Abstract
We have evaluated the participation of endothelium-derived hyperpolarizing factor (EDHF) in the endothelium-dependent relaxation of isolated human penile resistance arteries (HPRA) and human corpus cavernosum (HCC) strips. In addition, the effect of the angioprotective agent, calcium dobesilate (DOBE), on the endothelium-dependent relaxation of these tissues was investigated.
Combined inhibition of nitric oxide synthase (NOS) and cyclooxygenase (COX) nearly abolished the endothelium-dependent relaxation to acetylcholine (ACh) in HCC, while 60% relaxation of HPRA was observed under these conditions. Endothelium-dependent relaxation of HPRA resistant to NOS and COX inhibition was prevented by raising the extracellular concentration of K+ (35 mM) or by blocking Ca2+-activated K+ channels, with apamin (APA; 100 nM) and charybdotoxin (CTX; 100 nM), suggesting the involvement of EDHF in these responses.
Endothelium-dependent relaxation to ACh was markedly enhanced by DOBE (10 μM) in HPRA but not in HCC. The potentiating effects of DOBE on ACh-induced responses in HPRA, remained after NOS and COX inhibition, were reduced by inhibition of cytochrome P450 oxygenase with miconazole (0.3 mM) and were abolished by high K+ or a combination of APA and CTX.
In vivo, DOBE (10 mg kg−1 i.v.) significantly potentiated the erectile responses to cavernosal nerve stimulation in male rats.
EDHF plays an important role in the endothelium-dependent relaxation of HPRA but not in HCC. DOBE significantly improves endothelium-dependent relaxation of HPRA mediated by EDHF and potentiates erectile responses in vivo. Thus, EDHF becomes a new therapeutic target for the treatment of erectile dysfunction (ED) and DOBE could be considered a candidate for oral therapy for ED.
Keywords: Human corpus cavernosum, human penile resistance arteries, endothelium, endothelium-derived hyperpolarizing factor, calcium dobesilate
Introduction
The relaxation of arterial and trabecular penile smooth muscle is needed to achieve and maintain penile erection (Sáenz de Tejada et al., 1991). Penile smooth muscle tone is under the control of relaxing and contractile mediators that are released from autonomic nerve terminals and the endothelia of corpus cavernosum lacunar spaces and penile arteries. The endothelium seems to play a crucial role in facilitating the erectile response. In fact, in aging and vascular diseases associated with endothelial dysfunction such as hypercholesterolemia, hypertension, and diabetes, a high prevalence of erectile dysfunction (ED) is observed (Martín-Morales et al., 2001).
Several substances derived from the endothelium are involved in endothelium-dependent relaxation. Nitric oxide (NO) and prostacyclin (PGI2) were firstly identified as the main endothelial mediators participating in the local regulation of smooth muscle tone, but the existence of an additional unidentified endothelial factor that promotes smooth muscle hyperpolarization and is resistant to NO synthase (NOS) and cyclooxygenase (COX) inhibition has been clearly established (Garland et al., 1995). The functional relevance of this endothelium-derived hyperpolarizing factor (EDHF) depends on the specific vascular bed, being more prevalent in small than in large arteries. EDHF has been reported to be an important contributor to vasodilation in human coronary arteries (Miura et al., 1999) and resistance vessels (Coats et al., 2001; Halcox et al., 2001).
Calcium dobesilate (DOBE) has been extensively used as an orally administered angioprotective agent, specially in the treatment of diabetic retinopathy (Berthet et al., 1999). Although its mechanism of action is poorly understood, this compound has been shown to potentiate endothelium-dependent relaxation of rabbit aorta (Ruiz et al., 1997) and aorta from diabetic rats and to improve endothelial function in diabetic patients (Berthet et al., 1999; Tejerina et al., 1999).
The aims of the present study were: firstly, to determine the possible contribution of EDHF to the regulation of human penile smooth muscle contractility. Secondly, to determine the effects of DOBE on endothelium-dependent relaxation of penile smooth muscle, as well as the effects of this agent on the erectile responses, in vivo, in a rat model.
Methods
Human penile tissue
Human corpus cavernosum (HCC) biopsies (n=50) were obtained from men suffering from ED who gave informed consent at the time of penile prosthesis insertion. The causes of ED included failure of corporo-veno-occlusive mechanism, structural alterations, neurological dysfunction and vascular dysfunction. The study was approved by the local Ethics Committee. Tissues were maintained at 4–6°C in M-400 solution (composition per 100 ml: manitol, 4.19 g; KH2PO4, 0.205 g; K2HPO4·3H2O, 0.97 g; KCl, 0.112 g; NaHCO3, 0.084 g) until used, which was between 2 and 16 h from extraction. Penile resistance arteries and/or corpus cavernosum strips were obtained from these biopsies (Angulo et al., 2002).
Vascular reactivity of human penile resistance arteries (HPRA)
Human penile small arteries, helicine arteries (lumen diameter 150–400 μm), which are the terminal branches of deep penile arteries, were dissected by carefully removing the adhering trabecular tissue, and arterial ring segments (2 mm long) were subsequently mounted on two 40 μm wires on microvascular double Halpern–Mulvany myographs (J.P. Trading, Aarhus, Denmark) for isometric tension recordings (n=79 from 42 patients). The vessels were allowed to equilibrate for 30 min in physiological salt solution (PSS) of the following composition (mM): NaCl 119, KCl 4.6, CaCl2 1.5, MgCl2 1.2, NaHCO3 24.9, glucose 11, KH2PO4 1.2, EDTA 0.027 at 37°C continuously bubbled with 95% O2/5% CO2 mixture to maintain a pH of 7.4. Passive tension and internal circumference of vascular segments, when relaxed in situ under a transmural pressure of 100 mmHg (L100), were determined. The arteries were then set to an internal circumference equivalent to 90% of L100, at which the force development was close to maximal (Mulvany & Halpern, 1977). The preparations were then exposed to 125 mM K+ (KPSS, equimolar substitution of NaCl for KCl in PSS) and the contractile response was measured. The arteries were contracted with 1 μM norepinephrine (80% of KPSS induced contraction approximately) and relaxation responses were evaluated by cumulative additions of compounds to the chambers. The arterial segments considered as lacking functional endothelium did not relax to 10 μM acetylcholine.
