Abstract
Neuropeptide Y (NPY), peptide YY (PYY) and pancreatic polypeptide (PP) differentially activate three Y receptors (Y1, Y2 and Y4) in mouse and human isolated colon.
The aim of this study was to characterise Y2 receptor-mediated responses in colon mucosa and longitudinal smooth muscle preparations from wild type (Y2+/+) and knockout (Y2−/−) mice and to compare the former with human mucosal Y agonist responses. Inhibition of mucosal short-circuit current and increases in muscle tone were monitored in colonic tissues from Y2+/+ and Y2−/− mice±Y1 ((R)-N-[[4-(aminocarbonylaminomethyl)phenyl)methyl]-N2-(diphenylacetyl)-argininamide-trifluoroacetate (BIBO3304) or Y2 (S)-N2-[[1-[2-[4-[(R,S)-5,11-dihydro-6(6H)-oxodibenz[b,e]azepin-11-yl]-1-piperazinyl]-2-oxoethyl]cyclopentyl]acetyl]-N-[2-[1,2-dihydro-3,5(4H)-dioxo-1,2-diphenyl-3H-1,2,4-triazol-4-yl]ethyl]-argininamide (BIIE0246) antagonists.
Predictably, Y2−/− tissues were insensitive to Y2-preferred agonist PYY(3-36) (⩽100 nM), but unexpectedly Y4-preferred PP responses were right-shifted probably as a consequence of elevated circulating PP levels, particularly in male Y2−/− mice (Sainsbury et al., 2002).
BIBO3304 and BIIE0246 elevated mucosal ion transport, indicating blockade of inhibitory mucosal tone in Y2+/+ tissue. While BIBO3304 effects were unchanged, those to BIIE0246 were absent in Y2−/− mucosae. Neither antagonist altered muscle tone; however, BIIE0246 blocked NPY and PYY(3-36) increases in Y2+/+ basal tone. BIBO3304 abolished residual Y1-mediated NPY responses in Y2−/− smooth muscle.
Tetrodotoxin significantly reduced BIIE0246 and PYY(3-36) effects in Y2+/+ mouse and human mucosae, but had no effect upon Y-agonist contractile responses, indicating that Y2 receptors are located on submucosal, but not myenteric neurones.
Tonic activation of submucosal Y2 receptors by endogenous NPY, PYY or PYY(3-36) could indirectly reduce mucosal ion transport in murine and human colon, while direct activation of Y2 receptors on longitudinal muscle results in contraction.
Keywords: Pancreatic polypeptides, neuropeptide Y receptors, mouse and human colon, mucosal ion transport, smooth muscle contraction
Introduction
Neuropeptide Y (NPY) suppresses synaptic excitation in the central and peripheral nervous systems (King et al., 2000; Weiser et al., 2000; Silva et al., 2001) by activating distinct Y receptors, most commonly postsynaptic or postjunctional Y1 receptors and presynaptic Y2 receptors (Dumont et al., 1998; Weiser et al., 2000; Kaga et al., 2001; Bahn et al., 2002). In the hypothalamus, activation of Y2 receptors inhibits NPY-mediated tonic inhibition of adjacent pro-opiomelanocortin (POMC) neurones leading to satiety in mouse and man (Batterham et al., 2002). In the peripheral nervous system, Y2 receptors also provide significant presynaptic (or prejunctional) inhibition that can either be autoinhibitory (Malmström et al., 2002), inhibitory upon noradrenergic (Cunningham et al., 1994) or cholinergic neurotransmission (Smith-White et al., 2002), but little is known about Y2 receptor modulation of nonadrenergic, noncholinergic (NANC) neurotransmission.
Y2 receptors are not, however, always presynaptic, nor are Y1 receptors exclusively postsynaptic, or postjunctional (Cox & Cuthbert, 1990; McAuley & Westfall, 1992; Mannon et al., 1999; for reviews see, Cox, 1998; Michel et al., 1998). For example, epithelial Y2 receptors alone mediate NPY-, PYY- and PYY(3-36)-induced inhibition of Cl− secretion across rat jejunum mucosa (Cox et al., 1988; Cox & Cuthbert, 1990; Cox & Tough, 2000). In the rat colon mucosa, Y2 receptors are coactivated with Y1 receptors to inhibit anion secretion and in this tissue each receptor type exhibits pre- and postjunctional responses (Tough & Cox, 1996). The selective nonpeptide antagonists for Y1 (BIBO3304, Wieland et al., 1998) and Y2 receptors (BIIE0246, Doods et al., 1999; Dumont et al., 2000) have been crucial for the pharmacological characterisation of NPY, PYY and PYY(3-36) effects, especially in tissues coexpressing multiple Y receptor types. The two antagonists have enabled determination of the functional significance of Y1 and Y2 receptors in many isolated tissues and in vivo systems. For example in human isolated colon where NPY, and its hormone analogues, peptide YY (PYY), PYY(3-36) and pancreatic polypeptide (PP) are all antisecretory (and each agonist was equi-effective, Cox & Tough, 2002), only PP effects (i.e. Y4 receptor-mediated effects) were insensitive to treatment with a combination of a Y1 and Y2 receptor antagonist. The same was also true of PP responses in the 129Sv mouse colon mucosa where all four peptides were inhibitory (but Y1 responses predominated, Cox et al., 2001). Thus, in isolated human and murine colon mucosae, Y1, Y2 and Y4 receptors have been shown to differentially mediate Y agonist inhibition of ion transport, and Y5 receptors play no functional role in either tissue (Cox et al., 2001; Cox & Tough, 2002).
In the intestine, NPY released locally from enteric submucous secretomotor neurones innervating the mucosa (Ekblad et al., 1987;1988; Sang & Young, 1996; see also review by Furness, 2000) can be antisecretory by activating either Y2 or Y1 receptors, or both. In contrast, the endocrine peptides PYY, its fragment PYY(3-36) and PP, which are expressed and released from intestinal or pancreatic type F endocrine cells, respectively (Sundler et al., 1993; Arantes & Nogueira, 1997), will differentially activate Y1, Y2 or Y4 receptors in murine and human large bowel to provide further antisecretory, or proabsorptive, influences following ingestion of a meal. How these effects are coordinated with changes in the patterns of smooth muscle contraction and the modulatory roles that each Y receptor plays in the final integrated intestinal response, remains to be elucidated. Few studies of Y-agonist effects upon intestinal smooth muscle have actually included selective Y antagonists. However, from the agonist orders of potency, we may predict that tonic contractions stimulated by NPY and PYY (Pheng et al., 1999; Ferrier et al., 2000) are Y2-mediated, while those to PP are most likely Y4-mediated (in rat, Pheng et al., 1999; Ferrier et al., 2000; and rabbit intestine, Feletou et al., 1999). Neither the Y1 receptor (Feletou et al., 1999; Ferrier et al., 2000) nor the Y5 receptor (Feletou et al., 1999) appear to have a significant direct role on normal rodent intestine smooth muscle.
