Abstract
Three cannabinoid receptor agonists, anandamide (CB1 receptor-selective) and the aminoalkyl-indoles, JWH 015(2-methyl-1-propyl-1H-indol-3-yl)-1-napthalenylmethanone; (CB2 receptor-selective), R-(+)-WIN 55,212-2 (R-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolol[1,2,3-de]-1,4-benzoxazin-6-yl]-1-napthalenylmethanone; slightly CB2 receptor-selective), as well as the enantiomer S-(−)-WIN 55,212-3(S-(−)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolol[1,2,3-de]-1,4-benzoxazin-6-yl]-1-napthalenylmethanone; inactive at cannabinoid receptors), induced endothelium-independent relaxation of methoxamine-precontracted isolated small mesenteric artery of rat. KCL (60 mM) precontraction did not affect relaxation to the aminoalkylindoles, but reduced that to anandamide.
SR14176A (N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide; 3 μM; CB1 receptor antagonist) inhibited relaxation only to JWH 015 and anandamide. Neither AM 251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide; CB1 antagonist) nor SR 144528 (N-[(1S)-endo-1,3,3-trimethyl bicyclo[2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide; CB2 antagonist; both at 3 μM) affected any of the relaxations.
Vanilloid receptor desensitisation with capsaicin reduced anandamide relaxation; addition of SR 141716A (3 μM) then caused further inhibition. SR 141716A did not affect capsaicin-induced relaxation.
The aminoalkylindoles inhibited CaCl2-induced contractions in methoxamine-stimulated vessels previously depleted of intracellular Ca2+. These inhibitory effects were greatly reduced or abolished in ionomycin-(a calcium ionophore) contracted vessels. Anandamide also caused vanilloid receptor-independent, SR 141716A- (3 μM) insensitive, inhibition of CaCl2 contractions.
In conclusion, the aminoalkylindoles JWH 015, R-(+)-WIN 55,212-2 and S-(−)-WIN 55,212-3 relax rat small mesenteric artery mainly by inhibiting Ca2+ influx into vascular smooth muscle. Anandamide causes vasorelaxation by activating vanilloid receptors, but may also inhibit Ca2+ entry. Relaxation to JWH 015 and anandamide was sensitive to SR 141716A, but there is no other evidence for the involvement of CB1 or CB2 receptors in responses to these compounds.
Keywords: Mesenteric artery (rat), cannabinoids, anandamide, aminoalkylindoles, R-(+)-WIN 55212-2, JWH 015, cannabinoid receptor antagonists, SR 141716A, Ca2+ influx, ionomycin
Introduction
The endogenous cannabinoid anandamide (N-arachidonyl ethanolamide) is a vasorelaxant both in vivo and in vitro (see Hillard, 2000 for review). Activation of the cannabinoid CB1 receptors by anandamide is believed to mediate its hypotensive effects (Lake et al., 1997; Járai et al., 1999); however, recent reports indicate that anandamide might also modulate regional blood flow via multiple CB1 receptor-independent relaxant mechanisms. In particular, Zygmunt et al. (1999) demonstrated that anandamide is also an agonist of the vanilloid receptor (VR1), activation of which results in the release of CGRP, another vasorelaxant, from perivascular nerve endings. In isolated mesenteric arteries, anandamide-induced VR1 receptor activation probably underpins the endothelium-independent relaxation to anandamide (Zygmunt et al., 1999; White et al., 2001). However, there is evidence that a considerable component (50 – 60%) of anandamide-induced relaxation remains in rat mesenteric arteries pretreated with the VR1 agonist capsaicin, which causes functional desensitisation of the VR1 receptor system (White et al., 2001; Harris et al., 2002). While the relaxant mechanisms underlying this VR1- and endothelium-independent relaxation to anandamide are unknown, this observation emphasised the complex vasodilator actions of anandamide.
R-(+)-WIN55,212-2(R-(+)-[2,3-dihydro-5-methyl-3-(4-mor-pholinylmethyl)pyrrolol[1,2,3-de]-1,4-benzoxazin-6-yl]-1-nap-thalenylmethanone) is a potent CB1 and CB2 receptor agonist that is widely used to characterise cannabinoid receptors (Showalter et al., 1996; Griffin et al., 1998). Considering that this cannabinoid, which is inactive at VR1 receptors (Ross et al., 2001), has been shown to produce mesenteric vasorelaxation (White & Hiley, 1998a), the VR1-independent component of anandamide relaxations could be mediated by activation of cannabinoid receptors. However, R-(+)-WIN 55,212-2 was a much less effective vasorelaxant than expected from its affinities towards both CB1 and CB2 receptors and its relaxation was not affected by the CB1 receptor-selective antagonist SR 141716A(N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide) (White & Hiley, 1998a; Wagner et al., 1999). Interestingly, SR 141716A has been shown to attenuate anandamide relaxations, but this required higher concentrations of the antagonist than expected from its affinity for the classical CB1 receptor (White & Hiley, 1997; Zygmunt et al., 1997). Thus, although CB1 receptor mRNA and CB1-like immunoreactivity have been detected in rat mesenteric arteries (Darker et al., 1998), the involvement of cannabinoid receptors in relaxation to R-(+)-WIN 55,212-2 and anandamide remains unresolved. As a CB2-selective agonist, HU 308, has been shown to cause hypotension in vivo (Hanus et al., 1999), it is possible that CB2 or CB2-like receptors are involved in cannabinoid-induced mesenteric vasorelaxation.
In cat cerebral artery, relaxation to R-(+)-WIN 55,212-2 and anandamide appears to be the result of CB1-receptor-mediated inhibition of L-type, voltage-gated, Ca2+ channels (Gebremedhin et al., 1999). White & Hiley (1998a) also provided evidence that R-(+)-WIN 55,212-2 inhibited Ca2+ entry upon membrane depolarisation in rat mesenteric arteries. Ca2+ influx through L-type Ca2+ channels in vascular smooth muscle plays an important role in the contractile effects of many vasoconstrictors, including 5-hydroxytrptamine and noradrenaline (Criddle et al., 1996). However, it remains to be clarified if modulation of L-type Ca2+ channel activity contributes to the relaxant effects of cannabinoids, including R-(+)-WIN 55,212-2 and anandamide, in rat mesenteric artery.
In this study, relaxation to three aminoalkylindoles, R-(+)-WIN 55,212-2(R-(+)-[2,3-dihydro-5-methyl-3-(4-morpho-linylmethyl)pyrrolol[1,2,3-de]-1,4-benzoxazin-6-yl]-1-napthalenyl-methanone). S-(−)-WIN 55,212-3(S-(−)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolol[1,2,3-de]-1,4-benzoxazin-6-yl]-1-napthalenylmethanone) and JWH 015 (2-methyl-1-propyl-1H-indol-3-yl)-1-napthalenylmethanone) has been investigated in rat isolated small mesenteric artery. This defined group of compounds was chosen because R-(+)-WIN 55,212-2 and S-(−)-WIN 55,212-3 are enantiomers, with the former being a cannabinoid agonist, but the latter is reported to have no activity at CB1 or CB2 receptors (Felder et al., 1992; Slipetz et al., 1995), and JWH 015 is a cannabinoid showing higher CB2 receptor selectivity than R-(+)-WIN 55,212-2 (Showalter et al., 1996). In order to further investigate cannabinoid-induced mesenteric relaxation, cannabinoid receptor-selective antagonists in addition to SR 141716A were also used. The vasodilator actions of anandamide have also been compared to those of the aminoalkylindole cannabinoids and the role of Ca2+ influx in cannabinoid-induced relaxation investigated.
Methods
Myograph studies
Male Wistar rats (300–400 g; Tucks, Rayleigh, Essex or Charles River U.K. Ltd, Margate, Kent) were killed with an overdose of sodium pentobarbitone (120 mg kg−1, i.p., Sagatal, Rhone Mérieux, Harlow, Essex). Third-order branches of the superior mesenteric artery (250–400 μm) were removed and segments (2 mm) were mounted in a Mulvany–Halpern myograph (Model 500A, J.P. Trading, Aarhus, Denmark) containing gassed (95% O2/5% CO2) Krebs–Henseleit solution of the following composition (mM): NaCl 118, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, CaCl2 2.5, D-glucose 5.6 and indomethacin (10 μM), as described in White & Hiley (1997). After 30 min equilibration under zero tension in Krebs–Henseleit solution, vessels were normalised to a tension equivalent to that generated at 90% of the diameter of the vessel at 100 mmHg (Mulvany & Halpern, 1977). The vessels were left for another 30 min. The integrity of the endothelium was then assessed by precontracting the vessel with methoxamine (an α1-adrenoceptor agonist; 10 μM), followed by relaxation with carbachol (10 μM). Mesenteric arteries with greater than 90% relaxation to carbachol were designated as being endothelium-intact. In most experiments, endothelium was removed by rubbing the intima with a human hair and carbachol-induced relaxation of less than 10% indicated successful removal. As for previous studies, endothelial removal had no significant effect on methoxamine-induced tone in the same preparation (control: 14.2±0.4 mN; after removal of the endothelium: 14.1±0.4 mN; n=230).
