Abstract
The anticancer anthracycline doxorubicin (DOX) causes cardiomyopathy upon chronic administration. There is controversy about whether DOX acts directly or after conversion to its secondary alcohol metabolite DOXol. Here, the role of secondary alcohol metabolites was evaluated by treating rats with cumulative doses of DOX or analogues – like epirubicin (EPI) and the novel disaccharide anthracycline MEN 10755 – which were previously shown to form less alcohol metabolites than DOX when assessed in vitro.
DOX induced electrocardiographic and haemodynamic alterations, like elongation of QαT or SαT intervals and suppression of isoprenaline-induced dP/dt increases, which developed in a time-dependent manner and were accompanied by cardiomegaly, histologic lesions and mortality. EPI caused less progressive or severe effects, whereas MEN 10755 caused essentially no effect.
DOX and EPI exhibited comparable levels of cardiac uptake, but EPI formed ∼60% lower amounts of its alcohol metabolite EPIol at 4 and 13 weeks after treatment suspension (P<0.001 vs DOX). MEN 10755 exhibited the lowest levels of cardiac uptake; hence, it converted to its alcohol metabolite MEN 10755ol ∼40% less efficiently than did EPI to EPIol at either 4 or 13 weeks. Cardiotoxicity did not correlate with myocardial levels of DOX or EPI or MEN 10755, but correlated with those of DOXol or EPIol or MEN 10755ol (P=0.008, 0.029 and 0.017, respectively).
DOX and EPI inactivated cytoplasmic aconitase, an enzyme containing an Fe–S cluster liable to disassembly induced by anthracycline secondary alcohol metabolites. DOX caused greater inactivation of aconitase than EPI, a finding consistent with the higher formation of DOXol vs EPIol. MEN 10755 did not inactivate aconitase, which was because of both reduced formation and impaired reactivity of MEN 10755ol toward the Fe–S cluster. Aconitase inactivation correlated (P<0.01) with the different levels of cardiotoxicity induced by DOX or EPI or MEN 10755.
These results show that (i) secondary alcohol metabolites are important determinants of anthracycline-induced cardiotoxicity, and (ii) MEN 10755 is less cardiotoxic than DOX or EPI, a behaviour attributable to impaired formation and reactivity of its alcohol metabolite.
Keywords: Doxorubicin, epirubicin, MEN 10755, secondary alcohol metabolites, cardiotoxicity
Introduction
Clinical use of the anticancer anthracycline doxorubicin (DOX) is limited by the possible development of progressive cardiomyopathy, especially when the cumulative dose reaches or exceeds ∼500–550 mg m−2 (Singal & Iliskovic, 1998; Minotti et al., 1999). The mechanisms of anthracycline-induced cardiotoxicity remain a matter of debate, but some peculiar structure – activity relations have been clarified. As shown in Figure 1, DOX is composed of aglyconic and sugar moieties. The aglycone, called doxorubicinone, is composed of a quinone-containing tetracyclic ring, and of a short side chain with a carbonyl group at C-13 and a primary alcohol at C-14; the sugar, called daunosamine, is attached by a glycosidic bond to C-7 of the tetracyclic ring, and consists of a 3-amino-2,3,6-trideoxy-L-fucosyl moiety. One-electron redox cycling of the quinone moiety, mediated by NAD(P)H oxidoreductases of intracellular organelles, may contribute to inducing cardiotoxicity by converting oxygen to reactive species like superoxide anion (O2•−) and hydrogen peroxide (H2O2) while also releasing iron from ferritin. These processes eventually favour iron-catalysed formation of hydroxyl radicals or similar reactive species able to induce oxidative damage (Singal & Iliskovic, 1998; Minotti et al., 1999).
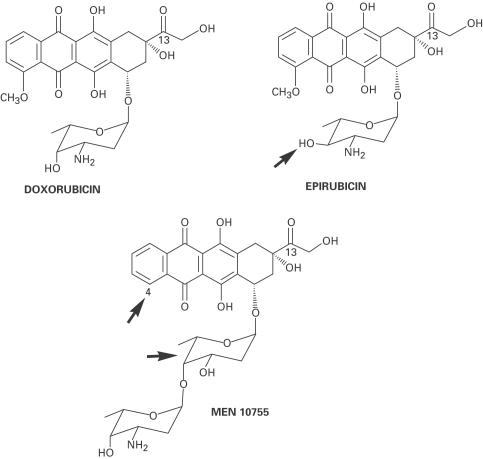
Figure 1.
Structures of doxorubicin, epirubicin and MEN 10755. The arrows indicate epimerization at C-4 in daunosamine (epirubicin); lack of a methoxy group at C-4 in the aglycone, and intercalation of 2,6-dideoxy-L-fucose between the aglycone and daunosamine (MEN 10755).
An additional determinant of cardiotoxicity has been identified in the enzymatic conversion of DOX to its secondary alcohol metabolite doxorubicinol (DOXol), mediated by cytoplasmic aldo/keto- or carbonyl-reductases, which add two electrons to the side chain carbonyl group (-CO-CH2OH→-CHOH-CH2OH). In fact, studies with transgenic mice bearing cardiac-restricted overexpression of carbonyl reductases have shown that an increased conversion of DOX to DOXol is accompanied by an accelerated course of development of cardiomyopathy (Forrest et al., 2000). Indirect evidence for the action of secondary alcohol metabolites is offered also by the different behaviour of analogues characterized by reduced levels of conversion to such metabolites. For example, there is clinical evidence that epirubicin (EPI), characterized by axial-to-equatorial epimerization of a hydroxyl group in daunosamine (Figure 1), induces cardiomyopathy at cumulative doses almost double those of DOX (Robert, 1993); this correlates with a ∼50% reduction in alcohol metabolite formation when EPI is compared to DOX in cytosolic fractions derived from human myocardium (Minotti et al., 2000). MEN 10755, an investigational anthracycline, is similar to EPI in producing ∼50% less alcohol metabolite than DOX when assessed in human cardiac cytosol (Minotti et al., 2000); however, MEN 10755 is characterized also by reduced cardiac uptake in mice (Gonzalez-Paz et al., 2001) or rat isolated heart (Minotti et al., 2001a). The behaviour of MEN 10755 reflects more extensive modifications of the anthracycline molecule, like absence of the methoxy group at C-4 in doxorubicinone and intercalation of 2,6-dideoxy-L-fucose between doxorubicinone and daunosamine (Figure 1). Previous studies showed that MEN 10755 induced less severe or progressive cardiomyopathy than DOX in the rat, but whether its improved tolerability reflected reduced cardiac uptake and/or impaired conversion to alcohol metabolite was not determined (Cirillo et al., 2000).
The present study was designed to improve our knowledge of the role of alcohol metabolites vs parent anthracyclines and to evaluate their impact on the development of cardiotoxicity induced by MEN 10755 vs approved anthracyclines like DOX or EPI. To obtain this information, rats were treated with cumulative doses of DOX or EPI or MEN 10755, and morphologic or functional indices of cardiomyopathy were correlated with the cardiac levels of unmetabolized anthracyclines or secondary alcohol metabolites, and with biochemical indices of alcohol metabolite reactivity.
