Abstract
Sildenafil (viagra) is a potent PDE5 inhibitor and thus a relaxant drug in corpus carvernosum smooth muscle. In the present work, we evidenced the presence of PDE5 isozyme and investigated the effect of sildenafil on the specific cyclic nucleotide phosphodiesterase (PDE) activity, smooth muscle tone and calcium signaling in the rat main pulmonary artery (MPA).
The PDE activity was measured in cytosolic and microsomal fractions. Total cAMP and cGMP-PDE activities were mainly present in the cytosolic fraction. Sildenafil (0.1 μM) reduced by 72% cGMP-PDE activity, whereas zaprinast (10 μM), a relatively selective PDE5 inhibitor, reduced this activity by 63%. Sildenafil (0.1 μM) also inhibited significantly (22%) the cAMP-PDE activity.
Western blot analysis revealed the expression of PDE5 mainly in the cytosolic fraction of MPA. Sildenafil concentration-dependently inhibited (IC50=3.4 nM) the activity of MPA PDE5 partially purified by HPLC.
Sildenafil (0.1 nM–50 μM) concentration-dependently relaxed MPA rings precontracted with phenylephrine (0.5 μM). The potency of sildenafil (IC50=11 nM) was similar to that of a nitric oxide donor, sodium nitroprusside, but higher than that of zaprinast (IC50=600 nM). The vasorelaxant effect of sildenafil was not altered by endothelium removal or in the presence of KT 5823 (1 μM) and H89 (1 μM), potent inhibitors of PKG and PKA, respectively.
In isolated MPA myocytes, which had been loaded with the calcium fluorophore indo-1, sildenafil (10–100 nM) antagonized ATP- and endothelin-1-induced calcium oscillations but had no effect on the transient caffeine-induced [Ca2+]i response.
This study demonstrates the presence of a functional and highly sildenafil-sensitive PDE5 isozyme in rat MPA. Inhibition of this isozyme mainly accounts for the potent pulmonary vasodilator action of sildenafil, which involves alteration in the inositol triphosphate-mediated calcium signaling pathway.
Keywords: Sildenafil, Cyclic nucleotide phosphodiesterase, Phosphodiesterase inhibitor, Pulmonary artery, Ca2+ signaling, Vascular tone
Introduction
Cyclic nucleotides (cAMP and cGMP) are involved in the control of smooth muscle tone, as an increase in their intracellular concentration generally leads to relaxation mainly via an alteration in calcium signaling (Polson & Strada, 1996; Carvajal et al., 2000). Cyclic nucleotide phosphodiesterases (PDEs), which hydrolyze cAMP and cGMP, represent the unique degradation pathway for these intracellular compounds (Stoclet et al., 1995). As a consequence, PDE activity is also implicated in the control of smooth muscle tone and PDE inhibition relaxes smooth muscle (Schoeffter et al., 1987; Komas et al., 1991; Wagner et al., 1997). Mammalian cells contain a variety of PDEs that have been classified into 11 gene families based on their primary structures, and their catalytic and regulatory properties (Soderling & Beavo, 2000; Francis et al., 2001). In mammalian smooth muscle, at least, five PDE isozymes (PDE1–PDE5) have been identified. Sildenafil citrate (Viagra™) has been shown as a potent (IC50≅1–4 nM) and reversible inhibitor of PDE5 (Moreland et al., 1998; Illarion et al., 1999). Moreover, it is highly selective for this PDE when compared with other PDE families (Ballard et al., 1998). In the corpus cavernosum, sildenafil increases cGMP concentration and causes smooth muscle relaxation of arteries and sinusoids, thus facilitating blood flow (Jeremy et al., 1997; Ballard et al., 1998). As a consequence, sildenafil has proved to be an effective, well-tolerated drug in the treatment of erectile dysfunction (Boolell et al., 1996; Ballard et al., 1998).
Pulmonary circulation develops a specific response to hypoxia, that is, vasoconstriction which can be maintained in patients suffering from pulmonary obstructive diseases (e.g., chronic bronchitis) (Rounds, 1989; Pierson, 2000), thus inducing, in turn, a sustained elevation in the pulmonary blood pressure that causes pulmonary hypertension (PAHT). PAHT leads to right ventricular hypertrophy, right heart failure and ultimately to death (Rabinovitch et al., 1979; Dawson, 1984). This pathophysiological adaptation of the pulmonary circulation to maintain hypoxia is a complicated process for which no curative treatment, except heart–lung transplantation, is currently available. Since PDEs are present in the pulmonary artery wall (Rabe et al., 1994; Maclean et al., 1997) and that cGMP-PDE activity is mainly because of the action of PDE5 (Rabe et al., 1994), some PDE5 inhibitors have been administered to counteract PAHT development both in animals and human (Eddahibi et al., 1998; Ziegler et al., 1998; Hanasato et al., 1999). Very recently, pioneer clinical trials with sildenafil have been performed in PAHT (Abrams et al., 2000; Ichinose et al., 2001; Zhao et al., 2001). However, to the best of our knowledge, no detailed study has been performed, at the cellular and molecular levels, to examine the interactions of sildenafil and cGMP-PDE signaling pathway in the pulmonary vascular smooth muscle.
Thus, the aims of the present study conducted in the rat pulmonary artery were: (1) to measure PDE5-specific activity in comparison with other cAMP- and cGMP-PDE activities both in cytosolic and microsomal fractions; (2) to examine the expression of PDE5 protein in the arterial wall; (3) to quantify the effect of sildenafil on the partially purified PDE5; (4) to determine the relaxant properties of sildenafil on pulmonary tone and its effect at the site of calcium signaling in vascular smooth muscle cells.
Methods
Materials
Acetylcholine, bovine serum albumin (BSA), EGTA, erythro-9-(2-hydroxy-3nonyl)adenine (EHNA), hydroxymethyl-aminomethane (Tris), 3-isobutyl-1-methylxanthine (IBMX), KT 5823, (N-2-[p-bromocinnamylamino]ethyl)-5-isoquinolinesulfonamide hydrochloride (H89), protease inhibitors, phenylephrine zaprinast, were purchased from Sigma (St-Quentin Fallavier, France). Cilostamide and rolipram were purchased from Tocris (Bristol, U.K.). Sildenafil was kindly provided by Pfizer (Sandwich, Kent, U.K.). All other materials were of reagent grade. All buffer solutions were prepared with deionized water from the Millipore Milli Ro-Milli-Q-UF system (18±0.2 cm2 −1). [8-3H]cAMP (30–50 Ci mmol−1) and [8-3H]cGMP (5–15 Ci mmol−1) were purchased from New England Nuclear (Boston, MA, U.S.A.) and purified by thin layer chromatography on silica gel, using isopropanol: NH4OH :H2O (70 : 15 : 15) as a solvent. Calmodulin was purified from bovine brain as previously described (Follénius & Gérard, 1984). PDE inhibitors were dissolved in either DMSO or water. The final DMSO concentrations were 0.5% in PDE assay and 0.1% in organ bath and they did not significantly affect hydrolytic or contractile activities.
