Abstract
Growth hormone secretagogues (GHS) exhibit potent growth hormone (GH)-releasing activity through the activation of a pituitary receptor. Here, we consider the possibility that GHS can target a specific receptor in rat skeletal muscle and have a role in the control of muscle function.
By means of the intracellular microelectrode technique, we found that in vitro application of hexarelin and L-163,255 dose dependently reduced resting chloride (gCl) and potassium (gK) conductances in rat skeletal muscle. These effects were prevented by the GHS-receptor antagonist [D-Lys-3]-GHRP-6, and by either phospholipase C or protein kinase C (PKC) inhibitors. Ghrelin, a natural ligand of GHS receptors, also induced a reduction of muscle gCl and gK, which was antagonised by [D-Lys-3]-GHRP-6.
Both GHS shifted the mechanical threshold for the contraction of muscle fibres towards more negative voltages. Accordingly, by means of FURA-2 fluorescent measurements, we demonstrated that L-163,255 induced a resting [Ca2+]i increase, which was reversible and not blocked by nifedipine or removal of external Ca2+.
Ageing is a condition characterised by a deficit of GH secretion, which in turn modifies the electrical and contractile properties of skeletal muscle. In contrast to GH, chronic treatment of aged rats with hexarelin or L-163,255 failed to restore the electrical and contractile muscle properties. Moreover, the two GHS applied in vitro were able to antagonise the beneficial effect on gCl and gK obtained through chronic treatment of aged animals with GH.
Thus, skeletal muscle expresses a specific GHS receptor able to decrease gCl and gK through a PKC-mediated intracellular pathway. This peripheral action may account for the lack of restoration of skeletal muscle function in long-term GHS-treated aged animals.
Keywords: Skeletal muscle, growth hormone secretagogues, ghrelin, ClC-1 chloride channel, chloride conductance, potassium conductance, protein kinase C, resting intracellular calcium, mechanical threshold for contraction, FURA-2 fluorescence measurements
Introduction
Growth-hormone secretagogues (GHS) comprise synthetic peptidic and nonpeptidic molecules, able to stimulate growth hormone (GH) secretion in both animals and human subjects (Ankersen et al., 1999; Müller et al., 1999). Their neuroendocrine activity is mediated by a specific G-protein-coupled receptor cloned from mammalian pituitary libraries (Howard et al., 1996), and an endogenous ligand, named ghrelin, has been recently identified in rat stomach (Kojima et al., 1999). It has been shown that the stimulation of GH release by GHS involves Ca2+- and phospholipid-dependent protein kinase (PKC), as well as potassium and calcium channels (Smith et al., 1997). Besides this stimulatory effect on GH secretion, it has been demonstrated that long-term treatment of GH-deficient and senescent rats with hexarelin protects the heart from ischemia and reperfusion damage (De Gennaro Colonna et al., 1997; Rossoni et al., 1998; Katugampola et al., 2001). Surprisingly, this protective effect is independent of any stimulation of the somatotropic axis (Locatelli et al., 1999), suggesting a direct action of hexarelin on specific cardiac receptors. Accordingly, binding and functional studies revealed the existence of cardiac receptors coupled to PKC-mediated pathways (Bodart et al., 1999).
Binding sites for GHS have also been found in human skeletal muscle, suggesting that specific receptors can mediate biological activities in this tissue, likely through PKC stimulation (Papotti et al., 2000). In rat skeletal muscle, PKC activation induces the reduction of the resting membrane chloride conductance (gCl), sustained by skeletal muscle chloride (ClC-1) channels (Steinmeyer et al., 1991), which is important in the control of resting potential, excitability and contraction (Conte Camerino et al., 1989; De Luca et al., 1994). Skeletal muscle is also a primary target of the action of GH, as shown by a wide series of biophysical and biochemical changes of ion channel function observed in aged rats, a condition characterised by GH deficiency. An ageing-related reduction of resting gCl is due to a down regulation of ClC-1 channel expression (Pierno et al., 1999), as well as due to a concomitant overactivity of PKC that inhibits chloride channels (De Luca et al., 1994). In aged animals, the activity of K+ and Na+ channels is also modified (De Luca et al., 1997; Tricarico et al., 1997; Desaphy et al., 1998a). Excitation–contraction coupling, the mechanism that links membrane depolarisation to calcium release and contraction, is also changed since the mechanical threshold (MT) for contraction is shifted in aged animals toward more negative voltages (De Luca & Conte Camerino, 1992; Pierno et al., 1998). Chronic administration of GH in aged animals significantly ameliorated the abnormally low gCl, the high potassium conductance (gK) and the changes in sodium channel function, as well as returned the excitability parameters toward adult values (De Luca et al., 1997; Desaphy et al., 1998b). GHS have been proposed for use in the treatment of elderly subjects because they stimulate a physiologically pulsatile GH release, without producing the adverse side effects because of continuous GH administration (Casanueva & Dieguez, 1999; Smith et al., 1999). Accordingly, previous studies reported a partial recovery of Na+ channel properties in aged rat skeletal muscle after chronic hexarelin treatment (Desaphy et al., 1998b).
The goal of the present study was to evaluate whether GHS can target a specific skeletal muscle receptor and exert a direct effect on muscle function, as well as to evaluate the therapeutic potential of these drugs. To achieve this aim, we tested the effects produced by in vitro application of hexarelin and of L-163,255, a nonpeptidic GHS (Chang et al., 1996), on the electrical and contractile properties of rat sarcolemma. Moreover, the same parameters were measured in skeletal muscle fibres obtained from GH-deficient, aged rats after in vivo chronic treatment with the two GHS.
Methods
In vivo studies
Young adult (4–6-month old) and aged (27–30-month old) male Wistar rats (Charles River Laboratories, Calco, Italy) were used in this study. Animals were maintained one per cage, having free access to food and water, at a constant room temperature (22–24°C) and exposed to a light cycle of 12 h day−1 (8.00 a.m.–8.00 p.m.). Different groups of aged rats were randomly selected for in vivo treatments. Five aged rats were treated with 80 μg kg−1 hexarelin s.c., 6 days a week for 4 weeks (Cattaneo et al., 1997). Nine aged rats were treated with 10 mg kg−1 L-163,255 s.c. 6 days a week for 8 weeks. Five aged rats were administered 150 μg kg−1 rat GH s.c., 6 days a week for 8 weeks. Six control aged and five control young adult rats received an equivalent volume of the vehicle (0.1 ml of distilled water) s.c. as placebo. Aged rats exhibited normal physical condition and motor activity during the treatment period. However, five out of 30 aged animals used in this study died before the end of the treatment for unknown sudden causes (one untreated, one GH-treated, one hexarelin-treated and two treated with L-163,255).
In vitro studies: electrophysiological measurements
The electrophysiological experiments were carried out in vitro on extensor digitorum longus (EDL) muscles removed under urethane anaesthesia (1.2 g kg−1, i.p.). Soon after dissection, the rats, still anaesthetised, were euthanaised by anaesthetic overdose. All experimental protocols were carried out in accordance with the Guide for the care and use of laboratory animals. Muscle preparations were immediately placed in a muscle chamber containing a physiological solution maintained at 30°C. This temperature has been shown not to modify either gCl or gK, when compared to the more physiological temperature of 37°C (Palade & Barchi, 1977).