Experiments in the presence of extracellular Ca2+
After the test for endothelial integrity, vessels were left for 30 min, and were then precontracted submaximally with methoxamine (10 μM), which allowed construction of cumulative concentration–relaxation curves to anandamide or an aminoalkylindole. Each preparation was only tested with a single agent.
To investigate relaxation mechanisms, cannabinoid antagonists, SR 141716A, AM 251 (N-(piperidin-1-yl)-5-(4-iodo-phenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide), and SR 144528 (N-[(1S)-endo-1,3,3-trimethyl bicyclo[2,2,1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide) or capsaicin were added to the myograph bath 30 min before, and were present during, construction of the concentration–response curve. Methoxamine-induced tone in any given preparation was not significantly different before and after these treatments. In experiments investigating the effects of the aminoalkylindoles or anandamide on voltage-gated Ca2+ channels, vessels were precontracted with high K+ (60 mM) Krebs–Henseleit solution, which was prepared by equimolar substitution of NaCl for KCl in the standard Krebs–Henseleit buffer described above. The mean tension generated by 60 mM KCl (15.0±0.8 mN) was similar to the tone induced by 10 μM methoxamine in the endothelial integrity test of the same vessels (15.9±0.8 mN; n=40).
In addition, vehicle controls were obtained by adding an appropriate volume of the vehicle (anandamide: water-dispersible emulsion; JWH 015: ethanol; R-(+)-WIN 55,212-2 or S-(−)-WIN 55,212-3: dimethyl sulphoxide) to methoxamine-precontracted vessels.
Ca2+-free experiments
Influx of extracellular Ca2+ through plasma membrane Ca2+ channels was examined in endothelium-denuded mesenteric arteries depleted of intracellular Ca2+ stores according to the methods described by White & Hiley (1998b). Briefly, extracellular Ca2+ was removed by washing vessels with Ca2+-free Krebs–Henseleit solution (composition the same as normal Krebs–Henseleit buffer, but with CaCl2 omitted). Ethylene glycol-bis (β-amino ethyl ether) tetraacetic acid (EGTA) (1 mM) was added to the organ bath, followed by a series of additions of 10 μM methoxamine in order to deplete the intracellular Ca2+ stores. Vessels were then washed with Ca2+-free Krebs–Henseleit solution, 10 μM methoxamine was added and a cumulative concentration–contraction curve to CaCl2 (10 μM–10 mM) was then obtained. As concentration–response curves to CaCl2 were found to be reproducible in any given vessel, after a control curve and the washing process, a second test curve was constructed in the presence of an aminoalkylindole or antagonist (all with 30 min preincubation).
When the effect of anandamide on CaCl2-induced contractions was examined, vessels were incubated with 10 μM capsaicin for 60 min in the presence of Ca2+ after the first control curve in order to avoid the involvement of anandamide-induced CGRP release through VR1 receptor activation, which requires extracellular Ca2+ (Maggi & Meli, 1988). The process of washing with Ca2+-free Krebs–Henseleit solution was then repeated and followed by construction of the second test curve in the presence of anandamide (with 30 min preincubation). Note that, in these experiments, capsaicin was incubated for 60 min as our previous study (White et al., 2001) showed that incubation with 10 μM capsaicin for 60 min followed by the washing-out process produced similar inhibitory effects on VR1 receptor-mediated relaxation as compared to 30 min incubation of capsaicin without washing.
The involvement of cannabinoid receptors was investigated by observing the effect of the presence of a cannabinoid antagonist (with 30 min preincubation) before the addition of an aminoalkylindole or anandamide. Contractions were expressed as a percentage of the maximum contraction induced by CaCl2 in the presence of 10 μM methoxamine alone.
To further examine the roles of voltage-gated Ca2+ channels, ionomycin (a Ca2+ ionophore) was used to facilitate Ca2+ entry. However, as the effects of ionomycin are irreversible, a modified protocol was used. Endothelium-denuded mesenteric arteries were depleted of intracellular Ca2+ stores as described above, and this was followed by incubation with 1 μM ionomycin for 30 min. Contraction was then induced by readmission of 2.5 mM CaCl2 and responses to an aminoalkylindole were recorded. For comparison, the relaxant effects of these agents in vessels stimulated with methoxamine (10 μM) under similar conditions were also determined. Effects of vehicle (dimethyl sulphoxide or ethanol) were also tested in ionomycin- and methoxamine-stimulated vessels.
Data and statistical analysis
All relaxation responses are expressed as the percentage relaxation of the tone induced by 10 μM methoxamine, 60 mM KCl or 1 μM ionomycin. Values are given as mean±s.e.m. and n represents the number of rats. As it was not usually possible to fully define concentration–response curves (solubility limitations prevented use of high enough concentrations to determine the maximum responses), potency is expressed as pEC50% (the negative logarithm of the concentration giving 50% relaxation of the induced tone); these values were determined directly from individual log concentration–response curves. Statistical analysis was performed by two-way analysis of variance, unless otherwise stated, of the entire concentration–response curves, followed by Bonferroni post hoc tests. For contractile responses to cumulative addition of CaCl2, data are expressed as percentage of the maximum contraction to CaCl2 in the presence of 10 μM methoxamine alone. Statistical comparison was performed by Student's paired t-test at individual concentrations. In some cases, Student's unpaired t-test was also used to make comparisons between treatment groups. Contractile responses to a single addition of 2.5 mM CaCl2 in the presence of methoxamine and ionomycin were also compared by Student's unpaired t-test. P-values of less than 0.05 were taken as statistically significant.
Drugs
Methoxamine hydrochloride and carbachol (Sigma Chemical Company, Poole, Dorset, U.K.) were dissolved in deionised water. Indomethacin (Sigma) was dissolved in 5% w v−1 NaHCO3 solution. Capsaicin (Sigma), JWH 015 (Tocris Cookson, Bristol, U.K.), SR 141716A, SR 144528, kind gifts from Sanofi-Synthélabo, France), were dissolved in 100% ethanol. Anandamide (Tocris) was supplied as a water-dispersible emulsion. R-(+)-WIN 55,212-2, S-(−)-WIN 55,212-3, ionomycin (Sigma) and AM 251 (Tocris) were dissolved in 100% dimethyl sulphoxide. EGTA (Sigma) was dissolved in Ca2+-free Krebs–Henseleit solution.
Results
Effect of endothelial removal on vasodilator actions of anandamide and the aminoalkylindoles
Anandamide, JWH 015 and R-(+)-WIN 55,212-2 (in descending order of potency) produced concentration-dependent relaxation of endothelium-intact, methoxamine-precontracted, rat isolated mesenteric arteries (P<0.01; Table 1). Removal of the endothelium had no significant effect on relaxations to any of these cannabinoids (Table 1). The enantiomer of R-(+)-WIN 55,212-2, S-(−)-WIN 55,212-3, also produced endothelium-independent relaxation with pEC50% values similar to those for R-(+)-WIN 55,212-2 (Table 1). The limited solubility of JWH 015, R-(+)-WIN 55,212-2 and S-(−)-WIN 55,212-3 in aqueous medium prevented the unequivocal determination of a maximum response; values given are those relaxations obtained at the highest concentrations that could be used. In view of the lack of influence of the endothelium, all subsequent experiments were performed in vessels denuded of the endothelium.
Table 1.
Relaxation to anandamide, JWH 015, R-(+)-WIN 55,212-2 and S-(−)-WIN 55,212-3 in endothelium-intact or -denuded rat isolated small mesenteric arteries
| Cannabinoid | Endothelium-intact | Endothelium-denuded | ||||
|---|---|---|---|---|---|---|
| pEC50% | % Relaxation (at concentration) | n | pEC50% | % Relaxation (at concentration) | n | |
| Anandamide | 6.7±0.1 | 93±2 (1 μM) | 6 | 6.7±0.1 | 90±5 (1 μM) | 7 |
| JWH 015 | 5.3±0.1 | 90±1 (30 μM) | 4 | 5.2±0.2 | 93±5 (30 μM) | 4 |
| R-(+)-WIN 55,212-2 | 4.9±0.1 | 87±2 (60 μM) | 3 | 4.7±0.1 | 78±4 (60 μM) | 5 |
| S-(−)-WIN 55,212-3 | 4.9±0.1 | 89±2 (60 μM) | 4 | 4.9±0.1 | 83±3 (60 μM) | 3 |
All vessels were precontracted with methoxamine. The concentrations given in parentheses are the highest that were used for a given compound. Data are expressed as mean±s.e.m. pEC50% values were obtained directly from the individual log concentration response curves. n indicates the number of animals used. No significant differences between relaxation in denuded and intact vessels were found.
The vehicle for anandamide (a water-dispersible emulsion; n=4) had no effect on methoxamine-induced tone, while the vehicles for JWH 015 (ethanol; n=6) and R-(+)-WIN 55,212-2 or S-(−)-WIN 55,212-3 (dimethyl sulphoxide; n=6) caused, respectively, 11±6 and 26±8% relaxation of the precontraction tone in the greatest volumes used.