Enhanced chemiluminescence (ECL) assay kit and Hybond-P PVDF membrane were from Amersham (Buckinghamshire, U.K.). Precision prestained protein standards, SDS–PAGE broad-range molecular weight standards and Tween 20 were from BioRad (Hercules, CA, U.S.A.). Methanol was from Carlo Erba (Val de Reuil, France). Glycine was from ICN (Orsay, France). Biomax-ML films were from Kodak (Paris, France). Anti-rabbit horseradish peroxidase (HRP) conjugate was from Promega (Charbonnieres, France). Acrylamide/bisacrylamide (29 : 1 mix ratio; 30% solution), bromophenol blue, glycerol and β-mercaptoethanol were from Sigma (St Quentin Fallavier, France). Sodium dodecyl sulfate (SDS) was from Quantum Biotechnologies (Montréal, Québec, Canada). I-Block (highly purified casein) was from Tropix (Bedford, MA, U.S.A.). Rabbit anti-bovine lung PDE5 polyclonal antisera was a kind gift of Dr J.D. Corbin (Vanderbilt University, Nashville, U.S.A.). Recombinant PDE5 (human heart) was a kind gift from Sanofi-Synthélabo (Paris, France).
Preparation of microsomal and cytosolic fractions
Wistar male rats aged from 8 to 10 weeks and weighing 280–340 g were anesthetized by intraperitoneal injection of 40 mg ethylcarbamate. Heart and lungs were removed en bloc. The extrapulmonary arteries were dissected in ice-cold Krebs solution containing 2 mM dithiothreitol and 1 mM ascorbic acid, as well as various protease inhibitors (1 μM pepstatin, 1 μM leupeptin, 2 UI m−1 aprotinin, 10 μM trypsin inhibitor), and they were quickly frozen in liquid nitrogen and stored at −85°C. Arteries were powdered in liquid nitrogen using a mortar/pestle and the tissue powder was homogenized in a buffer (pH 7.4) containing 20 mM Tris, 0.25 M sucrose, 5 mM K-EGTA, 2 mM Mg-acetate, 1 mM DTT, 0.2 mg ml−1 BSA, 2 UI ml−1 aprotinin, 0.2 mg ml−1 leupeptin, 0.33 mM Pefabloc and 10 mg ml−1 soybean trypsin inhibitor, with the aid of an Ultra-Turrax for six 10 s bursts. The homogenate was centrifuged at 105,000 × g for 45 min at 4°C and the supernatant containing the cytosolic enzymes was collected. The 105,000 × g pellet, containing the microsomal membranes, was washed three times and finally resuspended. The homogenate, supernatant and pellet fractions were stored at −85°C until use. The protein content of the subcellular fractions was determined by the method of Lowry et al. (1951) using BSA as standard.
PDE assay
PDE activities were measured by a two-step radioenzymatic assay, as previously described (Keravis et al., 1980). QAE-Sephadex A 25 columns were used to separate the final products (adenosine or guanosine) from the residual substrate (cAMP or cGMP). Assay was performed at a substrate concentration of 1 μM [3H]cAMP or [3H]cGMP, either with 1 mM EGTA or 10 μM Ca2+ plus an excess (20 nM) of calmodulin (Ca2+/CaM), with or without addition of PDE inhibitors. Results are expressed as means±standard error of the mean (s.e.m.) of at least three determinations in duplicate. In order to detect and gauge the relative activity of specific PDE isozymes in our subcellular preparations, we took advantage of the selective inhibition or activation of the enzymes by a variety of substances (Stoclet et al., 1995; Polson & Strada, 1996). Either 1 μM cilostamide or 5 μM cGMP were used to inhibit PDE3 activity, while 10 μM rolipram was added to inhibit PDE4 activity. Zaprinast (10 μM) was used to inhibit both PDE5 and PDE1 activities. All concentrations used were derived from previous experiments on purified smooth muscle PDE isozymes (Lugnier & Komas, 1993; Stoclet et al., 1995). The effect of these inhibitors was compared with that of sildenafil (0.1 μM).
Chromatographic resolution of PDE activities
The 105,000 × g supernatant containing the cytosolic enzymes was applied to a high-pressure liquid chromatography (HPLC) column (TSK DEAE-5PW, 7.5 × 75 mm2; Pharmacia). The elution was performed at a flow rate of 0.7 ml min−1 (30 bar pressure) with a linear gradient of NaCl (0.05–0.45 M) in Tris-HCl buffer (20 mM Tris-HCl, 2 mM Mg2+ acetate, 1 mM dithiothreitol, pH 7.5). Fractions (0.7 ml) were collected and assayed for cAMP- and cGMP-PDE activities. Appropriate fractions corresponding to distinct PDE activities were pooled separately, and stored in aliquots at −85°C.
Western blot analysis
Protein samples (20 μg of protein) were denatured and solubilized by heating for 5 min at 95°C in Laemmli buffer (0.005% bromophenol blue, 5% glycerol, 1% SDS, 2.5% β-mercaptoethanol, 50 mM Tris, pH 6.8), loaded along with recombinant PDE5 as positive control and molecular weight standards (precision prestained protein standards and SDS–PAGE broad-range molecular weight standards) on an SDS-8% acrylamide gel, topped with an SDS-4% stacking gel, electrophoresed in 0.1% SDS/Tris-glycine buffer (25 mM Tris, 200 mM glycine, pH 8.3), electrotransferred to PVDF membranes during 2 h at 100 V in 15% methanol/Tris-glycine buffer at 4°C. Membranes were processed for immunoblotting: washed twice with PBS (0.58 M Na2PHO4, 0.17 M NaH2PO4, 0.68 M NaCl), blocked overnight with blocking buffer (0.1% I-Block, 0.3% Tween 20 in PBS), incubated for 90 min at 20°C with the rabbit antibovine lung PDE5 polyclonal antisera at 1/10,000 as primary antibody (MacAllister-Lucas et al., 1995). Anti-rabbit HRP conjugate was used as secondary antibody (1/60,000 dilution) for 60 min at 20°C. The immobilized antigens were detected by chemiluminescence using an ECL assay kit following the manufacturer's instructions.