The gCl component recorded from EDL muscle fibres was calculated from the membrane resistance (Rm) values measured by standard cable analysis with the two intracellular microelectrode current-clamp method, in which a hyperpolarising square wave current pulse is applied through one electrode and the membrane voltage response is monitored at two distances from the current-passing electrode. Current pulse generation, acquisition of the voltage records and calculation of fibre constants were performed under computer control as detailed elsewhere (De Luca et al., 1997). The total membrane conductance (gm) was 1/Rm in the normal physiological solution, whereas gK was 1/Rm in chloride-free solution (Conte Camerino et al., 1989). Data were expressed as mean±s.e.m. from n fibres. The mean gCl was calculated as the mean gm minus the mean gK, while the s.e.m. and the n value for gCl were calculated from the variance and n value of gm and gK, as described by Green & Margerison (1978). Statistical differences between means was determined by analysis of variance (ANOVA) for multiple comparisons and by Student's t-test for single comparisons (Tallarida & Murray, 1987).
The MT of the fibres was determined using the two microelectrode ‘point' voltage-clamp method, in the presence of 3 μM tetrodotoxin, as previously described (Dulhunty, 1988; De Luca & Conte Camerino, 1992). The holding potential was set at −90 mV and depolarising current pulses of increasing duration (5–500 ms) were given repetitively at a rate of 0.3 Hz, while the impaled fibres were continuously viewed using a stereomicroscope. For each pulse duration, the command voltage was increased until contraction was just visible, and then backed down until the contraction disappeared. A digital sample-and-hold millivoltmeter stored the value of the threshold membrane potential at this point. We estimated the uncertainty of any single measurement for a given fibre to be 1–2 mV. The length of time required to determine the MT for each preparation was kept constant so as to exclude any effect that differences in the amount of citrate leaking from the electrodes might have on the results (Dulhunty, 1988). The threshold membrane potential V (mV) for each fibre was averaged at each pulse duration t (ms) and then the mean values plotted against duration giving a ‘strength–duration' relation. An estimate of the rheobase (R) and of the time constant to reach the rheobase was obtained by fitting the data points to a nonlinear least-squares algorithm using the equation V=[H−R exp (t/τ)]/[1−exp (t/τ)], where H is the holding potential (mV), R is the rheobase (mV) and τ is the time constant (De Luca & Conte Camerino, 1992). In the fitting algorithm, each data point was weighed by the reciprocal of the variance of that mean V. We used this procedure in order to incorporate all data points and their associated errors into the estimate of R under each set of conditions. The MT values are expressed as the fitted rheobase (R) parameter±standard error (s.e.), which was determined from the variance–covariance matrix in the nonlinear least-squares fitting algorithm. The statistical significance of the fitted rheobase values and their differences from each other were estimated by Student's t-distribution using a number of degrees of freedom equal to the total number of threshold values determining the curves minus the number of means minus two for the free parameters (De Luca & Conte Camerino, 1992).
Fluorescence measurements of resting intracellular Ca2+ ions
Fluorescence measurements were performed on EDL muscle extracted from adult rats. The muscle was pinned at tendons and small bundles of five to 10 fibres were dissected lengthwise, tendon to tendon, and arranged in a single layer. The dissection was carried out in normal physiological solution at room temperature (22°C).
Fluorescent measurements were made using a QuantiCell 900 integrated imaging system (VisiTech International Ltd, Sunderland, U.K.). The fura-2-acetoxymethyl ester (AM) (Molecular Probes, U.S.A.) fluorescent dye was used to measure cytosolic Ca2+. The muscle fibres were loaded with the dye by incubation for 45–60 min at 22°C in normal physiological solution containing 2.5 μM fura-2-AM and 10% (v/v) Pluronic F-127 (Molecular Probes). After the loading was completed, the muscle fibres were washed and mounted in a glass-bottomed RC-27NE experimental chamber (Warner Instrument Corp., Hamden, U.S.A.) on the stage of an inverted Eclipse TE300 microscope with an X40 Plan-Fluor objective (Nikon, Japan). The sarcomere length of the fibres in the field of view was adjusted to 2.5 μm using a micromanipulator. During experiments, pairs of background-subtracted images of the fura-2 fluorescence emission (510 nm) excited at 340 and 380 nm were acquired. The equation (Grynkiewicz et al., 1985) used to transform fluorescence ratio in Ca2+ concentration values was [Ca2+]i=(R−Rmin)/(Rmax−R)KDβ, where R is the ratio of fluorescence excited at 340 nm to that excited at 380 nm; KD=145 nM; β, Rmin and Rmax are constants according to Grynkiewicz et al. (1985) and were determined in situ in ionomycin-permeabilised muscle fibres. Rmin and Rmax were determined in muscle fibres incubated in Ca2+-free normal physiological solution containing 10 mM EGTA and in normal physiological solution, respectively.
Solutions and drugs
The normal physiological solution had the following composition (mM): NaCl 148; KCl 4.5; CaCl2 2.0; MgCl2 1.0; NaH2PO4 0.44; NaHCO3 12 and glucose 5.55. The chloride-free solution was prepared by equimolar replacement of methylsulphate salts for NaCl and KCl and nitrate salts for CaCl2 and MgCl2. Both solutions were continuously bubbled with 95% O2 and 5% CO2 to maintain the pH at 7.2–7.3 (Bryant & Conte Camerino, 1991). GH (provided by the National Hormone and Pituitary Program, NIDDK, N.I.H., Bethesda, MD, U.S.A.), hexarelin and L-163,255 (provided by Merck Res. Lab., Rahway, NJ, U.S.A.) were used for in vivo administration. For in vitro studies, hexarelin and L-163,255 as well as des-Gln14-Ghrelin (Tocris Cookson Ltd, Bristol, U.K.) (Hosoda et al., 2000) and the GHS antagonist [D-Lys-3]-GHRP-6 (Bachem AG, Bubendorf, Switzerland) were dissolved in normal or chloride-free physiological solutions. Staurosporine and U73122 (Sigma Chemicals, St Louis, U.S.A.) were dissolved in dimethylsulphoxide (DMSO). Chelerythrine chloride (Sigma) was dissolved in distilled water. These stock solutions were added to the normal physiological and chloride-free solutions to achieve the desired final concentration of each drug. The maximal concentration of DMSO used (0.5%) was without effect on the parameters studied (De Luca et al., 1994). EGTA and Nifedipine were from Sigma (St Louis, U.S.A.).
