Abstract
The aim of the present study was to verify a possible involvement of nitric oxide (NO) and of tachykinins in the contractile and relaxant effects caused by the activation of protease-activated receptor (PAR)-1 and PAR-2 in the longitudinal muscle of rat colon.
Mechanical responses to the PAR-1 activating peptides, SFLLRN-NH2 (10 nM–10 μM) and TFLLR-NH2 (10 nM–10 μM), and to the PAR-2-activating peptide, SLIGRL-NH2 (10 nM–10 μM), were examined in vitro in the absence and in the presence of different antagonists.
The relaxation induced by SFLLRN-NH2, TFLLR-NH2 and SLIGRL-NH2 was antagonised by the inhibitor of NO synthase L-Nω-nitroarginine methyl ester (300 μM), or by the inhibitor of the guanylyl cyclase, 1-H-oxodiazol-[1,2,4]-[4,3-a]quinoxaline-1-one (10 μM).
The contractile responses to PAR-1 and PAR-2 activation were concentration-dependently attenuated by SR140333 (0.1–1 μM), NK1 receptor antagonist, or by SR48968 (0.1–1 μM), NK2 receptor antagonist. The combined pretreatment with SR140333 (1 μM) and SR48968 (1 μM) produced additive suppressive effects on the contractile responses to PAR activation. Pretreatment of the preparation with capsaicin (10 μM) markedly reduced the contractions evoked by SFLLRN-NH2, TFLLR-NH2 and SLIGRL-NH2, while ω-conotoxin GVIA (0.2 μM) had no effect.
The present results suggest that in rat colonic longitudinal muscle, PAR-1 and PAR-2 activation can evoke (i) relaxation through the production of NO or (ii) contraction through the release of tachykinins, likely, from sensory nerves. These actions may contribute to motility disturbances during intestinal trauma and inflammation.
Keywords: PAR receptors, enteric nervous system, intestinal smooth muscle, nitric oxide, tachykinins
Introduction
During the last decade, there has been an exponential increase in data illustrating that the immune and nervous system are not separate entities. In particular, the interactions between the enteric nervous system and local immunocytes can be responsible for adaptive functional changes in the gastrointestinal tract, including motility and secretion. Several neuropeptides can affect immune reactions and, on the other hand, proinflammatory mediators may activate directly or indirectly the intrinsic neurones, which, in turn, releasing neurotransmitters, act on smooth muscle cells or on enterocytes (Bueno, 2000). There is now evidence that in addition to their well-established proteolytic roles, proteases such as trypsin, tryptase and thrombin may also act as cell-signalling molecules via protease-activated receptors (PARs). This family of G-protein-coupled receptors plays important roles in responses to injury, including inflammation and repair (Dery et al., 1998; Macfarlane et al., 2001) and includes four receptor subtypes (PAR-1, PAR-2, PAR-3 and PAR-4). PAR-1, PAR-3 and PAR-4, but not PAR-2, are activated by thrombin, whereas trypsin can activate PAR-2, and PAR-4 (Vu et al., 1991; Nystedt et al., 1994; Ishihara et al., 1997; Molino et al., 1997; Xu et al., 1998). The mechanism whereby proteases activate PARs involves the proteolytic cleavage and the unmasking of an N-terminal receptor sequence, which in turn acts as a tethered ligand, which activates the receptor itself (Dery et al., 1998). Short synthetic peptides (PAR-activating peptides), which have the same aminoacidic sequence of the tethered ligands of PAR-1, PAR-2 and PAR-4, are used to activate selectively the respective receptors, whereas PAR-3 does not respond to the presumed receptor-activating peptides (Ishihara et al., 1997).
The PARs, highly expressed in the gastrointestinal tract, have been hypothesised to play key roles throughout the digestive system, especially during inflammation and/or haemorrhage, where thrombin and/or mast cell tryptase become available as the endogenous agonists. A recent study in vivo has provided evidence for an increase of the mouse gastrointestinal transit in response to PAR-1 and PAR-2 activation (Kawabata et al., 2001). There is also in vitro evidence that PARs modulate smooth muscle mechanical activity and their activation can induce relaxant, contractile or biphasic responses (Al-Ani et al., 1995; Saifeddine et al., 1996; Corvera et al., 1997; Hollenberg et al., 1997,1999; Kawabata et al., 1999,2000; Cocks et al., 1999b; Mulè et al., 2002a).
PARs appear to be located on the enterocytes, myocytes and on myenteric neurones (Vergnolle, 2000). The activation of neuronal PARs in the intestine might be of particular importance because the intestinal tissues are exposed to proteases activating the PARs and the enteric nervous system plays an essential role in the regulation of intestinal functions, including motility. In general, PAR activation has been linked to proinflammatory responses (Dery et al., 1998; Vergnolle et al., 2001). Both agonists of PAR-1 (De Garavilla et al., 2001) and agonists of PAR-2 (Steinhoff et al., 2000) have been reported to cause inflammation by a neurogenic mechanism. PAR-1 and PAR-2 are expressed on guinea-pig myenteric neurones that also express excitatory (substance P) and inhibitory neurotransmitters (vasoactive intestinal peptide and nitric oxide) (Corvera et al., 1999). Activation of either of these receptors results in neuronal Ca2+ signals (Corvera et al., 1999), but it is not yet known whether it causes release of neurotransmitters.
Our previous study showed that PAR-1 and PAR-2 mediate changes of the motor activity, which might explain the alterations of colonic motility observed during inflammatory conditions (Mulè et al., 2002a). In particular, in the longitudinal smooth muscle of rat colon, the PAR activation induced contractile responses, which became biphasic at high concentrations. The effects were resistant to tetrodotoxin (TTX), but this not necessarily exclude involvement of neuronal mechanisms because TTX may not inhibit release of neurotransmitters following direct stimulation of receptors present in nerve terminals. Therefore, we hypothesised that the activation of PAR-1 and PAR-2 might cause the release of neurotransmitters known as participating in the regulation of intestinal motility.