Isometric contraction measurements
For contraction experiments, main pulmonary artery (MPA) rings (3 mM in length) were prepared and mounted in vertical 5 ml organ baths of a computerized isolated organ bath system (IOS, EMK Technologies, Paris, France) previously described (Pauvert et al., 2000). Baths were filled with Krebs Henseleit (KH) solution (composition in mM: 118.4 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 25 NaHCO3, 1.2 KH2PO4, 11.1 D-glucose, pH 7.4) maintained at 37°C and bubbled with a 95% O2− 5% CO2 gas mixture. Tissues were set at optimal length by equilibration against a passive load of 10 mN (Pauvert et al., 2000).
Two different experimental protocols were used to investigate the effect of sildenafil on MPA tone. In a first set of experiments, MPA rings were pretreated for 30 min in the presence of sildenafil (0.01–10 μM) before and throughout the construction of a cumulative concentration–response curve (CCRC) with phenylephrine (1 nM–50 μM). In a second set of experiments, sildenafil was applied on MPA rings precontracted with a unique submaximal concentration of phenylephrine (0.5 μM). Sildenafil was administered to one-half of the rings The unexposed rings served as control. The effect of sildenafil was compared to that of two other vasodilators, zaprinast and sodium nitroprusside (SNP). Finally, experiments were also performed in endothelium-denuded rings. In this respect, the MPA lumen was superfused with distilled water before mounting in the bath. Successful removal of the endothelium was confirmed by the inability of acetylcholine (1 μM) to induce more than 10% of relaxation in phenylephrine (1 μM)-contracted rings.
Cell preparation and [Ca2+]i measurement
Isolated MPA myocytes were obtained using an enzymatic dissociation method previously described (Guibert et al., 1996a; Pauvert et al., 2000). Freshly isolated myocytes were stored on glass coverslips at 4°C in PSS containing 0.8 mM Ca2+ and used on the same day. To assess the dynamic changes in [Ca2+]i of individual arterial myocytes, we used the [Ca2+]i-sensitive fluorophore indo-1. Cells were loaded with indo-1 by incubation in PSS containing 1 μM indo-1 penta-acetoxymethyl ester (indo-1 AM) for 25 min at room temperature and then washed in physiological saline solution (PSS) for 25 min. The coverslip with attached cells was then mounted in a perfusion chamber. The recording system included a Nikon Diaphot inverted microscope, fitted with epifluorescence (Nikon, Tokyo, Japan). A single cell among those on the coverslip was tested through a window slightly larger than the cell. The studied cell was illuminated at 360 nM and counted simultaneously at 405 and 480 nM by two photomultipliers (P100, Nikon, Tokyo, Japan). The fluorescence ratio (405/480) was calculated online and displayed with the two voltage signals on a monitor. [Ca2+]i was estimated from the 405/480 ratio (Grynkiewicz et al., 1985) using a calibration for indo-1 determined within cells (Guibert et al., 1997). The external PSS contained (in mM): 130 NaCl, 5.6 KCl, 1 MgCl2, 2 CaCl2, 11.1 D-glucose, 10 HEPES, pH 7.4 with NaOH. Ca2+-free PSS was prepared by replacing CaCl2 by 0.4 mM EGTA. Agonists were applied to the recorded cell by pressure ejection from a glass pipette located close to the cell for the period indicated on the records. It was verified, in control experiments, that no change in [Ca2+]i was observed during test ejections of PSS. Generally, each record of [Ca2+]i response to the different agonists was obtained from a different cell. Each type of experiment was repeated for the number of cells indicated in the text. Experiments were carried out at room temperature (20–22°C). ET-1, ATP and caffeine were used at concentrations of 100 nM, 100 μM and 5 mM, respectively, since we have previously shown that these concentrations induce [Ca2+]i responses in 90–100% of tested cells (Guibert et al., 1996a,1996b; Hyvelin et al., 1998).
Statistical analysis
All values are expressed as the mean±s.e.m. with n being the sample size. Significance was tested by means of Student's t-test at a P-value of 0.05. In contraction experiments, tension was expressed as a percentage of the response to K+-rich (80 mM) solution. EC50, the concentration of the phenylephrine inducing 50% of the maximal contractile response (first protocol), and IC50, the concentration of sildenafil (or zaprinast and SNP, second protocol) inducing 50% of the maximal relaxation, were graphically determined from the mean CCRC.
Results
Effect of sildenafil on PDE activities in rat pulmonary artery
cAMP- and cGMP-PDE-specific activities were determined in both microsomal and cytosolic fractions (Figure 1). cGMP-PDE-specific activity in microsomal fraction was four-fold lower than cAMP-PDE-specific activity, whereas it was 25% higher in cytosolic fraction. cGMP-and cAMP-PDE-specific activities were, respectively, 20- and 3.5-fold higher in cytosolic than in microsomal fraction.
Figure 1.
Total cAMP- and cGMP-PDE-specific activities in microsomal and cytosolic fractions derived from rat MPA. Hydrolytic activities were determined in the presence of 1 μM [3H]cAMP or 1 μM [3H]cGMP. Data are expressed as mean±s.e (n=4); *P<0.05.
The effect of sildenafil on both cGMP- and cAMP-PDE activities was investigated and compared to that of selective PDE inhibitors (Table 1 ). Sildenafil (0.1 μM) exhibited a potent inhibitory effect on cGMP-PDE activity, which was more pronounced in cytosolic (72%) than in microsomal (50%) fraction. Zaprinast, a relatively selective PDE5 inhibitor, displayed a similar effect but at a much higher concentration (10 μM). In an unexpected manner, sildenafil inhibited cAMP-PDE activity by about 20% in both subcellular fractions. Since previous studies in rat and bovine pulmonary arteries have revealed the presence of PDE3 and PDE4 as major cAMP-hydrolyzing isozymes (Maclean et al., 1997; Pauvert et al., 2002), we used cGMP (5 μM), cilostamide (1 μM) and rolipram (10 μM) to assess the participation of PDE3 (inhibited by cGMP and cilostamide) and PDE4 (specifically inhibited by rolipram) in cAMP-PDE activity. In microsomal fraction, rolipram inhibited cAMP-PDE activity (50%), whereas cGMP and cilostamide were less potent (respectively 21 and 31%), suggesting that PDE4 may represent the main isozyme in this fraction. In contrast, in cytosolic fraction, cGMP and cilostamide were more potent (respectively 40 and 56 %) than rolipram (33%), suggesting that PDE3 is the main isozyme in cytosolic fraction.
Table 1.
