Abstract
The effects on the whole-cell carbachol-induced muscarinic cationic current (mIcat) of antibodies against the α-subunits of various G proteins, as well as the effect of a Gβγ subunit, were studied in single guinea-pig ileal smooth muscle cells voltage-clamped at −50 mV. Ionized intracellular calcium concentration, [Ca2+]i, was clamped at 100 nM using a 1,2-bis(2-aminophenoxyl-ethane-N,N,N′,N′-tetraacetic acid)/Ca2+ mixture.
Application of ascending concentrations of carbachol (1–300 μM) activated mIcat (mean amplitude 0.83 nA at 300 μM carbachol; EC50 8 μM; Hill slope 1.0). A 20 min or longer intracellular application via the pipette solution of Gi3/Go or Go antibodies resulted in about a 70% depression of the maximum response without change in the EC50 value. In contrast, antibodies against α-subunits of Gi1, Gi1/Gi2, Gi3, Gq/G11 or Gs protein over a similar or longer period did not significantly reduce mIcat. Antibodies to common Gβ or infusion of the Gβγ subunit itself had no effect on mIcat.
If cells were exposed briefly to carbachol (50 or 100 μM) at early times (<3 min) after infusion of antibodies to Gαi3/Gαo or to Gαo had begun, carbachol responses remained unchanged even after 20–60 min; that is, the depression of mIcat by these antibodies was prevented.
These data show that Gαo protein couples the muscarinic receptor to the cationic channel in guinea-pig ileal longitudinal smooth muscle and that Gβγ is not involved. They also show that prior activation of the muscarinic receptor presumably causes a long-lasting postactivation change of the G protein, which is not reflected in mIcat, but acts to hinder antibody binding.
Keywords: Smooth muscle, GTP binding protein, muscarinic receptor, carbachol, cationic current, antibody
Introduction
Smooth muscles exhibit a number of cation currents. However, not only do these have different properties, but also the mechanisms by which they are gated are also different. In the longitudinal muscle of the guinea-pig small intestine, the cation current evoked by muscarinic receptor activation (mIcat) is strongly modulated by voltage, giving rise to a markedly U-shaped current–voltage (I–V) relation (Zholos & Bolton, 1994). A similar I–V relation is shown by mIcat in canine gastric smooth muscle cells; however, in complete contrast to the situation in guinea-pig small intestine, this current can also be evoked by caffeine and therefore is presumably gated by a rise in [Ca2+]i (Sims, 1992). In rabbit portal vein myocytes, the cation current evoked by noradrenaline shows similar properties to mIcat in guinea-pig small intestine at negative potentials but, unlike the latter, passes little current in the positive range (Helliwell & Large, 1996); it can be evoked by 1-oleoyl-2-acetyl-sn-glycerol (OAG) (Helliwell & Large, 1997), which is completely ineffective in evoking mIcat in the guinea-pig intestine (our unpublished observation). mIcat in guinea-pig small intestine is pertussis toxin (PTX)-sensitive (Inoue & Isenberg, 1990; Komori et al., 1992), as it is in equine tracheal smooth muscle cells (Wang et al., 1997). In the latter, the current was blocked by Gαi1/Gαi2 antibodies and by Gαi3/Gαo antibodies, but not by Gαq/Gα11 antibodies, suggesting that Gαi/Gαo was the link between muscarinic receptor and cation channel. However, this is in contrast to mouse gastric myocytes where mIcat was blocked by Gαq/Gα11 antibodies (Lee et al., 2002). It would appear that in smooth muscle muscarinic receptors link to a variety of cation channels, which are gated by the binding of different G proteins, and possibly other ligands.
We have also found that different muscarinic acetylcholine receptor (mAChR) subtypes provide (presumably different) concurrent signals for mIcat generation (Zholos & Bolton, 1997); activation of M2 receptor primarily opens cationic channels which are modulated by M3 muscarinic receptors (Bolton & Zholos, 1997). However, the identity of G proteins which link M2 and M3 subtype mAChRs to the cationic channel remains to be established.
In this paper, we describe the effects of antibodies against the α subunits of Gi1, Gi1/Gi2, Gi3, Gi3/Go, Go, Gq/G11 and Gs proteins, as well as antibodies against the Gβ (common) subunit and the effect of a βγ subunit, on mIcat generation in response to carbachol in longitudinal smooth muscle cells of the guinea-pig ileum.
Methods
Cell preparation and current recording
Adult male guinea pigs, weighing 300–400 g, were killed by dislocation of the neck followed by immediate exsanguination. Single smooth muscle myocytes from the longitudinal muscle layer of the ileum were obtained after collagenase and papain treatment as previously described (Komori et al., 1992; Zholos & Bolton, 1996a).
Patch pipettes were made of borosilicate glass (resistance 1–4 MΩ). Membrane current was recorded with either the voltage-clamp amplifier, Axopatch 200A (Axon Instruments Inc., Foster City, CA, U.S.A.) or SEZ-2300 (Nihon Kohden Inc., Tokyo, Japan), as described previously (Zholos & Bolton, 1997; Komori et al., 1998). Experiments were performed at room temperature (22–26°C).
Solutions
Patch pipettes were filled with the following solution (in mM): CsCl 80, adenosine 5′-trisphosphate magnesium salt (ATP) 1, guanosine 5′-trisphosphate sodium salt (GTP) 1, creatine 5, glucose 5 or 20, N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES) 10, 1,2-bis(2-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid (BAPTA) 10 and CaCl2 4.6, pH adjusted to 7.4 with CsOH (total Cs+ 124 mM). This solution was useful for blockade of K+ current, as Cs+ does not readily pass through K+ channels but freely moves through cationic channels (Zholos & Bolton, 1996b). The presence of 1 mM GTP in the pipette solution reduces desensitization to a minimum (Zholos & Bolton, 1996a). The use of a 10 mM BAPTA/4.6 mM CaCl2 combination to clamp [Ca2+]i at 100 nM effectively prevents mIcat modulation due to changes in [Ca2+]i (Pacaud & Bolton, 1991).
The basic external solution in which current responses to carbamylcholine chloride (carbachol) were recorded had the following composition (in mM): CsCl 120, glucose 12, HEPES 10, pH adjusted to 7.4 with CsOH (total Cs+ 124–126 mM). This solution contained no added Ca2+ and Mg2+ ions as their presence reduces mIcat (Zholos & Bolton, 1995). The cells until 10–20 s before their exposure to carbachol were kept in the following solution (in mM): NaCl 126, KCl 6, CaCl2 2, MgCl2 1.2, glucose 14 and HEPES 10.5, pH adjusted to 7.4 with NaOH. This solution was also used to wash the cell after carbachol application.
Detection of G-protein subtypes in longitudinal smooth muscle from the small intestine
The longitudinal smooth muscle layer was dissected from the guinea-pig small intestine and homogenized using an ultra turrax in nondenaturing buffer (50 mM Tris-HCl pH 7.4, 300 mM NaCl, 5 mM EDTA) containing a protease inhibitor cocktail (Roche). Triton-X-100 was then added to a final volume of 2% and the membranes incubated for 3 h at 4°C. The Triton-X-100 concentration was adjusted to 1% and the homogenate was centrifuged at 13,000 × g for 10 min and the proteins in the supernatant boiled for 5 min in denaturing solution. Denatured membrane proteins (50 μg) were separated using an 8% SDS–polyacrylamide gel and electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes (Biorad) using a Biorad Trans Blot semidry blotter. The membranes were then cut into strips and blocked by incubation in blocking buffer (5% Marvel, 0.5% Tween-20 in Tris-buffered saline (TBS)) for 1 h at room temperature, after which each strip was incubated with antibodies to either Gαs, Gαo, Gαi3, Gαi2 or Gαi1/Gαi2 (each at 1 : 1000 dilution) in 5% Marvel, 0.05% Tween-20 in TBS buffer overnight at 4°C with continuous rocking. The membranes were then washed three times in 2.5% Marvel, 0.5% Tween-20 in TBS, then TBS plus 0.5% Tween-20 and finally TBS followed by incubation with the secondary antibody, anti-rabbit IgG (1 : 5000) conjugated with horseradish peroxidase in 2.5% Marvel in TBS for 2 h at room temperature with continuous rocking. This was followed by three washes in 2.5% Marvel, 0.5% Tween in TBS, 1 wash in TBS plus 0.5% Tween and finally TBS. Immunoreactive bands were visualized using the enhanced chemiluminescence (ECL) kit (Amersham Corp.), followed by a 1 min exposure using ECL Hyperfilm (Amersham Corp.).
