Abstract
Neutrophil-derived elastase is an enzyme implicated in the pathogenesis of chronic obstructive pulmonary disease (COPD). Heparin inhibits the enzymatic activity of elastase and here we provide evidence for the first time that heparin can inhibit the release of elastase from human neutrophils.
Unfractionated and low molecular weight heparins (UH and LMWH, 0.01–1000 U ml−1) and corresponding concentrations (0.06–6000 μg ml−1) of nonanticoagulant O-desulphated heparin (ODH), dextran sulphate (DS) and nonsulphated poly-L-glutamic acid (PGA) were compared for their effects on both elastase release from and aggregation of neutrophils.
UH, ODH and LMWH inhibited (P<0.05) the homotypic aggregation of neutrophils, in response to both N-formyl-methionyl-leucyl-phenylalanine (fMLP, 10−6 M) and platelet-activating factor (PAF, 10−6 M), as well as elastase release in response to these stimuli, in the absence and presence of the priming agent tumour necrosis factor-alpha (TNF-α, 100 U ml−1).
DS inhibited elastase release under all the conditions of cellular activation tested (P<0.05) but had no effect on aggregation. PGA lacked efficacy in either assay, suggesting general sulphation to be important in both effects of heparin on neutrophil function and specific patterns of sulphation to be required for inhibition of aggregation.
Further investigation of the structural requirements for inhibition of elastase release confirmed the nonsulphated GAG hyaluronic acid and neutral dextran, respectively, to be without effect, whereas the IP3 receptor antagonist 2-aminoethoxydiphenylborate (2-APB) mimicked the effects of heparin, itself an established IP3 receptor antagonist, suggesting this to be a possible mechanism of action.
Keywords: Neutrophils, aggregation, elastase, heparin, fMLP, PAF, sulphation, polyanionic charge, nonanticoagulant
Introduction
The fortuitous discovery of heparin in 1916 and its anticoagulant properties (reviewed by McLean, 1959) has resulted in the widespread clinical use of this drug in the prevention and treatment of thrombosis. Heparin belongs to the glycosaminoglycan (GAG) family of molecules, a group of polysaccharides that also includes heparan sulphate, chondroitin sulphate and hyaluronic acid (Höök et al., 1984). GAGs are large polyanionic molecules expressed throughout the body, with diverse biological roles, that are usually associated with cell surfaces and extracellular matrices. Heparin is synthesized exclusively in mast cells, although its endogenous role remains to be defined (Page, 1991). It is produced as a proteoglycan with individual heparin polymers varying from 20 to 100 monosaccharide units in length.
In recent years, heparin has been found to possess numerous anti-inflammatory properties, many of which are independent of its anticoagulant nature and heparin has been successfully used clinically in a variety of inflammatory diseases, such as asthma, interstitial cystitis and ulcerative colitis (reviewed by Tyrell et al., 1999; Lever & Page, 2002).
Heparin inhibits the coagulation pathway through binding antithrombin III (AT III) in a specific manner via a unique pentasaccharide sequence, the consequence of which is to accelerate the inactivation of thrombin by AT III. By contrast, less is known about how heparin exerts anti-inflammatory properties although it is able to bind many important molecules involved in the inflammatory process and has been proposed to act as an endogenous anti-inflammatory molecule, serving to limit inflammation and associated tissue damage (Page, 1991; Lever & Page, 2002).
Among the many observations made regarding the anti-inflammatory properties of heparin are some that suggest heparin can inhibit inflammatory cell recruitment into tissues (reviewed by Tyrell et al., 1999 and Lever & Page, 2002). An important inflammatory disease involving the excessive recruitment and activation of neutrophils to the lung is chronic obstructive pulmonary disease (COPD), the fourth largest cause of death in the developed world. The release of elastase from neutrophils has been implicated in the pathogenesis of COPD and to date no drugs have been discovered that can inhibit this process, although heparin has been shown to bind to and inhibit elastase (Redini et al., 1988; Walsh et al., 1991) and limit the tissue-damaging activities of this enzyme (Rao et al., 1990; Lafuma et al., 1991). In addition, heparin can potentiate the neutralization of elastase by endogenous inhibitors (Ying et al., 1997).
Furthermore, since many of the anti-inflammatory activities of heparin are now known to be independent of anticoagulant activities, including inhibition of elastase action (Fryer et al., 1997) and adhesion of neutrophils to vascular endothelial cells (Lever et al., 2000), we have also investigated whether the ability of heparin to inhibit elastase release is independent of anticoagulant activity.
Heparin has been shown to modify many other aspects of inflammatory processes involving neutrophils. For example, heparin is able to inhibit the binding of interleukin-8 to neutrophils (Ramdin et al., 1998) and also L- and P-selectin to their ligands (Skinner et al., 1991; Asa et al., 1992; Koenig et al., 1998) as well as binding CD11b/CD18 (Mac-1) (Diamond et al., 1995). Heparin can also inhibit the adhesion of neutrophils to vascular endothelial cells, both in vitro (Lever et al., 2000) and in vivo (Xie et al., 1997), in addition to neutrophil accumulation in vivo (Sasaki et al., 1993). Neutrophil degranulation and the respiratory burst is inhibited by heparin as well as their homotypic aggregation (Bazzoni et al., 1993).
Thus, we wished to first extend observations made in previous studies regarding neutrophil aggregation by investigating the structural properties required to achieve this inhibition. Secondly, we were also interested in ascertaining whether the effects of heparin upon neutrophil degranulation were inclusive of elastase release, as this constitutes a more relevant marker of neutrophil activation with respect to diseases such as COPD, than those that have been previously measured. As tumour necrosis factor-α (TNF-α) is found to be elevated in the sputum of patients with COPD (Keatings et al., 1996), the effects of heparin upon TNF-α ‘primed' neutrophils were also studied.
In addition, since others have suggested heparin to be an antagonist of inositol 1,4,5-triphosphate (IP3) (Ghosh et al., 1988; Tones et al., 1989; Fasolato et al., 1993), we investigated whether the known IP3 antagonist 2-aminoethoxydiphenylborate (2-APB) was able to mimic the effects of heparin on elastase release from human neutrophils.