Methods
Study design
After 2 weeks of acclimatization, 6-week-old male Sprague – Dawley rats (Harlan, Corezzana, Milano, Italy) were injected through a tail vein with either vehicle or 1.5 mg kg−1 DOX, EPI or MEN 10755, once a week for five consecutive weeks. This dose regimen caused comparable myelotoxic effects, as evidenced by similar decreases in white blood cells at 3 days after the last administration of either drug (Mazué et al., 1995; Cirillo et al., 2000). At 3 days and 4 or 13 weeks after treatment completion, animals were anaesthetized with 0.3 ml kg−1 of Hypnorm (fentanyl citrate 0.315 mg ml−1 and fluanisone 10 mg ml−1) plus 40–60 mg kg−1 of sodium pentobarbitone, both given intraperitoneally, and ECG tracings were recorded. Following the induction of anaesthesia, the animals were subjected to surgical preparation for monitoring haemodynamic parameters. At the end of each experiment, hearts were arrested in diastole by cadmium chloride injection, excised and fixed in 10% buffered formol saline. Unfixed ventricle samples from 4 or 13 weeks post-treatment groups were stored at −80°C until used for biochemical analyses (anthracycline content and composition, aconitase activity). The schedule was modified for DOX-treated rats, which were examined at 12 rather than 13 weeks because of excessive mortality; nevertheless, for the sake of clarity, all final evaluations are referred to as performed at 13 weeks.
Electrocardiographic measurements
ECG tracings (lead II) were recorded by means of a BM 613 electrocardiograph (Biomedica Mangoni, Pisa, Italy) connected to an Astro-Med polygraph recorder (Astro-Med, West Warwick, U.S.A.). ECG tracings were analysed by measuring QRS voltage (from the isoelectric point to the apex of R wave) and QαT and SαT or QRS intervals (from the beginning of the first wave to the apex of the last wave).
Haemodynamic measurements
The animals, anaesthetized as indicated above, were placed in a homeothermic blanket system to maintain the body temperature at 37°C. The trachea was exposed and a polyethylene cannula was inserted to facilitate the spontaneous respiration. A catheter (angiocath 24 G, Becton Dickinson, U.S.A.) was inserted into a tail vein for infusion of sodium pentobarbitone (5 mg ml−1; 0.5–3 ml h−1) to maintain anaesthesia and/or inject other substances. Blood pressure (BP) was measured by means of a polyethylene cannula (PE 50) filled with heparinized saline (25 UI ml−1) inserted into the left femoral artery. The cannula was connected to a transducer (Transpack, Abbott, North Chicago, U.S.A.) and the signal was amplified by means of a BM 614/2 amplifier (Biomedica Mangoni, Pisa, Italy). Left ventricular systolic pressure (LVSP) was measured by means of a Millar mikro-tip transducer catheter inserted in the left ventricle via the right carotid artery, and the signal was amplified by means of a BM 614/2 amplifier (Biomedica Mangoni, Pisa, Italy). BP and LVSP were sent to, and recorded by a digital acquisition system (Ponemah, Gould G.N. Systems, Milano, Italy), which elaborated derived parameters like systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), heart rate (HR), left ventricular end diastolic pressure (LVEDP), maximum value of the first derivative of LVSP (dP/dt) and contractility index (CI). All parameters were recorded at baseline and following intravenous administration of increasing doses (0.01, 0.03, 0.1, 0.3 and 1 μg kg−1) of isoprenaline (administered at 10–15 min intervals).
Histology
After fixation, each heart was embedded in 5 μm thick paraffin sections and stained with hematoxylin/eosin/trichrome. Ventricles and atria were evaluated and scored separately. Cardiotoxicity was evaluated in terms of severity of lesions and extent of damage (Bertazzoli et al., 1979). Severity was scored grade 1 (sarcoplasmic microvacuolizations and/or inclusions and interstitial or cellular oedema) or grade 2 (as grade 1 plus sarcoplasmic macrovacuolization or atrophy, necrosis, fibrosis, endocardial lesions, and thrombi). The extent of damage was scored grade 0 (no lesions); grade 0.5 (<10 single altered myocytes in whole heart section); grade 1 (scattered single altered myocytes); grade 2 (scattered small groups of altered myocytes); grade 3 (widely spread small groups of altered myocytes); grade 4 (confluent groups of altered myocytes); and grade 5 (most cells damaged). Altered myocytes were those exhibiting grade 1 or 2 lesions, as described for severity. For each animal, the final cardiotoxicity score was calculated based upon a multiple of the numerical values assigned for the severity and extent of lesions.
Biochemical analyses
Left ventricle samples from 4 or 13 weeks post-treatment groups were split into two samples and assayed for anthracycline content and composition or cytoplasmic aconitase. For anthracycline measurements, ventricle samples were minced and washed in 2 ml of ice-cold 0.3 M NaCl, pH 7.0, homogenized and extracted with a four-fold excess of (1 : 1) CHCl3/CH3OH. The organic phases were recovered, pooled and dried under vacuum. Dry residues were dissolved in 200 μl of methanol and cleared of gross particulates by low-speed centrifugation. The supernatants were vacuum-dried and residues were suspended in 20–50 μl of methanol for analysis by two-dimensional TLC on 0.25 mm (20 × 20 cm) Silica Gel F254 plates (Merck, Darmstadt, Germany). Mobile phases and Rf values have been reported in detail (Minotti et al., 2000). After identification by co-chromatography with authentic standards, parent anthracyclines and secondary alcohol metabolites were eluted and quantified by fluorescence spectroscopy (Minotti et al., 2000). Values were expressed as nanomol per gram (wet weight). For cytoplasmic aconitase assays, ventricle samples were processed for cytosol preparation by sequential homogenization, ultracentrifugation and 65% ammonium sulphate precipitation of 105,000 × g supernatants; cis-aconitate (0.1 mM) was included throughout to stabilize the catalytic [4Fe–4S] cluster of this enzyme (Minotti et al., 2001b). After extensive dialysis against 0.3 M NaCl to remove contaminants and residual cis-aconitate, proteins were measured by the bicinchoninic acid method (Stoscheck, 1990), and aconitase activity was determined by reconstituting cytosol (15–30 μg ml−1) with excess cis-aconitate (100 μM) and by monitoring substrate consumption at 240 nm (ɛ=3.6 mM−1 cm−1), precisely as described (Minotti et al., 2000). Authentic aconitase activity was confirmed by suppression of cis-aconitate consumption by the pseudosubstrate-inhibitor d,l-fluorocitrate (100 μM). All measurements were performed before and after 5 min preincubation of cytosol with cysteine and ferrous ammonium sulphate at the final ratios of 1000 and 50 nmol mg protein−1, respectively. This treatment was previously shown to reconstitute [4Fe–4S] clusters, achieving maximal activation of aconitase (Minotti et al., 2000).
Statistical analyses
Data are expressed as means±s.e. Unless otherwise indicated, all differences were determined by one-way ANOVA followed by Bonferroni's test for comparisons between multiple groups. Correlations were determined by one-tailed nonparametric Spearman analysis; differences and correlations were considered to be significant when P<0.05.
Drugs and chemicals
MEN 10755 and MEN 10755ol were synthesized at the Chemistry Department of Menarini Ricerche (Pomezia, Italy). DOX and EPI were products of Pharmacia-Upjohn (Milan, Italy). DOXol and EPIol were purified by us after NaBH4 reduction of DOX or EPI (Minotti et al., 2000). Anthracyclines were dissolved in the vehicle (1% lactose in sterile saline) at a concentration of 0.15 mg ml−1. Both vehicle and anthracyclines were administered at a dose volume of 10 ml kg−1 body weight. Hypnorm was from Janssen-Cilag (Saunderton, Buckinghamshire, U.K.). Pentobarbital, isoprenaline and all other chemicals were from Sigma Aldrich (Milan, Italy).