Relaxation to aminoalkylindole compounds under 60 mM KCl-induced tone
Precontracting vessels with 60 mM KCl, instead of methoxamine, had no effect on relaxation to JWH 015 in endothelium-denuded vessels (methoxamine: pEC50%=5.5±0.1, relaxation at 30 μM=93±3%, n=6; KCl: pEC50%=5.6±0.1relaxation at 30 μM=92±3%, n=6).
Similarly, R-(+)-WIN 55,212-2 responses were not changed by precontraction with 60 mM KCl (methoxamine: pEC50%=4.7±0.1, relaxation at 60 μM=76±4%, n=6; KCl: pEC50=4.7±0.1, relaxation at 60 μM=74±6%, n=6). The same was also true for its enantiomer S-(−)-WIN 55,212-3 (methoxamine: pEC50%=4.8±0.1, relaxation at 60 μM=85±3%, n=6; KCl: pEC50%=4.9±0.1, relaxation at 60 μM=90±2%, n=4).
Effects of cannabinoid receptor antagonists on relaxation to JWH 015 in methoxamine- or KCl-precontracted vessels
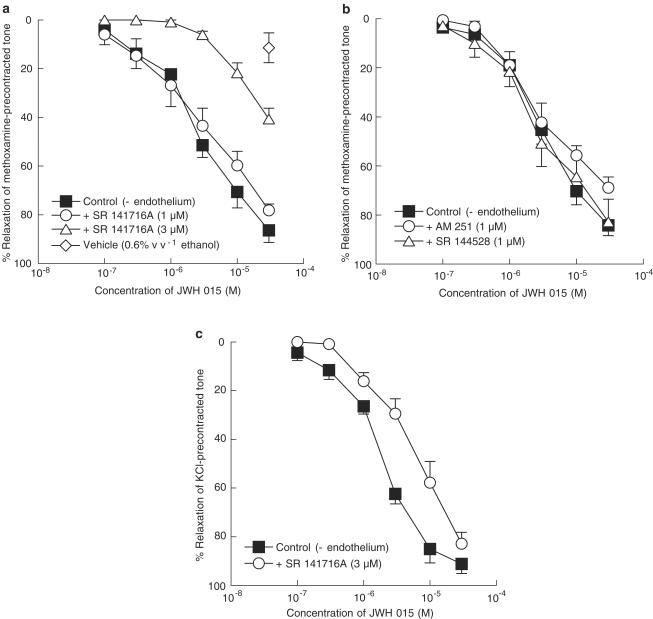
The cannabinoid CB1 receptor antagonist SR 141716A, at 1 μM, had no significant effect on relaxation induced by JWH 015 in endothelium-denuded vessels (control: pEC50%=5.4±0.1, n=8; 1 μM SR 141716A: pEC50%=5.5±0.2, n=4; Figure 1a). However, increasing the concentration of SR 141716A to 3 μM significantly inhibited (P<0.01) relaxations to JWH 015 and resulted in an apparent rightward shift of the concentration–response curve (Figure 1a). The effect of 0.6% v v−1 ethanol (vehicle for JWH 015; final concentration in myograph bath) is also shown in Figure 1a. Figure 1b shows that neither another CB1 receptor antagonist, AM 251 (1 μM) nor the CB2 receptor antagonist SR 144528 (1 μM) had any significant effect on relaxation to JWH 015 (control: pEC50%=5.6±0.2, n=9; AM 251: pEC50%=5.2±0.2, n=5; SR 144528: pEC50%=5.4±0.2, n=4). Increasing the concentrations of AM 251 or SR 144528 to 3 μM also revealed no inhibitory effect (control: pEC50%=5.7±0.1, relaxation at 30 μM=92±2%; AM 251: pEC50%=5.9±0.1, relaxation at 30 μM=85±2%; SR 144528: pEC50%=5.8±0.2, relaxation at 30 μM=96±1%, n=4–6).
Figure 1.
Concentration–response curves for JWH 015-induced relaxation of endothelium-denuded rat isolated mesenteric artery. (a) Relaxation was elicited by JWH 015 alone (n=8), and in the presence of 1 or 3 μM SR 141716A (n=4 for both), in methoxamine-induced tone. The effect of 0.6% v v−1 ethanol (the vehicle for JWH 015 at the highest concentration used) is also shown. n=6. (b) Relaxation was elicited by JWH 015 alone (n=9), and in the presence of 1 μM AM 251 (n=5) or 1 μM SR 144528 (n=4), in methoxamine-induced tone. (c) Relaxation was elicited by JWH 015 alone, and in the presence of 3 μM SR 141716A, in 60 mM KCl-induced tone, n=4 for both. Values are shown as means and vertical lines represent s.e.m.
Using KCl to induce tone, 3 μM SR 141716A caused an approximate four-fold rightward shift of the concentration–response curve for JWH 015 (control: pEC50%=5.7±0.1, n=4; SR 141716A: pEC50%=5.1±0.1, n=4; P<0.01; Figure 1c).
Effects of antagonists on relaxation to R-(+)-WIN 55,212-2 and S-(−)-WIN 55,212-3 in methoxamine-precontracted vessels
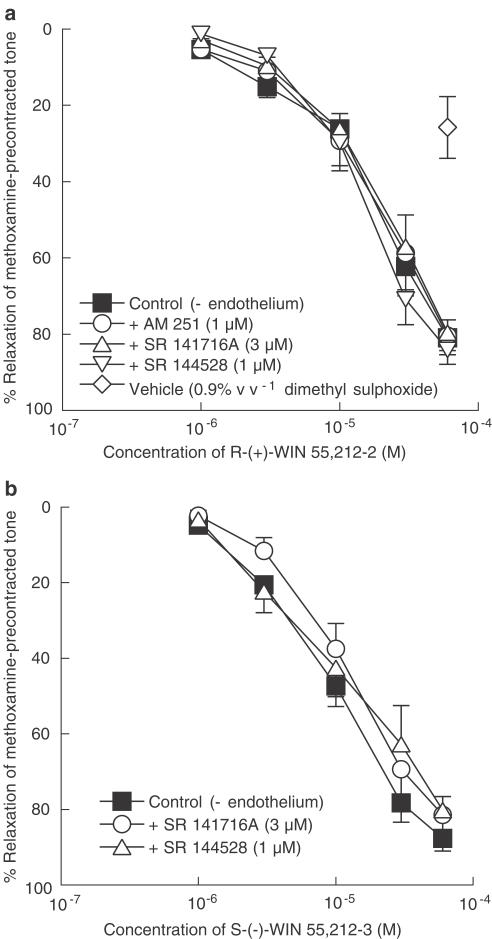
Relaxation to R-(+)-WIN 55,212-2 in endothelium-denuded vessels was not affected by the presence of any of the cannabinoid receptor antagonists, SR 141716A (3 μM), AM 251 (1 μM) or SR 144528 (1 μM) (control: pEC50%=4.7±0.1, n=7; SR 141716A: pEC50%=4.7±0.1, n=4; AM 251: pEC50%=4.6±0.1, n=5; SR 144528: pEC50%=4.8±0.1, n=4 for all; Figure 2a). The effect of 0.9% v v−1 dimethyl sulphoxide (vehicle for R-(+)-WIN 55,212-2 and S-(−)-WIN 55,212-3; final concentration in myograph bath) is also shown in Figure 2a. As in the case for JWH 015, increasing the concentration of AM 251 or SR 144528 to 3 μM revealed no inhibitory effect on relaxations to R-(+)-WIN 55212-2 (control: pEC50%=4.9±0.1, relaxation at 60 μM=90±4%; AM 251: pEC50%=4.8±0.1, relaxation at 60 μM=86±3%; SR 144528: pEC50%=5.0±0.1, relaxation at 60 μM=90±2%; n=5–6).
Figure 2.
Concentration–response curves for relaxation of endo-thelium-denuded rat isolated mesenteric artery. (a) Relaxation was elicited by R-(+)-WIN 55,212-2 alone, and in the presence of 3 μM SR 141716A, 1 μM AM 251 or 1 μM SR 144528, in methoxamine-induced tone, n=4–7. The effect of 0.9% v v−1 dimethyl sulphoxide (the vehicle for R-(+)-WIN 55,212-2 and S-(−)-WIN 55,212-3 at the highest concentration used) is also shown. n=6. (b) Relaxation was elicited by S-(−)-WIN 55,212-3 alone, and in the presence of 3 μM SR 141716A or 1 μM SR 144528, in methoxamine-induced tone. n=4–6. Values are shown as means and vertical lines represent s.e.m.