Thus, our initial aim was to elucidate the functional role(s) of Y2 receptors in isolated smooth muscle and mucosal preparations using the Y2 antagonist, BIIE0246 (Doods et al., 1999; Dumont et al., 2000) in wild-type (Y2+/+) murine tissue. The functional phenotype exhibited by these preparations were compared with those from germline Y2 receptor knockout (Y2−/−) mice and preliminary investigations have already been presented to the British Pharmacological Society (Hyland et al., 2002). Our second aim was to establish whether Y2 receptors were pre- or postjunctional and these studies were performed with murine and human colon mucosae. Thirdly, recent studies by Sainsbury et al. (2002) using the same germline Y2−/− mouse, described an unexpected gender-specific increase in circulating PP in male Y2−/− mice (together with complex changes in several parameters of energy homeostasis). How elevated circulating PP levels alter peripheral tissue sensitivity to Y agonists or to blockade of such responses by Y receptor antagonists, was of particular interest to us. We therefore set out to establish whether gender-specific differences in Y receptor responses were apparent in isolated intestinal tissue from germline Y2−/− in comparison with Y2+/+ mice.
Methods
Tissue preparation
Germline Y2−/− and Y2+/+ mice were generated and maintained on a mixed C57BL/6–129/SvJ background as described previously (Sainsbury et al., 2002). Age-matched adult mice (both sexes, 12 weeks or more) were asphyxiated with CO2, weighed, the ascending and descending colon taken and placed immediately in fresh Krebs–Henseleit (KH) solution with the following composition (in mM): NaCl 118, KCl 4.7, NaHCO3 25, KH2PO4 1.2, MgSO4 1.2, CaCl2 2.5, glucose 11.1 (pH 7.4). Luminal contents were removed by washing gently with KH.
Murine and human descending colon mucosal preparations
Human colon was obtained with the consent of patients undergoing elective bowel resection for primary intestinal carcinoma (the study was approved by the Guy's and St Thomas' Hospitals Research Ethics Committee; Cox & Tough, 2002). Mucosae were prepared by removing overlying circular and longitudinal smooth muscle layers by dissection under a microscope. Mouse and human colon routinely provided six or eight adjacent mucosal pieces, respectively. Either were placed between two halves of Perspex Ussing chambers (exposed area 0.2 cm2 for mouse or 0.6 cm2 for human tissues), bathed both sides with 5 ml of oxygenated (95% O2/5% CO2) KH and maintained at 37°C. Mucosae were voltage-clamped at 0 mV (using a DVC 1000 automatic voltage clamp, World Precision Instruments, Stevenage, U.K.) as previously described (Cox et al., 1988;2001). Once a stable short-circuit current (Isc) was obtained, agonists or antagonists were added to the basolateral reservoir only and the resultant changes in Isc were recorded continuously. Murine descending colon mucosae were pretreated with vasoactive intestinal polypeptide (VIP) (30 nM) and 15 min later with either a single concentration of a Y agonist or an appropriate Y receptor antagonist was added (for a further 10–15 min) prior to agonist addition. Tetrodotoxin (TTX) pretreatment was for 15 min prior to VIP addition in mouse mucosa and for 30 min once a stable basal Isc had been achieved with human mucosae. Maximal agonist-induced changes in Isc were pooled (and quoted as μA cm−2) and means±1 s.e.m. were calculated, where each mucosal or smooth muscle preparation provided a single n value.
Ascending colon longitudinal smooth muscle preparation
Each section of ascending colon provided two adjacent segments of longitudinal smooth muscle (each 1 cm long), which were cut distal to the caecal junction. Segments were washed with KH, attached with thread and suspended in an organ bath (10 ml) in oxygenated (95% O2/5% CO2) KH, maintained at 37°C. Tissues were stretched to a basal tension of 1 g and were allowed to equilibrate (for 45 min) with three intermittent KH washes. Isometric changes in basal tension were recorded in response to Y agonists in the absence or presence of specific Y antagonists (added 15 min prior to the agonist). Agonist-induced maximum increases in basal tone (within 5 min of agonist addition) were pooled and are quoted as increases in g tension throughout (mean±1 s.e.m.). Carbachol (CCh, 10 μM) was added as an internal contractile control at the end of each assay.
Statistical analysis
Each mucosal and smooth muscle preparation provided single observations, which were not paired but were pooled to provide means±1 s.e.m. For agonist–response curves, EC50 values (with 95% confidence limits) were calculated from pooled single agonist additions using GraphPad Prism (v. 3.0, GraphPad Software Inc., CA, U.S.A.). Student's unpaired t-test was used to compare responses±antagonist. Multiple comparisons between data groups were evaluated using one-way ANOVA with either Bonferroni's or Dunnett's post-test, where appropriate. In each case, a P-value of less than 0.05 was considered statistically significant.
Materials
Peptides were purchased from Bachem U.K. Ltd (Merseyside, U.K.) unless otherwise stated and aliquots were frozen and stored at −20°C, only undergoing a single freeze–thaw cycle. The porcine (p) sequences of PYY, NPY and PP (the latter from Eli Lilly Inc., IN, U.S.A.), plus the human (h) sequences of PP, PYY(3-36) and Pro34PYY were used as indicated. [(S)-N2-diphenylacetyl)-N-[(4-hydroxyphenyl)methyl]-argininamide] (acetate salt) (BIBP3435), ((R)-N-[[4-(aminocarbonylaminomethyl)-phenyl)methyl]-N2-(diphenyl acetyl)-argininamide-trifluoroacetate (BIBO3304) and (S)-N2-[[1-[2-[4-(R,S)-5, 11-dihydro-6(6H)-oxidobenz[b,e]azepin-11-y1]-1-piperazinyl]-2-oxoethyl]cyclopentyl]acetyl]-N-[2-[1,2-dihydro-3,5(4H)dioxo-1,2-diphenyl-3H-1,2,4-triazol-4-yl]ethyl]-argininamide (BIIE0246) were all obtained from Boehringer Ingelheim Pharma KG (Biberach an der Riss, Germany). [Ac-4NO2-Phe-c(D-Cys-Tyr-D-Trp-Lys-Thr-Cys)-D-Tyr-NH2 (CYN-154806) was a gift from Glaxo Institute of Applied Pharmacology (Cambridge, U.K.). 5-bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine (UK14,304) was purchased from Research Biochemical International (Natick, MA, U.S.A.) and CCh and TTX were from Sigma (Poole, U.K.).
Results
General features of Y2+/+ and Y2−/− mice and their descending colon mucosa
Age-matched female Y2+/+ and Y2−/− mice weighed significantly less than their male counterparts of similar age, and Y2−/− mice were leaner than Y2+/+ mice of a similar age (P-values ranging from 0.05 to 0.001, Table 1) as observed previously by Baldock et al. (2002). Since Sainsbury et al. (2002) described significantly elevated circulating PP levels in male germline Y2−/− (∼15 nM) compared with male Y2+/+ mice (∼5 nM), and lower PP levels in female mice of either genotype (∼2 nM in Y2−/− and 1 nM in Y2+/+ mice), we segregated our data accordingly.
Table 1.