Results
Effects of anthracyclines on mortality, body and heart weight and relative heart weight
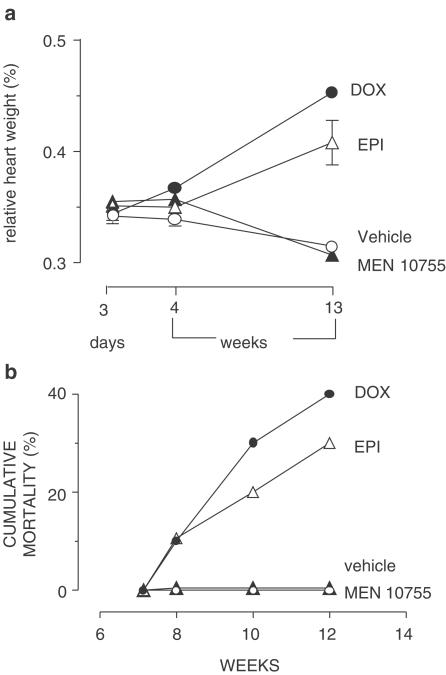
Rats exposed to chronic anthracycline regimens were examined for changes in body and heart weight. Table 1 shows that MEN 10755-treated rats exhibited the same gain in body weight as observed in the vehicle group, whereas DOX- or EPI-treated rats exhibited significant weight losses. In the case of DOX-treated rats, weight loss was P<0.001 vs vehicle or MEN 10755 groups at both 4 and 13 weeks; in the case of EPI-treated rats, a significant decrease was observed only at 13 weeks, reaching values intermediate between DOX and vehicle groups (P<0.001 vs either group). DOX-treated rats exhibited the lowest heart weights at all time points of post-treatment examination, whereas EPI-treated rats exhibited increasing heart weights, which essentially overlapped those of vehicle-treated rats (see also Table 1). In regard to MEN 10755-treated rats, there was a relatively minor but significant (P<0.05) decrease in heart weight at 13 weeks but not at 3 days or 4 weeks. Having determined changes in body and heart weight, we could calculate heart/body weight ratios (also referred to as relative heart weight) that serve an index of cardiomegaly induced by anthracyclines (Sacco et al., 2001). Figure 2a shows that MEN 10755-treated rats exhibited the same relative heart weight of vehicle-treated rats, whereas both DOX- and EPI-treated rats exhibited time-related increases. This pattern correlated with mortality in vehicle or anthracycline-treated groups. In fact, Figure 2b shows that there was time-related mortality among DOX- or EPI-treated groups (40 and 30%, respectively) but not among vehicle- or MEN 10755-treated groups. Thus, MEN 10755 seemed to induce less severe systemic and cardiac-specific toxicities than DOX or EPI.
Table 1.
Effects of anthracyclines on body and heart weight
| Day 3 | Week 4 | Week 13 | |
|---|---|---|---|
| Mean±s.e. (n) | Mean±s.e. (n) | Mean±s.e. (n) | |
| Body weight (g) | |||
| Vehicle | 383±6 (8) | 409±10 (10) | 460±8 (10) |
| DOX | 336±10 (10) | 338±14 (10)* | 274±18 (6)* |
| EPI | 356±4 (10) | 388±8 (10) | 355±16 (7)* |
| MEN 10755 | 344±5 (10) | 414±7 (10) | 433±6 (10) |
| Heart weight (g) | |||
| Vehicle | 1.31±0.03 (8) | 1.39±0.04 (10) | 1.45±0.03 (10) |
| DOX | 1.14±0.032 (10)** | 1.24±0.048 (10)** | 1.2±0.03 (6)** |
| EPI | 1.25±0.013 (10) | 1.35±0.017 (10) | 1.43±0.025 (7) |
| MEN 10755 | 1.23±0.033 (10) | 1.39±0.026 (10) | 1.33±0.025 (8)** |
P<0.001 vs vehicle or MEN 10755.
P<0.05 vs vehicle.
See Methods, and Results, for further details.
Figure 2.
Relative heart weight and mortality after treatment with DOX or EPI or MEN 10755. (a) Relative heart weights at 3 days and 4 or 13 weeks after treatment suspension. Values (means±s.e., n=6–10) were calculated based upon absolute body and heart weights reported in Table 1; those without vertical bars have s.e. within symbols. (b) Time-related mortality in the same groups.
Effects of anthracyclines on ECG
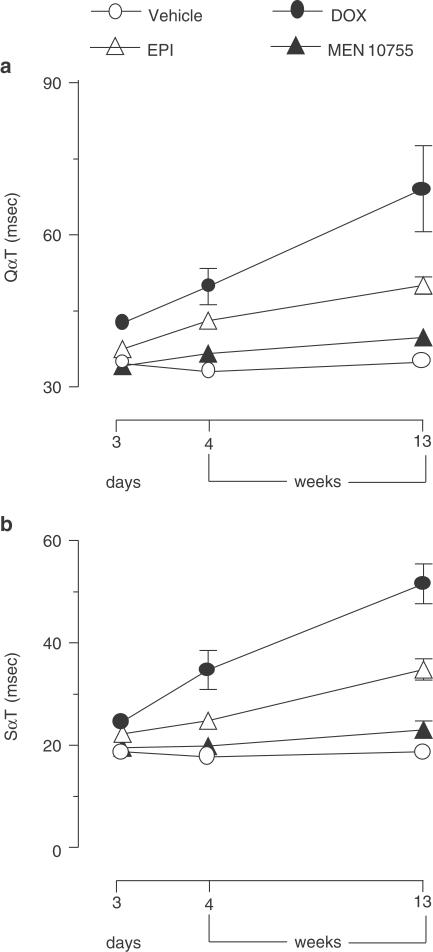
The anthracycline regimens adopted in this study caused no effects on heart rate or QRS duration and voltage (data not shown); however, there were important effects on QαT and SαT intervals. Significant (P<0.001) and progressive elongation of the QαT interval was observed in DOX-treated rats at both 3 days and 4 or 13 weeks of post-treatment observation, and an elongation of QαT was observed also with EPI at 4 or 13 weeks (P<0.05 vs vehicle), albeit less evident than that induced by DOX (P<0.05 at 13 weeks). MEN 10755 caused no effect on QαT at either time point (Figure 3a). The ECG-tolerability of MEN 10755 vs DOX and EPI was confirmed when the three anthracyclines were compared with regard to elongation of the SαT interval, considered the most sensitive and predictive ECG index of anthracycline-induced cardiotoxicity (Danesi et al., 1986). As shown in Figure 3b, DOX induced significant and progressive elongation of the SαT interval at all time points of observation (P<0.01 or 0.001 vs vehicle at 3 days and 4 or 13 weeks, respectively). EPI caused a significant elongation of SαT at both 3 days and 4 or 13 weeks (P<0.05 and <0.01 vs vehicle, respectively); however, the effects induced by EPI were significantly lower than those induced by DOX (P<0.025 and <0.05 at 4 and 13 weeks, respectively). Again, there was no appreciable elongation of the SαT interval in MEN 10755-treated rats at either 3 days, or 4 or 13 weeks. These results showed that anthracyclines induced ECG alterations according to a rank order DOX>EPI>MEN 10755.
Figure 3.
Time-dependent elongations of QαT or SαT intervals after treatment with DOX or EPI or MEN 10755. QαT or SαT intervals were determined at 3 days and 4 or 13 weeks after treatment suspension. Values are means±s.e. (n=5–10); those without vertical bars have s.e. within symbols.