The aim of the present study was to examine if the mechanical responses induced by PAR-1 or PAR-2 activation in rat colon longitudinal smooth muscle involve release and/or production of tachykinins as excitatory mediators or nitric oxide NO as inhibitory mediator.
Methods
Tissue preparation and mechanical recording
Experiments were authorised by the Ministero della Sanità (Rome, Italy). Adult male Wistar rats (250–400 g) were killed by cervical dislocation. The abdomen was immediately opened and the colon was removed distally to caecum. The colonic lumen was cleaned with Krebs solution of the following composition (mM): NaCl 119; KCl 4.5; MgSO4 2.5, NaHCO3 25, KH2PO4 1.2, CaCl2 2.5 and glucose 11.1. Segments of proximal colon of about 2 cm in length were cut. Each segment was suspended in a continuously perfused organ bath containing 5 ml of gassed (95% O2 and 5% CO2) Krebs solution that was maintained at 37°C. Preparations were then tied with silk thread to an isometric force transducer (DY2 Ugo Basile) for recording the longitudinal muscle mechanical activity, which was displayed on an ink writer recorder (Gemini, Ugo Basile). Segments were allowed to equilibrate for at least 30 min under 1 g load, before starting the experiment.
Design of study
After the equilibration period, the preparation was challenged with carbachol at 10 μM which, in preliminary experiments, was demonstrated to induce a maximal effect. Then, the responses of the preparations to cumulative concentrations of PAR-activating peptides were examined in the absence and in the presence of different inhibitors/antagonists. Peptides tested were: SFLLRN-NH2 (0.01–10 μM), a PAR-1 agonist, and SLIGRL-NH2 (0.01–10 μM), murine PAR-2-specific agonist (Nystedt et al., 1994). Although we previously demonstrated that the responses to SFLLRN-NH2 resulted from the activation of PAR-1 in rat colon (Mulè et al., 2002a), SFLLRN-NH2 has been reported to have a weak agonistic activity towards PAR-2 (Hollenberg et al., 1997). Therefore, in order to confirm the PAR-1 activation, we also performed some experiments using TFLLR-NH2, a highly specific PAR-1 agonist. The peptides were added into the bath in volumes of 50 μl after switching off the perfusion to give the concentrations indicated. Each concentration was left in contact with the tissue for 2 min. In preliminary experiments, two concentration–response curves were obtained in the same tissue to assess the reproducibility of the responses. In order to rule out any aspecific effect, the vehicle alone was added to the bath and it failed to enhance the tone of the preparation (Figure 1). The responses to PAR-1 and PAR-2 activation were tested in the absence and in the presence of the following drugs: L-Nω-nitroarginine methyl ester (L-NAME), inhibitor of NO synthase (300 μM); 1-H-oxodiazol-[1,2,4]-[4,3-a]quinoxaline-1-one (ODQ), inhibitor of NO-stimulated guanylyl cyclase (1–10 μM); SR140333, antagonist of NK1 receptors (0.1–1 μM); SR48968, antagonist of NK2 receptor (0.1–1 μM), capsaicin (10 μM), reported to cause depletion of tachykinins from sensory nerve fibres (Maggi, 1995), ω-conotoxin GVIA (0.2 μM), blocker of neuronal N-type Ca2+ channels. The antagonists were added to the perfusing solution at least 30 min before testing the PAR-activating peptides, except capsaicin. In fact, it caused an initial increase in the spontaneous contraction amplitude followed by a gradual decrease and, then, the mechanical activity stabilised after about 1 h. Therefore, the peptides were tested after 90 min capsaicin treatment.
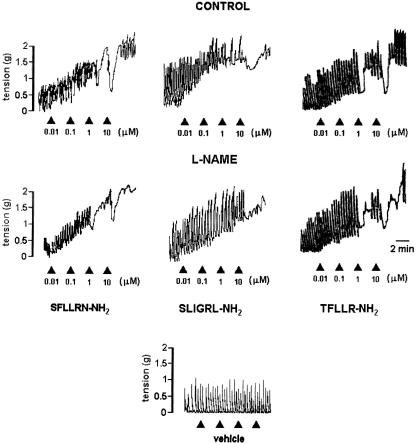
Figure 1.
Representative recordings of the effects induced by the PAR-1 agonists, SFLLRN-NH2 and TFLLR-NH2, and by the PAR-2 agonist, SLIGRL-NH2, on the longitudinal muscle of rat colon in the absence and in the presence of L-NAME, inhibitor of the NO synthesis. L-NAME (300 μM) markedly reduced the relaxation evoked by the highest concentrations of the PAR-activating peptides. The arrow indicates the application of the drug. In the same experimental conditions the cumulative addition of vehicle did not modify the spontaneous mechanical activity.
Data analysis and statistics
The contractile response of the longitudinal muscle was defined as the change in the resting tone (the bottom level of the tension oscillations) and was expressed as a percentage of the contraction caused by 10 μM carbachol. The amplitude values of the relaxant responses to PARs refer to the maximal peak obtained during the application period. All data are expressed as means ±s.e.m. n indicates the number of animals from which intestinal segment was taken. The half-maximal concentration (EC50) of the PAR-activating peptides was calculated by interpolation from the respective concentration–response curves. Statistical analysis was performed by means of Student's paired t-test or analysis of variance (ANOVA) followed by Bonferroni test, when appropriate. A probability value of less than 0.05 was regarded as significant.