Measurements and data analysis
mIcat was measured at a holding potential of −50 mV. In some experiments, its I–V relation was obtained by applying a slow 6 s duration voltage ramp from 80 to −120 mV before and after carbachol application with off-line correction for the background currents.
Concentration–effect curves were constructed by plotting mIcat amplitude against the carbachol concentration on a semilogarithmic scale. They were fitted by the Hill equation in the following form: I=Imax{1+([EC50]/[A])h}−1 where I is the mIcat amplitude at a given concentration of carbachol, [A], Imax is the amplitude of mIcat at a maximally effective carbachol concentration, EC50 is the carbachol concentration required to produce a current equal to 50% of Imax and h is the slope factor of the agonist curve.
Values are given as the means±s.e.m; n represents the number of cells tested. EC50 and slope factor were obtained by curve fitting of the averaged concentration–effect data points or individual values obtained from each cell. To determine the statistical significance of differences between the group means, a t-test (for two groups) or one-way analysis of variance (ANOVA) (for three or more groups) followed by a post hoc Dunnett multiple comparison test to compare all data vs control was used. The underlying assumptions that the data follow Gaussian distributions and have identical standard deviations (s.d.s) were tested using the method of Kolmogorov–Smirnov and Bartlett (the latter for ANOVA), respectively. It was verified that all measured or fitted variables (e.g., Imax, EC50, and Hill slope) passed a normality test with P>0.1 (note that logarithmic transformation of the data was used for Imax and EC50 values; see Figure 1b as an example). Also, when performing various t-tests, the differences between s.d. values were not significant in all cases (P ranging from 0.06 to 0.5; not presented for individual tests for simplicity). However, using an ANOVA for 10 groups of data, it was found that Barlett's test suggested significant differences between the s.d.s (the P-values were 0.0002, 0.0001 and 0.013 for fitted individual log(Imax), log(EC50) and h values, respectively). This was likely due to the large number of treatment groups. Thus, a Kruskal–Wallis test (nonparametric ANOVA) was used to test whether the median of a variable differs between the groups. This was followed by Dunn's multiple comparisons test. Since the nonparametric tests are robust, but not as powerful compared to the parametric tests, it is likely that only the major differences were revealed, and some smaller effects of the antibodies may have been undetected. Differences were judged to be statistically significant when P<0.05. Data in Table 2 refer to semilogarithmic plots in Figures 1d, 3,4b,d, 5b and 6d; these plots were constructed by measuring the maximum or steady-state values of mIcat attained during application of carbachol at the indicated concentrations. Responses on several cells held with pipettes containing the same antibody (or controls) were measured and were averaged for each concentration of carbachol (shown as mean±s.d.) and curves fitted by the Hill equation. Data in Table 3 refer to parameters of curves obtained in the following way: concentration–effect curves for mIcat on each cell were fitted individually using the Hill equation and the parameters (Imax, EC50 and h) averaged for the control group and for cells held with pipettes containing the same antibody.
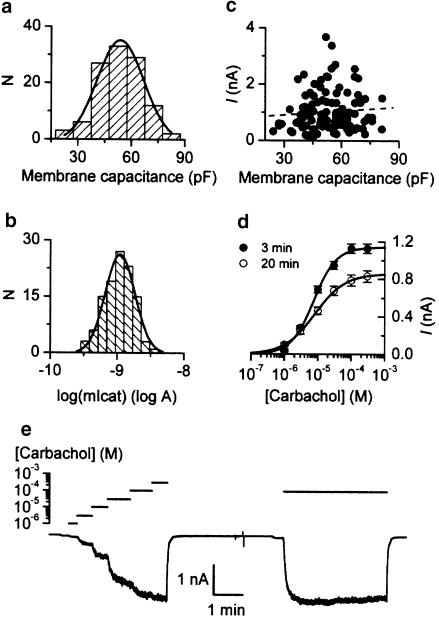
Figure 1.
(a) Frequency distribution of cell size expressed as membrane capacitance (n=112). (b) Frequency distribution of logarithm of maximum mIcat responses in 112 cells fitted by a normal curve. (c) Plot of size of cation current response against membrane capacitance; no correlation is seen (correlation coefficient R=0.08, dotted line). (d) Mean agonist curves measured in 113 cells at 3 min and in 53 cells at 20 min after breakthrough. Fitted parameters were: Imax 1.142 nA (3 min) and 0.857 nA (20 min); EC50 7.5 μM (3 min) and 8.1 μM (20 min); Hill slope 1.3 (3 min) and 1.0 (20 min). (e) Estimation of extent of mIcat desensitization: ascending concentrations of carbachol up to 300 μM were applied followed after washout by a longer application of 100 μM. The response to the latter is identical to that obtained at 100 μM when the ascending series of carbachol concentrations was applied.
Table 2.
The maximum amplitude (Imax), 50% effective concentration (EC50) and Hill slope (h) of the carbachol concentration–response curves for mIcat activation in control and anti-G protein antibody-treated cells and in Gβγ-treated cells
| Imax (nA) | EC50 (μM) | h | |
|---|---|---|---|
| Antibodies experiments | |||
| Control | 0.857 | 8.1 | 1.0 |
| Anti-Gαi3/Gαo (Calbiochem) | 0.338 | 4.3 | 1.18 |
| Anti-Gαi3/Gαo (NEN) | 0.165 | 8.1 | 0.79 |
| Anti-Gαo (Calbiochem) | 0.229 | 8.0 | 1.65 |
| Anti-Gαi1/Gαi2 (Calbiochem) | 0.783 | 4.4 | 2.15 |
| Anti-Gαi1/Gαi2 (NEN) | 0.891 | 6.2 | 0.54 |
| Anti-Gαi1 (Calbiochem) | 0.994 | 10.8 | 1.29 |
| Anti-Gαi3 (Calbiochem) | 0.914 | 8.0 | 1.30 |
| Anti-Gαs (Calbiochem) | 1.050 | 3.1 | 1.57 |
| Anti-Gαq/Gα11 (NEN) | 0.862 | 5.7 | 0.58 |
| Gβγ experiments | |||
| Control | 0.921 | 2.4 | 1.3 |
| Gβγ | 0.804 | 2.4 | 1.1 |
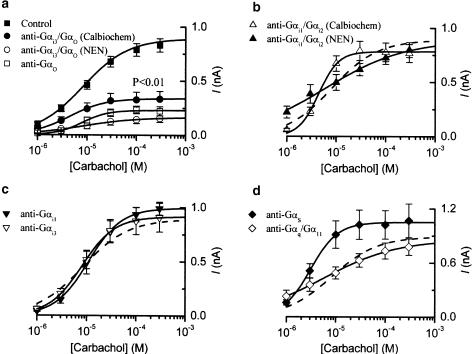
Figure 3.