Similarly, the enantiomer S-(−)-WIN 55,212-3 also caused endothelium-independent relaxation in methoxamine-precontracted vessels that was insensitive to either of the cannabinoid receptor antagonists SR 141716A (3 μM) or SR 144528 (1 μM) (control: pEC50%=4.9±0.1; SR 141716A: pEC50%=5.0±0.2; SR 144528: pEC50%=4.8±0.2; n=4–6; Figure 2b). SR 144528, at 3 μM, also had no effect on relaxation to S-(−)-WIN 55,212-3 (control: pEC50%=5.1±0.1, relaxation at 60 μM=91±2%, n=6; SR 144528: pEC50%=5.1±0.1, relaxation at 60 μM=94±1%, n=6).
Effects of aminoalkylindole compounds on CaCl2 contractions induced in the presence of methoxamine
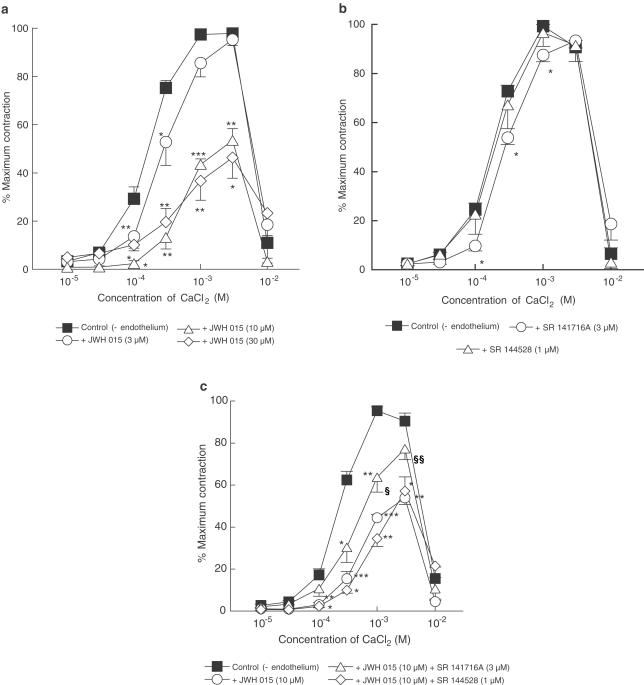
All experiments were performed in endothelium-denuded vessels depleted of intracellular Ca2+ and then exposed to methoxamine (10 μM) in the absence of extracellular Ca2+. Under these conditions, addition of CaCl2 (0.01–10 mM) caused concentration-dependent contractions up to 1–3 mM and produced relaxation at higher concentrations. JWH 015, at 3 μM only had a slight inhibitory effect on responses to CaCl2, but increasing its concentration to 10 and 30 μM inhibited maximal contractions to CaCl2 by approximately 50% (Figure 3a). Whereas 3 μM SR 141716A (CB1 receptor antagonist) alone slightly inhibited the contraction to CaCl2 (Figure 3b), it partially reversed the inhibitory effect of 10 μM JWH 015 (Figure 3c). However, SR 141716A (3 μM) had no significant effect on the inhibitory effect of 30 μM JWH 015 (CaCl2 contractions at 1 mM: control response in the presence of 30 μM JWH 015, 37±8%; with 30 μM JWH 015 and SR 141716A, 32±13%; CaCl2 contractions at 3 mM: in the presence of 30 μM JWH 015, 46±9%; with 30 μM JWH 015 and SR 141716A, 28±7%; n=4 for all). On the other hand, the CB2 receptor antagonist SR 144528 (1 μM) did not affect either the control contractions to CaCl2 or those obtained in the presence of 10 μM JWH 015 (Figure 3b,c).
Figure 3.
Concentration–response curves for CaCl2-induced contractions of methoxamine-stimulated rat isolated mesenteric arteries depleted of intracellular Ca2+ stores with EGTA as described in Methods. All vessels were denuded of endothelium. (a) Responses to CaCl2 in the absence (n=12) or presence of 3, 10 or 30 μM JWH 015 (n=4 each) in a given vessel. (b) Responses to CaCl2 in the absence (n=10) and presence of SR 141716A (3 μM; n=4) or SR 144528 (1 μM; n=6) in a given vessel. (c) Responses to CaCl2 in the absence (n=14) and presence of 10 μM JWH 015, or of 10 μM JWH 015 plus either 3 μM SR 141716A or 1 μM SR 144528 in a given vessel. n=4–6. In all panels, control curves were pooled for clarity and values are shown as means and vertical lines represent s.e.m. Statistical comparisons were made by Student's paired t-test at individual concentrations. *P<0.05, **P<0.01, ***P<0.001 show significant differences from control. In (c), statistical comparisons of CaCl2 responses in the presence of JWH 015 and JWH 015 plus a cannabinoid antagonist were also made by Student's unpaired t-test. §P<0.05, §§P<0.01 show significant differences from responses to CaCl2 in the presence of JWH 015.
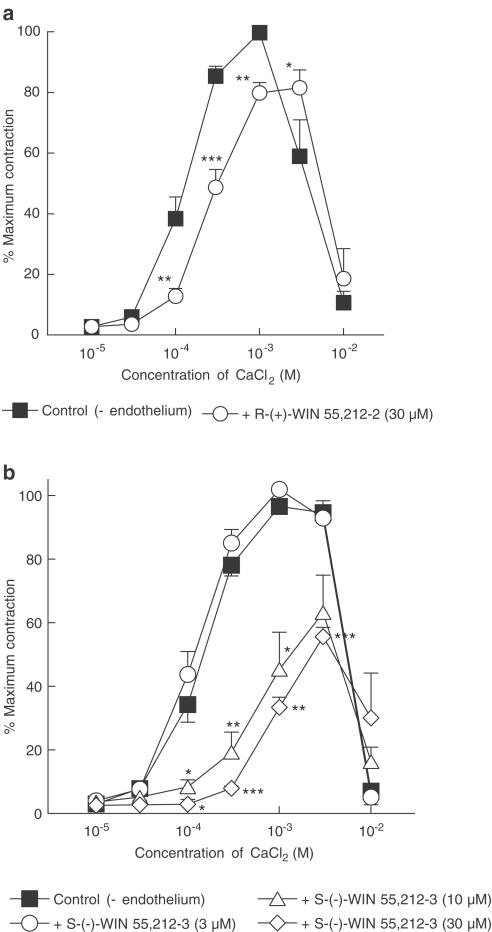
R-(+)-WIN 55,212-2 (30 μM) also significantly inhibited CaCl2-induced contractions (approximately 20% reduction in maximal CaCl2-induced contraction; Figure 4a). Its enantiomer, S-(−)-WIN 55,212-3 (at 10 and 30 μM but not 3 μM) also reduced the maximal contractions to CaCl2 by approximately 40% (Figure 4b). The presence of SR 141716A (3 μM) or SR 144528 (1 μM) did not reverse the inhibitory effects of R-(+)-WIN 55,212-2 (30 μM) or S-(−)-WIN 55,212-3 (10 μM) (data not shown).
Figure 4.
Concentration–response curves for CaCl2-induced contractions of methoxamine-stimulated rat isolated mesenteric arteries depleted of intracellular Ca2+ stores with EGTA as described in Methods. All vessels were denuded of endothelium. (a) Contractions to CaCl2 were determined in the absence and presence of 30 μM R-(+)-WIN 55,212-2 in a given vessel. n=6. (b) Contractions to CaCl2 were determined in the absence (n=12) and presence of 3, 10 or 30 μM S-(−)-WIN 55,212-3 in a given vessel. n=4 each. In both figures, control curves were pooled for clarity and values are shown as means and vertical lines represent s.e.m. Statistical comparisons were made by Student's paired t-test at individual concentrations. *P<0.05, **P<0.01, ***P<0.001 indicate significant differences from control values.
None of the aminoalkylindole compounds or the antagonists had any obvious effects on the relaxation to high concentrations of CaCl2. In addition, the vehicles (dimethyl sulphoxide or ethanol; 0.3% v v−1 final concentration in myograph bath for both) alone also had no effect on responses to CaCl2 (data not shown).