Results
Effects of in vitro application of hexarelin and L-163,255 on Cl− and K+ conductances of skeletal muscle fibres of adult and aged rats
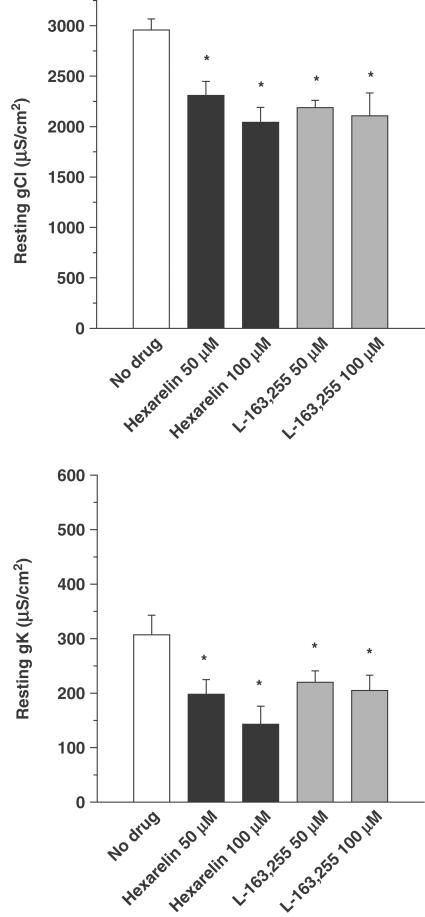
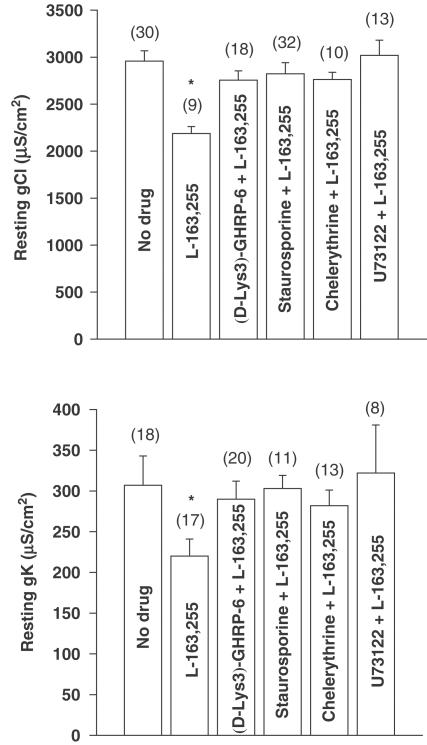
As shown in Figure 1, either hexarelin or L-163,255 applied in vitro on EDL muscle fibres of adult rats produced a significant and concentration-dependent reduction of both resting gCl and gK. Interestingly, the GHS receptor antagonist, [D-Lys-3]-GHRP-6, which by itself had no effect on gCl and gK (Table 1 ), was able to antagonise the effect of 50 μM L-163,255 on the conductances (Figure 2). To determine what intracellular components are involved in the GHS-induced reduction of ionic conductances, different enzyme inhibitors were tested. As previously reported, in vitro application of inhibitors of PKC, staurosporine (1 μM) and chelerythrine (1 μM) did not affect gCl and gK in the EDL muscle of young adult rats (Table 1) (De Luca et al., 1994). However, these compounds were able to prevent the decrease of both conductances because of L-163,255 application (Figure 2). Similarly, the reduction of gCl and gK because of 50 μM hexarelin was antagonised by staurosporine since gCl was 2992±91 μS cm−2 (n=15) and gK was 325±48 μS cm−2 (n=14). The reduction of both gCl and gK elicited by 50 μM L-163,255 was also abolished by preincubation with 10 μM U73122, an inhibitor of the phospholipase C (PLC) (Figure 2).
Figure 1.
Effects of in vitro application of different concentrations of hexarelin and L-163,255 on gCl (upper panel) and gK (lower panel) of adult rat EDL muscle fibres. Each bar represents the mean of gCl and gK (±s.e.m.) obtained from 10:30 fibres (two to three rats). *Significantly different with respect to the control value measured in the absence of drug (open bars) (P<0.05 or less).
Table 1.
Effects of GHS receptor antagonist and of different inhibitors of PKC and PLC on resting conductances in extensor digitorum longus muscle fibres of adult rats
| Experimental condition | Dose (μM) | n fibres | gCl (μS/cm2) | n fibres | gK (μS/cm2) |
|---|---|---|---|---|---|
| No drug | 30 | 2958±110 | 18 | 307±36 | |
| [D-Lys-3]-GHRP-6 | 100 | 14 | 2870±85 | 14 | 292±48 |
| Chelerythrine | 1 | 9 | 2905±71 | 17 | 288±13 |
| Staurosporine | 1 | 38 | 3023±67 | 12 | 322±19 |
| U73122 | 10 | 10 | 3355±122 | 10 | 274±33 |
No significant difference was found between drug and no-drug conditions using Student's t-test.
Figure 2.
Antagonism of the L-163,255-induced decrease in gCl (upper panel) and gK (lower panel) by [D-Lys-3]-GHRP-6 (GHS-receptor antagonist), staurosporine and chelerythrine (PKC inhibitors) and U73122 (PLC inhibitor) following in vitro application in the EDL muscle fibres of adult rats. The bars show the mean values of gCl and gK (±s.e.m.), from the number of fibres indicated within brackets (two to four rats), recorded in the absence and in the presence of L-163,255 (50 μM) alone and after the addition of L-163,255 to muscle preparations previously incubated for 30 min to 1 h with [D-Lys-3]-GHRP-6 (100 μM), staurosporine (1 μM), chelerythrine (1 μM), or U73122 (10 μM). *Significantly different with respect to the value recorded in the absence of drug (P<0.05 or less).
To verify whether the direct action of GHS on skeletal muscle is preserved throughout the lifespan of these animals, we tested the in vitro effects of GHS on EDL muscles dissected from 27-month-old, aged rats. Consistent with previous studies (De Luca et al., 1994; Pierno et al., 1999), gCl was reduced by about 24% (2238±190 μS cm−2, n=9, P<0.005) in aged animals as compared to the 4–6-month-old young adult rats (2958±110 μS cm−2, n=30). Yet, both GHS were still able to reduce gCl in the aged animals, since this parameter was significantly reduced to 1734±132 μS cm−2 (n=9, P<0.05 versus control aged rats) after application of 100 μM hexarelin and to 1759±27 μS cm−2 (n=8, P<0.05 versus control aged rats) in the presence of 100 μM L-163,255. Moreover, both compounds induced a more remarkable reduction of the high gK typical of aged muscle fibres. Indeed gK was reduced from 475±47 μS cm−2 (n=15) to 267±63 μS cm−2 (n=13, P<0.02) by 50 μM hexarelin and to 313±19 μS cm−2 (n=17, P<0.005) by 50 μM L-163,255. As in young adult animals, the effects of 50 μM L-163,255 in aged rat muscles were completely prevented by preincubation with 1 μM chelerythrine. Indeed, gCl and gK were 2272±295 (n=9) and 364±97 μS cm−2 (n=9) in the presence of chelerythrine alone, and were 2354±200 (n=12) and 350±104 μS cm−2 (n=12) when L-163,255 was added to the muscle bath.