Summary of results of experiments where antibodies to various G-protein α subunits were added to the pipette solution. mIcat was measured in the same way as shown in Figure 2, and its amplitude was plotted against the carbachol concentration on a logarithmic scale. Control data (n=53) are shown in (a) and as a dotted line in (b–d) for comparison. (a) Gαi3/Gαo antibodies (Calbiochem, n=9; NEN™, n=10) and Gαo antibodies (Calbiochem, n=7); (b) Gαi1/Gαi2 antibodies (Calbiochem, n=6; NEN™, n=10); (c) Gαi1 antibodies (Calbiochem, n=6) and Gαi3 antibodies (Calbiochem, n=8); (d) Gαs antibodies (Calbiochem, n=6) and Gαq/Gα11 antibodies (NEN™, n=13). All antibodies were used at 1 : 200 v v−1 dilution, except for Gαi1/Gαi2 and Gαq/Gα11 antibodies from NEN™ where 1 : 100 v v−1 dilution was used. Each point indicates the mean±s.e.m. (vertical bars). The individual sets of points were fitted by the Hill equation (see Methods) with the parameters shown in Table 2. Significant difference in the group mean from the control mean value occurred only for Gαi3/Gαo and Gαo antibodies (Table 3).
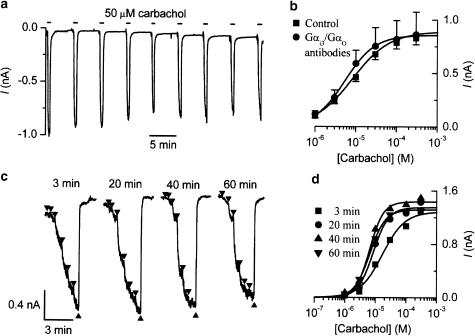
Figure 4.
The depression of mIcat by Gαi3/Gαo antibodies is prevented by carbachol application shortly after breakthrough to the whole-cell recording mode. (a) Current trace from a cell dialysed with anti-Gαi3/Gαo antibodies (Calbiochem; 1 : 200 v v−1) and repeatedly exposed to carbachol (50 μM) every 5 min starting 90 s after breakthrough. Note that the decline in mIcat was no more than the usual desensitization seen in control cells. (b) Averaged concentration–effect curves for carbachol-activated mIcat in cells dialysed with (n=4) or without (same data as shown in Figure 3a; n=53) the Gαi3/Gαo antibody. The former cells were exposed to carbachol (50 μM) for about 1 min at 1–3 min after the breakthrough. See text for details. (c) Current traces from a cell dialysed with Gαi3/Gαo antibodies and exposed to ascending carbachol concentrations (1–300 μM, shown by triangles) applied every 20 min starting 3 min after the breakthrough. (d) Concentration–response curves for the experiment shown in (c). Note that no inhibition of the agonist curve by Gαi3/Gαo antibody occurred using this protocol even at 60 min.
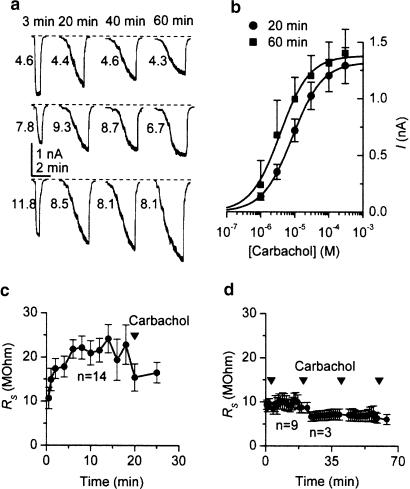
Figure 5.
Lack of mIcat inhibition in cells which were briefly exposed to 100 μM carbachol at 3 min after breakthrough and dialysed with Gαo antibodies. (a) Examples from three cells; the numbers next to each trace indicate Rs values (MΩ) which were measured shortly before. (b) Averaged concentration–effect curves measured at 20 min (n=6) and at 60 min (n=3) in the presence of the antibodies after carbachol exposure at 3 min. Fitted parameters were as follows: Imax 1.322 and 1.380 nA, EC50 8.5 and 3.7 μM and slope 1.0 and 1.0 at 20 and 60 min, respectively. (c) Changes in series resistance with time during whole-cell recording from 14 cells. The application of carbachol (1–300 μM) after 20 min is indicated by triangles. (d) Changes in series resistance with time of recording and following carbachol application (single 100 μM concentration at 3 min; later application 1–300 μM). Up to 20 min, nine cells were averaged; three cells were held for longer periods. Series resistance was measured before applications of carbachol and tended to be reduced in periods following an application.
Figure 6.
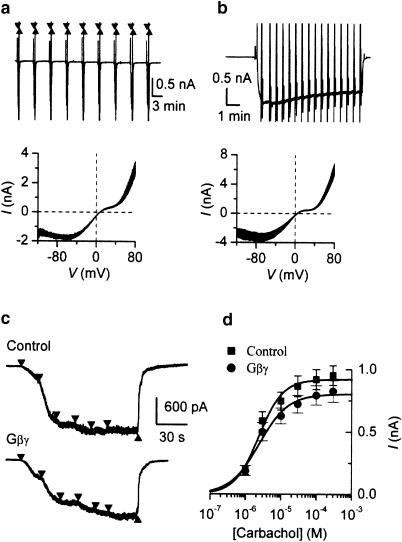
Gβ antibody and Gβγ subunit infusion did not affect mIcat properties. Carbachol in the presence of Gβ antibodies (1 : 200 v v−1) was applied at 50 μM concentration every 5 min (a) or for a long period (b) to measure steady-state I–V curves estimated by applying slow voltage ramp from 80 to −120 mV. Below are the superimposed I–V curves from the experiments in the panels above measured at 5 min intervals starting 20 min after breakthrough (a) and at 30 s interval starting at 35 min after breakthrough (b) For leak correction, the I–V curve measured immediately before carbachol application was subtracted. (c) Lack of effect of Gβγ dimer on mIcat generation obtained in a control (top) and in Gβγ -treated cell (bottom). (d) Mean data fitted by the Hill equation (control, n=17; Gβγ, n=13). Best-fit parameters were as follows: Imax 0.920 and 0.804 nA; EC50 2.4 and 2.4 μM; Hill slope 1.3 and 1.1 for control and Gβγ -treated groups, respectively.
Table 3.
Mean maximum amplitude, 50% effective concentration and Hill slope (h) of the carbachol concentration–response curves for mIcat activation in control and anti-G-protein antibody-treated cells and in Gβγ-treated cells
| log(Imax) (log A) | log(EC50) (log M) | h | n | |
|---|---|---|---|---|
| Antibodies experiments | ||||
| Control | −9.14±0.03 | −5.14±0.05 | 1.31±0.06 | 53 |
| Anti-Gαi3/Gαo (Calbiochem) | −9.56±0.11 | −5.32±0.09 | 1.26±0.14 | 9 |
| P<0.05 (<0.01) | ||||
| Anti-Gαi3/Gαo (NEN) | −9.93±0.14 | −4.77±0.25 | 1.57±0.19 | 10 |
| P<0.001 (<0.01) | ||||
| Anti-Gαo (Calbiochem) | −9.66±0.06 | −5.06±0.07 | 1.69±0.17 | 7 |
| P<0.01 (<0.01) | ||||
| Anti-Gαi1/Gαi2 (Calbiochem) | −9.12±0.06 | −5.38±0.05 | 2.05±0.03 | 6 |
| P<0.05 (<0.01) | ||||
| Anti-Gαi1/Gαi2 (NEN) | −9.11±0.03 | −5.36±0.15 | 0.95±0.09 | 10 |
| Anti-Gαi1 (Calbiochem) | −9.01±0.03 | −4.95±0.12 | 1.50±0.12 | 6 |
| Anti-Gαi3 (Calbiochem) | −9.07±0.07 | −5.09±0.11 | 1.73±0.15 | 8 |
| Anti-Gαs (Calbiochem) | −9.01±0.08 | −5.53±0.07 | 1.62±0.13 | 6 |
| P<0.05 (>0.05) | ||||
| Anti-Gαq/Gα11 (NEN) | −9.12±0.05 | −5.33±0.15 | 0.90±0.08 | 13 |
| P<0.05 (<0.05) | ||||
| Gβγ experiments | ||||
| Control | −9.05±0.04 | −5.59±0.09 | 1.41±0.13 | 17 |
| Gβγ | −9.13±0.05 | −5.62±0.08 | 1.33±0.14 | 13 |
| P=0.20 | P=0.080 | P=0.68 |
These parameters were determined by curve fitting of individual experiments (see Methods). Bold figures indicate statistically significant changes compared to control with the P-values shown below. The latter were calculated using Kruskal–Wallis nonparametric ANOVA test followed by Dunn's multiple comparisons test (in parentheses standard ANOVA test followed by Dunnett post hoc test values are shown for comparison). Note that Gβγ-treated cells showed no difference compared to control (in this case unpaired t-test was performed and hence the two-tailed P-values are indicated).