Drugs
The following drugs were used: SFLLRN-NH2, SLIGRL-NH2 (Bachem AG, Bubendorf, Switzerland), TFLLR-NH2, a kind gift by Dr N. Vergnolle (University of Calgary), carbamylcholine chloride (carbachol), L-NAME, ODQ, capsaicin, ω-cono-toxin GVIA (all purchased from Sigma, Chemical Corp., St Louis, MO, U.S.A.), (S)-N-methyl-N[4-(4-acetyl-amino-4-phenylpiperi-dino)-2-(3,4-dichloro-phenyl)butyl]benzamide, (SR48968) (S)-1-[2-[3-(3,4-dichlorophenyl)-1 (3-isopropoxy-phenylacetyl) piperidin-3yl] ethyl]-4-phenyl-1 azaniabicyclo[2.2.2] octane chloride (SR140333) (kind gifts from Sanofi Recherche, Montpellier Cédex, France). All drugs were dissolved in distilled water, except SR48968, SR140333 and ODQ, which were dissolved in DMSO; capsaicin was dissolved in absolute ethanol. The working solutions were prepared fresh on the day of the experiment by diluting the stock solutions. Control experiments using the different solvents alone showed that none had effects on the tissue responses studied.
Results
Rat colon showed spontaneous mechanical activity, consisting of spontaneous phasic contractions. PAR-1- and PAR-2-activating peptides, SFLLRN-NH2 (10 nM–10 μM), TFLLR-NH2 (10 nM–10 μM) and SLIGRL-NH2 (10 nM–10 μM), respectively, produced a contractile effect, consisting in an increase of the basal tone with the maintenance of the phasic contractions. At high concentrations used, the response became biphasic, a short-lived relaxation followed by contraction (Figure 1).
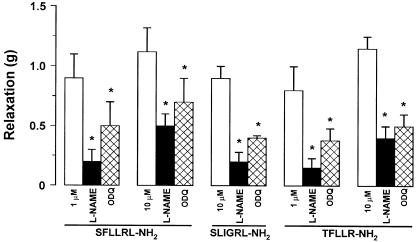
In order to determine if the effects of PAR-1 and PAR-2 agonists were because of the production of NO, we tested the responses to SFLLRN-NH2, TFLLR-NH2 and SLIGRL-NH2 in the presence of L-NAME, NO synthase inhibitor or ODQ, inhibitor of NO-dependent guanylyl cyclase. L-NAME (300 μM), which per se did not affect significantly the spontaneous mechanical activity, reduced the relaxation induced by PAR-1- or PAR-2-activating peptides, without affecting the contractile responses (Figures 1 and 2). ODQ at a concentration of 1 μM slightly reduced the relaxant responses to SFLLRN-NH2 or SLIGRL-NH2. When the concentration of ODQ was increased to 10 μM, a significant reduction of the relaxation to PAR-activating peptides was observed (Figure 2).
Figure 2.
Effects of L-NAME and ODQ on the longitudinal muscle relaxation evoked by PAR-1-and PAR-2-activating peptides in rat colon. The relaxation induced by SFLLRN-NH2 (1–10 μM), TFLLR-NH2 (1–10 μM) or SLIGRL-NH2 (10 μM) was significantly reduced by L-NAME (300 μM) or ODQ (10 μM). Data are means ±s.e.m. of at least four experiments. *P<0.05, compared to the control value.
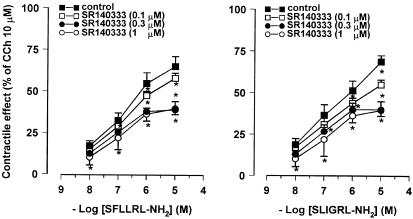
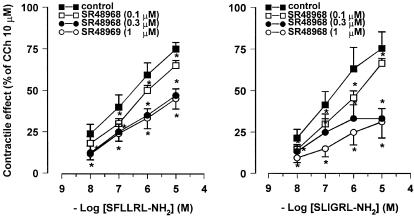
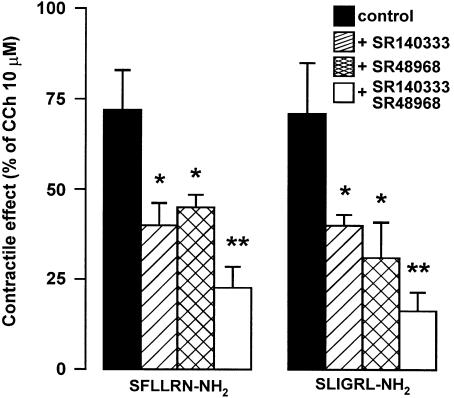
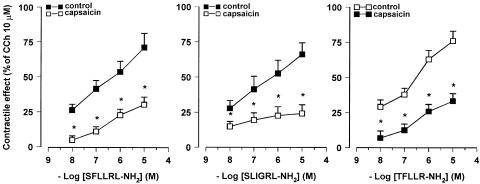
In order to determine if the responses to PAR-1- and PAR-2-activating peptides were because of the release of tachykinins, the effects evoked by SFLLRN-NH2, TFLLR-NH2 and SLIGRL-NH2 were tested in the presence of SR140333, NK1 receptor-selective antagonist, or SR48968, NK2 receptor-selective antagonist. The specificity of SR140333 and of SR48968 as NK1 and NK2 receptor antagonist, respectively, has been already demonstrated in our previous experiments on rat colon (Mulè et al., 2000). SR140333 (0.1–1 μM), which per se did not affect the spontaneous mechanical activity, produced a reduction of the contractile effects induced by PAR-1- and PAR-2-activating peptides, without affecting the relaxation. As shown in Figure 3, the concentration–response curves to peptides were displaced to the right with a significant reduction of the maximal contractile effect. Such a reduction reached a maximum in the presence of 0.3 μM SR140333. The EC50 values for the contractile effects of PAR-activating peptides before and after the different concentrations of SR140333 are shown in Table 1 . Also, in the presence of SR48968 (0.1–1 μM), which did not modify significantly the spontaneous mechanical activity, the contractile responses to SFLLRN-NH2, TFLLR-NH2 and SLIGRL-NH2 were decreased, whereas the relaxation persisted unaltered. Once more, the reduction by SR48968 resulted concentration-dependent and it reached the maximum at 0.3 μM (Figure 4 and Table 1). Furthermore, the suppressive effects of the two tachykinin receptor antagonists, on the contractile response evoked by SFLLRN-NH2 (10 μM) and SLIGRL-NH2 (10 μM) were additive when co-administered at the concentration of 1 μM for each (Figure 5). Moreover, in capsaicin (10 μM)-pretreated preparations, the contractile responses to SLFFRN-NH2, TFLLR-NH2 and SLIGRL-NH2 were markedly reduced, whereas the relaxation was not modified (Figure 6).