Effects of anthracyclines on basal or isoprenaline-stimulated haemodynamic parameters
Rats exposed to chronic anthracycline regimens were evaluated for changes in haemodynamic parameters. Measurements of HR, MBP, LVEDP and CI on day 3 and week 4 or 13 did not reveal significant effects induced by DOX or EPI and MEN 10755 as compared to vehicle (not shown). Sporadic changes were noticed in regard to LVSP and dP/dt. Rats treated with anthracyclines exhibited moderate but significant increase of LVSP at 3 days (mmHg: 135±5 (n=9), 145±4 (n=10) and 141±6 (n=9) vs 119±3 (n=8) for DOX, EPI and MEN 10755 vs vehicle, respectively; P<0.05 (DOX, MEN 10755) or P<0.01 (EPI)); however, there was no significant difference between vehicle and anthracycline groups when LVSP was evaluated at 4 and 13 weeks. Anthracyclines induced some effects also on dP/dt. At 3 days there was no significant difference between vehicle and anthracycline groups, but rats treated with DOX exhibited reduced dP/dt as compared to rats treated with EPI or MEN 10755 (mmHg/s: 6264±391 (n=9), 7667±232 (n=10) and 7428±229 (n=8) for DOX, EPI and MEN 10755, respectively; P<0.01 for DOX vs EPI, and P<0.05 for DOX vs MEN 10755). At 4 weeks, vehicle- and anthracycline-treated rats exhibited comparable dP/dt, but at 13 weeks DOX-treated rats exhibited reduced dP/dt vs vehicle- or MEN 10755-treated rats (mmHg/s: 4919±772 (n=6) vs 6829±281 mmHg (n=10) and 6862±162 (n=9); P<0.05). Collectively, these results showed that chronic anthracycline treatments perturbed haemodynamic parameters measured under basal conditions; however, the effects of anthracyclines seemed to vary from one parameter to another, nor did they exhibit clear-cut relations with post-treatment time.
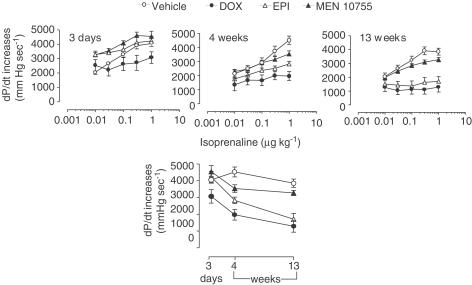
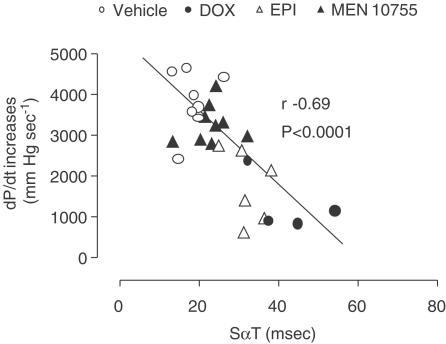
The influence of anthracyclines on haemodynamic parameters became more evident and consistent when these parameters were measured after injection of increasing doses of isoprenaline. Figure 4 shows data concerning isoprenaline-induced dose-dependent increases of dP/dt. At 3 days EPI- or MEN 10755-treated rats developed dP/dt increases, which were similar to those of vehicle-treated rats; in contrast, DOX-treated rats exhibited dP/dt increases of reduced amplitude as compared to vehicle-treated rats (P<0.001 at isoprenaline ⩾0.03 μg kg−1). Differences between vehicle and anthracyclines groups, and between DOX and EPI or MEN 10755 groups, became further evident at 4 weeks. In fact, EPI-treated rats exhibited impaired dP/dt increases vs vehicle (P<0.001 at 1 μg isoprenaline kg−1), whereas MEN 10755-treated rats developed dP/dt increases, which were not significantly different from controls. Isoprenaline-induced increases in dP/dt remained most remarkably suppressed in DOX-treated rats, significant differences (P<0.01) being observed vs MEN 10755-treated rats (but not vs EPI-treated rats) over the whole range of isoprenaline dosages. Evaluation at 13 weeks did not reveal any worsening of dP/dt increases in MEN 10755-treated rats; in contrast, EPI-treated rats exhibited further reductions in dP/dt increases, which were P<0.05 vs 4 weeks at all isoprenaline dosages. Therefore, EPI-treated rats became almost identical to DOX-treated rats in exhibiting the lowest dP/dt increases induced by isoprenaline, both DOX and EPI groups being P<0.01 vs MEN 10755 over the whole range of isoprenaline dosages. When assessed within such multiple comparisons, the differences between MEN 10755 and vehicle groups did not approach the level of significance. Collectively, these results showed that: (i) the effects induced by DOX on isoprenaline-induced dP/dt increases became evident immediately after treatment suspension; (ii) the effects induced by EPI developed more slowly, reaching their maximum at 13 weeks and (iii) MEN 10755 caused marginal and nonprogressive effects (see also Figure 4, bottom panel for comparisons between vehicle and anthracycline groups, at 3 days and 4 or 13 weeks, after isoprenaline 1 μg kg−1). These patterns of toxicity closely reproduced ECG changes induced by DOX or EPI or MEN 10755. Accordingly, a decline in dP/dt increases correlated in a highly significant manner with elongation of SαT intervals (Figure 5) or QαT intervals (not shown).
Figure 4.
Time-dependent isoprenaline-induced dP/dt increases after treatment with DOX or EPI or MEN 10755. dP/dt increases, induced by escalating doses of isoprenaline, were determined at 3 days and 4 or 13 weeks after treatment suspension, as described in Methods. Values are means±s.e. (n=4–10). Bottom panel shows time-dependent changes of dP/dt increases at a fixed dose of isoprenaline 1 μg kg−1.
Figure 5.
Correlations between SαT interval and isoprenaline-induced dP/dt increases after treatment with DOX or EPI or MEN 10755. Correlations were calculated using values determined at 13 weeks after treatment suspension; dP/dt increases were those induced with isoprenaline 1 μg kg−1.
Histopathological evaluation
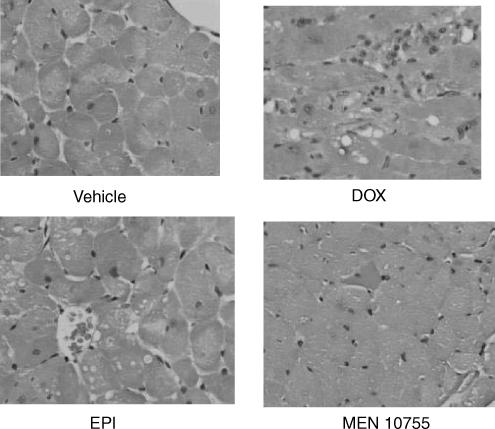
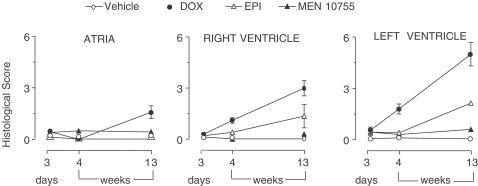
Examination of left ventricle from DOX-treated rats revealed the presence of important lesions like diffuse interstitial sclerosis and cytoplasmic macrovacuolization in residual myocytes (Figure 6). Micro- and macro-vacuolizations were seen also in the heart of EPI-treated rats, albeit less severe and diffuse than in the case of DOX-treated rats; in contrast, there were no appreciable lesions in the heart of MEN 10755-treated rats (see also Figure 6). The severity of histologic lesions was quantified based upon an established scoring procedure (Bertazzoli et al., 1979). As shown in Figure 7, DOX-induced myocardial damage developed in a time-dependent manner following treatment suspension and exhibited a peculiar pattern of localization. At 13 weeks, right ventricles were more severely damaged than atria (P<0.05), and the left ventricle was more severely damaged than the right ventricle (P<0.05). EPI did not cause any effect in atria but induced lesions in both right and left ventricles (P<0.05 and <0.001 vs vehicle at 13 weeks, respectively). There was no significant difference between DOX and EPI when cardiotoxicity scores were determined in the right ventricle, but DOX proved to be significantly more toxic than EPI when scores were determined in the left ventricle; in the latter, DOX-induced lesions developed at 4 weeks and worsened further at 13 weeks, whereas EPI-induced lesions were evident only at 13 weeks. These results showed that (i) ventricles, and especially the left ventricle, were predominant sites of anthracycline-induced myocardial damage and (ii) EPI-induced lesions developed more slowly than DOX-induced lesions. Importantly, MEN 10755 was essentially devoid of effects in either right or left ventricle throughout the post-treatment period (see also Figure 7). Histologic lesions induced by DOX or EPI or MEN 10755 therefore developed in a manner similar to that described for ECG changes or isoprenaline-induced dP/dt increases. Accordingly, histologic scores correlated in a highly significant manner with both elongation of the SαT interval and impairment of dP/dt increases induced by isoprenaline 1 μg kg−1 (P<0.0001 at 13 weeks).
