Abstract
Candoxin (MW 7334.6), a novel toxin isolated from the venom of the Malayan krait Bungarus candidus, belongs to the poorly characterized subfamily of nonconventional three-finger toxins present in Elapid venoms. The current study details the pharmacological effects of candoxin at the neuromuscular junction.
Candoxin produces a novel pattern of neuromuscular blockade in isolated nerve-muscle preparations and the tibialis anterior muscle of anaesthetized rats. In contrast to the virtually irreversible postsynaptic neuromuscular blockade produced by curaremimetic α-neurotoxins, the neuromuscular blockade produced by candoxin was rapidly and completely reversed by washing or by the addition of the anticholinesterase neostigmine.
Candoxin also produced significant train-of-four fade during the onset of and recovery from neuromuscular blockade, both, in vitro and in vivo. The fade phenomenon has been attributed to a blockade of putative presynaptic nicotinic acetylcholine receptors (nAChRs) that mediate a positive feedback mechanism and maintain adequate transmitter release during rapid repetitive stimulation. In this respect, candoxin closely resembles the neuromuscular blocking effects of d-tubocurarine, and differs markedly from curaremimetic α-neurotoxins that produce little or no fade.
Electrophysiological experiments confirmed that candoxin produced a readily reversible blockade (IC50∼10 nM) of oocyte-expressed muscle (αβγδ) nAChRs. Like α-conotoxin MI, well known for its preferential binding to the α/δ interface of the muscle (αβγδ) nAChR, candoxin also demonstrated a biphasic concentration–response inhibition curve with a high- (IC50∼2.2 nM) and a low- (IC50∼98 nM) affinity component, suggesting that it may exhibit differential affinities for the two binding sites on the muscle (αβγδ) receptor. In contrast, curaremimetic α-neurotoxins have been reported to antagonize both binding sites with equal affinity.
Keywords: Bungarus candidus, postsynaptic neurotoxin, three-finger toxin, neuromuscular junction, nicotinic acetylcholine receptors, train-of-four fade
Introduction
Snake venoms are complex mixtures of protein and polypeptide toxins that encompass an arsenal of lethal neurotoxins. These include curaremimetic or α-neurotoxins which target muscle (αβγδ) nicotinic acetylcholine receptors (nAChRs) with high affinity (Kd 10−9–10−11 M) to produce postsynaptic neuromuscular blockade (Endo & Tamiya, 1991; Servent & Menez, 2001; Hodgson & Wickramaratna, 2002). Based on the length of their polypeptide chains, α-neurotoxins have been classified as short-chain neurotoxins (e.g. erabutoxin-b (Laticauda semifasciata); toxin-α (Naja nigricollis)) that have 60–62 residues and four conserved disulphide bonds and long-chain neurotoxins (e.g. α-bungarotoxin (Bungarus multicinctus); α-cobratoxin (Naja kaouthia)) with 66–75 residues and five disulphide bonds, with the additional disulphide bridge located in the middle loop (loop II) (Endo & Tamiya, 1991). In common, these neurotoxins belong to the superfamily of three-finger proteins that are characterized by a common tertiary structure consisting of three loops extending from a globular core crosslinked by four conserved disulphide bonds (Tsetlin, 1999; Servent & Menez, 2001; Kini, 2002).
We have recently reported the isolation and purification of a novel three-finger toxin, candoxin, from the venom of the Malayan krait Bungarus candidus (Nirthanan et al., 2002a). Candoxin (MW 7334.6) consists of a single polypeptide chain of 66 amino acids with five disulphide bridges, including four that are conserved among all three-finger toxins (see Figure 6). The fifth disulphide bridge (Cys6-Cys11) in candoxin is located at the tip of loop I (N-terminus loop), instead of in loop II as present in conventional long-chain α-neurotoxins as well as in κ-neurotoxins that have a predilection for neuronal (α3β2) nAChRs. This cysteine motif is typical of the poorly characterized subfamily of nonconventional toxins, isolated exclusively from Elapid venoms (Servent & Menez, 2001; Nirthanan et al., 2003). This class of toxins is typically characterized by a lower order of toxicity (LD50 from ∼5 to 80 mg kg−1) as opposed to prototype α-neurotoxins (LD50∼0.04–0.3 mg kg−1) and, because of this, they have also been referred to as weak toxins (Utkin et al., 2001). Apart from toxicity studies, the nonconventional toxins have been poorly investigated in terms of their function or molecular targets. Recently, it has been reported that two nonconventional (‘weak') toxins from cobra venoms (WTX from Naja kaouthia and Wntx-5 from Naja sputatrix) produced a weak and irreversible inhibition of both, muscle (αβγδ) and α7 nAChRs, in micromolar inhibitory concentrations (Utkin et al., 2001; Poh et al., 2002). In contrast, candoxin has been shown to be a potent antagonist of muscle (αβγδ) (IC50∼10 nM) and α7 (IC50∼50 nM) nAChRs in electrophysiological experiments (Nirthanan et al., 2002a). Clearly therefore, nonconventional (weak) toxins do not appear to be a functionally homogeneous class of toxins. The present study provides a detailed account of the effects of a nonconventional toxin, candoxin, from krait venom at the neuromuscular junction.
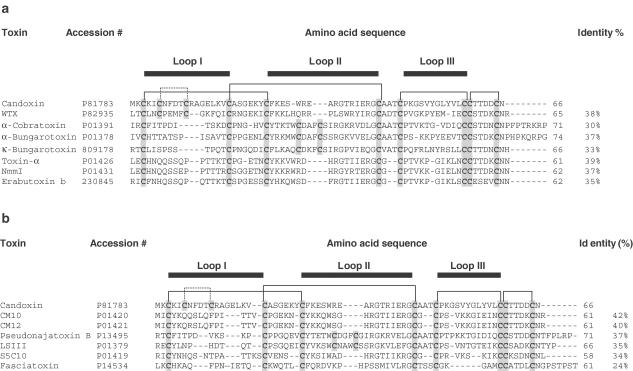
Figure 6.
Comparison of the amino-acid sequence of candoxin with the sequences of other (a) neurotoxins and (b) ‘reversible' neurotoxins and neurotoxin homologues. The number of amino-acid residues in each sequence is indicated at the end of the respective sequence. The percentage (%) identity of each sequence to the sequence of candoxin is also shown. The cysteine residues are shaded in grey. The four conserved disulfide linkages and the segments contributing to the three loops are outlined. The species names are as follows: WTX (nonconventional toxin) (Naja kaouthia), α-cobratoxin (Naja kaouthia), α-bungarotoxin (Bungarus multicinctus), κ-bungarotoxin (Bungarus multicinctus), toxin α (Naja nigricollis), NmmI (Naja mossambica mossambica), erabutoxin b (Laticauda semifasciata), CM10 and CM12 (Naja haje annulifera), pseudonajatoxin B (Pseudonaja textilis), LSIII (Laticauda semifasciata), S5C10 (Dendroaspis jamesoni) and fasciatoxin (Bungarus fasciatus). The protein database accession numbers are also stated for each toxin.
Methods
Animals
Swiss Albino mice, Sprague–Dawley rats and Hartley guinea pigs were purchased from the Laboratory Animals Centre, National University of Singapore, (Sembawang, Singapore) and housed in the Animal Holding Unit of the Department of Pharmacology, National University of Singapore, before use. Water and food (Glen Forrest Stockfeeders, WA, Australia) were provided ad libitum and a 12 h light–12 h dark cycle was maintained. Paper pellet bedding was also purchased from Glen Forrest Stockfeeders, WA, Australia. Locally bred chicks were purchased from a farm. These were delivered on the day of the experiment. The animals were handled according to the Guidelines of the National Medical Ethics Committee (Singapore), which conform to the World Health Organization's International Guiding Principles for Animal Research (The WHO Chronicle (1985); 39(2) 51–56: International Guiding Principles for Biomedical Research involving Animals), adapted by the Council for International Organizations of Medical Sciences in 1985.
Organ bath studies
Isolated tissue experiments were performed using conventional organ baths of various volumes (4, 6 or 8 ml) for different tissues. Kreb's physiological salt solution (pH 7.4) of the following composition (mM): NaCl (118); KCl (4.8); KH2PO4 (1.2); CaCl2 (2.5); NaHCO3 (25); MgSO4 (2.4) and D- (+) glucose (11) was used in all isolated tissue experiments. The solution was prepared in deionized water and aerated continuously with 5% carbon dioxide in oxygen. The temperature of the organ bath was maintained at 37°C. Isolated tissues were mounted in the organ bath under a resting tension of ∼1 g. The preparations were allowed to equilibrate for about 45–60 min with changes of Kreb's solution at 15 min intervals before beginning the experiments. Electrical field stimulation was carried out through platinum ring electrodes using a Grass stimulator S88 (Grass Instruments, MA, U.S.A.). The magnitude of the contractile responses of the tissues was measured in gram (g) tension. Data were recorded in a MacLab system/8 (AD Instruments, NSW, Australia) via a force–displacement transducer (model FT03, Grass Instruments, MA, U.S.A.).
Chick biventer cervicis muscle
Chicks (7–14 days old) were killed by exposure to 100% CO2 and the chick biventer cervicis muscle (CBCM) isolated as described by Ginsborg & Warriner (1960) and mounted in an 8 ml organ bath. Maximal twitch responses of the muscle were evoked by stimulating the motor nerve by electrical field stimulation (7–10 V) at a frequency of 0.2 Hz in supramaximal rectangular pulses of 0.1 ms duration. For direct muscle stimulation, the electrodes were lowered over the belly of the muscle and twitch responses evoked by electrical field stimulation (20–30 V, 1 ms, 0.2 Hz). d-Tubocurarine (d-TC) (10 μM) was added to the preparation to block neuromuscular transmission during direct muscle stimulation. The effects of candoxin (0.1–100 μg ml−1; 0.0136–13.6 μM) on the uninterrupted twitch responses of the CBCM to nerve or direct muscle stimulation were then investigated. The time taken to block the amplitude of control twitch responses by 90% (T90) was calculated in order to provide a quantitative measure of neurotoxicity. For comparison, the effects of 0.005–20 μg ml−1 of erabutoxin-b (0.733 nM–2.9 μM), α-bungarotoxin (0.625 nM–2.5 μM) or α-cobratoxin (0.626 nM–2.55 μM) on the nerve-evoked twitch responses of the CBCM were also investigated. Each preparation was exposed to one concentration of a test substance. Submaximal contractures to exogenously applied acetylcholine (ACh) 300 μM, carbachol (CCh) 8 μM or potassium chloride (KCl) 30 mM were obtained in the absence of electrical field stimulation. The contact time allowed for the agonists were 30, 60 and 90 s for ACh, KCl and CCh, respectively. The effects of candoxin (0.1–100 μg ml−1; 0.0136–13.6 μM) on the responses of the CBCM to exogenous ACh, CCh or KCl were investigated immediately after complete blockade of nerve-evoked twitch responses was established.
