Abstract
Studies were undertaken to determine the nature of the receptors mediating contractile effects of tachykinins in the uteri of nonpregnant women, and to analyse the expression of preprotachykinins (PPT), tachykinin receptors and the cell-surface peptidase, neprilysin (NEP), in the myometrium from pregnant and nonpregnant women.
The neurokinin B (NKB) precursor PPT-B was expressed in higher levels in the myometrium from nonpregnant than from pregnant women. Faint expression of PPT-A mRNA was detectable in the myometrium from nonpregnant but not pregnant women. PPT-C, the gene encoding the novel tachykinin peptide hemokinin-1 (HK-1), was present in trace amounts in the uteri from both pregnant and nonpregnant women.
Tachykinin NK2 receptors were more strongly expressed in tissues from nonpregnant than from pregnant women. NK1 receptor mRNA was present in low levels in tissues from both pregnant and nonpregnant women. A low abundance transcript corresponding to the NK3 receptor was present only in tissues from nonpregnant women.
The mRNA expression of the tachykinin-degrading enzyme NEP was lower in tissues from nonpregnant than from pregnant women.
Substance P (SP), neurokinin A (NKA) and NKB, in the presence of the peptidase inhibitors thiorphan, captopril and bestatin, produced contractions of myometrium from nonpregnant women. The order of potency was NKA≫SP≥NKB. The potency of NKA was unchanged in the absence of peptidase inhibitors.
The tachykinin NK2 receptor-selective agonist [Lys5MeLeu9Nle10]NKA(4–l0) was approximately equipotent with NKA, but the tachykinin NK1 and NK3 receptor-selective agonists [Sar9Met(O2)11]SP and [MePhe7]NKB were ineffective in the myometrium from nonpregnant women.
The uterotonic effects of [Lys5MeLeu9Nle10]NKA(4–10) were antagonized by the tachykinin NK2 receptor-selective antagonist SR48968. Neither atropine, nor phentolamine nor tetrodotoxin affected responses to [Lys5MeLeu9Nle10]NKA(4–10).
These data are consistent with a role of tachykinins in the regulation of human uterine function, and reinforce the importance of NK2 receptors in the regulation of myometrial contraction.
Keywords: Human myometrium, [Lys5MeLeu9Nle10]NKA(4–10), NK1 and NK2 receptors, neprilysin, neurokinin A, neurokinin B, substance P, tachykinins, uterine contractions, SR48968
Introduction
Tachykinins are members of a family of neuropeptides that includes substance P (SP), neurokinin A (NKA) and neurokinin B (NKB). Their biological actions are mediated through three receptors belonging to the family of G protein-coupled receptors (GPCR), denoted NK1, NK2 and NK3, that have highest affinity for SP, NKA and NKB, respectively (Henry, 1986; Regoli et al., 1994a,1994b; Lecci et al., 2000).
SP and NKA, but not NKB, are present within capsaicin-sensitive sensory nerves in the periphery and are locally released by a number of physical and chemical stimuli (Holzer, 1988; Maggi & Meli, 1988). SP and a new member of the tachykinin family, hemokinin-1 (HK-1), that has recently been cloned in the mouse (Zhang et al., 2000), rat and human (Kurtz et al., 2002) are also produced in non-neuronal cells (Joos & Pauwels, 2000; Zhang et al., 2000). This distribution suggests an important role for tachykinins in intercellular communication.
Previous studies from other laboratories and from ours indicate a role of tachykinins in the regulation of uterine function. Early immunohistochemical studies showed the presence of tachykinin-immunoreactive nerves supplying the uteri of several species including the mouse, rat, guinea-pig and human (Alm et al., 1978; Huang et al., 1984; Traurig et al., 1984; Papka et al., 1985; Samuelson et al., 1985; Heinrich et al., 1986; Alm & Lundberg, 1988; Reinecke et al., 1989; Traurig et al., 1991; Papka & Shew, 1994). The association of these nerves with smooth muscle indicates that tachykinins released from their peripheral terminals may influence uterine contractility. Preprotachykinin-A (PPT-A), the precursor of SP and NKA, is also expressed in non-neuronal cells such as monocytes and macrophages (Ho et al., 1997); these can be associated with the mammalian uterus (Cocchiara et al., 1997). SP has also recently been reported to participate in stress-induced abortion in the mouse and possibly in the human (Arck et al., 1995; Markert et al., 1997; Marx et al., 1999; Joachim et al., 2001). NKB has recently been reported to be expressed in the human placenta (Page et al., 2000) and the gene that encodes it, preprotachykinin-B, is expressed in the uterus of the rat and the human (Pinto et al., 2001;2002). NKB has been implicated in the symptoms associated with pre-eclampsia (Page et al., 2000). The major tachykinin-degrading enzyme neprilysin (NEP) (Matsas et al., 1983;1984; Hooper et al., 1985; Hooper & Turner, 1985) is also present in the uterus (Ottlecz et al., 1991; Head et al., 1993; Riley et al., 1995). This intrauterine distribution supports the view that tachykinins may be important intercellular signalling molecules within the female reproductive tract (Pinto et al., 2001; 2002).
To date, the majority of molecular (Pinto et al., 1999; Candenas et al., 2001), immunohistochemical (Traurig et al., 1984;1991; Papka et al., 1985) and functional (Fisher et al., 1993; Pennefather et al., 1993b; Fisher & Pennefather, 1997; Magraner et al., 1998; Patak et al., 2000a) studies of the uterine distribution of tachykinins, tachykinin receptors and/or degradation of tachykinins have been conducted using the rat. It is now emerging, however, that there are species (Patak et al., 2000a,2000b;2002a) as well as hormonal- and pregnancy-related differences in tachykinin expression and actions in the mammalian myometrium (Pinto et al., 1999;2001;2002; Candenas et al., 2001).
We have previously described the uterotonic effects of tachykinins on myometrium of late-pregnant women (Patak et al., 2000b). However, no systematic molecular studies of the expression of tachykinin precursor genes, neither of tachykinin receptors nor of NEP in either pregnant or nonpregnant human uterus, have been undertaken to date. Moreover, despite early reports that SP and eledoisin elicit contractions of the nonpregnant human uterus (Molina & Zappia, 1976; Ottesen et al., 1983), there have been no systematic functional studies of the actions of tachykinin peptides on myometrium from nonpregnant women.
For these reasons, the aim of the present study was to examine further the tachykinins, their receptors and NEP on the human myometrium. We have therefore performed molecular studies using myometrium from both nonpregnant and pregnant women, and functional studies to characterize the effects of tachykinins on uterine contraction of tissue from nonpregnant women.
A preliminary account of some of the results of this study has been presented previously (Patak et al., 2002b; Pinto et al., 2002).
Methods
Molecular studies
This component of the study was approved by the Ethics Committees of the Hospital Virgen del Rocío (Sevilla, Spain) and the Hospital Ntra. Sra. de Candelaria (Tenerife, Spain) and all patients gave informed consent.
Tissue preparation
Nonpregnant human uteri were obtained from five premenopausal patients (44–48 years old) who had undergone hysterectomy for benign uterine disease. Human pregnant uteri were obtained from five women (29–38 years old) undergoing elective lower uterine segment caesarean section (LUSCS) at 36–40 weeks gestation. Hysterectomies were performed under general anaesthetic induced by sevorane. LUSCSs were performed under epidural anaesthesia induced by bupivacaine. Uterine tissue obtained during LUSCS was excised from the upper edge of the incision.
Hysterectomy tissues were obtained from approximately the same location as specimens obtained at LUSCS. The myometrium was carefully removed, in the case of nonpregnant women from macroscopically normal uterine regions, and the outer layer dissected free from the adjoining inner layer, endometrium and connective tissue. Samples were immediately put on ice and stored at −80°C with a maximal delay of 2 h after excision (RT–PCR studies).