Figure 3.
Concentration–response curves for the contractile effects evoked by PAR-1 and PAR-2 agonists on the longitudinal muscle of rat colon in the absence and in the presence of SR140333 (0.1–1 μM), NK1 receptor antagonist. SR140333 reduced in a concentration–dependent manner, the responses to both SFLLRN-NH2 and SLIGRL-NH2. The contractile effects are expressed as a percentage of the maximal contraction to carbachol (10 μM). Each value is mean±s.e.m. of at least five experiments. *P<0.05, compared to the control value.
Table 1.
EC50 values of the PAR-1- and PAR-2-activating peptides before and after SR140333 or SR 48968, respectively, NK1 and NK2 receptor antagonists
| EC50(M) | ||||
|---|---|---|---|---|
| Control | After SR140333 | |||
| Peptide | 0.1 μM | 0.3 μM | 1 μM | |
| SFLLRN-NH2 | 1.0±0.2 × 10−7 | 3.3±0.3 × 10−7 | 5.0±0.8 × 10−7* | 8.0±0.3 × 10−7* |
| TFLLR-NH2 | 2.2±0.9 × 10−7 | 4.5±1.5 × 10−7 | 8.0±0.6 × 10−7* | 1.1±0.4 × 10−6* |
| SLIGRL-NH2 | 1.2±0.5 × 10−7 | 4.4±0.3 × 10−7* | 7.0±0.2 × 10−7* | 9.8±0.4 × 10−7 |
| After SR48968 | ||||
| 0.1 μM | 0.3 μM | 1 μM | ||
| SFLLRN-NH2 | 1.1±0.3 × 10−7 | 6.3±0.3 × 10−7* | 1.5±0.3 × 10−6* | 1.7±0.2 × 10−6* |
| TFLLR-NH2 | 2.1±0.3 × 10−7 | 7.3±0.3 × 10−7* | 2.0±0.3 × 10−6* | 3.9±0.9 × 10−6 |
| SLIGRL-NH2 | 1.2±0.3 × 10−7 | 7.0±0.7 × 10−7* | ND | ND |
Data are mean values ±s.e.m. of at least four experiments.
P<0.05, compared to the control value. ND, not determined.
Figure 4.
Concentration–response curves for the contractile effects evoked by PAR-1 and PAR-2 agonists on the longitudinal muscle of rat colon in the absence and in the presence of SR48968 (0.1–1 μM), NK2 receptor antagonist. SR48968 reduced in a concentration–dependent manner, the responses to both SFLLRN-NH2 and SLIGRL-NH2. The contractile effects are expressed as a percentage of the maximal contraction to carbachol (10 μM). Each value is mean±s.e.m. of at least five experiments. *P<0.05, compared to the control value.
Figure 5.
Histograms showing the effects of SR140333, NK1 receptor antagonist, SR48968, NK2 receptor antagonist, alone or in combination, on the contractile responses evoked by PAR-1 or PAR-2 activation on the longitudinal muscle of rat colon. The contractile responses to SFLLRN-NH2 (10 μM) and SLIGRL-NH2 (10 μM) were significantly reduced by SR48968 (1 μM) or SR140333 (1 μM) and even more by a combination of SR48968 (1 μM) and SR140333 (1 μM). The contractile effects are expressed as a percentage of the maximal contraction to carbachol (10 μM). Each value is mean ±s.e.m. of five experiments. *P<0.05, compared to the control value. **P<0.05, compared to the SR140333 or SR48968 alone.
Figure 6.
Effects of the capsaicin on the concentration–response curves for the contractile responses evoked by PAR-1 and PAR-2 agonists on the longitudinal muscle of rat colon. Capsaicin (10 μM) significantly reduced the contractile responses to PAR activation. The contractile effects are expressed as a percentage of the maximal contraction to carbachol (10 μM). Each value is mean ±s.e.m. of five experiments. *P<0.05, compared to the control value.
Lastly, pretreatment with ω-conotoxin GVIA (0.2 μM for 1 h), blocker of neuronal N-type Ca2+ channels, did not significantly modify the spontaneous contractile activity and it failed to affect the responses to SFLLRN-NH2, TFLLR-NH2 and SLIGRL-NH2 (data not shown).
Discussion
The major conclusion of this study is that PAR-1 and PAR-2 activation can evoke contraction or relaxation in the longitudinal smooth muscle of rat colon by releasing tachykinins, likely from sensory nerves, or by producing NO, respectively. These actions appear to occur independent of the action potential generation.
Although the mechanical effects induced by PAR-1 and PAR-2 activation are reported to be TTX- insensitive (Saifeddine et al., 1996; Corvera et al., 1997; Cocks et al., 1999b; Kawabata et al., 1999; Mulè et al., 2002a), recent studies have indicated that PAR-1 and PAR-2 can be present in the enteric neurones in guinea-pig and porcine small intestine (Corvera et al., 1999; Green et al., 2000). The previously reported lack of effect of TTX (Mulè et al., 2002a) supports the hypothesis that both inhibitory and contractile responses to PAR-1 and PAR-2 are independent by propagation of neural action potential, but it does not exclude that prejunctional PARs may directly induce release of mediators.
