Abstract
We have investigated the properties of native and haemagglutinin (HA)-tagged neuropeptide Y (NPY) Y1 receptors after mutation of the palmitoylation site Cys337 to Ser or Ala.
In Chinese hamster ovary cells expressing similar receptor levels, the C337A mutation abolished incorporation of [3H]palmitic acid into the HA-Y1 receptor.
Cys337 substitution did not alter the affinities of Y1 receptor agonists or antagonists, but it eliminated the ability of guanosine-5′-O-(3-thio)triphosphate (GTPγS) to displace [125I]PYY-specific binding (compared to approximately 50% inhibition in Y1 or HA-Y1 clones).
Stimulation of GTPγ[35S] binding by native and HA-Y1 receptors in standard incubation buffer (100 mM NaCl, 10 μM GDP) was prevented by Cys337 mutation. In this assay, the function of Y1(C337S) receptors could be partially rescued by reducing the Na+ concentration, and when overexpressed (Bmax: ∼10 pmol mg−1), both HA-Y1 and HA-Y1(C337A) receptors displayed similar responses to NPY and peptide YY (PYY).
In stably transfected adenocarcinoma cells expressing Y1 or Y1(C337S) receptors, PYY inhibited anion secretion stimulated by vasoactive intestinal peptide (VIP; measured as short-circuit current, ISC) with similar potency (EC50: 26–53 nM). In contrast to the transient Y1 receptor-mediated responses observed at maximal PYY concentrations, ISC reductions in both Y1(C337S) clones were sustained.
We conclude that nonpalmitoylation of the Y1 receptor reduces its coupling efficiency to G proteins, and may also indirectly influence desensitisation processes that depend on the formation of an active agonist-receptor conformation.
Keywords: Neuropeptide Y, Y1 receptor, palmitoylation, receptor efficacy, desensitisation, GTPγ35S binding, epithelial anion secretion
Introduction
Palmitic acid acylation of conserved C-terminal cysteine residues is a widespread post-translational modification of G protein-coupled receptors (GPCRs), which respond to both peptide and nonpeptide ligands (Qanbar & Bouvier, 2003). Studies using fluorescent palmitate analogues incorporated into bovine rhodopsin (Moench et al., 1994), and most recently its crystal structure (Palczewski et al., 2000), have directly supported the suggestion that the hydrophobic fatty acid tails anchor the palmitoylated residues to the lipid bilayer, creating a fourth intracellular loop. Moreover, the extent of acylation may be affected by agonist occupancy (Kennedy & Limbird, 1994; Loisel et al., 1996; Hayashi & Haga, 1997; Hawtin et al., 2001; Stevens et al., 2001; Ponimaskin et al., 2002), allowing dynamic structural modulation of the C terminus. Palmitoylation therefore has considerable potential to regulate both the nature and extent of GPCR signalling.
Many mutant receptors that lack the acylated cysteine residues exhibit reduced efficacy compared to wild-type proteins as a consequence of direct or indirect mechanisms. These include the prototypic GPCRs rhodopsin (after chemical reduction of the palmitoyl cysteines; Sachs et al., 2000) and the Gs-coupled β2 adrenoceptor (containing the C341G substitution; Moffett et al., 1996), and also GPCRs coupled to other G proteins, such as Gi (M2(C457A) muscarinic, the SST5 (C320A) somatostatin and CCR5 chemokine receptors; Hayashi & Haga, 1997; Hukovic et al., 1998; Kraft et al., 2001) or Gq and Gi (the palmitoyl-deficient endothelin ETA and ETB receptors; Doi et al., 1999). However, for a number of subtypes, G protein activation remains unaltered in the absence of palmitoylation (Kennedy & Limbird, 1993; Sadeghi et al., 1997; Tanaka et al., 1998; Gao et al., 1999; Hawtin et al., 2001; Stevens et al., 2001). In some instances receptor trafficking may be affected, resulting in changes in cell surface expression (Hayashi & Haga, 1997; Sadeghi et al., 1997; Tanaka et al., 1998; Gao et al., 1999; Percherancier et al., 2001) and positive or negative effects have also been reported on agonist-induced desensitisation, internalisation or downregulation (Eason et al., 1994; Moffett et al., 1996; Hukovic et al., 1998; Palmer & Stiles, 2000; Hawtin et al., 2001; Kraft et al., 2001). Thus the effects of GPCR palmitoylation appear more diverse than the conserved role of phosphorylation in regulating desensitisation of rhodopsin-like GPCRs (Ferguson, 2001), even among receptor subtypes activated by the same ligands or coupled to similar classes of G proteins.
Potentially palmitoylated cysteine residues are conserved in the C termini of all the five cloned Gi-linked Y receptors, for which the endogenous ligands are neuropeptide Y (NPY), peptide YY (PYY) and pancreatic polypeptide (PP), all sharing a common tertiary structure known as the PP-fold (Michel et al., 1998). The Y1 receptor (which exhibits high affinity for NPY and PYY, but not PP) was the first subtype to be cloned from rat and human tissue (Krause et al., 1992; Larhammar et al., 1992). Several Y1-selective antagonists have been synthesised, including the nonpeptides BIBP3226 and BIBO3304 (Wieland et al., 1998b), and these compounds have enabled characterisation of constitutively expressed antisecretory Y1 receptors in mucosal preparations of rodent and human descending colon (Tough & Cox, 1996; Cox & Tough, 2002) and in human adenocarcinoma cells (Tough & Cox, 1996).
In the current study, we have compared the wild-type rat Y1 receptor (which is 94% identical to its human counterpart; Larhammar et al., 1992) with mutants in which the palmitoylation site (Cys337) has been substituted by serine or alanine. To assess the relative efficacies of these nonpalmitoylated receptors in stably transfected Chinese hamster ovary (CHO) K1 cells, we have measured agonist-stimulated guanosine-5′-O-(3-thio)triphosphate (GTPγ[35S]) binding, a useful technique to directly determine GPCR activation of G proteins (particularly Gi; Williams et al., 1997). In addition, we have adopted a novel functional approach to investigate the Y1 and Y1(C337S) receptors expressed in a human epithelial cell line, by measuring their effects on anion secretion using short-circuit current (ISC) techniques (Holliday & Cox, 1996). In contrast to more conventional assays that determine the integrated changes in adenosine 3′ : 5′-cyclic monophosphate (cAMP) production (Kenakin, 1996), ISC responses may be followed in real time to allow indirect measurement of the temporal patterns of Y1 and Y1(C337S) receptor-mediated signalling.
Methods
Preparation of wild-type and mutated Y1 receptor cDNA constructs
The expression vector pTEJ8 (Johansen et al., 1990) containing wild-type rat Y1 or Y1(C337S) receptor cDNAs was kindly provided by Professor T. Schwartz (Laboratory of Molecular Pharmacology, The Panum Institute, University of Copenhagen, Denmark). To obtain the rat Y1 receptor modified at the N terminus with a haemagglutinin (HA) epitope tag (YPYDVPDYA), the coding sequence following the initial methionine was amplified by polymerase chain reaction using the primers: forward 5′-AAAAGTACTAATTCAACTCTGTTCTCCAGG-3′ and reverse 5′-AAAGGTACCTCAGATTTTTTCATTGTCATT-3′ (ScaI/KpnI sites underlined).
Following insertion of the product into pGEM-T (Promega, Southampton, U.K.), the ScaI/KpnI fragment was excised and subcloned into the pCruz HA eukaryotic expression vector (Santa Cruz Biotechnology, Santa Cruz, U.S.A.). The C337A substitution was introduced into this sequence using the Transformer mutagenesis kit (Clontech, Oxford, U.K.) with the following mutagenic primer: 5′-CTTCAACTTTGCTGACTTTCGAAGTCGAGACGACG-3′ (substituted nucleotides highlighted in bold, diagnostic SfuI site underlined). All cDNAs were sequenced in both directions to confirm the correct products.
Cell culture and transfection
CHO K1 cells (kindly provided by Professor S. Hill, University of Nottingham, U.K.) were grown in a 1 : 1 mixture of Ham's F12 and Dulbecco's modified Eagle's medium (DMEM) containing 10% (v v−1) heat-inactivated foetal calf serum and L-glutamine (2 mM). Colony 1 adenocarcinoma cells (a gift from Dr S. Kirkland, Imperial College London, U.K.) were routinely maintained in DMEM supplemented with 10% foetal calf serum, 100 μg ml−1 kanamycin and 1.2 μg ml−1 amphotericin B. Both cell lines were incubated at 37°C in a humidified atmosphere of 95% O2/5% CO2 and passaged when confluent by trypsinisation (0.25% w v−1 in versene). They were transfected with wild-type and mutated Y1 receptor cDNA constructs by coprecipitation with calcium phosphate followed by glycerol shock (15% v v−1 in phosphate-buffered saline) for 4 min. Stably transfected clones were identified by their resistance to the antibiotic G418 (1.0 mg ml−1), applied in the growth medium for 7–10 days, and isolated directly (Colony 1) or by dilution cloning (CHO). CHO clones were maintained in a medium containing 0.2 mg ml−1 G418 and were screened for Y1 receptor expression by [125I]PYY binding; suitable Colony 1 epithelial clones were determined by both binding and functional studies.