Experiments with HCC strips
Strips of corpus cavernosum tissue (3 × 3 × 7 mm3) were immersed in 8 ml organ chambers containing PSS, maintained at 37°C and aerated with 5% CO2/95% O2, pH 7.4. (n=15 from 12 patients). Each tissue strip was incrementally stretched to optimal isometric tension, as determined by maximal contractile response to 1 μM phenylephrine (Kim et al., 1991). Tissues were contracted with 0.5–3 μM phenylephrine (80% of KPSS-induced contraction) and relaxation responses were evaluated by cumulative additions of compounds to the chambers.
Evaluation of the effects of DOBE on endothelium-dependent relaxation of HCC and penile arteries
For evaluation of the effects of DOBE on endothelium-dependent relaxation, HPRA (from 36 patients) and corpus cavernosum (from 10 patients) were used. When a concentration–response curve to acetylcholine (ACh) was completed, tissues were washed and left to equilibrate. Then, DOBE (10 μM) was added and, after 30 min, tissues were recontracted and when a stable contraction was achieved, the responses to ACh were again evaluated. For evaluation of the effects of DOBE on EDHF-mediated responses, tissues that previously relaxed to ACh were treated with L-NNA (100 μM) and indomethacin (INDO) (5 μM) for 30 min and a concentration–response to ACh was evaluated. After a washout and equilibration period, tissues were cotreated with NG-nitro-L-arginine (L-NNA), INDO and DOBE (10 μM) and the relaxant response to ACh was again evaluated. For evaluation of the effects of other treatments on the effects induced by DOBE on endothelium-dependent relaxation of human penile arteries, experiments were conducted in parallel. An arterial segment was treated with DOBE (10 μM) alone, while another arterial segment from the same patient was treated with DOBE plus potassium (35 mM), DOBE plus L-NNA (100 μM) plus INDO (5 μM) plus apamin (APA) (0.1 μM) plus charybdotoxin (0.1 μM) or DOBE plus L-NNA plus INDO plus miconazole (0.3 mM).
Erectile responses to cavernosal nerve stimulation in anesthetized rats
Male Sprague–Dawley rats (n=8) were anesthetized with ketamine (60 mg kg−1). The surgical procedure consisted of dissection and isolation of the right cavernous nerve through an abdominal midline incision and exposure of penile crura through a transverse perineal incision. Intracavernosal pressure (ICP) measurements were accomplished by insertion into the right crus of a 23-gauge needle connected to a disposable pressure transducer (Abbott, Sligo, Ireland) and a data-acquisition system (ADInstruments, Castle Hill, Australia). Left carotid artery and right external jugular vein were catheterized for constant blood pressure measurement and saline or drug infusion, respectively. Electrical stimulation was applied by a delicate platinum bipolar hook electrode connected to a stimulator and current amplifier (Cibertec, Madrid, Spain). Parameters of electrical stimulation consisted of pulses with a duration of 1 ms and 1.5 mA of current intensity for 1 min. Frequency–response curves were performed by applying stimulation at 1, 3 and 10 Hz at 3 min intervals.
For evaluation of acute effects of DOBE on erectile responses, a control stimulation at 1, 3 and 10 Hz was performed and, after a stabilization period, DOBE (10 mg kg−1) dissolved in 20% hydroxy-propyl-β-cyclodextrin (HPβCD) (n=4) or the vehicle alone (n=4) was intravenously (i.v.) administered. The stimulation was repeated at 60 min after the administration of DOBE or vehicle. The 1 h period from administration of DOBE for the evaluation of the erectile responses was chosen based on results obtained in pilot studies, testing responses at 30 and at 60 min post-treatment. Although there were effects at 30 min after administration, these were partial, while at 60 min, the effects were always present.
Drugs and materials
Phenylephrine, norepinephrine (arterenol), ACh, INDO, L-NNA, APA, CTX and HPβCD were obtained from Sigma Chemical Co. (St Louis, MO, U.S.A.). Miconazole was obtained from RBI (Natick, MA, U.S.A.). DOBE (calcium dihydroxy-2,5 benzenesulfonate, Doxium®) was provided by Dr Esteve Laboratories (Barcelona, Spain).
Data analysis
Relaxation responses are expressed as percentage of total relaxation (loss in tone) induced by the addition of 0.1 mM.papaverine HCl to the chambers at the end of the experiment. All data are expressed as mean±s.e.m. Complete concentration–response or frequency–response curves were obtained and compared by a two-factor analysis of variance (ANOVA) statistical test using StatView software for Apple computers. Erectile responses to rat cavernosal nerve stimulation were determined by measuring the peak of ICP increase normalized by mean arterial pressure (MAP) values, the duration of the response and the area under the curve (AUC) of the ICP increases normalized by MAP values. The complete frequency–response curves were compared by a two-factor ANOVA test.
