Abstract
The purpose of this study was to synthesize novel valproyltaurine (VTA) derivatives including valproyltaurinamide (VTD), N-methyl-valproyltaurinamide (M-VTD), N,N-dimethyl-valproyltaurinamide (DM-VTD) and N-isopropyl-valproyltaurinamide (I-VTD) and evaluate their structure–pharmacokinetic–pharmacodynamic relationships with respect to anticonvulsant activity and teratogenic potential. However, their hepatotoxic potential could not be evaluated. The metabolism and pharmacokinetics of these derivatives in mice were also studied.
VTA lacked anticonvulsant activity, but VTD, DM-VTD and I-VTD possessed anticonvulsant activity in the Frings audiogenic seizure susceptible mice (ED50 values of 52, 134 and 126 mg kg−1, respectively).
VTA did not have any adverse effect on the reproductive outcome in the Swiss Vancouver/Fnn mice following a single i.p. injection of 600 mg kg−1 on gestational day (GD) 8.5. VTD (600 mg kg−1 at GD 8.5) produced an increase in embryolethality, but unlike valproic acid, it did not induce congenital malformations. DM-VTD and I-VTD (600 mg kg−1 at GD 8.5) produced a significant increase in the incidence of gross malformations. The incidence of birth defects increased when the length of the alkyl substituent or the degree of N-alkylation increased.
In mice, N-alkylated VTDs underwent metabolic N-dealkylation to VTD. DM-VTD was first biotransformed to M-VTD and subsequently to VTD. I-VTD's fraction metabolized to VTD was 29%. The observed metabolic pathways suggest that active metabolites may contribute to the anticonvulsant activity of the N-alkylated VTDs and reactive intermediates may be formed during their metabolism. In mice, VTD had five to 10 times lower clearance (CL), and three times longer half-life than I-VTD and DM-VTD, making it a more attractive compound than DM-VTD and I-VTD for further development. VTD's extent of brain penetration was only half that observed for the N-alkylated taurinamides suggesting that it has a higher intrinsic activity that DM-VTD and I-VTD.
In conclusion, from this series of compounds, although VTD caused embryolethality, this compound emerged as the most promising new antiepileptic drug, having a preclinical spectrum characterized by the highest anticonvulsant potential, lowest potential for teratogenicity and favorable pharmacokinetics.
Keywords: Valproic acid, taurine, valproyltaurinamides, anticonvulsant activity, audiogenic seizures, teratogenicity, pharmacokinetics, pharmacokinetics in brain, metabolism
Introduction
Valproic acid (VPA, Figure 1) is an efficacious first-line antiepileptic drug, useful against a variety of epileptic seizure types, including generalized and partial seizures, absence seizures and myoclonus (Perucca, 2002). The use of VPA is, however, limited by two rare but severe adverse effects, teratogenicity (1–2% incidence of spina bifida in exposed embryos) and hepatotoxicity (overall incidence 1 in 10,000). Maternal use of VPA is associated with an increased risk of neural tube defects (NTDs) and other congenital malformations (Lammer et al., 1987; Lindhout & Omtzigt, 1994; Kaneko et al., 1999). Empirical studies have shown that there are three entities in the VPA molecule that are necessary for the teratogenic properties of a VPA analog: (1) a carboxylic acid; (2) a hydrogen atom at C-2 (the α position to the carbonyl) and (3) branching at the C-2 position with at least two carbon atoms at each side chain (Nau et al., 1991; Bojic et al., 1998). Should any one of these requirements not be met, a VPA analog would not be expected to exert any teratogenic effects. Unlike teratogenicity, that is related to the parent compound (Nau, 1986; Nau and Hendrickx, 1987; Bojic et al., 1998), the hepatotoxicity of VPA is the result of VPA metabolite(s) with a terminal double bond, which depletes hepatic glutathione (GSH) and acyl-CoA (Tang et al., 1995; Grillo et al., 2001). The widespread use of VPA in neurology and psychiatry, its efficacy in epilepsy treatment and these two severe adverse events associated with its therapy, provide an excellent incentive for developing a second-generation VPA that would be a broad-spectrum antiepileptic and CNS drug, but without the teratogenic and hepatotoxic side effects (Isoherranen et al., 2003a).
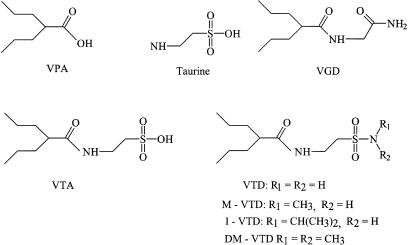
Figure 1.
Chemical structures of VPA (molecular weight (MW)=144), taurine, VTA (MW=273), VGD (valrocemide-TV-1901, MW=190), VTD (MW=250), M-VTD (MW=264), DM-VTD (MW=278) and I-VTD (MW=292).
Taurine (Figure 1), together with GABA and glycine, is one of the major inhibitory neurotransmitters found in the brain (Bonhaus et al., 1983; Kuriyama & Hashimoto, 1998). Brain taurine levels are reduced during metrazole-induced seizures (Li et al., 2000) and following amygdala kindling (Loscher et al., 1993), suggesting that decrease in taurine levels may be involved in epilepsy. In addition, the new AEDs, levetiracetam and lamotrigine, both increase taurine levels in the brain (Hassel et al., 2001; Tong & Patsalos, 2001). This may be related to one of their mechanisms of antiepileptic action. In epileptic patients, plasma and urinary taurine levels are reduced (Goodman et al., 1989; Hartley et al., 1989). Taurine is also an active anticonvulsant in a variety of animal models and in some epileptic patients (Bonhaus et al., 1983). In the past, structure–activity relation (SAR) studies have been performed on taurine, in order to create a CNS-active taurine derivative that would penetrate the brain better than taurine itself. Of these derivatives, γ-glutamyltaurine showed potent and long-lasting antiepileptic action in kindled rats (Uemura et al., 1992). The most successful of the taurine derivatives was taltrimide (phtalimidoethanesulfo-N-isopropylamide), that reached phase II clinical trials in epileptic patients, but development was stopped possibly because of the pharmacokinetic interactions with other AEDs and lack of efficacy (Kontro et al., 1983; Koivisto et al., 1986; Keranen et al., 1987).
In earlier studies, conjugation products of VPA, with glycine, GABA and gabamide, were found to be ineffective when used as anticonvulsants, whereas valproylglycinamide (VGD, valrocemide, TV1901) possessed significant anticonvulsant activity (Hadad & Bialer, 1995). Valrocemide (Figure 1) displays a broad spectrum of anticonvulsant activity in animal models, including efficacy in well-accepted standardized models of partial and generalized seizures (Isoherranen et al., 2001). This compound is currently in phase II clinical development (Bialer et al., 2002). As follow-up compounds to valrocemide, its N-alkyl derivatives were synthesized (Spiegelstein et al., 1999b). In preliminary anticonvulsant screening, these compounds were either inactive or less potent than valrocemide (Spiegelstein et al., 1999b).
The aims of this study were: (a) to synthesize a series of novel, lipophilic valproyltaurine (VTA) derivatives (Figure 1) that would cross the blood brain barrier (BBB), share the anticonvulsant activities of VPA and taurine, and act as drugs in their own right and not as prodrugs to VPA and/or taurine, (b) to characterize their anticonvulsant activity and document any teratogenic potential in comparison to VPA and valrocemide, and (c) to investigate the pharmacokinetics (PK), lipophilicity and metabolic pathways of the above derivatives and consequently to establish their pharmacokinetic–pharmacodynamic (PK–PD) relationships.