A comparison of basal current, basal resistance, in Isc following addition of 30 nM VIP and weight between Y2+/+ and Y2−/− mice
| Y2+/+ | Y2−/− | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Body weight (g) | 31.9±0.4 (25) | 25.6±0.4 (22)*** | 28.6±0.6 (37)+++ | 22.6±0.5 (32)***+ |
| Age (weeks) | 17.4±1.4 (16) | 18.8±1.0 (20) | 18.7±1.0 (31) | 19.4±1.6 (26) |
| Resistance (Ω cm2) | 31.9±1.7 (85) | 37.6±2.9 (65) | 34.3±1.9 (72) | 34.7±1.4 (88) |
| Basal current (μA cm−2) | 52.7±4.3 (138) | 41.0±2.3 (103) | 44.6±2.8 (167) | 36.6±3.0 (152) |
| VIP (30 nM) (μA cm−2) | 66.9±5.1 (105) | 67.8±4.4 (109) | 65.9±4.1 (173) | 70.4±4.2 (155) |
| EC50 PYY(3–36) (nM) | 10.1 (6.7–15.4) | 10.2 (4.3–23.9) | No response | No response |
| EC50 Pro 34PYY (nM) | 17.6 (9.2–33.8) | 6.2 (1.9–20.1) | 39.2 (25.0–61.4) | 18.3 (7.3–46.2) |
| EC50 hPP (nM) | 3.7 (0.3–41.7) | 9.9 (3.2–30.1) | 58.3 (43.0–79.2) | 16.3 (5.7–46.8) |
Values are ±1 s.e.m. with n values in parenthesis. All EC50 values (with 95% confidence limits) are calculated from the pooled agonist concentration–response curves. No responses were recorded with PYY(3–36) ⩽100 nM.
P⩽0.05
P⩽0.001 comparisons between Y2+/+ and Y2−/− mice and
P⩽0.001 for comparisons between males and females within each group.
Mucosal resistance and basal Isc levels were not significantly different in preparations from male and female Y2+/+ and Y2−/− mice (Table 1). Electrogenic responses to basolateral VIP (30 nM, which stimulates cAMP-mediated epithelial Cl− secretion and consequently elevates Isc) were unchanged. Neither the maxima (Table 1) nor the time courses (data not shown) of VIP responses were different between mucosae from Y2+/+ and Y2−/− tissue of either gender.
Mucosal Isc responses to the Y2 preferred agonist PYY(3-36) and to PYY± the Y1 antagonist, BIBO3304 or the Y2 antagonist, BIIE0246
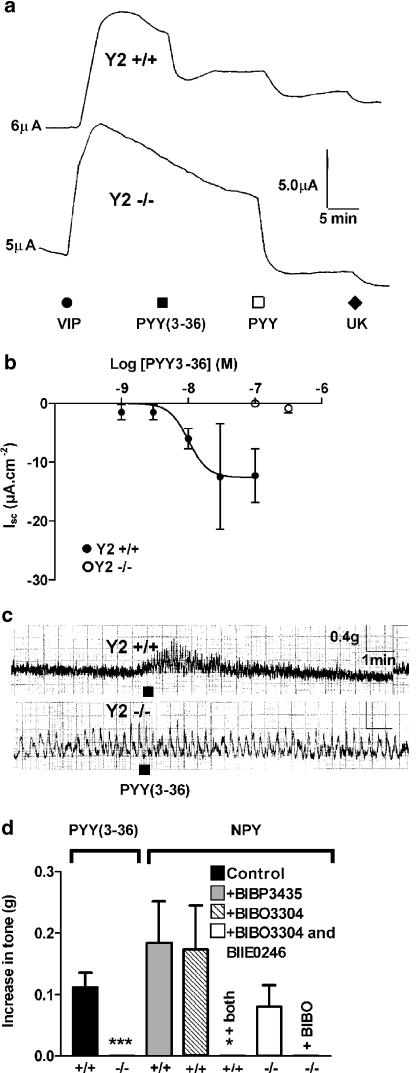
In Y2+/+ colon mucosae, PYY(3-36) attenuated VIP-elevated Isc in a concentration-dependent manner with similar potency in wild-type male and female tissue (Figure 1a, b; Table 1). No PYY(3-36) responses were observed (up to 100 nM, Figure 1b) in Y2−/− tissue from either gender and the small decrease in Isc observed with 300 nM PYY(3-36) (Figure 1b, male data only) was abolished by BIBO3304 (300 nM, data not shown, Hyland et al., 2002). PYY (10 nM) responses following PYY(3-36) (10 nM) addition were not significantly different in Y2−/− tissues (−25.8±6.6 μA cm−2, n=8) from those in Y2+/+ mucosa (−27.8±10.3 μA cm−2, n=4) indicating the predominance of Y1-mediated responses to the full-length peptide. Subsequent addition of the α2-adrenoceptor agonist, UK14,304 also elicited similar-sized reductions in Isc in wild-type and knockout groups (Figure 1a for representative trace, pooled data not shown). In Y2+/+ mucosa, the competitive Y2 receptor antagonist BIIE0246 (1 μM) raised Isc (by 2.5±0.4 μA cm−2, n=26 and 2.1± 0.3 μA cm−2, n=21 in male and female tissue respectively, compared with vehicle controls containing BIBP3435 (1 μM) of 0.3±0.2 μA cm−2, n=6 in male wild-type tissues) and this effect was lost from Y2−/− tissue (0.6±0.6 μA cm−2, n=6 and 0.3 μA cm−2, n=2, male and female tissues respectively). Thus, the inhibitory Y2 tone revealed by BIIE0246, as well as Y2-stimulated agonist responses were predictably absent from Y2−/− male and female colon mucosae, with no changes in non-Y receptor-stimulated effects. The combination of Y1 and Y2 antagonists to Y2+/+ mucosa resulted in a significant sustained elevation in Isc, indicating a combined Y1 and Y2 receptor-mediated inhibitory tone in wild-type colon (Figure 2a, b).
Figure 1.
The presence or absence of PYY(3-36) responses in Y2+/+ and Y2−/− mucosae (a, b) and longitudinal smooth muscle (c, d). (a) Representative responses to VIP (30 nM), PYY(3-36) (100 nM), PYY (10 nM) and UK14,304 (α2 adrenoceptor agonist) (1 μM) in Y2+/+ (upper trace) and Y2−/− (lower trace). Values to the left of each trace in (a) are the basal Isc prior to VIP addition. (b) Pooled PYY(3-36) data from male tissue in Y2+/+ and Y2−/− colon. Values are the mean±1 s.e.m., n=3 throughout (where n represents the number of preparations). (c) Contractile effects of PYY(3-36) (100 nM) on smooth muscle in Y2+/+ (upper trace) and Y2−/− (lower trace). (d) Pooled data showing PYY(3-36) or NPY (both at 100 nM) induced increases in tone in Y2+/+ and Y2−/− colon, respectively. Each bar is the mean+1 s.e.m. for between five and seven observations. Significant differences between NPY responses in the presence of both antagonists and vehicle control (BIBP3435, *P⩽0.05) and PYY(3-36) responses in Y2−/− compared with Y2+/+ tissue, ***P<0.001.