Figure 6.
Histologic lesions in the heart of rats treated with DOX, EPI or MEN 10755. Left ventricle samples were obtained from vehicle- or anthracycline-treated rats 13 weeks after treatment suspension, and haematoxylin/eosin/trichrome-stained trasverse sections were prepared and examined (× 40 magnification). See also Methods, and Results, for details and explanations.
Figure 7.
Time-dependent development of histologic scores of cardiotoxicity after treatment with DOX or EPI or MEN 10755. Histologic scores were determined at 3 days and 4 or 13 weeks after treatment suspension, precisely as described in Methods. Values are means±s.e. (n=6–10); those without vertical bars have s.e. within symbols.
Cardiac levels of carbonyl anthracyclines and secondary alcohol metabolites
Table 2 shows that the cardiac levels of DOX and EPI always exceeded those of MEN 10755. At 4 weeks, there was no difference between DOX and EPI, and both anthracyclines were significantly higher than MEN 10755 within multiple comparisons. At 13 weeks, there were minor changes in the levels of DOX or MEN 10755 but those of EPI decreased by approximately 31%; therefore, the cardiac levels of DOX became significantly higher than those of EPI or MEN 10755 when assessed within multiple comparisons, whereas the cardiac levels of EPI proved to be higher than those of MEN 10755 when the two anthracyclines were compared by unpaired Student's t-test (see also legend to Table 2). Collectively, the cardiac levels of carbonyl anthracyclines followed a rank order DOX=EPI>MEN 10755 at 4 weeks, and a rank order DOX>EPI>MEN 10755 at 13 weeks. This picture changed when the cardiac levels of secondary alcohol metabolites were measured. As shown in Table 3 , the levels of DOXol significantly exceeded those of EPIol and MEN 10755ol at both 4 and 13 weeks; moreover, the levels of DOXol significantly increased from 4 to 13 weeks, whereas those of EPIol and MEN 10755ol remained essentially unchanged. The levels of EPIol always exceeded those of MEN 10755ol, and differences were significant when assessed by unpaired Student's t-test (see also legend to Table 3). Thus, the cardiac levels of secondary alcohol metabolites followed a rank order DOXol>EPIol>MEN 10755ol at both 4 and 13 weeks. Based upon these findings, we determined whether functional or morphologic indices of cardiotoxicity, assessed at 4 or 13 weeks, correlated with the cardiac levels of carbonyl anthracyclines or secondary alcohol metabolites measured at the same time points. As shown in Table 4 , neither the elongation of QαT or SαT intervals, nor the impairment of isoprenaline-induced dP/dt increases or the development of histological scores correlated with the cardiac levels of carbonyl anthracyclines, but highly significant correlations always occurred with the levels of secondary alcohol metabolites. This suggested that the greater toxicity of DOX vs EPI or MEN 10755, and of EPI vs MEN 10755, reflected the different levels of formation of their alcohol metabolites.
Table 2.
Cardiac levels of carbonyl anthracyclines at 4 or 13 weeks after treatment suspension
| Carbonyl anthracycline | 4 weeks (nmol g−1) | 13 weeks (nmol g−1) |
|---|---|---|
| DOX | 0.091±0.008*,** | 0.084±0.008 ***,† |
| EPI | 0.067±0.007* | 0.046±0.004***,‡ |
| MEN 10755 | 0.026±0.005 | 0.033±0.007 |
Values are means±s.e. of 8–14 values.
P<0.001 vs MEN 10755
P>0.05 vs EPI.
P<0.001 vs MEN 10755
P<0.01 vs EPI.
P>0.05 vs MEN 10755 (P<0.025 by unpaired Student's t-test).
Table 3.
Cardiac levels of secondary alcohol metabolites at 4 or 13 weeks after treatment suspension
| Secondary alcohol metabolite | 4 weeks (nmol g−1) | 13 weeks (nmol g−1) |
|---|---|---|
| DOXol | 0.083±0.011*,** | 0.11±0.014† |
| EPIol | 0.031±0.004*** | 0.036±0.004‡ |
| MEN 10755ol | 0.019±0.002 | 0.021±0.002 |
Values are means±s.e. of 8–14 values.
P<0.001 vs EPIol and MEN 10755ol
P<0.01 vs DOXol at 13 weeks.
P>0.05 vs MEN 10755ol (P<0.025 by unpaired Student's t-test).
P<0.001 vs EPIol and MEN 10755ol
P>0.05 vs MEN 10755ol (P<0.01 by unpaired Student's t-test).
Table 4.
Correlations between functional or morphologic indices of cardiotoxicity and cardiac levels of carbonyl anthracyclines or secondary alcohol metabolites
| Index of cardiotoxicity | Correlation with carbonyl anthracyclines | Correlation with secondary alcohol metabolites |
|---|---|---|
| QαT elongation | r=0.69, P=0.070 (NS) | r=0.92, P=0.008 |
| SαT elongation | r=0.71, P=0.068 (NS) | r=0.94, P=0.008 |
| Impairment of isoprenaline-induced dP/dt increases | r=−0.71, P=0.068 (NS) | r=−0.84, P=0.029 |
| Histological scores | r=0.60, P=0.12 (NS) | r=0.89, P=0.017 |
Anthracycline secondary alcohol metabolite-dependent inactivation of cytoplasmic aconitase
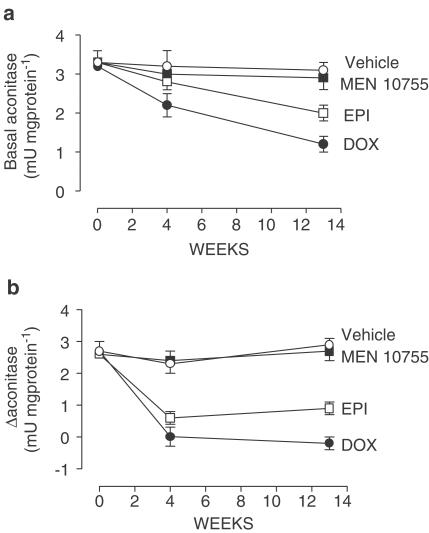
Previous studies have shown that anthracycline secondary alcohol metabolites are much more reactive than their parent anthracyclines toward cytoplasmic aconitase, causing disassembly of its catalytic Fe–S cluster (Minotti et al., 1999,2000); therefore, we measured changes in cytoplasmic aconitase activity after treatment with DOX or EPI or MEN 10755. In a first set of measurements aconitase activity was assayed under basal conditions, that is, without any sample manipulation. Figure 8a shows that basal aconitase activity was remarkably and time-dependently suppressed in the heart of DOX-treated rats but not in the heart of MEN 10755-treated rats, consistent with the fact that DOXol was higher than MEN 10755ol. Hearts of EPI-treated rats exhibited basal aconitase activities which were intermediate between those of DOX- or MEN 10755-treated rats, consistent with the fact that EPIol was lower than DOXol but higher than MEN 10755ol (cf. Table 3). Loss of basal aconitase activity correlated with cardiac levels of secondary alcohol metabolites (r=0.94, P=0.008) but not of carbonyl anthracyclines (r=0.71, P=0.068), confirming that the latter did not contribute to inducing disassembly of Fe–S clusters. Moreover, there were highly significant correlations between the loss of basal aconitase activity and the elongation of QαT or SαT intervals (r=0.97, P<0.001) and the impairment of isoprenaline-induced dP/dt increases or the development of histologic scores (r=0.94, P=0.008), offering evidence that alcohol metabolite reactivity was important in determining cardiotoxicity.