Methods
All reagents were purchased from Sigma-Aldrich Company, Poole, U.K., unless otherwise stated. Unfractionated heparin (UH) (Multiparin®) was obtained from CP Pharmaceuticals, Wrexham, U.K. A low-molecular-weight heparin (LMWH) (dalteparin sodium, mean molecular weight, 5.8 kDa) (Pharmacia-Laboratories Ltd, Milton Keynes, U.K.) was used as a control for molecular weight and the nonanticoagulant derivative of heparin, O-desulphated heparin (ODH) (a kind gift from Dr T. Kennedy, Carolinas Medical Health Care Foundation, Charlotte, NC, U.S.A.) was used to control for the anticoagulant activities of heparin. The nonsulphated GAG hyaluronic acid (HA), dextran sulphate (DS), which is not a GAG (mean molecular weight 5 kDa), nonsulphated dextran (mean molecular weight 10 kDa) and poly-L-glutamic acid (PGA) (molecular weight 15–50 kDa) were used to control for the effects of sulphation and charge. 2-APB was used as an IP3 antagonist. Mean anticoagulant activity of UH and LMWH heparins used is 180 and 125–180 IU mg−1, respectively.
Isolation of neutrophils
Ethical permission for these experiments was obtained from King's College Hospital Ethics Committee.
Peripheral venous blood was collected from healthy nonsmoking, nonallergic volunteers into tubes containing anticoagulant (1 volume in 10, acid citrate dextrose). Erythrocytes were removed by mixing the citrated blood with an equal volume of a 6% dextran in normal saline solution (Hespan®), (Du Pont Pharmaceuticals Ltd, Letchworth, U.K.), and left to sediment at room temperature for approximately 40 min. The leucocyte-rich supernatant was then removed and centrifuged at 175 × g for 7 min at 8°C, the supernatant discarded and the resultant white cell pellet mixed with 5–8 ml 55% Percoll® in phosphate-buffered saline (PBS). This suspension was carefully poured onto a gradient of 3 ml of 70% Percoll® upon 5 ml of 81% Percoll®. Tubes were then centrifuged for 25 min at 1750 × g at 8°C. Following this, the mononuclear cell layer, found at the 55 : 70% interface was removed in order to avoid contamination of the neutrophil layer, which was collected by careful aspiration, and resuspended in 0.83% cold ammonium chloride solution for 10 min at 4°C, to remove contaminating erythrocytes by hypotonic lysis. Neutrophils were then washed three times with modified Hanks' balanced salts solution (HBSS). Viability of the cell preparation was assessed using trypan blue dye exclusion and was always found to be in excess of 98%. Purity of the cell population was determined by microscopically examining cytospins that had been stained with Mayer's haematoxyllin followed by carbol chromotrope (Lendrum, 1944). The preparation consistently comprised greater than 95% neutrophils, the remainder of cells being eosinophils.
Neutrophil aggregation assay
Neutrophils were isolated as described above and resuspended in cell-free plasma at a concentration of 5 × 106 cells ml−1. Samples (250 μl) were placed in 1 ml glass vials, in addition to various concentrations of the UH or molecule of interest, immediately prior to their being placed in a Payton aggregometer, under constant stirring at 37°C. Cytochalasin B (5 μg ml−1) was included in the suspension to aid aggregation. After 1 min, N-formyl-methionyl-leucyl-phenylalanine (fMLP) (10−6 M) or platelet-activating factor (PAF) (10−6 M) was added in a volume of 2.5 μl normal saline, and the change in light transmission recorded over a 3 min period, against cell-free plasma as a blank.
Percentage aggregation of neutrophils was calculated as:
(Δx/x) × 100
where x=difference in light transmission between cell-free plasma and nonaggregated neutrophil suspension (t=0); Δx=x−difference in light transmission between cell-free plasma and aggregated neutrophil suspension (t=3 min).
Neutrophil elastase release
Neutrophils were obtained from whole blood in a slightly different way than that described above, to enable a more efficient isolation of mononuclear cells for a separate study. No differences in viability and purity of the preparation, nor the response to fMLP or PAF were found when neutrophils isolated using the two methods described were compared (data not shown).
Citrated venous blood was poured into leucocyte separation tubes containing Histopaque®-1077, and centrifuged at 1000 × g, for 10 min at room temperature. The cell suspension (containing mononuclear cells) above the porous barrier and the barrier itself were removed. The remaining cells were resuspended with an equal volume of Hespan® and left to sediment at room temperature for 40 min. The neutrophil-rich supernatant was placed into a fresh tube and contaminating erythrocytes removed as described above. Neutrophils were washed three times with modified HBSS and the cells then resuspended in complete HBSS. This solution (150 μl) was placed in duplicate into microfuge tubes containing different concentrations of UH, or related molecules as described above or HBSS alone (control cells), resulting in a final concentration of 2.5 × 106 cells ml−1. Cytochalasin B (5 μg ml−1) was present in all microfuge tubes. Cells were incubated for 30 min at room temperature. At this stage some cells were primed with TNF-α (100 U ml−1), (R & D Systems Ltd, U.K.), prior to stimulation of the neutrophils with fMLP (10−7 M) for 45 min, or PAF (10−6 M). At the end of this period, cells were microfuged for 1 min and 25 μl of sample placed in duplicate into wells of a flat-bottomed 96-well microplate. Tris-buffer (150 μl) (0.15 M Tris-hydrochloride, 0.3 M sodium chloride in deionized water) was added to each well in addition to 20 μl of neutrophil elastase substrate-N-methoxysuccinyl-ala-ala-pro-val p-nitroanilide (6 mM in 25% DMSO). After 1 h at room temperature, the coloured product was measured spectrophotometrically at 405 nm, using an Elx808 Ultra Microplate Reader (Bio-Tek Instruments, Inc., U.K.).
Statistical analysis
All data are expressed as a percentage of values obtained from stimulated neutrophils in the absence of UH and related molecules. In neutrophil elastase release experiments, data are first corrected for basal elastase release in the absence of stimulus, but in the presence of cytochalasin B. All data are reported as means±standard error of the mean (s.e.m.) from n experiments. Statistical analysis was made by repeated-measures ANOVA. For the elastase release inhibition experiments, statistical analysis was performed on normalized data due to donor variation.
