Abstract
Bisphenol A diglycidyl ether (BADGE) is a peroxisome proliferator-activated receptor-γ (PPAR-γ) antagonist, which is able to induce apoptosis in tumor cells independently of PPAR-γ in caspase-dependent and -independent manners. Additionally, BADGE promotes TRAIL-induced apoptosis.
We report that BADGE activates via Bax and caspases-2 and -8 both the intrinsic and extrinsic apoptotic pathways using Bid as a shunt.
BADGE stimulates the mitochondrial release of apoptosis-inducing factor (AIF), cytochrome c and second mitochondria-derived activator of caspase/direct IAP-binding protein with low pl (Smac/DIABLO). The release of cytochrome c could not be blocked by inhibitors of caspases-3, -8 and -9 indicating that BADGE acts upstream of caspases-3 and -9 and does not involve caspase-8 to release cytochrome c.
While the caspase-independent apoptotic effect might be mediated by AIF, the sensitizing effect of BADGE against other apoptotic substances is most likely mediated by the X-linked inhibitor of apoptosis inhibitor Smac/DIABLO.
Our data suggest that BADGE or BADGE derivatives could represent promising substances for the treatment of neoplasms improving the antitumoral activity of TRAIL.
Keywords: BADGE, Bax, Bid, AIF, Smac/DIABLO, cytochrome c, apoptosis, PPAR-γ
Introduction
Peroxisome proliferator-activated receptors (PPARs) are nuclear hormone receptors that share homology with the receptors for steroids, thyroid hormone and retinoic acid (Evans, 1988). There are three families of PPARs: PPAR-α, PPAR-β and PPAR-γ. Peroxisome proliferator-activated acceptor-γ (PPAR-γ) exists in two isoforms, PPAR-γ 1 and PPAR-γ 2, which are derived from the same gene but show different tissue distributions and functions (Tontonoz et al., 1994, 1995). While the expression of PPAR-γ 2 is limited to fat tissue PPAR-γ 1 is widely distributed. PPAR-γ is mainly involved in adipocyte differentiation, lipid metabolism and inflammation (Murphy & Holder, 2000). However, the wide distribution of this nuclear receptor suggests a role in multiple biological processes. There is convincing evidence that PPAR-γ exhibits a strong antitumoral activity by inhibiting tumor cell proliferation and inducing apoptosis in transformed cells (Murphy & Holder, 2000). Additionally, PPAR-γ agonists were shown to sensitize cells against apoptotic substances like TNF-related apoptosis-inducing ligand (TRAIL; Apo2 ligand) (Göke et al., 2000; Kim et al., 2002). This sensitization is mediated by a degradation of the survival factor FLICE-inhibitory protein (FLIP); however, this effect was independent from PPAR-γ expression suggesting the existence of a novel PPAR-γ agonist-regulated target that controls FLIP turnover (Kim et al., 2002). In order to investigate the biological functions of PPAR-γ and the signaling pathways involved, a specific PPAR-γ antagonist would be a worthful tool. Previously, bisphenol A diglycidyl ether (BADGE) was shown to be a PPAR-γ antagonist in 3T3-L1 and 3T3-F442A cells (Wright et al., 2000). In contrast, BADGE exhibited PPAR-γ agonistic activities in an ECV403 cell line (Bishop-Bailey et al., 2000). Furthermore, we reported that BADGE induces apoptosis in tumor cells independently of PPAR-γ in caspase-dependent and -independent manners (Fehlberg et al., 2002). BADGE-induced early apoptosis was inhibited by caspase inhibitors, late apoptosis was found to be caspase independent. While early apoptosis was mediated by the mitochondrial apoptotic pathway, the mechanisms of late apoptosis remained unclear. In the present study, we further elucidate the signaling pathways by showing that BADGE induces a translocation of Bax and a truncation of Bid. Furthermore, BADGE triggers the mitochondrial release of AIF and second mitochondria-derived activator of caspase/direct IAP-binding protein with low pl (Smac/DIABLO). While AIF seems to play an important role for BADGE-induced late apoptosis, the release of the IAP inhibitor Smac/DIABLO might explain the sensitization of tumor cells against apoptotic stimuli by BADGE.