Results
Role of EDHF in endothelium-dependent relaxation of HCC trabecular strips and penile resistance arteries
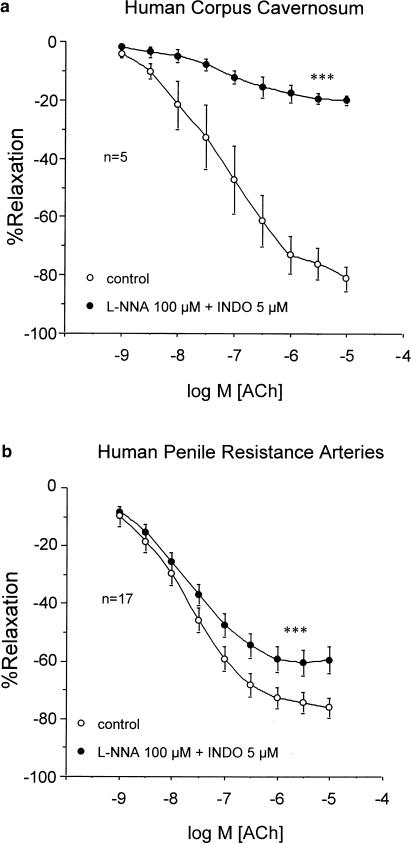
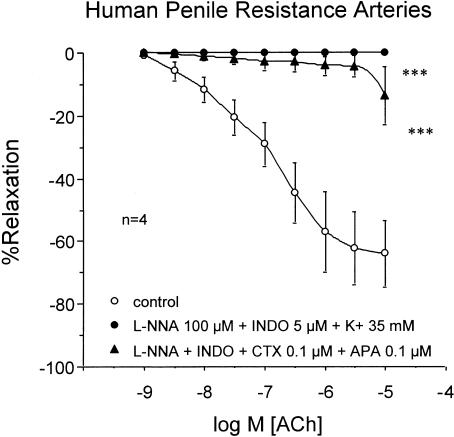
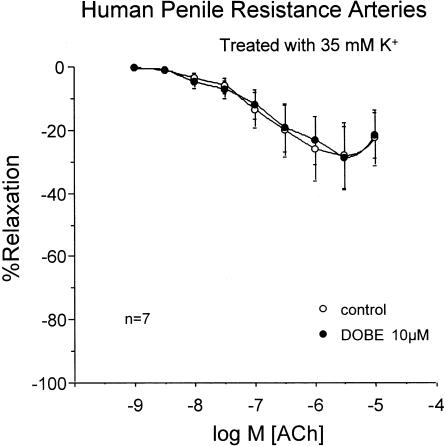
In strips of trabecular tissue (n=5), ACh; (1 nM–10 μM) produced concentration-dependent relaxation, which was nearly abolished after combined treatment with the NOS inhibitor, L-NNA (100 μM) and the COX inhibitor, INDO (5 μM) (Figure 1a). Conversely, in penile arteries (n=17), although the treatment with L-NNA (100 μM) and INDO (5 μM) significantly reduced ACh-induced relaxation, ACh produced approximately 60% relaxation of these arteries after NOS and COX inhibition (Figure 1b). The ACh-induced relaxations, in the presence of NOS and COX inhibition, were abolished by contracting the HPRA (n=4) with a high extracellular K+ concentration (35 mM) or by treating the arteries (n=4) with a combination of blockers of Ca2+-dependent K+-channel blockers, APA; (100 nM) and CTX 100 nM) (Figure 2).
Figure 1.
Effects of combined inhibition of NOS and COX with L-NNA; 100 μM) and INDO (5 μM), respectively, on the endothelium-dependent relaxation induced by ACh (1 nM–10 μM) on phenylephrine-contracted HCC strips (a) and norepinephrine-contracted HPRA (b). Data are expressed as mean±s.e.m. of the percentage of total relaxation induced by 0.1 mM papaverine. n Indicates the number of patients from whom the tissues were collected for the experiments. ***Indicates P<0.005 vs control responses by a two-factor ANOVA test.
Figure 2.
Effects of high extracellular K+ concentration (35 mM) and Ca2+-activated K+-channel blockade with APA; (100 nM) and CTX; (100 nM) in addition to combined inhibition of NOS and COX with L-NNA; (100 μM) and INDO (5 μM), respectively, on the endothelium-dependent relaxation induced by ACh (1 nM–10 μM) on norepinephrine-contracted HPRA. Data are expressed as mean±s.e.m. of the percentage of total relaxation induced by 0.1 mM papaverine. n Indicates the number of patients from whom the tissues were collected for the experiments. ***Indicates P<0.005 vs control responses by a two-factor ANOVA test.
Effects of DOBE on endothelium-dependent relaxation of human penile smooth muscle
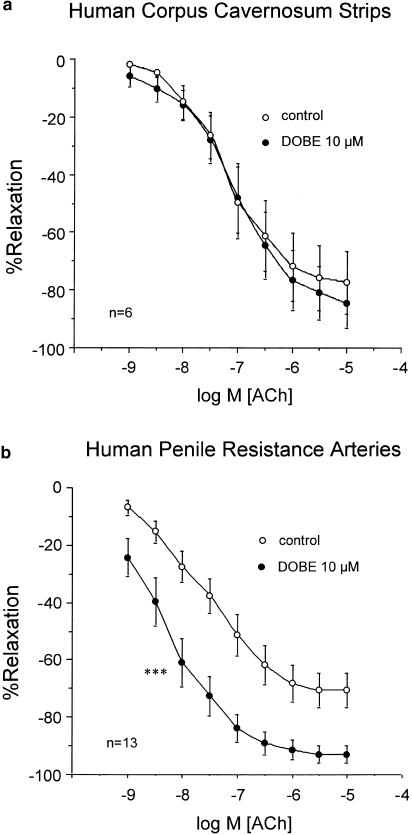
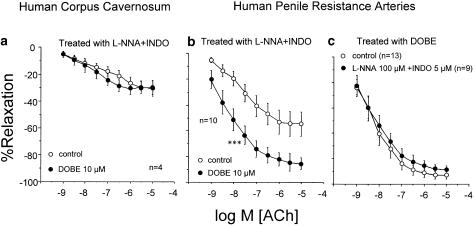
Endothelium-dependent relaxation of HCC trabecular strips induced by ACh was not modified by treatment with DOBE (10 μM) in the presence (n=4) or absence (n=6) of combined NOS and COX inhibition (Figures 3a and 4a). On the contrary, DOBE (10 μM) markedly potentiated ACh-induced relaxation of HPRA in the absence (n=13) (Figure 3b) or in the presence (n=10) (Figure 4b) of combined NOS and COX inhibition. Furthermore, in arteries treated with DOBE, combined treatment with L-NNA (100 μM) and INDO (5 μM) did not significantly affect ACh-induced relaxations (Figure 4c). However, when the arteries (n=7) were exposed to a high potassium concentration (35 mM), DOBE (10 μM) had no effect on ACh-induced relaxations (Figure 5).