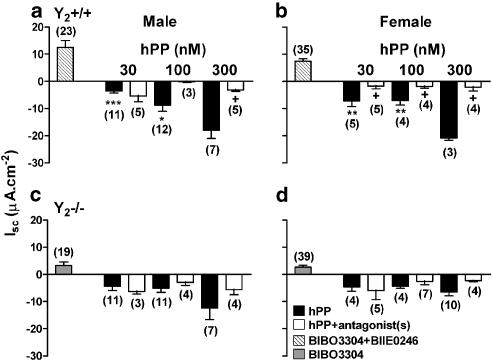
Figure 2.
The effects of BIBO3304 (grey bars) or a combination of BIBO3304 and BIIE0246 (hatched bars) upon inhibitory hPP responses (open bars, in the presence of antagonist(s)) compared with control responses (black bars, in the absence of antagonist(s)) in Y2+/+, male (a) and female (b), and Y2−/−, male (c) and female (d) colon. All values are the mean±1 s.e.m. with n numbers in parentheses. Each 300 nM hPP response, in male and female Y2+/+ tissue, was significantly larger (*P⩽0.05, **P⩽0.01, ***P⩽0.001; one-way ANOVA with Bonferonni's post-test) than the responses to 30 and 100 nM hPP in those groups. Unpaired Student's t-test was used to compare control hPP responses in the presence or absence of antagonist(s), where+P⩽0.05.
Smooth muscle responses to the Y2 preferred agonist PYY(3-36) and NPY± antagonists, BIBO3304 and BIIE0246
Longitudinal muscle contractile responses to PYY(3-36) (100 nM) were characterised (in Y2+/+ ascending colon) by an initial increase in basal tone and a concomitant increase in frequency and amplitude of spontaneous activity (Figure 1c). These effects were present for 3–5 min in Y2+/+, but were absent from Y2−/− colon (Figure 1c, d). PYY(3-36)-stimulated increases in Y2+/+ basal tone (0.12±0.02 g, n=5) were abolished by BIIE0246 (1 μM, 0.0±0.0 g, n=3, P<0.01) the antagonist doing nothing per se. NPY (100 nM) also stimulated Y2+/+ basal tone (Figure 1d) with associated increases in spontaneous activity (data not shown) and both components were unaffected by prior treatment with either the inactive enantiomer of Y1 antagonist BIBP3226, BIBP3435 (300 nM), or the more selective Y1 antagonist BIBO3304 (300 nM). NPY-stimulated Y2+/+ basal tone was abolished by BIIE0246 (1 μM added in combination with BIBO3304, 300 nM, Figure 1d), indicating that Y2 receptors predominantly mediate NPY-induced contraction in wild-type tissue. In Y2−/− colon, however, residual NPY-induced increases in basal tone (in the presence of BIBP3435) were abolished by BIBO3304 (Figure 1d), indicating that a Y1 receptor-mediated component is present in Y2 knockout longitudinal smooth muscle. CCh-stimulated contractions were not altered by any of the treatments above and there were no observable differences between muscarinic responses in male and female tissue (Figure 3a–d).
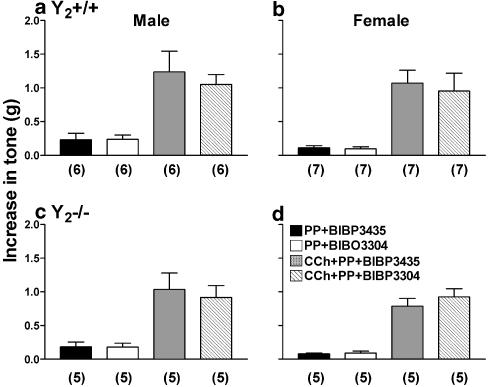
Figure 3.
The effect of pPP (30 nM) upon basal tone of ascending colon preparations obtained from male (a) Y2+/+ and female (b) Y2+/+ mice and Y2−/− tissue from male (c) and female (d) mice. Responses in the presence of either BIBP3435 (300 nM, an inactive enantiomer of the Y1 receptor antagonist, BIBP3226), or BIBO3304 (300 nM, a selective Y1 receptor antagonist) are shown. Each bar is the mean+1 s.e.m. with n numbers as shown in parenthesis. There are no significant differences between BIBP3435- and BIBO3304-pretreated pPP responses or between responses from male and female tissue.
Mucosal responses to Y4-preferring human pancreatic polypeptide (hPP) and Y1-preferred Pro34PYY±Y antagonists
At concentrations between 1 and 100 nM, human pancreatic polypeptide (hPP) was antisecretory with an EC50 of 3.7 nM in male and 9.9 nM in female Y2+/+ colon (Table 1). Within this concentration range, the sensitivity to exogenous hPP was approximately halved in female Y2−/− colon (where notably plasma PP levels doubled, Sainsbury et al., 2002) and in male Y2−/− mucosa the response curve was shifted an order of magnitude to the right compared with male Y2+/+ tissue (Table 1, and the former exhibited three-fold elevated PP levels compared with male Y2+/+ controls). In Figure 2, we show the effects of competitive antagonists (in combination, hatched bars or BIBO3304 alone in grey bars) followed by inhibitory responses to three hPP concentrations only (30, 100 and 300 nM) either in the absence or presence of Y1 and Y2 antagonists. In Y2+/+ colon treated with BIBO3304 (300 nM) and BIIE0246 (1 μM), Y4 receptors remain unaffected and can be stimulated by a Y4-preferring agonist hPP. Thus, the antagonist combination raised Isc levels (Figure 2a, b) indicating endogenous Y1 and Y2 receptor-mediated inhibitory tone in wild-type colon mucosae. Secondly, in male and female Y2+/+ tissues, control 300 nM hPP responses were significantly larger than those at 100 nM (and were excluded from EC50 calculations). Antagonist treatment significantly reduced these high concentration responses to the levels observed with 30 or 100 nM hPP (Figure 2a, b) showing that at 300 nM hPP is able to stimulate Y1 (and probably Y2) as well as Y4 receptors in this tissue.
In male and female Y2−/− colon, BIBO3304 alone caused a small but significant elevation in basal Isc (Figure 2c, d) indicating a similar degree of Y1 receptor-mediated endogenous tone as described for male 129Sv colon mucosa (Cox et al., 2001). In Y2−/− mucosae from either gender, BIBO3304 pretreatment did not significantly inhibit hPP effects (Figure 2c, d). Notably in male Y2−/− mucosa, the hPP concentration–response curve was shifted to the right (Table 1).
The potency of exogenous Y1-preferred agonist, Pro34PYY (0.3–300 nM) was not significantly reduced when comparing Y2−/− responses with those in respective wild types (Table 1). The Pro34PYY-response curve maxima (which were eight to 10 times greater than those for either hPP or PYY(3-36)) were unchanged in the four groups and BIBO3304 (300 nM) abolished Pro34PYY antisecretory effects (data not shown). Additionally, the inactive enantiomer of BIBP3226, BIBP3435 (1 μM) had no significant effect upon either Pro34PYY responses or other Y agonist effects in colon mucosal sheets (data not shown).
