Abstract
Taurine and GABA are recognized as endogenous cryogens. In a previous study, some structural analogues of taurine, namely 6-aminomethyl-3-methyl-4H-1,2,4-benzothiadiazine 1,1-dioxide (TAG), 2-aminoethylarsonic (AEA), 2-hydroxyethanesulfonic (ISE) and (±)cis-2-aminocyclohexane sulfonic acids (CAHS) have been shown to displace [3H]taurine binding from rabbit brain synaptic membrane preparations, without interacting either with GABA-ergic systems, nor with taurine uptake mechanism, thus behaving like direct taurinergic agents.
To answer the question whether the role of taurine as an endogenous cryogen depends on the activation of GABA receptors or that of specific taurine receptor(s), taurine or the above structural analogues were injected intracerebroventricularly in conscious, restrained rabbits singularly or in combination and their effects on rectal (RT)- and ear–skin temperature and gross motor behavior (GMB) were monitored.
Taurine (1.2 × 10−6–4.8 × 10−5 mol) induced a dose-related hypothermia, vasodilation at ear vascular bed and inhibition of GMB. CAHS, at the highest dose tested (4.8 × 10−5 mol) induced a taurine-like effect either on RT or GMB. On the contrary ISE, injected at the same doses of taurine, induced a dose-related hyperthermia, vasoconstriction and excitation of GMB. AEA and TAG caused a dose-related hyperthermia, but at doses higher than 1.2 × 10−7 mol caused death within 24 h after treatment.
CAHS (4.8 × 10−5 mol) antagonized the hyperthermic effect induced by TAG (1.2 × 10−6 mol), AEA (1.2 × 10−8 mol) or ISE (4.8 × 10−5 mol).
In conclusion, these findings may indicate the existence of a recognition site specific for taurine, responsible for its effects on thermoregulation.
Keywords: Taurine, taurine derivatives, thermoregulation, taurine binding site, taurine agonist, taurine antagonist
Introduction
Amino acids have recently received increased attention with regard to their role in thermoregulation as neurotransmitters within the central nervous system (CNS) (Zhang et al., 1995; Monda et al., 1998). This is particularly true for GABA and taurine as endogenous cryogens (Frosini et al., 2000). The pathways that down regulate body temperature are of overwhelming interest in view of the effectiveness of hypothermia in protecting from ischemia-reperfusion damage to the brain of patients surviving cardiac arrest (Safar & Kochanek, 2002). Nevertheless, the physical and pharmacological treatments so far adopted to attain a mild hypothermia have required such a long time (8–12 h) (The Hypothermia after Cardiac Arrest Study Group, 2002) that they may compromise its beneficial effects. It would therefore be valuable for emergency-care medicine to develop new drugs that could rapidly and safely induce hypothermia. To this end, it is important to improve the knowledge about the role of endogenous cryogens.
A recent study performed in the conscious rabbit has demonstrated that heat stress modifies brain metabolism of taurine and GABA so that increases in cerebrospinal fluid (CSF) concentrations of these amino acids were induced. This rise, which could not be ascribed to blood contamination resulting from a transient opening of the blood–CSF barrier, was probably aimed at counteracting the hyperthermia promoted by exposure to heat. Moreover, under these conditions, taurine appeared to play a prominent role in comparison to GABA. When the time course for heat-stress-induced changes in rectal temperature (RT) and CSF taurine contents were compared, in fact, it was found that RT decreased as soon as taurine CSF contents rose, while CSF GABA levels were significantly higher just before RT had regained basal values (Frosini et al., 2000). Furthermore, central or systemic injection of either GABA or agonists of either GABAA or GABAB receptors usually diminishes core temperature, whereas such injection of antagonists of either class induces hyperthermia (Serrano et al., 1985). Yakimova et al. (1996), however, have demonstrated that the GABA effect on thermoregulation is essentially mediated by GABAB receptors. This is confirmed by the fact that mice lacking the GABAB(1) subunit of the GABAB receptors did not become hypothermic when GABAB agonists were applied (Schuler et al., 2001). Taurine, as well as GABA, when injected intracerebroventricularly (i.c.v.) induces dose-related hypothermia accompanied by depression of gross motor behavior and peripheral vasodilation (Sgaragli et al., 1981), whereas the taurine antagonist 6-aminomethyl-3-methyl-4H-1,2,4-benzothiadiazine-1,1-dioxide (TAG), increases core temperature (Sgaragli et al., 1994). It is not clear, however, which receptor(s) mediates the hypothermic effect of taurine. Since taurine binds both to GABAA and GABAB receptors (Krogsgaard-Larsen et al., 1980; Kontro & Oja, 1990; Frosini et al., 2003), it may affect body temperature by interacting with GABA-ergic systems. However, there is a large body of evidence that taurine elicits a number of neurophysiological effects distinguishable from those afforded by GABA (Hayes et al., 1975; Huxtable, 1989; Kamisaki et al., 1993; Hussy et al., 2001; Tuz et al., 2001). Moreover, taurine desynchronizes while GABA, muscimol and baclofen synchronize motor and limbic cortex activity patterns, suggesting that their effects could depend on the interaction with different neuronal pathways afferent to the cortical areas monitored (Sgaragli et al., 1978; Sesti et al., 1999). Thus, it is conceivable that taurine exerts its biologic activity by interacting with a specific receptor. In a previous study, some direct taurinergic compounds were identified (Frosini et al., 2003). 2-Aminoethylarsonic acid (AEA), (±)cis-2-aminocyclohexane sulfonic acid (CAHS), 2-hydroxyethanesulfonic acid (ISE) and TAG proved to displace [3H]taurine binding without interacting either with both GABA receptors or taurine- and GABA-uptake systems and not affecting GABA-transaminase activity. The effects of these compounds on body temperature have been studied, and the findings so far collected may indicate that the effects of taurine on thermoregulation is mediated by a specific taurinergic pathway.