Figure 8.
Time-dependent changes in basal or Δ cytoplasmic aconitase activity after treatment with DOX or EPI or MEN 10755. Basal or Δ cytoplasmic aconitase activity was measured at 3 days and 4 or 13 weeks after treatment suspension, precisely as described in Methods. Values are means±s.e. (n=8–14).
Aconitase activity was assayed also after cytosol preincubation with cysteine and ferrous ammonium sulphate, a procedure known to increase enzyme activity by reconstituting Fe–S clusters (Minotti et al., 2000). Net increases in aconitase activities (referred to as Δ aconitase) therefore gave an indication of how effectively Fe-S clusters could be reincorporated in this enzyme after the disassembly induced by alcohol metabolites. As shown in Figure 8b, there was a substantial loss of Δ aconitase in DOX- or EPI-treated samples but not in MEN 10755-treated samples, confirming that MEN 10755 was the least toxic anthracycline tested in this study.
Discussion
We have shown that rats exposed to cumulative doses of DOX or EPI developed cardiomegaly, elongation of QαT and SαT intervals, suppression of isoprenaline-induced dP/dt increases, and morphologically documented lesions. In general, QαT/SαT intervals and histologic scores were altered by EPI less severely than by DOX. The two anthracyclines were similar in impairing isoprenaline-induced dP/dt increases at 13 weeks, but EPI was significantly less effective than DOX when assessed earlier at 4 weeks (cf. Figure 4). On balance, EPI induced cardiac effects, which developed either less severely or more slowly than in the case of DOX. Clinical studies have attributed the reduced cardiotoxicity of EPI to the fact that epimerization at C-4 in daunosamine improves glucuronidation and systemic clearance of the anthracycline molecule, limiting its cardiac accumulation (Robert, 1993). This mechanism may not explain a reduced cardiotoxicity of EPI in the rat, which lacks the ability to form EPI-glucuronides (Maessen et al., 1987). Consistent with this, we never detected EPI-glucuronides in our study nor were the cardiac levels of EPI significantly lower than those of DOX when assessed on week 4 after treatment suspension, a time long enough to unravel any possible difference between DOX or EPI in terms of cardiac uptake. The levels of EPI decreased and became significantly lower than those of DOX only at 13 weeks, likely because of processes like cardiac clearance or degradation of the anthracycline molecule. Nonetheless, DOX and EPI were found to generate different cardiac levels of their alcohol metabolites. DOXol increased from 4 to 13 weeks, as one would expect from a good substrate of cardiac reductases, but EPIol remained stable and lower than DOXol at both 4 and 13 weeks (cf. Table 3). This suggests that inherent resistance to alcohol metabolite formation might be a more relevant determinant of the reduced cardiotoxicity of EPI vs DOX. When examined in this context MEN 10755 proved to induce even less cardiac toxicity than EPI, regardless of whether cardiac damage was assessed in terms of cardiomegaly and mortality (cf. Figure 2), QαT or SαT elongation (cf. Figure 3), suppression of isoprenaline-induced dP/dt increases (cf. Figure 4), or development of histologic lesions (cf. Figures 6 and 7). In comparison to DOX or EPI, MEN 10755 was characterized by reductions in both cardiac uptake and alcohol metabolite formation, similar to what observed when these anthracyclines were delivered to rat ventricle strips (Minotti et al., 2001a). However, the prevailing importance of the reduced levels of alcohol metabolite formation was indicated by the fact that neither functional nor morphologic indices of cardiotoxicity correlated with the amounts of DOX or EPI or MEN 10755 recovered in the heart, whereas highly significant correlations always occurred with the levels of DOXol or EPIol or MEN 10755ol (cf. Table 4). Collectively, these results demonstrate that secondary alcohol metabolites are important determinants of cardiotoxicity; hence, the severity of cardiotoxicity (DOX>EPI>MEN 10755) reflects the levels of formation of secondary alcohol metabolites (DOXol>EPIol>MEN 10755ol). These results also demonstrate that MEN 10755, although exhibiting the same resistance as EPI to carbonyl reduction in vitro, generates lower myocardial levels of its alcohol metabolite in vivo because of a concomitant impairment in cardiac uptake.
The mode of action of secondary alcohol metabolites remains uncertain. These metabolites do not produce more free radicals than their parent drugs but actually exhibit reduced activity in forming O2•− and H2O2, presumably because the presence of a secondary alcohol moiety in the side chain decreases affinity of the anthracycline for one-electron quinone reductases (Gervasi et al., 1986). Some investigators have suggested that secondary alcohol metabolites may be more potent than their parent drugs at inactivating mitochondrial or sarcoplasmic ion pumps (Olson & Mushlin, 1990), but this mechanism has been questioned (Zucchi et al., 2000). We have suggested that secondary alcohol metabolites may act by inactivating cytoplasmic aconitase, counterpart of the mitochondrial aconitase which reversibly isomerizes citrate to isocitrate in the Krebs cycle (Minotti et al., 1999,2000). While not particularly sensitive to transcriptional regulation (Minotti et al., 2001b), cytoplasmic aconitase is highly sensitive to post-translational modifications induced by DOXol. Thus, in vitro studies have shown that the secondary alcohol moiety of DOXol promotes disassembly of the Fe–S cluster of cytoplasmic aconitase, causing enzyme inactivation and releasing iron ions. The latter might induce cardiac damage by catalysing free radical reactions or by occupying cellular sites important to the contraction – relaxation cycle (like e.g., the Ca2+ release channel-ryanodine receptor 2 of sarcoplasmic reticulum) (reviewed in Minotti et al., 1999). Moreover, inactivation of cytoplasmic aconitase reduces substrate supply to the cytoplasmic isoform of isocitrate dehydrogenase, decreasing formation of [NADH+H+] by this enzyme and altering the redox balance of the cell (Narahari et al., 2000). Our results demonstrate that anthracycline treatment was accompanied by aconitase inactivation, which correlated with the cardiac levels of secondary alcohol metabolites but not of their parent carbonyl anthracyclines. Therefore, aconitase inactivation followed a rank order DOX>EPI>MEN10755. It is worth noting that MEN 10755 caused essentially no effect on aconitase, in spite of the fact that the levels of MEN 10755ol were only in part lower than those of EPIol (cf. Figure 8 and Table 3). This is explained by keeping in mind that the presence of a disaccharide moiety not only impairs cardiac uptake of MEN 10755 and formation of MEN 10755ol but also diminishes reactivity of the latter toward the Fe–S cluster of cytoplasmic aconitase (Minotti et al., 2000). Overall, aconitase inactivation correlated in a highly significant manner with both QαT/SαT elongations, suppression of isoprenaline-induced dP/dt increases, and morphologic scores, providing a good link between the different levels of alcohol metabolite formation/reactivity and the different levels of cardiotoxicity induced by DOX and EPI or MEN 10755.