Chemicals and antibodies
Carbachol was purchased from Sigma Chemical Co. (Poole, Dorset, U.K.) or Wako (Osaka, Japan). ATP (magnesium salt), GTP (sodium salt), creatine, HEPES and BAPTA were from Sigma.
Six different antibodies specific for the following G-protein α subunits (Gαi1, Gαi1/Gαi2, Gαi3, Gαi3/Gαo, Gαo and Gαs) and antibodies against the Gβ subunit were obtained from Calbiochem-Novabiochem Ltd (Beeston, Nottingham, U.K.) (corresponding catalogue numbers 371720, 371723, 371729, 371726, 371728, 371732 and 371738). Antibodies to Gαi1/Gαi2, Gαi3/Gαo and Gαq/Gα11 were also obtained from Perkin-Elmer life sciences (NEN) (Boston, MA, U.S.A.) (corresponding catalogue numbers NEI-802, NEI-801 and NEI-809). The peptide antigens used to raise the antibodies are shown in Table 1 . With the exception of the antibody to Gαo, each antibody can recognize a specific sequence of the C-terminal of the specified α subunit or an internal sequence as indicated.
Table 1.
Peptide sequences against which the antibodies used in these experiments were raised
| Internal sequence | C-terminal sequence | |
|---|---|---|
| Gαi1 | LDRIAQPNYI (159–168) | |
| Gαi1/2 (Calbiochem) | KNNLKDCGLF | |
| Gαi1/2 (NEN*) | KENLKDCGLF | |
| Gαi3 | KNNLKECGLY | |
| Gαi3/o | KNNLKECGLY (Calbiochem) | |
| ANNLRGCGLY (NEN) | ||
| GαoGαq/11 | Full-length recombinant Gαo | QLNLKEYNLV |
| Gαs | RMHLRQYELL | |
| Gβ | KTREGNVRVSREL (127–139) |
The single letter code for amino acids is used. The same decapeptide (KNNLKECGLY) was used by Calbiochem to raise antibodies against Gαi3 or Gαi3/Gαo. Antibodies selective for either the Gαi3 or selective for both Gαi3 and Gαo were obtained. NEN, however, used a mixture of the C-terminal end of Gαo (ANNLRGCGLY) and the C-terminal end of Gαi3 (KNNLKECGLY) to produce an antibody which recognized both of these α subunits. The polyclonal anti-Gβ antibody recognizes both 35 and 36 kDa subunits.
Gαi1/Gαi2(NEN) was raised using the C-terminal sequence of the transducin α subunit (KENLKDCGLF).
The antibodies were each diluted to 1 : 200 or 1 : 100 v v−1 with pipette solution and included in the patch pipettes. The concentrations used were five to 10 times higher than typically employed for Western blotting. As a control, Gαi3/Gαo or Gαo antibodies diluted with pipette solution were heated at 80–85°C for 5 min to inactivate them, and then used at a concentration of 1 : 200 v v−1. Gβγ subunit was obtained from Calbiochem-Novabiochem Ltd (Beeston, Nottingham, U.K.) (catalogue number 371768) and was added to the pipette solution to give a final concentration of 500 ng ml−1.
Results
Cells were held under whole-cell recording mode using patch pipettes in which G-protein α subunit antibodies were either included (test) or not included (control) and, unless otherwise stated, 20–25 min were allowed for sufficient dialysis with the pipette solution before first application of carbachol.
In 112 cells tested using maximal (100–300 μM) or nearly maximal (50 μM) carbachol concentrations, membrane capacitance and mIcat amplitude were normally distributed with median values of 53.7 pF (arithmetic mean of 53.7±1.2 pF) and 0.851 nA (arithmetic mean of 1.020±0.066 nA) respectively (Figure 1a,b). No correlation was found between them (Figure 1c), indicating that factors other than the cell size account for the fairly large variations of maximal mIcat amplitude observed in different cells. Therefore, we analysed these amplitudes without normalizing them by membrane capacitance.
Since the experimental protocol employed in our tests of the effects of the antibodies required a long initial period for cell equilibration with these large molecules, it was verified that mIcat in controls was preserved. Figure 1d compares average concentration–effect curves obtained 3 and 20 min after breakthrough. Mean parameters obtained after fitting data from individual cells at 3 min (n=113) and 20 min (n=53) were, respectively: log(Imax) (log A) −9.00±0.02 and −9.14±0.03 (P=0.001); log(EC50) (log M) −5.13±0.03 and −5.14±0.05 (P=0.327); slope (h) 1.46±0.04 and 1.31±0.06 (P=0.06). Only maximal mIcat amplitude decreased significantly with time (by about 25%). Thus, time-matched controls were used when studying the effects of the antibodies.
Carbachol concentration–effect curve during the action of Gα antibodies
Figure 2b shows a typical example of mIcat in control cells evoked by an ascending series of six different carbachol concentrations (1, 3, 10, 30, 100 and 300 μM, down triangles) 20–25 min after achieving whole-cell recording mode. mIcat appeared as an inward current at −50 mV; its amplitude increased as carbachol concentration was increased and the mIcat response rapidly disappeared after agonist removal (up triangle). Maximal activation of mIcat was usually attained at 100 or 300 μM, and the mean value for mIcat amplitude at 300 μM was 0.830±0.062 nA (n=53). Figure 3a (closed squares) shows the averaged concentration–effect curve for mIcat activation by carbachol (see Table 2 for parameters of best-fit curves fitted to averaged responses at each carbachol concentration for controls and for cells treated with the same antibody, and Table 3 for averaged parameters of best-fit curves fitted to data obtained from individual cells). At lower carbachol concentrations, exposure to different carbachol concentrations for 25–40 s was sometimes not long enough for mIcat to reach a steady-state level but to increase this period may have resulted in an underestimation of maximal response at 300 μM due to desensitization. It should be emphasized that our conclusions are derived by comparing maximal responses, for example at 300 μM carbachol, at which concentration the steady-state level had been reached (see Figure 1e).
Figure 2.
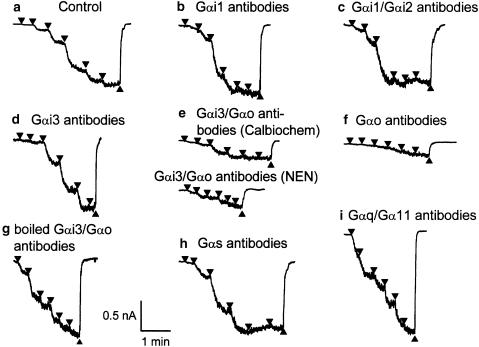
Examples of mIcat evoked by carbachol applied at stepwise increasing concentrations in cells untreated (control) (a) and treated with G-protein antibodies from Calbiochem or NEN™ (b–i). Cells were voltage-clamped at −50 mV and cumulative ascending carbachol concentrations from 1 up to 300 μM were applied sequentially at points indicated by the down triangles and washed out at points as indicated by the up triangles. Carbachol application was performed 20–25 min after establishment of the whole-cell recording mode. The indicated G-protein antibodies (1 : 200 v v−1) were applied intracellularly via patch pipettes. Note that in (g) the antibodies were boiled before use.