Methods
Cell culture
Jurkat cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum and 0.5% gentamycin. BADGE (Tocris, Bristol, U.K.) was dissolved in DMSO and added to the cell culture at a final concentration of 100–150 μM. Control cells received the same amount of DMSO, which never exceeded 0.5% of the culture medium.
Apoptosis assays
Cell death was determined using annexin-V–FITC. For this, cells were incubated for 6 h without and with the substances to be tested (indicated in the figures). Then, cells were harvested, washed with phosphate-buffered saline (PBS) (pH 7.4) and resuspended in binding buffer (10 mM HEPES, pH 7.4; 140 mM NaCl; 2.5 mM CaCl2) containing annexin-V–FITC followed by an incubation for 15 min in the dark. FITC fluorescence intensity was determined by flow cytometry with a FACScan cytometer. Analysis of the data was performed using Cell Quest software (Beckton-Dickinson).
Analysis of mitochondrial transmembrane potential
Jurkat cells were incubated for 6 h at 37°C in cell culture medium without and with the substances to be tested (indicated in the figures). Control samples were incubated with the K+ ionophore valinomycin (1 μM). Then JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide; final concentration 10 μg ml−1; Molecular Probes, Eugene, OR, U.S.A.) was added and incubation continued for 10 min at room temperature. Cells were washed with and resuspended in PBS (250 μl) and analyzed by flow cytometry with a FACScan cytometer. The photomultiplier values of FL-1 and FL-2 were set as described by Cossarizza et al. (1993). Analysis of the data was performed using Cell Quest software.
Western blot analysis
After treatment cells were pelleted, resuspended in ice-cold 10 mM Tris-HCl, pH 7.4 containing 1 mM EDTA and a cocktail of proteinase inhibitors (Protease Arrest, Alexis, Grünberg, Germany) and briefly sonicated. Equal amounts of protein were loaded onto a polyacrylamide gel and fractionated by electrophoresis. After transfer to a nitrocellulose membrane (pore size 0.2 μM), immunodetection was performed using antibodies in 50 mM Tris-HCl, pH 7.4; 150 mM NaCl, 0.1% Tween-20. The following antibodies were used: anticaspase 7 p20 (N17), antiactin (I-19), anti-AIF (E1), anti-Bax (N-20), anti-Bid (C-20) (all from Santa Cruz, Santa Cruz, CA, U.S.A.), anticytochrome c (Biosource, Camarillo, CA, U.S.A.) and anti-Smac/DIABLO (Calbiochem, San Diego, CA, U.S.A.). Secondary antibodies used were coupled to horseradish peroxidase and detection performed with an ECL kit (Amersham Bioscience). Cytosolic fractions of Jurkat cells were obtained as described (Jung et al., 2001). Briefly, cells were washed once in PBS and subsequently incubated in an appropriate volume of buffer A (20 mM HEPES-KOH; pH 7.5, 10 mM KCl, 1 mM sodium EGTA, 1 mM DTT, Protease Arrest (1 : 100)) for 15 min on ice. Cell disruption was achieved by passage through a 21-gauge needle three times. Lysate was spun at 1000 × g for 10 min at 4°C, followed by centrifugation of the supernatant at 100,000 × g for 30 min at 4°C. The supernatant was spun a second time applying the same conditions to yield the cytosolic fraction. Fractions were separated by PAGE and immunoblotted as mentioned above.
Indirect immunoflourescence
Cells were untreated or treated with BADGE for 16 h. After transfer of the cells onto microscopic slides by a cyto centrifuge cells were fixed for 5 min with ice-cold methanol and permeabilized for 5 min with ice-cold acetone. Cells were labeled with anti-AIF (E1) antibody and visualized with an Alexa Fluor 488 goat anti-mouse antibody. Photography was performed using a Zeiss microscope with epifluorescence optics.