Anthracycline treatments were also shown to impair Δ aconitase activity, that is an index of Fe–S cluster reassembly and recovery of enzymatic activity. Δ Aconitase decreases when cluster disassembly is followed by irreversible oxidation of cysteine residues required for coordinating iron in a cluster motif (e.g., cys 437) (reviewed in Minotti et al., 1999). Δ Aconitase activity was suppressed by anthracycline treatments in a fashion which was similar to that described for the loss of basal activity and reproduced the levels of formation of secondary alcohol metabolites (i.e., DOX was more inhibitory than EPI and the latter was more inhibitory than MEN 10755, cf. Figure 8a,b). This demonstrates that secondary alcohol metabolites may act not only by inducing cluster disassembly, but also by preventing cluster reassembly. Under such defined conditions of altered cluster reassembly, toxic iron increases further and induces cardiomyocyte apoptosis via free radical-dependent activation of NF-κB (Wang et al., 2002) or caspase 3 (Kotamraju et al., 2002). While providing correlates to explain the protective efficacy of iron chelators against cardiotoxicity (Myers, 1998) our findings demonstrate that MEN 10755 always induces less severe alterations of cluster assembly/disassembly than DOX or EPI.
In conclusion, we have shown that the cardiotoxicity of DOX or EPI or MEN 10755 depends on how effectively these anthracyclines distribute in the heart and convert to secondary alcohol metabolites with reactivity toward the Fe–S cluster of cytoplasmic aconitase. Whether similar mechanisms apply to other anthracyclines remains to be established. Daunorubicin, and especially 4-demethoxydaunorubicin (idarubicin), seem to be less cardiotoxic than DOX but the levels of their alcohol metabolites in plasma may be higher than those of DOXol (Danesi et al., 2002). In considering such inconsistency one should keep in mind that the expression and substrate specificity of anthracycline reductases may vary widely from blood cells to a given tissue or another, the yield of daunorubicinol or idarubicinol being higher or lower than that of DOXol depending on whether the parent anthracyclines are metabolized by specific families of carbonyl reductases or aldo/keto reductases, respectively (Forrest et al., 1991; Minotti et al., 1995). Moreover, circulating metabolites do not always exhibit good partitioning inside the heart, making it uncertain whether they actually contribute to inducing cardiotoxicity (Olson & Mushlin, 1990). An evaluation of the mechanisms of cardiotoxicity induced by daunorubicin or idarubicin vs DOX should therefore extend to comparing these anthracyclines in terms of cardiac uptake and alcohol metabolite formation or reactivity in appropriate animal models of chronic cardiomyopathy. Here we have shown that MEN 10755 exhibits both reduced cardiac uptake and impaired formation and reactivity of its alcohol metabolite, eventually inducing a pattern of chronic cardiotoxicity much less severe than that induced by DOX. Our studies suggest that MEN 10755 might offer advantages also in comparison to EPI, currently considered less toxic than DOX and of potential value for use in patients with cardiac risk factors. When examined in this context, MEN 10755 has the additional important feature of inducing less severe cardiotoxicity while also performing better than DOX in tumour cell lines or human tumour xenografts (Arcamone et al., 1997). This is observed in face of a reduced penetration of MEN 10755 in cancer cells (Gonzalez-Paz et al., 2001), similar to that observed in cardiomyocytes, and suggests that MEN 10755 is intrinsically superior to DOX at intercalating DNA or inhibiting topoisomerase II. It also suggests that secondary alcohol metabolites do not always contribute to the antitumour activity of anthracyclines; in cultured cells an excessive conversion of anthracyclines to secondary alcohol metabolites actually diminishes antitumour activity, as if the metabolites contributed to some undefined mechanism of resistance (Ax et al., 2000; Gonzalez et al., 1995). The unique behaviour of MEN 10755, inducing limited alcohol metabolite-dependent cardiotoxicity while also maintaining good antitumour activity, warrants characterization of its therapeutic index in clinical studies.
Acknowledgments
This work was supported by Istituto Mobiliare Italiano (IMI) (to Menarini Ricerche S.p.A.), and by AIRC, MURST COFIN 2001 and 2002, MURST ‘Center of Excellence on Aging at the University of Chieti' and FIRB RBNE 014HJ3-002 (to G.M.).
Abbreviations
- anthracycline-ol
anthracycline secondary alcohol metabolite
- CI
contractility index
- DBP
diastolic blood pressure
- DOX
doxorubicin
- dP/dt
maximum value of the first derivative of LVSP
- EPI
epirubicin
- H2O2
hydrogen peroxide
- HR
heart rate
- LVEDP
left ventricular end diastolic pressure
- LVSP
left ventricular systolic pressure
- MBP
mean blood pressure
- MEN 10755
(7-[(2,6-dideoxy-4-O-[3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)]-α-L-lyxohexopyranosyl]-4-demethoxydoxorubicinone)
- O2•−
superoxide anion
- SBP
systolic blood pressure
References
- ARCAMONE F., ANIMATI F., BERETTONI M., BIGIONI M., CAPRANICO G., CASAZZA A.M., CASERINI C, CIPOLLONE A., DE CESARE M., FRANCIOTTI M., LOMBARDI P., MADAMI A., MANZINI S., MONTEAGUDO E., POLIZZI D., PRATESI G., RIGHETTI S.C., SALVATORE C., SUPINO R., ZUNINO F. Doxorubicin disaccharide analogue: apoptosis-related improvement of efficacy in vivo. J. Natl. Cancer Inst. 1997;89:1217–1223. doi: 10.1093/jnci/89.16.1217. [DOI] [PubMed] [Google Scholar]
- AX W., SOLDAN M., KOCH L., MASER E. Development of daunorubicin resistance in tumour cells by induction of carbonyl reduction. Biochem. Pharmacol. 2000;59:293–300. doi: 10.1016/s0006-2952(99)00322-6. [DOI] [PubMed] [Google Scholar]
- BERTAZZOLI C., BELLINI O., MAGRINI U., TOSANA M.G. Quantitative experimental evaluation of adriamycin cardiotoxicity in the mouse. Cancer Treat. Rep. 1979;63:1877–1883. [PubMed] [Google Scholar]
- CIRILLO R., SACCO G., VENTURELLA S., BRIGHTWELL J., GIACHETTI A., MANZINI S. Comparison of doxorubicin- and MEN 10755-induced long term progressive cardiotoxicity in the rat. J. Cardiovasc. Pharmacol. 2000;35:100–108. doi: 10.1097/00005344-200001000-00013. [DOI] [PubMed] [Google Scholar]