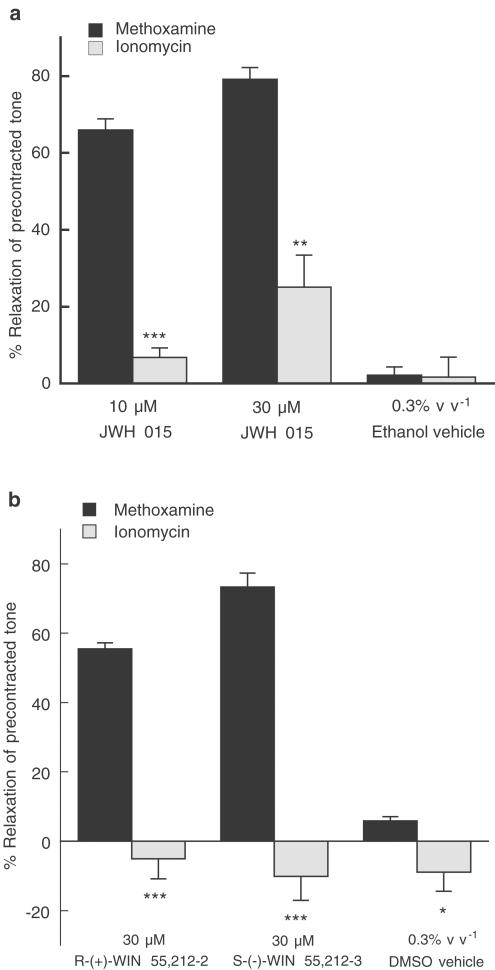
Effects of aminoalkylindole compounds on CaCl2 contractions induced in the presence of ionomycin
All vessels were denuded of endothelium, depleted of intracellular Ca2+ stores and initially maintained in the absence of extracellular Ca2+ as described in Methods. Preliminary experiments demonstrated that the L-type, voltage-gated, Ca2+ channel blocker verapamil (10 μM) had almost no effect on ionomycin-evoked contractions under the present protocol (reduction in tone: 4.3±2.2%; n=8), while the same concentration of verapamil abolished methoxamine-induced contraction (tension generated by 10 μM methoxamine: 20.5±2.6 mN; with verapamil: 0.7±0.1 mN; n=3). Figure 5a shows that the relaxant effects of JWH 015 (10 and 30 μM) were greatly reduced when the Ca2+ ionophore ionomycin (1 μM), instead of methoxamine (10 μM), was used to allow Ca2+ entry upon readmission of 2.5 mM CaCl2. The ethanol vehicle of JWH 015 (0.3% v v−1 final concentration in myograph bath) had very little effect in vessels stimulated with either methoxamine or ionomycin (Figure 5a).
Figure 5.
Cannabinoid-induced relaxation of contractions to 2.5 mM CaCl2 in ionomycin- or methoxamine-stimulated rat isolated mesenteric artery in the absence of extracellular Ca2+. All vessels were denuded of endothelium and depleted of intracellular Ca2+ stores with EGTA as described in Methods. (a) Relaxation was elicited by 10, 30 μM JWH 015 and 0.3% v v−1 ethanol (the vehicle for JWH 015). n=4–7. (b) Relaxation was elicited by 30 μM R-(+)-WIN 55,212-2, 30 μM S-(−)-WIN 55,212-3 and 0.3% v v−1 dimethyl sulphoxide (DMSO; the vehicle for both aminoalkylindoles). n=4–7. In both figures, values are shown as means and vertical lines represent s.e.m. Statistical comparisons were made by Student's unpaired t-test at individual concentrations. *P<0.05, **P<0.01, ***P<0.001 indicate significant differences from the corresponding values using methoxamine.
Similarly, the relaxation to R-(+)-WIN 55,212-2 (30 μM) and S-(−)-WIN 55,212-3 (30 μM) observed in methoxamine-stimulated vessels was absent in ionomycin-stimulated vessels (P<0.001, Figure 5b). In fact, under ionomycin-stimulated tone, these cannabinoids produced small contractions that were similar to that induced by their vehicle dimethyl sulphoxide (0.3% v v−1 final concentration in myograph bath; Figure 5b).
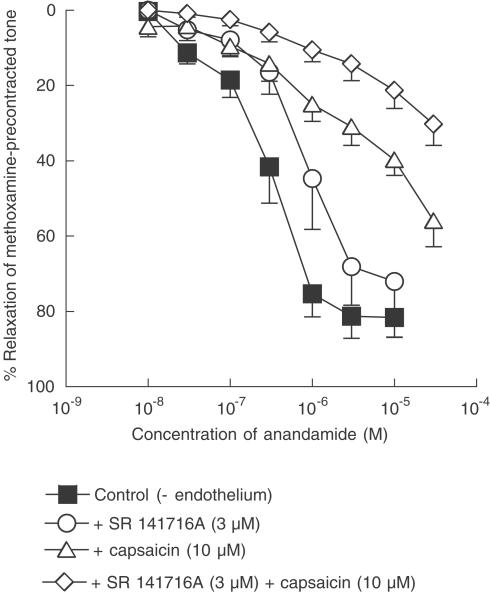
Effects of capsaicin pretreatment and cannabinoid receptor antagonists on relaxation to anandamide
Capsaicin pretreatment (10 μM for 30 min), which causes CGRP release by means of activation of the vanilloid receptor and results in functional desensitisation of the receptor system, inhibited relaxation to anandamide (P<0.01; Figure 6) and shifted the concentration – response curve to the right in a nonparallel manner. The control pEC50% was 6.4±0.1 (n=7) but, in the presence of capsaicin, a 50% relaxation was not always obtained, even at 30 μM (the mean response at this concentration was 56±7%, n=4), and a pEC50% could not be calculated. In contrast, the presence of the cannabinoid CB1 receptor antagonist SR 141716A (3 μM) alone caused an essentially parallel rightward displacement of the concentration–response curve for anandamide of approximately three-fold (SR 141716A: pEC50%=6.0±0.1, n=5; P<0.01; Figure 6). Importantly, addition of 3 μM SR 141716A to 10 μM capsaicin-pretreated vessels caused further inhibition of anandamide responses and, here, a 50% relaxation was not observed and the maximum relaxation at 30 μM anandamide was 30±6% (n=5; significance from capsaicin alone, P<0.01; Figure 6).
Figure 6.
Concentration–response curves for anandamide-induced relaxation of endothelium-denuded rat isolated mesenteric artery. (a) Relaxation was elicited by anandamide alone or in the presence of 3 μM SR 141716A, 10 μM capsaicin or 3 μM SR 141716A plus 10 μM capsaicin. n=4–7. Values are shown as means and vertical lines represent s.e.m.
A contrasting result was obtained with another CB1 receptor antagonist AM 251 (1 μM), which had no significant effect on relaxation to anandamide (control: pEC50%=6.6±0.1, relaxation at 10 μM=90±3%; n=8; AM 251: pEC50%=6.5±0.2, relaxation at 10 μM=86±5%; n=8). Increasing the concentration of AM 251 to 3 μM did not reveal an inhibitory effect (control: pEC50%=6.9±0.3, relaxation at 1 μM=93±2%; n=4; AM 251: pEC50%=7.1±0.2, relaxation at 1 μM=90±7%; n=4). Anandamide-induced relaxation (pEC50%=7.1±0.1, relaxation at 1 μM=92±2%; n=9) was also unaffected by the CB2 receptor antagonist SR 144528 (1 μM SR 144528: pEC50%=7.0±0.1, relaxation at 1 μM=98±1%; n=4; 3 μM SR 144528: pEC50%=6.9+0.1, relaxation at 1 μM=97±1%; n=6).
Effects of capsaicin pretreatment on relaxation to the aminoalkylindoles
Capsaicin pretreatment (10 μM for 30 min) caused a slight potentiation (P<0.01) of relaxation to JWH 015 (control: pEC50%=5.2±0.1, relaxation at 30 μM=81±2%; capsaicin: pEC50%=5.4±0.1, relaxation at 30 μM=90±2%; n=4 for both). Similarly, responses to R-(+)-WIN 55,212-2 (pEC50%=4.8±0.1, relaxation at 60 μM=86±6%; n=3) were potentiated (P<0.01) after capsaicin pretreatment (10 μM capsaicin for 30 min: pEC50%=5.0±0.1, relaxation at 60 μM=89±2%; n=4). Relaxation to S-(−)-WIN 55,212-3 was not significantly affected by capsaicin pretreatment (data not shown).
Effects of SR 141716A and 60 mM KCl precontraction on relaxation to anandamide and capsaicin
Unlike the aminoalkylindole compounds, anandamide-induced relaxation was greatly reduced in vessels precontracted with 60 mM KCl (methoxamine: pEC50%=6.5±0.1, relaxation at 10 μM=96±2%; n=4; KCl: relaxation at 10 μM=29±10%; n=6; P<0.01). Also, as opposed to the results with JWH 015, relaxation to anandamide under KCl-induced tone was not significantly affected by the presence of 3 μM SR 141716A (KCl-induced tone with SR 141716A: relaxation at 10 μM=26±7%; n=4).
In contrast to anandamide responses, capsaicin-induced relaxation was inhibited under 60 mM KCl-induced tone but not significantly affected by the presence of 3 μM SR 141716A (methoxamine-induced tone: pEC50%=5.3±0.1, relaxation at 100 μM=96±1%; n=6; KCl-induced tone: pEC50%=4.7±0.1, relaxation at 100 μM=89±2%; n=6; P<0.01; methoxamine-induced tone with SR 141716A: pEC50%=5.1±0.1, relaxation at 100 μM=95±1%; n=4).
Effects of anandamide and capsaicin on CaCl2 contractions induced in the presence of methoxamine
Capsaicin pretreatment (10 μM for 60 min) alone had no significant effect on CaCl2-induced contractions or relaxations (Figure 7). In capsaicin-pretreated vessels, anandamide at 10 μM, but not 1 μM, reduced contractions to CaCl2 (Figure 7). This inhibitory effect of anandamide (10 μM) was not affected by 3 μM SR 141716A (data not shown).
Figure 7.