Results
BADGE translocates Bax and induces truncation of Bid
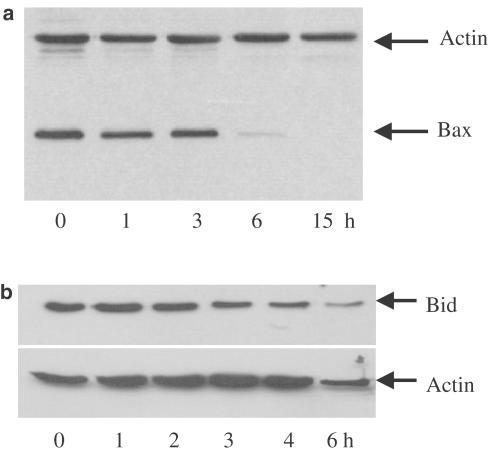
Treatment of cells with BADGE results in a decrease of the mitochondrial transmembrane potential (Fehlberg et al., 2002). In order to explore the signaling pathway upstream of the mitochondria, we investigated whether BADGE would have any impact on the proapoptotic Bcl2-family members Bax and Bid. Jurkat cells were treated with BADGE for up to 15 h at 37°C. Then, cytosol was prepared and analyzed for the presence of Bax and nontruncated Bid, respectively. As shown in Figure 1a, treatment of cells for 6 h with BADGE resulted in a marked decrease of Bax levels in the cytoplasm. After 15 h no Bax was detectable in the cytoplasm. Similarly, nontruncated Bid disappeared in response to BADGE, which was detectable already after 3 h of incubation (Figure 1b). These data indicate that BADGE depolarizes the potential of mitochondrial membranes via truncation of Bid, which stimulates the translocation of Bax to mitochondria and the formation of Bax pores in the outer mitochondrial transmembrane (Korsmeyer et al., 2000).
Figure 1.
Effect of BADGE on Bax and Bid. Jurkat cells were treated with 150 μM BADGE for up to 15 and 6 h, respectively. Western analysis of (a) Bax expression using cytosol or (b) Bid expression using whole-cell extract. Actin expression was used as control.
BADGE stimulates the mitochondrial release of apoptosis inducing factor (AIF)
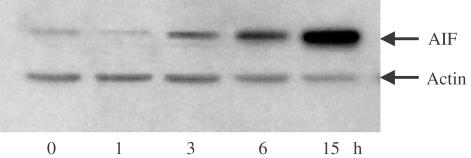
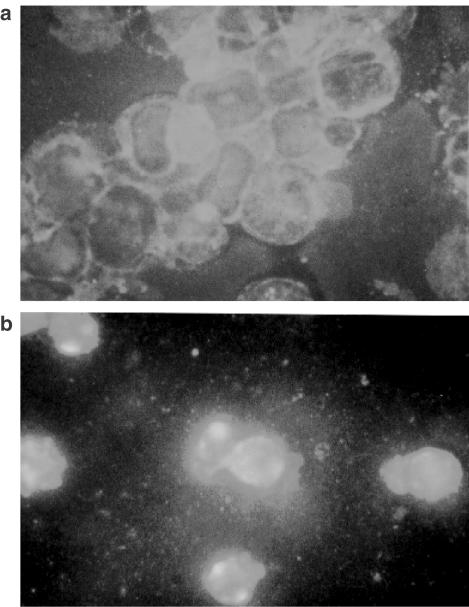
BADGE-induced late apoptosis is a caspase-independent process (Fehlberg et al., 2002). Since BADGE has an impact on the mitochondrial transmembrane potential we tested whether its caspase-independent proapoptotic effect might be mediated by apoptosis-inducing factor (AIF). For this, cells were treated for up to 15 h at 37°C with BADGE and cytosol was prepared and analyzed for the presence of AIF. Treatment of BADGE resulted in a marked increase of AIF levels in the cytoplasm, which was detectable as early as after 3 h of incubation (Figure 2). Immunostaining of AIF showed a bright staining pattern around the nuclei representing typical mitochondrial localization before BADGE treatment (Figure 3a). Stimulation by BADGE induced obvious redistribution of AIF. The staining pattern became diffuse in most cells suggesting that AIF was released into the cytosol (Figure 3b).
Figure 2.
Stimulation of AIF release by BADGE. Jurkat cells were treated with 100 μM BADGE for up to 15 h. Western analysis of AIF expression using cytosol. Actin expression was used as control.
Figure 3.
Immunostaining of AIF released after BADGE treatment. Jurkat cells were (a) untreated or (b) treated for 6 h with 100 μM BADGE, stained with AIF antibodies after fixing and visualized with an Alexa Fluor 488 labeled secondary antibody.