Results
Inhibition of neutrophil aggregation by UH and UH derivatives
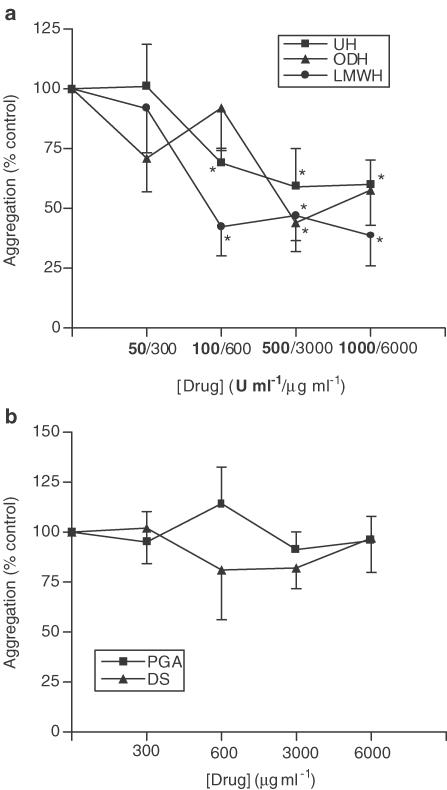
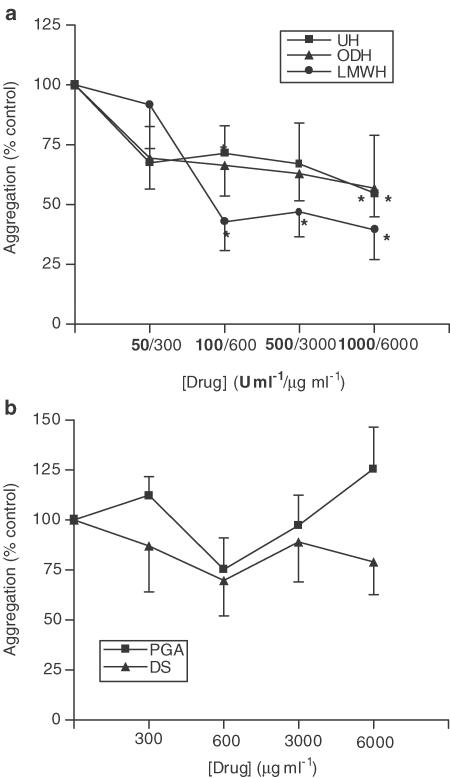
Addition of 10−6 M fMLP or PAF to neutrophil suspensions led to a 16.3±0.7% and 17.2±1.5% increase in light transmission, respectively (P<0.05), as a result of homotypic neutrophil aggregation, which could be inhibited by UH (Figure 1a and Figure 2a). Figure 1a shows that UH is able to inhibit fMLP-induced aggregation (maximum inhibition 40.0±10.3%, 1000 U ml−1, P<0.05) with a similar degree of inhibition being noted with PAF-induced aggregation (Figure 2a). A LMWH was found to mimic the effects of UH upon fMLP-induced aggregation, as was the nonanticoagulant derivative of heparin, ODH (max. inhibition achieved 61.3±12.7%, 1000 U ml−1, P<0.05 and 56.0±12.1%, 3000 μg ml−1, P<0.05 respectively). Similar results were observed with PAF-induced aggregation. Neither PGA nor DS were found to have any effect on aggregation induced by fMLP or PAF (Figure 1b and Figure 2b).
Figure 1.
Effects of UH, ODH, LMWH (a), DS and PGA (b) upon fMLP-induced homotypic aggregation of neutrophils. Neutrophils suspended at 5 × 106 cells ml−1 were placed in an aggregometer in the presence of 5 μg ml−1 cytochalasin B, in the absence and presence of UH or related molecules. After 3 min, 10−6 M fMLP was added and the change in light transmission measured over a 3 min period, against cell-free plasma as a blank. Results from experiments on cells from eight donors, each performed in duplicate, are expressed as mean±s.e.m. values, expressed as a percentage of control values from experiments carried out without drug (*P<0.05 when compared with media control).
Figure 2.
Effects of UH, ODH, LMWH (a), DS and PGA (b) upon PAF-induced homotypic aggregation of neutrophils. Neutrophils suspended at 5 × 106 cells ml−1 were placed in an aggregometer in the presence of 5 μg ml−1 cytochalasin B, in the absence and presence of UH or related molecules. After 3 min, 10−6 M PAF was added and the change in light transmission measured over a 3 min period, against cell-free plasma as a blank. Results from experiments on cells from eight donors, each performed in duplicate are expressed as mean±s.e.m. values, expressed as a percentage of control values from experiments carried out without drug (*P<0.05 when compared with media control).
Priming of neutrophils with TNF-α potentiates neutrophil elastase release
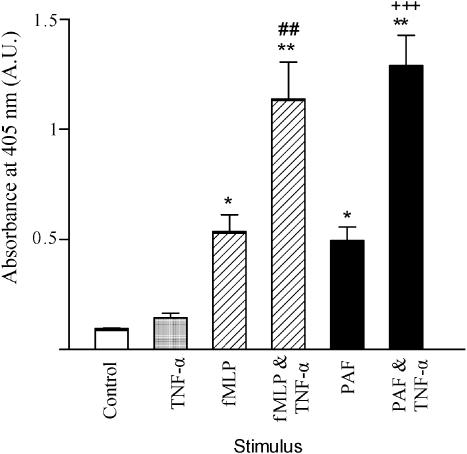
Figure 3 shows that both fMLP (10−7 M) and PAF (10−6 M) induce neutrophil elastase release (P<0.05 when compared to nonstimulated cells). Incubation of neutrophils with TNF-α for 30 min prior to fMLP stimulation potentiates fMLP-induced elastase release approximately two-fold (P<0.01). Likewise, the effects of PAF on neutrophil elastase release were potentiated following priming with TNF-α (P<0.001).
Figure 3.