Smooth muscle relaxation through the activation of PARs can be mediated by endothelial NO in blood vessels (Hollenberg et al., 1993,1996,1999; Saifeddine et al., 1996), by epithelial prostanoids in airways (Cocks et al., 1999a; Lan et al., 2000) and by activation of apamin-sensitive K+ channels in gastrointestinal tissues (Cocks et al., 1999b; Kawabata et al., 1999; Mulè et al., 2002a). Since NO is one of the main nonadrenergic noncholinergic inhibitory neurotransmitters to intestinal smooth muscle (Bennett, 1997), including rat colon (Serio et al., 1995; Mulè et al., 1999), we investigated its eventual involvement in the relaxation evoked by PAR-1 and PAR-2. Our data using L-NAME indicate that PAR-1 and PAR-2 activation induces NO production, responsible, at least in part, for the longitudinal muscle relaxation evoked by high concentrations of PARs. This hypothesis is supported by the observation that the relaxation to PAR-1- and PAR-2-activating peptides was also reduced by ODQ, inhibitors of NO-dependent guanylyl cyclase. Although PAR-1 and PAR-2 elicit release of endothelial NO, leading to vascular relaxation in vitro (Al-Ani et al., 1995; Saifeddine et al., 1996; Moffatt & Cocks, 1998; Hamilton & Cocks, 2000; Hamilton et al., 2001; Trottier et al., 2002), this is the first experimental evidence that NO is involved in the intestinal relaxation evoked by PAR-1 and PAR-2 activation. However, it remains to clarify the source of NO responsible for this action. In fact, in the intestinal tissue, several types of cells can release NO: nerve terminals (Ward et al., 1992), endothelial (Pollock et al., 1993), Cajal (Xue et al., 1994) and smooth muscle (Grider et al., 1992) cells. NO would act by opening of apamin-sensitive K+ channels, because the relaxation induced by PAR-1- and PAR-2-activating peptides is antagonised by apamin (Mulè et al., 2002a). On the other hand, it has already been reported that in this preparation NO can act through the apamin-sensitive K+ channels (Mulè et al., 1999).
The tachykinins contribute to the physiological regulation of various digestive functions, participating in the control of gastrointestinal motility, secretion, vascular permeability, immune function and pain sensitivity. Now there is mounting evidence that tachykinins are involved in inflammation-induced perturbations of digestive function (Holzer & Holzer-Petsche, 2001). NK2 receptors are expressed by the muscularis externa of the rat colon (Grady et al., 1996) and NK1 and NK2 receptors are both involved in the contractile responses of rat colon to endogenous tachykinins (Mulè et al., 2000). For these considerations, we verified the eventual involvement of tachykinins in the PAR-induced effects. We found that the contractile effects to PAR-1- and PAR-2-activating peptides were markedly attenuated by SR140333, NK1 receptor antagonist or by SR48968, NK2 receptor antagonist, indicating that both NK1 and NK2 receptors are involved in the action of the PARs. The observation that the suppressive effect of NK1 receptor-selective antagonist at a maximal concentration was additive with that due to the maximal concentration of NK2 receptor-selective antagonist strengthens the hypothesis that both types of NK receptors are involved in the response to PAR-activating peptides. Moreover, capsaicin also markedly reduced the ability of SFLLRN-NH2 TFLLR-NH2, and SLIGRL-NH2 to evoke contractile responses. Capsaicin may cause desensitisation of the receptor VR1, expressed by some enteric neurones (Nozawa et al., 2001), blockade of voltage-gated calcium channels and other effects (Maggi, 1995). However, it is likely that in our preparation, exposed to high concentration of capsaicin for a prolonged time, capsaicin acts on sensory nerve fibres to release, and then to deplete, biologically active substances, such as tachykinins (Maggi, 1995). The depletion of tachykinins from sensory nerves could account for the reduction of the contractile effect to SFLLRN-NH2 and SLIGRL-NH2, and it could strengthen the hypothesis that PAR-1 and PAR-2 activation can induce colonic smooth muscle contraction by stimulating the release of tachykinin peptides from sensory neurones within the colonic wall. Steinhoff and co-workers (2000) have recently reported that SLIGRL-NH2 can evoke the release of substance P from peripheral terminals of rat spinal sensory neurones. Interestingly, a similar system has also been described for trypsin-induced contraction of the guinea-pig bronchus, with the release of tachykinins from sensory nerves being responsible for the final contractile response (Carr et al., 2000; Ricciardolo et al., 2000).
We tested the effects of SFLLRN-NH2, TFLLR-NH2 and SLIGRL-NH2 in the presence of ω-conotoxin GVIA, in order to verify the hypothesis that the responses might be because of a prejunctional action of the PAR-1 and PAR-2-activating peptides. The observation that PAR-induced effects were insensitive to ω-conotoxin GVIA indicates that Ca2+ influx through N-type channels is not a required step in the mechanism of the responses to PAR activation. However, ω-conotoxin GVIA-sensitive Ca2+ channels are not uniformly represented on mammalian autonomic nerve terminals (De Luca et al., 1990). The transmitter release from nerve terminals in some tissues (e.g., rat bladder or duodenum) does not involve Ca2+ influx through ω-conotoxin GVIA-sensitive channels (Maggi et al., 1988). We ourselves have previously demonstrated that the N-type channels are not responsible for the mechanical responses induced by electrical field stimulation in rat proximal colon (Mulè & Serio, 1997).