Immunoprecipitation and western analysis
Stably transfected CHO cells in six-well plates were solubilised in immunoprecipitation buffer (RIPA; 50 mM Tris, 150 mM NaCl, 5 mM ethylenediaminetetraacetic acid (EDTA), 1% Nonidet P40, 0.5% sodium deoxycholate, 0.1% SDS, 0.5 mM 4-(2-aminoethyl) benzenesulphonyl fluoride, 10 μg ml−1 leupeptin and aprotinin; pH 8.0) and rotated end over end for 2 h at 4°C. Following clarification of the extracts by centrifugation (20,000 × g, 15 min, 4°C) and equalisation for protein content (BCA protein assay. Pierce, Cheshire, U.K.), receptors were immunoprecipitated overnight at 4°C by addition of directly conjugated anti-HA agarose (clone 3f10, Roche Molecular Biochemicals, Lewes, U.K.). Immunoprecipitates were washed (4°C, 15 min, rotating) twice with RIPA buffer, twice with high salt wash buffer (50 mM Tris, 500 mM NaCl, 5 mM EDTA, 0.1% Nonidet P40, 0.05% deoxycholate; pH 8.0) and once with low salt wash buffer (50 mM Tris, 5 mM EDTA, 0.1% Nonidet P40, 0.05% deoxycholate; pH 8.0). Bound proteins were eluted in 0.5% SDS, 1% β-mercaptoethanol and treated with peptide : N-glycosidase F (PNGase F; New England Biolabs, Hitchin, U.K.; 37°C, 1 h) as appropriate. Samples equalised for receptor number were denatured in Laemmli loading buffer (80°C, 3 min), resolved by SDS—polyacrylamide gel electrophoresis (PAGE; 7.5 or 10% Tris-HCl Ready Gels; Biorad, Hemel Hempstead, U.K.) and transferred to a polyvinyl difluoride membrane. Proteins were detected by the CT/2 rabbit polyclonal antibody directed against amino acids 362–382 of the rat Y1 C terminus (a gift of Prof A. Beck-Sickinger, University of Leipzig, Germany; Wieland et al., 1998a), and visualised with a horseradish peroxidase-conjugated secondary antibody and enhanced chemiluminescence (ECL plus; Amersham Biosciences, Buckinghamshire, U.K.).
[3H]palmitate labelling and detection of receptor palmitoylation
Prior to use, [3H]palmitic acid was dried under a stream of nitrogen and redissolved in a minimum volume of DMSO. CHO clones grown to 70% confluence in 75 cm2 flasks were incubated overnight in serum-free DMEM/F12 medium, after which the medium was replaced with 5 ml DMEM/F12 containing 1% bovine serum albumin (BSA) and 200 μCi ml−1 [3H]palmitic acid. After 4 h the cells were treated with 300 nM NPY for 10 min as appropriate and washed twice with PBS. Solubilisation and immunoprecipitation of HA-tagged receptors were carried out as described above. Immune complexes were eluted in sample buffer, equalised for receptor number and divided into two aliquots (80% : 20%) which were resolved by SDS–PAGE. Equal loading of receptor protein was confirmed by Western analysis of the gel containing 20% of the samples, while the remaining gel was treated with Amplify (Amersham Biosciences) and dried. [3H]labelled proteins were detected by fluorography of the gel exposed to preflashed Amersham Hyperfilm MP for 5–8 weeks at −70°C.
Membrane preparation and [125I]PYY binding
Membrane suspensions from Colony 1 (5–7 μg protein μl−1) and CHO cells (0.2–0.8 μg protein μl−1) were prepared in 10 mM N-Tris-(hydroxymethyl)-methyl-2-aminoethane-sulphonic acid (TES) and 0.1 mM phenylmethylsulphonyl fluoride (PMSF; pH 7.6), as described in detail in Holliday & Cox (1996). In [125I]PYY binding studies (Holliday & Cox, 1996), fresh membrane aliquots were incubated for 120 min at 21°C in buffer (4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid (HEPES) 8 mM, KCl 4 mM, NaHCO3 20 mM, MgSO4 1.0 mM, K3PO4 1.0 mM, CaCl2 2.0 mM, BSA 0.4% w v−1 and bacitracin (0.1 mg ml−1); pH 7.4) and [125I]PYY (17–25 pM, Colony 1; 10/15 pM, CHO), in the presence or absence of unlabelled ligand or GTPγS. Incubations were terminated by rapid filtration.
GTPγ[35S] assays
Fresh CHO membrane aliquots (30 μl, 0.5–1.0 μg protein μl−1) were diluted in an optimised 10 mM HEPES buffer (pH 7.4) containing 50 or 100 mM NaCl, 10 mM MgCl2, guanosine diphosphate (GDP, 10 μM unless otherwise stated), 0.1 mM EDTA, 0.2% BSA and 0.1 mg ml−1 bacitracin (final volume 480 μl), with or without agonist peptides (PYY, NPY 10 pM–1 μM) and 1 μM antagonist. Following a 90 min incubation period at 21°C to ensure equilibrium ligand binding, 20 μl GTPγ[35S] was added to a final concentration of 200 pM. After a further 20 min, reactions were terminated by filtration over GF/B filters and the bound membranes washed with 10 ml ice-cold incubation buffer (minus GDP). Filters were dried before addition of scintillant (Ultima Gold MV, Packard instruments, Berkshire, U.K.), and after 24 h the retained radioactivity was determined in a beta counter. Nonspecific binding in triplicate experiments was assessed in the presence of 10 μM unlabelled GTPγS and comprised less than 5% of the total counts.
ISC measurements
Colony 1 cells (passages 19–21) or transfected clones (passages 5–12) were trypsinised and seeded onto collagen-coated Millipore filters (1 × 106 cells per filter) as described by Holliday et al. (1997). Epithelial layers (area 0.2 cm2, reaching confluence after 6–7 days) were subsequently mounted between two halves of perspex Ussing chambers, bathed in oxygenated (95% O2/5% CO2) Krebs—Henseleit solution (composition in mM: NaCl 118, KCl 4.7, NaHCO3 25, KH2PO4 1.2, MgSO4 1.2, CaCl2 2.5 and glucose 11.1; pH7.4) at 37°C (15 ml in each reservoir) and voltage clamped at zero potential (WP Instruments, Florida, U.S.A.). The resulting short-circuit current (ISC) represents net electrogenic ion transport across the epithelial barrier. Following stimulation of cAMP-dependent chloride secretion (increased ISC) with vasoactive intestinal polypeptide (VIP), antisecretory ISC responses to basolateral additions of PYY and other agents were measured continuously on pen recorders.
Data evaluation
Displacement [125I]PYY binding curves and concentration–response curves in GTPγ[35S] binding studies were analysed as combined data groups from individual experiments using the program Graphpad Prism v. 3.0 (Graphpad software, San Diego, U.S.A.), to yield pIC50 or pEC50 values. For CHO clones, PYY displacements were also used to estimate total [125I]PYY binding sites (Bmax) according to Bmax=TSB × IC50/[L], where TSB is the total specific binding in the absence of agonist and [L] is the free radioligand concentration. Binding affinity (Ki) and Bmax estimates in Colony 1 membranes were obtained by saturation experiments and Scatchard analysis. Concentration–response curves in electrophysiological studies were constructed from responses to single additions of the desired agonist, expressed as the percentage inhibition of total ISC (including basal and VIP-stimulated components). Statistical comparisons between two data sets were made using unpaired Student's t-tests.
Materials
All enzymes used in site-directed mutagenesis were obtained from New England Biolabs or Roche Molecular Biochemicals. Cell culture materials were from the following origins: DMEM and G418 sulphate (Invitrogen, Paisley, U.K.); DMEM: Ham's F-12 and L-glutamine (Sigma-Aldrich, Poole, U.K.); foetal calf serum, kanamycin and amphotericin B (ICN Biomedicals, Oxford, U.K.); trypsin (Lorne Laboratories, Reading, U.K.). [9,10-3H]palmitic acid, [125I]PYY and GTPγ[35S] were purchased from Perkin-Elmer (Stevenage, U.K.), while unlabelled peptides of porcine (VIP) or rat origin (NPY and PYY) were from Bachem (Merseyside, U.K.); the Y1 selective antagonists BIBP3226 ((R)-N2-(diphenylacetyl)-N-[(4-hydroxyphenyl)methyl]-argininamide) and BIBO3304 ((R)-N2-(diphenylacetyl)-N-[(4-(aminocarbonylaminomethyl)phenyl)methyl]-argininamide) were gifts from Boehringer Ingelheim Pharma KG (Biberach, Germany). GTPγ[35S] was diluted in incubation buffer, and stored as aliquots at −70°C. All peptides, BIBP3226 and BIBO3304 were aliquoted and stored at −20°C until required. Piretanide was obtained from Hoechst Marion Roussel (Swindon, U.K.) and other reagents were from Sigma-Aldrich (Poole, U.K.) or VWR International (Poole, U.K.). UK14,304 (UK; 5-Bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine) was prepared as a 10 mM solution in dimethylsulphoxide and pertussis toxin was purchased as a solution in 50% glycerol containing 50 mM Tris, 10 mM glycine and 0.5 M NaCl (pH 7.5); other chemicals were made up as aqueous stock solutions.