RNA extraction and reverse transcription
Total RNA of approximately 30 mg of human myometrium was isolated by the method of Chomczynski & Sacchi (1987). Residual genomic DNA was removed by incubating the RNA samples with RNase-free, fast protein liquid chromatography pure DNase I (Amersham Biosciences, Essex, U.K.) and RNasin (Promega Corp., Madison, U.S.A.). The quantity of total RNA was determined by spectrophotometric measurement at 260 nM. DNase-treated total RNA (5 μg) was reverse transcribed using a first-strand cDNA synthesis kit (Amersham Biosciences).
PCR primers
Specific oligonucleotide primers for human preprotachykinins (PPTs), tachykinin receptors, NEP and β-actin were designed with the analysis software Primer 3 (Rozen & Skaletsky, 2000) and used for both end-point and real-time PCR. Amplification of the β-actin gene transcript was used as an internal control of RT–PCR reactions among the samples. The sequences of the primers, the size of the expected PCR fragments and appropriate references are shown in Table 1 . All primers were synthesized and purified by Sigma Genosys (Cambridge, U.K.).
Table 1.
Sequences of forward (F) and reverse (R) primers of indicated target genes and the size expected for each PCR-amplified product. Primers for β-actin, used as an internal control, are also shown
| Gene | Forward primer | Reverse primer | Amplicon size (bp) | Reference |
|---|---|---|---|---|
| PPT-A | 5′-TGTGTCAGCTACTGCGGAAA-3′ | 5′-CAATGAAATGAAGACATTAGAATCACA-3′ | 193 | Harmar et al. (1986) |
| PPT-B | 5′-CCCCCGAGAGCAGAATAGGT-3′ | 5′-CCAGGGTCAGGTAGAAAAAGATGG-3′ | 171 | Page et al. (2000) |
| PPT-C | 5′-CCTYGCCCTGYTTCTCCT-3′ | 5′-YCCACYCGCTTCCCCATC-3′ | 204 | Zhang et al. (2000) |
| NK1R | 5′-GCTGCCCTTCCACATCTTCTT-3′ | 5′-GTCCTCTGGCTCCTCCTCGT-3′ | 331 | Takeda et al. (1991) |
| NK2R | 5′-GCCCTACCACCTCTACTTCATCC-3′ | 5′-AGCAAACCATACCCAAACCA-3′ | 375 | Gerard et al. (1990) |
| NK3R | 5′-TTGCGGTGGACAGGTATATGG-3′ | 5′-GGCCATTGCACAAAGCAGAG -3′ | 178 | Takahashi et al. (1992) |
| NEP | 5′ -AGCCTCTCGGTCCTTGTCCT-3′ | 5′-GGAGCTGGTCTCGGGAATG-3′ | 219 | Shipp et al. (1988) |
| β-actin | 5′-TCCCTGGAGAAGAGCTACGA-3′ | 5′-ATCTGCTGGAAGGTGGACAG-3′ | 362 | Nakajima-Iijima et al. (1985) |
NEP, neprilysin.
End-point PCR
An end-point PCR assay was used to detect the mRNAs of PPTs, tachykinin receptors and NEP and to establish the identity of the amplified products. An aliquot of the resulting cDNA (corresponding to 100 ng of total RNA) was used as a template for PCR amplification using a DNA thermal cycler (MJ Research, Watertown, U.S.A.). The 25 μl volume PCR mixes also contained 0.2 μM primers, 1.5 U of Taq polymerase (Amersham Biosciences), the buffer supplied, 2.5 mM MgCl2 and 200 μM dNTPs. After a hot start (2 min at 94°C), the profile for each cycle was 94°C for 10 s; 60°C for 20 s and 72°C for 30 s. Cycle numbers were 36 for tachykinins and tachykinin receptors, 33 for NEP and 24 for β-actin. Serial half dilutions of cDNA were amplified at the indicated number of cycles for each target gene and β-actin to ensure analysis of products in the linear range of amplification. The PCR products were separated by agarose gel electrophoresis, stained with ethidium bromide and visualized and photographed under a UV transilluminator (Spectronics Corp., NY, U.S.A.). mRNA expression for all target genes and β-actin was analysed on each tissue. Amplicon sizes were verified by comparison with a DNA mass ladder and the identity of each PCR product was established by DNA sequence analysis, as previously described (Pinto et al., 1999). No PCR product was detectable when the samples were amplified without the RT step, suggesting that genomic DNA contamination was eliminated by DNase treatment. Similarly, no products were detected when the RT–PCR steps were carried out with no added RNA, indicating that all reagents were free of target sequence contamination.
Real-time PCR
A real-time PCR was used to quantify the expression of the genes encoding NKB, the tachykinin NK1 and NK2 receptors and NEP, using the iCycler iQ real-time detection system from Bio-Rad laboratories (CA, U.S.A.) and SYBR green (Molecular Probes, Leiden, The Netherlands). The PCR reaction mixture was identical to that used in the end-point PCR assay, adding SYBR green I (1 : 75,000 dilution of the 10,000 × stock solution). The PCR buffer also contained fluorescein (1 : 100,000 dilution) used as a reference dye for normalization of the reactions. Any fluctuation in the fluorescein signal is used to correct the sample signal. The reactions were performed in 96-well thin-wall PCR plates covered with a sheet of optical-quality sealing film. Thermal cycling conditions were the same as those described for end-point assays. Fluorescence measurements were recorded during each extension step. At the end of each PCR run, the data were automatically analysed by the system and an amplification plot was generated for each DNA sample. The real-time PCR data were plotted as the PCR baseline-subtracted relative fluorescence units (ΔRFU) versus the cycle number. The ΔRFU was calculated as the difference between the fluorescence signal of the product at any given time and the fluorescence signal of the baseline emission during cycles 2–18. From each of these plots, the iCycler software calculates the threshold cycle (CT), defined as the fractional cycle number at which the fluorescence reaches 10 × the standard deviation of the baseline. The fold change in expression of the target gene relative to β-actin was then calculated by the formula
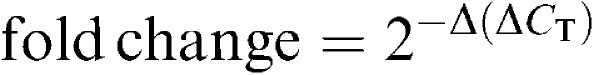 |
where ΔCT=CT target gene−CT β-actin and Δ(ΔCT)=ΔCT test sample−ΔCT control. Real-time PCR data from one of the samples were arbitrarily chosen as control and this sample was included in all PCR experiments to correct for possible interassay variations. Three serial dilutions of cDNA template were prepared from each tissue and each dilution was amplified in triplicate. The experimental approach was further validated by the observation that the differences between the CT for the target gene and β-actin remained essentially constant for each starting DNA amount.
Statistical analysis
Values are expressed as means±s.e.m. Differences between estimates in samples from pregnant and nonpregnant women were assessed by Student's unpaired t-test (GRAPHPAD PRISM 3.0, CA, U.S.A.). Statistical significance was accepted when P<0.05.
Functional studies with myometrium from nonpregnant women
Ethical approval was obtained from the Ethics Committee of the Royal Women's Hospital (Victoria, Australia). All patients gave informed consent.
Tissue preparation
Myometrium was obtained from 30 nonpregnant women (31–65 years old) who had undergone a hysterectomy for benign uterine disease. Hysterectomies were performed under general anaesthetic induced by propofol or thiopentane and maintained with isoflurane, sevoflurane or nitrous oxide either alone or in combination. Tissue obtained was from an area corresponding to that taken previously at LUSCS (Patak et al., 2000b).
The methods used to examine myometrial contractility have been described previously (Pennefather et al., 1993a; Patak et al., 2000b). Briefly, four preparations, 3 × 3 × 10 mm (mean weight 104.5±3.6 mg; n=98) preparations from 30 women, were obtained from the outer layer and mounted in 5 ml organ baths containing a modified Krebs–Henseleit solution of the following composition (in mM): NaCl 118.0; KC1 4.7; MgSO4·7H2O 1.1; KH2PO4 1.18; NaHCO3 25.0; glucose 11.66; CaCl2·2H2O 1.9, maintained at 37°C and bubbled with carbogen (5% CO2 in O2; pH=7.4). Preparations were set up under an initial resting force of approximately 15 g (Story et al., 1988) and isometric force produced by the longitudinally oriented smooth muscle measured using a Grass FT03 force transducer connected to a MACLAB data acquisition system.