Mouse phrenic nerve hemidiaphragm
Male Swiss Albino mice (20–30 g) were killed by cervical dislocation after exposure to 100% CO2. The mouse hemidiaphragm (MHD) with the associated phrenic nerve was isolated as described by Bulbring (1946) and mounted in a 4 ml organ bath. For indirect stimulation, the phrenic nerve was electrically stimulated at a frequency of 0.2 Hz in rectangular pulses of 0.2 ms duration and supra-maximal voltage (7–10 V). Direct muscle stimulation was achieved by electrical stimulation (0.2 Hz, 2 ms, 20–30 V) in the presence of d-TC (10 μM). The effects of candoxin (3, 10, 30 and 100 μg ml−1) on direct and indirect stimulation of the MHD were studied.
Train-of-four (TOF) stimulation
The effects of candoxin on the maximal twitch responses of the MHD evoked through TOF stimulation were also investigated. TOF stimulation (2 Hz for 2 s every 20 s) of the phrenic nerve was carried out as described by Cheah & Gwee (1988) and continued throughout the experiment. Control TOF twitches (T1, T2, T3, T4) were recorded for 20 min and conditions stabilized before the addition of candoxin (10–100 μg ml−1; 1.36–13.6 μM). The time course changes of the first (T1) and fourth (T4) twitch were noted during the onset of and recovery from neuromuscular blockade produced by candoxin. The corresponding TOF ratios (T4/T1) at 10, 25, 50, 75 and 90% block for the first twitch (T1) were calculated and the values obtained were plotted (T1 versus T4/T1 relation). The effects of α-bungarotoxin (3 μg ml−1; 0.37 μM), α-cobratoxin (3 μg ml−1; 0.38 μM), erabutoxin-b (3 μg ml−1; 0.44 μM) or d-TC (1 μM) on twitches evoked by TOF stimulation were also studied for comparison.
Guinea-pig diaphragm
Male Hartley guinea pigs (300–325 g) were killed by exposure to 100% CO2 and exsanguination. The guinea-pig diaphragm (GPD) was prepared as described for the rat diaphragm by Wolthuis et al. (1981). This method was preferred over the conventional phrenic nerve-hemidiaphragm preparation since it could be accommodated in a smaller organ bath, thereby minimizing the requirement of candoxin. Longitudinal strips (∼1.5 cm × 3 mm), cut parallel to the anatomical direction of the muscle fibres, were prepared from the isolated diaphragm. Ligatures were attached to the membranous and costal parts of the diaphragm strip and mounted vertically under 1 g tension, between two parallel platinum electrodes in a 6 ml organ bath. Twitch responses of the GPD were elicited by indirect field stimulation (7–10 V, 0.1 ms, 0.2 Hz). After an equilibration period of 30 min, d-TC (5 μM) was added to the preparation to confirm that the twitches elicited were as a result of nerve stimulation and not direct muscle stimulation (Hodgson & Wickramaratna, 2002). Twitches were then re-established by washing and following a further 45 min equilibration period, the effects of candoxin 3, 10, 30 and 100 μg ml−1 (in μM: 0.41, 1.36, 4.09 and 13.6) on the GPD were studied.
Reversal studies
The recovery of the CBCM, MHD and GPD from complete neuromuscular blockade produced by candoxin (10, 30 and 100 μg ml−1; 1.36, 4.08 and 13.6 μM, respectively) was assessed. In the case of other neurotoxins, recovery from 90% blockade produced by 0.05, 1 and 3 μg ml−1 erabutoxin-b (7.25 nM, 0.145 and 0.435 μM), α-bungarotoxin (6.25 nM, 0.125 and 0.37 μM) or α-cobratoxin (6.26 nM, 0.13 and 0.38 μM) was assessed. The toxins were removed from the organ bath by washing by bath overflow (16 ml min−1 for the first 5 min followed by a slow drip-wash at a flow rate of ∼8 ml min−1) with fresh Kreb's solution. The washing was continued until complete recovery or for a maximal period of 180 min. The effects of the anticholinesterase neostigmine 0.1, 1.0 or 3.0 μM on the reversal of neuromuscular blockade produced by candoxin or the effects of neostigmine added cumulatively up to 100 μM on the neuromuscular blockade produced by erabutoxin-b, α-bungarotoxin or α-cobratoxin were also studied.
In vivo experiments on anaesthetized rats
Experiments were designed to study the effects of candoxin (0.3–1 mg kg−1) on the twitch responses of the tibialis anterior muscle evoked by nerve stimulation in anaesthetized rats. Male Sprague–Dawley rats weighing 300–375 g were anaesthetized by intraperitoneal injections of thiobutabarbitone sodium (Inactin™) in a dose of 100 mg kg−1 (i.p.) to the point of a loss of eyelid reflex and lack of withdrawal from painful stimuli. The depth of anaesthesia was assessed by the stability of blood pressure and heart rate, absence of eyelid reflexes and by an absence of a cardiovascular response to paw pinch. Supplementary doses of thiobutabarbitone sodium (10–25 mg kg−1, i.v.) were given when necessary. The trachea was cannulated with a plastic endotracheal tube that was connected to a Harvard rodent ventilator (Model 683, Harvard Bioscience, MA, U.S.A.) and artificial ventilation provided throughout as described previously (Kleinman & Radford, 1964). For the i.v. administration of test substances, the right external jugular vein was cannulated with a Portex Fg2 nylon intravenous cannula that was connected to a three-way stopcock. For monitoring the blood pressure and heart rate, the left common carotid artery was cannulated with a Portex Fg2 nylon intravenous cannula and connected to a MacLab/8 recording system (AD Instruments, NSW, Australia) via a Gould Statham P23 pressure transducer.
Nerve-evoked maximal TOF twitches of the tibialis anterior muscle of the right lower limb of the rat were monitored throughout as described by Tran et al. (1982). The freed tendon of insertion of the tibialis anterior muscle was attached to a force–displacement transducer (model FT03, Grass Instruments), while the right lower limb was kept immobilized. The sciatic nerve in the popliteal space was stimulated (2 Hz for 2 s every 20 s) with a Harvard Dastre electrode connected to a Grass stimulator S88 to produce maximal twitches of the tibialis anterior muscle. The resting tension of the muscle was adjusted to give the greatest evoked twitch tension. Data were recorded on a MacLab/8 system. The effects of candoxin (0.3, 0.6 and 1 mg kg−1; 40.9, 81.8 and 136.3 nmol kg−1) (n=4), erabutoxin-b (100 μg kg−1; 14.6 nmol kg−1) (n=3) or dTC (10 nmol kg−1) (n=3) on the twitch responses of the muscle were studied. The ability of the muscle to recover spontaneously from twitch blockade produced by candoxin and the effects of neostigmine 0.4, 4 or 40 μM on the twitch blockade produced by candoxin were also evaluated. The recovery of the fourth twitch (T4) to its control twitch height was considered as complete recovery from neuromuscular blockade.
Assay for anticholinesterase activity
The effect of candoxin on the activity of acetylcholinesterase (AChE) was studied using the protocol of Ellman et al. (1961) as described in Nirthanan et al. (2002b). The initial rate of formation of thiocholine from acetylthiocholine by AChE was measured by the increase in optical density at 412 nm resulting from the reaction of thiocholine with dithiobisnitrobenzoic acid (DTNB). The control assay mixture contained 1 U of AChE (electric eel enzyme type V-S), 50 mM of DTNB and 0.5 mM acetylthiocholine in 200 μl of 0.1 M phosphate buffer (pH 7.8). The toxin assay mixture contained, in addition to the above, 10, 30 or 100 μM of candoxin, which was preincubated with the AChE for 30 or 60 min prior to the addition of acetylthiocholine. The enzyme kinetics of AChE in all the assay mixtures was assessed spectrophotometrically over a period of 5 min. An assay where the candoxin was replaced with neostigmine (0.3, 1 and 3 μM) was carried out as a positive control. All assays were performed in triplicates and repeated thrice.
Electrophysiological experiments
Electrophysiological experiments were carried out using the oocyte expression system as described in Nirthanan et al. (2002a). The effects of candoxin on currents evoked by ACh in the muscle (αβγδ) nAChR were studied. Concentration–response curves were adjusted using the empirical Hill equation: Y=1/(1+(x/IC50)∧nH) where Y=the fraction of remaining current, IC50=concentration of half inhibition, nH=the apparent cooperativity, x=antagonist concentration. The inhibition curve for muscle (αβγδ) nAChRs was fitted with a two-component Hill equation, and for comparison, a one-component Hill equation. When two Hill equations were employed, the sum of two identical equations was computed.
Drugs and chemicals
The drugs used in the pharmacological studies: acetylcholine iodide, AChE (electric eel enzyme type V-S), carbamylcholine chloride (CCh), d-Tc, neostigmine bromide and thiobutabarbitone (Inactin™) were obtained from Sigma Chemicals, St Louis, MO, U.S.A. All chemicals and reagents and α-conotoxin MI were also purchased from Sigma (St Louis, MO, U.S.A.). α-Bungarotoxin, α-cobratoxin and erabutoxin-b were from Latoxan (Valence, France). Candoxin was purified as described in Nirthanan et al. (2002a) from Bungarus candidus venom obtained from Venom Supplies Pvt. Ltd. (Tanunda, SA, Australia). The stock solutions of drugs and toxins were freshly made in deionized water and diluted to the required concentration with Kreb's solution for in vitro experiments or 0.9% saline for in vivo experiments.
Statistics
The data are expressed as mean±standard error of mean (s.e.m.) of at least four to six experiments unless stated otherwise. The experiments that required 100 μM or higher concentrations of candoxin were repeated thrice. Paired t-tests (two-tailed) were used to determine significance and P<0.05 was considered significant. The data were analysed using Fig P software version 2.2a (Fig P Software Corporation, Durham, NC, U.S.A.) or GraphPad Prism software (GraphPad Software Inc., San Diego, CA, U.S.A.).