Results
Generation of CHO clones
A number of G418-resistant CHO colonies were established, which stably expressed native or HA-tagged Y1 receptors, or their respective C337S or C337A palmitoylation site mutants. Following screening by [125I]PYY binding, the following clones were chosen for the study of the Y1 (Y1:Bmax: 1.2±0.2 pmol mg−1 membrane protein; n=6), Y1(C337S) (Y1C:Bmax:1.0±0.1 pmol mg−1; n=6), HA-tagged Y1 (HAY1-L:Bmax:1.9±0.2 pmol mg−1 (n=5) and HAY1-H: Bmax: 8.1±1.0 pmol mg−1; n=7) and HA-Y1(C337A) receptors (HAY1C-L: Bmax: 1.0±0.1 pmol mg−1 (n=5) and HAY1C-H: Bmax:17.2±2.9 pmol mg−1; n=5). As the summary of [125I]PYY competition experiments in Table 1 illustrates, neither the addition of the N-terminal HA sequence nor the mutation of Cys 337 substantially altered the affinity of the Y1 receptor for PYY (IC50 range: 0.24–0.42 nM), NPY (0.25–0.34 nM) or the antagonists BIBP3226 (3.21 nM) and BIBO3304 (0.28–0.48 nM).
Table 1.
Agonist and antagonist affinities for native and nonpalmitoylated Y1 receptors in CHO clones
| Y1 | Y1C | HAY1-L | HAY1C-L | HAY1-H | HAY1C-H | |
|---|---|---|---|---|---|---|
| PYY | 9.46±0.02 | 9.62±0.02 | 9.37±0.03 | 9.57±0.05 | 9.52±0.05 | 9.40±0.02 |
| PYY+GTPγS | 9.46±0.06 | 9.56±0.04 | 9.31±0.02 | 9.49±0.04 | 9.57±0.08 | 9.41±0.02 |
| NPY | 9.48±0.04 | 9.47±0.12 | 9.49±0.04 | 9.51±0.01 | 9.61±0.09 | 9.53±0.06 |
| BIBP3226 | 8.49±0.12 | 8.49±0.07 | ND | ND | ND | ND |
| BIBO3304 | 9.37±0.04 | ND | 9.49±0.04 | 9.55±0.01 | 9.32±0.08 | 9.42±0.05 |
pIC50 values (mean±s.e.m.) were calculated from triplicate competitive displacements of [125I]PYY binding, pooled from three to seven experiments. PYY displacements were conducted with or without 1 μM GTPγS. ND–not determined.
Palmitoylation of the rat Y1 receptor
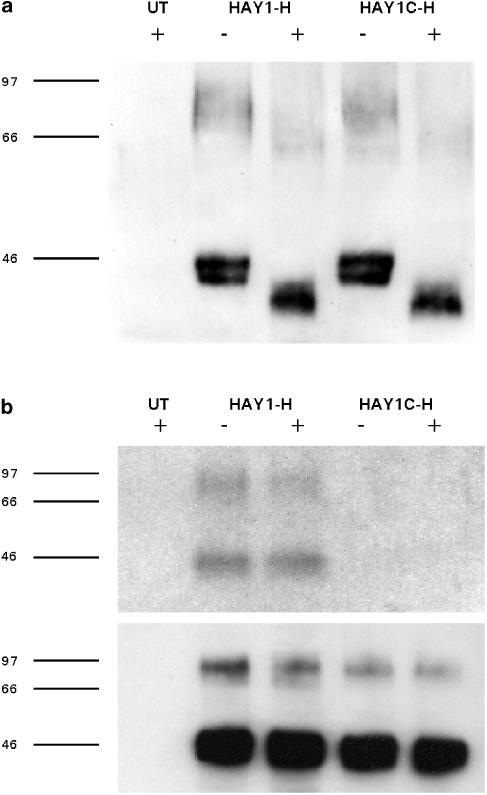
Anti-HA immunoprecipitates from solubilised extracts of HAY1-H and HAY1C-H whole cells were resolved by SDS–PAGE and probed on a Western blot with antibody CT/2 (Figure 1a). Both wild-type and C337A mutant receptors appeared as three principal bands that were absent from nontransfected CHO K1 cells, including a doublet of 37–42 and 40–47 kD, and a broad band of 64–93 kD (n=7). After PNGase F pretreatment, a single band of 34–41 kDa was predominant with a higher molecular weight form of 56–69 kDa also present (n=4). In immunoprecipitated extracts from HAY1-H cells labelled with [3H]palmitic acid for 4 h, two tritiated bands of approximately 40–45 and 73–115 kDa were detected by fluorography (Figure 1b). The intensity of the 73–115 kDa band was reduced following NPY (300 nM) pretreatment for 10 min, but NPY also decreased the immunoreactivity of this band on the accompanying Western blot (Figure 1b). No [3H]-labelled species were present in wild-type CHO K1 and the HAY1C-H samples (in which the same amount of receptor protein was detected in parallel Western blots), demonstrating that palmitoylation of the HA-tagged Y1 receptor is eliminated by the C337A mutation.
Figure 1.
(a) Anti-HA immunoprecipitates from untransfected CHO cells (UT), HAY1-H or HAY1C-H clones were resolved by 7.5% SDS–PAGE with (+) or without (−) prior treatment with PNGase F and transferred to a polyvinyl difluoride membrane. The blot illustrated (representative of four experiments) was probed with the primary antibody CT/2, raised against a Y1 receptor C terminal peptide. (b) Cells were labelled with [3H]palmitic acid and control (−) or 300 nM NPY-treated (+) samples were immunoprecipitated with directly conjugated anti-HA agarose. After resolution by 10% SDS–PAGE, gels were subjected to fluorography to detect [3H] incorporation (upper panel, one of two experiments). The lower panel illustrates the equivalent portion of a Western blot performed in parallel to confirm equal loading of HAY1-H and HAY1C-H receptors.
Effect of GTPγS on [125I]PYY binding
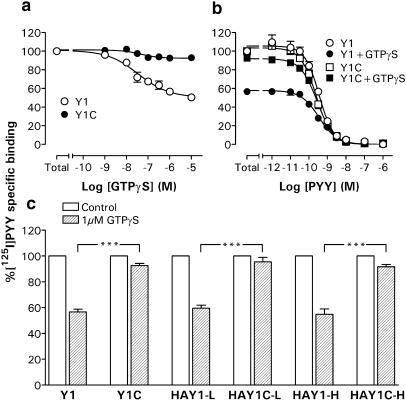
To assess the coupling of wild-type and Cys337 mutant Y1 receptors to G proteins, we first investigated the ability of the stable GTP analogue GTPγS to disrupt high-affinity [125I]PYY specific binding to membrane preparations from each CHO clone. GTPγS inhibited binding of the radiolabelled agonist to Y1 (pIC50: 7.41±0.25; n=3; Figure 2a) and HAY1-L (pIC50: 7.52±0.08; n=3) membranes, and was more potent in the HAY1-H clone exhibiting higher receptor expression levels (pIC50: 8.09±0.19; n=3). In each case, the maximal displacement afforded by 1 μM GTPγS was similar (Figure 2), equivalent to 40–45% of the total specific binding. This reduction equated almost entirely to a loss of [125I]PYY binding sites, since the affinities for unlabelled PYY in the presence of 1 μM GTPγS (Table 1; Figure 2b) were essentially unchanged compared to control displacements. In striking contrast, [125I]PYY binding to Y1(C337S) or HA-Y1(C337A) receptors was almost entirely resistant to GTPγS (only 5–9% inhibition at 1 μM; Figure 2c), independent of the 15-fold range in expression levels encompassed by the HAY1C-L and HAY1C-H clones.
Figure 2.
(a) Inhibition of [125I]PYY-specific binding by GTPγS in Y1 and Y1C membranes (both n=3). The curves fitted to combined triplicate experiments yielded a pIC50 of 7.41±0.25 in the Y1 clone. (b) Combined PYY displacements under control conditions or in the presence of 1 μM GTPγS in Y1 and Y1C membranes (n=3–6); pIC50 values are quoted in Table 1. (c) Total [125]PYY-specific binding in the absence (100%; n=5–7) or presence of 1 μM GTPγS (n=3). ***P<0.001 for comparisons between Y1 or HAY1 membranes and respective Cys mutant clones.
Agonist-stimulated GTPγ[35S] binding
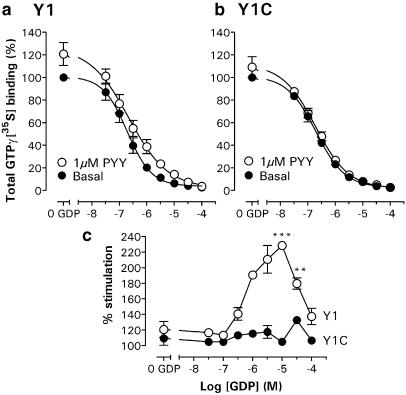
We next examined the ability of wild-type and mutant Y1 receptors to stimulate the binding of GTPγ[35S] to G proteins, following activation with PYY or NPY. Under optimised buffer conditions (100 mM NaCl and 10 μM GDP), similar basal initial GTPγ[35S] binding rates were observed in Y1 (3.2±0.1 fmol mg−1 protein min−1) and Y1C membranes (3.0±0.2 fmol mg−1 min−1; each n=3), which remained linear for incubation periods of up to 25 min. As a consequence, a 20 min GTPγ[35S] incubation was adopted (after the 90 min agonist equilibration time) for all subsequent experiments. As Figure 3 demonstrates, the presence of GDP decreased basal GTPγ[35S] binding to Y1 membranes (pIC50: 6.73±0.13; n=3), but at each concentration 1 μM PYY significantly increased binding above untreated levels. While the GDP dependence of basal GTPγ[35S] binding was very similar in the Y1C clone (pIC50: 6.70±0.08; n=3), the response to 1 μM PYY at each GDP concentration was substantially lower, particularly so at the GDP concentrations that yielded maximum stimulation in Y1 (10 μM) and Y1C membranes (30 μM; Figure 3c).