Concentration–response curves for CaCl2-induced contractions of methoxamine-stimulated rat-isolated mesenteric arteries depleted of intracellular Ca2+ stores with EGTA as described in Methods. All vessels were denuded of endothelium. Control responses to CaCl2 were determined in all vessels (n=12), then responses to CaCl2 were recorded after capsaicin pretreatment alone or after capsaicin treatment and in the presence of either 1 or 10 μM anandamide (n=4 each). Control curves were pooled for clarity and values are shown as means and vertical lines represent s.e.m. Statistical comparisons were made by Student's paired t-test at individual concentrations. *P<0.05, **P<0.01 indicate significant differences from control values.
Discussion
This study shows that the aminoalkylindole compounds, JWH 015, R-(+)-WIN 55,212-2 and S-(−)-WIN 55,212-3, all caused endothelium-independent relaxation of rat isolated small mesenteric artery notwithstanding the fact that S-(−)-WIN 55,212-3 is generally considered to be inactive at CB1 and CB2 receptors. The relaxation appears to be explicable in terms of the agents reducing Ca2+ entry into the vascular smooth muscle. The endogenous cannabinoid anandamide also affects Ca2+ entry into smooth muscle cells, although this appears to have a lesser role in the relaxation because of the action of anandamide in activating vanilloid receptors. Although responses to JWH 015 and anandamide were sensitive to the cannabinoid CB1 receptor antagonist SR 141716A, there is no other substantial evidence for the involvement of the classical CB1 and CB2 receptors in the vasorelaxation evoked by the agents in the rat isolated small mesenteric artery.
If it is accepted that the responses to the aminoalkylindoles are mediated through actions at a single target, then our results strongly suggest the absence of a functional role for ‘classical' CB1 and CB2 receptors in the relaxation response of the rat small mesenteric artery. Firstly, S-(−)-WIN 55,212-3, an enantiomer of R-(+)-WIN 55,212-2 that shows no activity at CB1 or CB2 receptors (Felder et al., 1992; Slipetz et al., 1995), produced endothelium-independent relaxation and was at least as potent as R-(+)-WIN 55,212-2. Secondly, relaxation to both R-(+)-WIN 55,212-2 and S-(−)-WIN 55,212-3 was not affected by the presence of the CB1 receptor-selective antagonist SR 141716A (3 μM) or the CB2 receptor-selective antagonist SR 144528 (1 or 3 μM), even though these antagonists were used at a concentration at least 240-fold greater than the Ki values reported for their preferred receptors (SR 141716A, CB1: Ki=12.3 nM, CB2: Ki=702 nM, Showalter et al., 1996; SR 1445258, CB1: Ki=437 nM, CB2: Ki=0.6 nM, Rinaldi-Carmona et al., 1998). Thirdly, JWH 015, which shows a higher selectivity for the CB2 receptors than R-(+)-WIN 55,212-2 (Showalter et al., 1996), caused endothelium-independent relaxation that was insensitive to SR 144528 (1 or 3 μM). Although vasorelaxation induced by JWH 015 was inhibited by SR 141716A (3 μM), suggesting potential involvement of CB1 receptors, another CB1 receptor-selective antagonist AM 251 (1 or 3 μM; CB1: Ki=7.5 nM, CB2: Ki=2290 nM, Lan et al., 1999) had no effect. Hence, the aminoalkylindole cannabinoids JWH 015, R-(+)-WIN 55,212-2 and the noncannabinoid S-(−)-WIN 55,212-3 apparently cause mesenteric vasorelaxation independently of the classical cannabinoid receptors. These observations also argue against the presence of functional CB1 or CB2 receptors in the smooth muscle cells of rat small mesenteric artery, although it remains possible that CB1-like receptors (Darker et al., 1998) that do not couple to vasorelaxation mechanisms might be present (Holland et al., 1999).
The present study has also examined the relaxant effects of the putative endocannabinoid anandamide in order to compare them with those of the aminoalkylindole compounds. Anandamide-induced relaxation was endothelium-independent and was greatly reduced in vessels pretreated with capsaicin (10 μM for 30 min), which results in functional desensitisation of the vanilloid receptor (VR1) system. In contrast, capsaicin pretreatment either slightly potentiated or had no significant effect on relaxation to the aminoalkylindoles. These results support the suggestion that anandamide, but not aminoalkylindoles, relaxes the rat small mesenteric artery mainly by activation of VR1 receptors on perivascular sensory nerves (Zygmunt et al., 1999; White et al., 2001). However, a significant component of anandamide-induced relaxation remained in capsaicin-pretreated vessels (50–60% relaxation: this study; White et al., 2001). Furthermore, longer incubation (10 μM 60 min) or neonatal treatment with capsaicin failed to cause further inhibition (White et al., 2001; Harris et al., 2002). These observations strongly suggest the presence of additional relaxant mechanisms of anandamide. Indeed, a previous study showed that relaxation to anandamide in the rat coronary artery is mediated entirely by VR1-independent mechanisms (White et al., 2001). It is noteworthy that endothelium-dependent relaxant mechanisms of anandamide, such as degradation of anandamide into vasoactive metabolites (porcine coronary artery: Pratt et al., 1998) and anandamide-induced release of nitric oxide (rat renal artery: Deutsch et al., 1997), are unlikely to explain anandamide-induced relaxation, since it is independent of a functional endothelium in the rat small mesenteric artery (White & Hiley, 1997).
The CB1 cannabinoid receptor antagonist, SR 141716A (3 μM), displaced the concentration–response curve for anandamide in methoxamine-precontracted vessels to the right with an estimated KD of about 1μM. This confirms our results from an earlier study (White & Hiley, 1998a). Interestingly, the inhibition by SR 141716A was additive to that caused by capsaicin pretreatment. This is unlikely to be a result of the antagonist inhibiting responses to VR1 receptor activation (De Petrocellis et al., 2001), as the same concentration of SR 141716A (3 μM) had no significant effect on relaxation to capsaicin. Therefore, as well as a VR1 receptor-mediated mechanism, an additional SR 141716A-sensitive pathway also contributes to anandamide-induced relaxation in methoxamine-precontracted vessels. The classical CB1 and CB2 receptors are, however, unlikely to be involved as AM 251 and SR 144528 had no significant effect on the responses to anandamide. Several studies have also reported a SR 141716A-sensitive component of anandamide relaxation; however, this response appeared to require the presence of a functional endothelium (rabbit isolated mesenteric artery: Chaytor et al., 1999; rabbit isolated aorta: Mukhopadhyay et al., 2002; rat-perfused mesenteric bed: Wagner et al., 1999). Reasons for this discrepancy remain unclear although species, as well as regional, differences are possible contributing factors. It is postulated that endothelium-dependent mechanisms might have a larger role in anandamide-induced relaxation measured using the perfused mesenteric bed preparation, where endothelium removal caused a modest inhibition of anandamide relaxations (Wagner et al., 1999; Harris et al., 2002).
Even though it is not possible to identify the receptors mediating responses to the agents investigated here, the possibility that they might share a common relaxant mechanism was further examined. Contractions to α1-adrenoceptor agonists, such as methoxamine, are initiated by Ca2+ release from intracellular stores, which is followed by activation of Ca2+-activated Cl− channels causing depolarisation of the vascular smooth muscle cell membranes and activation of voltage-gated Ca2+ channels (VGCC; Criddle et al., 1996). In contrast, precontraction with depolarising K+ solution (60 mM KCl) is mediated almost entirely through opening of VGCC (Karaki et al., 1997). Therefore, our observation that endothelium-independent relaxation to the aminoalkylindoles JWH 015, R-(+)-WIN 55,212-2 and S-(−)-WIN 55,212-3 was similar under methoxamine- and KCl-induced tone indicated that they might act by inhibiting Ca2+ entry via VGCC. The similarity of the responses under the two types of precontraction also suggests that these relaxations are independent of effects due to the K+ gradient or actions on α1-adrenoceptors, IP3-mediated release of intracellular Ca2+ stores or activation of Ca2+-activated Cl− channels.
In order to provide further evidence for the involvement of VGCC in relaxation to the aminoalkylindole compounds, we examined their effects on contractions induced by cumulative readdition of Ca2+ to methoxamine-stimulated vessels, which had been depleted of intracellular Ca2+ stores. Under the protocol employed, contractions to Ca2+ occur mainly through Ca2+ influx following activation of VGCC in the vascular smooth muscle (White & Hiley, 1998b). All the aminoalkylindole compounds inhibited CaCl2-induced contractions, between approximately 20% (R-(+)-WIN 55,212-2) and 40–50% (S-(−)-WIN 55,212-3 or JWH 015) of the maximal contractions to CaCl2. As with relaxation in methoxamine-precontracted tone, the effects of R-(+)-WIN 55,212-2 and S-(−)-WIN 55,212-3 on CaCl2-induced contractions were insensitive to the cannabinoid antagonists SR 141716A and SR 144528. It was noted that S-(−)-WIN 55,212-3 appeared to produce a larger inhibition of CaCl2 contractions than R-(+)-WIN 55,212-2, while the two enantiomers tended to show similar potencies in producing relaxation of precontracted vessels, indicating the presence of relaxant mechanisms other than inhibition of Ca2+ influx for R-(+)-WIN 55,212-2.