Release of excitatory and inhibitory mediators is not the only mechanism involved in the mechanical effects of PAR-1- and PAR-2-activating peptides in our preparation. In fact, although antagonism of NK1 and NK2 receptors and block of the NO synthesis strongly inhibited the contractile and relaxant responses, respectively, these responses were not abolished. Therefore, the residual responses could be explained assuming that the smooth muscle cells are directly stimulated. On the other hand, PAR-2 have been reported to be highly expressed by rat colonic smooth muscle (Corvera et al., 1997). Previously, we reported that the activation of a phospholipase C (PLC) triggered by PAR-1 and PAR-2 is responsible for the contractile actions (Mulè et al., 2002b). It is possible that the PAR-activating peptides act first via the release of tachykinins, which in turn trigger a pathway that involves the activation of PLC in the course of the contractile event.
In conclusion, to our knowledge, this study is the first demonstrating that the contractile and relaxant effects induced by PAR-1 and PAR-2 activation in intestinal smooth muscle can be because of release of neuronal tachykinins and production of NO. These findings once again support the hypothesis that PAR agonists play a role as proinflammatory mediators. Endogenous activators, such as thrombin or tryptase, might become accessible to the receptors present on the neurones or endothelial cells in the rat during inflammation or tissue injury and trigger release of tachykinins and NO resulting in changes of motility.
Acknowledgments
This work was supported by a grant from Ministero dell'Università e della Ricerca scientifica, Italy.
Abbreviations
- L-NAME
L-Nω-nitroarginine methyl ester
- NO
nitric oxide
- ODQ
1-H-oxodiazol-[1,2,4]-[4,3-a]quinoxaline-1-one
- PARs
protease-activated receptors
- PLC
phospholipase C
- TTX
tetrodotoxin
References
- AL-ANI B., SAIFEDDINE M., HOLLENBERG M.D. Detection of functional receptors for the proteinase-activated-re-ceptor-2-activating polypeptide, SLIGRL-NH2, in rat vascular and gastric smooth muscle. Can. J. Physiol. Pharmacol. 1995;73:1203–1207. doi: 10.1139/y95-172. [DOI] [PubMed] [Google Scholar]
- BENNETT M.R. Non-adrenergic non-cholinergic (NANC) transmission to smooth muscle: 35 years on. Progr. Neurobiol. 1997;52:159–195. doi: 10.1016/s0301-0082(97)00012-9. [DOI] [PubMed] [Google Scholar]
- BUENO L. Neuroimmune alterations of ENS functioning. Gut. 2000;47 suppl IV:63–65. doi: 10.1136/gut.47.suppl_4.iv63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARR M.J., SCHECHTER M., UNDEM B.J. Trypsin-induced, neurokinin-mediated contraction of guinea-pig bronchus. Am. J. Resp. Crit. Care Med. 2000;162:1662–1667. doi: 10.1164/ajrccm.162.5.9912099. [DOI] [PubMed] [Google Scholar]
- COCKS T.M., FONG B., CHOW J.M., ANDERSON G.P., FRAUMAN A.G., GOLDIE R.G., HENRY P.J., CARR M.J., HAMILTON J.R., MOFFATT J.D. A protective role for protease-activated receptors in the airways. Nature. 1999a;398:156–160. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- COCKS T.M., SOZZI V., MOFFATT J.D., SELEMIDIS S. Protease-activated receptors mediate apamin-sensitive relaxation of mouse and guinea pig gastrointestinal smooth muscle. Gastroenterology. 1999b;116:586–592. doi: 10.1016/s0016-5085(99)70180-0. [DOI] [PubMed] [Google Scholar]
- CORVERA C.U., DÉRY O., MCCONALOGUE K., BÖHM S.K., KHITIN L.M., CAUGHEY G.H., PAYAN D.G., BUNNETT N.W. Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J. Clin. Invest. 1997;100:1383–1393. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORVERA C.U., DÉRY O., MCCONALOGUE K., GAMP P., THOMA M., AL-ANI B., CAUGHEY G.H., HOLLENBERG M.D., BUNNETT N.W. Thrombin and mast cell tryptase regulate guinea-pig myenteric neurons through proteinase-activated receptors-1 and -2. J. Physiol. 1999;517:741–756. doi: 10.1111/j.1469-7793.1999.0741s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE GARAVILLA L., VERGNOLLE N., YOUNG S.H., ENNES H., STEINHOFF M., OSSOVSKAYA V.S., D'ANDREA M.R., MAYER E.A., WALLACE J.L., HOLLENBERG M.D., ANDRADE-GORDON P., BUNNETT N.W. Agonists of proteinase-activated receptor 1 induce plasma extravasation by a neurogenic mechanism. Br. J. Pharmacol. 2001;133:975–987. doi: 10.1038/sj.bjp.0704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LUCA A., LI C.G., RAND M.J., REID J.J., THAINA P., WONG-DUSTING H.K. Effects of ω-conotoxin GVIA on autonomic neuroeffector transmission in various tissues. Br. J. Pharmacol. 1990;101:437–447. doi: 10.1111/j.1476-5381.1990.tb12727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DERY O., CORVERA C.U., STEINHOFF M., BUNNETT N.W. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am. J. Physiol. 1998;274:C1429–C1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- GRADY E.F., BALUK P., BÖHM S., GAMP P.D., WONG H., PAYAN D.G., ANSEL J., PORTBURY A.L., FURNESS J.B., MCDONALD D.M., BUNNETT N.W. Characterization of antisera specific to NK1, NK2, and NK3 neurokinin receptors and their utilization to localize receptors in the rat gastrointestinal tract. J. Neurosci. 1996;16:6975–6986. doi: 10.1523/JNEUROSCI.16-21-06975.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN B.T., BUNNETT N.W., KULKARNI-NARLA A., STEINHOFF M., BROWN D.R. Intestinal type 2 proteinase-activated receptors: expression in opioid-sensitive secretomotor neural circuits that mediate epithelial ion transport. J. Pharmacol. Exp. Ther. 2000;295:410–416. [PubMed] [Google Scholar]