Smooth muscle responses to pPP in Y2+/+ and Y2−/− ± either BIBO3304 or BIBP3435
In smooth muscle preparations pPP (30 nM) increased basal tone in both Y2+/+ and Y2−/− tissue and these responses were not significantly altered by pretreatment with BIBO3304 or BIBP3435 (Figure 3). Neither compound altered basal tone per se (data not shown). Responses to pPP after either BIBO3304 or BIBP3435 were no different from those recorded in untreated male tissues (0.26±0.09 g, n=5 in Y2+/+ colon; 0.08±0.03 g, n=3, Y2−/− tissue). Subsequent CCh responses were unaffected either by BIBO3304 or BIBP3435 treatment, and were no different between male, female, wild-type or knockout groups (Figure 3).
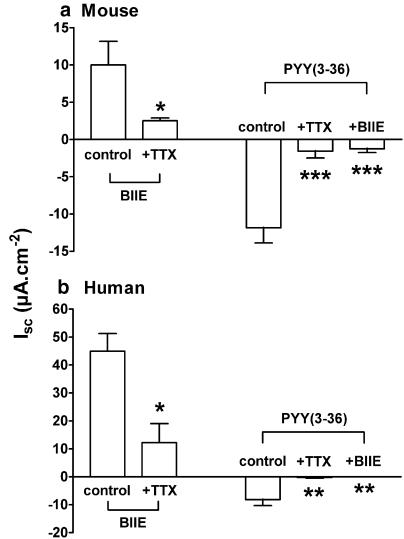
Effects of the Y2 antagonist BIIE0246 and TTX upon Y2-preferred PYY(3-36) responses in murine and human colon mucosae
The competitive Y2 antagonist, BIIE0246 (1 μM) increased Isc in both murine and human colon mucosae and these effects were significantly attenuated by TTX (100 nM, Figure 4a, b) indicating that Y2 receptors are present on inhibitory submucous neurones and that they are tonically active in both tissues. While TTX alone did not reduce mouse colon basal Isc (from 26.5±6.9 μA cm−2, n=9 to 24.7±6.7 μA cm−2, n=9), it did significantly inhibit subsequent antisecretory PYY(3-36) responses (by 87.2%, P<0.001, Figure 4a). This agonist activation of Y2 receptors was predominantly neurogenic although a residual ∼10% of the PYY(3-36) response (30 nM) was not TTX sensitive, indicating a minor postjunctional component, probably epithelial Y2 receptor expression in this tissue. The identity of the final effector inhibiting Isc across the wild-type mouse colon epithelium could be one of a number of inhibitory enteric neuropeptides, such as somatostatin (sst). A selective sst2 receptor antagonist, CYN-154806 (1 μM, Feniuk et al., 2000; Tough & Cox, 2002) was tested. However, it had no effect upon PYY(3-36) responses (controls −5.7± 1.1 μA cm−2, n=7; plus CYN-154806, −6.6±0.9 μA cm−2, n=7), but it significantly reduced sst (30 nM) responses (from −50.0±8.5 μA cm−2, n=7 to −10.2±3.4 μA cm−2, n=7, P<0.001).
Figure 4.
The effects of BIIE0246 (1 μM) and TTX (100 nM) per se and upon subsequent PYY(3-36) (30 nM in (a) and 100 nM in (b)) responses in (a); wild-type mouse descending colon mucosa and in (b), human colon mucosae. Each bar is the mean±1 s.e.m. from between three and six observations and *P⩽0.05, **P⩽0.01, ***P⩽0.001.
In human colon mucosa, TTX significantly reduced basal Isc (from 66.2±18.5 μA cm−2, n=6 to 17.8±8.9 μA cm−2, n=5; P<0.05, Figure 4b). Subsequent PYY(3-36) (100 nM) responses were abolished by BIIE0246 (as shown previously, Cox & Tough, 2002) and by TTX (P<0.01, Figure 4b). Thus, Y2 receptors in human colon mucosa are also predominantly prejunctional and reduce electrogenic epithelial ion transport, a mechanism similar to that observed in murine colon mucosa (Figure 4a).
Effects of TTX upon PYY(3-36)-induced contractile responses in mouse colon smooth muscle
In wild-type ascending colon, TTX (200 nM) caused an increase in basal tone (0.34±0.08 g, n=7) and spontaneous activity (data not shown), neither of which were significantly altered in Y2−/− tissue (basal tone; 0.21±0.03 g, n=7). PYY(3-36) effects following TTX were no different from those in naïve tissues (0.13±0.04 g, n=6; compared with controls of 0.12±0.02 g, n=5) and are therefore direct excitatory effects upon longitudinal smooth muscle.
Conclusion
General features
The reduced body weight observed in male and female Y2−/− mice is consistent with the lean phenotype observed previously in these germline knockout mice, where the long-term loss of hypothalamic Y2 receptors has been associated with elevations in orexigenic NPY and Agouti-related peptide (AgRP) and with coincident decreases in anorexic POMC and cocaine-and amphetamine-regulated transcript (CART) mRNA in the arcuate nucleus (Sainsbury et al., 2002). Y2 receptors in this nucleus are thought to mediate the inhibitory food intake response observed in humans and mice, that occurs for up to 12 h following elevated postprandial PYY(3-36) (Batterham et al., 2002). Male Y2−/− mice, in addition to being lean, also exhibit significantly increased circulating PP levels (Sainsbury et al., 2002) compared with Y2+/+ and a transgenic PP overexpressing mouse with elevated circulating levels of PP also exhibits a lean phenotype (Ueno et al., 1999). Conversely, human obesity syndromes and the genetically obese ob/ob mouse demonstrate reduced PP plasma levels. How raised PP occurs as a consequence of germ line Y2 receptor knockout remains unclear, although sexual dimorphism in the functioning of the hypothalamo-pituitary-adrenal axis is evidently partially responsible (Sainsbury et al., 2002). Such changes in circulating PP will not only alter hypothalamic mechanisms, but also peripheral tissue sensitivities to the hormone and potentially to other Y agonists with overlapping pharmacology (for example, hPP can activate murine Y1 as well as Y4 receptors).
Predicted losses in sensitivity to the Y2-preferred agonist, PYY(3-36) in Y2−/− compared with Y2+/+ colon mucosa and smooth muscle
Y2 receptors predominantly mediate PYY(3-36) responses (up to 100 nM) in colon mucosa and smooth muscle. This agonist's concentration–response curve in Y2+/+ mucosa was comparable with that from 129Sv mouse colon mucosa (Cox et al., 2001). No sensitivity to this fragment was observed in either female or male Y2−/− mucosae, up to concentrations of 100 nM. The small decreases in Isc seen to 300 nM PYY(3-36) in Y2−/− were abolished by BIBO3304 pretreatment, showing that the fragment can stimulate Y1 as well as Y2 receptors, albeit at high nM concentrations.