Results
Effects of candoxin on neuromuscular transmission
Chick biventer cervicis muscle
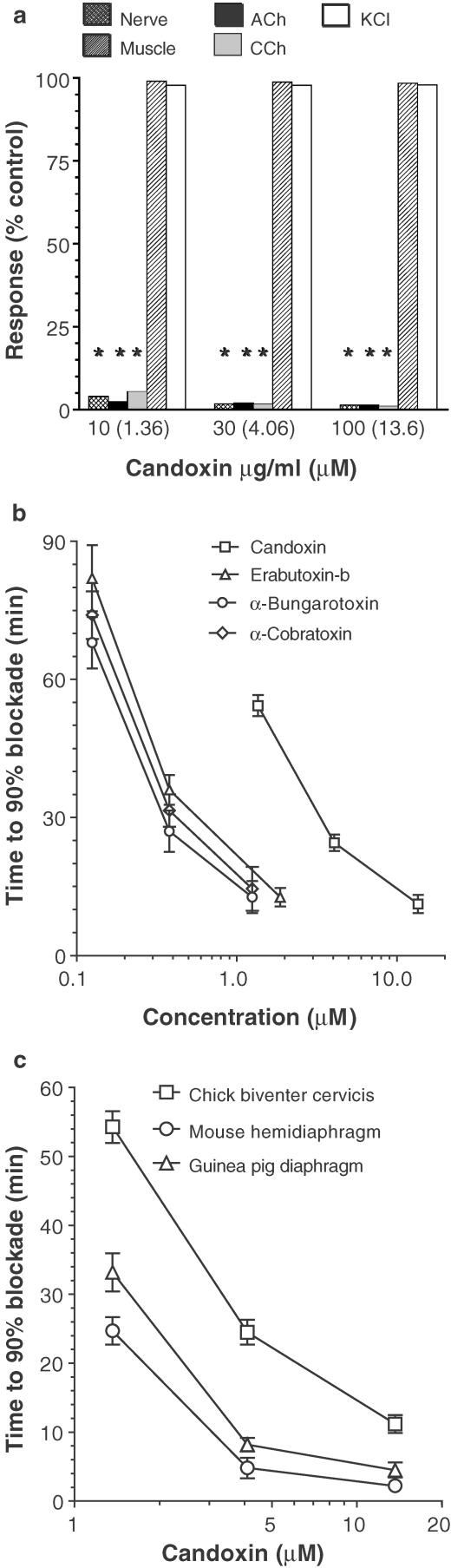
In control experiments, the twitch responses of the CBCM were 92±6% of control twitch height after 180 min of uninterrupted nerve stimulation at 0.2 Hz (mean±s.e.m., n=7). The twitches evoked by direct muscle stimulation were 96±3% of control twitch height after 180 min of uninterrupted stimulation (n=4). The responses to exogenously applied ACh, CCh and KCl were 95±3, 93±4 and 97±2% of control response height, respectively, at the end of 300 min of uninterrupted nerve stimulation (n=7). The muscle tone remained at baseline throughout this period of time. Candoxin produced a time-dependent blockade of the twitch responses of the CBCM to nerve stimulation as well as complete blockade of the responses to exogenously applied ACh and CCh (Figures 1a, b). Neither the twitch responses elicited by direct muscle stimulation nor the responses to exogenously applied KCl were affected by candoxin even at a concentration of 300 μg ml−1 (40.8 μM) (n=2). No contracture (or increase in muscle tone) was observed at any concentration of candoxin tested for up to ∼180 min following the addition of candoxin. α-Bungarotoxin, erabutoxin-b and α-cobratoxin also produced time-dependent blockade of nerve-evoked, but not directly stimulated, twitch responses of the CBCM as well as blockade of the responses elicited by ACh and CCh but not KCl. However, these neurotoxins were about 7–10-fold more potent than candoxin in producing neuromuscular blockade in the CBCM (Figure 1b).
Figure 1.
Pharmacological characterization of candoxin using the chick biventer cervicis muscle (a, b) and comparison of the blockade of nerve-evoked twitch responses produced by candoxin in various isolated nerve-muscle preparations (c). (a) The effects of candoxin (10, 30 and 100 μg ml−1) on twitch responses evoked by nerve stimulation (Nerve); twitch responses evoked by direct muscle stimulation (Muscle) and contractures produced by ACh (300 μM), CCh (8 μM) and KCl (30 mM) in the chick biventer cervicis muscle. The responses of the muscle to candoxin are expressed as a percentage of the respective control values in the absence of candoxin. Values are mean±s.e.m., n=6. Vertical bars represent the s.e.m. (b) Concentration-dependent blockade of nerve-evoked twitch responses of the chick biventer cervicis muscle produced by candoxin, erabutoxin-b, α-bungarotoxin and α-cobratoxin. The time taken to produce 90% twitch blockade is shown. Each data point is the mean of six experiments. The vertical bars represent the SEM. (c) The concentration-dependent blockade of nerve-evoked twitch responses produced by candoxin in the chick biventer cervicis muscle (n=10), mouse phrenic nerve hemidiaphragm (n=8) and GPD (n=8) is shown. The time to produce 90% twitch blockade in the respective nerve-muscle preparations is shown. Vertical bars represent the s.e.m. *P<0.001, significantly different from control.
Mouse phrenic nerve-hemidiaphragm
In control experiments, the twitch responses of the MHD were 97±1% (n=5) and 98±2% (n=4) of control twitch height after 90 min of uninterrupted nerve or direct muscle stimulation, respectively. The muscle tone remained at baseline throughout the entire duration of nerve or direct muscle stimulation. Candoxin 3, 10, 30 and 100 μg ml−1 (in μM: 0.41, 1.36, 4.09 and 13.6) produced complete blockade of nerve-evoked, but not directly stimulated, twitch responses in the MHD in a time-dependent manner (Figure 1c).
Guinea-pig diaphragm
In control experiments, the twitch responses of the GPD were 96±4% of control twitch height after 60 min of uninterrupted electrical field stimulation (n=4). The muscle tone remained at baseline throughout the entire duration of field stimulation. Nerve-evoked twitch responses of the GPD were completely blocked by candoxin 3, 10, 30 and 100 μg ml−1 (in μM: 0.41, 1.36, 4.09 and 13.6) in a time-dependent manner (Figure 1c).
Reversal of nerve-evoked twitch blockade produced by candoxin in vitro
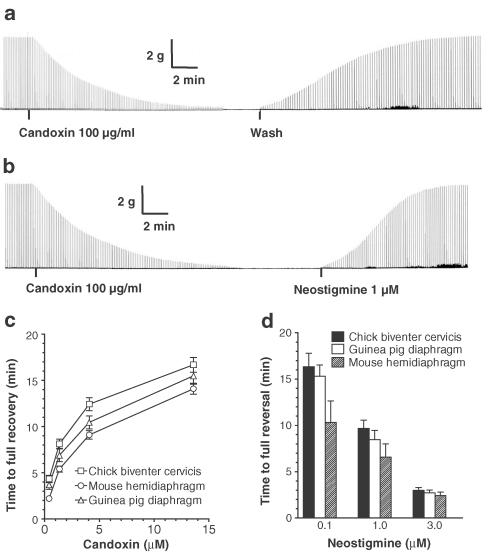
The twitch responses of the CBCM were rapidly and completely restored by washing out candoxin with fresh Kreb's solution following the blockade of nerve-evoked twitch responses produced by candoxin 3, 10, 30 and 100 μg ml−1 (in μM: 0.41, 1.36, 4.09 and 13.6) (Figures 2a, c). In another series of experiments, neostigmine 0.1, 1 and 3 μM produced complete reversal of the candoxin-induced blockade of nerve-evoked twitches in the CBCM in a concentration-dependent manner (Figures 2b, d). Likewise, the MHD and GPD were also able to recover rapidly and completely from the blockade of nerve-evoked twitch responses produced by candoxin (Figure 2c). Neostigmine 0.1, 1 and 3 μM also produced complete reversal of the twitch blockade produced by candoxin in the MHD and GPD (Figure 2d). In contrast, the twitch blockade produced by 0.05, 1 and 3 μg ml−1 α-bungarotoxin, erabutoxin-b and α-cobratoxin in the CBCM were virtually irreversible, even after prolonged washing for 180 min (n=3). The addition of the neostigmine (cumulatively up to 100 μM) failed to reverse the blockade of nerve-evoked twitches produced by α-bungarotoxin, erabutoxin-b or α-cobratoxin, once complete neuromuscular blockade had been established in the CBCM (n=3).
Figure 2.
Reversibility of nerve-evoked twitch blockade produced by candoxin. (a) Segments of tracing showing the complete blockade of nerve-evoked twitches of the chick biventer cervicis muscle produced by candoxin (100 μg ml−1; 13.6 μM). Candoxin was washed out at the point indicated. (b) Neostigmine (1 μM) was added to the bath at the point indicated. The vertical bar represents the magnitude of the twitch response in g tension. The horizontal bar depicts the time in min. The figures are representative of six experiments. (c) Comparison of the time to full recovery from complete blockade of nerve-evoked twitch responses produced by various concentrations of candoxin in the chick biventer cervicis muscle (n=6), mouse phrenic nerve hemidiaphragm (n=4) and GPD (n=4). Candoxin was washed out immediately following the establishment of complete twitch blockade. The time required for the restoration of nerve-evoked twitch responses to 100% of control twitch height is shown. (d) Comparison of the time to full reversal of blockade of nerve-evoked twitch responses produced by candoxin (100 μg ml−1; 13.6 μM) following the addition of various concentrations of neostigmine. Neostigmine was added 5 min after complete twitch blockade was established. Data is the mean of four experiments for all tissues. Vertical bars represent the s.e.m.
Effects of candoxin on TOF twitch responses in the MHD
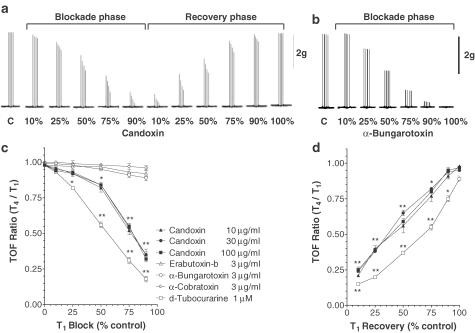
Figure 3a shows the typical effects of candoxin (100 μg ml−1; 13.6 μM) on maximal twitch responses of the MHD evoked by TOF nerve stimulation. During the onset of blockade of nerve-evoked twitches, candoxin progressively depressed all the four twitches (T1, T2, T3, T4) in each train, but at different rates (T4>T3>T2≫T1), as evidenced by the twitch heights recorded (Figure 3a). During recovery from twitch blockade produced by candoxin, T1 reached control levels faster than did T4. Thus, various concentrations of candoxin (10, 30 and 100 μg ml−1) (in μM: 1.36, 4.09 and 13.6) were found to produce significant TOF fade during the onset of and recovery from twitch blockade in the MHD (Figures 3c, d). The intensity of TOF fade produced by candoxin in the MHD was, however, less than that observed for d-TC (1 μM) at all stages of T1 block. In contrast, 3 μg ml−1 α-bungarotoxin (0.37 μM), erabutoxin-b (0.45 μM) and α-cobratoxin (0.38 μM) did not show significant TOF fade during their virtually irreversible blockade of nerve-evoked twitch responses (Figure 3c). For comparison, the absence of a fade response during the blockade of nerve-evoked TOF twitch responses of the MHD by α-bungarotoxin (3 μg ml−1) is shown in Figure 3b.