Effects of in vitro application of ghrelin on Cl− and K+ conductances of skeletal muscle fibres of adult rats
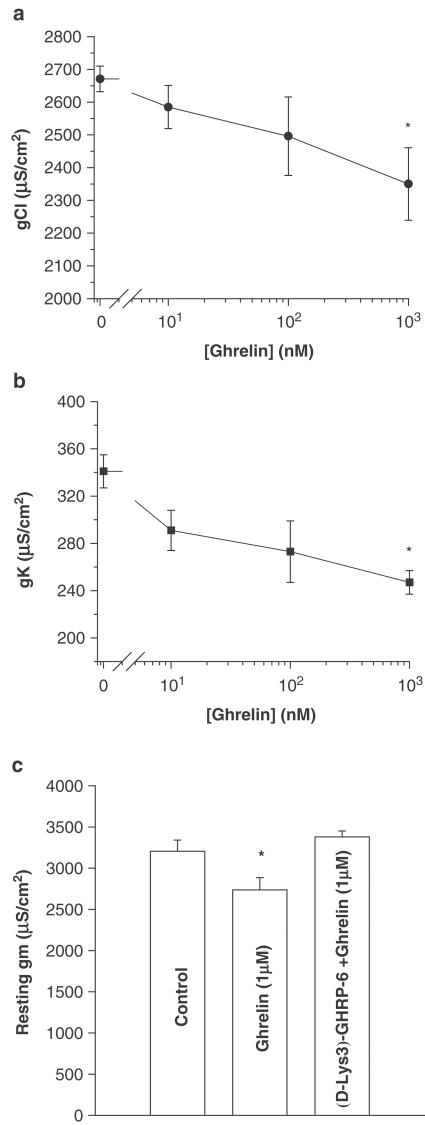
We decided to test in vitro the effects of the natural ligand of GHS receptor, namely ghrelin, on muscle resting conductances to support the presence of a specific skeletal muscle receptor. Ghrelin applied in vitro to EDL muscle fibres of adult rats dose dependently reduced the total gm, because of a reduction of both gCl and gK (Figure 3). In particular, in the presence of 1 μM ghrelin, the resting gCl and resting gK were significantly reduced by 12±3.8 and 27±3.0%, respectively. Moreover, prior incubation of EDL muscle with [D-Lys-3]-GHRP-6, the GHS receptor antagonist, suppressed the reduction of gm induced by 1 μM ghrelin (Figure 3).
Figure 3.
Effects of in vitro application of different concentrations of ghrelin on gCl (a) and gK (b) of adult EDL rat muscle fibres. Each point represents the mean±s.e.m. obtained from nine to 51 fibres of two to six rats. *Significantly different with respect to the control value measured in the absence of drug (P<0.005). (c) Antagonism by [D-Lys-3]-GHRP-6 (GHS-receptor antagonist) (100 μM), of the decrease in total gm induced by in vitro application of ghrelin (1 μM) in EDL muscle fibres. The bars show the absolute values of gm (±s.e.m.) from nine to 11 fibres of two animals. *Significantly different with respect to the value recorded in the absence of drug (P<0.05).
Effects of in vitro application of hexarelin and L-163,255 on the MT for contraction and cytosolic calcium concentration of skeletal muscle fibres of adult rats
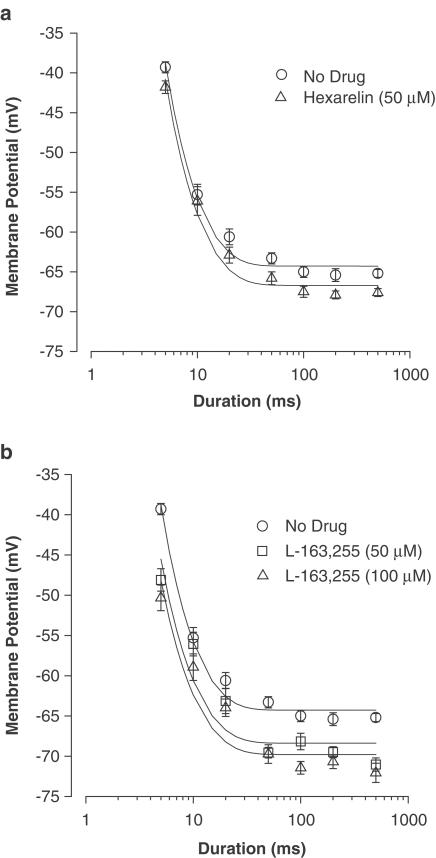
The MT for contraction is an integrative index of excitation–contraction coupling and allows the calculation of the rheobase (R), that is, the minimum voltage able to elicit contraction upon long-duration stimulation. Interestingly, the in vitro application of both GHS compounds to adult muscle fibres significantly shifted R towards more negative voltages (Figure 4). Indeed, the measured R value was –65.2±0.3 mV (n=17) in the absence of drug and –67.3±0.3 mV (n=10, P<0.001) in the presence of 50 μM hexarelin. Also, L-163,255 tested at 50 and 100 μM, produced a negative shift of the R voltage to –69.4±0.4 (n=17, P<0.001) and –70.3±0.45 mV (n=16, P<0.001), respectively.
Figure 4.
Strength–duration curves for the threshold potentials for mechanical activation of EDL muscle fibres from untreated adult rats before and after the in vitro application of 50 μM hexarelin (a) and 50 or 100 μM L-163,255 (b). Each point is the mean value±s.e.m. of the threshold potential (in mV) recorded at each pulse duration from six to 10 fibres. The curves fitting the experimental points have been obtained using the equation described in the ‘Methods section'. The rheobase (R, mV) obtained from the fits are given in the ‘Results section'.
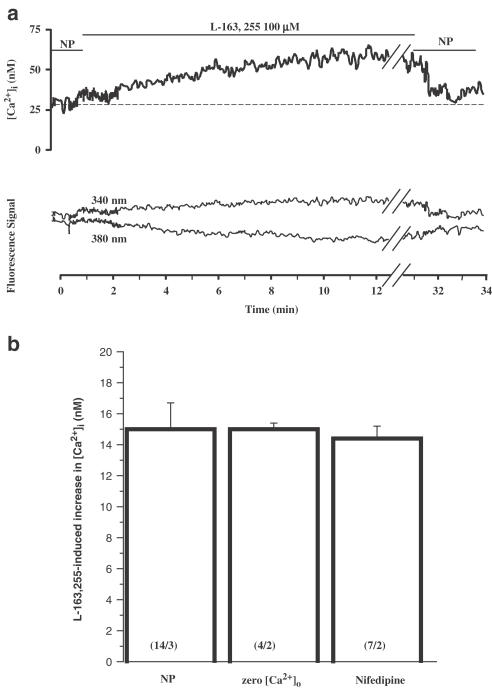
In order to verify whether GHS-induced MT lowering was related to a resting [Ca2+]i modification, we used the fluorescent calcium probe fura-2 (Figure 5). Application of 100 μM L-163,255 significantly increased [Ca2+]i from 34.1±3.3 to 51.4±5.0 nM (P<0.005, 14 muscle fibres from three rats). The GHS-induced calcium increase was characterised by a slow, 5 min long, rising phase reaching a plateau that lasted at least 30 min under continuous drug application. However, when the drug was removed, resting [Ca2+]i rapidly recovered to control value in approximately 1 min. Interestingly, the GHS-induced calcium increase was not altered by nifedipine (25 μM) or by withdrawal of Ca2+ from the perfusion solution (Figure 5b).