Endogenous PP is predicted to preferentially stimulate Y4 receptors, although costimulation of Y1 receptors may also occur (Cox et al., 2001). The consequence of either or both of these events would attenuate electrogenic anion secretion across mucosal preparations thereby lowering basal Isc levels. Such a pattern was observed with Y2−/− mucosae (Table 1) and correlates with the robust elevations in circulating PP levels established in Y2−/− mice of both genders compared to Y2+/+ mice (Sainsbury et al., 2002). The absence of differences in VIP-stimulated Isc responses and basal mucosal resistances, between the four tissues, argues against nonspecific mucosal changes in knockout tissues.
In Y2+/+ ascending colon longitudinal smooth muscle, Y2 receptors exclusively mediate PYY(3-36) and NPY contractile effects (Figure 1d). The effects of Y agonists that we observe in mouse ascending colon are similar to the tonic contractions recorded in rat ascending colon longitudinal smooth muscle by Ferrier et al. (2000) and Pheng et al. (1999). In the latter, NPY, PYY and PP responses were abolished by TTX and partially inhibited by atropine, indicating that activation of Y receptors (probably Y2 and Y4) resulted in ACh and NANC transmitter release to cause contraction indirectly. RT–PCR analysis of rat colon showed expression of Y1 and Y4 receptor mRNA (Ferrier et al., 2000) and there were no Y2-mediated NPY(13-36) effects. Y2 mRNA was also lacking from ‘nonepithelial' preparations of rat colon, where PCR-based detection identified Y1, Y4 and Y5 receptor mRNA (Goumain et al., 1998). It is important also to note that the Y6 receptor is not expressed in adult mouse tissue (Gregor et al., 1996). Thus, our data clearly demonstrate a functional role for Y2 receptors in mouse colon and indicate disparities between species, not only in terms of the Y receptor type(s) involved, but also the mechanism by which Y agonist-mediated excitation occurs. The TTX insensitivity of PYY(3-36) and also PP contractile responses (the latter were also atropine- and neurokinin-insensitive, Singh et al., 2002; Tough et al., 2002) indicate that both peptides act predominantly directly upon mouse longitudinal smooth muscle to cause contraction. In Y2−/− tissue, we also observed a Y1 receptor-mediated excitatory component, which was abolished with BIBO3304. Whether this represents a compensatory upregulation of the Y1 receptor type specifically in smooth muscle remains to be determined.
Wild-type colon longitudinal smooth muscle therefore contracts following stimulation of Y2 and Y4 receptors and there was no evidence of inhibitory tone in this preparation, in contrast with colonic mucosae from both human and mouse. It is clear, however, that in murine colon mucosa Y2 receptors are located predominantly on submucosal neurones (Figure 4) and that a significant level of Y2-mediated inhibitory tone is present in this tissue. Human colon mucosa also exhibits a marked Y2 tone which was also TTX-sensitive. Subsequent PYY(3-36) responses were abolished by either TTX or the Y2 antagonist, BIIE0246, indicating a solely prejunctional Y2-mediated mechanism.
Unpredicted functional changes in Y2−/− compared with Y2+/+ tissues
The increases in PP EC50 values in male Y2−/− mucosae could be a consequence of sustained Y4 receptor stimulation (this reducing basal epithelial Isc slightly in Y2−/− mucosa) by elevated endogenous PP (Sainsbury et al., 2002). At concentrations of 30 nM (and higher, Cox et al., 2001) hPP coactivates murine Y1 as well as Y4 receptors (Figure 2a, b, Tough et al., 2002). Thus, prolonged elevations of endogenous PP levels in male Y2−/− could also be manifested by reductions in Y1-agonist potency, which our observations with Pro34PYY do suggest. The EC50 values for Pro34PYY were greatest in male Y2−/− tissue (from mice where PP levels of ∼15 nM have been recorded), next highest in male Y2+/+ mucosa (where PP levels were ∼5 nM), followed by female Y2−/− and Y2+/+ tissue (where PP levels were ∼2 and ∼1 nM respectively, Sainsbury et al., 2002). In female Y2−/− tissues, hPP potency was reduced by half compared with that in female Y2+/+. The observation that both female groups exhibited reduced hPP sensitivity compared with those from males (Table 1) implicates additional gender-specific differences that have altered female tissue sensitivity to exogenous peptide.
In smooth muscle, PP responses (30 nM) were not sensitive to BIBO3304 in any group and there were no significant differences in the size of these peptides or CCh responses between Y2+/+ and Y2−/− preparations (Figure 3). The cellular mechanisms underpinning PP-mediated contractile effects in intestinal smooth muscle do involve voltage-gated Ca2+ channels since responses are abolished by nifedipine (Singh et al., 2002). All the Y receptor types are coupled to Gi/o, pertussis toxin (PT)-sensitive inhibition of adenylate cyclase (for review, see Michel et al., 1998), but in neuroblastoma cells (Lynch et al., 1994) a PT-insensitive, direct coupling to receptor-operated Ca2+ channels provides for depolarisation and resultant contraction. We have yet to determine the exact cellular mechanism(s) involved in these Y receptor-mediated smooth muscle responses.
From these functional studies, we conclude that Y2 receptors are differentially expressed, being present postjunctionally on murine smooth muscle (together with Y4 receptors), while in mucosal preparations Y2 receptors are predominantly prejunctional. Activation of the latter by the endogenous Y2 agonist PYY(3-36) results in intrinsic inhibitory Y2 tone and therefore attenuates epithelial ion transport indirectly in human and wild-type mouse colon. Germline knockout of Y2 receptors predictably led to loss of sensitivity to the preferred Y2 agonist, PYY(3-36) in isolated preparations, while the associated elevation in circulating PP levels in Y2−/− mice resulted in functional blunting, not just of exogenous PP (Y4-mediated) responses, but also of Pro34PYY (Y1-mediated) antisecretory effects.
Acknowledgments
Richard DeSouza contributed some of the data for PYY(3-36) and PP responses in smooth muscle. We thank Boehringer Ingelheim Pharma KG and Glaxo Institute of Applied Pharmacology for providing their respective selective receptor antagonists. This research was funded in part by the BBSRC, the Wellcome Trust and the Kimmel Cancer Foundation.