BADGE stimulates the mitochondrial release of cytochrome c
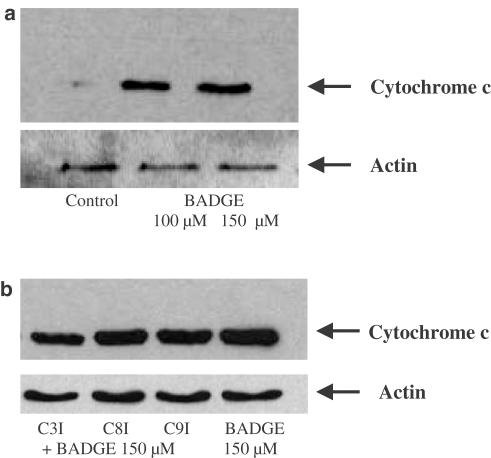
Bax promotes cytochrome c release (Jurgensmeier et al., 1998). Since BADGE translocates Bax, we investigated whether this would result in a cytochrome c release. Jurkat cells were incubated for 4 h in the absence and presence of BADGE. Then, the cytosol of the cells was prepared and analyzed for the presence of cytochrome c. As shown in Figure 4a, cytochrome c was increased in the cytosol after incubation with BADGE. This effect was not inhibited by inhibitors of caspases-3, -8 and -9 (Figure 4b).
Figure 4.
(a) Stimulation of cytochrome c release by BADGE. Jurkat cells were treated with 100 and 150 μM BADGE, respectively, for 4 h. Western analysis of cytochrome c expression using cytosol. Actin expression was used as control. (b) BADGE-induced cytochrome c release is not inhibited by caspase inhibitors-3, -8 and -9. Jurkat cells were pretreated for 30 min with inhibitors of caspases-3 (C3I), -8 (C8I) and -9 (C9I) (50 μM each), respectively, followed by incubation with 150 μM BADGE. Western analysis of cytochrome c expression using cytosol. Actin expression was used as control.
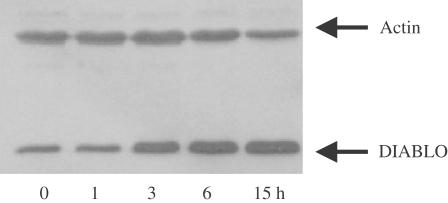
BADGE stimulates the mitochondrial release of Smac/DIABLO
An interesting phenomenon we observed was BADGE's ability to sensitize tumor cells against other proapoptotic substances (Fehlberg et al., 2002). Thus, preincubation of colon cancer or Jurkat cells with BADGE resulted in an increased sensitivity against TRAIL and indomethacin, respectively. In order to elucidate the mechanism of this effect, we treated Jurkat cells with BADGE for up to 15 h at 37°C. Then, the cytosol of the cells was prepared and analyzed for the presence of Smac/DIABLO. As shown in Figure 5, BADGE time dependently stimulated the mitochondrial release of Smac/DIABLO into the cytosol. This suggests that one mechanism by which BADGE sensitizes cells against proapoptotic substances is the translocation of Smac/DIABLO, which blocks the IAP-mediated inhibition of caspases-3, -7 and -9 (Goyal, 2001).
Figure 5.
Stimulation of Smac/DIABLO release by BADGE. Jurkat cells were treated with 100 μM BADGE for up to 15 h. Western analysis of Smac/DIABLO expression using cytosol. Actin expression was used as control.
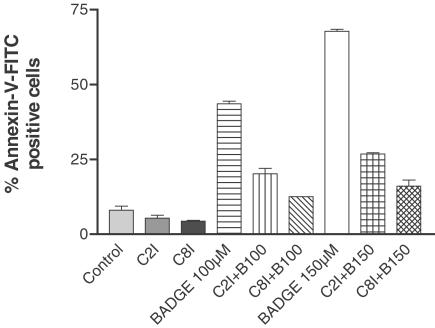
BADGE-induced activation of caspases
As shown above, BADGE initiates the truncation of Bid. It has been shown that Bid is substrate for both caspases-8 and -2 (Li et al., 1998; Guo et al., 2002). To investigate whether these caspases would be involved in the signal pathway of BADGE, we preincubated cells with inhibitors of caspases-2 (z-VDVAD-fmk; R&D Systems, Wiesbaden, Germany) and -8 (z-IETD-fmk; Calbiochem, Bad Soden, Germany), respectively, followed by treatment with BADGE for 6 h. As shown in Figure 6, both caspases-2- and -8 inhibitor inhibited BADGE-induced early apoptosis. In contrast hereto, both inhibitors had no effect on BADGE-induced depolarization of mitochondrial membranes, BADGE-induced late apoptosis or BADGE-induced mitochondrial AIF release (data not shown). Furthermore, the presence of caspases-2 and -8 inhibitors inhibited the BADGE-induced truncation of Bid (data not shown). These data suggest that the BADGE-stimulated truncation of Bid is mediated by caspases-2 and -8, which has an impact on early apoptosis. Late apoptosis is most likely mediated by BADGE-stimulated translocation of Bax and the mitochondrial release of AIF.