Effects of fMLP, PAF and TNF-α upon elastase release. Neutrophils were placed into microfuge tubes at a concentration of 2.5 × 106 cells ml−1, in the presence of 5 μg ml−1 cytochalasin B and in the presence or absence of 100 U ml−1 TNF-α. After 30 min, fMLP (10−7 M) or PAF (10−6 M) was added to some cells. After 45 min, cells were centrifuged and samples of supernatant taken and incubated for 1 h with an elastase substrate. After 1 h at room temperature, the coloured product was measured colorimetrically at 405 nm, using a microplate reader. Results from experiments on cells from six donors, each performed in duplicate, are expressed as absorbance mean±standard error of the mean values (*P<0.05 and **P<0.01, when compared with untreated cells, P<0.01 when fMLP- and TNF-α treated cells were compared with fMLP-only treated cells and +++P<0.001 when PAF- and TNF-α-treated cells were compared with PAF-only treated cells).
Inhibition of neutrophil elastase release by UH, UH derivatives and DS
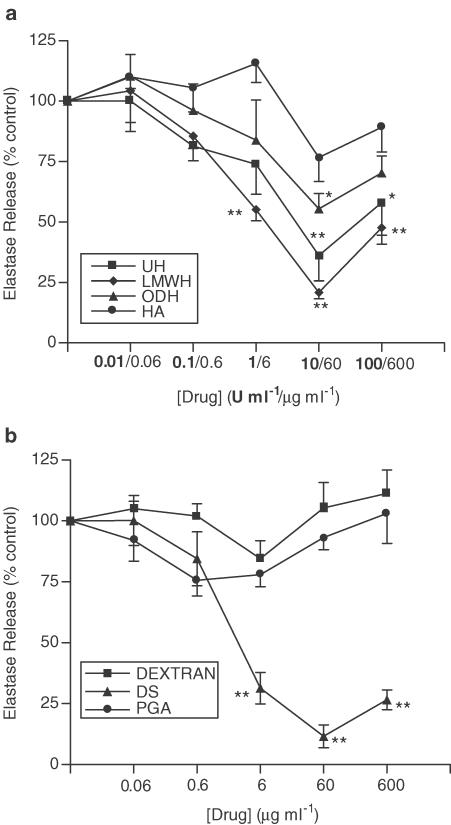
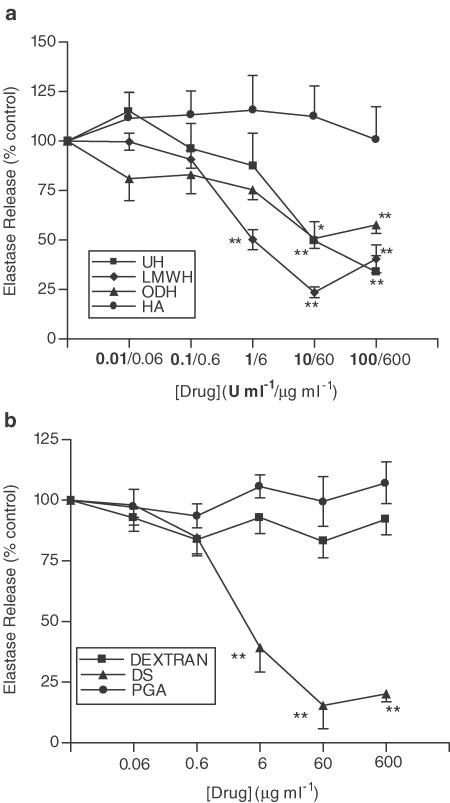
Figure 4a shows that UH inhibits fMLP-induced neutrophil elastase release from unprimed cells (max. inhibition 56.0±12.1%, 10 U ml−1, P<0.01), an effect shared by the LMWH (max. inhibition 79.1±6.8%, 10 U ml−1, P<0.01), and the nonanticoagulant derivative, ODH (max. inhibition 44.5±6.3%, 60 μg ml−1, P<0.05). The nonsulphated GAG, HA, was found to have no effect on neutrophil elastase release. However, in contrast to the lack of effect on neutrophil aggregation, DS was found to inhibit fMLP-induced elastase release in a concentration-dependent manner, similar to that of UH (Figure 4b) (DS, max. inhibition 88.4±4.7%, 60 μg ml−1, P<0.01). Dextran and PGA, which both lack sulphate groups, had no effect. Similar results were obtained in experiments where neutrophils were primed with TNF-α prior to stimulation with fMLP (Figures 5a and b).
Figure 4.
Effects of UH, ODH, LMWH, HA (a), DS, dextran and PGA (b) upon fMLP-induced elastase release. Neutrophils were placed into microfuge tubes at a concentration of 2.5 × 106 cells ml−1, in the presence of 5 μg ml−1 cytochalasin B and the presence or absence of inhibitor. After 30 min 10−7 M fMLP was added to the cells for 45 min, and cells were then centrifuged, samples of supernatant taken and incubated for 1 h with elastase substrate. After 1 h at room temperature, the coloured product was measured colorimetrically at 405 nm, using a microplate reader. Results from experiments on cells from at least six donors, each performed in duplicate are expressed as mean±s.e.m. values, expressed as a percentage of control values from experiments carried out without drug (*P<0.05 and **P<0.01, when compared with media control).
Figure 5.
Effects of UH, ODH, LMWH, HA (a), DS, dextran and PGA (b) upon fMLP-induced elastase release in the presence of TNF-α. Neutrophils were placed into microfuge tubes at a concentration of 2.5 × 106 cells ml−1, in the presence of 5 μg ml−1 cytochalasin B and the presence or absence of 100 U ml−1 TNF-α and inhibitor. After 30 min, 10−7 M fMLP was added to the cells for 45 min, and cells were then centrifuged, samples of supernatant taken and incubated for 1 h with elastase substrate. After 1 h at room temperature, the coloured product was measured colorimetrically at 405 nm, using a microplate reader. Results from experiments on cells from at least six donors, each performed in duplicate, are expressed as mean±s.e.m. values, expressed as a percentage of control values from experiments carried out without drug (*P<0.05 and **P<0.01, when compared with media control).