Figure 5.
(a) Typical calcium increase induced by application of 100 μM L-163,255 in an EDL muscle fibre (upper trace). Lower traces are fluorescence signals emitted at 510 nm after excitation of the preparation at 340 nm (fura-2 Ca2+-free form) and 380 nm (fura-2 Ca2+-bound form) (arbitrary units, for clarity 340 nm signal was scaled by a factor 1.2). NP=normal physiological solution. (b) The increase in cytosolic calcium induced by 100 μM L-163,255 was measured in NP as in (a), after removal of external calcium (zero [Ca2+]o), and in the presence of 25 μM nifedipine. The bars indicate the mean±s.e.m. from n fibres and N animals indicated within brackets as (n/N). Statistical analysis using ANOVA indicated no significant difference between the three experimental conditions (F=0.034, P>0.95).
Effects of in vivo chronic treatment with GH, hexarelin and L-163,255 on Cl− and K+ conductances and on the MT for contraction of muscle fibres of aged rats
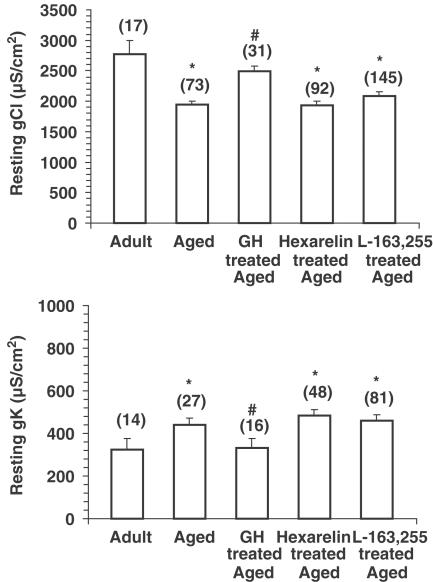
As previously described (De Luca et al., 1997), the chronic treatment of aged rats with GH was able to reverse the ageing-induced decrease in gCl observed in EDL muscle fibres. This parameter was increased by 28±4.3% (Figure 6) in preparations from GH-treated aged rats. Also, the resting gK, which underwent a significant increase in aged animals, recovered towards the adult value in response to GH treatment (Figure 6). In contrast, chronic treatment with either hexarelin or L-163,255 did not ameliorate the changes in either gCl nor gK, which remained similar to the values recorded in the preparations from untreated aged animals (Figure 6).
Figure 6.
gCl (upper panel) and gK (lower panel) measured in the EDL muscle fibres of untreated adult and untreated aged rats and aged rats chronically treated with the growth hormone secretagogues hexarelin (80 μg kg−1, daily for 4 weeks) and L-163,255 (10 mg kg−1, daily for 8 weeks). In comparison, gCl and gK have also been measured in EDL muscle of aged animals chronically treated with recombinant human GH (150 μg kg−1, daily for 8 weeks). Each column represents the mean±s.e.m. obtained from the number of fibres indicated within brackets (four to seven rats). The ANOVA showed significant differences in gCl (F=6.2; df=4/353; P<0.005) and gK (F=2.9; df=4/181; P<0.05) between untreated and treated groups. Significantly different with respect to *adult (P<0.05 or less) and #aged (P<0.05 or less) rats by Student's t-test.
To verify whether the lack of GHS effect in vivo was related to the direct action of these compounds on skeletal muscle fibres, we applied in vitro hexarelin on EDL muscle fibres of aged rats chronically treated with GH. We found that 100 μM hexarelin significantly decreased gCl and gK, from 2454±128 (n=12) to 1826±107 μS cm−2 (n=16, P<0.005) and from 336±82 (n=12) to 226±35 μS cm−2 (n=16). Thus, it appeared that the direct effects of GHS on skeletal muscle conductances can oppose the favourable effect obtained through GH treatment or the increased production of GH in GHS-treated animals.
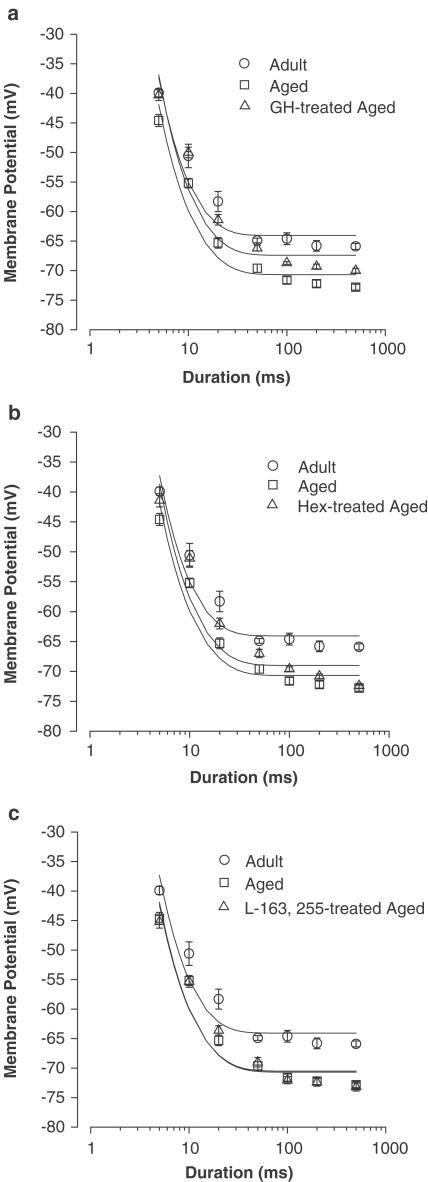
Finally, we investigated the effects of in vivo treatment with GH and GHS on the MT for the contraction of skeletal muscle fibres modified by ageing. In agreement with previous studies (De Luca & Conte Camerino, 1992), the EDL muscle fibres from aged animals needed significantly less depolarisation to contract with respect to the young adults; in fact the rheobase was shifted by 6 mV towards more negative potentials (Figure 7). The chronic treatment with GH produced a partial but significant recovery of the MT. Indeed, at each pulse duration, the threshold potential value was significantly different with respect to that found in the age-matched untreated rats (Figure 7). Consequently, the calculated value of R was intermediate between the values measured in untreated adult and aged animals (Figure 7).
Figure 7.
Strength–duration curves for the threshold potentials for mechanical activation of EDL muscle fibres from untreated adult and aged rats and aged rats chronically treated with (a) GH, (b) hexarelin (Hex) and (c) L-163,255. Each point is the mean value±s.e.m. of the threshold potential (in mV) recorded at each pulse duration from 10 to 52 fibres of four to seven animals. The rheobase values (R, mV) obtained from the fitting procedure in each experimental condition are as follows: untreated adult – 65.2±0.3; untreated aged – 71.4±0.3a; GH-treated aged – 68.6±0.2b; Hex-treated aged – 70.7±0.2a; L-163,255-treated aged – 71.2±0.3a. Significantly different with respect to auntreated adult (P<0.001) and to buntreated aged rats (P<0.05 or less).