Desensitization might reduce the accuracy of our measurements, particularly at higher agonist concentrations; so to estimate its extent the test illustrated in Figure 1e was performed. Shortly after completing the application of ascending concentrations of carbachol, which took nearly 4 min to complete, it was applied again at 100 μM for the period of time comparable to the time taken for application of the ascending concentrations. In control cells mIcat decline during this nearly 4 min carbachol (100 μM) exposure was on average 10.2±4.0 % (n=5). We also performed similar tests in the presence of anti-Gαi3/Gαo, one of the most efficient antibodies to inhibit mIcat (see below). In this case, desensitization was also found to be small (14.7±6.7%, n=3) and not significantly different from control (P>0.6).
Effects of G-protein antibodies
Effects of antibodies against various PTX-sensitive G proteins on the mIcat response to carbachol were investigated. After intracellular dialysis with Gαi1 or Gαi3 antibodies (obtained from Calbiochem; 1 : 200 v v−1) for 20–25 min, application of ascending carbachol concentrations induced mIcat similar to that seen in control cells (Figure 2b,d). The averaged concentration–effect curve for carbachol in cells treated with either antibody overlapped the control curve (Figure 3c). The mean amplitude of mIcat at 300 μM was 0.990±0.060 nA (n=6) in cells treated with Gαi1 antibodies and 0.908±0.133 nA (n=8) in those treated with Gαi3 antibodies. The differences between either of these mean values and the control Imax were not statistically significant. Essentially, the same results were obtained with Gαi1/Gαi2 antibodies (from Calbiochem and NEN™) (Figures 2c and 3b), although the slope of the carbachol curve was somewhat steeper in cells treated with antibodies from Calbiochem (1 : 200 v v−1) and less steep in cells treated with antibody from NEN™ (1 : 100 v v−1) (Figure 3b and Table 3). The mean amplitude of mIcat at 300 μM was 0.767±0.083 nA (n=6) for the former antibodies and 0.799±0.069 nA (n=10) for the latter ones; neither mean value was significantly different from the control.
In contrast, Gαi3/Gαo antibodies (1 : 200 v v−1) obtained from both suppliers significantly inhibited the current response to carbachol (Figures 2e and 3a). The mean mIcat amplitude at 300 μM was 0.334±0.071 nA (n=9) for the antibodies from Calbiochem and 0.156±0.037 nA (n=10) for those from NEN™. With antibodies from either source, the EC50 or slope factor remained unchanged (Figure 3a and Table 3). When NEN antibodies were used at 1 : 1000 and 1 : 500 v v−1, the mean mIcat amplitudes at 300 μM were 0.951±0.106 nA (n=9) and 0.525±0.077 nA (n=7), respectively, indicating the concentration dependency of the effect of these antibodies. Heat-inactivated Gαi3/Gαo antibody (NEN™, 1 : 200 v v−1) had no significant effect (Figure 2g); the mean amplitude of mIcat at 300 μM was 0.791±0.078 nA (n=5).
Cell dialysis with antibodies against Gαo (1 : 200 v v−1) resulted in a significant depression of mIcat by 73% (Figures 2f and 3a) and the EC50 or slope factor remained the same (Table 3). The mean amplitude of mIcat at 300 μM was 0.223±0.041 nA (n=7). After boiling, Gαo antibodies had no effect on mIcat. In five cells tested, the best-fit parameters were not different from control (log(Imax)=−9.12±0.04, P=0.86; log(EC50)=−5.10±0.07, P=0.84; h=1.32±0.28, P=0.95).
Antibodies against G-protein α subunits belonging to the PTX-insensitive family were also investigated. Gαq/Gα11 antibodies (NEN, 1 : 100 v v−1) or Gαs antibodies (Calbiochem, 1 : 200 v v−1) did not significantly reduce the carbachol-induced current (Figures 2h,i, 3d). The mean mIcat amplitude at 300 μM was 0.778±0.091 nA (n=13) for the Gαq/Gα11 antibodies and 1.068±0.186 nA (n=6) for the Gαs antibodies. These mean values did not significantly differ from the control. The EC50 was somewhat decreased by Gαs antibodies (on average from 7.3 to 2.9 μM) and Hill slope was decreased by Gαq/Gα11 antibodies (on average from 1.31 to 0.90); both effects were significant (P<0.05, Table 3).
Averaged parameters from individual cells in the control and antibody-treated groups (10 groups of cells in total) were subjected to ANOVA tests (Table 3). Cell-to-cell variations of Imax and EC50 values were reduced after log transformation of the data, but not sufficiently to use the more sensitive parametric tests. Nonetheless, we compared the results of the nonparametric/parametric tests and found a rather good agreement as both tests picked up essentially the same differences with the exception of the sensitizing action of Gαs antibodies (see Table 3). Only Gαi3/Gαo antibodies and Gαo antibodies produced a significant depression of mIcat and thus it is concluded that only Go is involved in transducing the opening of cation channels in response to muscarinic receptor activation. The effect of Gαs antibodies on EC50 was marginal and the effect on the slope factor of Gαi1/Gαi2 antibodies from Calbiochem and NEN™ was contradictory (increase or nonsignificant decrease, respectively).
Antibody block is prevented by early carbachol application
It was found that the inhibition of mIcat did not occur if even a brief application of carbachol was made at early times during the infusion of Gαi3/Gαo antibodies (Calbiochem or NEN™; 1 : 200 v v−1) via the patch pipette. This is illustrated in Figure 4a, where carbachol (50 μM) was first applied 1.5 min after breakthrough to the whole-cell recording mode using a pipette containing the antibodies and then applied for 30–40 s every 5 min. It can be seen that even after 30–40 min, mIcat decreased only by 10–15%, compared with its initial amplitude. This degree of current reduction was no more than the usual desensitization seen in control cells. In a series of experiments using Gαi3/Gαo antibody (NEN™), the response to carbachol at 20 min was 0.64±0.02 (n=6) of the response to the same concentration of carbachol applied at 3 min; this was greater (although not significantly; P=0.265) than in a control series done at the same time where, without antibodies in the pipette, the response at 20 min was 0.54±0.09 (n=5) of that at 3 min. Thus, application of carbachol at 3 min blocked the decline normally seen (Figures 2e and 3a) when Gαi3/Gαo antibodies were added to the pipette solution.
In another series of experiments using Gαi3/Gαo antibodies, carbachol was applied at 50 μM for about 1 min 1–3 min after the breakthrough, and 20 min later it was applied as usual at ascending concentrations (1–300 μM) to obtain the concentration–effect curve for mIcat activation. As shown in Figure 4b, the averaged concentration–effect curves from four cells tested using such a protocol lay close to the control curve obtained without both infusion of the antibodies and prior application of carbachol at early times. The mean amplitude of mIcat at 300 μM in four cells (0.863±0.150 nA) was close to that in control (0.830±0.062 nA; n=53). Also, fitting data from individual experiments and comparing the mean parameters using a t-test showed no change of Imax (P=0.75), EC50 (P=0.38) or h (P=0.85). When application of ascending carbachol concentrations was repeated every 20 min starting 3 min after the breakthrough, the response was not depressed but instead even slightly enhanced at 20, 40 and 60 min (Figure 4c,d).