Methods
Chemicals
For the chemical syntheses, the organic solvents were obtained from Frutarom, Israel, and were of analytical grade. All solvents used for chemical analyses were of analytical grade, and were purchased from J.T. Baker, The Netherlands. Double-distilled water was used throughout the study.
Chemical syntheses
The tested VTA derivatives and the chromatographic internal standard were synthesized using previously described methods (Isoherranen et al., 2003b). In brief, valproyl chloride was added to a basic aqueous solution of taurine. The resulting product, VTA, was isolated and the sulfonic acid moiety converted to sulfonylchloride using SOCl2. After distillation of the excess of the SOCl2, it was added to the aqueous solution of a suitable amine. The conjugation products were isolated and identified by elemental microanalysis and nuclear magnetic resonance spectroscopy.
Evaluation of anticonvulsant activity
For the anticonvulsant testing, the VTA derivatives were suspended in 0.5% methylcellulose and administered intraperitoneally (i.p.) in a volume of 0.01 ml g−1 body weight to the test animals. The anticonvulsant activity of the VTA derivatives was evaluated in both male and female Frings audiogenic seizure (AGS) susceptible mice, which were obtained from a breeding colony maintained by the animal resource center at the University of Utah. An 11 kHz, 20 s sound stimulus was used to induce the seizures, which are characterized by wild running, clonus and forelimb and hindlimb tonic extension (White et al., 1992). The severity of seizures was evaluated at 15, 30, 60, 120 and 240 min following i.p. drug administration, in order to determine the time of peak effect. The seizure severity was quantified by assigning a numerical score to the response according to the following criteria: stage 0, no response; stage 1, wild running for <10 s; stage 2, wild running for >10 s; stage 3, clonic seizure; stage 4, forelimb extension and hindlimb flexion; stage 5, tonic seizure (White et al., 1992). In order to determine the median effective dose for the test compounds, the dose of each of the compounds was varied until at least four points were established between the dose level providing 0% protection, and that providing 100% protection, as determined at the time of peak effect. Animals not displaying a tonic hindlimb extension were considered protected. The resulting data were then subjected to probit analysis (Finney, 1971), and the ED50 and 95% confidence interval (CI) were calculated. Any animal protected from the audiogenic seizures after drug treatment was challenged again 24 h after drug administration for tonic extension seizures. Animals not showing a stage 5 seizure at this time were excluded from the study. Historical data from the anticonvulsant screening project at the University of Utah have shown that vehicle administration does not have an effect on the audiogenic seizures.
Evaluation of teratogenicity
The ability of the VTA derivatives to induce NTDs was evaluated in the Swiss Vancouver (SWV)/Fnn mice. The mice were maintained in a breeding colony at the Institute of Biosciences and Technology, Texas A&M University System Health Science Center. Virgin females were mated overnight and were examined for the presence of vaginal plugs the following morning. The beginning of gestation (day 0, hour 0) was set at 10 p.m. of the previous evening, based upon the likely time of ovulation (Snell et al., 1948). Groups of at least six dams were randomly assigned to one of the following treatments: 600 mg kg−1 (2.20 mmol kg−1) VTA, 600 mg kg−1 (2.40 mmol kg−1) valproyltaurinamide (VTD), 600 mg kg−1 (2.15 mmol kg−1) N,N-dimethyl valproyltaurinamide (DM-VTD), 600 mg kg−1 (2.27 mmol kg−1) N-methyl valproyltaurinamide (M-VTD), 600 mg kg−1 (2.05 mmol kg−1) N-isopropyl valproyltaurinamide (I-VTD), 600 mg kg−1 (4.16 mmol kg−1) VPA in water or in chremophor EL (CEL) and vehicle control groups. At gestational day (GD) 8.5, a sensitive time point for neural tube closure, a single dose of the test compound dissolved in 25% CEL (polyether of castor oil and ethylene, Fluka, Switzerland) was administered by i.p. injection. For the M-VTD administered dams, a single blood sample was drawn 20 min after the drug administration in order to confirm drug exposure and possible metabolite formation.
At GD 18.5, the dams were killed by cervical dislocation, the abdomen opened and the uterine contents removed. The locations of all viable fetuses and resorption sites were recorded, and the fetuses were grossly examined for the presence of exencephaly, as well as other neural tube and associated gross malformations.
PK studies in mice
The PK of VTA, VTD, DM-VTD and I-VTD were studied in SWV/Fnn mice following a single i.p. injected dose of 300 mg kg−1 of the test compounds dissolved in 25% CEL. Male SWV mice weighing 22–30 g were randomly assigned to one of the four treatment groups. Three mice were killed at each of the following time points and blood samples (0.3 ml) were collected via the retro-orbital sinus at 5, 10, 20, 40, 60, 120, 180, 240, 300 and 360 min postinjection. The blood was centrifuged at 3000 × g for 5 min; the plasma separated, and stored at −20°C until analyzed. From the same mice, the brain was quickly removed, snap-frozen in liquid nitrogen and stored at −20°C until analyzed.
Analysis of the concentrations of VTDs
The analytical method used in these experiments has been described in detail in a separate publication (Isoherranen et al., 2003b). All analyzed concentrations in biological matrices were within the concentration range of the calibration curve. A Hewlett-Packard HP 5890 Series II gas chromatograph equipped with a 6860 autosampler, HP5971 mass selective detector and HPChemstation data analysis software was used. The chromatographic separation was obtained with the aid of a Quadrex 007 methyl 20% phenyl silicone capillary column (25 m, 0.25 μm film thickness, 0.25 mm i.d.; Quadrex Corp., New Haven, CT, U.S.A.) using the following temperature program: 2 min at an initial temperature of 50°C, a gradient of 40°C min−1 up to 180°C, hold time of 1 min and a second gradient of 0.5°C min−1 up to 192°C and then a third gradient of 10°C min−1 up to 220°C, which was maintained for 1 min. Injector temperature was maintained at 220°C, while the transfer line temperature was 280°C. Ions of m/z 162, 179, 208, 221, 234 and 264 were monitored for quantification. He (99.999%) with a 50 kPi head pressure was used as a carrier gas.
Plasma concentrations
To 50 μl of mouse plasma, 20 μl of the internal standard solution (N,N-diethyl-valproyltaurinamide in methanol) and 0.5 ml of water was added. The solid-phase extraction (SPE) cartridges (3 ml, 500 mg, Extra-Sep C18, Lida, Kenosha, WI, U.S.A.) were conditioned using 2 ml methanol followed by 1 ml of water. The sample was added and the cartridge was washed using 1 ml water. The analytes were eluted using 1 ml methanol, which was subsequently evaporated using a vortex evaporator. The dry residue was redissolved in 0.2 ml chloroform, and 1 μl was injected into the gas chromatograph. Calibration curves were prepared separately for all the analyzed compounds at a concentration range of 1–200 mg l−1. Additionally, for VTD only, the concentration range of the calibration curve was expanded between 100 and 500 mg l−1. The method was validated (Isoherranen et al., 2003b) according to published guidelines (Shah et al., 2000).