Agonist log concentration–response curves
After 60 min equilibration, discrete log concentration–response curves (increasing in 0.5 log increments) were constructed for SP, NKA, NKB, [Sar9Met(O2)11]SP, [Lys5MeLeu9Nle10]NKA(4–10) and [MePhe7]NKB. Each concentration of an agonist was washed out after 5 min contact, and a higher concentration added 15 min later. Only one concentration–response curve was constructed on each preparation. The peptidase inhibitors thiorphan (3 μM), captopril (10 μM) and bestatin (10 μM) were added 20 min before the first addition of SP, NKA and NKB and replaced after each wash. At the end of the experiment, tissues were exposed to a modified Kreb's solution (KPSS) in which 40 mM KCl replaced 40 mM NaCl.
The effects of the tachykinin NK2 receptor-selective antagonist SR48968 (1, 3 and 10 nM), or of atropine (0.3 μM), phentolamine (1 μM) or tetrodotoxin (1 μM) on the response to [Lys5MeLeu9Nle10]NKA(4–10) and of peptidase inhibitors on the response to NKA were also examined. SR48968, atropine, phentolamine and tetrodotoxin were added at the beginning of the equilibration period and replaced after each wash.
Data analysis
Responses to agonists were measured as area under the force–time curve (g s), for the 5 min period that the agonist was in contact with the tissue and expressed as a percentage of the corresponding response to KPSS. Results are presented as mean±s.e.m.; n values refer to the number of patients.
Mean log concentration–response curves were constructed by pooling data from individual log concentration–response curves. When mean log concentration curves reached a clear maximum, pD2 values were determined using nonlinear regression analysis in the GRAPHPAD PRISM (version 3.0) program. When log concentration–response curves did not reach a plateau, these estimates could not be made; but agonist potency ratios were determined as described previously (Patak et al., 2000b). Briefly, when there was significant regression of response with agonist concentration, least-squares regression lines were fitted to the linear portions (typically 15–85% of the maximum response to the reference agonists) of the log concentration–response curves. Analysis of variance (ANOVA) was undertaken to determine deviation from parallelism and coincidence as outlined in Geigy Scientific Tables (Lentner, 1982). Other statistical procedures used included one- and two-way analyses of variance followed by Student Newman Keuls' pairwise test for multiple comparisons and Student's unpaired t-tests to compare the means of two groups. Statistical significance was accepted when P<0.05.
Drugs and solutions
The drugs used were: atropine sulphate (Sigma); bestatin HCl (N-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutyryl]-L-leucine hydrochloride) (Sigma); captopril (D-3-mercapto-2-methyl propanoyl-L-proline) (Sigma); [Lys5MeLeu9Nle10]NKA(4–10) (RBI, lot ZIU-797A); NKA (AUSPEP, batch J20852 & J20647); NKB (AUSPEP, batch I20382); [N-MePhe7]NKB (AUSPEP, batch 120429); phentolamine HCl (Ciba-Geigy); [Sar9Met(O2)11]SP (AUSPEP, batch H40846); SR48968 ((S)-N-methyl-N[4-acetylamino-4-phenylpiperidino)-2-(3,4-dichlorophenyl)butyl]benzamide) (a generous gift from Sanofi Recherche); SP (AUSPEP, batch H10029 & J31170); DL-thiorphan (Sigma); tetrodotoxin (Sigma). The purity of all the peptides used in this study was confirmed by mass spectral analysis. Atropine, captopril, phentolamine and thiorphan were dissolved in distilled water. NKB and [MePhe7]NKB were dissolved in 0.1 M ammonia. SR48968 was dissolved in absolute ethanol. Tetrodotoxin was dissolved in citrate buffer. All remaining compounds were dissolved in dilute hydrochloric acid (0.01 M). Stock solutions of bestatin (10 mM), captopril (10 mM) and SR48968 (1 mM) were stored at 4°C. Standard solutions (1 mM) of all peptides, thiorphan and tetrodotoxin were aliquoted into Eppendorf tubes and stored at −20°C.
Results
Molecular studies
RT–PCR studies
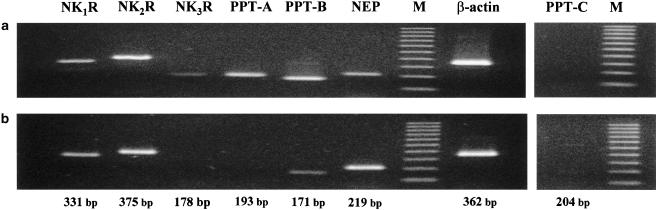
Figure 1 illustrates an example of an agarose gel showing RT–PCR products obtained by amplification of similar amounts of human uterine cDNA, as determined from the previous amplification of the β-actin sequence. By using end-point RT–PCR, we detected the presence of mRNA transcripts corresponding to the sizes expected for the tachykinin NK1R (331 bp) and the tachykinin NK2R (375 bp). These transcripts appeared in all the uteri assayed, both from pregnant (n=5) and from nonpregnant (n=5) women. The mRNA transcript expected for the tachykinin NK3 receptor (178 bp) appeared in uterine cDNA from nonpregnant women, but was undetectable in the uteri from pregnant women near term. The human myometrium from pregnant and nonpregnant women expressed significant amounts of preprotachykinin-B (PPT-B) mRNA (171 bp), the gene that encodes NKB. The expression of mRNA for PPT-A (193 bp), the gene that encodes SP and NKA, was clearly detectable only in the uteri from nonpregnant women (Figure 1). Preprotachykinin-C (PPT-C), the gene that encodes HK-1, was present in trace amounts that were detectable only after amplification of higher amounts of uterine cDNA from both pregnant and nonpregnant women. The 219 bp fragment expected for mRNA of NEP was detected in all the uteri assayed (Figure 1).
Figure 1.
Agarose gel showing products of reverse-transcriptase–polymerase chain reaction (RT–PCR) assay for cDNA from uterine samples from (a) a nonpregnant premenopausal woman and (b) a pregnant woman. Single transcripts corresponding to the sizes predicted for the NK1 receptor (NK1R), the NK2 receptor (NK2R), PPT-B and NEP were detected in all the tissues. The specific bands corresponding to the tachykinin NK3 receptor (NK3R) and PPT-A were only detectable in nonpregnant human uteri. The mRNA of PPT-C was not detected after amplification of the usual amount of uterine cDNA. β-actin was used as housekeeping gene. M, molecular size standards. Data are typical of results in five pregnant and five nonpregnant women.
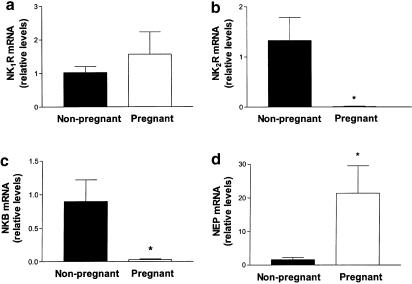
Real-time quantitative PCR was used to compare the relative abundance of tachykinin NK1R and NK2R, NKB and NEP mRNAs among pregnant and nonpregnant human uteri. It was, however, not possible to quantify the expression levels of the tachykinin NK3 receptor and PPT-A because of the disappearance of the specific transcripts in uterine cDNA from pregnant women.
Tachykinin NK1R mRNA levels were similar in the uteri from pregnant and nonpregnant women (Figure 2a). In contrast, the levels of NK2R and NKB mRNA were 95- and 30-fold higher, respectively, in nonpregnant human uteri (P<0.05, Figure 2b,c). By using real-time quantitative PCR, we observed that, among the genes assayed, the expression of NEP showed the highest interindividual variations among the different uterine samples. As a whole, NEP expression in the uteri from nonpregnant women was low except in one of the tissues assayed (n=1, not shown). If the expression value in this nonpregnant uterus is excluded, NEP mRNA levels were 14-fold higher in the uteri from pregnant women (P<0.05, n=5), compared with those from nonpregnant women (n=4, Figure 2d).