Figure 3.
GDP dependence of GTPγ[35S] binding in the absence and presence of 1 μM PYY. Combined data groups (n=3) for Y1 (a) and Y1C (b) membranes are expressed as a percentage (mean± s.e.m.) of the total basal binding in the absence of GDP (100 mM NaCl buffer; including negligible nonspecific counts), which was 1.67±0.33 pmol mg−1 (n=3) in Y1 and 1.26±0.14 pmol mg−1 (n=3) in the Y1C clone. (c) PYY stimulation following 90 min pretreatment in Y1 and Y1C clones is expressed as a percentage of basal GTPγ[35S] binding at each GDP concentration. Significant differences in Y1- and Y1C-mediated responses at 10 and 30 μM GDP are indicated as **P<0.01 and ***P<0.001.
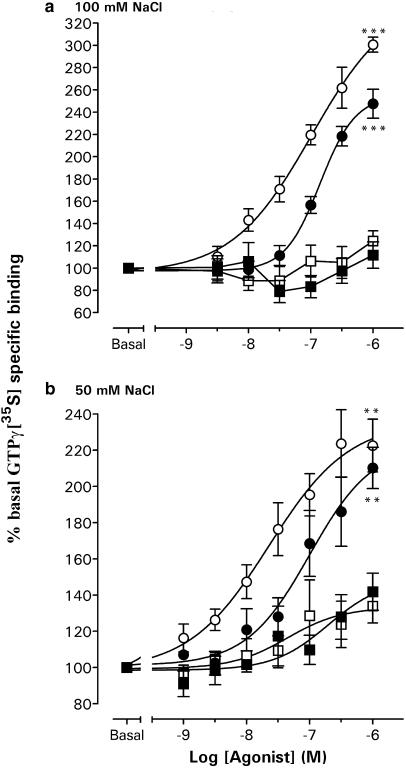
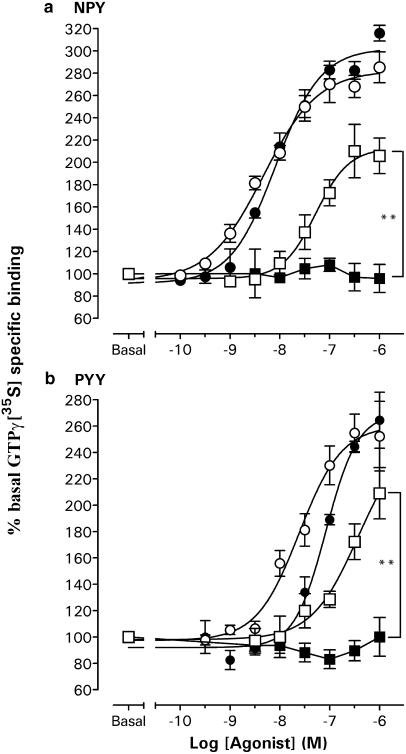
In a buffer containing 100 mM NaCl and 10 μM GDP, NPY and PYY stimulated GTPγ[35S] binding to Y1 membranes with similar potency (respective pEC50 values 6.98±0.13 and 6.85±0.02), although the maximal stimulation by 1 μM agonist was significantly elevated for NPY compared to PYY (P<0.05; Figure 4a). The presence of 1 μM BIBP3226, which itself did not significantly alter GTPγ[35S] binding (96.2±5.8%; n=4), abolished responses to 1 μM PYY (107.6±7.7%; n=4) and NPY (125.0±9.6; n=4; each P<0.001 compared to control minus antagonist of 247.6±13.0% (PYY) and 300.7±6.7% (NPY), n=4). Under these stringent buffer conditions, no concentration-dependent agonist responses were observed in Y1C membranes (Figure 4a). In a reduced NaCl concentration (50 mM), Y1 membranes displayed elevated basal GTPγ[35S] binding (81.6±4.3 fmol mg−1; n=4 compared to 64.8±4.9 fmol mg−1; n=4) and, as a consequence, lower maximum responses to NPY (1 μM; 222.6±14.6%; n=5) and to PYY (1 μM; 210.2±11.4%, n=5); in this case, the potency of NPY (pEC50: 7.65±0.26; n=5) was four-fold higher than for PYY (pEC50: 7.00±0.34; n=5; Figure 4b). In this buffer, agonists were also able to stimulate GTPγ[35S] binding in Y1C membranes, with similar potencies to the Y1 clone (NPY pEC50: 7.36±0.53 and PYY pEC50: 6.71±0.45; both n=3) but significantly reduced 1 μM responses (Figure 4b). Agonist stimulation was prevented by 1 μM BIBP3226 (e.g. 1 μM PYY; 87.3±13.1% (n=3), P<0.05 compared to control responses of 141.8±10.2%, n=3) and was therefore Y1(C337S) receptor-mediated.
Figure 4.
Concentration–response relations for agonist-stimulated GTPγ[35S] binding in Y1 and Y1C membranes, in buffers containing 10 μM GDP and (a) 100 or (b) 50 mM NaCl. Individual responses were expressed as a percentage stimulation over basal GTPγ[35S] binding; the graphs show pooled data groups (three to five triplicate experiments) for responses to NPY and PYY fitted with sigmoid curves where appropriate (Hill slopes of 0.9–1.1; pEC50 values given in text). Significant differences are indicated by ***P<0.001 and **P<0.01 for Y1 vs Y1C 1 μM agonist responses. Basal specific binding for each data group was as follows: 64.8±4.9 fmol mg−1 (Y1 100 mM NaCl; n=4), 84.7±8.8 fmol mg−1 (Y1C 100 mM NaCl; n=5), 81.6±4.3 fmol mg−1 (Y1 50 mM NaCl; n=5) and 99.3±1.3 fmol mg−1 (Y1C 50 mM NaCl; n=3).
The C337A mutation also abolished agonist-stimulated GTPγ[35S] responses mediated by the HA-tagged Y1 receptor, when comparing (in 100 mM NaCl buffer) clones, HAY1-L and HAY1C-L, that exhibited similar receptor expression levels to Y1 and Y1C membranes (Bmax: 1.0–2.0 pmol mg−1; Figure 5). The potencies of NPY (pEC50: 8.33±0.10, n=4) and PYY (pEC50: 7.61±0.13, n=4) in HAY1-H membranes were approximately 10-fold greater than in the HAY1-L clone (pEC50's 7.28±0.05 and 6.71±0.07, respectively, n=4), and also exhibited a higher maximal percentage stimulation to 1 μM agonist (Figure 5). When, in the HAY1C-H clone, HA-Y1(C337A) receptors were expressed at similar density to HAY1-H cells (Bmax: 8–17 pmol mg−1), they were able to stimulate GTPγ[35S] binding almost as efficiently as wild-type receptors, with only a small (two- to three-fold) reduction in the potency of NPY (pEC50: 8.08±0.09, n=3) or PYY (pEC50: 7.07±0.07, n=4; Figure 5).
Figure 5.
GTPγ[35S] responses to NPY (a) and PYY (b) under standard conditions (100 mM NaCl, 10 μM GDP) in HAY1 and HAY1C high and low expressing clones. Combined data groups (n=3–5) are illustrated with sigmoid curve fits (Hill slopes 1.0–1.4) that yielded the pEC50 values in the text. Significant differences between HAY1-L and HAY1C-L 1 μM peptide responses are shown by **P<0.01. Basal specific binding for NPY data groups was as follows: 83.0±2.9 fmol mg−1 (HAY1-H; n=5), 80.5±5.2 fmol mg−1 (HAY1C-H; n=3), 59.7±16.0 fmol mg−1 (HAY1-L; n=3) and 58.2±10.0 fmol mg−1 (HAY1C-L; n=3).
Identification of Colony 1 C1Y1 and C1Y1C clones and [125I]PYY binding
In order to extend the investigation of the nonpalmitoylated Y1(C337S) receptor using ISC techniques, we also obtained two stably transfected Colony 1 epithelial clones which expressed the wild-type Y1 receptor (C1Y1-6 and C1Y1-15) and two which expressed the cysteine mutant (C1Y1C-3 and C1Y1C-5), identified on the basis of functional and/or binding studies. In contrast to nontransfected Colony 1 cell membranes, membrane preparations from both C1Y1 and C1Y1C clones exhibited saturable specific binding, with similar [125I]PYY affinities (pKi; n=3–4) of 9.02±0.14 (C1Y1-6), 8.70±0.10 (C1Y1-15), 8.69±0.09 (C1Y1C-3) and 8.58±0.04 (C1Y1C-5). Bmax estimates (n=3–4) were most similar in C1Y1-6 (145±9 fmol mg−1 membrane protein) and C1Y1C-3 membranes (232±47 fmol mg−1); they were lower in C1Y1-15 cells (80±24 fmol mg−1) and higher in the C1Y1C-5 clone (533±102 fmol mg−1). BIBP3226 displaced [125I]PYY binding from all the four clones with pIC50 values (each n=4) of 8.54±0.09 in C1Y1-6, 8.38±0.14 in C1Y1-15, 8.67±0.09 in C1Y1C-3 and 8.63±0.04 in C1Y1C-5. The presence of GTPγS in the incubation buffer inhibited total specific [125I]PYY binding without altering the pIC50 values for the displacing PYY significantly in C1Y1-6 (pIC50 in 10 μM GTPγS: 7.92±0.10 vs control pIC50: 8.11±0.04; n=4) and C1Y1C-5 membranes (10 μM pIC50: 8.25±0.06 vs control pIC50: 8.43±0.09; n=4). However, the maximal inhibition afforded by 10 μM GTPγS was significantly greater in C1Y1-6 membranes (43.6±5.3% reduction in total specific counts; n=4) than in the C1Y1C-5 clone (29.4±2.6%; n=5; P<0.05), as observed (to a much larger extent) in the CHO Y1 and Y1C clones.