Brain concentrations
To 50 mg of brain tissue, 20 μl of the internal standard solution (100 mg l−1 N,N-diethyl-valproyltaurinamide in methanol) and 2 ml of 2.5 M acetate buffer (pH 2.5) were added. The SPE cartridges were conditioned as described above, and the sample was added. The cartridge was washed using 1 ml water, and the analytes were eluted using 1 ml methanol followed by centrifugation. The methanol phase was evaporated to dryness using a vortex evaporator and the dry residue was redissolved in 0.2 ml chloroform and 1 μl was injected into the gas chromatograph. Calibration curves were prepared separately for all the analyzed compounds at a concentration range 0.5–200 mg kg−1. The method was validated according to published guidelines (Shah et al., 2000), and was found to have a precision of <15% at brain concentrations of 1, 2.5, 25, 50, 100 and 150 mg kg−1 and an accuracy of 86–114% at the same brain concentrations.
Urine concentrations
To 50 μl of mouse urine, 200 μl of water and 20 μl of internal standard solution (DE-VTD 1 mg ml−1) were added. The mixture was extracted using 0.5 ml chloroform, the phases separated by centrifugation and the chloroform phase evaporated to dryness. The dry residue was reconstituted in 0.25 ml of chloroform and injected into the gas chromatograph. The calibration curve was prepared at a concentration range of 25–1500 mg l−1 and the accuracy and precision were within the recommended limits (Shah et al., 2000).
Analysis of VTA in plasma
A Shimadzu model 10AT HPLC system equipped with SPD-10A UV–Vis detector, SCL-10A system controller and SIL 10 Axl autosampler were used for these analyses. The separation was obtained using a Lichrospher 250-4 RP-18 column with a particle size of 5 μm, and a mobile phase of 30% acetonitrile and 70% 0.75 mM phosphate buffer titrated to pH 2.5 using triethylamine. A flow rate of 0.7 ml min−1 was used. To 50 μl of mouse plasma, 1 ml of methanol was added, the mixture was vigorously vortexed and centrifuged at 2000 × g. The methanol phase was separated and evaporated to dryness in a vortex evaporator. The dry residue was redissolved to 50 μl of methanol and 30 μl was injected to the HPLC system. Calibration curves were prepared at a concentration range of 10–500 mg l−1 and the method was validated according to published guidelines (Shah et al., 2000) and met the criteria set for bioanalytical methods.
Determination of lipophilicity of the VTDs
The lipophilicity of VTD, M-VTD, DM-VTD and I-VTD was evaluated by use of log P-values as determined by their water–octanol partitioning. Each compound was analyzed at two different concentrations, and three replicates were evaluated at each concentration. The test compounds (0.100 or 0.250 mg) were added to a test tube and dissolved in 1 ml aqueous potassium phosphate buffer at pH 7.4. Octanol (1 ml) was added and the test tube was stirred vigorously. Mixing was continued at 37°C for 1 h, at which point a 0.5 ml sample was taken for analysis from the water phase and 50 μl of internal standard solution (DE-VTD at 0.1 mg ml−1) was added. The buffer phase was extracted with 2 ml chloroform. A 1 μl sample was injected to the gas chromatograph from the chloroform phase and the sample analyzed using the above-described chromatographic method.
PK calculations
PK parameters were calculated, assuming complete bioavailability following i.p. administration, by the classical noncompartmental methods based on the statistical moment theory (Gibaldi & Perrier, 1982). The plasma and brain concentrations for each time point were obtained as an arithmetic mean of three individual mice. The area under the plasma or brain concentration versus time curve (AUC) was calculated by the linear trapezoidal method with extrapolation to infinity. The mean residence time of the compounds in plasma or brain was calculated from the quotient AUMC/AUC, where AUMC is the area under concentration time product versus time curve from zero to infinity. The clearance (CL) was calculated from the quotient dose/AUC and the volume of distribution (Vβ) was calculated from the quotient D/AUC*β−1, in which β is obtained from the linear fit of the log plasma concentration versus time curve. The terminal half-life (t1/2) was calculated from the quotient ln 2/β. The fraction metabolized (fm) of I-VTD to VTD, was calculated from the quotient AUCMD/AUCM in which AUCM is the AUC of the metabolite following its administration and AUCMD is the AUC of the metabolite after administration of the drug. The AUC was normalized to the dose and molecular weight of the drug and the metabolite. The fm of DM-VTD to VTD was calculated from urine data by dividing the fraction of the drug excreted as the metabolite by the fraction of the metabolite excreted in urine after its administration. Renal clearance (CLr) was calculated from the quotient of cumulative amount excreted unchanged in the urine (Ae) over plasma AUC, and the fraction excreted unchanged (fe) was calculated from the ratio between Ae and dose.
Statistical analysis
Results of the anticonvulsant activity are presented as ED50 values and 95% CIs. Significance in the reduction of seizure score in the Frings mice was evaluated by ANOVA and Dunnett's test as a post hoc test. The reproductive outcomes (resorption rates and the incidence of gross malformations) were evaluated using arcsine transformation and χ2 analysis with a Tukey-type q-test. A P-value <0.05 was considered statistically significant.
Results
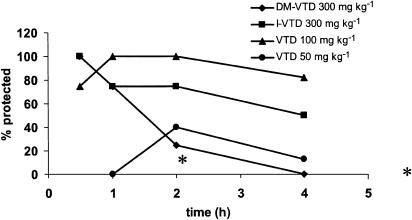
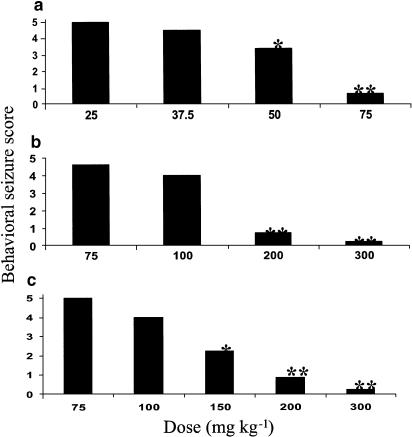
The ability of the VTDs to block sensory-evoked generalized seizures was evaluated in the Frings AGS mice. The Frings mouse is a reliable model of generalized epilepsy and a genetic model of reflex epilepsy (Rogers et al., 1998; Skradski et al., 2001). The mutation causing the Frings mice to be susceptible to the audiogenic seizures has been linked to a similar mutation in humans causing febrile seizures (Nakayama et al., 2002). The time course of the anticonvulsant effect of VTD, DM-VTD and I-VTD in the Frings mice is shown in Figure 2. As shown in Figure 2, all the three compounds were active against sound-induced seizures at the doses tested. In contrast, VTA was inactive at doses of 300 mg kg−1. In terms of the duration of action, VTD and I-VTD both continued to show good anticonvulsant activity at 4 h. Table 1 shows the quantitative results with respect to anticonvulsant activity in comparison to VPA and VGD. VTD was the most potent compound among the derivatives tested. It was 2.5 to three times more potent than I-VTD, DM-VTD and VPA (five times more potent than VPA on molar basis) but equipotent with VGD (slightly more potent than VGD on molar basis). All of the compounds were active at doses that showed no behavioral impairment. VTA was inactive in this model throughout the entire time interval that was investigated. As can be seen from the results summarized in Figure 3, VTD, DM-VTD and I-VTD all reduced the seizure score from 5 to <1 in a dose-dependent manner. Therefore, all the three compounds were effective in preventing tonic extension, clonus and wild running.
Figure 2.