Selective inhibition of cGMP- and cAMP-PDE activity in rat pulmonary artery subcellular fractions
| cGMP-PDE activity (percentage of inhibition) | |||
|---|---|---|---|
| Microsomal | Cytosolic | ||
| Sildenafil | 0.1 μM | 50.7±1.4 (4) | 72.3±2.3 (4)* |
| Zaprinast | 10 μM | 56.2±1.9 (4) | 63.2±1.2 (4)* |
| cAMP-PDE activity (% of inhibition) | |||
| Microsomal | Cytosolic | ||
| Sildenafil | 0.1 μM | 20.4±1.2 (4) | 22.8±1.6 (4) |
| Cilostamide | 1 μM | 31.1±1.9 (4)* | 56.6±2.6 (4) |
| cGMP | 5 μM | 21.1±1.5 (4)* | 40.2±2.1 (4) |
| Rolipram | 10 μM | 50.0±2.2 (4)* | 33.6±3.2 (4) |
Data are shown as mean±s.e.m. (n). Statistical comparisons were carried out using Student's t-test
P< 0.05, comparing the inhibition in the microsomal membrane and cytosolic fraction.
Inhibition by sildenafil of the partially purified cytosolic PDE5
Figure 2 shows the HPLC resolution of cGMP hydrolytic activity of rat MPA cytosolic fraction. Under basal condition in the assay, that is, in the presence of EGTA, two major peaks were obtained, the main peak (fractions 21–28) and a second minor peak (fractions 30–34). Sildenafil (0.1 μM) inhibited the first peak, especially fractions 24–26, but had no effect on the second peak (Figure 2a). Since both PDE1 and PDE5 hydrolyze cGMP, but only PDE1 is activated by calmodulin (Lugnier et al., 1986, Polson & Strada, 1996), we characterized their activity in the presence of Ca2+/CaM, which induced a 10-fold increase in the PDE activity of fractions 21–25 (Figure 2b). Comparison of Figures 2a and b thus reveals the presence of PDE1, especially in fractions 21–23, and the presence of PDE5 in fractions 24–26. Another cGMP hydrolytic activity was detected in fractions 30–34 and was shown to be totally unaffected by sildenafil (Figure 2a) and poorly sensitive to calmodulin (Figure 2b). When HPLC fractions were assessed for cAMP hydrolytic activity, fractions 30–34 displayed cAMP-PDE activity stimulated by cGMP (Figure 3), thus suggesting the presence of PDE2 in these fractions.
Figure 2.
Effect of sildenafil on cGMP-PDE activity resolution following HPLC chromatography of cytosolic PDE isozymes from rat pulmonary artery. (a) cGMP-PDE activities were determined in control condition (EGTA) in the absence or presence of sildenafil. (b) cGMP-PDE activities were determined in control condition and in the presence of Ca2+/CaM. cGMP-PDE activities were determined using 1 μM [3H]cGMP. Note the difference in the ordinate scale between panels (a) and (b).
Figure 3.
Effect of sildenafil on cAMP-PDE activity resolution following HPLC chromatography of cytosolic PDE isozymes from rat pulmonary artery. cAMP-PDE activities were determined in control condition (EGTA) in the absence and in the presence of rolipram or cGMP. cAMP-PDE activities were determined using 1 μM [3H]cAMP.
The effect of sildenafil on cGMP-PDE activity was then tested on the partially purified PDE5 corresponding to the pool of fractions 25–26. Sildenafil induced a concentration dependent inhibition of PDE5 activity (Figure 4). IC50 value was 3.4 nM and maximal inhibition of the cGMP hydrolytic activity values was obtained for 1 μM sildenafil.
Figure 4.
Concentration–response curve for sildenafil effect on PDE5 activity. Fractions 25 and 26 from HPLC were pooled and used to assess the effect of sildenafil on partially purified PDE5.
Expression of PDE5 in rat MPA subcellular fractions
The presence of PDE5 in rat MPA was further investigated by Western blot analysis of the different subcellular fractions (Figure 5). Homogenate and cytosolic fractions show a doublet signal of 90/92 kDa, whereas only a tiny 92 kDa signal was detected in microsomal fraction. These results are in agreement with the data of Figure 1, showing that the major part of cGMP-PDE activity is present in cytosolic fraction, and with the data of Figure 2, showing the presence of PDE5 activity. Thus, PDE5 is essentially cytosolic.
Figure 5.
Expression of PDE5 in rat pulmonary artery subcellular fractions. Western blot analysis was performed on cytosolic (C), microsomal (M) and homogenate (H) fractions as described in Methods. Recombinant PDE5 (rPDE5) was used as positive control and ran as 90 and 110 kDa signals.
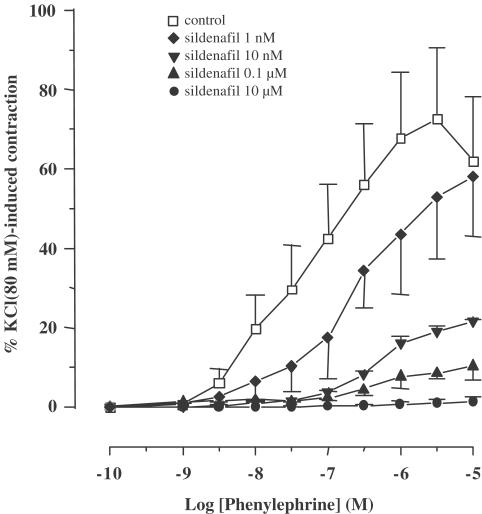
Effect of sildenafil on MPA tone
In the subsequent series of experiments, we investigated the functional effect of sildenafil on pulmonary vascular tone. Figure 6 shows the effect of sildenafil on the CCRC for the α1-adrenoceptor agonist, phenylephrine, in MPA rings. Pretreatment of rings with sildenafil (1 nM–10 μM) for 30 min, concentration dependently decreased the contraction induced by phenylephrine. The effect was observed throughout the range of phenylephrine concentrations (1 nM–50 μM) and was accompanied by an increase in EC50 (Table 2 ). Sildenafil (10 μM) fully inhibited maximal phenylephrine-induced contraction. In another set of experiments, the relaxant effect of sildenafil (0.1 nM–100 μM) on MPA rings precontracted by phenylephrine (0.5 μM) was compared to that of two other potent pulmonary vasodilators, SNP, an NO donor, and zaprinast, a relatively selective inhibitor of PDE5. All the three compounds concentration dependently relaxed MPA rings (Figure 7a). SNP and sildenafil exhibited the same potency (IC50=11 nM), which was 60-fold higher than that of zaprinast (IC50=600 nM). CCRC for sildenafil appeared less sigmoidal than that for SNP and zaprinast. The relaxant effect of sildenafil on MPA rings was not altered by endothelium removal (Figure 7b). Since sildenafil is expected to increase cGMP level and since cross-talk between cGMP and cAMP signaling pathway has been demonstrated in vascular wall (Lugnier et al., 1999; Carvajal et al, 2000), we finally investigated the action of KT5823 and H89 compounds, respectively, specific inhibitors of PKG and PKA, on the relaxant effect of sildenafil. The presence of 1 μM KT5823 alone or the additional presence of 1 μM H89 in the bath did not modify the relaxant effect of sildenafil on MPA rings (Figure 7c).