- GRIDER J.R., MURTHY K.S., JIN J.G., MAKHLOUF G.M. Stimulation of nitric oxide from muscle cells by VIP: prejunctional enhancement of VIP release. Am. J. Physiol. 1992;262:G774–G778. doi: 10.1152/ajpgi.1992.262.4.G774. [DOI] [PubMed] [Google Scholar]
- HAMILTON J.R., COCKS T.M. Heterogeneous mechanisms of endothelium-dependent relaxation for thrombin and peptide activators of protease-activated receptor-1 in porcine isolated coronary artery. Br. J. Pharmacol. 2000;130:181–188. doi: 10.1038/sj.bjp.0703146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON J.R., MOFFATT J.D., FRAUMAN A.G., COCKS T.M. Protease-activated receptor (PAR) 1 but not PAR2 or PAR4 mediates endothelium-dependent relaxation to thrombin and trypsin in human pulmonary arteries. J. Cardiovasc. Pharmacol. 2001;38:108–119. doi: 10.1097/00005344-200107000-00012. [DOI] [PubMed] [Google Scholar]
- HOLLENBERG M.D., LANIYONU A.A., SAIFEDDINE M., MOORE G.J. Role of the amino- and carboxyl-terminal domains of thrombin receptor-derived polypeptides in biological activity in vascular endothelium and gastric smooth muscle: evidence for receptor subtypes. Mol. Pharmacol. 1993;43:921–930. [PubMed] [Google Scholar]
- HOLLENBERG M.D., SAIFEDDINE M., AL ANI B. Proteinase-activated receptor-2 in rat aorta: structural requirements for agonist activity of receptor-activating peptides. Mol. Pharmacol. 1996;49:229–233. [PubMed] [Google Scholar]
- HOLLENBERG M.D., SAIFEDDINE M., AL-ANI B., GUI Y. Proteinase-activated receptor 4 (PAR4): action of PAR4-activating peptides in vascular and gastric tissue and lack of cross-reactivity with PAR1 and PAR2. Can. J. Physiol. Pharmacol. 1999;77:458–464. [PubMed] [Google Scholar]
- HOLLENBERG M.D., SAIFEDDINE M., AL-ANI B., KAWABATA A. Proteinase activated receptor: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor-activating peptides. Can. J. Physiol. Pharmacol. 1997;75:832–841. [PubMed] [Google Scholar]
- HOLZER P., HOLZER-PETSCHE U. Tachykinin receptors in the gut: physiological and pathological implications. Curr. Opin. Pharmacol. 2001;1:583–590. doi: 10.1016/s1471-4892(01)00100-x. [DOI] [PubMed] [Google Scholar]
- ISHIHARA H., CONNOLLY A.J., ZENG D., KAHN M.L., ZHENG Y.W., TIMMONS C., TRAM T., COUGHLIN S.R. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature. 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., KUROKI N., NISHIKAWA H., KAWAI K. Dual modulation by thrombin of the motility of rat oesophageal muscularis mucosae via two distinct protease-activated receptors (PARs): a novel role for PAR-4 as opposed to PAR-1. Br. J. Pharmacol. 2000;131:578–584. doi: 10.1038/sj.bjp.0703590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., NAGATA N., KAWAO N., MASUKO T., NISHIKAWA H., KAWAI K. In vivo evidence that protease-activated receptors 1 and 2 modulate gastrointestinal transit in the mouse. Br. J. Pharmacol. 2001;133:1213–1218. doi: 10.1038/sj.bjp.0704211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., NISHIKAWA H., KAWAI K. Modulation by protease-activated receptors of the rat duodenal motility in vitro: possible mechanisms underlying the evoked contraction and relaxation. Br. J. Pharmacol. 1999;128:865–872. doi: 10.1038/sj.bjp.0702755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAN R.S., STEWART G.A., HENRY P.J. Modulation of airway smooth muscle tone by protease activated receptor-1, -2, -3 and –4 in trachea isolated from influenza A virus-infected mice. Br. J. Pharmacol. 2000;129:63–70. doi: 10.1038/sj.bjp.0703007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACFARLANE S.R., SEATTER M.J., KANKE T., HUNTER G., PLEVIN R. Proteinase-activated receptors. Pharmacol. Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- MAGGI C.A. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog. Neurobiol. 1995;45:1–98. doi: 10.1016/0301-0082(94)e0017-b. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., PATACCHINI R., SANTICIOLI P., LIPPE T., GIULIANI S., GEPPETTI P., DEL BIANCO E., SELLERI S., MELI A. The effect of ω-conotoxin GVIA, a peptide modulator of the N-type voltage sensitive calcium channels, on motor responses produced by activation of efferent and sensory nerves in mammalian smooth muscle. Naunyn-. Scmiedebergs Arch. Pharmacol. 1988;334:172–180. doi: 10.1007/BF00174856. [DOI] [PubMed] [Google Scholar]