Time course of the anticonvulsant effect in the Frings mice following i.p. administration of two doses of VTD (a), I-VTD (b) and DM-VTD (c). *Indicates significant difference in seizure protection when compared to the response at 30 min.
Table 1.
Anticonvulsant activity of the valproyltaurinamides in the Frings mice
| Compound | ED50 (mg kg−1) (95% CI) | Time of test (h)a |
|---|---|---|
| VTA | >300 | 0.25–4 |
| VTD | 52 (43–62) | 2 |
| I-VTD | 126 (96–174) | 0.5 |
| DM-VTD | 134 (104–168) | 0.5 |
| VPAb | 155 (110–216) | 0.25 |
| VGDc | 52 (39–64) | 0.5 |
Also, the time of peak anticonvulsant effect.
Data from White et al. (2002).
Data from Isoherranen et al. (2001).
Figure 3.
Dose-dependent reduction in the seizure score in the Frings audiogenic seizure susceptible mice following i.p. administration of VTD (a), I-VTD (b) and DM-VTD (c). * and ** indicate significant differences with P<0.05 and P<0.01 respectively from the lowest dose values (ANOVA and Dunnett's test).
The ability of the VTA derivatives to induce NTDs and other gross malformations was evaluated in the SWV/Fnn mice, which are known to be highly sensitive to AED-induced teratogenicity (Bennett et al., 1997; Finnell et al., 1988; 1995; Wlodarczyk et al., 1996). The effect of a single dose of the VTDs, during the period of neural tube closure, on the reproductive outcome in the SWV/Fnn mice is summarized in Table 2. Comparisons of the results obtained with the VTDs are made to VPA, valrocemide and valproylglycine (Table 2). The teratogenic potential of the VTDs increased with the increase in the degree of alkylation in the sulfonamide (DM-VTD>I-VTD>VTD). The most teratogenic compound was DM-VTD, followed by I-VTD. Nonetheless, DM-VTD and I-VTD were still less teratogenic than VPA in this model. M-VTD had a very low teratogenic potential with only 1% of the live-born fetuses (not significant; P>0.05) expressing NTDs. VTD and VTA were completely free from any indication of inducing external malformations. However, the resorption rates were significantly increased (P<0.01) following a single dose of VTD, DM-VTD and I-VTD at GD 8.5, indicating that these compounds may be embryotoxic.
Table 2.
Reproductive outcome in the SWV/Fnn mice following administration of 600 mg kg−1 of the valproyltaurine derivatives at GD 8.5
| Compound | Litters (N) | Implants (N) | Resorptions (N) (%) | Live births (N) | Malformed (N) (%) |
|---|---|---|---|---|---|
| VTA | 10 | 132 | 11 (8) | 121 | 0 |
| VTD | 9 | 108 | 27 (25)** | 81 | 0 |
| M-VTDa | 9 | 116 | 6 (5) | 110 | 1 (0.9) |
| DM-VTD | 9 | 130 | 45 (35)** | 85 | 14 (17)** |
| I-VTD | 8 | 104 | 23 (22)** | 81 | 6 (7)** |
| VPA (in H2O)b | 10 | 120 | 5 (4) | 115 | 59 (82)** |
| VPA (in CEL)a | 12 | 152 | 3d (2) | 119 | 89 (75)** |
| VPA (in H2O)a | 18 | 237 | 50b (21) | 181 | 109 (60)** |
| CEL controla | 10 | 120 | 5 (4) | 115 | 0 |
| CEL controla | 16 | 212 | 14f (7) | 196 | 0 |
| H2O controla | 12 | 152 | 3 (2) | 146 | 0 |
| VGDc | 15 | 178 | 1 (1) | 177 | 0 |
| VGAc | 7 | 97 | 6 (6) | 91 | 1 (1) |
| VPAc | 13 | 148 | 13 (9) | 135 | 99 (73)** |
| H2O controlc | 8 | 123 | 1 (1) | 122 | 0 |
Control for M-VTD treatment group
control for all other groups except M-VTD
VPA and H2O respective controls
Three stillborn fetuses
six stillborn fetuses
three stillborn fetuses
Significantly different from the respective vehicle-treated control group, P<0.05. VTA–valproyltaurine, VTD–valproyltaurinamide, M-VTD–N-methyl-valproyltaurinamide, DM-VTD–N,N-dimethyl-valproyltaurinamide, I-VTD–N-isopropyl valproyltaurinamide, VPA–valproic acid, CEL–cremophor EL.
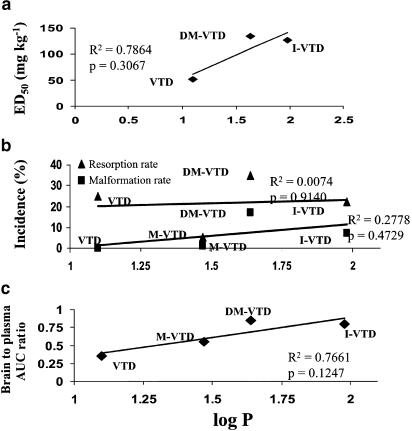
The anticonvulsant activity of the investigated VTDs may be because of the active metabolites, and formation of reactive metabolites is one of the possible suggested mechanisms of antiepileptic drug (AED) teratogenicity (Bennett et al., 1997; Wells et al., 1997; Parman et al., 1998). In order to evaluate the possible contribution of metabolites to the differences observed in the anticonvulsant and teratogenic potency of these compounds, a PK study was undertaken. In addition, the lipophilicity (log P) of the experimental compounds was determined (Table 3). No significant correlation was found between the lipophilicity of the drug and their teratogenic potency, as shown in Figure 4b. The greater lipophilicity of DM-VTD and I-VTD may facilitate their better penetration to the fetus, but the two-fold increase in the incidence of NTDs between I-VTD and DM-VTD and the lack of NTDs after VTD administration cannot be explained by lone differences in drug lipophilicity. There was also no positive correlation between the potency of the VTDs and their log P-values, as the compound with lowest log P (lowest lipophilicity) turned out to be the most potent. However, a correlation appeared to be between the brain-to-plasma AUC ratios and log P (Figure 4c).
Table 3.
Lipophilicity profile of the valproyltaurinamides (VTD, M-VTD, DM-VTD and I-VTD)
| log P at 100 mg l−1 | log P at 250 mg l−1 | log P | |
|---|---|---|---|
| VTD | 1.05±0.16 | 1.13±0.10 | 1.1 |
| M-VTD | 1.46±0.02 | 1.47±0.05 | 1.47 |
| DM-VTD | 1.61±0.02 | 1.67±0.04 | 1.64 |
| I-VTD | 2.00±0.03 | 1.95±0.03 | 1.98 |
Figure 4.
Correlation between log P and anticonvulsant activity (a), teratogenic potency (b) and brain penetration as expressed by brain to plasma AUC ratio (c).