Figure 3.
TOF fade produced by candoxin in the MHD. Segments of a typical tracing showing the effects of (a) candoxin (100 μg ml−1) and (b) α-bungarotoxin (3 μg ml−1) on the twitch responses elicited by TOF nerve stimulation in the mouse phrenic nerve hemidiaphragm. The TOF fade response at 10, 25, 50, 75 and 90% block and/or recovery (for the first twitch, T1) is shown. The data presented in (a) and (b) is representative of six experiments. The vertical bar depicts the magnitude of the twitch response in g tension. The neuromuscular blockade produced by α-bungarotoxin was irreversible. (c, d) Comparison of the TOF fade produced by candoxin, d-tubocurarine and other α-neurotoxins in vitro. The fade response produced during the onset of nerve-evoked twitch blockade (c) and recovery from twitch blockade (d) by various concentrations of candoxin, d-tubocurarine, α-bungarotoxin, α-cobratoxin and erabutoxin-b during TOF stimulation of the mouse phrenic nerve hemidiaphragm. The nerve-evoked twitch blockade produced by α-bungarotoxin, α-cobratoxin and erabutoxin-b were irreversible. The TOF ratio (T4/T1) at 10, 25, 50, 75 and 90% block (for the first twitch, T1) is compared. Each data point is the mean of six experiments. The vertical bars represent the s.e.m. *P< 0.01; **P<0.001, significantly different from control.
Neuromuscular blockade produced by candoxin in vivo
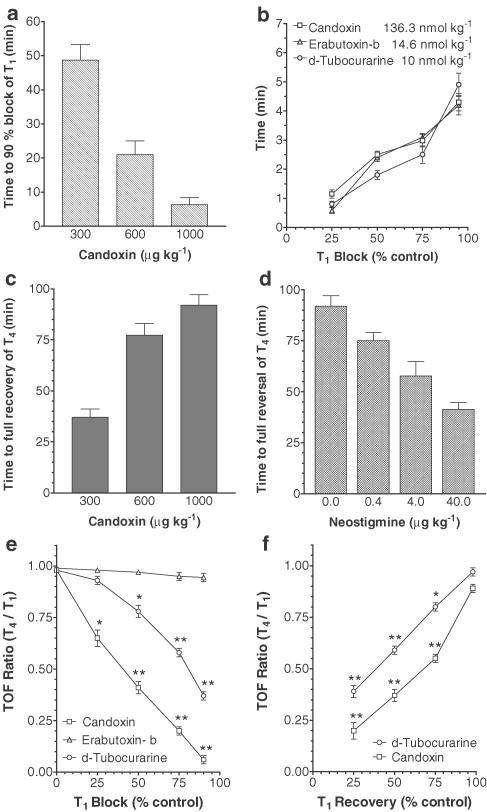
Control twitch responses of the rat tibialis anterior muscle to nerve stimulation were 16±1.2 g (n=6). Candoxin 0.3, 0.6 and 1 mg kg−1 produced complete blockade of the nerve-evoked twitch responses of the muscle in a time-dependent manner (Figure 4a). Furthermore, 1 mg kg−1 (136.3 nmol kg−1) of candoxin produced complete neuromuscular blockade in the tibialis anterior muscle in about the same time (∼4 min) as that mediated by 0.1 mg kg−1 (14.6 nmol kg−1) of erabutoxin-b (Figure 4b). The twitch responses of the muscle recovered spontaneously and completely; the time to complete spontaneous recovery was longer with increasing doses of candoxin (Figure 4c). The blockade of twitch responses produced by 1 mg kg−1 candoxin was also completely reversed by the addition of neostigmine (0.4, 4 or 40 μg kg−1) in a dose-dependent manner (Figure 4d). Candoxin (1 mg kg−1; 136.3 nmol kg−1) also produced significant TOF fade during the onset of and spontaneous recovery from twitch blockade in vivo (Figures 4e, f). Candoxin did not appear to affect the arterial blood pressure, heart rate and cardiac conductivity of the anaesthetized rat during neuromuscular blockade (data not shown).
Figure 4.
Neuromuscular blockade produced by candoxin in vivo. (a) Concentration-dependent candoxin-induced blockade of twitch responses evoked by TOF nerve stimulation in the tibialis anterior muscle in the anaesthetized rat. The time taken to produce 90% blockade of the first twitch (T1) is shown. (b) Comparison of the blockade of nerve-evoked twitch responses of the tibialis anterior muscle produced by candoxin (1 mg kg−1; 136.3 nmol kg−1), erabutoxin-b (100 μg kg−1; 14.6 nmol kg−1) and d-tubocurarine (10 nmol kg−1). The time taken to produce 25, 50, 75 and 90% blockade of the first twitch response (T1) is shown. (c) Time-dependent spontaneous recovery of the fourth twitch response (T4) of the tibialis anterior muscle from the nerve-evoked twitch blockade produced by candoxin 0.3, 0.6 or 1 mg kg−1. The time to complete recovery of the fourth twitch response (T4) is shown. (d) Dose-dependent reversal of nerve-evoked twitch blockade produced by candoxin (1 mg kg−1) in the anterior tibialis muscle by neostigmine (0.4, 4 or 40 μg kg−1). The time to complete recovery of the fourth twitch response (T4) is shown. All values are mean±s.e.m. of four experiments. Vertical bars indicate the s.e.m. (e, f) Comparison of the TOF fade produced by candoxin, d-tubocurarine and erabutoxin-b in vivo. The fade response produced during the onset of nerve-evoked twitch blockade (e) and recovery from twitch blockade (f) by candoxin, d-tubocurarine and erabutoxin-b during TOF stimulation of the tibialis anterior muscle in the anaesthetized rat. The nerve-evoked twitch blockade produced by erabutoxin-b was irreversible. The TOF ratio (T4/T1) at 25, 50, 75 and 90% block (for the first twitch, T1) is compared. Each data point is the mean of four experiments. The vertical bars represent the s.e.m. *P<0.01; **P<0.001, significantly different from control.
Erabutoxin-b (0.1 mg kg−1; 14.6 nmol kg−1) produced rapid neuromuscular blockade in the tibialis anterior muscle that did not recover spontaneously. The injection of neostigmine, in bolus doses cumulatively up to 80 μg kg−1, did not reverse the erabutoxin-induced blockade. No TOF fade was observed during the twitch blockade produced by erabutoxin-b (Figure 4e). In contrast, d-TC (10 nmol kg−1) produced reversible twitch blockade accompanied by significant TOF fade during the onset of and spontaneous recovery from twitch blockade (Figures 4e, f).
Biochemical assay for anticholinesterase activity
The rate of change in optical density for the control assay was 7.14±0.1 mAU min−1 (n=9). The rate of change in optical density for the candoxin assay with 10, 30 and 100 μM of candoxin was 7.05±0.11, 6.96±0.2 and 07.07± 0.14 mAU min−1, respectively, after 30 min incubation and 6.97±0.16, 7.11±0.12 and 7.09±0.08 mAU min−1, respectively, after 60 min incubation. Thus, candoxin did not inhibit AChE activity. Neostigmine 1 μM completely inhibited the AChE activity.
Effects of candoxin on muscle (αβγδ) nicotinic ACh receptors
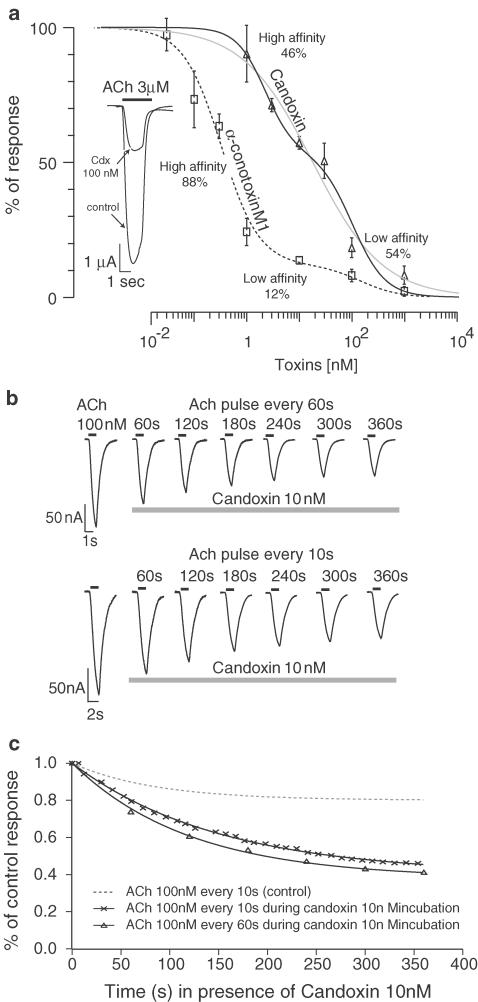
Candoxin strongly inhibited ACh-evoked currents in the rat muscle (αβγδ) nAChRs expressed on oocytes with a half inhibitory concentration (IC50) of 10 nM. The inhibition of muscle (αβγδ) nAChRs by candoxin was also rapidly and completely reversed by washing (Nirthanan et al., 2002a). The inhibition concentration–response over a broad range of candoxin concentrations is shown in Figure 5a. Attempts to describe these points with a single Hill equation yielded a low Hill coefficient (nH=0.64) (Figure 5a, grey line), whereas the fit obtained with the sum of two Hill equations showed a high- (2.2 nM, nH=1.6) and a low-affinity (98 nM, nH=1.4) component. The high- and low-affinity components contributed almost equivalently to the inhibition (46 and 54%, respectively). The blockade of muscle (αβγδ) nAChRs produced by α-conotoxin MI (which has high selectivity for the α/δ interface of mammalian muscle nAChRs), measured under the same experimental conditions, also yielded a biphasic concentration–response inhibition curve with a high (0.33 nM, nH=1.2) and low affinity (150 nM, nH=1.2) contributing to 88 and 12% to the blockade, respectively (Figure 5a).
Figure 5.