Tests were done using Gαo antibodies with similar results. When carbachol was briefly applied at 2 min after breakthrough (at 100 μM), the subsequent inhibition of mIcat was completely prevented (see examples from three different cells in Figure 5a). Averaged agonist curves are plotted in Figure 5b (circles for six cells at 20 min and squares for three cells where recordings could be made up to 60 min). There was no reduction of the current compared to control cells and even some increase at 60 min perfusion with the antibodies (P=0.02 when comparing best-fit Imax values from individual cells). Other best-fit parameters remained unchanged (EC50, P=0.72; h, P=0.27). Also, a paired t-test for three cells where concentration–effect curves were measured at 20 and 60 min in the presence of Gαo antibodies showed no difference of Imax (P=0.36), EC50 (P=0.13) or h (P=0.88).
The possibility was examined that early carbachol application might somehow increase the access resistance, thus impairing the diffusion of the antibodies into the cytosol. Thus, the series resistance (Rs) was measured before each agonist curve was measured. The values (MΩ) are plotted near the corresponding traces in Figure 5a. No consistent increase of Rs was found in these experiments, but on the contrary, Rs even decreased after carbachol application at 20 min if no early agonist application was made (during the initial period, it gradually increased from 10.7±2.4 to 22.9±4.4 MΩ but dropped to 16.5±2.3 MΩ after the first carbachol application) (Figure 5c). Moreover, if an early carbachol application had been made, this completely prevented an increase in Rs during the initial 20 min, and subsequent carbachol applications only decreased the Rs value (Figure 5d).
No role of Gβγ subunits in mIcat generation
The inhibition of the cation current response by the antibodies raised against the Gαo subunits does not necessarily exclude a possibility that the βγ dimer is also involved in the response (Morel et al., 1997). When α-subunit of a G protein is blocked by the antibody, this disrupts the interaction of the corresponding G protein with the receptor rather than with the effector (see Discussion). In this situation, Gβγ subunits also will not be released. Their important role in many signal transduction pathways has been established (Clapham & Neer, 1997) but not tested in previous studies of the muscarinic receptor cationic current in smooth muscles.
Thus, Gβ (common) antibody (1 : 200 v v−1) or purified Gβγ subunits was applied via inclusion in the pipette solution. In cells treated with Gβ antibody, repetitive carbachol applications (50 μM) at 5 min intervals, beginning 20 min after breakthrough, evoked large and robust mIcat responses and the I–V relation retained its characteristic shape (Figure 6a). In another cell, 35 min after the beginning of cell perfusion with Gβ antibody, carbachol was applied for a long period while I–V curves were measured at 30 s interval (seen as vertical deflections in the top trace in Figure 6b). Again, I–V curves showed no unusual properties. Reduction of the current was within normal desensitization limits. Similar results were obtained from five cells.
In support of these findings which indicated no role of Gβ in mIcat generation, we also found that intracellular application of Gβγ dimer caused no noticeable current during 25 min recording. Furthermore, mIcat similar to control could be evoked 20–25 min after breakthrough with Gβγ subunit in the pipette solution (Figure 6c). The agonist curve (Imax 0.825±0.087 nA, EC50 2.4 μM, h 1.1; n=13) remained similar to the time-matched control curve (Imax 0.952±0.079 nA, EC50 2.4 μM, h 1.3; n=17) as shown in Figure 6d and Table 2 (mean Imax two-tail unpaired t-test P>0.29). In these experiments, a new control group of cells was used by alternating test and control conditions on the same day. There was no statistically significant difference between responses in both control groups (cf. Figure 3a squares and Figure 6d squares; P>0.3). All best-fit parameters derived from individual cells also remained unchanged (Table 3).
Discussion
The use of antibodies to G-protein α subunits to investigate signal pathways depends critically upon the site at which they bind and the importance of this binding site for the interaction of the G-protein α subunit with the receptor and/or its effector (e.g., cation channel). Nothing is known about the binding of the G-protein α subunit to the cation channel, if indeed direct binding occurs, rather than there being some intermediate step. The C-terminal region of Gαi/Gαo is important for binding to receptors (Liu et al., 1995; Sprang, 1997; Hamm, 1998) and a peptide corresponding to its last 10 peptide residues was used to raise antibodies against Gαi3/Gαo (Table 1). These antibodies were able to reduce the cation current response to near-maximal concentrations of carbachol by around 60–80%. Given that more than one site on the G protein is believed to bind to the M2 receptor (Sprang, 1997; Hamm, 1998; Kostenis et al., 1999) and that the receptor competes with the antibody for binding to the α subunit, less than complete inhibition is to be expected. The Gαo antibody was raised against a full-length recombinant Gαo subunit (Table 1) and produced 73% inhibition of the carbachol-evoked current (Figure 3a). Since specific Gαi3 antibody was ineffective, as was boiled Gαi3/Gαo antibody, these results provide strong evidence that it is the Gαo subunit which is important for linking M2 muscarinic receptor activation to cation channel opening. This was supported by the failure of Gαi1, Gαi1/Gαi2, Gαq/Gα11 and Gαs antibodies to reduce the cation current response. These results are also consistent with the block of the cation current response to M2 receptor activation by PTX (Inoue & Isenberg, 1990; Komori et al., 1992; Unno et al., 1995; Pucovsky et al., 1998). The failure of Gαq /Gα11 antibodies to inhibit mIcat is significant because Wang et al. (1997) recently showed that dialysis of the same antibody inhibited muscarinic-receptor ICl(Ca) in tracheal smooth muscle cells, although it had no effect on mIcat. The significant decrease in the EC50 produced by Gαs antibody may reflect its interaction with some system mediated by Gαs inhibitory for mIcat.
Our result differs from that in tracheal smooth muscle where Gαi1/Gαi2, as well as Gαi3/Gαo antibodies, blocked the muscarinic-evoked cation current implying a Gαi/Gαo link (Wang et al., 1997) and in mouse gastric myocytes where the muscarinic cation current was blocked by antibodies to Gαq/Gα11 (Lee et al., 2002). However, a similar result was obtained in guinea-pig gastric myocytes where antibodies against Gαo were effective in blocking the muscarinic cation current (Kim et al., 1998).
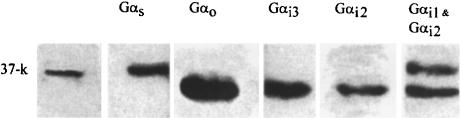
The failure of Gαi1, Gαi1/Gαi2, Gαq/Gα11 and Gαs antibodies to significantly reduce mIcat was not due to their inability to bind to these subunits in the guinea pig as the antibodies are raised to epitopes highly conserved across mammalian species. Moreover, it was also shown (Figure 7) that Gαi1, Gαi1/Gαi2, Gαi3 and Gαs antibodies bind to the G-protein subunits solubilized from guinea-pig longitudinal smooth muscle membranes, confirming results by Murthy et al. (1996), who in addition also used Gβ and Gαq/Gα11 antibodies. We have recently shown that Gαq/Gα11 antibody will block IK(Ca) evoked by muscarinic receptor activation in guinea-pig intestinal smooth muscle cells as used in the present experiments (Yan et al., 2000). Thus, the inability of antibodies other than Gαo and Gαi3/Gαo to block mIcat was not due to their inability to bind to their respective α subunits in the guinea pig.
Figure 7.
Binding of antibodies to various G-protein α subunits obtained from membranes isolated from the longitudinal muscle layer of the small intestine of guinea pig. Membrane proteins were solubilized with 2% Triton-X-100 in Tris buffer. The proteins were resolved using SDS–PAGE, electrophoretically transferred to PVDF membranes and probed with specific antibodies to the alpha subunits of various G proteins used in the electrophysiological experiments. The immunoreactive bands were identified using an anti-rabbit IgG antibody conjugated with horseradish peroxidase and detected using enhanced chemiluminescence.