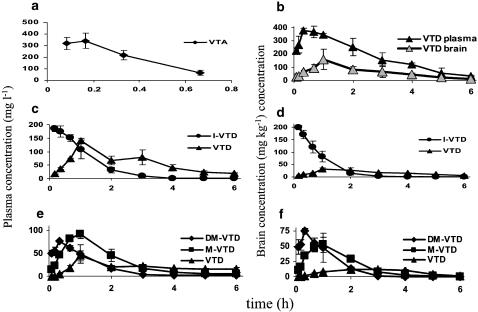
The plasma and brain concentration versus time curves of the taurine derivatives of VPA and their selected metabolites are shown in Figure 5. The analyzed taurine derivatives of VPA differed significantly in their PK, as shown by the obtained PK parameters in Table 4. High CL, very short half-life, and relatively small volume of distribution characterized the PK of VTA. The brain concentrations of VTA were not analyzed because of the lack of a suitable analytical method, and very low VTA brain concentrations. VTD had the lowest CL, and consequently, a longest half-life of all tested compounds. Approximately half of VTD's CL was through the renal route (fe=39%) and therefore, based on the additive nature of clearances, approximately half of the total body CL of VTD is via the hepatic route (metabolic CL). VTD's metabolic CL equals about 2.5% of the hepatic blood flow in the mouse and therefore, VTD has a low hepatic extraction ratio and is not likely to be subject to hepatic first pass effect following oral dosing. I-VTD had a five times higher CL than VTD (one-fourth of the liver blood flow) and DM-VTD had twice as high CL as I-VTD. As DM-VTD was hardly cleared by the kidney (CLr=0.09 ml h−1), majority of its CL constitutes of metabolic CL. DM-VTD's metabolic CL was approximately half of the liver blood flow in mice. As such DM-VTD and I-VTD are likely to be subjected to hepatic first pass effect, DM-VTD and I-VTD had nearly equal half-lives (0.5 h), as the V and CL of DM-VTD were both 2.5-fold higher than that of I-VTD. The mean residence time (MRT) values of DM-VTD, I-VTD and M-VTD were 20–30% shorter in the brain than in the plasma, whereas VTD had a slightly longer MRT in the brain.
Figure 5.
Brain (mg kg−1) and plasma (mg l−1) concentration versus time courses of the VTA derivatives and their metabolites: (a) plasma concentration versus time course of VTA, brain concentrations were not measured for VTA; (b) plasma and brain concentration versus time curves of VTD in mg l−1 and mg kg−1 respectively; (c) plasma and (d) brain concentration versus time curves of I-VTD and VTD following administration of I-VTD and (e) plasma and (f) brain concentration versus time curves of DM-VTD, M-VTD and VTD following administration of DM-VTD. All compounds were administered at 300 mg kg−1 i.p.
Table 4.
The pharmacokinetic parameters obtained for the studied valproyltaurinamides in SWV/Fnn mice
| VTA | VTD | DM-VTD | I-VTD | |
|---|---|---|---|---|
| CL (L h−1 kg−1) | 2.03 | 0.26 | 2.7 | 1.26 |
| Vβ (L kg−1) | 0.57 | 0.54 | 2.5 | 1.00 |
| t1/2 (h) | 0.19 | 1.44 | 0.63 | 0.55 |
| MRT (h) | 0.33 | 2.33 | 1.2 | 0.94 |
| AUCplasma (mg l−1 h) | 148 | 1159 | 110 | 238 |
| tmax (h) | 0.17 | 0.33 | 0.33 | 0.17 |
| Cmax (mg l−1) | 338 | 375 | 76.4 | 185 |
| AUCbrain (mg kg−1 h−1) | 407 | 93 | 187 | |
| t1/2brain (h) | 1.4 | 0.5 | 0.52 | |
| MRTbrain (h) | 2.5 | 0.89 | 0.74 | |
| tmax brain (h) | 1.0 | 0.33 | 0.17 | |
| Cmax brain (mg l−1) | 159 | 74.4 | 199 | |
| Brain to plasma AUC ratio | 0.35 | 0.85 | 0.79 | |
| CLr (ml h−1) | 2.9 | 0.1 | ||
| fe | 39 | <0.5% |
CL–clearance; Vβ–volume of distribution during the β phase; t1/2–half life; MRT–mean residence time; AUC–area under concentration curve; tmax–time to reach maximal concentration; Cmax–maximal concentration; CLr–renal clearance; fe–fraction of the dose excreted unchanged.
N-dealkylation was an observed metabolic pathway for the VTDs. DM-VTD was metabolized to M-VTD and subsequently to VTD (fm to VTD was 10% based on urine data). Following administration of DM-VTD, less than 0.5% of the dose was recovered in urine as DM-VTD, with 1.7% excreted as M-VTD and 3.9% as VTD. As for I-VTD, the N-dealkylation was a significant metabolic pathway (fm to VTD based on plasma AUCs was 28%). The PK parameters obtained for selected metabolites are shown in Table 5. VTD had a much larger CLr than the two other compounds, DM-VTD and I-VTD, and consequently a higher fraction of the dose excreted unchanged (fe=39%). The PK parameters obtained for M-VTD and VTD as metabolites of DM-VTD and for VTD after I-VTD administration suggested that their CL as metabolites is elimination rate-limited, that is, the formation CL of these metabolites is higher than their elimination CL and therefore, the metabolites reside in the body longer than their parent compound. The rate-limiting elimination of the metabolites (M-VTD and VTD) could be observed in their MRT and t1/2 values that were longer than the MRT and t1/2 of DM-VTD or I-VTD.
Table 5.
Pharmacokinetic parameters of valproyltaurinamide and N-methyl-valproyltaurinamide as metabolites of the investigated N-alkyl-valproyltaurinamides
| PK parameter | VTD from DM-VTD | M-VTD from DM-VTD | VTD from I-VTD |
|---|---|---|---|
| t1/2 metabolite (h) | 3.6 | 0.83 | 1.43 |
| MRTmetabolite (h) | 6.1 | 1.83 | 2.90 |
| AUCplasma (mg l−1 h) | 191 | 188 | 386 |
| AUCbrain (mg kg−1 h) | 46.7 | 103 | 115 |
| MRTbrain (h) | 2.9 | 1.45 | 3.4 |
| AUCbrain/AUCplasma | 0.24 | 0.55 | 0.30 |
| CLr (ml h−1) | 1.5 | 0.69 | |
| fem | 3.9 | 1.7 |
t1/2 metabolite–half-life of the metabolite; MRT–mean residence time; AUC–area under concentration curve; CLr–renal clearance; fem–fraction of the dose excreted in urine as the specific metabolite.
Discussion
The purpose of this study was to develop new CNS-active VTA derivatives that would cross the BBB, possess anticonvulsant properties and would be free of any teratogenic potential. Three of the four VTDs tested, VTD, I-VTD and DM-VTD (Figure 1), displayed in the Frings mouse model of reflex seizures equivalent or greater potency than VPA. However, DM-VTD and I-VTD also induced a significant rate of NTDs. In the Frings mice, VTD was 2.5 to three times more potent than VPA, I-VTD and DM-VTD, but equipotent with VGD. DM-VTD and I-VTD displayed a peak anticonvulsant effect during the first 30 min after their administration. In contrast, the time of peak effect for VTD was 2 h after dosing. All of the tested VTDs displayed anticonvulsant activity at various time points between 15 and 240 min after dosing (Figure 2). The difference between the times of peak effect of VTD and the N-alkylated taurinamides (2 h versus 30 min) may be explained by their PK in brain. VTD reached its maximal brain concentrations 60 min after dosing, whereas DM-VTD and I-VTD had a brain tmax of 20–40 min. After reaching the Cmax, VTD concentrations declined much slower than did the other two compounds. However, the better potency of VTD is unlikely to be solely a result of different PK, as I-VTD had a similar brain Cmax as VTD. DM-VTD, despite its equal potency with I-VTD, had a brain Cmax half of that of I-VTD.