Methods
Materials
Taurine and ISE were purchased by Sigma. AEA was prepared by periodate oxidation of 2-[(2-hydroxyethyl)amino]ethylarsonic acid, itself made by treating 2-chloroethylarsonic acid with ethanolamine (Geoghegan & Dixon, 1989). CAHS was prepared from 2-aminobenzenesulfonic acid by catalytic hydrogenation as reported by Egli & Eugster (1975). TAHS was prepared from cyclohexene by sulfur monochloride addition, followed by oxidation to 2-chlorosulfonic acid and substitution of chlorine as reported by Machetti et al. (2000). β-alanine (β-ALA) was purchased from Sigma Chemical Co (St Louis, MA, U.S.A.). TAG, synthesized as described by Girard et al. (1982), was a generous gift of Dr G.G. Yarbrough from Merk Frosst Laboratories (Quebec, Canada). The purity of all compounds were evaluated above 95% by 1H NMR or high-perfomance liquid chromatography.
Animals
Adult male New Zealand albino rabbits (Charles River, Calco, Como, Italy) weighing 2.0–2.5 kg were kept in large individual cages under a 12 : 12 h day–night cycle at 20°C ambient temperature (Ta). Drinking water and conventional laboratory rabbit food were available ad libitum. Before the experimental session, the animals were habituated to restraint and to the rectal probe in order to minimize the stress response.
Surgery
All the experiments were performed in strict compliance with the recommendations of the EEC (86/609/CEE) for the care and use of laboratory animals and were approved by the Animal Care and Ethics Committee of the University of Siena, Italy.
At least 3 weeks before the experiment, animals were implanted with a cannula for i.c.v. injection at the level of the lateral ventricle. The stereotaxic technique has been described elsewhere (Palmi et al., 1994). Anesthesia was induced by intramuscular (i.m.) injection of xylazine chloride (10 mg kg−1, Rompun® Vet., Bayer AG, Germany) and ketamine hydrochloride (35 mg kg−1, Ketavet®, Parke Davis/Warner-Lambert, U.S.A.). Under sterile precautions, the dorsal structure of the skull was exposed by a midline skin incision and, after drilling a hole near the bregma according to stereotaxic coordinates (Sawyer et al., 1954), a thin stainless-steel cannula was inserted. Spontaneously, CSF outflow indicated the correct position of the cannula. The cannula was fixed to the skull with stainless-steel screws and dental acrylic cement, occluded with an obturator, and the skin incision was sutured around the cannula.
After surgery, rabbits were injected for at least 5 days with the following drugs: prednisolone acetate (Novosterol®, Vetem S.p.A., Italy), 10 mg day−1 i.m.; enrofloxacin, (Baytril®, Bayer AG, Germany) 25 mg day−1 i.m. The animals were allowed to recover for at least 15 days.
Experimental protocol
Conscious animals were individually housed in a thermostatted chamber set at neutral temperature (20°C). Concurrently, ear–skin temperature (EST) and RT were measured to an accuracy of 0.1°C, using thermocouples attached to the ear and inserted 10 cm deep into the rectum, respectively, every 5 min. The thermocouple thermometer was connected to a personal computer with an Isothermex program (Columbus Instr., Columbus, OH, U.S.A.). Temperature was monitored for at least 1 h before the experimental session and up to 325 min after the treatment. RT was again monitored 24 h after i.c.v. injection.
To isolate any experimental artefacts arising from animal restraint, manipulation or i.c.v. injection, all the rabbits used in the present study were injected with 10 μl pyrogen-free water (vehicle) and RT was monitored as previously described. Only those animals that did not exhibit significant changes in RT were used in the subsequent experiments. Among all the rabbits used in this study, six were randomly selected to form the control group in which the vehicle was injected i.c.v. and RT monitored.
Compounds dissolved in pyrogen-free water were administered i.c.v. (range of doses: 1.2 × 10−8–4.8 × 10−5 mol) in a final volume of 10 or 20 μl with an Agla micrometer-operated syringe (Burroughs Wellcome and Co., London, U.K.) to randomly selected rabbits (four to six animals/group/dose).
After i.c.v. injections, gross motor behavior (GMB) was observed during the entire experimental session. The following arbitrary scale to describe GMB changes was chosen: 0=no effect. Depression of GMB: −, sedation and light motor incoordination; −−, sedation, drowsiness and motor incoordination; −−−, sedation, drowsiness and long-lasting motor incoordination; −−−− sedation, drowsiness, severe and long-lasting motor incoordination and loss of righting reflex. Excitation of GMB: +, alertness; ++, alertness and increased panting rate; +++, alertness and elevated panting rate, ++++, alertness, sustained panting rate and episodic convulsive attacks.
Statistical analysis
Values are expressed as mean±s.e.m. and reported as changes in RT or EST (ΔRT and ΔEST). The area under the experimental curve (AUC) relative to RT was calculated by a combined linear logarithmic trapezoidal method using Graphpad-Prism II program (GraphPad Software Inc., San Diego, CA, U.S.A.). The comparison between AUC(0–24 h) of control (vehicle-injected) vs treated (taurine- or its derivatives-injected) animals or between the AUC(0–24 h) relative to different doses of the compounds, was performed using Student's t-test. A P-value <0.05 was considered significant.
Results
Effects of i.c.v. injection of pyrogen-free water on RT and EST
To isolate any experimental artefacts arising from animal restraint, manipulation or i.c.v. injection, in a group of six rabbits treated by i.c.v. injection of 10 μl pyrogen-free water, RT and EST were monitored. RT and EST basal values were, respectively, 39.0±0.1 and 20.8±0.2°C. Pre- and postinjection values of temperatures showed no statistically significant differences (data not shown). GMB was not affected by the treatment.