Once again, the chronic administration of hexarelin as well as L-163,255 were ineffective in the restoration of the MT modified by the ageing process. Indeed, the threshold membrane potential values measured in muscle fibres of treated aged rats were similar to those found in the untreated aged animals, and consequently, the R value remained unchanged (Figure 7).
Discussion
This study shows for the first time that GHS directly affect skeletal muscle function. We demonstrate that peptidic and nonpeptidic GHS, as well as ghrelin, the endogenous ligand of GHS receptor, applied in vitro to rat skeletal muscle produce a concentration-dependent reduction of gCl and gK. This reduction is totally suppressed by [D-Lys-3]-GHRP-6 (Kojima et al., 1999), indicating the presence of a specific GHS receptor in skeletal muscle. At the moment, we cannot say whether the GHS receptor of rat skeletal muscle is the same as that found in the pituitary (GHS-R Ia) (Howard et al., 1996), or whether it is a receptor subtype like that described in the heart (Bodart et al., 1999), or perhaps one still to be identified (Muccioli et al., 2002). Some authors have supposed that the GHS binding sites found in human skeletal muscle are different from the first one cloned in the pituitary because they showed lower affinity for ghrelin and for some nonpeptidic GHS (Papotti et al., 2000). Recent studies have shown expression of the mRNA for ghrelin but not that of GHS-R Ia in human skeletal muscle, suggesting a role for ghrelin in this tissue and its possible interaction with a still unknown GHS-R subtype (Gnanapavan et al., 2002).
The finding that the block of gCl and gK was prevented by inhibitors of both PLC and PKC supports the involvement of these enzymes in their response to GHS. This is in line with the mechanism of action of GHS described in the pituitary (Smith et al., 1997). The activity of GHS in the CNS is mediated by a specific G-protein-coupled receptor whose stimulation generates GH release through a series of events, including IP3-dependent release of Ca2+ and activation of PKC (Bresson-Bepoldin & Dufy-Barbe, 1994; Adams et al., 1995; Chen et al., 1996). A similar mechanism may be responsible for GHS action in rat skeletal muscle. Although we cannot completely exclude that the PKC involved in muscle gCl modulation by GHS was independent of calcium, we suggest that the binding of GHS or ghrelin to the skeletal muscle receptor activates the PLC signalling pathway, producing both IP3, responsible for the release of Ca2+ from intracellular stores, and DAG, which together with the high cytosolic Ca2+ induces persistent stimulation of PKC, which in turn closes Cl− channels and reduces gCl. The increase of Ca2+ release in this tissue is confirmed by the observation that the GHS shifted the rheobase towards more negative potentials, and by the direct demonstration that L-163,255 increases resting [Ca2+]i in muscle fibres most probably through the release from IP3-dependent stores, since this effect is insensitive to external calcium removal and nifedipine. Moreover, we demonstrated in a precedent study that in vitro application of the calcium ionophore A23187 reduces muscle gCl in a manner redundant to activators of PKC (De Luca et al., 1994). Our study also shows that the block of gK by GHS in adult muscle is mediated by PKC and probably involves the phosphorylation of inwardly rectifying K+ channels that contribute to gK, since these channels are being inhibited by PKC in other tissues (Stevens et al., 1999). Similar effects are elicited acutely by GHS in the skeletal muscle of aged rats, suggesting that aged muscle tissues are also sensitive to direct GHS action.
For therapeutic aims, such results prompted us to query if during in vivo treatment with GHS, a direct modulation of skeletal muscle function could interfere with the restorative effects because of GH release. Here we find that GH chronic treatment ameliorates the contractile function in aged skeletal muscle fibres by shifting the rheobase towards more positive values, typical of adults. This effect may be related to the GH-induced production of muscle insulin-like growth factor-1 (IGF-1), which in turn normalises intracellular Ca2+ level by the restoration of the expression, turnover and activity of proteins involved in the contractile machinery (Narayanan et al., 1996; Renganathan et al., 1997; 1998; Ferrington et al., 1998; Wang et al., 1999). We also confirm the efficacy of long-term GH administration in the restoration of gCl and gK (De Luca et al., 1997). The effect on gCl was shown to result from the IGF-1-mediated activation of an okadaic acid-sensitive phosphatase that counteracts the ageing-related abnormally elevated PKC activity responsible for the inhibition of Cl− channels (De Luca et al., 1998). Moreover, the higher gK measured in aged rats is mainly because of increased activity of Ca2+-activated K+ channels at rest (Tricarico et al., 1997), and the normalisation of Ca2+ homeostasis through the GH/IGF-1 axis probably contributes to the recovery of resting gK towards adult values.
In contrast to GH, chronic treatment with doses of hexarelin and L-163,255, able to stimulate GH release (Chang et al., 1996; Cattaneo et al., 1997), failed to ameliorate the low gCl, the high gK or the negative rheobase, typical of aged subjects. Thus, the direct interaction of GHS with its skeletal muscle receptor may counteract the effects induced by GH release. We hypothesise that chronic treatment with GHS may shift the phosphorylation/dephosphorylation balance of Cl− channels in favour of phosphorylation, because of the direct activation of PKC at the muscle level over the indirect activation of phosphatase sustained by IGF-1. This hypothesis is strongly supported by the finding that in vitro exposure to GHS of GH-treated aged muscle is able to reverse the effect of chronic exposure to GH. In addition, the increased intracellular Ca2+ concentration induced by GHS may counteract the beneficial effect on gK obtained through stimulation of the GH/IGF-1 axis.
In conclusion, our study confirms the beneficial effect of GH supplementation in the elderly but indicates that the GHS may not be able to correct the ageing-induced impairment of skeletal muscle. Although GHS stimulate the release of GH at the pituitary level, the activation of a specific G-protein-coupled receptor at the skeletal muscle level may counteract the amelioration brought about through the GH/IGF-1 axis. The knowledge of muscle GHS receptors likely different from those expressed in the CNS level opens the way to design more specific drugs with peripheral or central activity.
Acknowledgments
This work has been supported by a grant from the European Community (C11*-CT94-0037). We thank the American National Hormone and Pituitary Program of the N.I.H. for providing the rat growth hormone and Merck Research Laboratories, Rahway, NJ (U.S.A.), for providing L-163,255 and for the generous support. We also thank Dr Gianpatrizio Bianco for computer assistance.