Figure 6.
Effect of sildenafil on phenylephrine-induced contraction. CCRCs for the effect of phenylephrine in the absence (control) and in the presence of different concentrations of sildenafil. MPA rings were exposed to sildenafil 30 min before and throughout the duration of the CCRC. Ordinate, contraction expressed as a percentage of the KCl (80 mM)-induced response obtained at the beginning of the experiments. Data points are means±s.e.m. (n=6).
Table 2.
EC50 and Emax values of concentration – response curves for the contractile effectof phenylephrine on MPA rings in the absence (control) and in the presence of increasing concentration of sildenafil
| Phenylephrine | ||
|---|---|---|
| EC50 (nM) | Emax (% KCl 80 mM) | |
| Control | 48 | 72.6±17.9 |
| Sildenafil 1 nM | 200 | 57.8±14.9 |
| Sildenafil 10 nM | 490 | 21.6±0.4 |
| Sildenafil 0.1 μM | 530 | 10.2±3.5 |
| Sildenafil 10 μM | N.D | 1.25±0.2 |
Data for Emax are mean±s.e.m, (n=6).
Figure 7.
Effect of sildenafil on MPA rings precontracted with phenylephrine. (a) CCRCs for the effect of sildenafil, SNP and zaprinast. (b) CCRC for the effect of sildenafil in intact and endothelium-denuded rings. (c) CCRC for the effect of sildenafil alone (control) and with the additional presence of KT5823 and KT5823+H89. MPA rings were precontracted with 0.5 μM phenylephrine. Data points are means±s.e.m. (n=6).
Effect of sildenafil on calcium signaling in freshly isolated myocytes
Since an increase in cytosolic calcium concentration ([Ca2+]i) is the main determinant of vascular smooth muscle contraction, the effect of sildenafil on calcium response induced by agonists was investigated in isolated pulmonary vascular myocytes. As previously shown (Guibert et al., 1996b; Hyvelin et al., 1998), short (40–60 s) application of agonists acting on seven transmembrane domain receptors, such as ATP (100 μM) or ET-1 (0.1 μM), induced a complex [Ca2+]i response composed of four to six oscillations of decreasing amplitude in about 90% of tested cells (Figure 8Aa). Preincubation of MPA myocytes with sildenafil (10 and 100 nM) for 10 min did not alter the resting [Ca2+]i value but, concentration dependently, decreased both the amplitude and the frequency of ATP-induced [Ca2+]i oscillations (Figure 8Ab and 8Ac and Table 3 ). Similar results were obtained with ET-1 (0.1 μM, not shown). In contrast, sildenafil (100 nM) did not alter the caffeine-induced transient [Ca2+]i response (Figure 8b and Table 3).
Figure 8.
Sildenafil effect on agonist-induced [Ca2+]i responses in rat pulmonary artery smooth muscle cells. (A) ATP-induced [Ca2+]i response was recorded without (a) and with 10 nM (b) or 100 nM (c) sildenafil; each trace was recorded from a different cell and is typical of 10–20 cells. (B) Caffeine-induced [Ca2+]i response was recorded without (a) and with 100 nM (b) sildenafil; each trace was recorded from a different cell and is typical of five to eight cells.
Table 3.
Effect of sildenafil on resting and ATP- or caffeine-induced [Ca 2+]i responses in MPA myocytes
| n | Resting[Ca]i (nM) | Peak [Ca2+]i, (nM) | Responding cells (%) | Oscillations frequency (min−1) | |
|---|---|---|---|---|---|
| ATP (100 μM) | 41 | 54.9±2.9 | 598±37 | 80 | 4.6±0.5 |
| Control | |||||
| ATP (100 μM | 42 | 48.7±2.8 | 552±74 | 50* | 1.8±0.6* |
| Sildenafil 10 nM | |||||
| ATP (100 μM) | 18 | 41.3±6.1 | 234±81 | 38* | 0.5±0.6* |
| Sildenafil 100 nM | |||||
| Caffeine 5 mM | 10 | 74.5±4.1 | 814.6±57 | 90 | |
| Control | |||||
| Caffeine 5 mM | 10 | 68±4.9 | 837.6±80 | 80 | |
| Sildenafil 100 nM |
Values are means±s.e.m; n, number of myocytes in a sample. [Ca2+]i, intracellular Ca2+ concentration; MPA, main pulmonary artery.
P<0.05, comparing the effect of ATP alone and ATP in the presence of sildenafil.
Discussion
The present study shows that sildenafil acts as a potent pulmonary vasorelaxant and that this effect is mainly related to its inhibitory effect on PDE5 which is expressed in the pulmonary artery wall and which is essentially cytosolic. Sildenafil-induced vasodilation involves alteration in calcium signaling.
Both cAMP- and cGMP-PDE activities are present in rat MPA and are significantly higher in cytosolic than microsomal fractions. Cytosolic PDE-specific activities in rat MPA (1000 and 800 pmol mg−1 min−1, respectively, for cGMP and cAMP) are much higher than those previously reported in bovine or human pulmonary arteries (Rabe et al., 1994; Pauvert et al., 2002). Sildenafil inhibited the cGMP-PDE activity in both subcellular fractions. This inhibitory effect appears mainly related to PDE5 inhibition for the following reasons: (1) sildenafil inhibited the cGMP-PDE activity at a concentration (0.1 μM) 100-fold lower than that of zaprinast, a relatively selective PDE5 inhibitor (Stoclet et al., 1995); (2) chromatographical resolution of cGMP-PDE activity revealed the presence of a peak of activity sensitive to sildenafil (0.1 μM); (3) pooling the fractions corresponding to this peak provided a partially purified PDE5, the activity of which was highly sensitive to sildenafil (IC50=3.4 nM); (4) Western blot analysis demonstrated the expression of PDE5 protein in rat MPA. Finally, we show, for the first time, that the potency of sildenafil on PDE5 from pulmonary vascular smooth muscle is similar to that observed on PDE5 from other smooth muscles, especially the corpus cavernosum (IC50=4 nM; Ballard et al., 1998). Another original finding of the present work is the 20% significant inhibitory effect of 0.1 μM sildenafil on cAMP-PDE activity in both subcellular fractions from rat MPA. The following arguments should be taken into account: (1) the cAMP-PDE activity is inhibited by rolipram and cilostamide and this activity can be ascribed to the presence of PDE3 and PDE4, as is the case in the other pulmonary preparations (bovine and human); (2) PDE3 and PDE4 are resolved by HPLC, (3) the concentration of sildenafil used (0.1 μM) is ineffective on PDE3 and PDE4 (Ballard et al., 1998). It can be speculated that sildenafil may be active on another PDE isozyme such as PDE10 or PDE11, which displays affinity for both cAMP and cGMP and inhibition by zaprinast of cGMP hydrolysis (Fujishige et al., 1999, Fawcett et al., 2000). The combined effect of sildenafil on cGMP- and cAMP-PDE activity may potentiate its ability to increase cyclic nucleotide level in MPA myocytes, and thus to vasodilate the pulmonary vasculature.