Effects of i.c.v. injection of taurine, TAG, AEA, ISE, CAHS, TAHS and β-ALA on RT and EST
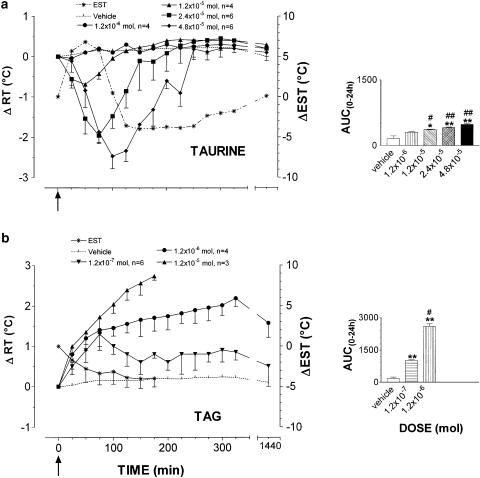
As reported in Figure 1, (panel a) taurine induced a dose-related hypothermia, accompanied by muscle tone reduction and vasodilation in the vascular bed of the ear. Hypothermia was significant at 1.2 × 10−5 mol dose and, at the highest dose of 4.8 × 10−5 mol, it was marked and long lasting, peaked with a −ΔRT value of 2.5±0.3°C at 100 min after injection and was accompanied by increase in EST. This peaked at 50 min with a ΔEST value of 6.3±2.8°C. Subsequently, EST decreased owing to a steady vasocostriction beyond basal values with a −ΔEST value of 3.6±2.1°C at 125 min, then remained almost stable during the entire experimental session. EST shift marked the start of the slow recovery of RT to baseline values that were attained at 300 min. After 24 h, all taurine-treated animals were normothermic. Taurine was shown to induce depression of GMB. This depression depended on the dose of taurine and was characterized by sedation and light motor incoordination (1.2 × 10−5 mol dose; arbitrary scale, −−), sedation, drowsiness and motor incoordination (2.4 × 10−5 mol dose; arbitrary scale, −−−) and, finally, sedation, drowsiness, long-lasting motor incoordination and loss of righting reflex (4.8 × 10−5 mol dose; arbitrary scale, −−−−). After 24 h, the behavior was comparable to that of controls.
Figure 1.
Mean (± s.e.m.) changes in rabbit rectal- (RT) and ear–skin temperature (EST) following i.c.v. administration of taurine (panel a) and TAG (panel b). The doses injected for taurine were 1.2 × 10−6 (•, n=4), 1.2 × 10−5 (▴, n=4), 2.4 × 10−5 (▪, n=6) and 4.8 × 10−5 mol (⧫, n=6); for TAG 1.2 × 10−7 (▾, n=6), 1.2 × 10−6 (•, n=4), 1.2 × 10−5 (▴, n=3) mol. Vehicle alone (pyrogen-free water, dotted line) was injected at the same volume used for the compounds to a group of six rabbits. EST was measured in animals treated with the highest dose (*, dotted line). To improve clarity, s.e.m. of vehicle RT values and EST values are not depicted. Arrows indicate time of i.c.v. injection. In the right panels, the area under the experimental curve (AUC(0–24 h)) of RT changes relative to each dose/compound is shown. The comparison between AUC(0–24 h) of vehicle- vs taurine- or TAG-injected rabbits or between AUC(0–24 h) of the different doses of each compound was performed by using Student's t-test. *P<0.05, **P<0.01, ***P<0.001 vs vehicle. #P<0.05, ##P<0.01 vs the previous dose.
As shown in panel b, TAG, at the dose of 1.2 × 10−7 mol, did modify RT by inducing hyperthermia. RT increased regularly peaking with an increment of 1.3±0.3°C at 100 min (AUC(0–24 h) TAG vs control, P<0.01, n=6). After 24 h, RT had regained basal values. The 1.2 × 10−6 mol dose (n=4) produced a sustained and long-lasting hyperthermia that peaked with a ΔRT value of 2.2±0.2°C at 325 min, and was still maintained after 24 h (ΔRT value of 1.6±0.4°C); at 48 h after treatment, however, RT had returned to normal values (data not shown). At the highest dose tested of 1.2 × 10−5 mol, TAG induced a sharp rise in RT, increasing by 2.7±0.1°C at 175 min. At this time, however, all rabbits of this group (n=3) died. TAG did not modify GMB at the dose of 1.2 × 10−7mol, while at 1.2 × 10−6 mol dose, during the hyperthermic phase, alertness and sustained panting rate were observed (arbitrary scale, +++). At 1.2 × 10−5 mol dose, alertness, sustained panting rate and episodic seizures together with intense kicking were observed (arbitrary scale, ++++).