Abbreviations
- ClC-1
skeletal muscle chloride channel
- EDL
extensor digitorum longus
- gCl
resting chloride conductance
- GH
growth hormone
- GHS
GH secretagogues
- gK
resting potassium conductance
- gm total
membrane conductance
- IGF-1
Insulin-like growth factor-1
- MT
mechanical threshold
- PKC
protein kinase C
- PLC
phospholipase C
References
- ADAMS E.F., PETERSEN B., LEI T., BUCHFELDER M., FAHLBUSCH R. The growth hormone secretagogue, L-692,429 induces phosphatidylinositol hydrolysis and hormone secretion by human pituitary tumors. Biochem. Biophys. Res. Commun. 1995;208:555–561. doi: 10.1006/bbrc.1995.1374. [DOI] [PubMed] [Google Scholar]
- ANKERSEN M., HANSEN T.K., AHNFELT-RONNE I., KAPPELGAARD A.M. Growth hormone secretagogues: recent advances and applications. Drug Discov. Today. 1999;4:497–506. doi: 10.1016/s1359-6446(99)01415-4. [DOI] [PubMed] [Google Scholar]
- BODART V., BOUCHARD J.F., MCNICOLL N., ESCHER E., CARRIÈRE P., GHIGO E., SEJLITZ T., SIROIS M.G., LAMONTAGNE D., ONG H. Identification and characterization of a new growth hormone-releasing peptide receptor in the heart. Circ. Res. 1999;85:796–802. doi: 10.1161/01.res.85.9.796. [DOI] [PubMed] [Google Scholar]
- BRESSON-BEPOLDIN L., DUFY-BARBE L. GHRP-6 induces a biphasic calcium response in rat pituitary somatotrophs. Cell Calcium. 1994;15:247–258. doi: 10.1016/0143-4160(94)90064-7. [DOI] [PubMed] [Google Scholar]
- BRYANT S.H., CONTE CAMERINO D. Chloride channel regulation in the skeletal muscle of normal and myotonic goats. Pflügers Arch. 1991;417:605–610. doi: 10.1007/BF00372958. [DOI] [PubMed] [Google Scholar]
- CASANUEVA F.F., DIEGUEZ C. Growth hormone secretagogues: physiological role and clinical utility. Trends Endocrinol. Metab. 1999;10:30–38. doi: 10.1016/s1043-2760(98)00116-7. [DOI] [PubMed] [Google Scholar]
- CATTANEO L., LUONI M., SETTEMBRINI B., MÜLLER E.E., COCCHI D. Effect of long-term administration of hexarelin on the somatotrophic axis in aged rats. Pharmacol. Res. 1997;36:49–54. doi: 10.1006/phrs.1997.0203. [DOI] [PubMed] [Google Scholar]
- CHANG C.H., RICKES E.L., MCGUIRE L., FRAZIER E., CHEN H., BARAKAT K., NARGUND R., PATCHETT A., SMITH R.G., HICKEY G.J. Growth hormone (GH) and insulin-like growth factor-I responses after treatments with an orally active GH secretagogue L-163,255 in swine. Endocrinology. 1996;137:4851–4856. doi: 10.1210/endo.137.11.8895356. [DOI] [PubMed] [Google Scholar]
- CHEN C., WU D., CLARKE I.J. Signal transduction systems employed by synthetic GH-releasing peptides in somatotrophs. J. Endocrinol. 1996;148:381–386. doi: 10.1677/joe.0.1480381. [DOI] [PubMed] [Google Scholar]
- CONTE CAMERINO D., DE LUCA A., MAMBRINI M., VRBOVÀ G. Membrane ionic conductances in normal and denervated skeletal muscle of the rat during development. Pflügers Arch. 1989;413:568–570. doi: 10.1007/BF00594192. [DOI] [PubMed] [Google Scholar]
- DE GENNARO COLONNA V., ROSSONI G., BERNAREGGI M., MÜLLER E.E., BERTI F. Cardiac ischemia and impairment of vascular endothelium function in hearts from growth hormone-deficient rats: protection by hexarelin. Eur. J. Pharmacol. 1997;334:201–207. doi: 10.1016/s0014-2999(97)01178-3. [DOI] [PubMed] [Google Scholar]
- DE LUCA A., CONTE CAMERINO D. Effect of aging on the mechanical threshold of rat skeletal muscle fibers. Pflügers Arch. 1992;420:407–409. doi: 10.1007/BF00374477. [DOI] [PubMed] [Google Scholar]
- DE LUCA A., PIERNO S., COCCHI D., CONTE CAMERINO D. Effects of chronic growth hormone treatment in aged rats on the biophysical and pharmacological properties of skeletal muscle chloride channels. Br. J. Pharmacol. 1997;121:369–374. doi: 10.1038/sj.bjp.0701129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LUCA A., PIERNO S., LIANTONIO A., CAMERINO C., CONTE CAMERINO D. Phosphorylation and IGF-1 mediated dephosphorylation pathways control the activity and the pharmacological properties of skeletal muscle chloride channels. Br. J. Pharmacol. 1998;125:477–482. doi: 10.1038/sj.bjp.0702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LUCA A., TRICARICO D., PIERNO S., CONTE CAMERINO D. Aging and chloride channel regulation in rat fast-twitch muscle fibers. Pflügers Arch. 1994;427:80–85. doi: 10.1007/BF00585945. [DOI] [PubMed] [Google Scholar]
- DESAPHY J.-F., DE LUCA A., IMBRICI P., CONTE CAMERINO D. Modification by ageing of the tetrodotoxin-sensitive sodium channels in rat skeletal muscle fibres. Biochim. Biophys. Acta. 1998a;1373:37–46. doi: 10.1016/s0005-2736(98)00085-6. [DOI] [PubMed] [Google Scholar]
- DESAPHY J.-F., DE LUCA A., PIERNO S., IMBRICI P., CONTE CAMERINO D. Partial recovery of skeletal muscle sodium channel properties in aged rats chronically treated with growth hormone or the GH-secretagogue hexarelin. J. Pharmacol. Exp. Ther. 1998b;286:903–912. [PubMed] [Google Scholar]
- DULHUNTY A. Internal citrate ions reduce the membrane potential for contraction threshold in mammalian skeletal muscle fibers. Biophys. J. 1988;53:609–615. doi: 10.1016/S0006-3495(88)83139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRINGTON D.A., KRAINEV A.G., BIGELOW D.J. Altered turnover of calcium regulatory proteins of the sarcoplasmic reticulum in aged skeletal muscle. J. Biol. Chem. 1998;273:5885–5891. doi: 10.1074/jbc.273.10.5885. [DOI] [PubMed] [Google Scholar]
- GNANAPAVAN S., KOLA B., BUSTIN S.A., MORRIS D.G., MCGEE P., FAIRCLOUGH P., BHATTACHARYA S., CARPENTER R., GROSSMAN A.B., KORBONITS M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 2002;87:2988–2991. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- GREEN J.R., MARGERISON D. Statistical Treatment of Experimental Data. New York: Elsevier; 1978. pp. 86–88. [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- HOSODA H., KOJIMA M., MATSUO H., KANGAWA K. Purification and characterization of rat des-Gln14-Ghrelin, a second endogenous ligand for the growth hormone secretagogue receptor. J. Biol. Chem. 2000;275:1995–2000. doi: 10.1074/jbc.M002784200. [DOI] [PubMed] [Google Scholar]