- MOFFATT J.D., COCKS T.M. Endothelium-dependent and –independent responses to protease-activated receptor-2 (PAR-2) activation in mouse isolated renal arteries. Br. J. Pharmacol. 1998;125:591–594. doi: 10.1038/sj.bjp.0702157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLINO M., BARNATHAN E.S., NUMEROF R., CLARK J., DREYER M., CUMASHI A., HOXIE J.A., SCHECTER N., WOOLKALIS M., BRASS L.F. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J. Biol. Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- MULÈ F., BAFFI M.C., CERRA M.C. Dual effect mediated by protease-activated receptors on the mechanical activity of rat colon. Br. J. Pharmacol. 2002a;136:367–374. doi: 10.1038/sj.bjp.0704746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULÈ F., BAFFI M.C., FALZONE M., CERRA M.C. Signal transduction pathways involved in the mechanical responses to the protease-activated receptors in rat colon. J. Pharmacol. Exp. Therap. 2002b;303:1–8. doi: 10.1124/jpet.102.041301. [DOI] [PubMed] [Google Scholar]
- MULÈ F., D'ANGELO S., SERIO R. Tonic inhibitory action by nitric oxide on spontaneous mechanical activity in rat proximal colon: involvement of cyclic GMP and apamin-sensitive K+ channels. Br. J. Pharmacol. 1999;127:514–520. doi: 10.1038/sj.bjp.0702537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULÈ F., D'ANGELO S., TABACCHI G., SERIO R. Involvement of tachykinin NK2 receptors in the modulation of spontaneous motility in rat proximal colon. Neurogastroenterol. Mot. 2000;12:459–466. doi: 10.1046/j.1365-2982.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- MULÈ F., SERIO R. Inhibition of mechanical activity by neurotensin in rat proximal colon: involvement of nitric oxide. Am. J. Physiol. 1997;273:G491–G497. doi: 10.1152/ajpgi.1997.273.2.G491. [DOI] [PubMed] [Google Scholar]
- NOZAWA Y., NISHIHARA K., YAMAMOTO A., NAKANO M., AJIOKA H., MATSUURA N. Distribution and characterization of vanilloid receptors in the rat stomach. Neurosci. Lett. 2001;309:33–36. doi: 10.1016/s0304-3940(01)02021-3. [DOI] [PubMed] [Google Scholar]
- NYSTEDT S., EMILSSON K., WAHLESTEDT C., SUNDELIN J. Molecular cloning of a potential proteinase activated receptor. Proc. Nat. Acad. Sci. U.S.A. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK J.S., NATANE M., BUTTERY L.D., MARTINEZ A., SPRINGALL D., POLAK J.M. Characterization and localization of endothelial nitric oxide synthase using specific monoclonal antibodies. Am. J. Physiol. 1993;265:C1379–C1387. doi: 10.1152/ajpcell.1993.265.5.C1379. [DOI] [PubMed] [Google Scholar]
- RICCIARDOLO F.L., STEINHOFF M., AMADESI S., GUERRINI R., TOGNETTO M., TREVISANI M., CREMINON C., BERTRAND C., BUNNETT N.W., FABBRI L.M., SALVATORI S., GEPPETTI P. Presence and bronchomotor activity of protease-activated receptor-2 in guinea pig airways. Am. J. Resp. Crit. Care Med. 2000;161:1672–1680. doi: 10.1164/ajrccm.161.5.9907133. [DOI] [PubMed] [Google Scholar]
- SAIFEDDINE M., AL-ANI B., CHENG C.H., WANG L., HOLLENBERG M.D. Rat proteinase-activated receptor-2 (PAR-2): cDNA sequence and activity of receptor-derived peptides in gastric and vascular tissue. Br. J. Pharmacol. 1996;118:521–530. doi: 10.1111/j.1476-5381.1996.tb15433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERIO R., MULÈ F., POSTORINO A. Non adrenergic, non cholinergic inhibitory junction potentials in rat proximal colon: role of nitric oxide. Can. J. Physiol. Pharmacol. 1995;73:79–84. doi: 10.1139/y95-011. [DOI] [PubMed] [Google Scholar]
- STEINHOFF M., VERGNOLLE N., YOUNG S.H., TOGNETTO M., AMADESI S., ENNES H.S., TREVISANI M., HOLLENBERG M.D., WALLACE J.L., CAUGHEY G.H., MITCHELL S.E., WILLIAMS L.M., GEPPETTI P., MAYER E.A., BUNNETT N.W. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat. Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- TROTTIER G., HOLLENBERG M., WANG X., GUI Y., LOUTZENHISER K., LOUTZENHISER R. PAR-2 elicits afferent arteriolar vasodilation by NO-dependent and NO-independent actions. Am. J. Physiol. Renal Physiol. 2002;282:F891–F897. doi: 10.1152/ajprenal.00233.2001. [DOI] [PubMed] [Google Scholar]
- VERGNOLLE N. Proteinase-activated receptors – novel signals for gastrointestinal pathophysiology. Aliment. Pharmacol. Ther. 2000;14:257–266. doi: 10.1046/j.1365-2036.2000.00690.x. [DOI] [PubMed] [Google Scholar]
- VERGNOLLE N., WALLACE J.L., BUNNETT N.W., HOLLENBERG M.D. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol. Sci. 2001;22:146–152. doi: 10.1016/s0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- VU T.K., HUNG D.T., WHEATON V.I., COUGHLIN S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- WARD S.M., XUE C., SHUTTLEWORTH C.W.R., BREDT D.S., SNYDER S.H., SANDERS K.M. NADPH diaphorase and nitric oxide synthase colocalization in enteric neurons of canine proximal colon. Am. J. Physiol. 1992;263:G277–G284. doi: 10.1152/ajpgi.1992.263.2.G277. [DOI] [PubMed] [Google Scholar]
- XU W.F., ANDERSEN H., WHITMORE T.E., PRESNELL S.R., YEE D.P., CHING A., GILBERT T., DAVIE E.W., FOSTER D.C. Cloning and characterization of human protease-activated receptor 4. Proc. Natl. Acad. Sci. USA. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XUE C., POLLOCK J., SCHMIDT H.H.H.W., WARD S.M., SANDERS K.M. Expression of nitric oxide synthase by interstitial cells of the canine proximal colon. J. Auton. Nerv. Syst. 1994;49:1–14. doi: 10.1016/0165-1838(94)90015-9. [DOI] [PubMed] [Google Scholar]