Figure 3.
Effects of the treatment with DOBE (10 μM) on the endothelium-dependent relaxation induced by ACh (1 nM–10 μM) on phenylephrine-contracted HCC strips (a) and norepinephrine-contracted HPRA (b). Data are expressed as mean±s.e.m. of the percentage of total relaxation induced by 0.1 mM papaverine. n Indicates the number of patients from whom the tissues were collected for the experiments. ***Indicates P<0.005 vs control responses by a two-factor ANOVA test.
Figure 4.
Effects induced by DOBE (10 μM) on the endothelium-dependent relaxation induced by ACh (1 nM–10 μM) on phenylephrine-contracted HCC strips (a) and norepinephrine-contracted HPRA (b) after combined inhibition of NOS and COX with L-NNA (100 μM) and INDO (5 μM), respectively. (c) shows the comparison of ACh-induced responses in HPRA treated with DOBE (10 μM) in the presence or the absence of L-NNA (100 μM) plus INDO (5 μM). Data are expressed as mean±s.e.m. of the percentage of total relaxation induced by 0.1 mM papaverine. n Indicates the number of patients from whom the tissues were collected for the experiments. ***Indicates P<0.005 vs control curve by a two-factor ANOVA test.
Figure 5.
Effects induced by DOBE (10 μM) on the endothelium-dependent relaxation induced by ACh (1 nM–10 μM) on norepinephrine-contracted HPRA exposed to high extracellular K+ concentration (35 mM). Data are expressed as mean±s.e.m. of the percentage of total relaxation induced by 0.1 mM papaverine. n Indicates the number of patients from whom the tissues were collected for the experiments.
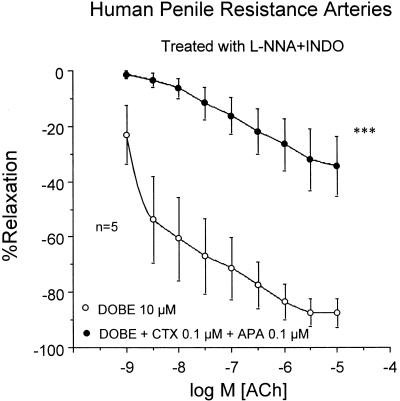
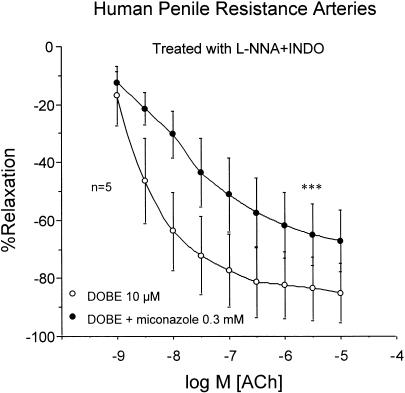
Exposure of HPRA (n=5) to a combination of APA (100 nM) and CTX (100 nM) that blocked ACh-induced relaxation resistant to NOS and COX inhibition, abolished the potentiating effects of DOBE (Figure 6). Finally, miconazole (0.3 mM) significantly reduced the potentiating effects of DOBE of ACh-induced relaxation of HPRA (n=5) resistant to NOS and COX inhibition (Figure 7).
Figure 6.
Effects of Ca2+-activated K+-channel blockade with APA (100 nM) and CTX (100 nM) on the effects induced by DOBE (10 μM) on the endothelium-dependent relaxation induced by ACh (1 nM–10 μM) on norepinephrine-contracted HPRA in the presence of L-NNA (100 μM) plus INDO (5 μM). Data are expressed as mean±s.e.m. of the percentage of total relaxation induced by 0.1 mM papaverine. n Indicates the number of patients from whom the tissues were collected for the experiments. ***Indicates P<0.005 vs DOBE alone by a two-factor ANOVA test.
Figure 7.
Influence of cytochrome P450 oxygenase inhibition with miconazole (0.3 mM) on the effects induced by DOBE (10 μM) on the endothelium-dependent relaxation induced by ACh (1 nM–10 μM) on norepinephrine-contracted HPRA in the presence of L-NNA (100 μM) plus INDO (5 μM). Data are expressed as mean±s.e.m. of the percentage of total relaxation induced by 0.1 mM papaverine. n Indicates the number of patients from whom the tissues were collected for the experiments. ***Indicates P<0.005 vs DOBE alone by a two-factors ANOVA test.
Effects of DOBE on erectile responses in anesthetized rats
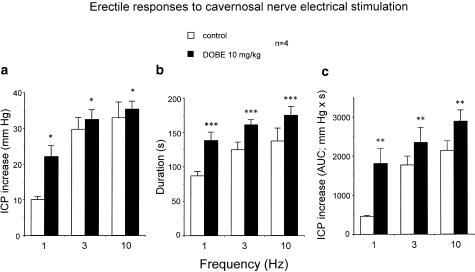
Electrical stimulation of the cavernosal nerve in anesthetized rats produced frequency-dependent ICP increases, which were not modified by the treatment with vehicle (20% HPβCD) (n=4). I.v. administration of DOBE (10 mg kg−1) (n=4) produced a rapid and transitory increase in systemic blood pressure (15–20 mmHg), which recovered to basal levels after 4–6 min. Treatment with DOBE significantly enhanced the erectile responses to cavernosal nerve stimulation (Figure 8). Statistically significant potentiating effects by DOBE were observed when erectile responses were expressed as the peak of ICP increase (Figure 8a), the duration of the response (Figure 8b) or the AUC of the ICP increase (Figure 8c).