- HOWARD A.D., FEIGHNER S.D., CULLY D.F., ARENA J.P., LIBERATOR P.A., ROSENBLUM C.I., HAMELIN M., HRENIUK D.L., PALYHA O.C., ANDERSON J., PARESS P.S., DIAZ C., CHOU M., LIU K.K., MCKEE K.K., PONG S.-S., CHAUNG L.-Y., ELBRECHT A., DASHKEVICZ M., HEAVENS R., RIGBY M., SIRINATHSINGHJI D.J.S., DEAN D.C., MELILLO D.G., PATCHETT A.A., NARGUND R., GRIFFIN P.R., DEMARTINO J.A., GUPTA S.K., SCHAEFFER J.M., SMITH R.G., VAN DER PLOEG L.H.T. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- KATUGAMPOLA S.D., PALLIKAROS Z., DAVENPORT A.P. [125I-His9]–Ghrelin, a novel radioligand for localizing GHS orphan receptors in human and rat tissue; up-regulation of receptors with atherosclerosis. Br. J. Pharmacol. 2001;134:143–149. doi: 10.1038/sj.bjp.0704228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOJIMA M., HOSODA H., DATE Y., NAKAZATO M., MATSUO H., KANGAWA K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- LOCATELLI V., ROSSONI G., SCHWEIGER F., TORSELLO A., DE GENNARO COLONNA V., BERNAREGGI M., DEGHENGHI R., MÜLLER E.E., BERTI F. Growth hormone independent cardioprotective effects of hexarelin in the rat. Endocrinology. 1999;140:4024–4031. doi: 10.1210/endo.140.9.6948. [DOI] [PubMed] [Google Scholar]
- MUCCIOLI G., TSCHÖP M., PAPOTTI M., DEGHENGHI R., HEIMAN M., GHIGO E. Neuroendocrine and peripheral activities of ghrelin: implications in metabolism and obesity. Eur. J. Pharmacol. 2002;440:235–254. doi: 10.1016/s0014-2999(02)01432-2. [DOI] [PubMed] [Google Scholar]
- MÜLLER E.E., LOCATELLI V., COCCHI D. Neuroendocrine control of growth hormone secretion. Physiol. Rev. 1999;79:511–607. doi: 10.1152/physrev.1999.79.2.511. [DOI] [PubMed] [Google Scholar]
- NARAYANAN N., JONES D.L., XU A., YU J.C. Effects of aging on sarcoplasmic reticulum function and contraction duration in skeletal muscles of the rat. Am. J. Physiol. 1996;271:C1032–C1040. doi: 10.1152/ajpcell.1996.271.4.C1032. [DOI] [PubMed] [Google Scholar]
- PALADE P.T., BARCHI R.L. Characteristics of the chloride conductance in muscle fibers of the rat diaphragm. J. Gen. Physiol. 1977;69:325–342. doi: 10.1085/jgp.69.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPOTTI M., GHE' C., CASSONI P., CATAPANO F., DEGHENGHI R., GHIGO E., MUCCIOLI G. Growth hormone secretagogue binding sites in peripheral human tissues. J. Clin. Endocrinol. Metab. 2000;85:3803–3807. doi: 10.1210/jcem.85.10.6846. [DOI] [PubMed] [Google Scholar]
- PIERNO S., DE LUCA A., BECK C.L., GEORGE A.L., JR, CONTE CAMERINO D. Aging-associated down-regulation of ClC-1 expression in skeletal muscle: phenotypic-independent relation to the decrease of chloride conductance. FEBS Lett. 1999;449:12–16. doi: 10.1016/s0014-5793(99)00202-1. [DOI] [PubMed] [Google Scholar]
- PIERNO S., DE LUCA A., CAMERINO C., HUXTABLE R.J., CONTE CAMERINO D. Chronic administration of taurine to aged rats improves the electrical and contractile properties of skeletal muscle fibers. J. Pharmacol. Exp. Ther. 1998;286:1183–1190. [PubMed] [Google Scholar]
- RENGANATHAN M., MESSI M.L., DELBONO O. Overexpression of IGF-I exclusively in skeletal muscle prevents age-related decline in the number of dihydropyridine receptors. J. Biol. Chem. 1998;273:28845–28851. doi: 10.1074/jbc.273.44.28845. [DOI] [PubMed] [Google Scholar]
- RENGANATHAN M., SONNTAG W.E., DELBONO O. L-type Ca2+ channel-insulin-like growth factor-1 receptor signaling impairment in aging rat skeletal muscle. Biochem. Biophys. Res. Commun. 1997;235:784–789. doi: 10.1006/bbrc.1997.6881. [DOI] [PubMed] [Google Scholar]
- ROSSONI G., DE GENNARO COLONNA V., BERNAREGGI M., POLVANI G.L., MÜLLER E.E., BERTI F. Protectant activity of Hexarelin or growth hormone against post-ischemic ventricular dysfunction in hearts from aged rats. J. Cardiovasc. Pharmacol. 1998;32:260–265. doi: 10.1097/00005344-199808000-00013. [DOI] [PubMed] [Google Scholar]
- SMITH R.G., FEIGHNER S., PRENDERGAST K., GUAN X., HOWARD A. A new orphan receptor involved in pulsatile growth hormone release. Trends Endocrinol. Metab. 1999;10:128–135. doi: 10.1016/s1043-2760(98)00132-5. [DOI] [PubMed] [Google Scholar]
- SMITH R.G., VAN DER PLOEG L.H.T., HOWARD A.D., FEIGHNER S.D., CHENG K., HICKEY G.J., WYVRATT M.J., FISHER M.H., NARGUND R.P., PATCHETT A.A. Peptidomimetic regulation of growth hormone secretion. Endocr. Rev. 1997;18:621–645. doi: 10.1210/edrv.18.5.0316. [DOI] [PubMed] [Google Scholar]
- STEINMEYER K., ORTLAND C., JENTSCH T.J. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature (Lond.) 1991;354:301–304. doi: 10.1038/354301a0. [DOI] [PubMed] [Google Scholar]
- STEVENS E.B., SHAH B.S., PINNOCK R.D., LEE K. Bombesin receptors inhibit G protein-coupled inwardly rectifying K+ channels expressed in Xenopus oocytes through a protein kinase C-dependent pathway. Mol. Pharmacol. 1999;55:1020–1027. [PubMed] [Google Scholar]
- TALLARIDA R.J., MURRAY R.B. Manual of Pharmacologic Calculations with Computer Programs. New York: Springer-Verlag; 1987. [Google Scholar]
- TRICARICO D., PETRUZZI R., CONTE CAMERINO D. Changes of the biophysical properties of calcium-activated potassium channels of rat skeletal muscle fibres during aging. Pflügers Arch. 1997;434:822–829. doi: 10.1007/s004240050471. [DOI] [PubMed] [Google Scholar]
- WANG Z.-M., MESSI M.L., RENGANATHAN M., DELBONO O. Insulin-like growth factor-1 enhances rat skeletal muscle charge movement and L-type Ca2+ channel gene expression. J. Physiol. 1999;516:331–341. doi: 10.1111/j.1469-7793.1999.0331v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]