Figure 6.
BADGE-induced apoptosis is inhibited by caspase-2 and -8 inhibitors. Jurkat cells were untreated or treated for 6 h with BADGE (B 100 and 150 μM, respectively) after preincubation without or with caspase-2 (C2I) and -8 (C8I) inhibitors, respectively. Then cell aliquots were stained with annexin-V-FITC and analyzed by FACScan. The experiment was repeated twice with identical results. Data presented are means±s.e.m. Control, cells were incubated with vehicle alone.
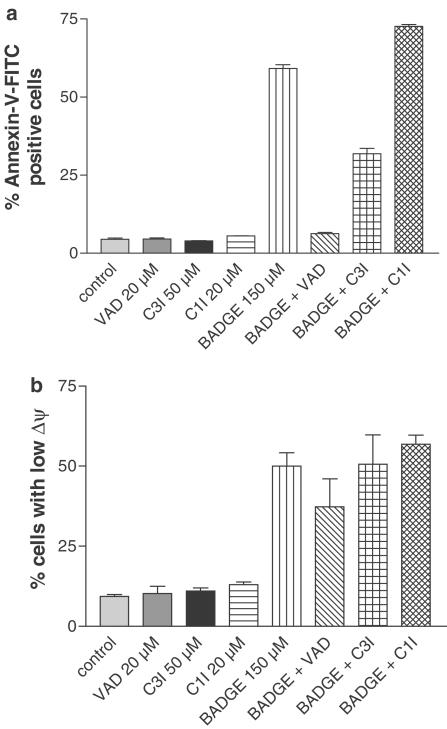
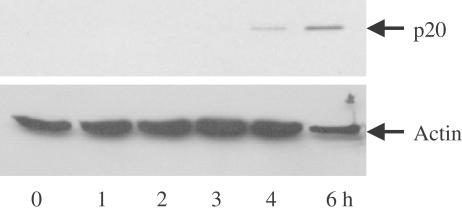
BADGE-induced apoptosis is inhibited by the caspase inhibitor z-VAD-fmk, which inhibits the activation of caspases-1, -3, -4 and -7. In order to investigate which of these caspases are involved in the mediation of cell death, we incubated Jurkat cells without and with BADGE in the absence or presence of specific inhibitors of caspase-3 (z-DQMD-fmk; Calbiochem) and caspases-1 and -4 (YVAD-CHO; Calbiochem). While caspase-3 inhibitor partially decreased the percentage of apoptotic cells, inhibition of caspases-1 and -4 showed no effect (Figure 7a). None of the inhibitors showed any effect on BADGE-induced depolarization of mitochondrial transmembrane potential (Figure 7b). This suggested that beside caspase-3, caspase-7 might be activated after stimulation of cells with BADGE. To our knowledge there is no specific caspase-7 inhibitor available. Therefore, to test whether treatment with BADGE would lead to an activation of caspase-7, we incubated cells for up to 6 h at 37°C with BADGE and analyzed cell extract for the appearance of caspase cleavage product p20. As shown in Figure 8, after 4 h the p20 cleavage product was detectable indicating that treatment with BADGE results in caspase-7 activation.
Figure 7.
Caspase-3 but not caspases-1 and -4 are involved in BADGE-induced apoptosis. Jurkat cells were untreated or treated for 6 h with BADGE (B 150 μM) after preincubation without or with inhibitor of caspases –1, -3, -4 -7 (VAD), inhibitor of caspases-1 and –4 (C1I) or inhibitor of caspase-3 (C3I). Then cell aliquots were stained with (a) annexin-V–FITC or (b) JC-1 and analyzed by FACScan to determine percentage of apoptotic cells and cells with low mitochondrial membrane potential, respectively. The experiment was repeated twice with identical results. Data presented are means±s.e.m. Control, cells were incubated with vehicle alone.