Figure 8.
Effects of i.v. administration of DOBE (10 mg kg−1) on erectile responses to cavernosal nerve electrical stimulation (CNES) in anesthetized male rats. Data are expressed as the mean±s.e.m. of the peak of ICP increase to CNES normalized by MAP values (a), the duration of the response to CNES (b) and the AUC ( mmHgs) of ICP increase to CNES normalized by MAP values (c). *P<0.05, **P<0.01, ***P<0.005 vs control frequency–response curve by a two-factors ANOVA test.
Discussion
Preserved endothelial function seems to be fundamental for penile erection. Indeed, diseases in which endothelial dysfunction develops, such as in diabetes mellitus, hypertension, hypercholesterolemia or aging, are associated with a high prevalence of ED (Martin-Morales et al., 2001) In animal models of these diseases, impaired endothelium-dependent relaxation of corpus cavernosum (Azadzoi and Sáenz de Tejada (1991); Haas et al., 1998; Keegan et al., 2000) and reduced in vivo erectile responses (Azadzoi et al., 1996; Champion et al., 1999; Escrig et al., 2002) have been demonstrated. Further evidence of the relevance of the endothelium in penile erection is that mice lacking the neuronal NO synthase (nNOS) gene have normal erectile function, possibly due to the overexpression of endothelial NOS (eNOS) (Burnett et al., 1996), although the existence of splice variant genes or residual activity of the gene for nNOS could also account for erectile function in these animals. In addition, the transfection in corpus cavernosum of aged rats of the eNOS gene reversed the ED observed in these animals (Champion et al., 1999)
NO is a key mediator of endothelium-dependent relaxation (Moncada et al., 1987), but it is not the only relaxant factor derived from the endothelium. It is well known that, in response to various stimuli, the human endothelium is able to produce prostacyclin (Osanai et al., 2000; Yamasaki et al., 2000), which, in turn, has the ability to relax a large number of vascular beds, including penile arteries (Angulo et al., 2002). Furthermore, the endothelium-dependent relaxation of some human resistance arteries has a component resistant to the inhibition of NO and prostacyclin production, which is attributed to EDHF (Miura et al., 1999; Coats et al., 2001). In horse penile arteries, the existence of a hyperpolarizing factor contributing to endothelium-dependent dilation has been demonstrated (Prieto et al., 1998). The results of the present study show that a factor resistant to NOS and COX inhibition, likely EDHF, participates in endothelium-dependent relaxation of HPRA but not in trabecular tissue. The involvement of EDHF in the endothelium-dependent relaxation of HPRA is strongly suggested by the fact that consistent relaxation to ACh remains after NOS and COX inhibition that is abolished by a high extracellular K+ concentration that prevents hyperpolarization of smooth muscle and the endothelium. Another characteristic feature of NOS and COX inhibition-resistant responses attributed to EDHF and present in HPRA is that relaxation is blocked by a combination of APA and CTX. These toxins most probably act on small (SK(Ca)), intermediate (IK(Ca)) and possibly large conductance (BK(Ca)) Ca2+-activated K+ channels (K(Ca)), localized in the endothelium (Busse et al., 2002). Agonist-induced (e.g. ACh) hyperpolarization of the endothelium, apparently an essential step in the production/action of EDHF, is prevented by the combined action of APA (blocking SK(Ca)) and CTX (blocking IK(Ca) and BK(Ca)).
Here we also report that DOBE is able to improve endothelium-dependent relaxation of HPRA, while not affecting these responses in corpus cavernosum tissue. This observation suggests that the mechanisms of endothelium-dependent relaxation differ in arterial and corpus cavernosum trabecular tissues. Functional differences between penile arterial and trabecular tissues have been reported affecting the receptor profile and hence the responses to vasoactive drugs (Hedlund & Andersson, 1985; Angulo et al., 2002).
Dobesilate has been reported to reduce capillary permeability, to inhibit platelet aggregation and to reduce blood viscosity (Berthet et al., 1999). In addition, DOBE is able to potentiate endothelium-dependent relaxation in the aorta of rabbit (Ruiz et al., 1997) and diabetic rats (Tejerina et al., 1999). DOBE has been reported to enhance endothelial NOS activity (Suschek et al., 1997), but it is unlikely that this mechanism is involved in the effects of DOBE in human penile arteries, since DOBE was active despite NOS inhibition with L-NNA. In addition, it has been reported that DOBE has antioxidant capacity in vitro (Brunet et al., 1998b) and in vivo (Brunet et al., 1998a), which could be related to its inhibitory effects on aldose reductase activity (Schmidt & Michal, 1989). Since oxidative stress could affect endothelial function, this capacity of DOBE could be relevant to its effects on endothelium-dependent relaxation.
We propose that DOBE enhances the release, the availability or the action of EDHF. This proposal is based on three facts: first, DOBE is able to potentiate ACh-induced responses when NOS and COX are both inhibited; second, if the concentration of potassium ions is increased, precluding the hyperpolarization of the tissue, the potentiating effect of DOBE disappears; and third, K(Ca) channels are required for the action of DOBE. This mechanism of action proposed for DOBE would explain the differential effects exerted by the compound on human penile arterial and corpus cavernosum trabecular tissues, because the participation of EDHF in endothelium-dependent relaxation of corpus cavernosum seems to be, at most, marginal.