Abbreviations
- BIBO3304
((R)-N-[[4-(aminocarbonylaminomethyl)phenyl)methyl]-N2-(diphenylacetyl)-argininamide-trifluoroacetate
- BIBP3226
N2-(diphenylacetyl)-N-[(4-hydroxy-phenyl)methyl]-D-arginine amide
- BIBP3435
[(S)-N2-diphenylacetyl)-N-[(4-hydroxyphenyl)methyl]-argininamide] (acetate salt)
- BIIE0246
(S)-N2-[[1-[2-[4-[(R,S)-5, 11-dihydro-6(6H)-oxodibenz[b,e]azepin-11-yl]-1-piperazinyl]-2-oxoethyl]cyclopentyl]acetyl]-N-[2-[1,2-dihydro-3,5(4H)- dioxo-1,2-diphenyl-3H-1,2,4-triazol-4-yl]ethyl]-argininamide
- CCh
carbachol
- CYN-154806
Ac-4NO2-Phe-c(D-Cys-Tyr-D-Trp-Lys-Thr-Cys)-D-Tyr-NH2
- hPP
human pancreatic polypeptide
- KH
Krebs Henseleit
- NPY
neuropeptide Y
- Pro34PYY
human [Leu31, Pro34]PYY
- PYY
peptide YY
- PYY(3-36)
peptide YY(3-36)
- sst
somatostatin 14-28
- TTX
tetrodotoxin
- UK14,304
5-bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine
- VIP
vasoactive intestinal polypeptide
References
- ARANTES R.M.E., NOGUEIRA A.M.M.F. Distribution of enteroglucagon- and peptide YY-immunoreactive cells in the intestinal mucosa of germ-free and conventional mice. Cell Tissue Res. 1997;290:61–69. doi: 10.1007/s004410050908. [DOI] [PubMed] [Google Scholar]
- BAHN B., CAO J.Q., BECK-SICKINGER A., COLMERS W.F. Blockade of neuropeptide Y2 receptors and suppression of NPY's anti-epileptic actions in the rat hippocampal slice by BIIE0246. Br. J. Pharmacol. 2002;136:502–509. doi: 10.1038/sj.bjp.0704751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALDOCK P.A., SAINSBURY A., COUZENS M., ENRIQUEZ R.F., THOMAS G.P., GARDINER E.M., HERZOG H. Hypothalamic Y2 receptors regulate bone formation. J. Clin. Invest. 2002;109:915–921. doi: 10.1172/JCI14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATTERHAM R.L., COWLEY M.A., SMALL C.J., HERZOG H., COHEN M.A., DAKIN C.L., WREN A.M., BRYNES A.E., LOW M.J., GHATEI M.A., CONE R.D., BLOOM S.R. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- COX H.M. Peptidergic regulation of intestinal ion transport: a major role for pancreatic polypeptide family. Digestion. 1998;59:395–399. doi: 10.1159/000007496. [DOI] [PubMed] [Google Scholar]
- COX H.M., CUTHBERT A.W. The effects of NPY and its fragments upon basal and electrically stimulated ion secretion in rat jejunum mucosa. Br. J. Pharmacol. 1990;101:247–252. doi: 10.1111/j.1476-5381.1990.tb12695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COX H.M., CUTHBERT A.W., HÅKANSON R., WAHLESTEDT C. The effect of NPY and PYY on electrogenic ion transport in rat intestinal epithelia. J. Physiol. 1988;398:65–80. doi: 10.1113/jphysiol.1988.sp017029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COX H.M., POLLOCK E.L., TOUGH I.R., HERZOG H. Multiple Y receptors mediate pancreatic polypeptide responses in mouse colon mucosa. Peptides. 2001;22:445–452. doi: 10.1016/s0196-9781(01)00355-2. [DOI] [PubMed] [Google Scholar]
- COX H.M., TOUGH I.R. Functional studies with a novel neuropeptide Y Y2 receptor antagonist, BIIE0246 in isolated preparations from the rat gastrointestinal tract. Br. J. Pharmacol. 2000;129:89. [Google Scholar]
- COX H.M., TOUGH I.R. Neuropeptide Y Y1, Y2, and Y4 receptors mediate Y agonist responses in isolated human colon mucosa. Br. J. Pharmacol. 2002;135:1505–1512. doi: 10.1038/sj.bjp.0704604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNNINGHAM S.M.C., MIHARA S., LEES G.M. Y2-receptor-mediated selective inhibition of slow, inhibitory postsynaptic potential in submucous neurones of guinea-pig caecum. Br. J. Pharmacol. 1994;113:883–888. doi: 10.1111/j.1476-5381.1994.tb17075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOODS H., GAIDA W., WIELAND H.A., DOLLOINGER H., SCHNORRENBERG G., ESSER F., ENGEL W., EBERLEIN W., RUDOLF K. BIIE0246: a selective and high affinity neuropeptide Y Y2 receptor antagonist. Eur. J. Pharmacol. 1999;384:R3–R5. doi: 10.1016/s0014-2999(99)00650-0. [DOI] [PubMed] [Google Scholar]
- DUMONT Y., CADIEUX A., DOODS H., PHENG L.H., ABOUNDER R., HAMEL E., JACQUES D., REGOLI D., QUIRION R. BIIE0246, a potent and highly selective non-peptide neuropeptide Y Y2 receptor antagonist. Br. J. Pharmacol. 2000;129:1075–1088. doi: 10.1038/sj.bjp.0703162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUMONT Y., JACQUES D., BOUCHARD P., QUIRION R. Species differences in the expression and distribution of neuropeptide Y Y1, Y2, Y4 and Y5 receptors in rodents, guinea pig and primate brain. J. Comp. Neurol. 1998;402:372–384. [PubMed] [Google Scholar]
- EKBLAD E., EKMAN R., HÅKANSON R., SUNDLER F. Projections of peptide-containing neurons in rat colon. Neuroscience. 1988;27:655–674. doi: 10.1016/0306-4522(88)90296-5. [DOI] [PubMed] [Google Scholar]
- EKBLAD E., WINTER C., EKMAN E., HÅKANSON R., SUNDLER F. Projections of peptide-containing neurons in rat small intestine. Neuroscience. 1987;20:169–188. doi: 10.1016/0306-4522(87)90010-8. [DOI] [PubMed] [Google Scholar]
- FELETOU M., NICOLAS J.-P., RODRIGUEZ M., BEAUVERGER P., GALIZZI J.-P., BOUTIN J.A., DUHAULT J. NPY receptor subtype in the rabbit isolated ileum. Br. J. Pharmacol. 1999;127:795–801. doi: 10.1038/sj.bjp.0702594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENIUK W., JARVIE E., LUO J., HUMPHREY P.P.A. Selective somatostatin sst2 receptor blockade with the novel cyclic octapeptide, CYN-154806. Neuropharmacology. 2000;39:1443–1450. doi: 10.1016/s0028-3908(00)00035-6. [DOI] [PubMed] [Google Scholar]
- FERRIER L., SEGAIN J.-P., PACAUD P., CHERBUT C., LOIRAND G., GALMICHE J.-P., BLOTTIERE H.M. Pathways and receptors involved in peptide YY induced contraction of rat proximal colonic muscle in vitro. Gut. 2000;46:370–375. doi: 10.1136/gut.46.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURNESS J.B. Types of neurons in the enteric nervous system. J. Autonom. Nerv. Syst. 2000;81:87–96. doi: 10.1016/s0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- GOUMAIN M., VOISIN T., LORINET A.-M., LABURTHE M. Identification and distribution of mRNA encoding the Y1, Y2, Y4 and Y5 receptors for peptides of the PP-fold family in the rat intestine and colon. Biochem. Biophys. Res. Commun. 1998;247:52–56. doi: 10.1006/bbrc.1998.8647. [DOI] [PubMed] [Google Scholar]