Figure 2.
Relative gene expression, obtained by real-time quantitative PCR, of (a) NK1R; (b) NK2R; (c) NKB and (d) NEP in cDNA from the uteri of pregnant and nonpregnant women. Values for NK1R, NK2R, NKB and NEP mRNA are shown in arbitrary units, relative to β-actin mRNA expression. Each bar represents the mean±s.e.m. of at least 18 uterine cDNA samples from each of four to five nonpregnant and four to five pregnant women. *P<0.05, significant difference versus mRNA levels in nonpregnant uteri, Student's t-test for unpaired data.
Functional studies with myometrium from nonpregnant women
Agonist studies
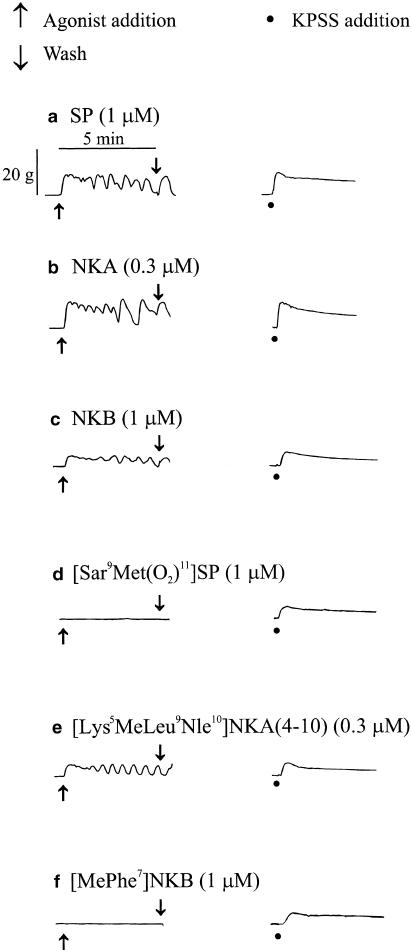
Only tissues that contracted in response to KPSS, indicating their viability, were included in this study. The mean response to KPSS was 1613+110.6 g s (n=98 preparations). No significant differences in the mean responses to high potassium-containing modified physiological saline solution (KPSS) were observed in experiments in which the potencies of agonists were compared (one-way ANOVA, P>0.05). Figure 3 shows that SP, NKA, NKB and [Lys5MeLeu9Nle10]NKA(4–10) were able to induce contractions of the isolated myometrium obtained from nonpregnant women, while [Sar9Met(O2)11]SP and [MePhe7]NKB were without effect.
Figure 3.
Representative traces showing contractile activity elicited by the tachykinins (a) SP, (b) NKA, (c) NKB, (d) [Sar9Met(O2)11]SP, (e) [Lys5MeLeu9Nle10]NKA(4–10) and (f) [MePhe7]NKB, together with their corresponding response to KPSS on myometrial preparations obtained from two nonpregnant women. Responses shown in (a)–(c) were on tissue obtained from a 41-year old woman. Responses shown in (d)–(f) were on tissue obtained from a 38-year old woman. Responses to SP, NKA and NKB are in the presence of peptidase inhibitors thiorphan (3 μM), captopril (10 μM) and bestatin (10 μM).
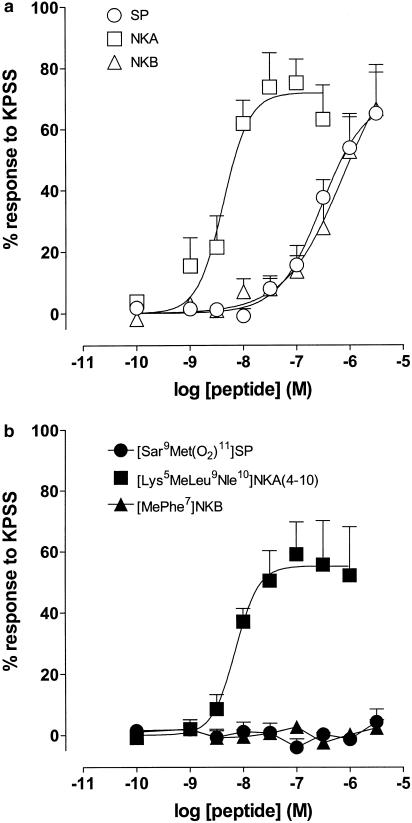
Figure 4a shows log concentration–response curves to SP, NKA and NKB in the myometrium from nonpregnant women. In the presence of the peptidase inhibitors thiorphan (3 μM), captopril (10 μM) and bestatin (10 μM), NKA was the most potent mammalian tachykinin (two-way ANOVA, P<0.05) with a mean pD2 value of 8.39±0.12 (n=6). It was 82- and 94-fold more potent than SP (95% confidence limits (CL)=35, 218; degrees of freedom (d.f.=57) and NKB (95% CL=36, 293; d.f.=56), respectively. Of the receptor-selective agonists, only the NK2 receptor-selective [Lys5MeLeu9Nle10]NKA(4–10) acted as an agonist with a mean pD2 value of 8.15±0.12 (n=6). In contrast, the NK1 and NK3 receptor-selective agonists [Sar9Met(O2)11]SP and [MePhe7]NKB, respectively, elicited no response as shown in Figure 4b.
Figure 4.
Log concentration–response curves to tachykinin peptides on myometrial preparations from nonpregnant women. Data points are mean responses±s.e.m. and are expressed as a percentage of the response to KPSS. (a) Mean responses to SP, NKA and NKB in the presence of the peptidase inhibitors thiorphan (3 μM), captopril (10 μM) and bestatin (10 μM) (n=6). (b) Mean responses to [Sar9Met(O2)11]SP, [Lys5MeLeu9Nle10]NKA(4–10) and [MePhe7]NKB (n=5–6).
Antagonist studies
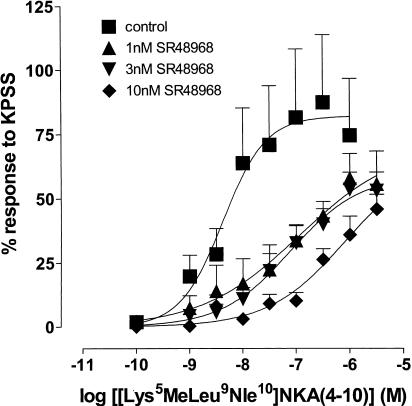
The effect of the NK2 receptor-selective antagonist SR48968 (Emonds-Alt et al., 1992) on the response to [Lys5MeLeu9Nle10]NKA(4–10) was examined. Figure 5 shows that SR48968 produced significant concentration-related rightward shifts in the position of the log concentration–response curve to [Lys5MeLeu9Nle10]NKA(4–10) at 1 nM (90-fold, 95% CL=9, 1965; d.f.=26), 3 nM (154-fold, 95% CL=20, 1873; d.f.=27) and 10 nM (641-fold, 95% CL=92, 14706; d.f.=30). Analysis by one-way ANOVA indicated no significant difference in the maximum response to [Lys5MeLeu9Nle10]NKA(4–10) in either the absence or presence of an antagonist; however, inspection of the curves indicates a trend towards depression of the maximum response as the antagonist concentration was increased.
Figure 5.
Log concentration–response curves to [Lys5MeLeu9Nle10]NKA(4–10) in the absence and presence of 1, 3 and 10 nM SR48968 on myometrial preparations from nonpregnant women. Data points are mean responses±s.e.m. and are expressed as a percentage of the response to KPSS (n=5–7).