The nature of EDHF remains unclear, although several candidates have been proposed. The ion potassium induces hyperpolarization and relaxation of smooth muscle and has been proposed as a putative EDHF in rat arteries (Edwards et al., 1998; Dora & Garland, 2001). Hydrogen peroxide (H2O2) has also been suggested to be an EDHF in human arteries (Matoba et al., 2001). However, prevention of H2O2 formation with catalase (1000 U ml−1) did not affect endothelium-dependent relaxation of HPRA resistant to NOS and COX inhibition and catalase did not modify the effect of DOBE in our preparations (data not shown). Finally, endothelial cytochrome P450 oxygenase (CYP) activity has been considered to be responsible for EDHF production in different human vascular beds (Miura et al., 1999; Coats et al., 2001; Halcox et al., 2001). Epoxyeicosatrienoic acids (EET), derived from arachidonic acid through CYP activity, act as the EDHF in bovine coronary arteries (Campbell et al., 1996). These compounds elicit hyperpolarization and relaxation of vascular smooth muscle with activation of K(Ca) channels (Zhang et al., 2001). In human penile arteries, the treatment with miconazole, which nonselectively inhibits the CYP activities (Rodrigues et al., 1987; Ballard et al., 1988), significantly attenuated the effect of DOBE on EDHF-mediated relaxations, suggesting that the effect of this agent is related to the enhancement of the production or the action of CYP derivatives. However, while miconazole significantly reduced, it did not abolish the DOBE-induced enhancement of the responses attributed to EDHF in our preparations. This could be due to the existence of a pool of EETs associated with the membrane phospholipids that are released independently of CYP activity (Weintraub et al., 1997). Nevertheless, we cannot exclude the coexistence of more than one factor, possibly of different chemical nature, acting as EDHF in human penile arteries. To our knowledge, DOBE is the first agent proposed to acutely potentiate EDHF-mediated relaxation of human arteries. β-naphthoflavone and the calcium antagonist, nifedipine, have been reported to potentiate EDHF-mediated responses by increasing expression of the CYP2C enzyme in bovine coronary arteries (Fisslthaler et al., 2000). This is a mechanism that requires time; thus, it is unlikely that the acute effects of DOBE in human penile arteries were mediated by augmenting CYP2C expression.
Finally, the enhancement by DOBE of in vivo erectile responses in the rat confers additional relevance to the in vitro effects of this compound on human penile arteries. Potentiation of erectile responses by DOBE affected the amplitude and the duration of the response, and was specially marked at the lowest frequency used (1 Hz). This is not surprising since we are using healthy rats that are supposed to have normal erectile function, and the treatment with DOBE facilitates near to maximal responses at low frequencies, lowering the stimulation threshold. Similar finding has been reported with phosphodiesterase 5 inhibition (Gemalmaz et al., 2001). Furthermore, the fact that an enhancement of penile arterial relaxation could improve erections provides the rationale to study the effects of therapeutic candidates for ED on penile arteries, and not only in corpus cavernosum tissue. It is important to note that the concentration of DOBE that we used for in vitro experiments is in the range of the plasma levels achieved after the oral dose of 500 mg (∼20 μM) (Benakis et al., 1974), although higher doses of DOBE are commonly administered (Berthet et al., 1999). In addition, the dose of DOBE used in the in vivo experiments is consistent with those used by other investigators in the rat model (Brunet et al., 1998a).
In conclusion, EDHF plays an important role in the endothelium-dependent relaxation of HPRA. DOBE is able to clearly potentiate endothelium-dependent relaxation of HPRA likely by enhancing the production or the action of EDHF in these vessels. The effect of dobesilate results in enhanced erectile responses, as observed in the rat model. These observations make EDHF a possible therapeutic target for the treatment of ED, and DOBE a candidate for the treatment of ED. The use of DOBE as an oral therapeutic agent for the treatment of other diseases allows us to hypothesize its oral use for ED. Although the acute effects of DOBE observed in this study would lead us to speculate with an on-demand administration, further research is needed to support this speculation or to evaluate possible additional benefits of chronic administration.
Acknowledgments
This work was partially supported by a grant from Laboratorios Dr Esteve.