In the case of JWH 015, its inhibitory effect on CaCl2 contractions was also unaffected by SR 144528. However, SR 141716A (3 μM) partially reversed the inhibition of CaCl2 contractions induced by JWH 015, at 10 μM but not 30 μM, consistent with our observation that 3 μM SR 141716A reduced the vasorelaxant potency of JWH 015 in KCl-precontracted vessels. It follows that JWH 015 might evoke vasorelaxation by multiple mechanisms in the rat mesenteric artery and that only one component of JWH 015-induced inhibition of Ca2+ entry into vascular smooth muscle is sensitive to SR 141716A (Figure 1). Nevertheless, it is likely that the SR 141716A-insensitive component of JWH 015 responses, which probably also involves negative modulation of VGCC, is shared with R-(+)-WIN 55,212-2 and S-(−)-WIN 55,212-3.
The L-type VGCC is the likely channel involved in the relaxation to the aminoalkylindole compounds as the L-type channel blocker, verapamil, inhibits contractions to methoxamine, KCl and CaCl2 (White & Hiley, 1998b; Ho & Hiley, 2003; this study). Importantly, the aminoalkylindoles, as well as verapamil, caused either little or no relaxation in vessels contracted by the Ca2+ ionophore iomomycin (Liu & Hermann, 1978), excluding the possibility that aminoalkylindoles act by reducing the Ca2+ sensitivity of contractile proteins in vascular smooth muscle. Together, our results support the proposal that these cannabinoids in some way reduce Ca2+ entry via L-type channels. Nevertheless, as VGCC-independent effects have also been reported for verapamil (e.g. modulation of muscarinic receptors, Ellis & Seidenberg, 2000; inhibition of epithelial Na+ transport, Segal et al., 2002), direct studies of voltage-dependent Ca2+ currents in the smooth muscle cells are necessary to confirm the role of L-type VGCC in cannabinoid-induced relaxation of rat mesenteric arteries.
Cannabinoids, through activation of CB1 receptors, are known to modulate the activity of several ion channels. They cause inhibition of P/Q-, N- or L-type, voltage-gated, Ca2+ channels as well as activation of inwardly rectifying K+ channels in neurons (see Pertwee, 1997 for review). In vascular smooth muscle cells, however, CB1 receptor-mediated inhibition of the L-type Ca2+ channel has only been reported in cat cerebral artery (Gebremedhin et al., 1999). Here, we have found that the actions of the aminoalkylindole agents in the rat mesenteric artery could be explained by modulation of Ca2+ channels, probably L-type Ca2+ channels, via non-CB1, and non-CB2 receptor mechanisms. In fact, R-(+)-WIN 55,212-2 and S-(−)-WIN 55,212-3, at concentrations higher than 1 μM, also inhibit N- and P/Q-type Ca2+ channels by cannabinoid receptor-independent mechanisms in rat hippocampal neurons (Shen & Thayer, 1998). Interestingly, another structurally unrelated cannabinoid receptor agonist, CP 55,940, might also inhibit mesenteric contractions due to activation of VGCC (White & Hiley, 1998a). In fact, abnormal-cannabidiol (Ho & Hiley, 2003), which is a cannabinoid derivative without activities at CB1 or CB2 receptors, and, paradoxically, the cannabinoid antagonist SR 141716A (>3 μM; White & Hiley, 1998b) have also been reported to inhibit Ca2+ influx activities in the rat mesenteric artery. At present, the mechanisms by which these structurally diverse cannabinoids reduce Ca2+ influx are unclear. Importantly, it remains to be determined if they act via common or similar pathways; indeed, direct binding of cannabinoids to VGCC has been proposed by Johnson et al. (1993) and Shen & Thayer (1998).
The possibility that anandamide also reduces Ca2+ influx is supported by the observation that, at a concentration of 10 μM, it inhibited contractions to CaCl2 in methoxamine-stimulated vessels which had been previously treated with capsaicin and depleted of intracellular Ca2+. This might be explained by the ability of anandamide to bind directly to the L-type Ca2+ channels with low affinity (Ki=15 μM; Johnson et al., 1993). However, in contrast to the case of the aminoalkylindoles, modulation of VGCC would apparently have a minor role in anandamide-induced relaxation of methoxamine-precontracted vessels as anandamide responses were markedly inhibited under KCl-induced tone. In addition, the relaxation of KCl-precontracted vessels and the inhibition of CaCl2 contractions induced by anandamide (unlike that induced by JWH 015) were not affected by SR 141716A (3 μM). Thus, the SR 141716A-sensitive component of anandamide relaxation might involve actions on any contractile mechanism that is bypassed by direct membrane depolarisation, such as Ca2+ release from intracellular stores and opening of Ca2+-activated Cl− channels (Zygmunt et al., 1997; White & Hiley, 1998a), or activation of K+ channels, although earlier reports indicated that K+ channels were unlikely to be involved (White & Hiley, 1997; Zygmunt et al., 1997). These observations suggest that there are both SR 141716A-sensitive and -insensitive vasorelaxation mechanisms which mediate the relaxation to anandamide in capsaicin-pretreated, small mesenteric artery of the rat.
Our results suggest that VR1 receptor activation cannot explain fully the relaxant effects of anandamide in the rat small mesenteric artery, and hence provide further evidence for the complex vasodilator actions of anandamide, which are dissimilar to those of the aminoalkylindoles. Of note, a recent report showed that anandamide-induced hypotension, which was partially inhibited by SR 141716A, also involved activation of VR1 receptors (Malinowska et al., 2001). In fact, the presence of multiple vasorelaxation mechanisms for anandamide might, at least in part, explain its different systemic or regional haemodynamic profile from other synthetic cannabinoids (Wagner et al., 2001).
To conclude, the present study shows that the amino-alkylindole compounds, JWH 015, R-(+)-WIN 55,212-2 and its enantiomer S-(−)-WIN 55,212-3, cause endothelium-independent relaxation of rat small mesenteric artery mainly by reducing Ca2+ entry into the vascular smooth muscle, which is independent of the recognised cannabinoid CB1 and CB2 receptors. A likely target of these cannabinoids is the L-type VGCC in the vascular smooth muscle cells. In addition, the putative endocannabinoid anandamide, at high concentrations, might also modify Ca2+ entry in smooth muscle by vanilloid receptor-independent pathways. However, activation of the vanilloid receptors and, to a lesser extent, a SR 141716A-sensitive pathway also appear to be the mechanisms by which anandamide causes vasorelaxation in this preparation.
Acknowledgments
We thank Sanofi-Synthélabo (France) for their generous gifts of SR 141716A and SR 144528. W-SVH is supported by the Cambridge Overseas Trust (Hong Kong) and New Hall, Cambridge.