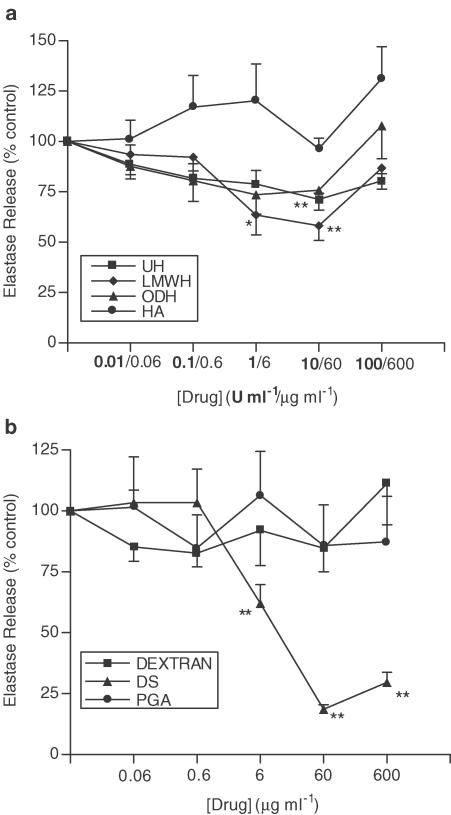
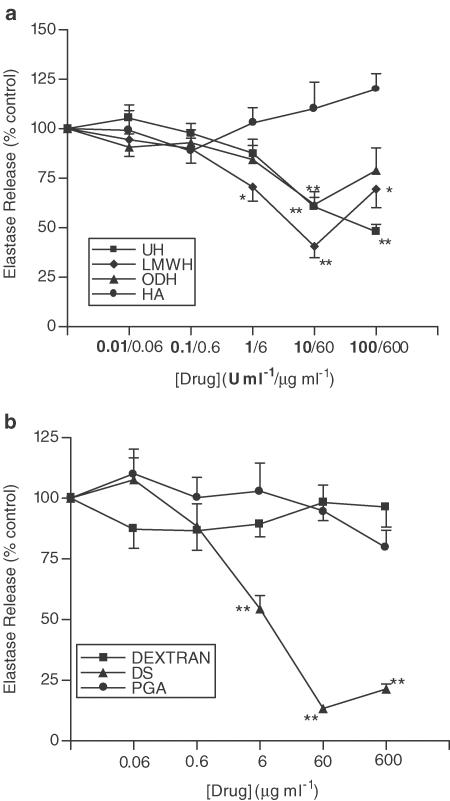
A similar pattern of inhibition occurs when cells are stimulated with PAF instead of fMLP. Figure 6a shows the effects of UH, ODH, LMWH and HA on PAF-induced neutrophil elastase release. The extent of the inhibition is smaller in magnitude than that seen with fMLP, with the decrease observed with ODH not reaching significance. However, UH and LMWH do achieve a small but significant inhibition of PAF-induced neutrophil elastase release (max. inhibition 28.7±2.9% and 41.8±7.3% respectively, 10 U ml−1, P<0.01). DS inhibits PAF-induced neutrophil elastase release to a similar extent to that observed when cells are stimulated with fMLP (Figure 6b) (max. inhibition 81.4±1.9%, 60 μg ml−1, P<0.01). The maximum inhibitory effects of UH and its derivatives are greater when cells are primed with TNF-α prior to PAF stimulation (Figure 7a). Under these conditions, the inhibitory effect of ODH reached significance, achieving a maximum inhibition of 38.3±6.5% (60 μg ml−1, P<0.01). Again, HA has no effect. As in previous experiments, DS attains the greatest inhibition in comparison with UH or its derivatives (Figure 7b), (86.6±1.6%, 60 μg ml−1, P<0.01), while PGA and dextran are without effect.
Figure 6.
Effects of UH, ODH, LMWH, HA (a), DS, dextran and PGA (b) upon PAF-induced elastase release. Neutrophils were placed into microfuge tubes at a concentration of 2.5 × 106 cells ml−1, in the presence of 5 μg ml−1 cytochalasin B and the presence or absence of inhibitor. After 30 min, 10−6 M PAF was added to the cells for 45 min, and cells were then centrifuged, samples of supernatant taken and incubated for 1 h with elastase substrate. After 1 h at room temperature, the coloured product was measured colorimetrically at 405 nm, using a microplate reader. Results from experiments on cells from at least six donors, each performed in duplicate, are expressed as mean±s.e.m. values, expressed as a percentage of control values from experiments carried out without drug (*P<0.05 and **P<0.01, when compared with media control).
Figure 7.
Effects of UH, ODH, LMWH, HA (a), DS, dextran and PGA (b) upon PAF-induced elastase release in the presence of TNF-α. Neutrophils were placed into microfuge tubes at a concentration of 2.5 × 106 cells ml−1, in the presence of 5 μg ml−1 cytochalasin B and the presence or absence of 100 U ml−1 TNF-α and inhibitor. After 30 min, 10−6 M PAF was added to the cells for 45 min, and cells were then centrifuged, samples of supernatant taken and incubated for 1 h with elastase substrate. After 1 h at room temperature, the coloured product was measured colorimetrically at 405 nm, using a microplate reader. Results from experiments on cells from at least six donors, each performed in duplicate, are expressed as mean ± s.e.m. values, expressed as a percentage of control values from experiments carried out without drug (*P<0.05 and **P<0.01, when compared with media control).
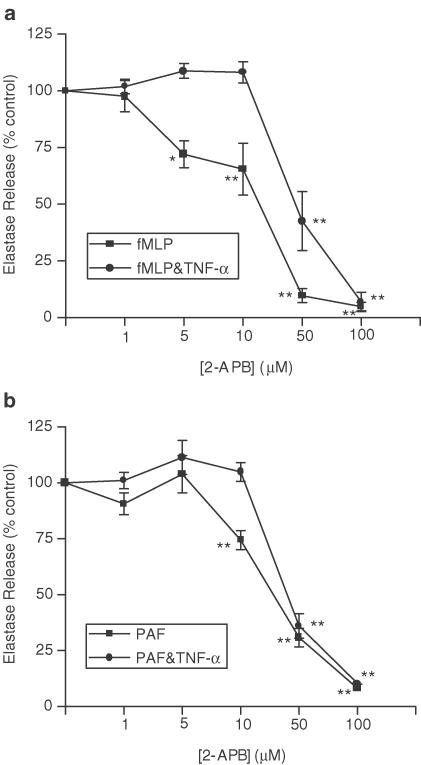
Figures 8a and b show the concentration-dependent inhibitory effects of the IP3 receptor antagonist, 2-APB upon fMLP and PAF-induced neutrophil elastase release. In control experiments, none of the molecules investigated had any effect on the detection of neutrophil elastase activity when added to wells at the end of each experiment (data not shown), that is, when UH or related molecules are added to the assay once neutrophil elastase has been released, immediately prior to the addition of chromogenic substrate to sample wells.