Effect of peptidase inhibitors on the response to NKA
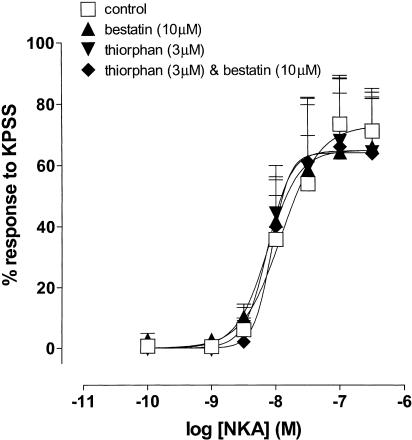
The effects of the peptidase inhibitors thiorphan (3 μM) and bestatin (10 μM) on the response to NKA in the nonpregnant uterus were investigated. Figure 6 shows that neither thiorphan (potency ratio=1.1, 95% CL=0.3, 4.1; d.f.=36), nor bestatin (potency ratio=1.1, 95% CL=0.3, 4.3; d.f.=36) nor thiorphan in combination with bestatin (potency ratio=1.0, 95% CL=0.3, 3.6; d.f.=36) potentiated the response to NKA.
Figure 6.
Log concentration–response curves to NKA in the absence and presence of thiorphan (3 μM), bestatin (10 μM) and thiorphan (3 μM) in combination with bestatin (10 μM) on myometrial preparations from nonpregnant women. Data points are mean responses±s.e.m. and are expressed as a percentage of the response to KPSS (n=5).
Effect of atropine, phentolamine and tetrodotoxin on the response to [Lys5MeLeu9Nle10]NKA(4–10)
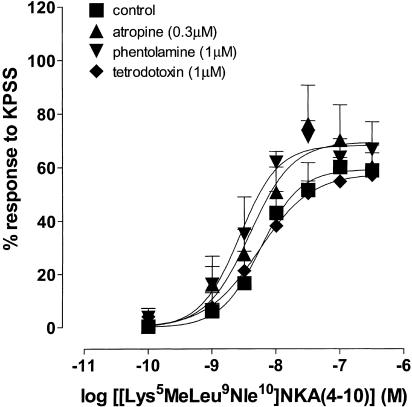
Figure 7 shows that responses to [Lys5MeLeu9Nle10]NKA(4–10) in the nonpregnant uterus were not affected by atropine (0.3 μM; potency ratio=2.4, 95% CL=1.1, 6.1; d.f.=38), phentolamine (1 μM; potency ratio=3.0, 95% CL=1.4, 7.7; d.f.=34) nor tetrodotoxin (1 μM; potency ratio=1.0, 95% CL=0.5, 2.0; d.f.=35).
Figure 7.
Log concentration–response curves to [Lys5MeLeu9Nle10]NKA(4–10) in the absence and presence of atropine (0.3 μM), phentolamine (1 μM) and tetrodoxin (1 μM) on myometrial preparations from nonpregnant women. Data points are mean responses±s.e.m. and are expressed as a percentage of the response to KPSS (n=5–6).
Discussion
The present results demonstrate that tachykinins can induce contraction of uteri from nonpregnant women by stimulating smooth muscle NK2 receptors. PPT-A, PPT-B, and the three tachykinin receptors are expressed in the human uterus and their levels of expression show differential and high variation in tissue from pregnant and nonpregnant women. NKB is the predominant tachykinin expressed in the myometrium, with mRNA levels significantly higher in myometrium from nonpregnant than from pregnant women. These data are consistent with a role of tachykinins, and, in particular, NKB and the tachykinin NK2 receptor in the regulation of human uterine function.
Capsaicin-sensitive primary afferent neurones supplying the female urogenital tract (Traurig & Papka, 1993) are generally regarded as an important source of SP and NKA. Consistent with our molecular findings of the relatively sparse expression of PPT-A, a number of studies (Samuelson et al., 1985; Heinrich et al., 1986; Fried et al., 1990; Buttler-Manuel et al., 2002) indicate only a modest level of SP-like immunoreactivity in sensory neurones innervating uterus in the human. However, it should be noted that, in sensory neurones, the tachykinin precursor PPT-A is processed into SP/NKA in the cell soma; SP/NKA are then transported along the axon to sites of release in the nerve terminals (Maggi, 1997). This distribution of precursor PPT-A may also explain why in the nonpregnant myometrium the mRNA levels of the tachykinin precursor PPT-A we observed were low, since peripheral axons but not cell bodies are present.
The absence of PPT-A from myometrium from late-pregnant women may also reflect fetus-dependent sensory neurodegeneration or hormonal regulation of SP/NKA expression within uterine neurones or other cells. In the mammalian uterus, including that of the human, the uterine noradrenergic innervation degenerates as pregnancy progresses (Thorbert et al., 1979; Owman, 1981; Wikland et al., 1984). Alm & Lundberg (1988) also reported the absence of SP and NKA-like immunoreactivity in the late pregnant guinea-pig uterus. No clear data are yet available for the human uterus. However, there are species differences, since no comparable degeneration of the afferent innervation occurs in the rat uterus during pregnancy (Traurig et al., 1984). Indeed, Amira et al. (1995) have reported hypertrophy of the afferent innervation in the rat uterus during pregnancy.
The present molecular data show that PPT-B, the gene that encodes NKB, is expressed in the human uterus and indicate that NKB is the predominant tachykinin associated with the human uterus. However, PPT-B levels are about 30-fold higher in the myometrium from nonpregnant women, compared to that from pregnant women. These findings are reminiscent of those previously obtained in the rat uterus showing that PPT-B expression is regulated by ovarian steroids (Pinto et al., 2001). Since there are few, if any, examples of NKB expression in sensory nerve terminals (Maggi, 1997), it is highly probable that NKB may be synthesized and released from uterine non-neuronal cells.
The present molecular investigations strongly reinforce the idea that the tachykinin NK2 receptor is the predominant tachykinin receptor expressed in the human uterus. Our previous (Patak et al., 2000b) and present functional data strongly suggest that tachykinins, including NKB, most probably act at an NK2 receptor to cause contraction of the human uterus. The order of potency of the mammalian tachykinins (NKA>SP⩾NKB); the actions of receptor-selective agonists; and the potent antagonistic effect of the tachykinin NK2 receptor-selective antagonist, SR48968 (Emonds-Alt et al., 1992), all pointed towards the notion that the NK2 receptor mediates the uterotonic effects of tachykinins. These findings are similar to those in rat uterus (Fisher et al., 1993; Pennefather et al., 1993b; Fisher & Pennefather, 1997; Magraner et al., 1998), but are in contrast to the nonpregnant mouse in which NK1 receptors are important in mediating uterine contraction (Patak et al., 2002a).
The levels of the expression of the NK2 receptor are higher in the uterus of nonpregnant women compared to pregnant women. Consistent with this, the two most effective tachykinins, NKA and [Lys5MeLeu9Nle10]NKA(4–10), were each more potent in tissues from nonpregnant as compared to pregnant women (present data; Patak et al., 2000b). This suggests that changes in the steady-state mRNA levels were accompanied by changes in the amount of functional receptor protein. Our findings that the uterotonic action of [Lys5MeLeu9Nle10]NKA(4–10) was not blocked by tetrodotoxin, atropine or phentolamine strongly support the probability that the receptors are located on uterine smooth muscle rather than on autonomic nerves. The tachykinin NK1 and NK3 receptors are also expressed in human myometrium, but do not appear to be involved in mediating uterine contractility. This is particularly evident in the case of the tachykinin NK3 receptor, which virtually disappeared in the uteri obtained from pregnant women at term. This tachykinin NK3 receptor is mainly found in the central nervous system and is absent or present only in small amounts in peripheral tissues (Tsuchida et al., 1990). In good agreement with previous findings in the rat (Pinto et al., 1999; Candenas et al., 2001), the uterus represents one of the few human peripheral tissues that express the tachykinin NK3 receptor. The precise physiological role played by the tachykinin NK1 and NK3 receptors in the human uterus remains unknown. We have previously shown that the contractile effects induced by mammalian tachykinins in the uteri from pregnant women are enhanced by the inhibition of NEP by thiorphan (Patak et al., 2000b). Our present molecular data show that NEP mRNA levels were generally lower in nonpregnant myometrium than in the myometrium from pregnant women. Low expression of NEP could explain the inability of thiorphan to potentiate the contractile effects of NKA on tissues from nonpregnant women. The high interindividual variation in the levels of NEP mRNA in nonpregnant women that we observed is consistent with reports that NEP protein varied among different uterine diseases being very low or negative in women with cellular leiomyomas (McCluggage et al., 2001).