Contractile experiments in MPA rings, either pretreated with sildenafil or precontracted with phenylephrine and subsequently exposed to sildenafil, demonstrate the potent pulmonary relaxant effect of this compound. In precontracted rings, the IC50 value (11 nM) is close to that obtained for sildenafil with the purified PDE5. Sildenafil appears 60-fold more potent than zaprinast on precontracted MPA rings (Figure 7), a result in good agreement with previously reported differences between these two PDE5 inhibitors in corpus carvenosum (Ballard et al., 1998) or in bovine lung (Illarion et al., 1999). In rat MPA, this difference is related to (i) the more important inhibitory effect of sildenafil on the cGMP-PDE activity (Table 1) and (ii) the combined inhibitory effect of sildenafil on cGMP- and cAMP-PDE activities. Furthermore, the differential effect of sildenafil on CCRC, depending on the range of concentration (10−10–10−7 and 10−7–10−5 M), is in agreement with a possible additive inhibitory effect of sildenafil on another PDE isoform. Sildenafil vasorelaxant effect was not altered by endothelium removal, suggesting that PDE activities are mainly present in the smooth muscle cells of the MPA ring. Interestingly, sildenafil appears as potent as the NO donor SNP to relax pulmonary arteries. Therefore, it would be of interest to examine the effect of a combination of PDE5 inhibitor and an NO donor in the treatment of PAHT.
In rat MPA, the main action of sildenafil thus appears to be the inhibition of PDE5 which secondarily may lead to an increase in the cGMP content. Most of the cGMP effects are mediated via the activation of cGMP-dependent protein kinase (PKG) (Carvajal et al., 2000). However, in the present work, addition of KT 5823, a potent PKG inhibitor (Ito & Karachot, 1992), did not alter the relaxant effect of sildenafil (Figure 7). Such a cGMP-dependent, but PKG-independent, effect has been previously reported in the pulmonary vascular bed and tracheal smooth muscle of the rat for the action of a variety of NO donors (Fouty et al., 1998; Waniishi et al., 1998; Pauvert et al., 2000).
cGMP is known to interact at different steps of Ca2+ signaling including Ca2+-ion channels, pumps, inositol triphosphate (IP3) pathway and regulatory proteins of the contractile apparatus (Carvajal et al., 2000). In freshly isolated MPA myocytes, sildenafil concentration-dependently inhibits Ca2+ oscillations induced by agonists acting on G-protein-coupled transmembrane receptors such as ATP or ET-1. It is again noteworthy that sildenafil is active on Ca2+ oscillations in a range of concentrations similar to that inducing its relaxant effect. In MPA myocytes, we have previously demonstrated that such Ca2+ oscillations are because of a cyclic release of Ca2+ ions stored in the sarcoplasmic reticulum (SR), via an IP3-dependent pathway and participate in the amplitude of the contractile response induced by these agonists (Guibert et al., 1996a,1996b; Guibert et al., 1997; Hyvelin et al., 1998). In contrast, sildenafil, even at high concentration (0.1 μM), had no effect on the caffeine-induced [Ca2+]i response, which is dependent on the Ca2+ -induced Ca2+ -release mechanism via the ryanodine receptor. We have previously shown that the permeant cGMP analog 8-Br-cGMP also inhibits Ca2+ oscillations, but has no effect on the transient caffeine-induced [Ca2+]i response (Pauvert et al., 2000). Collectively, these data suggest that the relaxant effect of sildenafil is mainly because of a cGMP-dependent mechanism that alters the Ca2+ signaling involving the IP3 pathway.
In conclusion, our study shows that sildenafil is a potent pulmonary vascular relaxant and that its action is mainly mediated by the inhibition of the PDE5. At the cellular level, sildenafil alters calcium signaling in pulmonary vascular myocytes through the inhibition of agonist-induced calcium oscillations. A complementary study performed in pulmonary arteries obtained from animals suffering from PAHT could provide the fundamental basis for the mechanism of action of sildenafil.
Acknowledgments
This work was supported by a grant from the ‘Conseil Régional d'Aquitaine' (20000301114). We thank Dr J.D. Corbin for the anti-PDE5 antisera and Sanofi-Synthélabo for the recombinant PDE5. We acknowledge Ms H. Basaran and Dr A. Le Bec for technical assistance. ER. is a National Scholar and a member of the Health Respiratory Network of the ‘Fond de la Recherche en Santé du Québec (FRSQ)'.