- GREGOR P., FENG Y., DeCARR L.B., CORNFIELD L.J., McCLEB M.L. Molecular characterisation of a second mouse pancreatic polypeptide receptor and its inactivated human homologue. J. Biol. Chem. 1996;271:27776–27781. doi: 10.1074/jbc.271.44.27776. [DOI] [PubMed] [Google Scholar]
- HYLAND N.P., HERZOG H., COX H.M. Decreased sensitivity to pancreatic polypeptide in colonic mucosa from Y2 receptor knockout mice. Br. J. Pharmacol. 2002;135:43. [Google Scholar]
- KAGA T., FUJIMIYA M., INUI A. Emerging functions of neuropeptide Y Y2 receptors in the brain. Peptides. 2001;22:501–506. doi: 10.1016/s0196-9781(01)00362-x. [DOI] [PubMed] [Google Scholar]
- KING P.J., WILLIAMS G., DOODS H., WIDDOWSON P.S. Effect of a selective neuropeptide Y Y2 receptor antagonist, BIIE0246 on neuropeptide Y release. Eur. J. Pharmacol. 2000;396:R1–R3. doi: 10.1016/s0014-2999(00)00230-2. [DOI] [PubMed] [Google Scholar]
- LYNCH J.W., LEMOS V.S., BUCHER B., STOCLET J.-C., TAKEDA K. A pertussis toxin-insensitive calcium influx mediated by neuropeptide Y2 receptors in a human neuroblastoma cell line. J. Biol. Chem. 1994;269:8226–8233. [PubMed] [Google Scholar]
- MALMSTRÖM R., LUNDBERG J.O.N., WEITZBERG E. Autoinhibitory function of the sympathetic prejunctional neuropeptide Y Y2 receptor evidenced by BIIE0246. Eur. J. Pharmacol. 2002;439:113–119. doi: 10.1016/s0014-2999(02)01371-7. [DOI] [PubMed] [Google Scholar]
- MANNON P.J., KANUNGO A., MANNON R.B., LUDWIG K.A. Peptide YY/neuropeptide Y Y1 receptor expression in the epithelium and mucosal nerves of the human colon. Regul. Pept. 1999;83:11–19. doi: 10.1016/s0167-0115(99)00035-x. [DOI] [PubMed] [Google Scholar]
- McAULEY M.A., WESTFALL T.C. Possible location and function of neuropeptide Y receptor subtypes in the rat mesenteric arterial bed. J. Pharmacol. Exp. Ther. 1992;261:863–868. [PubMed] [Google Scholar]
- MICHEL M.C., BECK-SICKINGER A., COX H.M., DOODS H.N., HERZOG H., LARHAMMAR D., QUIRION R., SCHWARTZ T.W., WESTFALL T. XVI. IUPHAR Recommendations for the nomenclature of neuropeptide Y, peptide YY and pancreatic polypeptide receptors. Pharmacol. Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- PHENG L.-H., PERRON A., QUIRION R., CADIEUX A., FAUCHERE J.L., DUMONT D., REGOLI D. Neuropeptide Y-induced contraction is mediated by neuropeptide Y Y2 and Y4 receptors in the rat colon. Eur. J. Pharmacol. 1999;374:85–91. doi: 10.1016/s0014-2999(99)00296-4. [DOI] [PubMed] [Google Scholar]
- SAINSBURY A., SCHWARZER C., COUZENS M., FETISSOV S., FURTINGER S., JENKINS A., COX H.M., SPERK G., HÖKFELT T., HERZOG H. Important role of hypothalamic Y2 receptors in bodyweight regulation revealed in conditional knockout mice. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8938–8943. doi: 10.1073/pnas.132043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANG Q., YOUNG H.M. Chemical coding of neurons in the myenteric plexus and external muscle of the small and large intestine of the mouse. Cell Tissue Res. 1996;284:39–53. doi: 10.1007/s004410050565. [DOI] [PubMed] [Google Scholar]
- SILVA A.P., CARVALHO A.P., CARVALHO C.M., MALVA J.O. Modulation of intracellular calcium changes and glutamate release by neuropeptide Y1 and Y2 receptors in the rat hippocampus: differential effects in CA1, CA3 and dentate gyrus. J. Neurochem. 2001;79:286–296. doi: 10.1046/j.1471-4159.2001.00560.x. [DOI] [PubMed] [Google Scholar]
- SINGH N., BRAIN S.D., COX H.M. A study of muscarinic and neurokinin components underlying pancreatic polypeptide motor responses in mouse proximal colon. Br. J. Pharmacol. 2002;135:232. [Google Scholar]
- SMITH-WHITE M.A., HERZOG H., POTTER E.K. Role of neuropeptide Y Y2 receptors in modulation of cardiac parasympathetic neurotransmission. Regul. Pept. 2002;103:105–111. doi: 10.1016/s0167-0115(01)00368-8. [DOI] [PubMed] [Google Scholar]
- SUNDLER F., BÖTTCHER G., EKBLAD E., HÅKANSON R.PP, PYY AND NPY: occurrence and distribution in the periphery The Biology of NPY and Related Peptides 1993Totowa, NJ, U.S.A.: Humana Press Inc.ed. COLMERS, W.F. & WAHLESTEDT, C [Google Scholar]
- TOUGH I.R., COX H.M. Selective inhibition of neuropeptide Y Y1 receptors by BIBP3226 in rat and human epithelial preparations. Eur. J. Pharmacol. 1996;310:55–60. doi: 10.1016/0014-2999(96)00372-x. [DOI] [PubMed] [Google Scholar]
- TOUGH I.R., COX H.M. Functional studies with the somatostatin (SRIF) sst2 receptor antagonist, CYN-154806, in rat colonic mucosa. Br. J. Pharmacol. 2002;129:172. [Google Scholar]
- TOUGH I.R., De SOUZA R.J., HERZOG H., COX H.M. Pancreatic polypeptide responses in colonic mucosal and smooth muscle preparations from wild type and Y4 receptor knockout mice. Br. J Pharmacol. 2002;135:44. [Google Scholar]
- UENO N., INUI A., IWAMOTO M., KAGA T., ASAKAWA A., OKITA M., FUJIMIYA M., NAKAJIMA Y., OHMOTO Y., OHNAKA M., NAKAYA Y., MIYAZAKI J.-I., KASUGA M. Decreased food intake and body weight in pancreatic polypeptide-overexpressing mice. Gastroenterology. 1999;117:1427–1432. doi: 10.1016/s0016-5085(99)70293-3. [DOI] [PubMed] [Google Scholar]
- WIELAND H.A., ENGEL W., EBERLEIN W., RUDOLF K., DOODS H.N. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO3304 and its effect on feeding in rodents. Br. J. Pharmacol. 1998;125:549–555. doi: 10.1038/sj.bjp.0702084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISER T., WEILAND H.A., DOODS H.N. Effects of the neuropeptide Y Y2 receptor antagonist BIIE0246 on presynaptic inhibition of neuropeptide Y in rat hippocampal slices. Eur. J. Pharmacol. 2000;404:133–136. doi: 10.1016/s0014-2999(00)00478-7. [DOI] [PubMed] [Google Scholar]