Despite the existence of active metabolites, there was a good correlation between the brain concentration of N-alkyl-VTD and its anticonvulsant effect. Still, the possibility that metabolites of I-VTD and DM-VTD (i.e. M-VTD and VTD) contribute to their anticonvulsant activity and prolonged efficacy cannot be ruled out. For example, the weak correlation between the log P and ED50 values may be partially because of the additional contribution of active metabolites. Both M-VTD and VTD were detected in the mouse brain at significant concentrations following DM-VTD administration, and VTD was also detected in the mouse brain after administration of I-VTD (Figure 5).
The fact that no correlation was found between the obtained log P value and teratogenic potency of DM-VTD and I-VTD, suggests that their induced teratogenicity is more likely a response to the formation of reactive intermediates or metabolites and/or specific receptor interactions, than the result of better partitioning to the embryo. It should be mentioned that the two- to five-fold higher plasma concentrations of VTD would allow an embryonic exposure comparable to that which occurs after DM-VTD and I-VTD administration with much lower partitioning. DM-VTD's higher teratogenic potency may involve reactive metabolic intermediates. The lack of teratogenicity by VTD, an analog that does not undergo metabolic N-dealkylation, would further support this hypothesis. Reactive metabolites and/or intermediates and free radicals have been previously shown to contribute to the teratogenicity of carbamazepine, phenytoin (Finnell et al., 1995; Parman et al., 1998) and ethanol (Kotch et al., 1995) and to general chemical teratogenesis (Juchau et al., 1992).
Simple amide derivatives of VPA have been found to be nonteratogenic in mice (Radatz et al., 1998; Spiegelstein et al., 1999a). The contribution of substituents of hydrogens on the nitrogen of a VPA amide derivative was not, however, evaluated. In this study, the teratogenicity of taurine and glycine derivatives of VPA was evaluated. Valproylglycine and VTA were free of teratogenic potential as were VTD and VGD (valrocemide). VTD, however, induced an increase in the resorption rates and therefore, it appears less safe during gestation than valrocemide. Teratogenic end points are: malformation, growth retardation, developmental delay and death. VPA induced an elevated rate of resorption and of the surviving fetuses; there is an extremely high rate of NTDs. With VTD, there is an increased resorption rate, but it is not as alarming when compared to VPA in the same animal model system. The alkyl substituents of hydrogens on the nitrogen of the sulfonamide of VTD to yield DM-VTD and I-VTD did, however, induce NTDs in mice and had significant embryotoxic potential. The fact that the N-alkyl amide derivatives of VPA, such as DM-VTD and I-VTD, induced NTD suggests that additional structural requirements may need to be evaluated in order to fully understand the SAR behind the teratogenicity of VPA derivatives. This study also suggests that despite the belief that VPA itself and not one of its metabolites is the teratogenic entity (Nau, 1986; Nau and Hendrickx, 1987; Bojic et al., 1998), with VPA derivatives metabolism may contribute to teratogenicity. When reliable animal models of hepatotoxicity become available, it will be interesting to investigate the possible hepatotoxic potential of the nonteratogenic VPA derivatives as well as the VTD derivatives presented in this study.
This study emphasizes the importance of monitoring serial brain concentrations (AUC) of analogous CNS-active compounds as part of any comprehensive program evaluating differences in potency, and conducting PK–PD calculations. VTD had a (plasma) Cmax that was twice as high as the (plasma) Cmax of I-VTD; yet, no differences were observed in their brain Cmax. Based solely on the plasma data, one could have misinterpreted that the greater potency of VTD is because of the higher effective concentration (i.e. Cmax). The overall AUC for VTD in plasma and brain, however, was twice as high as that of I-VTD and five to 10 times higher than those of DM-VTD, giving a partial explanation for VTD's better potency. The late time of peak effect of VTD was explained by the brain PK parameters but, without the PK data, it could wrongly be assumed that the late effect is because of active metabolites and not because of the parent drug itself.
This series of taurine derivatives of VPA and previous data on taltrimide (Kontro et al., 1983; Oja et al., 1983) can be compared to an analogous series of glycine derivatives. As previously observed with glycinamide derivatives such as phtaloylglycinamide (Abu Salach et al., 1994) and valrocemide (Spiegelstein et al., 1999b), the phthaloyl derivative of taurine (taltrimide) and VTD appeared the most promising derivatives as anticonvulsants. No difference in the anticonvulsant activity between VTD and VGD could be detected in the Frings mice. Unlike the N-alkyl derivatives of VGD, the N-alkyl derivatives of VTD demonstrated anticonvulsant activity, but they were less promising than VTD. The metabolic N-dealkylation of I-VTD is in agreement with previous data on taltrimide. Similar to I-VTD, taltrimide underwent metabolic N-dealkylation of the isopropyl moiety to a nonalkylated phthalyol taurinamide (Koivisto et al., 1986; Keranen et al., 1987).
In conclusion, from the studied compounds, VTD, although caused embryolethality, overall displayed the most promising preclinical spectrum because of its more potent anticonvulsant activity, lack of capacity to induce NTDs and favorable PK. However, it was not superior to VGD in terms of anticonvulsant activity or teratogenicity. The results of this study support further evaluation of VTD as a new AED to assess whether it is equally effective in other seizure and epilepsy models.