Inhibition of ACh currents in oocyte-expressed muscle (αβγδ) nicotinic ACn receptors by candoxin and α-conotoxin M1. The inhibition curve of candoxin was fitted by the sum of two Hill equations, with a high (2.2 nM, nH=1.6) and a low affinity (98 nM, nH=1.4) component, contributing to 46 and 54%, respectively. For comparison, the fit using a single Hill equation that yielded a low Hill coefficient (IC50 of 18 nM, nH=0.64) is also shown (grey line). The inhibition curve of α-conotoxin M1 was also biphasic with a high- (0.33 nM, nH=1.2) and a low-affinity (150 nM, nH=1.2) component, contributing to 88 and 12%, respectively. Inset: typical current evoked by 3 μM ACh before and after 30 min exposure to 100 nM candoxin. (b) Candoxin blockade is use independent. Currents evoked by 100 nM ACh were recorded at successive intervals of either 60 (upper traces) or 10 (lower traces) seconds in presence of 10 nM candoxin. Values above the traces indicate the incubation time in candoxin. (c) Plot of the current amplitude as a function of incubation time yields a single exponential decay that is fitted by an exponential in the form y=a*e−t/τ+b, where a=0.6, τ=140 and b=0.4 for the 10 s interval and a=0.5, τ=120 and b=0.5, for the 60 s interval.
Discussion
Neuromuscular blockade produced by candoxin
Candoxin produces a novel pattern of neuromuscular blockade: the onset of blockade is rapid and the offset is also rapid and completely reversible. In the CBCM, candoxin produced concentration- and time-dependent blockade of uninterrupted twitch responses of the muscle to nerve stimulation. This blockade can be attributed to a postsynaptic action since candoxin also blocks the contractile responses of the CBCM to the exogenously applied cholinoceptor agonists ACh and CCh. As candoxin did not block the twitch responses elicited by direct muscle stimulation or the responses elicited by KCl as a consequence of direct muscle depolarization, the postsynaptic activity of candoxin cannot be attributed to a direct inhibitory effect on muscle contractility but, rather, more specifically to its blockade of postsynaptic nAChRs at the neuromuscular junction. These effects closely resemble the neuromuscular blockade produced by curaremimetic α-neurotoxins such as erabutoxin-b, α-bungarotoxin and α-cobratoxin, which are well documented to have high selectivity and high affinity for postsynaptic nAChRs (Lee, 1972; Chang, 1979). However, the neuromuscular blockade produced by candoxin in the CBCM was ∼7–10-fold less potent than that produced by erabutoxin-b, α-bungarotoxin or α-cobratoxin. A direct myotoxic effect of candoxin was further excluded by the observation that candoxin did not produce a slow-onset and irreversible contracture that is usually, but not invariably (Harris, 1991) seen as a characteristic feature of neuromuscular blockade associated with venom components such as phospholipase A2 that cause myotoxicity (Rowan et al., 1989; Geh et al., 1992; Hodgson & Wickramaratna, 2002). Moreover, candoxin did not show any phospholipase A2 activity in a reliable and sensitive biochemical assay described by Kawuachi et al. (1971) (data not shown).
Candoxin also produced concentration- and time-dependent neuromuscular blockade in some mammalian nerve-muscle preparations, including the phrenic nerve hemidiaphragm of the mouse (MHD) and the GPD. The MHD and GPD were more sensitive (EC50∼0.8 μM) to neuromuscular blockade by candoxin than the CBCM (EC50∼1.5 μM). It has been reported that the determinant residues for toxin binding are different between the ligand-binding domains of the α subunits of the chick and rat nicotinic receptors (Arias, 2000) and therefore, it may be likely that snake toxins have evolved to show species selectivity to effectively target their intended prey (Dufton et al., 1989), which in the case of Bungarus candidus consists of reptiles and rodents (Lim, 1990). The results obtained from in vitro experiments were corroborated by in vivo pharmacological studies performed on anaesthetized rats. Candoxin produced dose- and time-dependent blockade of twitch responses in the tibialis anterior muscle evoked by electrical stimulation of the sciatic nerve. Doses less than 0.3 mg kg−1 produced only partial (45–85% of control) blockade, whereas 0.3, 0.6 and 1 mg kg−1 candoxin produced rapid and complete neuromuscular blockade. Furthermore, like in the in vitro studies, erabutoxin-b was about 10-fold more potent than candoxin in vivo in producing neuromuscular lockade.
Reversibility of neuromuscular blockade induced by candoxin
In contrast to most α-neurotoxins that produce virtually irreversible neuromuscular blockade (Lee, 1972; Chicheportiche et al., 1975; Lee et al., 1977; Chang, 1979; Harris, 1984), candoxin produced a blockade of nerve-evoked twitch responses in vitro and in vivo that was rapidly and completely reversed by washing or by the addition of the anticholinesterase neostigmine. Hence, the neuromuscular blockade produced by candoxin closely resembled the neuromuscular effects of d-TC, a reversible and competitive antagonist of ACh acting selectively at postsynaptic nAChRs. The rapid recovery of the candoxin-induced neuromuscular blockade can be attributed to the rapid removal of the toxin from its postsynaptic target sites by washing. The reversal of neuromuscular blockade by neostigmine is likely because of AChE inhibition and ACh preservation at the synapse, possibly resulting in competitive displacement of candoxin.
α-Conotoxins MI and GI, short (∼12–30 residues) disulphide-rich peptides from marine cone snail venoms, are well known to produce reversible postsynaptic neuromuscular blockade in vitro and in vivo (Marshall & Harvey, 1990). There are also a few other neurotoxins and neurotoxin homologues isolated from snake venoms that reportedly exhibit reversible or partially reversible neuromuscular blockade in isolated nerve-muscle preparations. These include neurotoxins: LSIII (a postsynaptically acting neurotoxin isolated from the venom of the sea snake Laticauda semifasciata; Maeda & Tamiya, 1974), fasciatoxin (a weak postsynaptic neurotoxin from Bungarus fasciatus; Liu et al., 1989) and pseudonajatoxin b (a highly lethal neurotoxic polypeptide from the Australian common brown snake Pseudonaja textilis; Tyler et al., 1987). Neurotoxin homologues CM10, CM12 (Naja haje annulifera; Joubert, 1975) and S5C10 (Dendroaspis jamesoni; Joubert & Taljaard, 1979) are proteins that are structurally similar to neurotoxins, but with greatly reduced toxicity (LD50∼5–60 mg kg−1) (Dufton & Hider, 1983). Candoxin showed very little sequence homology to all these ‘reversible' toxins as well as to other more conventional neurotoxins (Figure 6).
The neuromuscular blockade induced by toxin LSIII (Laticauda semifasciata) in the CBCM was reported to be significantly reversed by washing (Maeda et al., 1974; Harvey & Rodger, 1978). However, the time to recovery from neuromuscular blockade produced by LSIII increased with increasing concentrations of the toxin used for blockade. In contrast, the recovery of twitch responses of the CBCM or MHD or GPD from blockade produced by low (3–10 μg ml−1) or high (100 μg ml−1) concentrations of candoxin occurred well within 15–20 min. The concentration-dependent pattern of recovery shown by LSIII is unusual, since it has been proposed that although a higher concentration of toxin spreads faster into the neuromuscular junction within the muscle and produces a more rapid twitch blockade, once the preparation is completely blocked, the ‘effective' toxin concentration is similar and independent of the initial concentration added to the bath, and consequently, the pattern of recovery is similar (Harvey & Rodger, 1978). Functional studies on the neurotoxin homologues, CM10, CM12 and S5C10 showed that these proteins also exhibit a postsynaptic site of action as do curaremimetic α-neurotoxins, but with reduced affinity and that in addition, the neuromuscular blockade produced by CM10, CM12 and S5C10 were easily reversed by washing or by the addition of neostigmine (Harvey et al., 1984). The nature of the reversibility of neuromuscular blockade induced by fasciatoxin (Bungarus fasciatus) and by pseudonajatoxin b (Pseudonaja textilis) has not been investigated in detail.
It could be argued that the reversibility of neuromuscular blockade induced by some toxins, as opposed to the irreversible blockade attributed to others, may just result from their weak binding affinity to the receptors. However, in electrophysiological studies, α-bungarotoxin produced an irreversible block of muscle (αβγδ) receptors with an IC50 of ∼5 nM (Johnson et al., 1995), which is just two-fold lower than the IC50 (∼10 nM) of candoxin, which produced reversible blockade of the same receptor (Nirthanan et al., 2002a). Utkin et al. (2001) have also found that WTX (Naja kaouthia), a toxin that is structurally similar to candoxin but a 1000-fold weaker antagonist at muscle (αβγδ) receptors, is almost irreversible in its action. Clearly, therefore, the reversibility of toxin action at the neuromuscular junction is not always a reflection of their binding affinity to the receptor. It has already been suggested that reversibility of neurotoxin action may be associated with a specific area of interaction on the toxin molecule, distinct from the receptor recognition site (Harvey & Rodger, 1978). For instance, it was recently reported that the mutation of a single residue (Phe38) in a three-finger toxin m1-Toxin 1 (Dendroaspis angusticeps) that binds specifically and irreversibly to M1 muscarinic receptors, resulted in completely reversible binding of the toxin to M1 receptors (Krajewski et al., 2001). Harvey et al. (1984) also made the observation that in contrast to most α-neurotoxins, an aspartate at position 31 was conspicuously absent in both CM10 and CM12, as well as in toxin LSIII, all of which were reported to be reversible in their action. The authors postulated that the absence of an aspartate at position 31 may be associated with easy reversibility of neuromuscular blockade produced by these toxins. Interestingly, candoxin also lacks an aspartate at a position homologous to position 31 in CM10 and CM12.
Effects of candoxin on oocyte-expressed muscle (αβγδ) nAChRs
Candoxin effectively blocked currents evoked by ACh in muscle (αβγδ) nAChRs expressed in Xenopus oocytes with a low half-inhibitory concentration of ∼10 nM (Nirthanan et al., 2002a). In agreement with the neuromuscular blockade produced by candoxin in in vitro and in vivo, complete recovery from the candoxin-induced block of muscle (αβγδ) nAChRs was observed following washing. In contrast, the functional block of oocyte-expressed muscle (αβγδ) nAChRs produced by α-bungarotoxin (Johnson et al., 1995), erabutoxin-a (Servent et al., 1997) and WTX (Utkin et al., 2001) were virtually irreversible. Candoxin is therefore functionally distinct from other curaremimetic short- and long-chain α-neurotoxins as well as other nonconventional toxins from cobra venoms.