Activation of the M3 muscarinic receptor has been suggested to provide a potentiating role on the M2-cation current system, other than by an increase in [Ca2+]i linked to an increase in phospholipase C (PLC) activity (Bolton & Zholos, 1997; Zholos & Bolton, 1997). The antibody against Gαq/Gα11 was raised by injection of a decapeptide corresponding to the C-terminal sequence important for the binding at the M3 receptor (Shenker et al., 1991; Kostenis et al., 1997) and evidence in support of this was provided by Yan et al. (2000), who found in guinea-pig intestinal myocytes under conditions similar to our present experiments that calcium store release, as measured by the evoked calcium-activated potassium current, was reduced by these antibodies. Since these antibodies were an effective blocker of the InsP3/Ca store link, it is significant that they did not affect the cation current response apart from some decrease of the Hill slope (Figure 3d and Table 3), which argues that any potentiating effect of M3 receptor activation on the M2 system is not exerted via the Gαq/PLC pathway.
Activation of M2 receptors in smooth muscle from the guinea-pig small intestine inhibits adenylyl cyclase probably via a Gαi link (Thomas et al., 1993) and the carboxy terminus of this α-subunit has been suggested to be important for its binding to various G-protein-coupled receptors (Goldsmith et al., 1987; Gilchrist et al., 1998; Hamm, 1998). However, antibodies against Gαi1/Gαi2, or Gαi1 or Gαi3 alone, did not alter the cation current response arguing against any involvement of Gαi proteins (Figures 3b,c and Table 3). Consistent with this, there was no effect of 8-Br-cAMP on mIcat (our unpublished observations).
Our experiments also show for the first time that the G-protein βγ dimer, which on present knowledge would be expected to be freed from its binding to the Gα subunit upon receptor activation, has no role in the mIcat response in smooth muscle; addition of Gβγ subunits to the pipette solution did not evoke any cation current and the cation current response to carbachol was unchanged, which implies that the Gβγ subunits play no role in the opening of cation channels in response to M2 muscarinic receptor activation. The Gβ antibody, which was ineffective in reducing the carbachol-evoked cation current, was raised against a peptide corresponding to amino acids 127–139 of Gβ (Table 1). This region is within that (75–165) proposed to be important for binding of the β subunit to its effectors (Weng et al., 1996; Panchenko et al., 1998) and the binding of an antibody (Murthy et al., 1996) within this region might be expected to reduce any interaction of the β subunit with the cation channel. Overall, the results support the hypothesis that, following M2 muscarinic receptor stimulation, Gαo is involved in the signal pathway for the opening of the cation channels, and that it is the αo subunit which binds to the cation channel resulting in a large increase in probability of the open state; the β subunit does not seem to be involved.
It was also found that application of carbachol at early times after breakthrough prevented the depression by anti-Gαi3/Gαo and Gαo antibodies of the mIcat response (Figures 4a–d, 5a,b). This finding indicates that activation of muscarinic receptors before G-protein antibodies can bind somehow blocks subsequent binding of Gαi3/Gαo or Gαo antibodies. How long this protection may last was not determined, but it was at least 1 h and holding single myocytes and obtaining reliable carbachol responses for longer periods is difficult. The observation does not seem to have a trivial explanation, such as blockage of the patch pipette (Figure 5c,d), and so has interesting implications for how M2 muscarinic receptors interact with G-protein α subunits. One possibility is that, following activation, the Gαo subunits may adopt a conformation which occludes the antibody binding site(s), while allowing the receptor to bind, possibly because of the relative importance of different binding sites on the α subunit for the two peptides. It is also possible that agonist dissociation from the ternary complex agonist-receptor-G protein (Weiss et al., 1996) leaves receptor-G protein dimer not accessible to the antibodies. These and other possibilities remain to be evaluated, but the paradoxical finding in the present study provides a warning that the blocking ability of G-protein antibodies could depend on the experimental protocol employed.
No consistent change in the EC50 values was observed in our experiments when severe depression of the maximal response by Gαo or Gαi3/Gαo antibodies was produced. This indicates that it is probably the number of functional channels which is reduced. This appears as a noncompetitive antagonism by analogy with the action of receptor antagonists. When a fraction of the receptors is inactivated and a reduction of the maximal response is observed without a rightward shift of the agonist curve, this is often interpreted as a lack of ‘spare' or ‘reserve' receptors. By analogy, our present results might imply the lack of ‘spare' G proteins to activate mIcat in ileal myocytes when near-maximal concentrations of carbachol are applied.
In conclusion, our data provide evidence to favour the idea that the Gαo subunit, and not the Gβ subunit, transduces signals from the M2 muscarinic receptor to cationic channel opening in guinea-pig ileal smooth muscle cells. For a complete picture of the M2 transduction mechanisms, it will be necessary also to identify the components of the system linking the receptor to the adenylyl cyclase (Candell et al., 1990; Thomas et al., 1993).
Acknowledgments
This work was supported by the Wellcome Trust (grants 051162 and 062926) and by Grant-in-Aid Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (grants 13460141 and 14760188). Dr Tsytsyura was supported by a Junior Fellowship from The Physiological Society International Program.
Abbreviations
- ATP
adenosine 5′-trisphosphate magnesium salt
- BAPTA
1,2-bis(2-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid
- [Ca2+]i
ionized intracellular Ca2+ concentration
- GTP
guanosine 5′-trisphosphate sodium salt
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid
- I–V relation
current–voltage relation
- mAChR
muscarinic acetylcholine receptor
- mIcat
muscarinic receptor cationic current
- PLC
phospholipase C
- PTX
pertussis toxin
- PVDF
polyvinylidene difluoride
- Rs
series resistance
- TBS
Tris-buffered saline
References
- BOLTON T.B., ZHOLOS A.V. Activation of M2 muscarinic receptor in guinea-pig ileum opens cationic channels modulated by M3 muscarinic receptors. Life Sci. 1997;60:1121–1128. doi: 10.1016/s0024-3205(97)00056-8. [DOI] [PubMed] [Google Scholar]
- CANDELL L.M., YUN S.H., TRAN L.L.P., EHLERT F.J. Differential coupling of subtypes of the muscarinic receptor to adenylate cyclase and phosphoinositide hydrolysis in the longitudinal muscle of the rat ileum. Mol. Pharmacol. 1990;38:689–697. [PubMed] [Google Scholar]
- CLAPHAM D.E., NEER E.J. G protein βγ subunits. Ann. Rev. Pharmacol. Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- GILCHRIST A., MAZZONI M.R., DINEEN B., DICE A., LINDEN J., PROCTOR W.R., LUPICA C.R., DUNWIDDIE T.V., HAMM H.E. Antagonists of the receptor-G protein interface block Gi-coupled signal transduction. J. Biol. Chem. 1998;273:14912–14919. doi: 10.1074/jbc.273.24.14912. [DOI] [PubMed] [Google Scholar]