Figure 8.
Effects of 2-APB upon fMLP (a) and PAF (b)-induced elastase release, with and without the presence of TNF-α. Neutrophils were placed into microfuge tubes at a concentration of 2.5 × 106 cells ml−1, in the presence of 5 μg ml−1 cytochalasin B and the presence or absence of 100 U ml−1 TNF-α and inhibitor. After 30 min, 10−7 M fMLP or 10−6 M PAF was added to the cells for 45 min, and cells were then centrifuged, samples of supernatant taken and incubated for 1 h with elastase substrate. After 1 h at room temperature, the coloured product was measured colorimetrically at 405 nm, using a microplate reader. Results from experiments on cells from at least six donors, each performed in duplicate, are expressed as mean±s.e.m. values, expressed as a percentage of control values from experiments carried out without drug (*P<0.05 and **P<0.01, when compared with media control).
Discussion
Previous studies have shown that heparin is able to inhibit many functions of neutrophils (see Tyrell et al., 1999; Lever & Page, 2002). In the present studies, we report for the first time the ability of UH to inhibit the release of neutrophil elastase from human neutrophils over a similar concentration–response curve to that reported for human neutrophil homotypic aggregation, the latter effect supporting the observations of Bazzoni et al. (1993). This effect of UH on neutrophil elastase release was mimicked by ODH and an LMWH heparin preparation and while this effect was also shared by DS, this molecule was without effect on neutrophil aggregation, suggesting that the mechanism of action of heparin on elastase release from human neutrophils may not be via the same mechanism as the effect of heparin on homotypic neutrophil aggregation.
The inhibition of aggregation by UH is likely to be a result of modifying interactions between adhesion molecules at the initial stage of aggregation, rather than the effect of anionic charge per se since this effect of heparin was not mimicked by PGA. The integrin CD11b/CD18 is a component of neutrophil–neutrophil adhesion. Heparin and heparan sulphate, but not other sulphated GAGs, bind CD11b/CD18 (Diamond et al., 1995) and account for the inhibitory effect of heparin on leucocytes in vivo (Salas et al., 2000); so it is likely that UH, ODH and LMWH inhibit aggregation through preventing CD11b/CD18 binding to its ligand. This specificity is also emphasized by the lack of activity of DS on homotypic neutrophil aggregation in our experiments.
The inhibition of neutrophil elastase release by heparin may involve several mechanisms. It would appear to be sulphate dependent since the effect of UH on neutrophil elastase release was mimicked by DS, but not by the nonsulphated molecules PGA or HA in this system. Our observations support earlier findings that other neutrophil functions can be inhibited by heparin via a sulphate dependent mechanism (Matzner et al., 1984; Bazzoni et al., 1993; Lever et al., 2000). Moreover, the rank order of activity of the different drugs used in this study against elastase release was DS, LMWH, UH and finally ODH. DS is more sulphated than UH and its derivatives, and ODH is less sulphated than UH and LMWH, providing further evidence that the inhibitory effect upon elastase release is sulphate dependent. Also, the effect may correlate inversely with molecular size, since LMWH is more effective than UH at equivalent concentrations, and DS of a larger molecular weight (10 kDa) was less effective than the DS used in this study (data not shown).
Heparin has been suggested to be an anti-inflammatory molecule as a result of its ability to bind to many different molecules implicated in the inflammatory process (Tyrell et al., 1999). However, it has been established that heparin does not bind fMLP (Matzner et al., 1984; Bazzoni et al., 1993) and there is no evidence it can bind to PAF. Therefore, it is unlikely that the effects of heparin in our studies are because of direct interference with the stimuli, although heparin does appear to be more active against fMLP than PAF-induced activation of neutrophils. Interestingly, heparin and related molecules also inhibit the release of neutrophil elastase from human neutrophils primed with TNF-α, an effect mimicked by ODH, the nonanticoagulant derivative of heparin. These results suggest that this novel effect of heparin on neutrophil function is independent of anticoagulant activities supporting earlier work that other actions of heparin are dissociated from the well-described anticoagulant activities of this molecule.
A variety of cell types have been observed to bind and internalize heparin (Wright et al., 1989; Akimoto et al., 1996) and heparin has been shown to competitively antagonize the receptor for the signalling molecule IP3 in both permeabilized (Ghosh et al., 1988; Tones et al., 1989; Fasolato et al., 1993) and intact cells (Jonas et al., 1997). The inhibitory effects of 2-APB (Figures 8a and b) confirm that IP3 is an essential component of fMLP- and PAF-induced neutrophil elastase release, raising the possibility that antagonism of IP3 receptors in neutrophils by heparin may contribute to its ability to inhibit neutrophil elastase release.
Also, Thourani et al. (2000) demonstrated that heparin is able to inhibit activation of the transcription factor nuclear factor-kappaB (NF-κB). It would be expected that smaller heparin molecules are internalized more readily than larger molecules. It is of interest, therefore, that an inverse relationship between the molecular weight and antiallergic activity of fractionated heparin in allergic sheep has been reported (Martinez-Salas et al., 1998; Ahmed et al., 2000), suggesting that the anti-inflammatory activity of heparin may reside in the smaller fractions. Whether these intracellular effects are specific to heparin, or can be achieved by other sulphated molecules, such as dextran sulphate, remains to be evaluated.
Although much effort has gone into understanding the causes of inflammation, endogenous factors that limit inflammation in the nonpathological state have largely been ignored. On the basis of the vast array of evidence regarding the anti-inflammatory nature of heparin (Tyrell et al., 1999; Lever & Page, 2002), it is feasible that heparin and/or other glycosaminoglycans exist as endogenous anti-inflammatory molecules (Page, 1991), a concept that can be extended to the inhibition of neutrophil elastase release from human neutrophils. Heparin is a molecule that occurs only in mast cells and it is of interest therefore that mast cell numbers have been found to be elevated in the epithelium and bronchial glands of subjects with COPD (Pesci et al., 1994), a disease where TNF-α primed neutrophils and their products, in particular elastase, are found in the sputum and bronchoalveolar lavage fluid of these patients (Martin et al., 1985; Thompson et al., 1989; Keatings et al., 1996). Thus, the combined ability of heparin to not only inhibit the enzymatic activity of neutrophil elastase, but to also inhibit neutrophil activation and release of products including neutrophil elastase, as we report here for the first time, may provide a novel approach to controlling COPD.