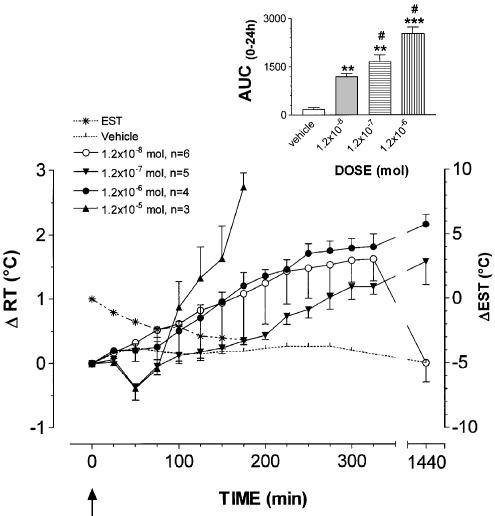
As reported in Figure 2, AEA at 1.2 × 10−8, 1.2 × 10−7, 1.2 × 10−6 and 1.2 × 10−5 mol dose did remarkably affect thermoregulation, inducing hyperthermia and vasoconstriction at ear vascular bed. After the lowest dose injected, RT increased regularly peaking with an increment of 1.6±0.3°C at 325 min (AUC(0–24 h) AEA vs control, P<0.01, n=6). After 24 h, RT had regained basal values. The 1.2 × 10−7 mol dose produced a bimodal effect. Immediately after the injection RT decreased peaking with a ΔRT value of 0.4±0.2°C at 50 min and rose gradually thereafter. At 325 min rabbits were hyperthermic with a ΔRT value of 1.2±0.2°C. After 24 h, hyperthermia was even greater with a ΔRT value of 1.6±0.4°C; after 48 h, however, RT had returned to basal values (data not shown). None of the five animals injected with this dose died. The statistical analysis demonstrated a significant difference of the AUC(0–24 h) of AEA vs control and vs the lowest dose tested (P<0.01 and <0.05, respectively). The dose of 1.2 × 10−6 mol, injected in four rabbits, induced a marked rise in RT. The increase observed at the end of the experimental session was 1.8±0.1°C. With this dose two rabbits died during the subsequent night. The autoptic examination of their brain did not reveal macroscopic modifications. The other surviving animals were still hyperthermic 24 h after treatment showing a ΔRT value of 2.2±0.2°C, but returned normothermic within 48 h (data not shown). At the highest dose tested (1.2 × 10−5 mol), AEA induces a biphasic effect. After i.c.v injection, RT decreased by 0.4±0.1°C after 50 min and sharply rose thereafter showing a ΔRT value of 2.7±0.1°C at 175 min. All animals (n=3) injected with this dose died after reaching a ΔRT value of 2.7±0.2°C. AEA did not modify GMB at the doses of 1.2 × 10−8, 1.2 × 10−7, 1.2 × 10−6 mol, while after 1.2 × 10−5 mol dose, during the hyperthermic phase, it caused alertness, sustained panting rate and episodic seizures (arbitrary scale, ++++).
Figure 2.
Mean (± s.e.m.) changes in rabbit rectal- (RT) and ear–skin temperature (EST) following i.c.v. administration of AEA. The doses injected were 1.2 × 10−8 (○, n=6), 1.2 × 10−7 (▾, n=5), 1.2 × 10−6 (•, n=4), 1.2 × 10−5 (▴, n=3) mol. Vehicle alone (pyrogen-free water, dotted line) was injected at the same volume used for the compounds to a group of six rabbits. EST was measured in animals treated with the highest dose (*, dotted line). To improve clarity, s.e.m. of vehicle RT values and EST values are not depicted. Arrows indicate time of i.c.v. injection. In the inset the area under the experimental curve (AUC(0–24 h)) of RT changes relative to each dose is shown. The comparison between AUC(0–24 h) of vehicle- vs AEA-injected rabbits or between AUC(0–24 h) of the different doses was performed by using Student's t-test. *P<0.05, **P<0.01, ***P<0.0001 vs vehicle. #P<0.05, ##P<0.01 vs the previous dose.
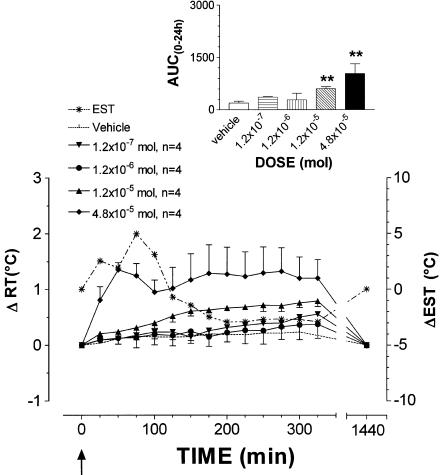
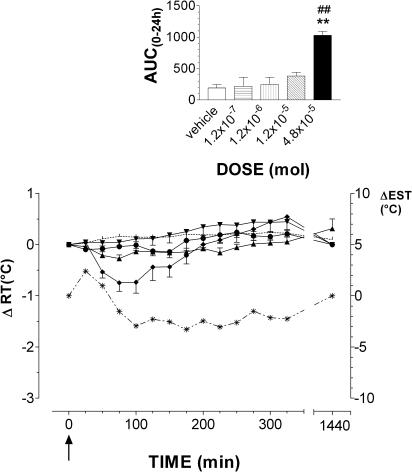
ISE was tested at the doses range of 1.2 × 10−7, 1.2 × 10−6, 1.2 × 10−5 and 4.8 × 10−5 mol (Figure 3, panel b). Both doses of 1.2 × 10−7 and 1.2 × 10−6 mol did not modify RT (AUC(0–24 h) ISE vs control, NS, n=4/dose). On the contrary, at either 1.2 × 10−5 and 4.8 × 10−5 mol doses, it induced a significant rise in body temperature that peaked with a ΔRT value of 0.8±0.1°C at 325 min and 1.3±0.4°C at 275 min, respectively (AUC(0–24 h) ISE vs control, P<0.01, n=4/dose). At 4.8 × 10−5 mol dose, EST increased, peaking with a Δ value of 1.3±0.4°C, which was maintained constant until the end of the experimental session. After 24 h, RT had gained basal values. It was only at the highest dose that ISE slightly stimulated GMB (arbitrary scale, +). CAHS did not affect significantly RT at the doses of 1.2 × 10−7, 1.2 × 10−6 and 1.2 × 10−5 mol (Figure 4). At the 4.8 × 10−5 mol dose, however, it induced hypothermia that peaked with a −ΔRT value of 0.8±0.1°C after 100 min (AUC(0–24 h) CAHS vs control and vs 1.2 × 10−5 mol, P<0.01, n=6). Hypothermia was accompanied by vasodilation at ear vascular bed, which caused increase in EST, subsequently followed by a moderate decrease which peaked with a −Δ value of 3.3±1.4°C at 225 min. RT regained basal values at 150 min. CAHS slightly affected GMB with sedation and light motor incoordination during the decrease of RT. These effects were comparable to those induced by 1.2 × 10−5 mol dose of taurine (arbitrary scale, −).
