAUMANN A.J. Comparison of the affinity of β-blockers for the two states of the β1-adrenoceptor in ferret ventricular myocardium. Br. J. Pharmacol. 2002;135:451–461. doi: 10.1038/sj.bjp.0704450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.C., RANDALL R.J. Protein measurements with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MCDONNELL J., LATIF M.L., REES E.S., BEVAN N.J., HILL S.J. Influence of receptor number on the stimulation by salmeterol of gene transcription in CHO-K1 cells transfected with the human β2-adrenoceptor. Br. J. Pharmacol. 1998;125:717–726. doi: 10.1038/sj.bjp.0702139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGRATH J.C., ARRIBAS S.M., DALY C.J. Fluorescent ligands for the study of receptors. Trends Pharmacol. Sci. 1996;17:393–399. doi: 10.1016/s0165-6147(96)40004-9. [DOI] [PubMed] [Google Scholar]
- MOHELL S., DICKER A. The beta-adrenergic radioligand 3H-CGP-12177, generally classified as an antagonist, is a thermogenic agonist in brown adipose tissue. Biochem. J. 1989;261:401–405. doi: 10.1042/bj2610401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUZZIN P., REVELLI J.P., FRASER C.M., GIACOBINO J.P. Radioligand binding studies of the atypical beta 3-adrenergic receptor in rat brown adipose tissue using 3H-CGP-12177. FEBS Lett. 1992;298:162–164. doi: 10.1016/0014-5793(92)80046-j. [DOI] [PubMed] [Google Scholar]
- PAK M.D., FISHMAN P.H. Anomalous behaviour of CGP 12177A on beta 1-adrenergic receptors. J. Recept. Signal Transduct. Res. 1996;16:1–23. doi: 10.3109/10799899609039938. [DOI] [PubMed] [Google Scholar]
- REES S., BROWN S., STABLES J.Reporter gene systems for the study of G protein-coupled receptor signal transduction in mammalian cells Signal Transduction: A Practical Approach 1999Oxford: Oxford University Press; 171–222.2nd edn., ed. Milligan, G. pp [Google Scholar]
- STAEHELIN M., SIMONS P., JAEGGI K., WIGGER N. CGP-12177 a hydrophilic β-adrenergic receptor radioligand reveals high affinity binding of agonist to intact cells. J. Biol. Chem. 1983;258:3496–3502. [PubMed] [Google Scholar]
- STEINKRAUS V., NOSE M., MENSING H., KORNER C. Radioligand binding characteristics of beta 2-adrenoceptors of cultured melanoma cells. Br. J. Dermatol. 1990;123:163–170. doi: 10.1111/j.1365-2133.1990.tb01843.x. [DOI] [PubMed] [Google Scholar]
- STEPHENSON R.P. A modification of receptor theory. Br. J. Pharmacol. 1956;11:5109–5116. doi: 10.1111/j.1476-5381.1997.tb06784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THEDE-REYNOLDS K.R., MOTULSKY H.J., FELDMAN R.D. Temperature-dependent binding of hydrophilic beta-adrenergic receptor ligands to intact human lymphocytes. Life Sci. 1986;39:1325–1334. doi: 10.1016/0024-3205(86)90330-9. [DOI] [PubMed] [Google Scholar]
- THORLIN T., PERSSON P.A.I., ERIKSON P.S., RONNBACK L., HANSSON E. Astrocyte β1-adrenergic receptor immunoreactivity and agonist induced increases in [Ca2+]I: differential results indicative of a modified membrane receptor. Life Sci. 2000;67:1285–1296. doi: 10.1016/s0024-3205(00)00726-8. [DOI] [PubMed] [Google Scholar]
- WILKINSON M., WILKINSON D.A. Beta-adrenergic 3H-CGP-12177 binding to brain slices and single intact pineal glands. Neurochem. Res. 1985;10:829–839. doi: 10.1007/BF00964540. [DOI] [PubMed] [Google Scholar]