- DANESI R., DEL TACCA M., SOLDANI G. Measurement of the SαT segment as the most reliable electrocardiogram parameter for the assessment of adriamycin-induced cardiotoxicity in the rat. J. Pharmacol. Methods. 1986;16:251–258. doi: 10.1016/0160-5402(86)90046-x. [DOI] [PubMed] [Google Scholar]
- DANESI R., FOGLI R., GENNARI A., CONTE P., DEL TACCA M. Pharmacokinetic-pharmacodynamic relationships of the anthracycline anticancer drugs. Clin. Pharmacokinet. 2002;41:431–444. doi: 10.2165/00003088-200241060-00004. [DOI] [PubMed] [Google Scholar]
- FORREST G.L., AKMAN S., DOROSHOW J., RIVERA H., KAPLAN W.D. Genomic sequence and expression of a cloned human carbonyl reductase gene with daunorubicin reductase activity. Mol. Pharmacol. 1991;40:502–507. [PubMed] [Google Scholar]
- FORREST G.L., GONZALEZ B., TSENG W., LI X., MANN J. Human carbonyl reductase overexpression in the heart advances the development of doxorubicin-induced cardiotoxicity in transgenic mice. Cancer Res. 2000;60:5158–5164. [PubMed] [Google Scholar]
- GERVASI P.G., AGRILLO M.R., CITTI L., DANESI R., DEL TACCA M. Superoxide anion production by adriamycinol from cardiac sarcosomes and by mitochondrial NADH dehydrogenase. Anticancer Res. 1986;6:1231–1235. [PubMed] [Google Scholar]
- GONZALEZ B., AKMAN S., DOROSHOW J., RIVERA H., KAPLAN W.D., FORREST G.L. Protection against daunorubicin cytotoxicity by expression of a cloned human carbonyl reductase cDNA in K562 leukemia cells. Cancer Res. 1995;55:4646–4650. [PubMed] [Google Scholar]
- GONZALEZ-PAZ O., POLIZZI D., DE CESARE M., ZUNINO F., BIGIONI M., MAGGI C.A., MANZINI S., PRATESI G. Tissue distribution, antitumour activity and in vivo apoptosis induction by MEN 10755 in nude mice. Eur. J. Cancer. 2001;37:431–437. doi: 10.1016/s0959-8049(00)00414-7. [DOI] [PubMed] [Google Scholar]
- KOTAMRAJU S., CHITAMBAR C.R., KALIVENDI S.V., JOSEPH J., KALAYANARAMAN B. Transferrin receptor-dependent iron uptake is responsible for doxorubicin-mediated apoptosis in endothelial cells: role of oxidant-induced iron signaling in apoptosis. J. Biol. Chem. 2002;277:17179–17187. doi: 10.1074/jbc.M111604200. [DOI] [PubMed] [Google Scholar]
- MAESSEN P.A., MROSS K.B., PINEDO H.M., van der VIJGH WJ. Metabolism of epidoxorubicin in animals: absence of glucuronidation. Cancer. Chemother. Pharmacol. 1987;20:85–87. doi: 10.1007/BF00252968. [DOI] [PubMed] [Google Scholar]
- MAZUÉ G., IATROPOULOS M., IMONDI A., CASTELLINO S., BRUGHERA M., PODESTÁ A., DELLA TORRE P., MONETA D. Anthracyclines: a review of general and special toxicity. Int. J. Oncol. 1995;7:713–726. [PubMed] [Google Scholar]
- MINOTTI G., CAIRO G., MONTI E. Role of iron in anthracycline cardiotoxicity: new tunes for an old song. FASEB J. 1999;13:199–212. [PubMed] [Google Scholar]
- MINOTTI G., CAVALIERE A.F., MORDENTE A., ROSSI M., SCHIAVELLO R., ZAMPARELLI R., POSSATI G.F. Secondary alcohol metabolites mediate iron delocalization in cytosolic fractions of myocardial biopsies exposed to anticancer anthracyclines. J. Clin. Invest. 1995;95:1595–1605. doi: 10.1172/JCI117833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINOTTI G., LICATA S., SAPONIERO A., MENNA P., CALAFIORE A.M., DI GIAMMARCO G., LIBERI G., ANIMATI F., CIPOLLONE A., MANZINI S., MAGGI C.A. Anthracycline metabolism and toxicity in human myocardium: comparisons between doxorubicin, epirubicin, and a novel disaccharide analogue with a reduced level of formation and [4Fe–4S] reactivity of its secondary alcohol metabolite. Chem. Res. Toxicol. 2000;13:1336–1341. doi: 10.1021/tx000143z. [DOI] [PubMed] [Google Scholar]
- MINOTTI G., PARLANI M., SALVATORELLI E., MENNA P., CIPOLLONE A., ANIMATI F., MAGGI C.A., MANZINI S. Impairment of myocardial contractility by anticancer anthracyclines: role of secondary alcohol metabolites and evidence of reduced toxicity by a novel disaccharide analogue. Br. J. Pharmacol. 2001a;134:1271–1278. doi: 10.1038/sj.bjp.0704369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINOTTI G., RONCHI R., SALVATORELLI E., MENNA P., CAIRO G. Doxorubicin irreversibly inactivates iron regulatory proteins 1 and 2 in cardiomyocytes: evidence for distinct metabolic pathways and implications for iron-mediated cardiotoxicity of antitumor therapy. Cancer Res. 2001b;61:8422–8428. [PubMed] [Google Scholar]
- MYERS C. The role of iron in doxorubicin-induced cardiomyopathy. Semin. Oncol. 1998;25 Suppl. 10:10–14. [PubMed] [Google Scholar]
- NARAHARI J., MA R., WANG M., WALDEN W.E. The aconitase function of iron regulatory protein 1. Genetic studies in yeast implicate its role in iron-mediated redox regulation. J. Biol. Chem. 2000;275:16227–16234. doi: 10.1074/jbc.M910450199. [DOI] [PubMed] [Google Scholar]
- OLSON R.D., MUSHLIN P.S. Doxorubicin cardiotoxicity: analysis of prevailing hypotheses. FASEB J. 1990;4:3076–3086. [PubMed] [Google Scholar]
- ROBERT J. Epirubicin. Clinical pharmacology and dose – effect relationship. Drugs. 1993;45 Suppl. 2:20–30. doi: 10.2165/00003495-199300452-00005. [DOI] [PubMed] [Google Scholar]
- SACCO G., BIGIONI M., EVANGELISTA S., GOSO C., MANZINI S., MAGGI C.A. Cardioprotective effects of zofenopril, a new angiotensin-converting enzyme inhibitor, on doxorubicin-induced cardiotoxicity in the rat. Eur. J. Pharmacol. 2001;414:71–78. doi: 10.1016/s0014-2999(01)00782-8. [DOI] [PubMed] [Google Scholar]
- SINGAL P.K., ILISKOVIC N. Doxorubicin-induced cardiomyopathy. N. Engl. J. Med. 1998;339:900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- STOSCHECK C.M. Quantitation of protein. Methods Enzymol. 1990;182:50–68. doi: 10.1016/0076-6879(90)82008-p. [DOI] [PubMed] [Google Scholar]
- WANG S., KOTAMRAJU S., KONOREV, E, KALIVENDI S., JOSEPH J., KALYANARAMAN B. Activation of nuclear factor kappaB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic – The role of hydrogen peroxide. Biochem. J. 2002;367:729–740. doi: 10.1042/BJ20020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZUCCHI R., YU G., GHELARDONI S., RONCA F., RONCA-TESTONI S. Effect of MEN 10755, a new disaccharide analogue of doxorubicin, on sarcoplasmic reticulum Ca(2+) handling and contractile function in rat heart. Br. J. Pharmacol. 2000;131:342–348. doi: 10.1038/sj.bjp.0703575. [DOI] [PMC free article] [PubMed] [Google Scholar]