ISC responses to endogenous agonists
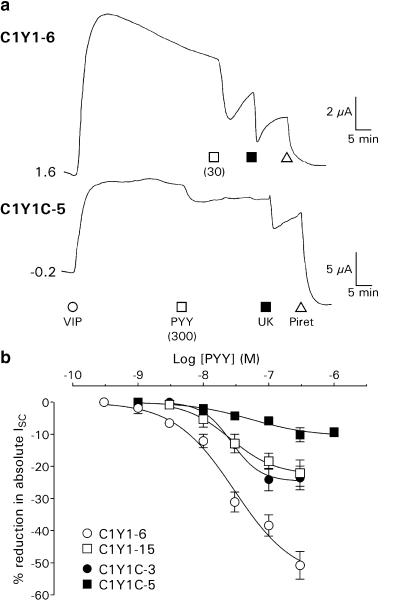
Under voltage-clamp conditions, basal resistances and levels of ISC in the four clones were, respectively, 45.8±1.0 Ω cm2 and 18.8±0.8 μA cm−2 in C1Y1-6 (n=309), 65.9±2.4 Ω cm2 and 24.6±2.2 μA cm−2 in C1Y1-15 (n=81), 133.4±4.7 Ω cm2 and 4.8±0.4 μA cm−2 in C1Y1C-3 (n=95), and 82.0±2.1 Ω cm2 and 39.6±2.0 μA cm−2 in C1Y1C-5 epithelial layers (n=187); these values being significantly different (P<0.001) in all cases from the parent Colony 1 cell line (39.2±0.6 Ω cm2 and 11.0±0.4 μA cm−2; n=531). Addition of a maximally effective concentration of VIP (30 nM) to each cell type resulted in sustained elevations in ISC that were sensitive to the loop diuretic piretanide (Figure 6a). The potency of this secretory agonist was similar in each case (EC50 values of 3.3–8.9 nM), although the size of the peak responses differed (particularly in the C1Y1C-3 clone; Table 2). Similar differences were observed following stimulation with 10 μM forskolin, a direct activator of cAMP-mediated secretion (e.g. peak responses were obtained of +84.3±6.3 μA cm−2 (n=4) in C1Y1-6 cells and +10.5±0.8 μA cm−2 (n=5) in C1Y1C-3 epithelial layers; P<0.001). This indicated variation in the ability of each clone to form polarised epithelial layers (hence the different transepithelial resistances) rather than changes in VIP receptor expression. The stimulation of endogenous α2A adrenoceptors by UK14,304 (UK) inhibited VIP-stimulated ISC, with EC50 values of 48.5–77.4 nM. Maximal reductions to 1 μM UK were in part dependent on the prior absolute levels of ISC, comprising 20–50% of the total ISC prior to UK addition (Table 2).
Figure 6.
(a) Example ISC traces from the C1Y1 and C1Y1C clones. C1Y1-6 or C1Y1C-5 epithelial layers (0.2 cm2 area, on different vertical scales) were treated with 30 nM VIP, followed by PYY (at the concentration (nM) indicated), 1 μM UK and 200 μM piretanide (Piret). (b) PYY concentration–response curves in C1Y1-6 (n=3–8), C1Y1-15 (n=3–6), C1Y1C-3 (n=3–7) and C1Y1C-5 (n=2–8) epithelial layers prestimulated with 30 nM VIP. Each point represents the mean±1 s.e.m. of n individual peak responses to a single concentration of PYY, expressed as a percentage of the total ISC (including basal and VIP-induced components). Fitted sigmoid curves yielded the pEC50 values presented in Table 2 and Hill coefficients of −0.9 (C1Y1-6, C1Y1C-5), −1.3 (C1Y1-15) and −1.9 (C1Y1C-3).
Table 2.
Functional responses in Colony 1 cells and clones expressing the Y1 or Y1(C337S) receptors
| Cell line | VIP receptors | α2A-Adrenoceptors | Transfected Y1 receptors | |||
|---|---|---|---|---|---|---|
| pEC50 | Max (μA cm−2) | UK pEC50 | Max (% total ISC) | PYY pEC50 | Max (% total ISC) | |
| COLONY 1 | 8.18±0.17 | +29.6±1.2 (2 0 0) | 7.10±0.11 | −28.3±3.2 (4) | — | — |
| C1Y1-6 | 7.92±0.03 | +51.6±1.6 (2 1 5) | 7.09±0.15 | −49.1±7.0 (3) | 7.52±0.16 | −50.8±4.3 (4) |
| C1Y1-15 | 8.11±0.19 | +37.0±2.1 (54) | 7.07±0.06 | −35.5±3.9 (5) | 7.59±0.06 | −22.1±4.1 (4) |
| C1Y1C-3 | 8.05±0.16 | +10.5±0.7 (38) | 7.18±0.19 | −22.6±2.6 (3) | 7.57±0.05 | −23.6±3.7 (4) |
| C1Y1C-5 | 8.49±0.29 | +49.4±2.8 (66) | 6.97±0.14 | −19.2±2.0 (4) | 7.28±0.21 | −10.2±2.2 (4) |
Concentration–response curves were constructed from single basolateral additions of the desired agonist to otherwise unstimulated cells (VIP; n=3–215) or to epithelial layers pretreated with 30 nM VIP (UK; n=2–5 and PYY; n=2–8, see Figure 6b); nontransfected Colony 1 epithelia did not respond to 100 nM PYY (n=21). PEC50 values (mean±s.e.m.) were calculated from fitted sigmoidal curves to the pooled data. The peak responses (Max) to maximally effective concentrations of VIP (30 nM), UK (1 μM) or PYY (300 nM) are given as absolute changes in ISC (μA cm−2) or as a percentage decrease in total ISC recorded (including basal and VIP-stimulated contributions); in both cases, the number of observations is indicated in brackets.
Effects of Y1-agonists and antagonists under VIP-stimulated conditions
In contrast to the absence of Y1-receptor-mediated responses in Colony 1 untransfected cells (Holliday et al., 1997), PYY significantly reduced VIP-elevated ISC in both C1Y1 and C1Y1C clones (Figure 6; Table 2). Treatment with BIBP3226 or BIBO3304 inhibited the effect of 30 nM PYY added 10 min later in the C1Y1-6 clone, with respective pIC50 values of 7.15±0.13 (n=3–5) and 8.08±0.11 (n=3–7); 1 μM BIBP3226 also abolished the responses to 100 nM PYY in C1Y1-15, C1Y1C-3 and C1Y1C-5 epithelial layers (each n=3, data not shown). Preincubation of C1Y1-6 or C1Y1C-5 epithelial layers with pertussis toxin (100 ng ml−1) for 24 h preserved the secretory responses to 30 nM VIP, but eliminated the subsequent reductions in VIP-stimulated ISC to 100 nM PYY and 1 μM UK (each n=3, data not shown).
PYY concentration–response curves under VIP-elevated conditions were derived by expressing the peak ISC reduction after each peptide addition as a percentage of the absolute ISC levels, in order to mitigate the effects of variation between the basal and VIP-stimulated ISC components in the different clones. PYY pEC50 values were equivalent in C1Y1-6, C1Y1-15 and C1Y1C-3 cells and slightly lower in the C1Y1C-5 clone (Figure 6b; Table 2), while the percentage peak responses to 300 nM PYY were greatest in the C1Y1-6 clone compared to C1Y1-15, C1Y1C-3 and C1Y1C-5 epithelial layers (Table 2).