Abbreviations
- AED
antiepileptic drug
- AGS
audiogenic seizure susceptible
- AUC
area under concentration–time curve
- BBB
blood brain barrier
- CI
confidence interval
- CL
clearance
- CLr
renal clearance
- Cmax
maximal plasma or barin concentrations
- DM-VTD
N,N-dimethyl valproyltaurinamide
- ED50
mean effective dose
- fe
fraction excreted unchanged
- fm
fraction metabolized
- GSH
glutathione
- I-VTD
N-isopropyl valproyltaurinamide
- M-VTD
N-methyl valproyltaurinamide
- MRT
mean residence time
- NTD
neural tube defect
- PK
pharmacokinetics
- SAR
structure–activity relationships
- SWV
Swiss Vancouver
- t1/2
half life
- tmax
time to reach Cmax
- V
volume of distribution
- VGA
valproylglycine
- VGD
valproylglycinamide
- VPA
valproic acid
- VTA
valproyltaurine
- VTD
valproyltaurinamide
References
- ABU SALACH O., HADAD S., HAJ-YEHIA A., SUSSAN S., BIALER M. Comparative pharmacokinetic and pharmacodynamic analysis of phthaloyl glycine derivatives with potential antiepileptic activity. Pharm. Res. 1994;11:1429–1434. doi: 10.1023/a:1018943906510. [DOI] [PubMed] [Google Scholar]
- BENNETT G.D., LAU F., CALVIN J.A., FINNELL R.H. Phenytoin-induced teratogenesis: a molecular basis for the observed developmental delay during neurulation. Epilepsia. 1997;38:415–423. doi: 10.1111/j.1528-1157.1997.tb01730.x. [DOI] [PubMed] [Google Scholar]
- BIALER M., JOHANNESSEN S.I., KUPFERBERG H.J., LEVY R.H., LOISEAU P., PERUCCA E. Progress report on new antiepileptic drugs: a summary of the sixth EILAT conference on new antiepileptic drugs (EILAT VI) Epilepsy Res. 2002;51:31–71. doi: 10.1016/s0920-1211(02)00106-7. [DOI] [PubMed] [Google Scholar]
- BOJIC U., EHLERS K., ELLERBECK U., BACON C.L., O'DRISCOLL E., O'CONNELL C., BEREZIN V., KAWA A., LEPEKHIN E., BOCK E., REGAN C.M., NAU H. Studies on the teratogen pharmacophore of valproic acid analogues: evidence of interactions at a hydrophobic center. Eur. J. Pharmacol. 1998;354:289–299. doi: 10.1016/s0014-2999(98)00462-2. [DOI] [PubMed] [Google Scholar]
- BONHAUS D.W., LAIRD H., MIMAKI T., YAMAMURA H.I., HUXTABLE R.J.Possible base for anticonvulsant action of taurine Sulfur Amino Acids; Biochemical and Clinical Aspects 1983New York: Alan R. Liss; 195–209.ed. Kuriyama, K., Huxtable, R.J. & Iwata, H. pp [PubMed] [Google Scholar]
- FINNELL R.H., BENNETT G.D., KARRAS S.B., MOHL V.K. Common hierarchies of susceptibility to the induction of neural tube defects by valproic acid and its 4-propyl-4-pentenoic acid metabolite. Teratology. 1988;38:313–320. doi: 10.1002/tera.1420380403. [DOI] [PubMed] [Google Scholar]
- FINNELL R.H., BENNETT G.D., SLATTERY J.T., AMORE B.M., BAJPAJ M., LEVY R.H. Effect of treatment with phenobarbital and stiripentol on carbamazepine-induced teratogenicity and reactive metabolite formation. Teratology. 1995;52:324–332. doi: 10.1002/tera.1420520603. [DOI] [PubMed] [Google Scholar]
- FINNEY D.J. Probit Analysis 1971Cambridge, U.K.: Cambridge University Press; 3rd edn [Google Scholar]
- GIBALDI M., PERRIER D. Pharmacokinetics 1982New York: Marcel-Dekker; 2nd edn [Google Scholar]
- GOODMAN H.O., SHIHABI Z., OLES K.S. Antiepileptic drugs and plasma and platelet taurine in epilepsy. Epilepsia. 1989;30:201–207. doi: 10.1111/j.1528-1157.1989.tb05455.x. [DOI] [PubMed] [Google Scholar]
- GRILLO M.P., CHIELLINI G., TONELLI M., BENET L.Z. Effect of alpha-fluorination of valproic acid on valproyl-S-acyl-CoA formation in vivo in rats. Drug Metab. Dispos. 2001;29:1210–1215. [PubMed] [Google Scholar]
- HADAD S., BIALER M. Pharmacokinetic analysis and antiepileptic activity of N-valproyl derivatives of GABA and glycine. Pharm. Res. 1995;12:905–910. doi: 10.1023/a:1016277507865. [DOI] [PubMed] [Google Scholar]
- HARTLEY S.G., GOODMAN H.O., SHIHABI Z. Urinary excretion of taurine in epilepsy. Neurochem. Res. 1989;14:149–152. doi: 10.1007/BF00969630. [DOI] [PubMed] [Google Scholar]
- HASSEL B., TAUBOELL E., GJERSTAD L. Chronic lamotrigine treatment increases rat hippocampal GABA shunt activity and elevates cerebral taurine levels. Epilepsy Res. 2001;43:153–163. doi: 10.1016/s0920-1211(00)00196-0. [DOI] [PubMed] [Google Scholar]
- ISOHERRANEN N., WOODHEAD J., WHITE H.S., BIALER M. Anticonvulsant profile of valrocemide (TV1901): a new antiepileptic drug. Epilepsia. 2001;42:831–836. doi: 10.1046/j.1528-1157.2001.042007831.x. [DOI] [PubMed] [Google Scholar]
- ISOHERRANEN N., YAGEN B., BIALER M. New CNS-active drugs which are second-generation valproic acid: can they lead to the development of a magic bullet. Curr. Opin. Neurol. 2003a;16:203–211. doi: 10.1097/01.wco.0000063774.81810.30. [DOI] [PubMed] [Google Scholar]
- ISOHERRANEN N., YAGEN B., SPIEGELSTEIN O., STEINMAN A., FINNELL R.H., BIALER M. Gas chromatographic determination of novel valproyl taurinamide derivatives in mouse and dog plasma. J. Chromatogr. B. 2003b;788:125–136. doi: 10.1016/s1570-0232(02)01034-6. [DOI] [PubMed] [Google Scholar]
- JUCHAU M.R., LEE Q.P., FANTEL A.G. Xenobiotic biotransformation/bioactivation in organogenesis-stage conceptal tissues: implications for embryotoxicity and teratogenesis. Drug Metab. Rev. 1992;24:195–238. doi: 10.3109/03602539208996293. [DOI] [PubMed] [Google Scholar]
- KANEKO S., BATTINO D., ANDERMAN E., WADA K., KAN R., TAKEDA A., NAKANE Y., OGAWA Y., AVANZINI G., FUMAROLA C., GRANATA T., MOLTENI F., PARDI G., MINOTTI L., CANGER R., DANSKY L., OGUNI M., LOPES-CENDAS I., SHERWIN A., ANDERMAN F., SENI M.-H., OKADA M., TERANISHI T. Congenital malformations due to antiepileptic drugs. Epilepsy Res. 1999;33:145–158. doi: 10.1016/s0920-1211(98)00084-9. [DOI] [PubMed] [Google Scholar]
- KERANEN T., PARTANEN V.S.J., KOIVISTO K., TOKOLA O., NEUVONEN P.J., RIEKKINEN P.J. Effects of taltrimide, an experimental taurine derivative, on photoconvulsive response in epileptic patients. Epilepsia. 1987;28:133–137. doi: 10.1111/j.1528-1157.1987.tb03638.x. [DOI] [PubMed] [Google Scholar]
- KOIVISTO K., SIVENIUS J., KERANEN T., PARTANEN J., RIEKKINEN P., GOTHONI G., TOKOLA O., NEUVONEN P.J. Clinical trial with an experimental taurine derivative, taltrimide, in epileptic patients. Epilepsia. 1986;27:87–90. doi: 10.1111/j.1528-1157.1986.tb03506.x. [DOI] [PubMed] [Google Scholar]
- KONTRO P., LINDEN I.-B., GOTHONI G., OJA S.S. Sulfur Amino Acids: Biochemical and Clinical Aspects. New York: Alan R. Liss; 1983. Novel anticonvulsant taurine derivatives; pp. 211–220. [PubMed] [Google Scholar]