Abbreviations
- APA
apamin
- COX
cyclooxygenase
- CTX
charybdotoxin
- DOBE
calcium dobesilate
- EDHF
endothelium-derived hyperpolarizing factor
- HCC
human corpus cavernosum
- HPRA
human penile resistance arteries
- HPβCD
hydroxy-propyl-β-cyclodextrin
- INDO
indomethacin
- L-NNA
NG-nitro-L-arginine
- NOS
nitric oxide synthase
References
- ANGULO J., CUEVAS P., LA FUENTE J.M., POMEROL J.M., RUIZCASTAÑE E., PUIGVERT A., GABANCHO S., FERNÁNDEZ A., NEY P., SÁENZ DE TEJADA I. Regulation of human penile smooth muscle tone by prostanoid receptors. Br. J. Pharmacol. 2002;136:23–30. doi: 10.1038/sj.bjp.0704675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AZADZOI K.M., SÁENZ DE TEJADA I. Hypercholesterolemia impairs endothelium-dependent relaxation of rabbit corpus cavernosum smooth muscle. J. Urol. 1991;146:238–240. doi: 10.1016/s0022-5347(17)37759-5. [DOI] [PubMed] [Google Scholar]
- AZADZOI K.M., SIROKY M.B., GOLDSTEIN I. Study of etiologic relationship of arterial atherosclerosis to corporal veno-occlusive dysfunction in the rabbit. J. Urol. 1996;155:1795–1800. [PubMed] [Google Scholar]
- BALLARD S.A., LODOLA A., TARBIT M.H. A comparative study of 1-substituted imidazole and 1,2,4-triazole antifungal compounds as inhibitors of testosterone hydroxylations catalysed by mouse hepatic microsomal cytochromes P-450. Biochem. Pharmacol. 1988;37:4643–4651. doi: 10.1016/0006-2952(88)90333-4. [DOI] [PubMed] [Google Scholar]
- BENAKIS A., GLASSON B., BOUVIER C.A., RITSCHARD J., KRAHENBUHL B., JUNG A., HACHEN J.H. Metabolisme et pharmacocinetique du dobesilate de calcium chez l'homme. Therapie. 1974;29:211–219. [PubMed] [Google Scholar]
- BERTHET P., FARINE J.C., BARRAS J.P. Calcium dobesilate: pharmacological profile related to its use in diabetic retinopathy. Int. J. Clin. Pract. 1999;53:631–636. [PubMed] [Google Scholar]
- BRUNET J., FARINE J.C., GARAY R.P., HANNAERT P. Angioprotective action of calcium dobesilate against reactive oxygen species-induced capillary permeability in the rat. Eur. J. Pharmacol. 1998a;358:213–220. doi: 10.1016/s0014-2999(98)00604-9. [DOI] [PubMed] [Google Scholar]
- BRUNET J., FARINE J.C., GARAY R.P., HANNAERT P. In vitro antioxidant properties of calcium dobesilate. Fundam. Clin. Pharmacol. 1998b;12:205–212. doi: 10.1111/j.1472-8206.1998.tb00943.x. [DOI] [PubMed] [Google Scholar]
- BURNETT A.L., NELSON R.J., CALVIN D.C., LIU J.X., DEMAS G.E., KLEIN S.L., KRIEGSFELD L.J., DAWSON V.L., DAWSON T.M., SNYDER S.H. Nitric oxide-dependent penile erection in mice lacking neuronal nitric oxide synthase. Mol. Med. 1996;2:288–296. [PMC free article] [PubMed] [Google Scholar]
- BUSSE R., EDWARDS G., FÉLÉTOU M., FLEMING I., VANHOUTTE P.M., WESTON A. EDHF: bringing the concepts together. Trends Pharmacol. Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- CAMPBELL W.B., GEBREMEDHIN D., PRATT P.F., HARDER D.R. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- CHAMPION H.C., BIVALACQUA T.J., HYMAN A.L., IGNARRO L.J., HELLSTROM W.J.G., KADOWITZ P.J. Gene transfer of endothelial nitric oxide synthase to the penis augments erectile responses in the aged rat. Proc. Natl. Acad. Sci. U.S.A. 1999;96:11648–11652. doi: 10.1073/pnas.96.20.11648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COATS P., JOHNSTON F., MACDONALD J., MCMURRAY J.J.V., HILLIER C. Endothelium-derived hyperpolarizing factor. Identification and mechanisms of action in human subcutaneous resistance arteries. Circulation. 2001;103:1702–1708. doi: 10.1161/01.cir.103.12.1702. [DOI] [PubMed] [Google Scholar]
- DORA K.A., GARLAND C.J. Properties of smooth muscle hyperpolarization and relaxation to K+ in the rat isolated mesenteric artery. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2424–H2429. doi: 10.1152/ajpheart.2001.280.6.H2424. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., DORA K.A., GARDENER M.J., GARLAND C.J., WESTON A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- ESCRIG A., MARIN R., ABREUP, GONZALEZ-MORA L.J., MAS M. Changes in mating behavior, erectile function, and nitric oxide levels in penile corpora cavernosa in streptozotocin-diabetic rats. Biol. Reprod. 2002;66:185–189. doi: 10.1095/biolreprod66.1.185. [DOI] [PubMed] [Google Scholar]
- FISSLTHALER B., HINSCH N., CHATAIGNEAU T., POPP R., KISS L., BUSSE R., FLEMING I. Nifedipine increases cytochrome P4502C expression and endothelium-derived hyperpolarizing factor-mediated responses in coronary arteries. Hypertension. 2000;36:270–275. doi: 10.1161/01.hyp.36.2.270. [DOI] [PubMed] [Google Scholar]
- GARLAND C.J., PLANE F., KEMP B.K., COCKS T.M. Endothelium-dependent hyperpolarization: a role in the control of vascular tone. Trends Pharmacol. Sci. 1995;16:23–30. doi: 10.1016/s0165-6147(00)88969-5. [DOI] [PubMed] [Google Scholar]
- GEMALMAZ H., WALDECK K., CHAPMAN T.N., TUTTLE J.B., STEERS W.D., ANDERSSON K.E. In vivo and in vitro investigation of the effects of sildenafil on rat cavernous smooth muscle. J. Urol. 2001;165:1010–1014. [PubMed] [Google Scholar]
- HAAS C.A., SEFTEL A.D., RAZMJOUEI K., GANZ M.B., HAMPEL N., FERGUSON K. Erectile dysfunction in aging: upregulation of endothelial nitric oxide synthase. Urology. 1998;51:516–522. doi: 10.1016/s0090-4295(97)00715-2. [DOI] [PubMed] [Google Scholar]