Figure 3.
Mean (± s.e.m.) changes in rabbit rectal- (RT) and ear–skin temperature (EST) following i.c.v. administration of ISE. The doses injected for ISE were 1.2 × 10−7 (n=4), 1.2 × 10−6 (•, n=4), 1.2 × 10−5 (▴, n=4) and 4.8 × 10−5 mol (⧫, n=4). Vehicle alone (pyrogen-free water, dotted line) was injected at the same volume used for the compounds to a group of six rabbits. EST was measured in animals treated with the highest dose (*, dotted line). To improve clarity, s.e.m. of vehicle RT values and EST values are not depicted. Arrows indicate time of i.c.v. injection. In the inset, the area under the experimental curve (AUC(0–24 h)) of RT changes relative to each dose is shown. The comparison between AUC(0–24 h) of vehicle- vs ISE-injected rabbits or between AUC(0–24 h) of the different doses was performed by using Student's t-test. *P<0.05, *ast;P<0.01, ***P<0.001 vs vehicle. #P<0.05, ##P<0.01 vs the previous dose.
Figure 4.
Mean (± s.e.m.) changes in rabbit rectal- (RT) and ear–skin temperature (EST) following i.c.v. administration of CAHS. The doses injected were 1.2 × 10−7 mol (▾, n=4), 1.2 × 10−6 (•, n=4), 1.2 × 10−5 (▴, n=4) and 4.8 × 10−5 mol (⧫, n=6) mol. Vehicle alone (pyrogen-free water, dotted line) was injected at the same volume used for the compounds to a group of six rabbits. EST was measured in animals treated with the highest dose (*, dotted line). To improve clarity, s.e.m. of vehicle RT values and EST values are not depicted. Arrows indicate time of i.c.v. injection. In the inset, the area under the experimental curve (AUC(0–24 h)) of RT changes relative to each dose is shown. The comparison between AUC(0–24 h) of vehicle- vs CAHS-injected rabbits or between AUC(0–24 h) of the different doses was performed by using Student's t-test. *P<0.05, **P<0.01, ***P<0.001 vs vehicle. #P<0.05, ##P<0.01 vs the previous dose.
β-ALA and TAHS did not induce significant changes of both RT or GMB at all the doses injected (1.2 × 10−8, 1.2 × 10−7, 1.2 × 10−6, 1.2 × 10−5, 4.8 × 10−5mol) (data not shown).
Effects of coadministration of CAHS with TAG, ISE or AEA
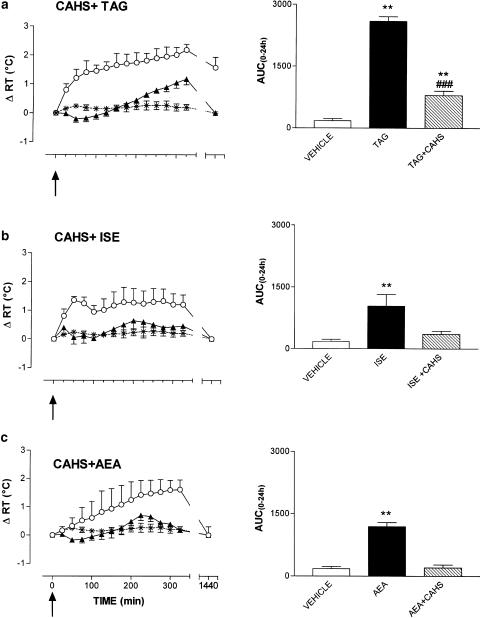
CAHS, injected at the dose of 4.8 × 10−5 mol partially antagonized the hyperthermic effect induced by 1.2 × 10−6 molTAG. As reported in Figure 5, panel a, in fact, after the coadministration of the two compounds, RT decreased slightly in the first 75 min after the treatment, and rose slowly thereafter, peaking to 1.2±0.2°C at the end of the experimental session. After 24 h, RT regained basal values. During the experimental session, GBM was comparable to that of control animals. The AUC(0–24 h) relative to CAHS+TAG treatment was significantly lower (P<0.001) than that of TAG injected alone at the same dose as that used in combination with CAHS. However, as reported in the inset of Figure 5, the AUC(0–24 h) relative to the coadministration of the two above-mentioned drugs was significantly higher than that relative to the injection of vehicle (P<0.01).
Figure 5.
Effects of CAHS on TAG-, ISE- and AEA-induced hyperthermia. Data are reported as mean ±s.e.m. of changes in rabbit RT. Black triangles represent the effects of CAHS (4.8 × 10−5 mol) injected 10 min before TAG (1.2 × 10−6 mol, n=4, panel a), ISE (4.8 × 10−5 mol, n=4, panel b) or AEA (1.2 × 10−8 mol, n=4, panel c), while open circles represent the hyperthermia elicited by TAG, ISE or AEA injected alone. Arrows indicate time of TAG, ISE or AEA i.c.v. injection. In the right panels, the area under the experimental curve (AUC(0–24 h)) of RT changes relative to the different treatments is shown. The comparison between AUC(0–24 h) relatives to the different treatments was performed by using Student's t-test. **P<0.01 vs vehicle. ###P<0.001 vs TAG, ISE or AEA alone.