We suggested previously that an excitatory effect of tachykinins on the near-term gravid uterus could indicate a role for these peptides in the initiation of parturition, where the release of tachykinins could contribute to the cascade of events leading to labour (Patak et al., 2000b). The finding by Page et al. (2000) that the placenta also provides a source of NKB in late pregnancy seems consistent with this possibility. However, it is possible that the pregnancy-associated decreases in the level of uterine NKB precursor gene and of the NK2 receptor we have now observed may comprise, to the contrary, a regulatory effect that could contribute to uterine quiescence during normal pregnancy. The relatively high expression of the tachykinin degrading enzyme NEP in the uterus during late pregnancy could also contribute to this relative quiescence. It may, however, be that tachykinins may enhance contractility in the nonpregnant human uterus. Indeed, taken together, the high expression of PPT-B, and of tachykinin NK2 receptors, and the generally low expression of NEP in uterus from nonpregnant women raises the possibility that tachykinins including neurokinin B may be candidates for a pathophysiological role in painful inflammatory menstrual and menopausal uterine disorders. Whether any such actions might also be mediated by NK1 or NK3 receptors merit further investigation.
Acknowledgments
We are very grateful to Dr J.M. Loizaga and Dr M.T. Pereda, from Hospital Virgen del Rocío (Sevilla, Spain), for their invaluable help in the management of human samples. This work was supported in part by grants from NH&MRC to J.N. Pennefather and from the Royal Women's Hospital to M.E. Story. SR48968 was a generous gift from Dr Xavier Emonds-Alt.
Abbreviations
- HK-1
hemokinin-1
- KPSS
high potassium-containing modified physiological saline solution
- LUSCS
lower uterine segment caesarean section
- NEP
neprilysin
- NKA
neurokinin A
- NKB
neurokinin B
- PPT
preprotachykinin
- ΔRFU
relative fluorescence units
- SP
substance P
- SR48968
((S)-N-methyl-N[4-acetyl-amino-4-phenylpiperidino-2-(3,4-dichlorophenyl)butyl]benzamide)
References
- ALM P., ALUMETS J., BRODIN E., HAKANSON R., NILSSON G., SJOBERG N.O., SUNDLER F. Peptidergic (substance P) nerves in the genitourinary tract. Neuroscience. 1978;3:419–425. doi: 10.1016/0306-4522(78)90044-1. [DOI] [PubMed] [Google Scholar]
- ALM P., LUNDBERG L.M. Co-existence and origin of peptidergic and adrenergic nerves in the guinea pig uterus. Retrograde tracing and immunocytochemistry, effects of chemical sympathectomy, capsaicin treatment and pregnancy. Cell Tissue Res. 1988;254:517–530. doi: 10.1007/BF00226501. [DOI] [PubMed] [Google Scholar]
- AMIRA S., MORRISON J.F., RAYFIELD K.M. The effects of pregnancy and parturition on the substance P content of the rat uterus: uterine growth is accompanied by hypertrophy of its afferent innervation. Exp. Physiol. 1995;80:645–650. doi: 10.1113/expphysiol.1995.sp003874. [DOI] [PubMed] [Google Scholar]
- ARCK P.C., MERALI F.S., STANISZ A.M., STEAD R.H., CHAOUAT G., MANUEL J., CLARK D.A. Stress-induced murine abortion associated with substance P-dependent alteration in cytokines in maternal uterine decidua. Biol. Reprod. 1995;53:814–819. doi: 10.1095/biolreprod53.4.814. [DOI] [PubMed] [Google Scholar]
- BUTTLER-MANUEL S.A., BUTTERY L.D., A'HERN R.P., POLAK J.M., BARTON D.P. Pelvic nerve plexus trauma at radical and simple hysterectomy: a quantitative study of nerve types in the uterine supporting ligaments. J. Soc. Gynecol. Investig. 2002;9:47–56. doi: 10.1016/s1071-5576(01)00145-9. [DOI] [PubMed] [Google Scholar]
- CANDENAS M.L., MAGRANER J., ARMESTO C.P., ANSELMI E., NIETO P.M., MARTIN J.D., ADVENIER C., PINTO F.M. Changes in the expression of tachykinin receptors in the rat uterus during the course of pregnancy. Biol. Reprod. 2001;65:538–543. doi: 10.1095/biolreprod65.2.538. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidium thiocyanate–phenol–chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- COCCHIARA R., BONGIOVANNI A., ALBEGGIANI G., AZZOLINA A., GERACI D. Substance P selectively activates TNF-alpha mRNA in rat uterine immune cells: a neuroimmune link. NeuroReport. 1997;8:2961–2964. doi: 10.1097/00001756-199709080-00031. [DOI] [PubMed] [Google Scholar]
- DE FELIPE C., HERRERO J.F., O'BRIEN J.A., PALMER J.A., DOYLE C.A., SMITH A.J., LAIRD J.M., BELMONTE C., CERVERO F., HUNT S.P. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- EMONDS-ALT X., VILAIN P., GOULAOUIC P., PROIETTO V., VAN BROECK D., ADVENIER C., NALINE E., NELIAT G., LE FUR G., BRELIERE J.C. A potent and selective non-peptide antagonist of the neurokinin A (NK2) receptor. Life Sci. 1992;50:L101–L106. doi: 10.1016/0024-3205(92)90352-p. [DOI] [PubMed] [Google Scholar]
- FISHER L., PENNEFATHER J.N. Potencies of agonists acting at tachykinin receptors in the oestrogen-primed rat uterus: effects of peptidase inhibitors. Eur. J. Pharmacol. 1997;335:221–226. doi: 10.1016/s0014-2999(97)01229-6. [DOI] [PubMed] [Google Scholar]
- FISHER L., PENNEFATHER J.N., HALL S. Tachykinin receptors in the rat isolated uterus. Regul. Peptides. 1993;46:396–398. doi: 10.1016/0167-0115(93)90098-s. [DOI] [PubMed] [Google Scholar]
- FRIED G., MEISTER B., RADESTAD A. Peptide-containing nerves in the human pregnant uterine cervix: an immunohistochemical study exploring the effect of RU 486 (mifepristone) Hum. Reprod. 1990;5:870–876. doi: 10.1093/oxfordjournals.humrep.a137200. [DOI] [PubMed] [Google Scholar]
- GERARD N.P., EDDY R.L., Jr, SHOWS T.B., GERARD C. The human neurokinin A (substance K) receptor. Molecular cloning of the gene, chromosome localization, and isolation of cDNA from tracheal and gastric tissues. J. Biol. Chem. 1990;265:20455–20462. [PubMed] [Google Scholar]
- HARMAR A.J., ARMSTRONG A., PASCALL J.C., CHAPMAN K., ROSIE R., CURTIS A., GOING J., EDWARDS C.R., FINK G. cDNA sequence of human beta-preprotachykinin, the common precursor to substance P and neurokinin A. FEBS Lett. 1986;208:67–72. doi: 10.1016/0014-5793(86)81534-4. [DOI] [PubMed] [Google Scholar]
- HEAD J.R., MACDONALD P.C., CASEY M.L. Cellular localization of membrane metalloendopeptidase (enkephalinase) in human endometrium during the ovarian cycle. J. Clin. Endocrinol. Metab. 1993;76:769–776. doi: 10.1210/jcem.76.3.8445036. [DOI] [PubMed] [Google Scholar]
- HEINRICH D., REINECKE M., FORSSMANN W.G. Peptidergic innervation of the human and guinea pig uterus. Arch. Gynecol. 1986;237:213–219. doi: 10.1007/BF02133783. [DOI] [PubMed] [Google Scholar]