Figure 8.
BADGE stimulates cleavage of caspase-7. Jurkat cells were treated with 150 μM BADGE for up to 6 h. Western analysis of the expression of the caspase-7 cleavage product p20 using whole-cell extract. Actin expression was used as control.
Discussion
PPAR-γ is a nuclear hormone receptor that is involved in the regulation of lipid metabolism, adipocyte differentiation and inflammation (Murphy & Holder, 2000). Additionally, PPAR-γ agonists show antineoplastic, antiproliferative and proapoptotic activities in different tumors (Göke et al., 2000; Kim et al., 2002). However, there is emerging evidence that PPAR-γ agonists exert PPAR-γ-independent functions. To further elucidate the physiological functions of PPAR-γ and the signal transduction of PPAR-γ agonists, a specific PPAR-γ antagonist would be a very useful tool. BADGE was identified as a specific PPAR-γ antagonist in 3T3-L1 and 3T3-F442A cells (Wright et al., 2000). However, Bishop-Bailey et al. (2000) reported an agonistic activity in ECV304 cells in which BADGE exerted a proapoptotic function. Recently, we showed that BADGE induces apoptosis in different tumor cell lines PPAR-γ independently in caspase-dependent and -independent manners (Fehlberg et al., 2002). Treatment of tumor cells with BADGE resulted in an activation of the apoptotic mitochondrial pathway involving a decrease of the mitochondrial transmembrane potential and activation of caspases-9 and -3. While early BADGE-induced apoptosis is a caspase-dependent process, late apoptosis was found to be caspase independent. Furthermore, BADGE-induced apoptosis was totally independent from PPAR-γ expression. Interestingly, BADGE is able to sensitize cells against other apoptotic substances. The present study was undertaken to further elucidate the signaling pathways of BADGE.
Bcl-2-family proteins are either antiapoptotic (for example, Bcl-2 and BclxL) or proapoptotic (for example, Bax and Bak). Antiapoptotic family members like Bcl-2 are localized mainly in mitochondrial membranes where they block membrane permeabilization. Proapoptotic members like Bax are translocated from the cytosol to mitochondria facilitating the membrane permeabilization (for review see Ferri & Kroemer, 2001). Bax translocation and permeabilization of membranes is blocked by antiapoptotic proteins like Bcl-2 and promoted by proapoptotic proteins like Bid. One function by which these proteins affect cell death is the regulation of mitochondrial cytochrome c release. While Bax promotes cytochrome c release Bcl-2 prevents cytochrome c release in response to apoptotic substances. Investigating how BADGE's impact on mitochondria is mediated, we found that BADGE stimulates the translocation of Bax from cytosol to mitochondria and causes the truncation of Bid. Furthermore, BADGE induced the release of cytochrome c from the mitochondria into the cytosol. This is in accordance with our previous observation that treatment of Jurkat cells with BADGE leads to activation of caspase-9 (Fehlberg et al., 2002). Inhibitors of caspases-3, -8 and -9 could not block BADGE-induced cytochrome c release indicating that this effect is mediated upstream of caspases-3 and -9 and does not involve the activation of caspase-8.
Since Bid is substrate for activated caspases-8 and -2, it represents a link between the extrinsic apoptotic pathway (initiated by death receptors) and the intrinsic mitochondrial apoptotic pathway. As our experiments show, BADGE-induced apoptosis is inhibited by inhibitors of caspases-8 and -2 indicating that BADGE activates both apoptotic pathways using Bid as a shunt.