The inhibition concentration–response curve of muscle (αβγδ) receptors by candoxin was fitted by the sum of two Hill equations with a high- (2.2 nM, nH=1.6) and low- (98 nM, nH=1.4) affinity component that contributed almost equivalently, 46 and 54%, respectively, to the inhibition. Attempts to fit the data points using a single Hill equation yielded a low Hill coefficient (IC50 of 18 nM, nH=0.64), which cannot adequately describe the cooperativity between the subunits in the allosteric model of the nAChR (Changeux & Edelstein, 1998). Even if candoxin showed equal affinity for the two binding sites and acted independently upon them, the Hill coefficient would be expected to be close to one. Taking this into consideration, we were of the opinion that using the sum of two Hill equations to describe the inhibition curve of candoxin for the muscle receptor was a better representation of physiological reality. Furthermore, this apparent dual affinity inhibition of muscle αβγδ nAChRs by candoxin also revealed the presence of a plateau phase between the high- and low-affinity dose–response inhibition curves. These data suggested that candoxin might exhibit different affinities for the two nonidentical binding sites present on muscle αβγδ nAChRs although further experiments are needed to confirm this finding. The existence of two binding sites, a high-affinity site at the α/γ subunit interface and a low-affinity site at the α/δ subunit interface, in the mammalian muscle and Torpedo receptors has been previously established based on the ability of d-TC to bind to the two sites with dissociation constants that differed by ∼100-fold (Neubig & Cohen, 1979; Pedersen & Cohen, 1990). This difference has been attributed to the influence of the nonequivalent subunits (i.e. γ and δ) on the conformation of the binding sites at their respective interfaces with the α subunit (Neubig & Cohen, 1979).
More recently, α-conotoxin MI (Conus magus) has been reported to display unique specificity for the α/δ site over the α/γ site (Jacobsen et al., 1999). The current electrophysiological study on α-conotoxin MI demonstrated that the presence of a high- and a low-affinity site on muscle αβγδ nAChRs can be functionally revealed by the observation of a biphasic concentration–response inhibition curve with a plateau phase. Specifically, α-conotoxin MI revealed the existence of a high- and a low-affinity site for which it displayed IC50 values of ∼0.33 nM (nH=1.2) and ∼150 nM (nH=1.2), respectively. In contrast, α-neurotoxins such as erabutoxin-b, α-bungarotoxin and α-cobratoxin have been reported to antagonize both binding sites with equal affinity (Taylor et al., 1998; Jacobsen et al., 1999) and such a dual affinity curve with a plateau phase has not been observed in their binding or functional studies. However, site-directed mutagenesis studies on α-cobratoxin revealed that the mutation of Lys23 and Lys49 to Glu23 and Glu49, respectively, caused a differential lowering of binding affinity at the two binding sites of the muscle αβγδ nAChRs (Antil-Delbeke et al., 2000). This observation was verified for a short-chain α-neurotoxin Naja mossamica mossambica (NmmI), whereby the mutation of Lys27 to Glu27 affected binding at the α/γ site more than the α/δ site (Ackermann & Taylor, 1997). Interestingly, position 29 in candoxin (homologous to Lys23 and Lys27 in α-cobratoxin and NmmI, respectively) is occupied by a glutamic acid (Glu29) instead of a lysine (Figure 6). The possible role of Glu29 in candoxin in conferring differential selectivity for the two binding sites of the muscle αβγδ nAChR warrants further investigation.
Effects of candoxin on twitch responses evoked by TOF stimulation
Candoxin, like d-TC, produced significant TOF fade during the rapid onset of and recovery from neuromuscular blockade in the mouse diaphragm in vitro and in anaesthetized rats. In contrast, under the same experimental conditions, erabutoxin-b, α-cobratoxin and α-bungarotoxin did not produce such a fade during neuromuscular blockade. It has long been recognized that d-TC and related compounds, in addition to producing neuromuscular blockade, also produced an independent ‘fade' phenomenon characterized by rapidly waning tetanic tension or rundown in the successive twitch responses to TOF nerve stimulation (Paton & Zaimis, 1952; Cheah and Gwee, 1988; Gibb & Marshall, 1986; Bowman et al., 1988). In contrast, curaremimetic α-neurotoxins such as α-bungarotoxin do not display prominent fade response during TOF stimulation (Gibb & Marshall, 1986; Bowman et al., 1988; Cheah & Gwee, 1988; Blount et al., 1992; Wilson & Nicholson, 1997; Oliveira & Oliveira, 1999). Nonetheless, it has been reported that under certain experimental conditions, α-cobratoxin (Chang & Hong, 1987; Hong & Chang, 1991) and erabutoxin-b (Bradley et al., 1987), but not α-bungarotoxin (Bradley et al., 1990), also produced fade when tested in low (<5 nM) concentrations and after prolonged (>5–8 h) incubation periods, the fade being more pronounced following the washout of the toxin (Bradley et al., 1990).
The phenomenon of TOF fade has been ascribed to a presynaptic event at the neuromuscular junction involving putative autofacilitatory nAChRs. Bowman et al. (1986; 1988) hypothesized that these prejunctional nicotinic receptors serve as ‘autoreceptors' that sustain a positive feedback mechanism, which mobilizes ‘reserve' stores of ACh to a ‘releasable' store and thus maintain adequate transmitter release during high-frequency nerve activity. Consequently, blockade of this autoreceptor would inhibit the positive feedback control of ACh release resulting in a fade in muscle tension during rapid, repetitive nerve stimulation (Wessler et al., 1986; Gibb & Marshall, 1987; Bowman et al., 1988; Prior et al., 1995; Singh & Prior, 1998). Other studies have reported that d-TC, but not snake α-neurotoxins, produced a reduction in nerve-evoked 3H-ACh release from rat nerve terminals during rapid and repetitive nerve stimulation, providing direct evidence that a presynaptic modulation of ACh release may be involved in the fade response produced by d-TC (Vizi & Somogyi, 1989; Wessler, 1992; Apel et al., 1995).
An alternate hypothesis suggests that the presynaptic nicotinic receptors normally function to reduce ACh release via a negative feedback mechanism and consequently, a block of these presynaptic receptors would enhance ACh release (Wilson, 1982). Subsequently, it has been suggested that another distinct population of prejunctional ‘inhibitory' nicotinic autoreceptors may coexist with the ‘facilitatory' autoreceptors involved in positive feedback modulation of transmitter release (Tian et al., 1994; 1997). The negative feedback mechanism, however, has been proposed to act to reduce the number of vesicles docked at the release sites during passive leakage or spontaneous release of transmitter from the nerve terminal (Prior et al., 1995). Therefore, it is unlikely that these inhibitory autoreceptors, which operate at low frequencies of motor nerve stimulation, are involved in contributing to the fade response observed during rapid nerve stimulation.
Other workers have attributed the fade response to a use-dependent failure of postsynaptic receptor function under conditions of repetitive nerve stimulation (Colquhoun et al., 1979; Bradley et al., 1990). Specifically, it was suggested that the fade-producing nicotinic receptor antagonists exhibit slow dissociation rates from the postsynaptic nicotinic receptors with resultant accumulation of blocked channels during repetitive stimulation. Consequently, fewer receptors are available for activation with each consecutive depolarization, thereby producing a fade in endplate currents. To assess if the TOF fade produced by candoxin could be attributed to a progressive blockade of the postsynaptic receptors, use-dependent experiments were designed at neuromuscular junction receptors expressed in Xenopus oocytes. Application of low ACh test pulses at periodic intervals (10 s) showed little time dependence when recorded in control conditions. In contrast, incubation with 10 nM candoxin caused a progressive reduction of the ACh-evoked currents (Figure 5b, c) and the time course of the blockade was independent of the frequency of the ACh test pulses (Figure 5c). Comparable results were obtained in five cells (data not shown). This clearly indicates that the fade produced by candoxin cannot be attributed to an open-channel blocking effect of the toxin.
Taken together, the present data strongly suggest that, in addition to its established competitive antagonistic action at postsynaptic nAChRs, candoxin also mediates a presynaptic action at the neuromuscular junction as evidenced by the significant TOF fade produced during the onset of and offset from neuromuscular blockade. This is a novel and unusual functional characteristic of candoxin, not previously reported for snake toxins that exert a curaremimetic effect at the neuromuscular junction.
Effect of candoxin at other cholinergic sites
We also carried out a biochemical assay to investigate the effects of candoxin on AChE since it was important to ensure that candoxin did not possess an intrinsic anticholinesterase activity such as that shown by fasciculin, a three-finger toxin isolated from Dendroaspis spp. (Cervenansky et al., 1991). Such an inherent anticholinesterase activity of candoxin would result in a ‘self-cancellation' effect (Kenakin, 1993) as a consequence of its neuromuscular blocking action being opposed by the inherent anticholinesterase activity. Studies using an established in vitro biochemical assay showed that candoxin did not produce inhibition of AChE activity even at concentrations as high as 100 μM. Furthermore, candoxin, like other snake α-neurotoxins, did not mediate any effects on muscarinic receptors in isolated tissue preparations such as the guinea-pig ileum, rat anococcygeus muscle and rat isolated atria nor block ganglionic transmission mediated by ganglionic nAChRs in the guinea-pig ileum in concentrations that were 250-fold higher than that required to produce complete neuromuscular blockade in the GPD (data not shown).
Conclusions
In summary, candoxin produces a novel pattern of neuromuscular blockade at the neuromuscular junction, not usually associated with curaremimetic neurotoxins from snake venoms: (1) the blockade of nerve-evoked twitches was rapidly and completely reversed by washing or by the addition of an anticholinesterase, (2) a significant TOF fade was observed during the onset of and recovery from neuromuscular blockade, and (3) the inhibition of the muscle (αβγδ) nAChR in electrophysiological studies was characterized by different apparent affinities for the two distinct binding sites on the receptor.