- HENRY J.L.Discussion of nomenclature for tachykinins and tachykinin receptors Substance P and Neurokinins. 1986New York: Springer-Verlag; xvii–xviii.ed. Henry, J.L., Couture, R., Cuello, A.C., Pelletier, G., Quirion, R. & Regoli, D. pp [Google Scholar]
- HO W.Z., LAI J.P., ZHU X.H., UVAYDOVA M., DOUGLAS S.D. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J. Immunol. 1997;159:5654–5660. [PubMed] [Google Scholar]
- HOLZER P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- HOOPER N.M., KENNY A.J., TURNER A.J. The metabolism of neuropeptides. Neurokinin A (substance K) is a substrate for endopeptidase-24.11 but not for peptidyl dipeptidase A (angiotensin-converting enzyme) Biochem. J. 1985;231:357–361. doi: 10.1042/bj2310357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOOPER N.M., TURNER A.J. Neurokinin B is hydrolysed by synaptic membranes and by endopeptidase-24.11 (enkephalinase) but not by angiotensin converting enzyme. FEBS Lett. 1985;190:133–136. doi: 10.1016/0014-5793(85)80443-9. [DOI] [PubMed] [Google Scholar]
- HUANG W.M., GU J., BLANK M.A., ALLEN J.M., BLOOM S.R., POLAK J.M. Peptide-immunoreactive nerves in the mammalian female genital tract. Histochem. J. 1984;16:1297–1310. doi: 10.1007/BF01003727. [DOI] [PubMed] [Google Scholar]
- JOACHIM R.A., HILDEBRANDT M., ODER J., KLAPP B.F., ARCK P.C. Murine stress-triggered abortion is mediated by increase of CD8+ TNF-alpha+ decidual cells via substance P. Am. J. Reprod. Immunol. 2001;45:303–309. doi: 10.1111/j.8755-8920.2001.450506.x. [DOI] [PubMed] [Google Scholar]
- JOOS G.F., PAUWELS R.A. Pro-inflammatory effects of substance P: new perspectives for the treatment of airway diseases. Trends Pharmacol. Sci. 2000;21:131–133. doi: 10.1016/s0165-6147(00)01458-9. [DOI] [PubMed] [Google Scholar]
- KURTZ M.M., WANG R., CLEMENTS M., CASCIERI M., AUSTIN C., CUNNINGHAM B., CHICCHI G., LIU Q. Identification, localization and receptor characterization of novel mammalian substance P-like peptides. Gene. 2002;296:205–222. doi: 10.1016/s0378-1119(02)00861-2. [DOI] [PubMed] [Google Scholar]
- LECCI A., GIULIANI S., TRAMONTANA M., CARINI F., MAGGI C.A. Peripheral actions of tachykinins. Neuropeptides. 2000;34:303–313. doi: 10.1054/npep.2000.0825. [DOI] [PubMed] [Google Scholar]
- LENTNER C.Introduction to statistics, statistical tables, mathematical formulae Geigy Scientific Tables. 19822Switzerland: Ciba-Geigy Ltd; 214ed. Lentner C. Vol8th edn. Bosle, p [Google Scholar]
- MAGGI C.A. Tachykinins as peripheral modulators of primary afferent nerves and visceral sensitivity. Pharmacol. Res. 1997;36:153–169. doi: 10.1006/phrs.1997.0219. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., MELI A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen. Pharmacol. 1988;19:1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- McCLUGGAGE W.G, SUMATHI V.P., MAXWELL P. CD10 is a sensitive and diagnostically useful immunohistochemical marker of normal endometrial stroma and of endometrial stromal neoplasms. Histopathology. 2001;39:273–278. doi: 10.1046/j.1365-2559.2001.01215.x. [DOI] [PubMed] [Google Scholar]
- MAGRANER J., PINTO F.M., ANSELMI E., HERNANDEZ M., PEREZ-AFONSO R., MARTIN J.D., ADVENIER C., CANDENAS M.L. Characterization of tachykinin receptors in the uterus of the oestrogen-primed rat. Br. J. Pharmacol. 1998;123:259–268. doi: 10.1038/sj.bjp.0701613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKERT U.R., ARCK P.C., McBEY B.A., MANUEL J., CROY B.A., MARSHALL J.S., CHAOUAT G., CLARK D.A. Stress triggered abortions are associated with alterations of granulated cells into the decidua. Am. J. Reprod. Immunol. 1997;37:94–100. doi: 10.1111/j.1600-0897.1997.tb00197.x. [DOI] [PubMed] [Google Scholar]
- MARX L., ARCK P., KIESLICH C., MITTERLECHNER S., KAPP M., DIETL J. Decidual mast cells might be involved in the onset of human first-trimester abortion. Am. J. Reprod. Immunol. 1999;41:34–40. doi: 10.1111/j.1600-0897.1999.tb00073.x. [DOI] [PubMed] [Google Scholar]
- MATSAS R., FULCHER I.S., KENNY A.J., TURNER A.J. Substance P and [Leu]enkephalin are hydrolyzed by an enzyme in pig caudate synaptic membranes that is identical with the endopeptidase of kidney microvilli. Proc. Natl. Acad. Sci. U.S.A. 1983;80:3111–3115. doi: 10.1073/pnas.80.10.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSAS R., KENNY A.J., TURNER A.J. The metabolism of neuropeptides. The hydrolysis of peptides, including enkephalins, tachykinins and their analogues, by endopeptidase-24.11. Biochem. J. 1984;223:433–440. doi: 10.1042/bj2230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLINA E., ZAPPIA L. In vitro activity of some natural and synthetic substances on non-pregnant human myometrium. Farm. Ed. Prat. 1976;31:329–336. [PubMed] [Google Scholar]
- NAKAJIMA-IIJIMA S., HAMADA H., REDDY P., KAKUNAGA T. Molecular structure of the human cytoplasmic beta actin gene: interspecies homology of sequences in the introns. Proc. Natl. Acad. Sci. U.S.A. 1985;82:6133–6137. doi: 10.1073/pnas.82.18.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTTESEN B., SONDERGAARD F., FAHRENKRUG J. Neuropeptides in the regulation of female genital smooth muscle contractility. Acta. Obstet. Gynecol. Scand. 1983;62:591–592. doi: 10.3109/00016348309156254. [DOI] [PubMed] [Google Scholar]
- OTTLECZ A., WALKER S., CONRAD M., STARCHER B. Neutral metalloendopeptidase associated with the smooth muscle cells of pregnant rat uterus. J. Cell. Biochem. 1991;45:401–411. doi: 10.1002/jcb.240450414. [DOI] [PubMed] [Google Scholar]
- OWMAN C. Pregnancy induces degenerative and regenerative changes in the autonomic innervation of the female reproductive tract. Ciba Found. Symp. 1981;83:252–279. doi: 10.1002/9780470720653.ch13. [DOI] [PubMed] [Google Scholar]
- PAGE N.M., WOODS R.J., GARDINER S.M., LOMTHAISONG K., GLADWELL R.T., BUTLIN D.J., MANYONDA I.T., LOWRY P.J. Excessive placental secretion of neurokinin B during the third trimester causes pre-eclampsia. Nature. 2000;405:797–800. doi: 10.1038/35015579. [DOI] [PubMed] [Google Scholar]
- PAPKA R.E., COTTON J.P., TRAURIG H.H. Comparative distribution of neuropeptide tyrosine-, vasoactive intestinal polypeptide-, substance P-immunoreactive, acetylcholinesterase-positive and noradrenergic nerves in the reproductive tract of the female rat. Cell Tissue Res. 1985;242:475–490. doi: 10.1007/BF00225412. [DOI] [PubMed] [Google Scholar]