CAHS was able to fully antagonize the hyperthermic effect of both AEA and ISE. As reported in Figure 5, panels b and c, respectively, the injection of 4.8 × 10−5 mol of CAHS together with 4.8 × 10−5 mol ISE or 1.2 × 10−8 mol AEA did not significantly modify RT (AUC(0–24 h) CAHS+ISE and CAHS+AEA vs AUC(0–24 h) controls, NS) that remained around basal values during the entire experimental session. Also GMB was not affected by these treatments.
Discussion
The results of the present study when combined with data relative to the interaction of these compounds with GABA-ergic or taurinergic systems of the rabbit brain (Frosini et al., 2003), can shed light on the question whether the role of taurine as an endogenous cryogen depends on the activation of GABA or specific taurine receptor(s).
We have demonstrated that AEA, ISE and CAHS, and the taurine antagonist TAG were able to modify RT of conscious rabbit. In particular, while CAHS induced hypothermia, TAG, AEA and ISE caused hyperthermia, TAG and AEA being effective even at a dose of 1.2 × 10−8 mol, but were also very neurotoxic causing death of animals few hours after administration. All the above-mentioned compounds, as described by Frosini et al. (2003) did not interact with GABA-ergic systems, but were able to inhibit [3H]taurine binding to washed synaptic membranes of rabbit brain. Furthermore, CAHS was able to antagonize the hyperthermia induced by AEA, ISE and TAG, suggesting that the effects of the latter compounds are mediated by taurine binding sites. In the light of these results, the hypothesis of the existence of a specific taurinergic pathway involved in the central mechanisms of thermoregulation in the rabbit which parallels a GABA-ergic system, can be advanced.
AEA and ISE that induced hyperthermia similar to TAG, can be considered as novel taurine antagonists. Interestingly, it has been demonstrated that AEA antagonizes taurine-stimulated calcium uptake by rat liver mitochondria in a competitive fashion, suggesting the presence of a common recognition site for both compounds (Palmi et al., 1999). CAHS, which induced a taurine-like effect on body temperature, can be considered a taurine agonist. Liebowitz et al. (1987) have demonstrated that this compound, like taurine, is able to stimulate ATP-dependent calcium uptake in rat retina.
Taurine was able to bind sites in rabbit whole brain synaptic membrane preparations with a higher affinity than that shown for GABAB receptors (Frosini et al., 2003). This suggests that taurine-induced hypothermia depends on its interaction with taurine binding sites and, to a certain extent, also with GABAB receptors. The hypothesis that part of the effect of taurine is mediated by GABAB receptors, however, is hampered by the finding that β-ALA did not affect thermoregulation in the rabbit, although it exhibited [3H]GABA displacing activity at micromolar concentrations from GABAB receptors in rabbit brain synaptic membrane preparations (Frosini et al., 2003).
At physiological pH, taurine is present as a zwitterion, while alkylsulfonic acids such as ISE are present almost in the anion form. Aminoalkylarsonic acids might be expected to have pK values of about 4 and about 9. Hence, at neutral pH, they will be almost in the charged form, being monoanion predominating over the dianion form. These considerations, together with the effects elicited by these compounds on body temperature, suggest that both sulfonic and amine groups are essential for taurine to induce the hypothermic effect. In fact, the substitution of sulfonic group by one with similar tetrahedral shape and size such as the arsonate group gives rise to AEA that induced a hyperthermia. Also the substitution of the entire amine with an hydroxy group shifted the biological activity of the resulting ISE toward hyperthermia. The substitution of sulfonic with a planar carboxylic group, moreover, gives rise to β-ALA which, in contrast with its effectiveness in the rat (Sgaragli & Pavan, 1972), was inactive in the rabbit.
Taurine is a highly flexible molecule and both amine and sulfonic moiety have free rotation around the central methylene linkage; therefore, it can attain many low-energy conformations that allow its binding either to GABA receptors or taurine binding sites (Liebowitz et al., 1987; Lombardini et al., 1989). Since the configuration of taurine at its binding sites is unknown, more restricted analogues of taurine, like its cyclic derivatives, have been considered. In CAHS, the amine and sulfonic groups must have equatorial and axial position, respectively (Liebowitz et al., 1987). Since CAHS and taurine exert similar effects in vivo, we conclude that the amine and sulfonic groups need to be in the gauche position for the compound to induce hypothermia. It is worth noting that THAS, in which these two groups are both equatorial, is inactive. The difference in pharmacological activity of TAHS and CAHS suggests that CAHS effect is mediated by a specific recognition site. The lower potency of CAHS in comparison to taurine in inducing hypothermia, could be explained by considering that CAHS was administered as a racemate. Since the difference in pharmacological activity between enantiomers could be great, in some cases, the resolution of CAHS racemates might lead to an optical isomer provided with considerable potency. Braghiroli et al. (1996), have shown, in fact, that the hypotensive effect elicited by i.c.v. injection of the 2-methyltaurine racemate was mainly because of (S)-enantiomer, the (R)-form being inactive.
To explain the different effects of taurine derivatives on thermoregulation, we can postulate the existence of a recognition site specific for taurine in rabbit brain. Taking into account the conformation of the compounds used in the present study, we can hypothesize a receptor possessing two regions, one negatively and the other positively charged, which interact with the amine and the sulfonic group, respectively, of taurine. The distance between these two sites corresponds to that of two bonded carbon atoms. The interaction of taurine with the receptor at both groups is essential, but the receptor binding of the sulfonate group is specific for its tetrahedral shape, since β-ALA, with its planar carboxylate group, had no effect.
The hypothesis of a taurine receptor might help to explain why some of the taurine analogues (i.e. ISE, AEA and TAG) elicited hyperthermia. If we admit that they are able to block the receptor without activating it, then they will prevent endogenous taurine from exerting a tonic influence on thermoregulatory centers that are directed towards dissipation of body heat. Without this influence the system will be unbalanced and hyperthermia will develop.