Evidently, further investigations regarding the use of heparin or its derivatives in neutrophil-mediated airway diseases such as COPD are required, but our observations that demonstrate an inhibitory effect of heparin on neutrophil elastase release, that is independent of the anticoagulant activity of this molecule, may provide the basis for the design of novel therapeutics for the treatment of this and other inflammatory diseases that involve neutrophil recruitment and activation.
Abbreviations
- 2-APB
2-aminoethoxydiphenylborate
- AT III
antithrombin III
- COPD
chronic obstructive pulmonary disease
- DMSO
dimethyl sulphoxide
- DS
dextran sulphate
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- GAG
glycosaminoglycan
- HA
hyaluronic acid
- HBSS
Hanks' balanced salts solution
- IL
interleukin
- IP3
inositol 1,4,5-trisphosphate
- LMWH
low-molecular-weight heparin
- NF-κB
nuclear factor-kappaB
- ODH
O-desulphated heparin
- PAF
platelet-activating factor
- PBS
phosphate-buffered saline
- PGA
poly-L-glutamic acid
- PMA
phorbol myristate acetate
- TNF-α
tumour necrosis factor-α
- UH
unfractionated heparin
References
- AHMED T., UNGO J., ZHOU M., CAMPO C. Inhibition of allergic late airway responses by inhaled heparin-derived oligosaccharides. J. Appl. Physiol. 2000;88:1721–1729. doi: 10.1152/jappl.2000.88.5.1721. [DOI] [PubMed] [Google Scholar]
- AKIMOTO H., ITO H., TANAKA M., ADACHI S., HATA M., LIN M., FUJISAKI H., MARUMO F., HIROE M. Heparin and heparan sulfate block angiotensin-II-induced hypertropy in cultured neonatal rat cardiomyocytes A possible role of intrinsic heparin-like molecules in regulation of cardiomyocyte hypertrophy. Circulation. 1996;93:810–816. doi: 10.1161/01.cir.93.4.810. [DOI] [PubMed] [Google Scholar]
- ASA D., GANT T., ODA Y., BRANDLEY B.K. Evidence for two classes of carbohydrate binding sites on selectins. Glycobiology. 1992;2:395–400. doi: 10.1093/glycob/2.5.395. [DOI] [PubMed] [Google Scholar]
- BAZZONI G., NUNEZ A.B., MASCELLANI G., BIANCHINI P., DEJANA E., DEL MASCHIO A. Effect of heparin, dermatan sulfate, and related oligo-derivatives on human polymorphonuclear leukocyte functions. J. Lab. Clin. Med. 1993;121:268–275. [PubMed] [Google Scholar]
- DIAMOND M.S., ALON R., PARKOS C.A., QUINN M.T., SPRINGER T.A. Heparin is an adhesive ligand for the leukocyte Mac-1 (CD11b/CD18) J. Cell. Biol. 1995;130:1473–1482. doi: 10.1083/jcb.130.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FASOLATO C., HOTH M., MATTHEWS G., PENNER R. Ca2+ and Mn2+ influx through receptor-mediated activation of nonspecific cation channels in mast cells. Proc. Natl. Acad. Sci. U.S.A. 1993;90:3068–3072. doi: 10.1073/pnas.90.7.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRYER A., HUANG Y.C., RAO G., JACOBY D., MANCILLA E., WHORTON R., PIANTADOSI C.A., KENNEDY T., HOIDAL J. Selective O-desulfation produces nonanticoagulant heparin that retains pharmacological activity in the lung. J. Pharmacol. Exp. Ther. 1997;282:208–219. [PubMed] [Google Scholar]
- GHOSH T.K., EIS P.S., MULLANEY J.M., EBERT C.L., GILL D.L. Competitive, reversible, and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. J. Biol. Chem. 1988;263:11075–11079. [PubMed] [Google Scholar]
- HOOK M., KJELLEN L., JOHANSSON S., ROBINSON J. Cell-surface glycosaminoglycans. Annu. Rev. Biochem. 1984;53:847–869. doi: 10.1146/annurev.bi.53.070184.004215. [DOI] [PubMed] [Google Scholar]
- JONAS S., SUGIMORI M., LLINAS R. Is low molecular weight heparin a neuroprotectant. Ann. N. Y. Acad. Sci. 1997;825:389–393. doi: 10.1111/j.1749-6632.1997.tb48449.x. [DOI] [PubMed] [Google Scholar]
- KEATINGS V.M., COLLINS P.D., SCOTT D.M., BARNES P.J. Differences in interleukin-8 and tumour necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1996;153:530–534. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- KOENIG A., NORGARD-SUMNICHT K., LINDHARDT R., VARKI A. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins. J. Clin. Invest. 1998;101:877–889. doi: 10.1172/JCI1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAFUMA C., FRISDAL E., HARF A., ROBERT L., HORNEBECK W. Prevention of leucocyte elastase-induced emphysema in mice by heparin fragments. Eur. Respir. J. 1991;4:1004–1009. [PubMed] [Google Scholar]
- LENDRUM A.C. The staining of eosinophil polymorphs and enterochromaffin cells in histological sections. J. Pathol. Bacteriol. 1944;56:441–445. [Google Scholar]
- LEVER R., HOULT J.R.S., PAGE C.P. The effects of heparin and related molecules upon the adhesion of polymorphonuclear leukocytes to vascular endothelium in vitro. Br. J. Pharmacol. 2000;129:533–540. doi: 10.1038/sj.bjp.0703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVER R., PAGE C.P. Novel drug development opportunities for heparin. Nat. Rev. Drug Discov. 2002;1:140–148. doi: 10.1038/nrd724. [DOI] [PubMed] [Google Scholar]