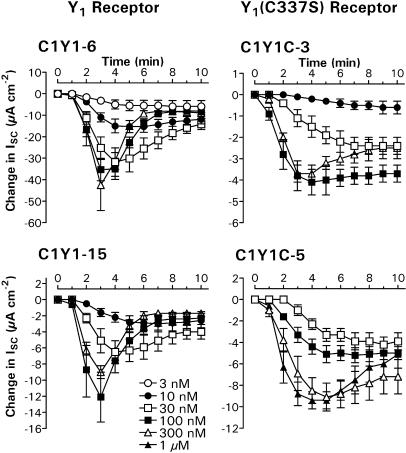
PYY time courses after VIP pretreatment
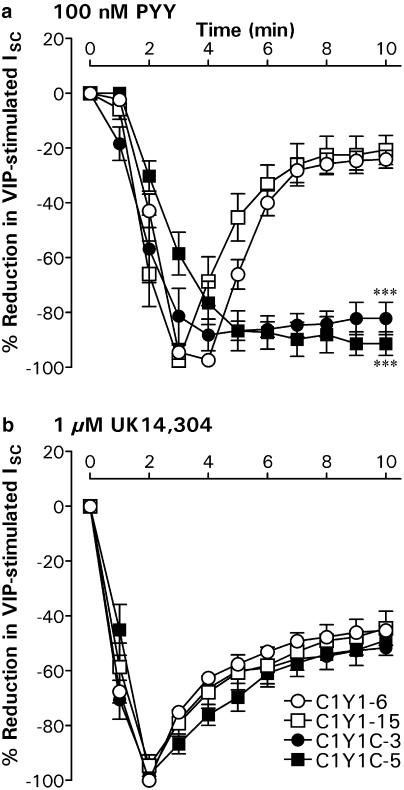
PYY responses in C1Y1-6 and C1Y1-15 epithelial layers stimulated with 30 nM VIP became markedly more transient as the peptide concentration was increased, with a concomitant reduction in the time to peak from 6 to 3 min (Figure 7). In contrast, the reductions in VIP-elevated ISC recorded in both C1Y1C clones remained essentially sustained even at the highest agonist concentrations (300 nM PYY in C1Y1C-3 and 1 μM PYY in C1Y1C-5 cells). When expressed as a percentage of the peak response at 3–5 min (Figure 8), the residual reduction in VIP-stimulated ISC afforded by 100 nM PYY was significantly greater (P<0.001) 10 min after agonist addition in the C1Y1C-3 (−82.2±5.9%; n=7) and C1Y1C-5 clones (−91.4±4.3%; n=8) compared to either C1Y1-6 (−24.1±3.2%; n=8) or C1Y1-15 epithelia (−20.7±5.3%; n=6). This difference in time profile was specific for the decreases in ISC mediated by the transfected receptors, since the time courses of subsequent responses to 1 μM UK were essentially identical in all the four clones (Figure 8), decaying to 50–60% of the peak at 2 min.
Figure 7.
Y1 and Y1(C337S) receptor-mediated responses in epithelial layers stimulated with 30 nM VIP. Mean PYY-induced changes in ISC are shown over a 10 min period for each of the four clones, following agonist addition (t=0) at concentrations of 3 nM (n=4), 10 nM (n=4–5), 30 nM (n=4–8), 100 nM (n=5–8), 300 nM (n=3–4) or 1 μM (n=4). Note the changes in vertical scale for each graph.
Figure 8.
Time course comparison for antisecretory responses in C1Y1 and C1Y1C clones. In all, 100 nM PYY (a) and 1 μM UK (b) were added sequentially to C1Y1-6, C1Y1-15, C1Y1C-3 or C1Y1C-5 epithelial layers pretreated for 30 min with 30 nM VIP. Graphs show the subsequent changes in ISC following the addition of each agonist at time zero; each point represents the mean±s.e.m. of four to eight normalised responses. Significant differences at t=10 min between the C1Y1C clones and either C1Y1-6 or C1Y1-15 epithelial layers are indicated by ***P<0.001. Absolute peak responses after 100 nM PYY were −35.1±4.8 μA cm−2 (C1Y1-6, n=8), −12.5±3.0 μA cm−2 (C1Y1-15, n=6), −4.6±0.5 μA cm−2 (C1Y1C-3, n=7) and −5.6±0.8 μA−cm−2 (C1Y1C-5, n=8). After 1 μM UK, they were −27.8±5.0 μA cm−2 (C1Y1-6, n=7). −20.8±5.9 μA cm−2 (C1Y1-15, n=6), −5.8±0.4 μA cm−2 (C1Y1C-3, n=7) and −18.7±3.0 μA cm−2 (C1Y1C-5, n=8).
Discussion
We compared wild-type and HA-tagged Y1 receptors with mutants in which Cys337 has been replaced by serine or alanine. This mutation abolished incorporation of [3H]palmitate into the HAY1 receptor (as predicted from its conserved location for acylation sites; Qanbar & Bouvier, 2003). The distinctive properties of the Y1(C337S) and HAY1(C337A) receptors are thus likely to arise from structural changes caused by the absence of palmitoylation.
HA-Y1 and HA-Y1(C337A) receptors were detected as multiple molecular weight species in CHO immunoprecipitates, of which bands of about 43 and 93 kDa were labelled by [3H]palmitate. This complex pattern may result partly from immunoprecipitation of differentially glycosylated receptors (revealed by PNGase F treatment) at different stages of maturity (Petäjä-Repo et al., 2002) and perhaps also from the presence of Y1 receptor dimers (Dinger et al., 2003) resistant to denaturation. Although NPY stimulation reduced the labelling intensity of the 93 kDa tritiated protein, we were unable to attribute this effect solely to a change in receptor palmitoylation. The C337A mutation did not alter the glycosylation profile of the receptor and we obtained stably transfected clones that expressed HAY1(C337A) and Y1(C337S) receptors at similar or higher levels, compared to their wild-type counterparts. This is in contrast to the reduction in surface density in some nonpalmitoylated GPCRs (Moffett et al., 1996; Hayashi & Haga, 1997; Sadeghi et al., 1997; Palmer & Stiles, 2000; Percherancier et al., 2001); however, our results do not exclude consequences on plasma membrane targeting which may not affect overall expression in stably transfected clones (e.g. Tanaka et al., 1998; Gao et al., 1999).
High-affinity binding sites of Y1 agonists and antagonists were preserved in Cys mutant Y1 receptors expressed in CHO K1 or human adenocarinoma (Colony 1) cell lines. [125I]PYY binding to Y1 or HA-Y1 receptors in CHO and Colony 1 clones was similarly sensitive (approximately 50% inhibition) to maximal GTPγS concentrations, a stable GTP analogue which disrupts agonist-GPCR-G-protein complexes (Kenakin, 1996). Increasing numbers of receptors four-fold (HAY1-H clone) did not change the total proportion of sites inhibited by GTPγS. In wild-type and nonpalmitoylated GPCRs that show no changes in G-protein coupling, this GTPγS sensitivity is maintained for the mutant receptor (e.g. the Gi-coupled α2A-adrenergic and A1 adenosine receptors; Kennedy & Limbird, 1993; Gao et al., 1999). However, [125I]PYY binding to Y1C and HAY1C membranes was insensitive to GTPγS, regardless of the level of receptor expression. Although less pronounced, a significant reduction in the effect of maximal GTPγS concentrations was also observed in Colony 1 C1Y1C-5 membranes compared to C1Y1-6. Thus, the Cys mutations increase the proportion of transfected Y1 receptors that are uncoupled from heterotrimeric G proteins.
NPY and PYY elicited a robust stimulation of GTPγ[35S] binding in Y1 and HAY1 membranes, mediated through the transfected Y1 receptors under conditions known to favour agonist-mediated GPCR–Gi protein interactions (100 mM NaCl and the presence of GDP; Williams et al., 1997). NPY and PYY potencies were considerably lower than their almost identical affinities in [125I]PYY competition studies in the same membranes (as for SST5; Williams et al., 1997), probably reflecting the low-affinity state of the Y1 receptor. This conformation is difficult to study using available Y1-agonist radioligands, but would be favoured by the high Na+ and GDP concentrations (Kenakin, 1996). NPY was significantly more potent (HAY1-L, HAY1-H) or exhibited increased maximal responses (Y1) compared to PYY, suggesting that under these conditions it is the more efficacious agonist. Increasing the numbers of available receptors increased the potency and maximum response to both peptides in the HAY1-H clone.
In 100 mM NaCl, neither PYY nor NPY stimulated GTPγ[35S] binding in HAY1C-L or Y1C membranes. The similar basal-binding characteristics observed suggest that the absence of a receptor-mediated effect was a consequence of a loss of receptor function and not differences in clonal G protein levels. Our results are thus consistent with previous GTPγ[35S] studies, which have proved reliable determinants of whether mutant receptors have reduced efficacy (Hayashi & Haga, 1997; Doi et al., 1999) or equivalent G protein coupling compared to wild-type GPCRs (Kennedy & Limbird, 1993; Stevens et al., 2001). However, we demonstrated some coupling of Cys mutant Y1 receptors to G proteins in two ways. First, on reduction of the NaCl concentration to 50 mM (which facilitates conversion between inactive and active GPCR states; Williams et al., 1997), Y1(C337S) receptor-stimulated GTPγ[35S] binding in Y1C membranes could be observed. This response remained substantially lower than that demonstrated for the wild-type Y1 receptor under similar conditions. Second, in the high expressing HAY1C-H clone, NPY and PYY responses became indistinguishable from those in the HAY1-H clone. While inhibition of [125I]PYY binding by GTPγS depends on the proportion of receptors coupled to G proteins (and did not vary between HAY1C-L and HAY1C-H), GTPγ[35S] studies indicate the numbers of coupled receptors and are more likely to saturate from limited availability of G protein as total receptor levels increase. This results in the loss of a difference between wild-type and mutant receptors at high expression levels. Nevertheless, our combined approach using both techniques provides strong evidence that the nonpalmitoylated mutant receptors are characterised by low coupling efficacy.