- HALCOX J.P.J., NARAYANAN S., CRAMER-JOYCE L., MINCEMOYER R., QUYYUMI A.A. Characterization of endothelium-derived hyperpolarizing factor in the human forearm microcirculation. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2470–H2477. doi: 10.1152/ajpheart.2001.280.6.H2470. [DOI] [PubMed] [Google Scholar]
- HEDLUND H., ANDERSSON K.E. Comparison of the responses to drugs acting on adrenoreceptors and muscarinic receptors in human isolated corpus cavernosum and cavernous artery. J. Auton. Pharmacol. 1985;5:81–88. doi: 10.1111/j.1474-8673.1985.tb00568.x. [DOI] [PubMed] [Google Scholar]
- KEEGAN A., JACK A.M., COTTER M.A., CAMERON N.E. Effects of aldose reductase inhibition on responses of the corpus cavernosum and mesenteric vascular bed of diabetic rats. J. Cardiovasc. Pharmacol. 2000;35:606–613. doi: 10.1097/00005344-200004000-00014. [DOI] [PubMed] [Google Scholar]
- KIM N., AZADZOI K.M., GOLDSTEIN I., SÁENZ DE TEJADA I. A nitric oxide-like factor mediates nonadrenergic–noncholinergic neurogenic relaxation of penile corpus cavernosum smooth muscle. J Clin Invest. 1991;88:112–118. doi: 10.1172/JCI115266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN-MORALES A., SANCHEZ-CRUZ J.J., SAENZ DE TEJADA I., RODRIGUEZ-VELA L., JIMENEZ-CRUZ J.F., BURGOS-RODRIGUEZ R. Prevalence and independent risk factors for erectile dysfunction in Spain: results of the Epidemiologia de la Disfuncion Erectil Masculina study. J. Urol. 2001;166:569–574. doi: 10.1016/s0022-5347(05)65986-1. [DOI] [PubMed] [Google Scholar]
- MATOBA T., SHIMOKAWA H., KUBOTA H., MORIKAWA K., FUJIKI T., KUNIHIRO I., MUKAI Y., HIRAKAWA Y., TAKESHITA A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem. Biophys. Res. Commun. 2001;290:909–913. doi: 10.1006/bbrc.2001.6278. [DOI] [PubMed] [Google Scholar]
- MIURA H., LIU Y., GUTTERMAN D.D. Human coronary arteriolar dilation to bradykinin depends on membrane hyperpolarization: contribution of nitric oxide and Ca2+-activated K+ channels. Circulation. 1999;99:3132–3138. doi: 10.1161/01.cir.99.24.3132. [DOI] [PubMed] [Google Scholar]
- MONCADA S., HERMAN A.G., VANHOUTTE P.M. Endothelium-derived relaxing factor is identified as nitric oxide. Trends Pharmacol. Sci. 1987;8:365–368. [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small resistance arteries in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- OSANAI T., FUJITA N., FUJIWARA N., NAKANO T., TAKAHASHI K., GUAN W., OKUMURA K. Cross talk of shear-induced production of prostacyclin and nitric oxide in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H233–H238. doi: 10.1152/ajpheart.2000.278.1.H233. [DOI] [PubMed] [Google Scholar]
- PRIETO D., SIMONSEN U., HERNÁNDEZ M., GARCÍA-SACRISTÁN A. Contribution of K+ channels and ouabain-sensitive mechanisms to the endothelium-dependent relaxations of horse penile small arteries. Br. J. Pharmacol. 1998;123:1609–1620. doi: 10.1038/sj.bjp.0701780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUES A.D., GIBSON G.G., IOANNIDES C., PARKE D.V. Interactions of imidazole antifungal agents with purified cytochrome P-450 proteins. Biochem Pharmacol. 1987;36:4277–4281. doi: 10.1016/0006-2952(87)90670-8. [DOI] [PubMed] [Google Scholar]
- RUIZ E., LORENTE R., TEJERINA T. Effects of calcium dobesilate on the synthesis of endothelium-dependent relaxing factors in rabbit isolated aorta. Br. J. Pharmacol. 1997;121:711–716. doi: 10.1038/sj.bjp.0701184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SÁENZ DE TEJADA I., MOROUKIAN P., TESSIER J., KIM J.J., GOLDSTEIN I., FROHRIB D. Trabecular smooth muscle modulates the capacitor function of the penis Studies on a rabbit model. Am. J. Physiol. 1991;260:H1590–H1595. doi: 10.1152/ajpheart.1991.260.5.H1590. [DOI] [PubMed] [Google Scholar]
- SCHMIDT M., MICHAL M. Inhibition of sorbitol formation in human erythrocytes by calcium dobesilate. Arzneimittelforschung. 1989;39:493–495. [PubMed] [Google Scholar]
- SUSCHEK C., KOLB H., KOLB-BACHOFEN V. Dobesilate enhances endothelial nitric oxide synthase-activity in macro- and microvascular endothelial cells. Br. J. Pharmacol. 1997;122:1502–1508. doi: 10.1038/sj.bjp.0701512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEJERINA T., RUIZ E., SANZ M., GANADO P. Study of calcium dobesilate in diabetic rats. Int. J. Angiol. 1999;8:16–20. doi: 10.1007/BF01619844. [DOI] [PubMed] [Google Scholar]
- WEINTRAUB N.L., FANG X., KADUCE T.L., VANROLLINS M., CHATTERJEE P., SPECTOR A.A. Potentiation of endothelium-dependent relaxation by epoxyeicosatrienoic acids. Circ. Res. 1997;81:258–267. doi: 10.1161/01.res.81.2.258. [DOI] [PubMed] [Google Scholar]
- YAMASAKI S., SAWADA S., KOMATSU S., KAWAHARA T., TSUDA Y., SATO T., TORATANI A., KONO Y., HIGAKI T., IMAMURA H., TADA Y., AKAMATSU N., TAMAGAKI T., TSUJI H., NAKAGAWA M. Effects of bradykinin on prostaglandin I2 synthesis in human vascular endothelial cells. Hypertension. 2000;36:201–207. doi: 10.1161/01.hyp.36.2.201. [DOI] [PubMed] [Google Scholar]
- ZHANG Y., OLTMAN C.L., LU T., LEE H.-C., DELLSPERGER K.C., VANROLLINS M. EET homologs potently dilate coronary microvessels and activate BKCa channels. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2430–H2440. doi: 10.1152/ajpheart.2001.280.6.H2430. [DOI] [PubMed] [Google Scholar]