- MARTIN T.R., RAGHU G., MAUNDER R.J., SPRINGMEYER S.C. The effects of chronic bronchitis and chronic airflow obstruction on lung cell populations recovered by bronchoalveolar lavage. Am. Rev. Respir. Dis. 1985;132:254–260. doi: 10.1164/arrd.1985.132.2.254. [DOI] [PubMed] [Google Scholar]
- MARTINEZ-SALAS J., MENDELSSOHN R., ABRAHAM W., HSIAO B., AHMED T. Inhibition of allergic airway responses by inhaled low-molecular weight heparins: molecular weight dependence. J. Appl. Physiol. 1998;84:222–228. doi: 10.1152/jappl.1998.84.1.222. [DOI] [PubMed] [Google Scholar]
- MATZNER Y., MARX G., DREXLER R., ELDOR A. The inhibitory effect of heparin and related glycosaminoglycans on neutrophil chemotaxis. Thromb. Haemost. 1984;52:134–137. [PubMed] [Google Scholar]
- MCLEAN J. The discovery of heparin. Circulation. 1959;19:75–78. doi: 10.1161/01.cir.19.1.75. [DOI] [PubMed] [Google Scholar]
- PAGE C.P. One explanation of the asthma paradox: inhibition of natural anti-inflammatory mechanism by beta 2-agonists. Lancet. 1991;337:717–720. doi: 10.1016/0140-6736(91)90289-2. [DOI] [PubMed] [Google Scholar]
- PESCI A., ROSSI G.A., BERTORELLI G., AUFIERO A., ZANON P., OLIVIERI D. Mast cells in the airway lumen and bronchial mucosa of patients with chronic bronchitis. Am. J. Respir. Crit. Care Med. 1994;152:260–266. doi: 10.1164/ajrccm.149.5.8173772. [DOI] [PubMed] [Google Scholar]
- RAMDIN L., PERKS B., SHERON N., SHUTE J.K. Regulation of interleukin-8 binding and function by heparin and α2-macroglobulin. Clin. Exp. Allergy. 1998;28:616–624. doi: 10.1046/j.1365-2222.1998.00283.x. [DOI] [PubMed] [Google Scholar]
- RAO N.V., KENNEDY T.P., RAO G., KY N., HOIDAL J.R. Sulfated polysaccharides prevent human leukocyte-induced acute lung injury and emphysema in hamsters. Am. Rev. Respir. Dis. 1990;142:407–412. doi: 10.1164/ajrccm/142.2.407. [DOI] [PubMed] [Google Scholar]
- REDINI F., TIXIER J.-M., PETITOU M., CHOAY J., ROBERT L., HORNEBECK W. Inhibition of leucocyte elastase by heparin and its derivatives. Biochem. J. 1988;252:515–519. doi: 10.1042/bj2520515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALAS A., SANS M., SORIANO A., REVERTER J.C., ANDERSON D.C., PIQUE J.M., PANES J. Heparin attenuates TNF-alpha induced inflammatory response through a CD11b dependent mechanism. Gut. 2000;47:88–96. doi: 10.1136/gut.47.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SASAKI M., HERD C.M., PAGE C.P. Effect of heparin and a low-molecular weight heparinoid on PAF-induced airway responses in neonatally immunized rabbits. Br. J. Pharmacol. 1993;110:107–112. doi: 10.1111/j.1476-5381.1993.tb13778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKINNER M.P., LUCAS C.M., BURNS G.F., CHESTERMAN C.N., BERNDT M.C. GMP-140 binding to neutrophils is inhibited by sulfated glycans. J. Biol. Chem. 1991;226:5371–5374. [PubMed] [Google Scholar]
- THOMPSON P.B., DAUGHTON D., ROBBINS G.A., GHAFOURI M.A., OEHLERKING M., RENNARD S.I. Intraluminal airway inflammation in chronic bronchitis. Am. Rev. Respir. Dis. 1989;140:1527–1537. doi: 10.1164/ajrccm/140.6.1527. [DOI] [PubMed] [Google Scholar]
- THOURANI V.H., BRAR S.S., KENNEDY T.P., THORNTON L.R., WATTS J.A., RONSON R.S., ZHAO Z.Q., STURROCK A.L., HOIDAL J.R., VINTEN- JOHANSEN J. Nonanticoagulant heparin inhibits NF-kappaB activation and attenuates myocardial reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H2084–H2093. doi: 10.1152/ajpheart.2000.278.6.H2084. [DOI] [PubMed] [Google Scholar]
- TONES M.A., BOOTMAN M.D., HIGGINS B.F., LANE D.A., PAY G.F., LINDAHL U. The effect of heparin on the inositol 1,4,5-trisphosphate receptor in rat liver microsomes. Dependence on sulphate content and chain length. FEBS Lett. 1989;252:105–108. doi: 10.1016/0014-5793(89)80898-1. [DOI] [PubMed] [Google Scholar]
- TYRELL D.J., HORNE A.P., HOLME K.R., PREUSS J.M.H., PAGE C.P. Heparin in inflammation: Potential therapeutic applications beyond anticoagulation. Adv. Pharmacol. 1999;46:151–208. doi: 10.1016/s1054-3589(08)60471-8. [DOI] [PubMed] [Google Scholar]
- WALSH R.L., DILLON T.J., SCICCHITANO R., MCLENNAN G.B. Heparin and heparan sulphate are inhibitors of human leucocyte elastase. Clin. Sci. 1991;81:341–346. doi: 10.1042/cs0810341. [DOI] [PubMed] [Google Scholar]
- WRIGHT T.C., CASTELLOT J.J., DIAMOND J.R., KARNOVSKY M.J.Regulation of cellular proliferation by heparin and heparan sulfate Heparin Chemical and Biological Properties. Clinical Applications 1989London: Edward Arnold; 295–316.ed. Lane, D.A., & Lindahl, U. pp [Google Scholar]
- XIE X., THORLACIUS H., RAUD J., HEDQVIST P., LINDBOM L. Inhibitory effect of locally administered heparin on leukocyte rolling and chemoattractant-induced firm adhesion in rat mesenteric venules in vivo. Br. J. Pharmacol. 1997;122:906–910. doi: 10.1038/sj.bjp.0701454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YING Q.-L., KEMME M., SAUNDERS D., SIMON S.R. Glycosaminoglycans regulate elastase inhibition by oxidized secretory leukoprotease inhibitor. Am. J. Physiol. 1997;272:L533–L541. doi: 10.1152/ajplung.1997.272.3.L533. [DOI] [PubMed] [Google Scholar]