Two explanations are available for the reduced efficacy of many nonpalmitoylated GPCRs. Cys341 substitution in the β2-adrenoceptor resulted in increased phosphorylation of proximal serine residues, resulting in its constitutive desensitisation (Moffett et al., 1996). A similar mechanism may exist in other nonpalmitoylated GPCRs (e.g. A3 adenosine receptor; Palmer & Stiles, 2000), and the Cys337 substitution could enhance basal Y1 receptor phosphorylation in a similar manner, leading to a nonpalmitoylated receptor which exhibits high-affinity agonist binding (the agonist-GPCR–arrestin complex; Martini et al., 2002) but reduced G-protein coupling. However, we do not favour this mechanism because consequent downregulation would result in reduced expression levels (Moffett et al., 1996; Palmer & Stiles, 2000). We prefer a reduction in intrinsic receptor efficacy after Cys337 mutation, as demonstrated in experiments which analysed the decreased coupling of ‘nonpalmitoylated' GPCRs (the M2 muscarinic and endothelin receptor mutants) in reconstituted systems in the absence of kinase (Hayashi & Haga, 1997; Doi et al., 1999). In rhodopsin, the fourth intracellular loop forms a short helix (H8; Palczewski et al., 2000; Lu et al., 2002) which when disrupted by chemical depalmitoylation reduces signalling efficacy (Sachs et al., 2000). The conserved nature of this structural motif among GPCRs may therefore be a key to alterations in intrinsic efficacy associated with changes in the palmitoylation state.
Confluent C1Y1 and C1Y1C epithelial layers retained the capability of the parent Colony 1 cells for cAMP-stimulated chloride secretion in ISC studies. They thus allowed real-time measurement of inhibitory PYY responses, mediated by the transfected Y1 receptors coupled to Gi/o proteins. Owing to the intervening amplification and saturation steps in this signalling pathway, differences in receptor efficacy were less clearcut, particularly as maximal responses to the endogenous α2-adrenoceptor agonist UK also varied between clones. However, C1Y1 epithelial layers exhibited functional desensitisation of the Y1 receptor in the form of increasingly transient PYY responses at higher agonist concentrations. It is most likely that this desensitisation is initiated by G-protein receptor kinase (GRK) phosphorylation of the Y1 receptor and subsequent arrestin binding (Ferguson, 2001). This would be consistent with the promiscuous ability of GRKs and arrestins to regulate many GPCRs (Ferguson, 2001) and the recent demonstration that the Y1 receptor exhibits agonist-promoted endocytosis (Gicquiaux et al., 2002), a subsequent stage in the GRK—arrestin pathway. GRKs phosphorylate activated receptors specifically; for the well-characterised β2-adrenoceptor, this results in a concentration dependence for GRK-promoted events which follows the predicted agonist receptor occupancy (i.e. EC50 close to binding affinity) independent of amplification by the signalling cascade (and thus receptor levels; Clark et al., 1999). These characteristics are reflected in C1Y1 PYY responses, which become short-lived at relatively high agonist concentrations and show similar time profiles in C1Y1-6 and C1Y1-15 cells, encompassing a 1.8-fold range in receptor expression levels.
Strikingly, Y1(C337S) receptor-mediated responses in C1Y1C-3 and C1Y1C-5 cells (1.6- and 3.6-fold higher receptor levels than C1Y1-6, respectively) were more sustained than their counterparts in wild-type C1Y1 clones. Such time-dependent differences (despite identical UK response profiles) suggest that the Y1(C337S) receptor may be resistant to desensitisation, a predicted consequence of its reduced efficacy (proportionately fewer agonist-occupied Y1(C337S) receptors would adopt the active GPCR substrate for GRK-mediated phosphorylation). This indirect mechanism has a parallel in the reduced ability of partial agonists to initiate GPCR desensitisation (e.g. January et al., 1997; Clark et al., 1999) and similar correlations between lower efficacy and reduced desensitisation exist for SST5 (Hukovic et al., 1998) and CCR5 nonpalmitoylated receptors (Kraft et al., 2001).
We have demonstrated that nonpalmitoylation of the Y1 receptor by mutating the acylation site dramatically reduces its coupling to G proteins. Our results suggest that similar endogenous regulation of Y1 receptor acylation may be a significant mechanism by which its efficacy, and thus the balance between G protein activation and desensitisation, is controlled.
Acknowledgments
We thank Dr S. Mø11er-Nielsen and Professor T. Schwartz for the provision of the rat Y1 and Y1(C337S) receptor cDNAs, Dr H. Doods for the antagonists BIBP3226 and BIBO3304, Professor A. Beck-Sickinger for the CT/2 antibody, Dr Delyth Morgan for expert assistance in molecular biology and Professor M. Bouvier for helpful discussion. This work was supported by the BBSRC, the Kimmel Cancer Foundation and the Special Trustées of St Thomas' Hospital.
Abbreviations
- CHO
chinese hamster ovary
- DMEM
Dulbecco's modified Eagle's medium
- GPCR
G-protein-coupled receptor
- GRK
G-protein-coupled receptor kinase
- GTPγS
Guanosine-5′-O-(3-thio)triphosphate
- HA
haemagglutinin
- HAY1 or HAY1C clones
CHO clones expressing HA-tagged Y1 or Y1(C337A) receptors at low (L) or high (H) expression levels
- ISC
short-circuit current
- NPY
neuropeptide Y
- PNGase F
peptide : N-glycosidase F
- PP
pancreatic polypeptide
- PYY
peptide YY
- UK, UK14
304
- VIP
vasoactive intestinal polypeptide
- (C1)Y1 or (C1)Y1C clones
CHO or Colony 1 (C1) cells expressing the Y1 or Y1(C337S) receptors
References
- CLARK R.B., KNOLL B.J., BARBER R. Partial agonists and G protein-coupled receptor desensitization. Trends Pharmacol. Sci. 1999;20:279–286. doi: 10.1016/s0165-6147(99)01351-6. [DOI] [PubMed] [Google Scholar]
- COX H.M., TOUGH I.R. Neuropeptide Y, Y1, Y2 and Y4 receptors mediate Y agonist responses in isolated human colon mucosa. Br. J. Pharmacol. 2002;135:1505–1512. doi: 10.1038/sj.bjp.0704604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINGER M.C., BADER J.E., KOBOR A.D., KRETZSCHMAR A.K., BECK-SICKINGER A.G. Homodimerization of neuropeptide Y receptors investigated by fluorescence resonance energy transfer in living cells. J. Biol. Chem. 2003;278:10562–10571. doi: 10.1074/jbc.M205747200. [DOI] [PubMed] [Google Scholar]
- DOI T., SUGIMOTO H., ARIMOTO L., HIROAKI Y., FUJIYOSHI Y. Interactions of endothelin subtypes A and B with Gi, Go and Gq in reconstituted phospholipid vesicles. Biochemistry. 1999;38:3090–3099. doi: 10.1021/bi981919m. [DOI] [PubMed] [Google Scholar]
- EASON M.G., JACINTO M.T., THEISS C.T., LIGGETT S.B. The palmitoylated cysteine of the cytoplasmic tail of α2A-adrenergic receptors confers subtype-specific downregulation. Proc. Natl. Acad. Sci. USA. 1994;91:11178–11182. doi: 10.1073/pnas.91.23.11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERGUSON S.S.G. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signalling. Pharmacol. Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- GAO Z., YAJUN N.I., SZABO G., LINDEN J. Palmitoylation of the recombinant human A1 adenosine receptor: enhanced proteolysis of palmitoylation-deficient mutant receptors. Biochem. J. 1999;342:387–395. [PMC free article] [PubMed] [Google Scholar]
- GICQUIAUX H., LECAT S., GAIRE M., DIETERLEN A., MELY Y., TAKEDA K., BUCHER B., GALZI J.L. Rapid internalization and recycling of the human neuropeptide Y Y1 receptor. J. Biol. Chem. 2002;277:6645–6655. doi: 10.1074/jbc.M107224200. [DOI] [PubMed] [Google Scholar]
- HAWTIN S.R., TOBIN A.B., PATEL S., WHEATLEY M. Palmitoylation of the vasopressin V1a receptor reveals different conformational requirements for signaling, agonist-induced receptor phosphorylation, and sequestration. J. Biol. Chem. 2001;276:38139–38146. doi: 10.1074/jbc.M106142200. [DOI] [PubMed] [Google Scholar]
- HAYASHI M.K., HAGA T. Palmitoylation of muscarinic acetylcholine receptor m2 subtypes: reduction in their ability to activate G proteins by mutation of a putative palmitoylation site, cysteine 457, in the carboxy-terminal tail. Arch. Biochem. Biophys. 1997;340:376–382. doi: 10.1006/abbi.1997.9906. [DOI] [PubMed] [Google Scholar]