- GOLDSMITH P., GIERSCHIK P., MILLIGAN G., UNSON C.G., VINITSKY R., MALECH H.L., SPIEGEL A.M. Antibodies directed against synthetic peptides distinguish between GTP-binding proteins in neutrophil and brain. J. Biol. Chem. 1987;262:14683–14688. [PubMed] [Google Scholar]
- HAMM H.E. The many faces of G protein signalling. J. Biol. Chem. 1998;273:669–672. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- HELLIWELL R.M., LARGE W.A. Dual effects of external Ca2+ on noradrenaline-activated cation current in rabbit portal vein smooth muscle cells. J. Physiol. 1996;492:75–88. doi: 10.1113/jphysiol.1996.sp021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLIWELL R.M., LARGE W.A. α1-Adrenoceptor activation of a non-selective cation current in rabbit portal vein by 1,2-diacyl-sn-glycerol. J. Physiol. 1997;499:417–428. doi: 10.1113/jphysiol.1997.sp021938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE R., ISENBERG G. Acetylcholine activates nonselective cation channels in guinea-pig ileum through a G protein. Am. J. Physiol. 1990;258:C1173–C1178. doi: 10.1152/ajpcell.1990.258.6.C1173. [DOI] [PubMed] [Google Scholar]
- KIM Y.C., KIM S.J., SIM J.H., CHO C.H., JUHNN Y.-S., SUH S.H., SO I., KIM K.W. Suppression of the carbachol-activated nonselective cationic current by antibody against α subunit of Go protein in guinea-pig gastric myocytes. Pflügers Arch. 1998;436:494–496. doi: 10.1007/s004240050663. [DOI] [PubMed] [Google Scholar]
- KOMORI S., KAWAI M., TAKEWAKI T., OHASHI H. GTP-binding protein involvement in membrane currents evoked by carbachol and histamine in guinea-pig ileal muscle. J. Physiol. 1992;450:105–126. doi: 10.1113/jphysiol.1992.sp019118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMORI S., UNNO T., NAKAYAMA T., OHASHI H. M2 and M3 muscarinic receptors couple, respectively, with activation of nonselective cationic channels and potassium channels in intestinal smooth muscle cells. Jpn. J. Pharmacol. 1998;76:213–218. doi: 10.1254/jjp.76.213. [DOI] [PubMed] [Google Scholar]
- KOSTENIS E., GOMEZA J., LERCHE C., WESS J. Genetic analysis of receptor–Gαq coupling selectivity. J. Biol. Chem. 1997;272:23675–23681. doi: 10.1074/jbc.272.38.23675. [DOI] [PubMed] [Google Scholar]
- KOSTENIS E., ZENG F.-Y., WESS J. Structure–function analysis of muscarinic receptors and their associated G proteins. Life Sci. 1999;64:355–362. doi: 10.1016/s0024-3205(98)00574-8. [DOI] [PubMed] [Google Scholar]
- LEE Y., KIM B.J., KIM H.J., YANG I.S., SO I., KIM K.W. Transient receptor potential channel 5 as a candidate for nonselective cation current activated by carbachol. Biophys. J. 2002;82:638a. [Google Scholar]
- LIU J., CONKLIN B.R., BLIN N., YUN J., WESS J. Identification of a receptor/G-protein contact site critical for signalling specificity and G-protein activation. Proc. Natl. Acad. Sci. U.S.A. 1995;92:11642–11646. doi: 10.1073/pnas.92.25.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOREL J.L., MACREZ N., MIRONNEAU J. Specific Gq protein involvement in muscarinic M3 receptor-induced phosphatidylinositol hydrolysis and Ca2+ release in mouse duodenum myocytes. Br. J. Pharmacol. 1997;121:451–458. doi: 10.1038/sj.bjp.0701157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURTHY K.S., COY D.H., MAKHLOUF G.M. Somatostatin receptor-mediated signaling in smooth muscle. J. Biol. Chem. 1996;271:23458–23463. doi: 10.1074/jbc.271.38.23458. [DOI] [PubMed] [Google Scholar]
- PACAUD P., BOLTON T.B. Relation between muscarinic receptor cationic current and internal calcium in guinea-pig jejunal smooth muscle cells. J. Physiol. 1991;441:477–499. doi: 10.1113/jphysiol.1991.sp018763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANCHENKO M.P., SAXENA K., LI Y., CHARNECKI S., STERNWEIS P.M., SMITH T.F., GILMAN A.G., KOZASA T, NEER E.J. Sites important for PLC β2 activation by the G protein βγ subunit map to the sides of the β propeller structure. J. Biol. Chem. 1998;273:28298–28304. doi: 10.1074/jbc.273.43.28298. [DOI] [PubMed] [Google Scholar]
- PUCOVSKY V., ZHOLOS A.V., BOLTON T.B. Muscarinic cation current and suppression of Ca2+ current in guinea pig ileal smooth muscle cells. Eur. J. Pharmacol. 1998;346:323–330. doi: 10.1016/s0014-2999(98)00059-4. [DOI] [PubMed] [Google Scholar]
- SHENKER A., GOLDSMITH P., UNSON C.G., SPIEGAL A.M. The G protein coupled to the thromboxane A2 receptor in human platelets is a member of the novel Gq family. J. Biol. Chem. 1991;266:9309–9312. [PubMed] [Google Scholar]
- SIMS S.M. Cholinergic activation of a non-selective cation current in canine gastric smooth muscle is associated with contraction. J. Physiol. 1992;449:377–398. doi: 10.1113/jphysiol.1992.sp019091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPRANG S.R. G protein mechanisms: insights from structural analysis. Ann. Rev. Biochem. 1997;66:639–678. doi: 10.1146/annurev.biochem.66.1.639. [DOI] [PubMed] [Google Scholar]
- THOMAS E.A., BAKER S.A., EHLERT F.J. Functional role for the M2 muscarinic receptor in smooth muscle of guinea pig ileum. Mol. Pharmacol. 1993;44:102–110. [PubMed] [Google Scholar]
- UNNO T., KOMORI S., OHASHI H. Inhibitory effect of muscarinic receptor activation on Ca2+ channel current in smooth muscle cells of guinea-pig ileum. J. Physiol. 1995;484:567–581. doi: 10.1113/jphysiol.1995.sp020687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Y.-X., FLEISCHMANN B.K., KOTLIKOFF M.I. M2 receptor activation of nonselective cation channels in smooth muscle cells: calcium and Gi/Go requirements. Am. J. Physiol. 1997;273:C500–C508. doi: 10.1152/ajpcell.1997.273.2.C500. [DOI] [PubMed] [Google Scholar]
- WEISS J.M., MORGAN P.H., LUTZ M.W., KENAKIN T.P. The cubic ternary complex receptor-occupancy model. I. Model description. J. Theor. Biol. 1996;178:151–167. doi: 10.1006/jtbi.1996.0139. [DOI] [PubMed] [Google Scholar]
- WENG G., LI J., DINGUS J., HILDEBRANDT J.D., WEINSTEIN H., IYENGAR R. Gβ subunit interacts with a peptide encoding region 956–982 of adenylyl cyclase 2. J. Biol. Chem. 1996;271:26445–26448. doi: 10.1074/jbc.271.43.26445. [DOI] [PubMed] [Google Scholar]
- YAN H.-D., KOMORI S., UNNO T., OHASHI H. Effects of anti-G protein α subunit antibodies on muscarinic activation of membrane currents in intestinal smooth muscle cells. Jpn. J. Pharmacol. 2000;82:98. [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. G-protein control of voltage dependence as well as gating of muscarinic metabotropic channels in guinea-pig ileum. J. Physiol. 1994;478:195–202. doi: 10.1113/jphysiol.1994.sp020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. Effects of divalent cations on muscarinic receptor cationic current in smooth muscle from guinea-pig small intestine. J. Physiol. 1995;486:67–82. doi: 10.1113/jphysiol.1995.sp020791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. A novel GTP-dependent mechanism of ileal muscarinic metabotropic channel desensitization. Br. J. Pharmacol. 1996a;119:997–1005. doi: 10.1111/j.1476-5381.1996.tb15770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. Effect of protons on muscarinic receptor cationic current in single visceral smooth muscle cells. Am. J. Physiol. 1996b;272:G215–G223. doi: 10.1152/ajpgi.1997.272.2.G215. [DOI] [PubMed] [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. Muscarinic receptor subtypes controlling the cationic current in guinea-pig ileal smooth muscle. Br. J. Pharmacol. 1997;122:885–893. doi: 10.1038/sj.bjp.0701438. [DOI] [PMC free article] [PubMed] [Google Scholar]