Abbreviations
- BSA
Bovine serum albumin
- CCRC
Cumulative concentration–response curve
- DMSO
Dimethyl sulphoxide
- EHNA
Erythro-9-(2-hydroxy-3nonyl)adenine
- HPLC
High-pressure liquid chromatography
- IBMX
3-isobutyl-1-methylxanthine
- MPA
Main pulmonary artery
- PDE
Cyclic nucleotide phosphodiesterase
- SDS
Sodium dodecyl sulfate
- Tris
hydroxymethylaminomethane
References
- ABRAMS D., SCHULZE-NEICK I., MAGEE A.G. Sildenafil as a selective vasodilator in childhood primary pulmonary hypertension. Heart. 2000;84:E4. doi: 10.1136/heart.84.2.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALLARD S.A., GINGELL C.J., TANG K., TURNER L.A., PRICE M.E., NAYLOR A.M. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isosymes. J. Urol. 1998;159:2164–2171. doi: 10.1016/S0022-5347(01)63299-3. [DOI] [PubMed] [Google Scholar]
- BOOLELL M., GEPI-ATTEE S., GINGELL J.C., ALLEN M.J. Sildenafil, a novel effective oral therapy for male erectile dysfunction. Br. J. Urol. 1996;78:257–261. doi: 10.1046/j.1464-410x.1996.10220.x. [DOI] [PubMed] [Google Scholar]
- CARVAJAL J.A., GERMAIN A.M., HUIDOBRO-TORO J.P., WEINER C.P. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J. Cell. Physiol. 2000;184:409–420. doi: 10.1002/1097-4652(200009)184:3<409::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- DAWSON C.A. Role of pulmonary vasomotion in physiology of the lung. Physiol. Rev. 1984;64:544–616. doi: 10.1152/physrev.1984.64.2.544. [DOI] [PubMed] [Google Scholar]
- EDDAHIBI S., RAFFESTIN B., LE MONNIER DE GOUVILLE A.C., ADNOT S. Effect of DMPPO, a phosphodiesterase type 5 inhibitor, on hypoxic pulmonary hypertension in rats. Br. J. Pharmacol. 1998;125:681–688. doi: 10.1038/sj.bjp.0702124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAWCETT L., BAXENDALE R., STACEY P., MCGROUTHER C., HARROW I., SODERLING S., HETMAN J., BEAVO J.A., PHILLIPS S.C. Molecular cloning and characterization of a distinct human phosphodiesterase gene family: PDE11A. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3702–3707. doi: 10.1073/pnas.050585197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLLÉNIUS A., GÉRARD D. Fluorescence investigations of calmodulin hydrophobic sites. Biochem. Biophys. Res. Commun. 1984;119:1154–1160. doi: 10.1016/0006-291x(84)90896-9. [DOI] [PubMed] [Google Scholar]
- FOUTY B., KOMALAVILAS P., MURAMATSU M., COHEN A., MCMURTRY I.F., LINCOLN T.M., RODMAN D.M. Protein kinase G is not essential to NO-cGMP modulation of basal tone in rat pulmonary circulation. Am J Physiol. 1998;274:H672–H678. doi: 10.1152/ajpheart.1998.274.2.H672. [DOI] [PubMed] [Google Scholar]
- FRANCIS S.H., TURKO I.V., CORBIN J.D. Cyclic nucleotide phosphodiesterases: relating structure function. Progr. Nucleic Acid Res. Mol. Biol. 2001;65:1–52. doi: 10.1016/s0079-6603(00)65001-8. [DOI] [PubMed] [Google Scholar]
- FUJISHIGE K., KOTERA J., MICHIBATA H., YUASA K., TAKABAYASHI S-I., OKUMURA K., OMORI K. Cloning and characterization of a novel human phosphodiesterase that hydrolyses both cAMP and cGMP (PDE10A) J. Biol. Chem. 1999;274:18438–18445. doi: 10.1074/jbc.274.26.18438. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- GUIBERT C., MARTHAN R., SAVINEAU J.P. Angiotensin II-induced Ca(2+)-oscillations in vascular myocytes from the rat pulmonary artery. Am. J. Physiol. 1996a;270:L637–L642. doi: 10.1152/ajplung.1996.270.4.L637. [DOI] [PubMed] [Google Scholar]
- GUIBERT C., MARTHAN R., SAVINEAU J.P. Oscillatory Cl− current induced by angiotensin II in rat pulmonary arterial myocytes: Ca2+ dependence and physiological implication. Cell Calcium. 1997;21:421–429. doi: 10.1016/s0143-4160(97)90053-1. [DOI] [PubMed] [Google Scholar]
- GUIBERT C., PACAUD P., LOIRAND G., MARTHAN R., SAVINEAU J.P. Effect of extracellular ATP on cytosolic Ca2+ concentration in rat pulmonary artery myocytes. Am. J. Physiol. 1996b;271:L450–L458. doi: 10.1152/ajplung.1996.271.3.L450. [DOI] [PubMed] [Google Scholar]
- HANASATO N., OKA M., MURAMATSU M., NISHINO M., ADACHI H., FUKUCHI Y. E-4010, a selective phosphodiesterase 5 inhibitor, attenuates hypoxic pulmonary hypertension in rats. Am. J. Physiol. 1999;277:L225–L232. doi: 10.1152/ajplung.1999.277.2.L225. [DOI] [PubMed] [Google Scholar]
- HYVELIN J.M., GUIBERT C., MARTHAN R., SAVINEAU J.P. Cellular mechanisms and role of endothelin-1-induced calcium oscillations in pulmonary arterial myocytes. Am. J. Physiol. 1998;275:L269–L282. doi: 10.1152/ajplung.1998.275.2.L269. [DOI] [PubMed] [Google Scholar]
- ICHINOSE F., ERANA-GARCIA J., HROMI J., RAVEH Y., JONES R., KRIM L., CLARK M.W., WINKLER J.D., BLOCH K.D., ZAPOL W.M. Nebulized sildenafil is a selective pulmonary vasodilator in lambs with acute pulmonary hypertension. Crit. Care Med. 2001;29:1000–1005. doi: 10.1097/00003246-200105000-00024. [DOI] [PubMed] [Google Scholar]
- ILLARION V., BALLARD S.A., FRANCIS S.H., CORBIN J.D. Inhibition of cyclic GMP-binding cyclic GMP-specific phosphodiesterase (Type 5) by sildenafil and related compounds. Mol. Pharmacol. 1999;56:124–130. doi: 10.1124/mol.56.1.124. [DOI] [PubMed] [Google Scholar]
- ITO M., KARACHOT L. Protein kinases and phosphatase inhibitors mediating long-term desensitization of glutamate receptors in cerebellar Purkinje cells. Neurosci. Res. 1992;14:27–38. doi: 10.1016/s0168-0102(05)80004-5. [DOI] [PubMed] [Google Scholar]
- JEREMY J.Y., BALLARD S.A., NAYLOR A.M., MILLER M.A.W., ANGELINI G.D. Effects of sildenafil, a type-5 cGMP phosphodiesterase inhibitor and papaverine on cyclic GMP and cyclic AMP levels in the rabbit corpus cavernosum in vitro. Br. J. Urol. 1997;79:958–963. doi: 10.1046/j.1464-410x.1997.00206.x. [DOI] [PubMed] [Google Scholar]