Mitochondria act as a crossover point between caspase-dependent and -independent apoptotic pathways. AIF, which is located in the mitochondrial intermembrane space and is released to the cytosol and to the nucleus in response to death stimuli, is a key trigger of caspase-independent apoptosis (Susin et al., 1999; Daugas et al., 2000). Release of AIF results in generation of apoptotic phenotypes like chromatin condensation and phosphatidylserin exposure. Since Bax was reported to induce mitochondrial AIF release (Antonsson, 2001) and since BADGE has an impact on mitochondria via Bax, we tested whether treatment of cells with BADGE would lead to a translocation of AIF to the cytosol. We found that incubation of cells with BADGE resulted in a time-dependent increase of AIF levels in the cytosol. While BADGE-induced early apoptosis was caspase dependent, late apoptosis was not. This suggests that late apoptosis caused by BADGE might be mediated by AIF. Recently, it was reported that caspase-2 via truncation of Bid induces the release of apoptotic mitochondrial proteins like cytochrome c, which could be blocked by Bcl-2 and Bcl-xL (Guo et al., 2002). Interestingly, caspase-2 can also induce directly the release of cytochrome c, AIF and other factors independent from Bid. In our experiments, caspase-2 inhibitor z-VDVAD-fmk was able to block BADGE-induced early apoptosis but failed to inhibit BADGE-induced AIF release and late apoptosis, respectively. This suggests that caspase-2 is involved in BADGE-induced early apoptosis most likely via Bid and cytochrome c release, but has no impact on processes that mediate late apoptosis.
Using the broad-range caspase inhibitor z-VAD-fmk (inhibits caspases-1, -3, -4 and -7), we were able to block BADGE-induced early apoptosis (Fehlberg et al., 2002). We now tested which of these caspases are involved in BADGE signaling. A specific inhibitor of caspases-1 and -4 had no effect and a specific caspase-3 inhibitor only partially inhibited BADGE-induced apoptosis suggesting that caspase-7 might also be involved in the signaling. Confirming this assumption, we could demonstrate the appearance of the p20 cleavage product of caspase-7 after the incubation of Jurkat cells with BADGE. Summarizing our previous and present findings, caspases-2, -3, -7, -8 an -9 are activated in Jurkat cells in response to BADGE.
An interesting feature of BADGE is its ability to sensitize tumor cells against other apoptotic stimuli like TRAIL and indomethacin (Fehlberg et al., 2002). Since BADGE exerts an effect on mitochondria, we investigated whether BADGE would stimulate the release of Smac/DIABLO. Smac/DIABLO is a mitochondrial factor that binds to X-linked inhibitor of apoptosis (XIAP) inhibiting the XIAP blockade of activation of caspase-3 by caspases-8 and -9 (Deng et al., 2002). We found that BADGE caused a Smac/DIABLO release to the cytosol suggesting that the sensitizing effect of BADGE is based on the inhibition of XIAP by Smac/DIABLO. TRAIL, an important new potential tool for the treatment of tumors, has the exciting feature that it selectively kills transformed cells leaving nontransformed cells unharmed. Recently, it was reported that cooperation between caspase-8 activation and mitochondrial signaling is required for complete processing and activation of caspase-3 (Deng et al., 2002). Impairment of mitochondrial function is often associated with tumor development. Therefore, substances like BADGE or BADGE derivatives targeting Smac/DIABLO and XIAP could represent promising new substances for the treatment of neoplasms improving the antineoplastic activity of TRAIL.
Acknowledgments
This work was supported by grants of the Deutsche Forschungsgemeinschaft and the Konrad-Adenauer-Stiftung which is gratefully acknowledged. S.F. and C.G. contributed equally to this work.