- KOTCH L.E., CHEN S.-Y., SULIK K.K. Ethanol-induced teratogenesis: free radical damage as a possible mechanism. Teratology. 1995;52:128–136. doi: 10.1002/tera.1420520304. [DOI] [PubMed] [Google Scholar]
- KURIYAMA K., HASHIMOTO T.Interrelationship between taurine and GABA Taurine 3. Cellular and Regulatory Mechanisms 1998New York: Plenum Press; 329–337.ed. Schaffer, S., Lombardini, J.B. & Huxtable R.J. pp [DOI] [PubMed] [Google Scholar]
- LAMMER E.J., SEVER L.E., OAKLEY G.P. Teratogen update: valproic acid. Teratology. 1987;35:465–473. doi: 10.1002/tera.1420350319. [DOI] [PubMed] [Google Scholar]
- LI Z-Q., YAMAMOTO Y., MORIMOTO T., ONO J., OKADA S., YAMATODANI A. The effect of pentylenetetrazole-kindling on the extracellular glutamate and taurine levels in the frontal cortex in rats. Neurosci. Lett. 2000;282:117–119. doi: 10.1016/s0304-3940(00)00838-7. [DOI] [PubMed] [Google Scholar]
- LINDHOUT D., OMTZIGT J.G.C. Teratogenic effects of antiepileptic drugs: implications for the management of epilepsy in women of childbearing age. Epilepsia. 1994;35 Suppl. 4:S19–S28. doi: 10.1111/j.1528-1157.1994.tb05952.x. [DOI] [PubMed] [Google Scholar]
- LOSCHER W., HORSTERMANN D., HONACK D., RUNDFELDT C., WAHNSCHAFFE U. Transmitter amino acid levels in rat brain regions after amygdala-kindling or chronic electrode implantation without kindling: evidence for a pro-kindling effect of prolonged electrode implantation. Neurochem. Res. 1993;18:775–781. doi: 10.1007/BF00966772. [DOI] [PubMed] [Google Scholar]
- NAKAYAMA J., FU Y.-H., CLARK A.M., NAKAHARA S., HAMANO K., IWASAKI N., MATSUI A., ARINAMI T., PTACEK L.J. A nonsense mutation of the MASS1 gene in a family with febrile and afebrile seizures. Ann. Neurol. 2002;52:654–657. doi: 10.1002/ana.10347. [DOI] [PubMed] [Google Scholar]
- NAU H. Valproic acid teratogenicity in mice after various administration and phenobarbital-pretreatment regimens. The parent drug and not one of the metabolites assayed is implicated as teratogen. Fundam. Appl. Toxicol. 1986;6:662–668. doi: 10.1016/0272-0590(86)90179-x. [DOI] [PubMed] [Google Scholar]
- NAU H., HAUCK R.-S., EHLERS K. Valproic acid-induced neural tube defects in mouse and human: aspects of chirality, alternative drug development, pharmacokinetics and possible mechanisms. Pharmacol. Toxicol. 1991;69:310–321. doi: 10.1111/j.1600-0773.1991.tb01303.x. [DOI] [PubMed] [Google Scholar]
- NAU H., HENDRICKX A.G. Valproic acid teratogenesis. ISI Atlas Sci. Pharm. 1987;1:52–56. [Google Scholar]
- OJA S.S., KONTRO P., LINDEN I.-B., GOTHONI G. Anticonvulsant activity of some 2-aminoethanesulphonic acid (taurine) derivatives. Eur. J. Pharmacol. 1983;87:191–198. doi: 10.1016/0014-2999(83)90329-1. [DOI] [PubMed] [Google Scholar]
- PARMAN T., CHEN G., WELLS P.G. Free radical intermediates in phenytoin and related teratogens. J. Biol Chem. 1998;273:25079–25088. doi: 10.1074/jbc.273.39.25079. [DOI] [PubMed] [Google Scholar]
- PERUCCA E. Pharmacological and therapeutic properties of valproate. A summary after 35 years of clinical experience. CNS Drugs. 2002;16:695–714. doi: 10.2165/00023210-200216100-00004. [DOI] [PubMed] [Google Scholar]
- RADATZ M., EHLERS K., YAGEN B., BIALER M., NAU H. Valnoctamide, valpromide and valnoctic acid are much less teratogenic in mice than valproic acid. Epilepsy Res. 1998;30:41–48. doi: 10.1016/s0920-1211(97)00095-8. [DOI] [PubMed] [Google Scholar]
- ROGERS S.W., GAHRNG L.C., WHITE H.S. Glutamate receptor GluR1 expression is altered selectively by chronic audiogenic seizures in the Frings mouse brain. J. Neurobiol. 1998;35:209–216. [PubMed] [Google Scholar]
- SPIEGELSTEIN O., BIALER M., RADATZ M., NAU H., YAGEN B. Asymmetric synthesis and teratogenicity of propylisopropyl acetamide–a CNS-active chiral amide analogue of valproic acid. Chirality. 1999a;11:645–650. doi: 10.1002/(SICI)1520-636X(1999)11:8<645::AID-CHIR6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- SPIEGELSTEIN O., YAGEN B., BIALER M. Structure–pharmacokinetic–pharmacodynamic relationships of N-alkyl derivatives of the new antiepileptic drug valproyl glycinamide. Epilepsia. 1999b;40:545–552. doi: 10.1111/j.1528-1157.1999.tb05555.x. [DOI] [PubMed] [Google Scholar]
- SHAH V.P., MIDHA K.K., FINDJAY J.W.A., HILL J.D., HULSE I.J., MCILVERAY I.J., MCKAY G., MILLER K.J., PATNAIK R.N., POWELL M.L., TONELL A., VISWANATHAN C.T., YACOBI A. Bioanalytical method validation–a revisit with a decade of progress. Pharm. Res. 2000;17:1551–1557. doi: 10.1023/a:1007669411738. [DOI] [PubMed] [Google Scholar]
- SKRADSKI S.K., CLARK A.M., JIANG H., WHITE H.S., FU Y.-H., PTACEK L.J. A novel gene causing a Mendelian audiogenic mouse epilepsy. Neuron. 2001;31:537–544. doi: 10.1016/s0896-6273(01)00397-x. [DOI] [PubMed] [Google Scholar]
- SNELL G.D., FEKETE E., HUMMEL K.P. The relation of mating, ovulation and the estrus smear in the house mouse to the time of day. Anat. Rec. 1948;76:30–54. [Google Scholar]
- TANG W., BOREL A.G., FUJIMIYA T., ABBOTT F.S. Fluorinated analogues as mechanistic probes in valproic acid hepatotoxicity: hepatic microvesicular steatosis and glutathione status. Chem. Res. Toxicol. 1995;8:671–682. doi: 10.1021/tx00047a006. [DOI] [PubMed] [Google Scholar]
- TONG X., PATSALOS P.N. A microdialysis study of the novel antiepileptic drug levetiracetam: extracellular pharmacokinetics and effect on taurine in the rat brain. Br. J. Pharmacol. 2001;133:867–874. doi: 10.1038/sj.bjp.0704141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UEMURA S., IENAGA K., HIGAHIURA K., KIMURA H. χ-Glutamyltaurine has potent and long-lasting antiepileptic action as demonstrated by intra-amygdaloid injection in amygdala-kindled rats. Brain Res. 1992;594:347–350. doi: 10.1016/0006-8993(92)91150-d. [DOI] [PubMed] [Google Scholar]
- WELLS P.G., KIM P.M., LAPOSA R.R., NICOL C.J., PARMAN T., WINN L.M. Oxidative damage in chemical teratogenesis. Mutat. Res. 1997;396:65–78. doi: 10.1016/s0027-5107(97)00175-9. [DOI] [PubMed] [Google Scholar]
- WHITE H.S., PATEL S., MELDRUM B.S. Anticonvulsant profile of MDL 27,266: an orally active, broad-spectrum anticonvulsant agent. Epilepsy Res. 1992;12:217–226. doi: 10.1016/0920-1211(92)90076-6. [DOI] [PubMed] [Google Scholar]
- WLODARCZYK B.C., CRAIG J.C., BENNETT G.D., CALVIN J.A., FINNELL R.H. Valproic acid-induced changes in gene expression during neurulation in a mouse model. Teratology. 1996;54:284–297. doi: 10.1002/(SICI)1096-9926(199612)54:6<284::AID-TERA3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]