In conclusion, the existence of a specific taurinergic system that is involved in the central mechanisms of thermoregulation in the rabbit can be advanced. The future development of new taurine derivatives will be crucial for the characterization of the brain receptor responsible for the physio-pharmacological activity of taurine. This is the premise for the development of new drugs, able to induce rapidly and safely hypothermia, to be used in the emergency care medicine.
Acknowledgments
This work was supported by contributions of Ministero degli Affari Esteri (Rome, Italy) under law 212/92 and by MURST, Cofin. ‘98, EU COST Action D13 (WG number 13-0011-00) and Fondazione Monte dei Paschi di Siena. The technical assistance of R. Borghi is gratefully acknowledged. A preliminary account of this study was presented at the International Taurine Symposium 1999 held in Siena, Italy, on August 4–8, 1999 and published as short report in Della Corte et al. (2000).
Abbreviations
- AEA
2-aminoethylarsonic acid
- β-ALA
β-alanine
- CAHS
(±)cis-2-aminocyclohexane sulfonic acid
- ISE
2-hydroxyethanesulfonic acid
- TAG
6-aminomethyl-3-methyl-4H-1,2,4-benzothiadiazine 1,1-dioxide
- TAHS
(±)trans-2-aminocyclohexane sulfonic acid
References
- BRAGHIROLI D., TRUZZI C., BRANDOLI C., BARALDI M., GAMBERONI G., DI BELLA M. Effect of 2-methyltaurine and its enantiomers on arterial blood pressure. Pharmacol. Res. 1996;34:33–36. doi: 10.1006/phrs.1996.0060. [DOI] [PubMed] [Google Scholar]
- DELLA CORTE L., HUXTABLE R.J., SGARAGLI G.P., TIPTON K.F. Taurine 4: Taurine and Excitable Tissues 2000New York: Plenum Press; (eds) [Google Scholar]
- EGLI VON R., EUGSTER C.D. Über die selektive katalytische Reduktion von substituierten Anilinen zu substituierten Cyclo-hexylaminen und von Benzol-bwz. Phenyl-alkan-sulfonsäuren zu Cyclohexan-bzw. Cyclohexylalkan-sulfonsäuren. Helv. Chim. Acta. 1975;58:2321–2346. [Google Scholar]
- FROSINI M., SESTI C., DRAGONI S., VALOTI M., PALMI M., DIXON H.B.F., MACHETTI F., SGARGLI G.P. Interaction of taurine and structurally related analogues with the GABAergic system and taurine binding sites of rabbit brain. Br. J. Pharmacol. 2003;138:1163–1171. doi: 10.1038/sj.bjp.0705134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FROSINI M., SESTI C., PALMI M., VALOTI M., FUSI F., MANTOVANI P., BIANCHI L., DELLA CORTE L., SGARAGLI G.P. Heat-stress-induced hyperthermia alters CSF osmolality and composition in conscious rabbits. Am. J. Physiol. – Regul. Integ. Comp. Physiol. 2000;279:2095–2103. doi: 10.1152/ajpregu.2000.279.6.R2095. [DOI] [PubMed] [Google Scholar]
- GEOGHEGAN K.F., DIXON H.B. Synthesis of 2-amino-ethylarsonic acid. A new synthesis of primary amines. Biochem. J. 1989;15:295–296. doi: 10.1042/bj2600295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRARD Y., ATKINSON J.G., HAUBRICH D.R., WILLIAMS M, YARBROUGH G.G. Aminomethyl-1,2,4-benzothiadiazines as potential analogues of gamma-aminobutyric acid. Unexpected discovery of a taurine antagonist. J. Med. Chem. 1982;25:113–116. doi: 10.1021/jm00344a004. [DOI] [PubMed] [Google Scholar]
- HAYES K.C., CAREY R.E., SCHMIDT S.Y. Retinal degeneration associated with taurine deficiency in the cat. Science. 1975;188:949–951. doi: 10.1126/science.1138364. [DOI] [PubMed] [Google Scholar]
- HUSSY N., BRES V., ROCHETTE M., DUVOID A., ALONSO G., DAYANITHI G., MOOS F.C. Osmoregulation of vasopressin secretion via activation of neurohypophysial nerve terminals glycine receptors by glial taurine. J. Neurosci. 2001;21:7110–7116. doi: 10.1523/JNEUROSCI.21-18-07110.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXTABLE R.J. Taurine in the central nervous system and the mammalian actions of taurine. Progr. Neurobiol. 1989;32:471–533. doi: 10.1016/0301-0082(89)90019-1. [DOI] [PubMed] [Google Scholar]
- KAMISAKI Y., MAEDA K., ISHIMURA M., OMURA H., ITOH T. Effects of taurine on depolarization-evoked release of amino acids from rat cortical synaptosomes. Brain Res. 1993;627:181–185. doi: 10.1016/0006-8993(93)90318-h. [DOI] [PubMed] [Google Scholar]
- KONTRO P., OJA S.S. Interactions of taurine with GABAB binding sites in mouse brain. Neuropharmacology. 1990;29:243–247. doi: 10.1016/0028-3908(90)90008-f. [DOI] [PubMed] [Google Scholar]
- KROGSGAARD-LARSEN P., FALCH E., SCHOUSBOE A., CURTIS D.R., LODGE D. Piperidine-4-sulphonic acid, a new specific GABA agonist. J. Neurochem. 1980;34:756–759. doi: 10.1111/j.1471-4159.1980.tb11211.x. [DOI] [PubMed] [Google Scholar]
- LIEBOWITZ S.M., LOMBARDINI J.B., SALVA P.S. Cyclic taurine analogs. Synthesis and effects on ATP-dependent Ca2+ uptake in rat retina. Biochem. Pharmacol. 1987;36:2109–2114. doi: 10.1016/0006-2952(87)90138-9. [DOI] [PubMed] [Google Scholar]
- LOMBARDINI J.B., LIEBOWITZ S.M., CHOU T.C. Analogues of taurine as stimulators and inhibitors of ATP-dependent calcium ion uptake in rat retina: combination kinetics. Mol. Pharmacol. 1989;36:256–264. [PubMed] [Google Scholar]
- MACHETTI F., CACCIARINI M., CATRAMBONE F., CORDERO F.M., ROMOLI S., DE SARLO F. Synthesis of taurine analogues. Part 1: 2-aminosulfonic acids from alkene – sulfur monochloride adducts. J. Chem. Res. 2000. pp. 120–121.