Abbreviations
- AIF
apoptosis-inducing factor
- BADGE
bisphenol A diglycidyl ether
- PPAR-γ
Peroxisome proliferator-activated receptor-γ
- Smac/DIABLO
Second mitochondria-derived activator of caspase/direct IAP-binding protein with low pl
- TRAIL
TNF-related apoptosis-inducing ligand
- XIAP
X-linked inhibitor of apoptosis
References
- ANTONSSON B. Bax and other pro-apoptotic Bcl-2 family ‘killer-proteins' and their victim the mitochondrion. Cell and Tissue Research. 2001;306:347–361. doi: 10.1007/s00441-001-0472-0. [DOI] [PubMed] [Google Scholar]
- BISHOP-BAILEY D., HLA T., WARNER T.D. Bisphenol A diglycidyl ether (BADGE) is a PPARgamma agonist in an ECV304 cell line. Br. J. Pharmacol. 2000;131:651–654. doi: 10.1038/sj.bjp.0703628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSSARIZZA A., BACCARANI_CONTRI M., KALASHNIKOVA G., FRANCESCHI C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1) Biochem. Biophys. Res. Commun. 1993;197:40–45. doi: 10.1006/bbrc.1993.2438. [DOI] [PubMed] [Google Scholar]
- DAUGAS E., SUSIN S.A., ZAMZAMI N., FERRI K.F., IRINOPOULOU T., LAROCHETTE N., PREVOST M.C., LEBER B., ANDREWS D., PENNINGER J., KROEMER G. Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. FASEB J. 2000;14:729–739. [PubMed] [Google Scholar]
- DENG Y., LIN Y., WU X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 2002;16:33–45. doi: 10.1101/gad.949602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS R.M. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEHLBERG S., TRAUTWEIN S., GÖKE A., GÖKE R. Bisphenol A diglycidyl ether induces apoptosis in tumour cells independently of peroxisome proliferator-activated receptor-gamma, in caspase-dependent and -independent manners. Biochem. J. 2002;362:573–578. doi: 10.1042/0264-6021:3620573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRI K.F., KROEMER G. Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 2001;3:E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- GÖKE R., GÖKE A., GÖKE B., CHEN Y. Regulation of TRAIL-induced apoptosis by transcription factors. Cell. Immunol. 2000;201:77–82. doi: 10.1006/cimm.2000.1650. [DOI] [PubMed] [Google Scholar]
- GOYAL L. Cell death inhibition: keeping caspases in check. Cell. 2001;104:805–880. doi: 10.1016/s0092-8674(01)00276-8. [DOI] [PubMed] [Google Scholar]
- GUO Y., SRINIVASULA S.M., DRUILHE A., FERNANDES_ALNEMRI T., ALNEMRI E.S. Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria. J. Biol. Chem. 2002;277:13430–13437. doi: 10.1074/jbc.M108029200. [DOI] [PubMed] [Google Scholar]
- JUNG Y.S., KIM K.S., KIM K.D., LIM J.S., KIM J.W., KIM E. Apoptosis-linked gene 2 binds to the death domain of Fas and dissociates from Fas during Fas-mediated apoptosis in Jurkat cells. Biochem. Biophys. Res. Commun. 2001;288:420–426. doi: 10.1006/bbrc.2001.5769. [DOI] [PubMed] [Google Scholar]
- JURGENSMEIER J.M., XIE Z., DEVERAUX Q., ELLERBY L., BREDESEN D., REED J.C. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM Y., SUH N., SPORN M., REED J.C. An inducible pathway for degradation of FLIP protein sensitizes tumor cells to TRAIL-induced apoptosis. J. Biol. Chem. 2002;277:22320–22329. doi: 10.1074/jbc.M202458200. [DOI] [PubMed] [Google Scholar]
- KORSMEYER S.J., WEI M.C., SAITO M., WEILER S., OH K.J., SCHLESINGER P.H. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- LI H., ZHU H., XU C.J., YUAN J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- MURPHY G.J., HOLDER J.C. PPAR-gamma agonists: therapeutic role in diabetes, inflammation and cancer. Trends Pharmacol. Sci. 2000;21:469–474. doi: 10.1016/s0165-6147(00)01559-5. [DOI] [PubMed] [Google Scholar]
- SUSIN S.A., LORENZO H.K., ZAMZAMI N., MARZO I., SNOW B.E., BROTHERS G.M., MANGION J., JACOTOT E., COSTANTINI P., LOEFFLER M., LAROCHETTE N., GOODLETT D.R., AEBERSOLD R., SIDEROVSKI D.P., PENNINGER J.M., KROEMER G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- TONTONOZ P., HU E., DEVINE J., BEALE E.G., SPIEGELMAN B.M. PPAR gamma 2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Mol. Cell Biol. 1995;15:351–357. doi: 10.1128/mcb.15.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONTONOZ P., HU E., GRAVES R.A., BUDAVARI A.I., SPIEGELMAN B.M. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- WRIGHT H.M., CLISH C.B., MIKAMI T., HAUSER S., YANAGI K., HIRAMATSU R., SERHAN C.N., SPIEGELMAN B.M. A synthetic antagonist for the peroxisome proliferator-activated receptor gamma inhibits adipocyte differentiation. J. Biol. Chem. 2000;275:1873–1877. doi: 10.1074/jbc.275.3.1873. [DOI] [PubMed] [Google Scholar]