- PAPKA R.E., SHEW R.L.Neural input to the uterus and influence on uterine contractility Control Of Uterine Contractility. 1994Boca Raton, FL: CRC Press; 375–399.ed. Garfield, R.E. & Tabb, T.N. pp [Google Scholar]
- PATAK E.N., PENNEFATHER J.N., STORY M.E. Effects of tachykinins on uterine smooth muscle. Clin. Exp. Pharmacol. Physiol. 2000a;27:922–927. doi: 10.1046/j.1440-1681.2000.03362.x. [DOI] [PubMed] [Google Scholar]
- PATAK E.N., PENNEFATHER J.N., FLEMING A., STORY M.E. Functional characterisation of tachykinin NK1 receptors in the mouse uterus. Br. J. Pharmacol. 2002a;137:1247–1254. doi: 10.1038/sj.bjp.0704996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATAK E., PENNEFATHER J.N., STORY M.E., PINTO F.M., CANDENAS M.L., ZICCONE S., LILLEY A. Tachykinin receptors in human myometrium. Placenta. 2002b;23:A37. [Google Scholar]
- PATAK E.N., ZICCONE S., STORY M.E., FLEMING A.J., LILLEY A., PENNEFATHER J.N. Activation of neurokinin NK(2) receptors by tachykinin peptides causes contraction of uterus in pregnant women near term. Mol. Hum. Reprod. 2000b;6:549–554. doi: 10.1093/molehr/6.6.549. [DOI] [PubMed] [Google Scholar]
- PENNEFATHER J.N., PAULL J.D., STORY M.E., ZICCONE S.P. Supersensitivity to the stimulant action of noradrenaline in human myometrium near term. Reprod. Fertil. Dev. 1993a;5:39–48. doi: 10.1071/rd9930039. [DOI] [PubMed] [Google Scholar]
- PENNEFATHER J.N., ZENG X.P., GOULD D., HALL S., BURCHER E. Mammalian tachykinins stimulate rat uterus by activating NK-2 receptors. Peptides. 1993b;14:169–174. doi: 10.1016/0196-9781(93)90025-c. [DOI] [PubMed] [Google Scholar]
- PINTO F.M., ARMESTO C.P., MAGRANER J., TRUJILLO M., MARTÍN J.D., CANDENAS M.L. Tachykinin receptor and neutral endopeptidase gene expression in the rat uterus: characterization and regulation in response to ovarian steroid treatment. Endocrinology. 1999;140:2526–2532. doi: 10.1210/endo.140.6.6695. [DOI] [PubMed] [Google Scholar]
- PINTO F.M., CANDENAS M.L., VENTURA S., PATAK E., ZICCONE S., LILLEY A., STORY M.E., PENNEFATHER J.N. Tachykinin expression in human uterus. Placenta. 2002;23:A37. [Google Scholar]
- PINTO F.M., CINTADO C.G., DEVILLIER P., CANDENAS M.L. Expression of preprotachykinin-B, the gene that encodes neurokinin B, in the rat uterus. Eur. J. Pharmacol. 2001;425:R1–R2. doi: 10.1016/s0014-2999(01)01186-4. [DOI] [PubMed] [Google Scholar]
- REGOLI D., BOUDON A., FAUCHERE J.L. Receptors and antagonists for substance P and related peptides. Pharmacol. Rev. 1994a;46:551–599. [PubMed] [Google Scholar]
- REGOLI D., NGUYEN Q.T., JUKIC D. Neurokinin receptor subtypes characterized by biological assays. Life Sci. 1994b;54:2035–2047. doi: 10.1016/0024-3205(94)00712-8. [DOI] [PubMed] [Google Scholar]
- REINECKE M., GAUWERKY J.F., SCHNEIDER K. Peptidergic (NPY, NT, VIP, SP, CGRP) innervation of the functional systems of the uterus and fallopian tube in the human. Arch. Gynecol. Obstet. 1989;245:399–401. doi: 10.1007/BF02417342. [DOI] [PubMed] [Google Scholar]
- RILEY S.C., WONG E., FINDLAY J.K., SALAMONSEN L.A. Localization of neutral endopeptidase in the ovine uterus and conceptus during the oestrous cycle and early pregnancy. Reprod. Fertil. Dev. 1995;7:27–33. doi: 10.1071/rd9950027. [DOI] [PubMed] [Google Scholar]
- ROZEN S., SKALETSKY H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- SAMUELSON U.E., DALSGAARD C.J., LUNDBERG J.M., HOKFELT T. Calcitonin gene-related peptide inhibits spontaneous contractions in human uterus and fallopian tube. Neurosci. Lett. 1985;62:225–230. doi: 10.1016/0304-3940(85)90359-3. [DOI] [PubMed] [Google Scholar]
- SHIPP M.A., RICHARDSON N.E., SAYRE P.H., BROWN N.R., MASTELLER E.L., CLAYTON L.K., RITZ J., REINHERZ E.L. Molecular cloning of the common acute lymphoblastic leukemia antigen (CALLA) identifies a type II integral membrane protein. Proc. Natl. Acad. Sci. U.S.A. 1988;85:4819–4823. doi: 10.1073/pnas.85.13.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STORY M.E., HALL S., ZICCONE S.P., PAULL J.D. Effects of adrenaline, isoprenaline and forskolin on pregnant human myometrial preparations. Clin. Exp. Pharmacol. Physiol. 1988;15:703–713. doi: 10.1111/j.1440-1681.1988.tb01130.x. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI K., TANAKA A., HARA M., NAKANISHI S. The primary structure and gene organization of human substance P and neuromedin K receptors. Eur. J. Biochem. 1992;204:1025–1033. doi: 10.1111/j.1432-1033.1992.tb16724.x. [DOI] [PubMed] [Google Scholar]
- TAKEDA Y., CHOU K.B., TAKEDA J., SACHAIS B.S., KRAUSE J.E. Molecular cloning, structural organization and functional expression of the human substance P receptor. Biochem. Biophys. Res. Commun. 1991;179:1232–1240. doi: 10.1016/0006-291x(91)91704-g. [DOI] [PubMed] [Google Scholar]
- THORBERT G., ALM P., BJORKLUND A.B., OWMAN C., SJOBERG N.O. Adrenergic innervation of the human uterus. Disappearance of the transmitter and transmitterforming enzymes during pregnancy. Am. J. Obstet. Gynecol. 1979;135:223–226. doi: 10.1016/0002-9378(79)90348-x. [DOI] [PubMed] [Google Scholar]
- TRAURIG H., PAPKA R.E.Autonomic efferent and visceral sensory innervation of the female reproductive system Nervous Control of the Urogenital System. 1993Switzerland: Harwood Academic Publishers; 423–466.ed. Maggi, C.A. pp [Google Scholar]
- TRAURIG H.H., PAPKA R.E., SHEW RL. Substance P and related peptides associated with the afferent and autonomic innervation of the uterus. Ann. N.Y. Acad. Sci. 1991;632:304–313. doi: 10.1111/j.1749-6632.1991.tb33118.x. [DOI] [PubMed] [Google Scholar]
- TRAURIG H., SARIA A., LEMBECK F. Substance P in primary afferent neurons of the female rat reproductive system. Naunyn-Schmiedebergs Arch. Pharmacol. 1984;326:343–346. doi: 10.1007/BF00501440. [DOI] [PubMed] [Google Scholar]
- TSUCHIDA K., SHIGEMOTO R., YOKOTA Y., NAKANISHI S. Tissue distribution and quantitation of the mRNAs for three rat tachykinin receptors. Eur. J. Biochem. 1990;193:751–757. doi: 10.1111/j.1432-1033.1990.tb19396.x. [DOI] [PubMed] [Google Scholar]
- WIKLAND M., LINDBLOM B., DAHLSTROM A., HAGLID K.G. Structural and functional evidence for the denervation of human myometrium during pregnancy. Obstet. Gynecol. 1984;64:503–509. [PubMed] [Google Scholar]
- ZHANG Y., LU L., FURLONGER C., WU G.E., PAIGE C.J. Hemokinin is a hematopoietic-specific tachykinin that regulates B lymphopoiesis. Nat. Immunol. 2000;1:392–397. doi: 10.1038/80826. [DOI] [PubMed] [Google Scholar]