- MONDA M., VIAGGIANO A., SULLO A., DE LUCA V. Aspartic and glutamic acids increase in the frontal cortex during prostaglandin E1 hyperthermia. Neuroscience. 1998;83:1239–1243. doi: 10.1016/s0306-4522(97)00448-x. [DOI] [PubMed] [Google Scholar]
- PALMI M., FROSINI M., BECHERUCCI C., SGARAGLI G.P., PARENTE L. Increase of extracellular brain calcium involved in interleukin-1 beta-induced pyresis in the rabbit: antagonism by dexamethasone. Br. J. Pharmacol. 1994;112:449–452. doi: 10.1111/j.1476-5381.1994.tb13093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALMI M., YOUMBI T.G., FUSI F., FROSINI M., SGARAGLI G.P., DIXON H.B.F., TIPTON K.F. Taurine potentiates Ca2+ sequestration by rat liver mitochondria. Biochem. Pharmacol. 1999;58:1123–1131. doi: 10.1016/s0006-2952(99)00183-5. [DOI] [PubMed] [Google Scholar]
- SAFAR P.J., KOCHANEK P.M. Therapeutic hypothermia after cardiac arrest. N. Engl. J. Med. 2002;346:612–613. doi: 10.1056/NEJM200202213460811. [DOI] [PubMed] [Google Scholar]
- SAWYER C.H., EVERETT J.W., GREEN J.D. The rabbit diencephalons in stereotaxic coordinates. J. Comp. Neurol. 1954;101:801–824. doi: 10.1002/cne.901010307. [DOI] [PubMed] [Google Scholar]
- SCHULER V., LUSCHER C., BLANCHET C., KLIX N., SANSIG G., KLEBS K., SCHMUTZ M., HEID J., GENTRY C., URBAN L., FOX A., SPOOREN W., JATON A.L., VIGOURET J., POZZA M., KELLY P.H., MOSBACHER J., FROESTL W., KASLIN E., KORN R., BISCHOFF S., KAUPMANN K., VAN DER PUTTEN H., BETTLER B. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)) Neuron. 2001;31:47–58. doi: 10.1016/s0896-6273(01)00345-2. [DOI] [PubMed] [Google Scholar]
- SERRANO J.S., MINANO F.J., SANCIBRIAN M., DURAN J.A. Involvement of bicuculline-insensitive receptors in the hypothermic effect of GABA and its agonists. Gen. Pharmacol. 1985;16:505–508. doi: 10.1016/0306-3623(85)90012-6. [DOI] [PubMed] [Google Scholar]
- SESTI C., FROSINI M., SGARAGLI G.P. Rabbit rectal temperature and ECoG spectrum changes induced by intracerebroventricular injection of GABA, taurine and analogues. Pharmacol. Res. 1999;39:61. [Google Scholar]
- SGARAGLI G.P., CARLÀ V., MAGNANI M., GALLI A. Hypothermia induced in rabbits by intracerebroventricular taurine: specificity and relationship with central serotonin (5-HT) systems. J. Pharmacol. Exp. Ther. 1981;219:778–785. [PubMed] [Google Scholar]
- SGARAGLI G.P., CARLÀ V., MAGNANI M., GIOTTI A. Homotaurine and muscimol mimic taurine and GABA effects on muscle tone and temperature regulation. Naunyn-Schmiedebergs Arch. Pharmacol. 1978;305:155–158. doi: 10.1007/BF00508286. [DOI] [PubMed] [Google Scholar]
- SGARAGLI G.P., FROSINI M., PALMI M., BIANCHI L., DELLA CORTE L. Calcium and taurine interaction in mammalian brain metabolism. Adv. Exp. Med. Biol. 1994;359:299–308. doi: 10.1007/978-1-4899-1471-2_30. [DOI] [PubMed] [Google Scholar]
- SGARAGLI G.P., PAVAN F. Effects of amino acid compounds injected into cerebrospinal fluid spaces, on colonic temperature, arterial blood pressure and behaviour of the rat. Neuropharmacology. 1972;11:45–56. doi: 10.1016/0028-3908(72)90056-1. [DOI] [PubMed] [Google Scholar]
- The Hypothermia After Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- TUZ K., ORDAZ B., VAA L., QUESADA O., PASANTES-MORALES H. Isovolumetric regulation mechanisms in cultured cerebellar granule neurons. J. Neurochem. 2001;79:143–151. doi: 10.1046/j.1471-4159.2001.00546.x. [DOI] [PubMed] [Google Scholar]
- YAKIMOVA K., SANN H., SCHMID H.A., PIERAU F.K. Effects of GABA agonists and antagonists on temperature-sensitive neurones in the rat hypothalamus. J. Physiol. (London) 1996;494:217–230. doi: 10.1113/jphysiol.1996.sp021486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG Y.H., YANASE-FUJIWARA M., HOSONO T., KANOSUE K. Warm and cold signals from the preoptic area: which contribute more to the control of shivering in rats. J. Physiol. (London) 1995;485:195–202. doi: 10.1113/jphysiol.1995.sp020723. [DOI] [PMC free article] [PubMed] [Google Scholar]