Abbreviations
- ACh
acetylcholine
- AChE
acetylcholinesterase
- CBCM
chick biventer cervicis muscle
- CCh
carbachol
- d-TC
d-tubocurarine
- DTNB
dithiobisnitrobenzoic acid
- GPD
guinea-pig diaphragm
- KCl
potassium chloride
- MHD
mouse hemidiaphragm
- nAChR
nicotinic acetylcholine receptors
- TOF
train-of-four
References
- ACKERMANN E.J., TAYLOR P. Non-identity of the α-neurotoxin binding sites on the nicotinic acetylcholine receptor revealed by modification in α-neurotoxin and receptor structures. Biochemistry. 1997;36:12836–12844. doi: 10.1021/bi971513u. [DOI] [PubMed] [Google Scholar]
- ANTIL-DELBEKE S., GAILLARD C., TAMIYA T., CORRINGER P.J., CHANGEUX J.P., SERVENT D., MENEZ A. Molecular determinants by which a long chain toxin from snake venom interacts with the neuronal α7-nicotinic acetylcholine receptor. J. Biol. Chem. 2000;275:29594–29601. doi: 10.1074/jbc.M909746199. [DOI] [PubMed] [Google Scholar]
- APEL C., RICNY J., WAGNER G., WESSLER I. α-Bungarotoxin, κ-bungarotoxin, α-cobratoxin and erabutoxin-b do not affect [3H] acetylcholine release from the rat isolated left hemidiaphragm. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;352:646–652. doi: 10.1007/BF00171324. [DOI] [PubMed] [Google Scholar]
- ARIAS H.R. Localization of agonist and competitive antagonist binding sites on nicotinic acetylcholine receptors. Neurochem. Int. 2000;36:595–645. doi: 10.1016/s0197-0186(99)00154-0. [DOI] [PubMed] [Google Scholar]
- BLOUNT K., JOHNSON A., PRIOR C., MARSHALL I.G. α-Conotoxin GI produces tetanic fade at the rat neuromuscular junction. Toxicon. 1992;30:835–842. doi: 10.1016/0041-0101(92)90381-e. [DOI] [PubMed] [Google Scholar]
- BOWMAN W.C., GIBB A.G., HARVEY A.L., MARSHALL I.G.Prejunctional effects of cholinoceptors agonists and antagonists, and of anticholinesterase drugs Neuromuscular Blocking Agents 1986Berlin: Springer-Verlag; 141–168.ed. Kharkevich, D.A. pp [Google Scholar]
- BOWMAN W.C., MARSHALL I.G., GIBB A.J., HARBORNE A.J. Feedback control of transmitter release at the neuromuscular junction. Trends Pharmacol. Sci. 1988;9:16–20. doi: 10.1016/0165-6147(88)90236-2. [DOI] [PubMed] [Google Scholar]
- BRADLEY R.J., EDGE M.T., CHAU W.-C. The α-neurotoxin erabutoxin b causes fade at the rat end plate. Eur. J. Pharmacol. 1990;176:11–21. doi: 10.1016/0014-2999(90)90127-r. [DOI] [PubMed] [Google Scholar]
- BRADLEY R.J., PAGALA M.K., EDGE M.T. Multiple effects of α-toxins on the nicotinic acetylcholine receptor. FEBS Lett. 1987;244:277–281. doi: 10.1016/0014-5793(87)80469-6. [DOI] [PubMed] [Google Scholar]
- BULBRING E. Observations on the isolated phrenic nerve diaphragm preparations of the rat. Br. J. Pharmacol. 1946;1:38–61. doi: 10.1111/j.1476-5381.1946.tb00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CERVENANSKY C., DAJAS F., HARVEY A.L., KARLSSON E.Fasciculins, anticholinesterase toxins from mamba venoms: biochemistry and pharmacology Snake Toxins 1991New York: Pergamon Press; 303–321.ed. Harvey, A.L. pp [Google Scholar]
- CHANG C.C.The action of snake venoms on nerve and muscle Snake Venoms, Handbook of Experimental Pharmacology 197952Berlin: Springer-Verlag; 309–376.ed. Lee, C.-Y [Google Scholar]
- CHANG C.C., HONG S.J. Dissociation of the end-plate potential run down and the tetanic fade from the postsynaptic inhibition of the acetylcholine receptor by α-neurotoxins. Exp. Neurol. 1987;98:509–517. doi: 10.1016/0014-4886(87)90260-3. [DOI] [PubMed] [Google Scholar]
- CHANGEUX J-P., EDELSTEIN S.J. Allosteric receptors after 30 years. Neuron. 1998;21:959–980. doi: 10.1016/s0896-6273(00)80616-9. [DOI] [PubMed] [Google Scholar]
- CHEAH L.S., GWEE M.C.E. Train-of-four fade during neuromuscular blockade induced by tubocurarine, succinylcholine or α-bungarotoxin in the rat isolated hemidiaphragm. Clin. Exp. Pharmacol. Physiol. 1988;15:937–943. doi: 10.1111/j.1440-1681.1988.tb01039.x. [DOI] [PubMed] [Google Scholar]
- CHICHEPORTICHE R., VINCENT J.P., KOPEYAN C., SCHWEITZ H., LAZDUNSKI M. Structure–function relationship in the binding of snake neurotoxins to the torpedo membrane receptor. Biochemistry. 1975;14:2081–2091. doi: 10.1021/bi00681a007. [DOI] [PubMed] [Google Scholar]
- COLQUHOUN D., DREYER F., SHERIDAN R.E. The actions of tubocurarine at the frog neuromuscular junction. J. Physiol. 1979;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUFTON M.J., BLADON P., HARVEY A.L. Identification of a locality in snake venom alpha-neurotoxins with a significant compositional similarity to marine snail alpha-conotoxins: implications for evolution and structure/activity. J. Mol. Evol. 1989;29:355–366. doi: 10.1007/BF02103622. [DOI] [PubMed] [Google Scholar]
- DUFTON M.J., HIDER R.C. Conformational properties of the neurotoxins and cytotoxins isolated from elapid snake venoms. CRC Crit. Rev. Biochem. 1983;14:113–171. doi: 10.3109/10409238309102792. [DOI] [PubMed] [Google Scholar]
- ELLMAN G.L., COURTNEY K.D., ANDRES V., FEATHERSTONE R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- ENDO T., TAMIYA N.Structure–function relationships of postsynaptic neurotoxins from snake venoms Snake Toxins 1991New York: Pergamon Press; 165–222.ed. Harvey, A.L. pp [DOI] [PubMed] [Google Scholar]
- GEH S.L., ROWAN E.G., HARVEY A.L. Neuromuscular effects of three phospholipases A2 from the venom of Pseudechis australis, the Australian king brown snake. Toxicon. 1992;30:1051–1057. doi: 10.1016/0041-0101(92)90050-f. [DOI] [PubMed] [Google Scholar]
- GIBB A.J., MARSHALL I.G. Nicotinic antagonists produce differing amounts tetanic fade in the isolated diaphragm of the rat. Br. J. Pharmacol. 1986;89:619–624. doi: 10.1111/j.1476-5381.1986.tb11164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBB A.J., MARSHALL I.G. Examination of the mechanisms involved in tetanic fade produced by vecuronium and related analogues in the rat diaphragm. Br. J. Pharmacol. 1987;90:511–521. doi: 10.1111/j.1476-5381.1987.tb11200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBORG B.L., WARRINER J.N. The isolated chick biventer cervicis nerve-muscle preparation. Br. J. Pharmacol. 1960;15:410–411. doi: 10.1111/j.1476-5381.1960.tb01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS J.B. Polypeptides from snake venoms which act on nerve and muscle. Prog. Med. Chem. 1984;21:63–110. doi: 10.1016/s0079-6468(08)70407-7. [DOI] [PubMed] [Google Scholar]
- HARRIS J.B.Phospholipases in snake venoms and their effects on nerve and muscle Snake Toxins 1991New York: Pergamon Press; 91–129.ed. Harvey, A.L. pp [Google Scholar]
- HARVEY A.L., HIDER R.C., HODGES S.J., JOUBERT F.J. Structure–activity studies of homologues of short chain neurotoxins from Elapid snake venoms. Br. J. Pharmacol. 1984;82:709–716. doi: 10.1111/j.1476-5381.1984.tb10810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY A.L., RODGER I.W. Reversibility of neuromuscular blockade produced by toxins isolated from the venom of the sea snake Laticauda semifasciata. Toxicon. 1978;16:219–255. doi: 10.1016/0041-0101(78)90082-x. [DOI] [PubMed] [Google Scholar]
- HODGSON W.C., WICKRAMARATNA J.C. In vitro neuromuscular activity of snake venoms. Clin. Exp. Pharmacol. Physiol. 2002;29:807–814. doi: 10.1046/j.1440-1681.2002.03740.x. [DOI] [PubMed] [Google Scholar]
- HONG S.J., CHANG C.C. Run-down of neuromuscular transmission during repetitive nerve activity by nicotinic antagonists is not due to desensitisation of the postsynaptic receptor. Br. J. Pharmacol. 1991;102:817–822. doi: 10.1111/j.1476-5381.1991.tb12258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSEN R.B., DELA-CRUZ R.G., GROSE J.H., MCINTOSH J.M., YOSHIKAMI D., OLIVERA B.M. Critical residues influence the affinity and selectivity of α-conotoxin MI for nicotinic acetylcholine receptors. Biochemistry. 1999;38:13310–13315. doi: 10.1021/bi9907476. [DOI] [PubMed] [Google Scholar]
- JOHNSON D.S., MARTINEZ J., ELGOYHEN A.B., HEINEMANN S.F., MCINTOSH J.M. α-Conotoxin ImI exhibits subtype-specific nicotinic acetylcholine receptor blockade: preferential inhibition of homomeric α7 and α9 receptors. Mol. Pharmacol. 1995;48:194–199. [PubMed] [Google Scholar]
- JOUBERT F.J. The amino acid sequences of three toxins (CM-10, CM-12 and CM-14) from Naja haje annulifera (Egyptian cobra) venom. Hoppe-Seyler's Z. Physiol. Chem. 1975;356:53–72. [PubMed] [Google Scholar]
- JOUBERT F.J., TALJAARD N. Some properties and the complete primary structures of two reduced and S-carboxymethylated polypeptide (S5C1 and S5C10) from Dendroaspis jamesoni Kaimosae (Jameson's mamba) venom. Biochim. Biophys. Acta. 1979;579:228–233. doi: 10.1016/0005-2795(79)90101-6. [DOI] [PubMed] [Google Scholar]
- KAWUACHI S., IWANAGA S., SAMEJIMA Y., SUZUKI T. Isolation and characterisation of two phospholipase A's from the venom of Agkistrodon lays blomhoffii. Biochim. Biophys. Acta. 1971;236:142–160. doi: 10.1016/0005-2795(71)90159-0. [DOI] [PubMed] [Google Scholar]
- KENAKIN T.Pharmacological Analysis of Drug Receptor Interaction 1993New York: Raven Press; 2nd edn [Google Scholar]