- HOLLIDAY N.D., COX H.M. The functional investigation of a human adenocarcinoma cell line, stably transfected with the neuropeptide Y Y1 receptor. Br J Pharmacol. 1996;119:321–329. doi: 10.1111/j.1476-5381.1996.tb15989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLIDAY N.D., TOUGH I.R., COX H.M. Inhibition of cAMP-dependent chloride secretion by PP receptors and α2-adrenoceptors in a human colonic epithelial cell line. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;355:183–189. doi: 10.1007/pl00004930. [DOI] [PubMed] [Google Scholar]
- HUKOVIC N., PANETTA R., KUMAR U., ROCHEVILLE M., PATEL Y.C. The cytoplasmic tail of the human somatostatin receptor type 5 is crucial for interaction with adenylyl cyclase and in mediating desensitization and internalization. J. Biol. Chem. 1998;273:21416–21422. doi: 10.1074/jbc.273.33.21416. [DOI] [PubMed] [Google Scholar]
- JANUARY B., SEIBOLD A., WHALEY B., HIPKIN R.W., LIN D., SCHONBRUNN A., BARBER R., CLARK R.B. β2-adrenergic receptor desensitization, internalization, and phosphorylation in response to full and partial agonists. J. Biol. Chem. 1997;272:23871–23879. doi: 10.1074/jbc.272.38.23871. [DOI] [PubMed] [Google Scholar]
- JOHANSEN T.E., SCHØLLER M.S., TOLSTOY S., SCHWARTZ T.W. Biosynthesis of peptide precursors and inhibitors using new constitutive and inducible eukaryotic expression vectors. FEBS Lett. 1990;355:165–170. doi: 10.1016/0014-5793(90)80947-h. [DOI] [PubMed] [Google Scholar]
- KENAKIN T. The classification of seven transmembrane receptors in recombinant expression systems. Pharmacol Rev. 1996;48:413–463. [PubMed] [Google Scholar]
- KENNEDY M.E., LIMBIRD L.E. Mutations of the α2A-adrenergic receptor that eliminate detectable palmitoylation do not perturb receptor-G-protein coupling. J. Biol. Chem. 1993;268:8003–8011. [PubMed] [Google Scholar]
- KENNEDY M.E., LIMBIRD L.E. Palmitoylation of the α2A-adrenergic receptor. J. Biol. Chem. 1994;269:31915–31922. [PubMed] [Google Scholar]
- KRAFT K., OLBRICH H., MAJOUL I., MACK M., PROUDFOOT A., OPPERMANN M. Characterization of sequence determinants within the carboxyl-terminal domain of chemokine receptor CCR5 that regulate signaling and receptor internalization. J. Biol. Chem. 2001;276:34408–34418. doi: 10.1074/jbc.M102782200. [DOI] [PubMed] [Google Scholar]
- KRAUSE J., EVA C., SEEBURG P.H., SPRENGEL R. Neuropeptide Y1 subtype pharmacology of a recombinantly expressed neuropeptide receptor. Mol. Pharmacol. 1992;41:817–821. [PubMed] [Google Scholar]
- LARHAMMAR D., BLOMQVIST A.G., YEE F., JAZIN E., YOO H., WAHLESTEDT C. Cloning and functional expression of a human neuropeptide Y/peptide YY receptor of the Y1 type. J. Biol. Chem. 1992;267:10935–10938. [PubMed] [Google Scholar]
- LOISEL T.P., ADAM L., HERBERT T., BOUVIER M. Agonist stimulation increases the turnover rate of β2AR-bound palmitate and promotes receptor depalmitoylation. Biochemistry. 1996;35:15923–15932. doi: 10.1021/bi9611321. [DOI] [PubMed] [Google Scholar]
- LU Z.L., SALDANHA J.W., HULME E.C. Seven-transmembrane receptors: crystals clarify. Trends Pharmacol. Sci. 2002;23:140–146. doi: 10.1016/S0165-6147(00)01973-8. [DOI] [PubMed] [Google Scholar]
- MARTINI L., HASTRUP H., HOLST B., FRAILE-RAMOS A., MARSH M., SCHWARTZ T.W. NK1 receptor fused to beta-arrestin displays a single-component, high-affinity molecular phenotype. Mol. Pharmacol. 2002;62:30–37. doi: 10.1124/mol.62.1.30. [DOI] [PubMed] [Google Scholar]
- MICHEL M.C., BECK-SICKINGER A.G., COX H.M., DOODS H.N., HERZOG H., LARHAMMAR D., QUIRION R., SCHWARTZ T.W., WESTFALL T. XVI, international punion of pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY and pancreatic polypeptide receptors. Pharmacol. Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- MOENCH S.J., MORELAND J., STEWARD D.H., DEWEY T.G. Fluorescence studies of the location and membrane accessibility of the palmitoylation sites of rhodopsin. Biochemistry. 1994;33:5791–5796. doi: 10.1021/bi00185a017. [DOI] [PubMed] [Google Scholar]
- MOFFETT S., ADAM L., BONIN H., LOISEL T.P., BOUVIER M., MOUILLAC B. Palmitoylated cysteine 341 modulates phosphorylation of the β2-adrenergic receptor by the cAMP-dependent protein kinase. J. Biol. Chem. 1996;271:21490–21497. doi: 10.1074/jbc.271.35.21490. [DOI] [PubMed] [Google Scholar]
- PALCZEWSKI K., KUMASAKA T., HORI T., BEHNKE C.A., MOTOSHIMA H., FOX B.A., LE TRONG I., TELLER D.C., OKADA T., STENKAMP R.E., YAMAMOTO M., MIYANO M. Crystal structure of rhodopsin: a G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- PALMER T.M., STILES G.L. Identification of threonine residues controlling the agonist-dependent phosphorylation and desensitization of the rat A3 adenosine receptor. Mol. Pharmacol. 2000;57:539–545. [PubMed] [Google Scholar]
- PERCHERANCIER Y., PLANCHENAULT T., VALENZUELA-FERNANDEZ A., VIRELIZIER J.L., ARENZANA-SEISDEDOS F., BACHELERIE F. Palmitoylation-dependent control of degradation, life span, and membrane expression of the CCR5 receptor. J. Biol. Chem. 2001;276:31936–31944. doi: 10.1074/jbc.M104013200. [DOI] [PubMed] [Google Scholar]
- PETÄJÄ-REPO U.E., HOGUE M., BHALLA S., LAPERRIÈRE A., MORELLO J.-P., BOUVIER M. Ligands act as pharmacological chaperones and increase the efficiency of δ opioid receptor maturation. EMBO. J. 2002;21:1628–1637. doi: 10.1093/emboj/21.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PONIMASKIN E.G., HEINE M., JOUBERT L., SEBBEN M., BICKMEYER U., RICHTER D.W., DUMUIS A. The 5-hydroxytryptamine(4a) receptor is palmitoylated at two different sites, and acylation is critically involved in regulation of receptor constitutive activity. J. Biol. Chem. 2002;277:2534–2546. doi: 10.1074/jbc.M106529200. [DOI] [PubMed] [Google Scholar]
- QANBAR R., BOUVIER M. Role of palmitoylation/depalmitoylation reactions in G-protein-coupled receptor function. Pharmacol. Ther. 2003;97:1–33. doi: 10.1016/s0163-7258(02)00300-5. [DOI] [PubMed] [Google Scholar]
- SACHS K., MARETZKI D., MEYER C.K., HOFMANN K.P. Diffusible ligand all-trans-retinal activates opsin via a palmitoylation-dependent mechanism. J. Biol. Chem. 2000;275:6189–6194. doi: 10.1074/jbc.275.9.6189. [DOI] [PubMed] [Google Scholar]
- SADEGHI H.M., INNAMORATI G., DAGARAG M., BIRNBAUMER M. Palmitoylation of the V2 vasopressin receptor. Mol. Pharmacol. 1997;52:21–29. doi: 10.1124/mol.52.1.21. [DOI] [PubMed] [Google Scholar]
- STEVENS P.A., PEDIANI J., CARILLO J.J., MILLIGAN G. Coordinated agonist regulation of receptor and G protein palmitoylation and functional rescue of palmitoylation-deficient mutants of the G protein G11α following fusion to the α1b-adrenoreceptor. J. Biol. Chem. 2001;276:35883–35890. doi: 10.1074/jbc.M103816200. [DOI] [PubMed] [Google Scholar]
- TANAKA K., NAGAYAMA Y., NISHINARA E., NAMBA H., YAMASHITA S., NIWA M. Palmitoylation of human thyrotropin receptor: slower intracellular trafficking of the palmitoylation-defective mutant. Endocrinology. 1998;139:803–806. doi: 10.1210/endo.139.2.5911. [DOI] [PubMed] [Google Scholar]
- TOUGH I.R., COX H.M. Selective inhibition of neuropeptide Y Y1 receptors by BIBP3226 in rat and human epithelial preparations. Eur. J. Pharmacol. 1996;310:55–60. doi: 10.1016/0014-2999(96)00372-x. [DOI] [PubMed] [Google Scholar]
- WIELAND H.A., ECKARD C.P., DOODS H.N., BECK-SLCKINGER A.G. Probing of the neuropeptide Y-Y1-receptor's interaction with anti-receptor antibodies. Eur. J. Biochem. 1998a;255:595–603. doi: 10.1046/j.1432-1327.1998.2550595.x. [DOI] [PubMed] [Google Scholar]
- WIELAND H.A., ENGEL W., EBERLEIN W., RUDOLF K., DOODS H.N. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO3304 and its effect on feeding in rodents. Br. J. Pharmacol. 1998b;125:549–555. doi: 10.1038/sj.bjp.0702084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS A.J., MICHEL A.D., FENIUK W., HUMPHREY P.P.A. Somatostatin5 receptor-mediated [35S]guanosine-5′-O-(3-thio)triphosphate binding: agonist potencies and the influence of sodium chloride on intrinsic activity. Mol. Pharmacol. 1997;51:1060–1069. doi: 10.1124/mol.51.6.1060. [DOI] [PubMed] [Google Scholar]