- KERAVIS T.M., WELLS J.N., HARDMAN J.G. Cyclic nucleotide phosphodiesterase activities from pig coronary arteries: lack of interconvertibility of major forms. Biochim. Biophys. Acta. 1980;613:116–129. doi: 10.1016/0005-2744(80)90198-9. [DOI] [PubMed] [Google Scholar]
- KOMAS N., LUGNIER C., STOCLET J.C. Endothelium-dependent and independent relaxation of the rat aorta by cyclic nucleotide phosphodiesterase inhibitors. Br. J. Pharmacol. 1991;104:495–503. doi: 10.1111/j.1476-5381.1991.tb12457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- LUGNIER C., KERAVIS T., ECKLY-MICHEL A. Cross-talk between NO and cyclic nucleotide phosphodiesterase in the modulation of signal transduction in blood vessel. J. Physiol. Pharmacol. 1999;50:639–652. [PubMed] [Google Scholar]
- LUGNIER C., KOMAS N. Modulation of vascular cyclic nucleotide phosphodiesterases by cGMP: role in vasodilatation. Eur. Heart J. 1993;14 Suppl 1:141–148. [PubMed] [Google Scholar]
- LUGNIER C., SCHOEFFTER P., LE BEC A., STROUTHOU E., STOCLET J.C. Selective inhibition of cyclic nucleotide phosphodiesterases of human, bovine and rat aorta. Biochem. Pharmacol. 1986;35:1743–1751. doi: 10.1016/0006-2952(86)90333-3. [DOI] [PubMed] [Google Scholar]
- MACALLISTER-LUCAS L.M., HAIK T.L., COLBRAN J.L., SONNENBOURG W.K., SERGER D., TURKO I.V., BEAVO J.A., FRANCIS S.H., CORBIN J.D. An essential aspartic acid at each of two allosteric cGMP-binding sites of a cGMP-specific phosphodiesterase. J. Biol. Chem. 1995;270:30671–30679. doi: 10.1074/jbc.270.51.30671. [DOI] [PubMed] [Google Scholar]
- MACLEAN M.R., JOHNSTON E.D., MCCULLOCH K.M., POOLEY L., HOUSLAY M.D., SWEENEY G. Phospho-diesterase isoforms in the pulmonary arterial circulation of the rat: changes in pulmonary hypertension. J. Pharmacol. Exp. Ther. 1997;283:619–624. [PubMed] [Google Scholar]
- MORELAND R.B., GOLDSTEIN I., TRAISH A. Sildenafil, a novel inhibitor of phosphodiesterase type 5 in human corpus cavernosum smooth muscle cells. Life Sci. 1998;62:309–318. doi: 10.1016/s0024-3205(98)00158-1. [DOI] [PubMed] [Google Scholar]
- PAUVERT O., MARTHAN R., SAVINEAU J.P. NO-induced modulation of calcium-oscillations in pulmonary vascular smooth muscle. Cell Calcium. 2000;27:329–338. doi: 10.1054/ceca.2000.0123. [DOI] [PubMed] [Google Scholar]
- PAUVERT O., SAVAIL D., ROUSSEAU E., LUGNIER C., MARTHAN R., SAVINEAU J.P. Characterization of cyclic nucleotide phosphodiesterase isoforms in the media layer of the main pulmonary artery. Biochem. Pharmacol. 2002;63:1763–1772. doi: 10.1016/s0006-2952(02)00919-x. [DOI] [PubMed] [Google Scholar]
- PIERSON D.J. Pathophysiology and clinical effects of chronic hypoxia. Respir. Care. 2000;45:39–51. [PubMed] [Google Scholar]
- POLSON J.B., STRADA S.J. Cyclic nucleotide phosphodiesterases and vascular smooth muscle. Ann. Rev. Pharmacol. Toxicol. 1996;36:403–427. doi: 10.1146/annurev.pa.36.040196.002155. [DOI] [PubMed] [Google Scholar]
- RABE K.F., TENOR H., DENT G., SCHUDT C., NAKASHIMA M., MAGNUSSEN H. Identification of PDE isoenzymes in human pulmonary artery and effect of selective PDE inhibitors. Am. J. Physiol. 1994;266:L536–L543. doi: 10.1152/ajplung.1994.266.5.L536. [DOI] [PubMed] [Google Scholar]
- RABINOVITCH M., GAMBLE W., NADAS A.S., MIETTINEN O.S., REID L. Rat pulmonary circulation after chronic hypoxia: hemodynamic and structural features. Am. J. Physiol. 1979;236:H818–H827. doi: 10.1152/ajpheart.1979.236.6.H818. [DOI] [PubMed] [Google Scholar]
- ROUNDS S.I.S.Pulmonary circulatory control in lung injury Pulmonary Vascular Physiology and Pathophysiology. 198938New York: Marcel-Dekker; 403–468.ed. Weir, E.K. & Reeves, J.T. Vol [Google Scholar]
- SCHOEFFTER P., LUGNIER C., DEMESY-WAELDELE F., STOCLET J.C. Role of cyclic AMP- and cyclic GMP-phosphodiesterases in the control of cyclic nucleotide levels and smooth muscle tone in rat isolated aorta. A study with selective inhibitors. Biochem. Pharmacol. 1987;36:3965–3972. doi: 10.1016/0006-2952(87)90465-5. [DOI] [PubMed] [Google Scholar]
- SODERLING S.H., BEAVO J.A. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr. Opin. Cell. Biol. 2000;12:174–179. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- STOCLET J.C., KERAVIS T., KOMAS N., LUGNIER C. Cyclic nucleotide phosphodiesterases as therapeutic targets in cardiovascular diseases. Exp. Opin. Invest. Drugs. 1995;4:1081–1100. [Google Scholar]
- WAGNER R.S., SMITH C.J., TAYLOR A., ROADES R.A. Phosphodiesterase inhibition improves agonist-induced relaxation of hypertensive pulmonaries arteries. J. Pharmacol. Exp. Ther. 1997;282:1650–1657. [PubMed] [Google Scholar]
- WANIISHI Y., INOUE R., MORITA H., TERAMOTO N., ABE K., ITO Y. Cyclic GMP-dependent but G-kinase-independent inhibition of Ca2+-dependent Cl− currents by NO donors in cat tracheal smooth muscle. J. Physiol. 1998;511:719–731. doi: 10.1111/j.1469-7793.1998.719bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAO L., MASON N.A., MORRELL N.W., KOJONAZAROV B., SADYKOV A., MARIPOV A., MIRRAKHIMOV M.M., ALDASHEV A., WILKINS M.R. Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation. 2001;24:424–428. doi: 10.1161/hc2901.093117. [DOI] [PubMed] [Google Scholar]
- ZIEGLER J.W., IVY D.D., WIGGINS J.W., KINSELLA J.P., CLARKE W.R., ABMAN S.H. Effect of dipyridamole and inhaled nitric oxide in pediatric patients with pulmonary hypertension. Am. J. Respir. Crit. Care. Med. 1998;158:1388–1395. doi: 10.1164/ajrccm.158.5.9710117. [DOI] [PubMed] [Google Scholar]