Abbreviations
- AM 251
N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- JWH 015
(2-methyl-1-propyl-1H-indol-3-yl)-1-naphthalenylmethanone
- pEC50%
negative logarithm of the concentration causing 50% relaxation
- R-(+)-WIN 55,212-2
R-(+)-[2,3-dihydro5-methyl-3-(4-morpholinylmethyl)pyrrolol[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone
- SR 141716A
N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- SR 144528
N-[(1S)-endo-1,3,3-trimethyl bicyclo [2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide
- S-(−)-WIN 55,212-3
S-(−)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolol[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone
- VGCC
voltage-gated Ca2+ channels
References
- CHAYTOR A.T., MARTIN P.E., EVANS W.H., RANDALL M.D., GRIFFITH T.M. The endothelial component of cannabinoid-induced relaxation in rabbit mesenteric artery depends on gap junctional communication. J. Physiol. 1999;520:539–550. doi: 10.1111/j.1469-7793.1999.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRIDDLE D.N., DE MOURA R.S., GREENWOOD I.A., LARGE W.A. Effect of niflumic acid on noradrenaline-induced contractions of the rat aorta. Br. J. Pharmacol. 1996;118:1065–1071. doi: 10.1111/j.1476-5381.1996.tb15507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARKER I.T., MILLNS P.J., SELBIE L., RANDALL M.D., S-BAXTER G., KENDALL D.A. Cannabinoid (CB1) receptor expression is associated with mesenteric resistance vessels but not thoracic aorta in the rat. Br. J. Pharmacol. 1998;12:95. [Google Scholar]
- DE PETROCELLIS L., BISOGNO T., MACCARRONE M., DAVIS J.B., FINAZZI-AGRO A., DI MARZO V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J. Biol. Chem. 2001;276:12856–12863. doi: 10.1074/jbc.M008555200. [DOI] [PubMed] [Google Scholar]
- DEUTSCH D.G., GOLIGORSKY M.S., SCHMID P.C., KREBSBACH R.J., SCHMID H.H.O., DAS S.K., DEY S.K., ARREAZA G., THORUP C., STEFANO G., MOORE L.C. Production and physiological actions of anandamide in the vasculature of the rat kidney. J. Clin. Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS J., SEIDENBERG M. Interactions of alcuronium, TMB-8, and other allosteric ligands with muscarinic acetylcholine receptors: studies with chimeric receptors. Mol. Pharmacol. 2000;58:1451–1460. doi: 10.1124/mol.58.6.1451. [DOI] [PubMed] [Google Scholar]
- FELDER C.C., VELUZ J.S., WILLIAMS H.L., BRILEY E.M., MASUDA L.A. Cannabinoid agonists stimulate both receptor- and non-receptor-mediated signal transduction pathways in cells transfected with and expressing cannabinoid receptor clones. Mol. Pharmacol. 1992;42:838–845. [PubMed] [Google Scholar]
- GEBREMEDHIN D., LANGE A.R., CAMPELL W.B., HILLARD C.J., HARDER D.R. Cannabinoid CB1 receptor of rat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am. J. Physiol. 1999;276:H2085–H2093. doi: 10.1152/ajpheart.1999.276.6.H2085. [DOI] [PubMed] [Google Scholar]
- GRIFFIN G., ATKINSON P.J, SHOWALTER V.M., MARTIN B.R., ABOOD M.E. Evaluation of cannabinoid receptor agonist and antagonists using the guanosine-5′-O-(3-[35S]thio)−triphosphate binding assay in rat cerebellar membranes. J. Pharmacol. Exp. Ther. 1998;285:553–560. [PubMed] [Google Scholar]
- HANUS L., BREUER A., TCHILIBON S., SHILOAH S., GOLDENBERG D., HOROWITZ M., PERTWEE R.G., ROSS R.A., MACHOULAM R., FRIED E. HU308: a specific agonist for CB2, a peripheral cannabinoid receptor. Proc. Natl. Acad. Sci. U.S.A. 1999;25:14228–14233. doi: 10.1073/pnas.96.25.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS D., MCCULLOCH A.I., KENDALL D.A., RANDALL M.D. Characterization of vasorelaxant responses to anandamide in the rat mesenteric arterial bed. J. Physiol. 2002;539:893–902. doi: 10.1113/jphysiol.2001.013489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLARD C.J. Endocannabinoids and vascular function. J. Pharmacol. Exp. Ther. 2000;294:27–32. [PubMed] [Google Scholar]
- HO W.-S.V., HILEY C.R. Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery. Br. J. Pharmacol. 2003;138:1320–1332. doi: 10.1038/sj.bjp.0705160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND M., CHALLISS R.A.J., STANDEN N.B., BOYLE J.P. Cannabinoids CB1 receptors fail to cause relaxation, but couple via Gi/Go to the inhibition of adenylyl cyclase in carotid artery smooth muscle. Br. J. Pharmacol. 1999;128:59–64. doi: 10.1038/sj.bjp.0702842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JÁRAI Z., WAGNER J.A., VARGA K., LAKE K.D., COMPTON D.R., MARTIN B.R., ZIMMER A.M., BONNER T.I., BUCKLEY N.E., MEZEY E., RAZDAN R.K., ZIMMER A., KUNOS G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc. Natl. Acad. Sci. U.S.A. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON D.E., HEALD S.L., DALLY R.D., JANIS R.A. Isolation, identification and synthesis of an endogenous arachidonic amide that inhibits calcium channel antagonist 1,4-dihydropyridine binding. Prostagland. Leukot. Essent. Fatty Acids. 1993;48:429–437. doi: 10.1016/0952-3278(93)90048-2. [DOI] [PubMed] [Google Scholar]
- KARAKI H., OZAKI H., HORI M., MITSUSAITO M., AMINO K.I., HARADA K.I., MIYAMOTO S., NAKAZAWA H., WON K.J., SATO K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol, Rev. 1997;49:157–230. [PubMed] [Google Scholar]
- LAKE K.D., COMPTON D.R., VARGA K., MARTIN B.R., KUNOS G. Cannabinoid-induced hypotension and bradycardia in rats is mediated by CB1-like cannabinoid receptors. J. Pharmacol. Exp. Ther. 1997;281:1030–1037. [PubMed] [Google Scholar]
- LAN R., LIU Q., FAN P., LIN S., FERNANDO S.R., MCCALLION D., PERTWEE R., MAKRIYANNIS A. Structure–activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J. Med. Chem. 1999;42:769–776. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- LIU C., HERMANN T.E. Characterization of ionomycin as a calcium ionophore. J. Biol. Chem. 1978;253:5892–5894. [PubMed] [Google Scholar]
- MALINOWSKA B., KWOLEK G., GOTHERT M. Anandamide and methanandamide induce both vanilloid VR1- and cannabinoid CB1 receptor-mediated changes in heart rate and blood pressure in anaesthetized rats. Naunyn Schmiedeberg's Arch. Pharmacol. 2001;364:562–569. doi: 10.1007/s00210-001-0498-6. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., MELI A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen. Pharmacol. 1988;19:1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- MUKHOPADHYAY S., CHAPNICK B.M., HOWLETT A.C. Anandamide-induced vasorelaxation in rabbit aortic rings has two components: G protein dependent and independent. Am. J. Physiol. 2002;282:H2046–H2054. doi: 10.1152/ajpheart.00497.2001. [DOI] [PubMed] [Google Scholar]
- MULVANY M.L.J., HALPERN W. Contractile responses of small resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- PRATT P.F., HILLARD C.J., EDGEMOND W.S., CAMPBELL W.B. N-arachidonylethanolamide relaxation of bovine coronary artery is not mediated by CB1 cannabinoid receptor. Am. J. Physiol. 1998;274:H375–H381. doi: 10.1152/ajpheart.1998.274.1.H375. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., MILLAN J., DEROCQ J.M., CASELLAS P., CONGY C., OUSTRIC D., SARRAN M., BOUABOULA M., CALANDRA B., PORTIER M., SHIRE D., BRELIERE J.C., LE FUR G. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- ROSS R.A., GIBSON T.M., BROCKIE H.C., LESLIE M., PASHMI G., CRAIB S.J., DI MARZO V., PERTWEE R.G. Structure–activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Br. J. Pharmacol. 2001;132:631–640. doi: 10.1038/sj.bjp.0703850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEGAL A.S., HAYSLETT J.P., DESIR G.V. On the natriuretic effect of verapamil: inhibition of ENaC and transepithelial sodium transport. Am. J. Physiol. 2002;283:F765–F770. doi: 10.1152/ajprenal.00253.2001. [DOI] [PubMed] [Google Scholar]
- SHEN M., THAYER S.A. The cannabinoid agonist Win55,212-2 inhibits calcium channels by receptor-mediated and direct pathways in cultured rat hippocampal neurons. Brain Res. 1998;783:77–84. doi: 10.1016/s0006-8993(97)01195-5. [DOI] [PubMed] [Google Scholar]
- SHOWALTER V.M., COMPTON D.R., MARTIN B.R., ABOOD M.E. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J. Pharmacol. Exp. Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- SLIPETZ D.M., O'NEILL G.P., FAVREAU L., DUFRESNE C., GALLANT M., GAREAU Y., GUAY D., LABELLE M., METTERS K.M. Activation of the human peripheral cannabinoid receptor results in inhibition of adenylyl cyclase. Mol. Pharmacol. 1995;48:352–361. [PubMed] [Google Scholar]
- WAGNER J.A., JÁRAI Z., BÁTKAI S., KUNOS G. Hemodynamic effects of cannabinoids: coronary and cerebral vasodilation mediated by cannabinoid CB1 receptors. Eur. J. Pharmacol. 2001;423:203–210. doi: 10.1016/s0014-2999(01)01112-8. [DOI] [PubMed] [Google Scholar]
- WAGNER J.A., VARGA K., JÁRAI Z., KUNOS G. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension. 1999;33:429–434. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. Br. J. Pharmacol. 1997;122:1573–1584. doi: 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. The actions of some cannabinoid receptor ligands in rat isolated mesenteric artery. Br. J. Pharmacol. 1998a;125:533–541. doi: 10.1038/sj.bjp.0702111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. The actions of the cannabinoid receptor antagonist, SR 141716A, in the rat isolated mesenteric artery. Br. J. Pharmacol. 1998b;125:689–696. doi: 10.1038/sj.bjp.0702127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., HO W.-S.V., BOTTRILL F.E., FORD W.R., HILEY C.R. Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. Br. J. Pharmacol. 2001;134:921–929. doi: 10.1038/sj.bjp.0704333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., HÖGESTÄTT E.D., WALDECK K., EDWARDS G., KIRKUP A.J., WESTON A.H. Studies on the effects of anandamide in rat hepatic artery. Br. J. Pharmacol. 1997;122:1679–1686. doi: 10.1038/sj.bjp.0701601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SØRGÅRD M., DI MARZO V., JULIUS D., HÖGESTÄTT E.D. Vanilloid receptors on sensory nerves mediate vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]