- KINI R.M. Molecular moulds with multiple missions: functional sites in three-finger toxins. Clin. Exp. Pharmacol. Physiol. 2002;29:815–822. doi: 10.1046/j.1440-1681.2002.03725.x. [DOI] [PubMed] [Google Scholar]
- KLEINMAN L.I., RADFORD E.P., JR Ventilation standards for small mammals. J. Appl. Physiol. 1964;19:733–743. doi: 10.1152/jappl.1964.19.2.360. [DOI] [PubMed] [Google Scholar]
- KRAJEWSKI J.L., DICKERSON I.M., POTTER L.T. Site-directed mutagenesis of m1-Toxin1: two amino acids responsible for stable toxin binding to M1 muscarinic receptors. Mol. Pharmacol. 2001;60:725–731. [PubMed] [Google Scholar]
- LEE C.Y. Chemistry and pharmacology of polypeptide toxins in snake venoms. Annu. Rev. Pharmacol. 1972;12:265–286. doi: 10.1146/annurev.pa.12.040172.001405. [DOI] [PubMed] [Google Scholar]
- LEE C., CHEN D., KATZ R.L. Characteristics of non-depolarizing neuromuscular block (1) Post-junctional block by α-bungarotoxin. Can. Anaesth. Soc. J. 1977;24:212–218. doi: 10.1007/BF03006234. [DOI] [PubMed] [Google Scholar]
- LIM B.L.Venomous land snakes of Malaysia Snakes of Medical Importance (Asia-Pacific Region) 1990Singapore: Singapore University Press; 387–417.ed. Gopalakrishnakone, P. & Chou, L.M. pp [Google Scholar]
- LIU C.-S., HSIAO P.-W., CHANG C.-S., TZENG M.-C., LO T.-B. Unusual amino acid sequence of fasciatoxin, a weak reversibly acting neurotoxin in the venom of the banded krait, Bungarus fasciatus. Biochem. J. 1989;259:153–158. doi: 10.1042/bj2590153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAEDA N., TAMIYA N. The primary structure of toxin Laticauda semifasciata III, a weak and reversibly acting neurotoxin from the venom of a sea snake Laticauda semifasciata. Biochem. J. 1974;141:389–400. doi: 10.1042/bj1410389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAEDA N., TAKAGI K., TAMIYA N., CHEN Y.-M., LEE C.-Y. The isolation of an easily reversible post-synaptic toxin from the venom of a sea snake Laticauda semifasciata. Biochem. J. 1974;141:383–387. doi: 10.1042/bj1410383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHALL I.G., HARVEY A.L. Selective neuromuscular blocking properties of α-conotoxins in vivo. Toxicon. 1990;28:231–234. doi: 10.1016/0041-0101(90)90417-6. [DOI] [PubMed] [Google Scholar]
- NEUBIG R.R., COHEN J.B. Equilibrium binding of [3H] tubocurarine and [3H] acetylcholine by Torpedo postsynaptic membranes: stoichiometry and ligand interactions. Biochemistry. 1979;18:5464–5475. doi: 10.1021/bi00591a032. [DOI] [PubMed] [Google Scholar]
- NIRTHANAN S., CHARPANTIER E., GOPALAKRISHNAKONE P., GWEE M.C.E., KHOO H.E., CHEAH L.S., BERTRAND D., KINI R.M. Candoxin, a novel toxin from Bungarus candidus is a reversible antagonist of muscle (αβγδ) but a poorly reversible antagonist of neuronal α7 nicotinic acetylcholine receptors. J. Biol. Chem. 2002a;277:17811–17820. doi: 10.1074/jbc.M111152200. [DOI] [PubMed] [Google Scholar]
- NIRTHANAN S., GAO R., GOPALAKRISHNAKONE P., GWEE M.C.E., KHOO H.E., CHEAH L.S., KINI R.M. Pharmacological characterization of mikatoxin, an α-neurotoxin isolated from a snake (Micropechis ikaheka) venom. Toxicon. 2002b;40:863–871. doi: 10.1016/s0041-0101(01)00268-9. [DOI] [PubMed] [Google Scholar]
- NIRTHANAN S., GOPALAKRISHNAKONE P., GWEE M.C.E., KHOO H.E., KINI R.M. Non-conventional toxins from Elapid venoms. Toxicon. 2003;41:397–407. doi: 10.1016/s0041-0101(02)00388-4. [DOI] [PubMed] [Google Scholar]
- OLIVEIRA M., OLIVEIRA A.C. Mechanisms underlying the vecuronium-induced tetanic fade in the isolated rat muscle. Pharmacol. Toxicol. 1999;85:282–287. doi: 10.1111/j.1600-0773.1999.tb02023.x. [DOI] [PubMed] [Google Scholar]
- PATON W.D.M., ZAIMIS E.J. The methonium compounds. Pharmacol. Rev. 1952;4:219–231. [PubMed] [Google Scholar]
- PEDERSEN S.E., COHEN J.B. d-Tubocurarine binding sites are located at the α–γ and α–δ subunit interfaces of the nicotinic acetylcholine receptor. Proc. Natl. Acad. Sci. U.S.A. 1990;87:2785–2789. doi: 10.1073/pnas.87.7.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POH S.L., MOURIER G., THAI R., ARMUGAM A., MOLGO J., SERVENT D., JEYASEELAN K., MENEZ A. A synthetic weak neurotoxin binds with low affinity to Torpedo and chicken alpha7 nicotinic acetylcholine receptors. Eur. J. Biochem. 2002;269:4247–4256. doi: 10.1046/j.1432-1033.2002.03113.x. [DOI] [PubMed] [Google Scholar]
- PRIOR C., TIAN L., DEMPSTER J., MARSHALL I.G. Prejunctional actions of muscle relaxants: synaptic vesicles and transmitter mobilization as sites of action. Gen. Pharmacol. 1995;26:659–666. doi: 10.1016/0306-3623(94)00246-j. [DOI] [PubMed] [Google Scholar]
- ROWAN E.G., HARVEY A.L., TAKASAKI C., TAMIYA N. Neuromuscular effects of three phospholipase A2 from the venom of the Australian king brown snake Pseudechis australis. Toxicon. 1989;27:551–560. doi: 10.1016/0041-0101(89)90116-5. [DOI] [PubMed] [Google Scholar]
- SERVENT D., MENEZ A.Snake neurotoxins that interact with nicotinic acetylcholine receptors Handbook of Neurotoxicology 20011Totowa, NJ: Humana; 385–425.ed. Massaro, E.J [Google Scholar]
- SERVENT D., WINCKLER-DIETRICH V., HU H.Y., KESSLER P., DREVET P., BERTRAND D., MENEZ A. Only snake curaremimetic toxins with a fifth disulfide bond have high affinity for the neuronal alpha7 nicotinic receptor. J. Biol. Chem. 1997;272:24279–24286. doi: 10.1074/jbc.272.39.24279. [DOI] [PubMed] [Google Scholar]
- SINGH S., PRIOR C. Prejunctional effects of the nicotinic ACh receptor agonist dimethylphenylpiperazinium at the rat neuromuscular junction. J. Physiol. 1998;511:451–460. doi: 10.1111/j.1469-7793.1998.451bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR P., OSAKA H., MOLLES B.E., SUGIYAMA N., MARCHOT P., ACKERMANN E.J., MALANY S., MCARDLE J.J., SINE S.M., TSIGELNY I. Toxins selective for subunit interfaces as probes of nicotinic acetylcholine structure. J. Physiol. Paris. 1998;92:79–83. doi: 10.1016/S0928-4257(98)80142-3. [DOI] [PubMed] [Google Scholar]
- TIAN L., PRIOR C., DEMPSTER J., MARSHALL I.G. Nicotinic antagonist-produced frequency-dependent changes in acetylcholine release from rat motor nerve terminals. J. Physiol. 1994;476:517–529. doi: 10.1113/jphysiol.1994.sp020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIAN L., PRIOR C., DEMPSTER J., MARSHALL I.G. Hexamethonium- and methyllycaconitine-induced changes in acetylcholine release from rat motor nerve terminals. Br. J. Pharmacol. 1997;122:1025–1034. doi: 10.1038/sj.bjp.0701481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAN D.Q., AMAKI Y., OHTA Y., NAGASHIMA H., DUNCALF D., FOLDES F.F. Simultaneous in vivo measurement of neuromuscular block on three muscles. Anaesthesiology. 1982;57:A276. [Google Scholar]
- TSETLIN V. Snake venom alpha-neurotoxins and other ‘three-finger' proteins. Eur. J. Biochem. 1999;264:281–286. doi: 10.1046/j.1432-1327.1999.00623.x. [DOI] [PubMed] [Google Scholar]
- TYLER M.I., SPENCE I., BARNETT D., HOWDEN M.E.H. Pseudonajatoxin b: unusual amino acid sequence of a lethal neurotoxin from the venom of the Australian common brown snake, Pseudonaja textiles. Eur. J. Biochem. 1987;166:139–143. doi: 10.1111/j.1432-1033.1987.tb13493.x. [DOI] [PubMed] [Google Scholar]
- UTKIN Y.N., KUKHTINA V.V., KRYUKOVA E.V., CHIODINI F., BERTRAND D., METHFESSEL C., TSETLIN V.I. ‘Weak toxin' from Naja kaouthia is a nontoxic antagonist of α7 and muscle-type nicotinic acetylcholine receptors. J. Biol. Chem. 2001;276:15810–15815. doi: 10.1074/jbc.M100788200. [DOI] [PubMed] [Google Scholar]
- VIZI E.S., SOMOGYI G.T. Prejunctional modulation of acetylcholine release from the skeletal neuromuscular junction: link between positive (nicotinic)- and negative (muscarinic)-feedback modulation. Br. J. Pharmacol. 1989;97:65–70. doi: 10.1111/j.1476-5381.1989.tb11924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESSLER I. Acetylcholine at motor nerves: storage release, and presynaptic modulation by autoreceptors and adrenoreceptors. Int. Rev. Neurobiol. 1992;34:283–384. doi: 10.1016/s0074-7742(08)60100-2. [DOI] [PubMed] [Google Scholar]
- WESSLER I., HALANK M., RASBACH J., KILBINGER H. Presynaptic nicotinic receptors mediating a positive feedback on transmitter release from the rat phrenic nerve. Naunyn-Schmiedeberg's Arch. Pharmacol. 1986;334:365–372. doi: 10.1007/BF00569371. [DOI] [PubMed] [Google Scholar]
- WILSON D.F. Influence of presynaptic receptors on neuromuscular transmission in the rat. Am. J. Physiol. 1982;242:C366–C372. doi: 10.1152/ajpcell.1982.242.5.C366. [DOI] [PubMed] [Google Scholar]
- WILSON H.I., NICHOLSON G.M. Presynaptic snake β-neurotoxins produce tetanic fade and endplate potential run-down during neuromuscular blockade in mouse diaphragm. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:626–634. doi: 10.1007/pl00005099. [DOI] [PubMed] [Google Scholar]
- WOLTHUIS O., VANWERSCH R.A.P., VAN DER WIEL H.J. The efficacy of some bis-pyridinium oximes as antidotes to soma in isolated muscle of several species including man. Eur. J. Pharmacol. 1981;70:355–369. doi: 10.1016/0014-2999(81)90169-2. [DOI] [PubMed] [Google Scholar]