Abstract
Two cannabinoid receptors, CB1 and CB2, have been identified. The CB1 receptor is preferentially expressed in brain, and the CB2 receptor in cells of leukocyte lineage. We identified the mRNA for the CB1 receptor in human neuroblastoma SH-SY5Y cells, and the mRNA and protein for the CB2 receptor in human microglia and THP-1 cells.
Δ9-and Δ8-tetrahydrocannabinol (THC) were toxic when added directly to SH-SY5Y neuroblastoma cells. The toxicity of Δ9- THC was inhibited by the CB1 receptor antagonist SR141716A but not by the CB2 receptor antagonist SR144528. The endogenous ligand anandamide was also toxic, and this toxicity was enhanced by inhibitors of its enzymatic hydrolysis.
The selective CB2 receptor ligands JWH-015 and indomethacin morpholinylamide (BML-190), when added to THP-1 cells before stimulation with lipopolysaccharide (LPS) and IFN-γ, reduced the toxicity of their culture supernatants to SH-SY5Y cells. JWH-015 was more effective against neurotoxicity of human microglia than THP-1 cells. The antineurotoxic activity of JWH-015 was blocked by the selective CB2 receptor antagonist SR144528, but not by the CB1 receptor antagonist SR141716A. This activity of JWH-015 was synergistic with that of the 5-lipoxygenase (5-LOX) inhibitor REV 5901.
Cannabinoids inhibited secretion of IL-1β and tumor necrosis factor-α (TNF-α) by stimulated THP-1 cells, but these effects could not be directly correlated with their antineurotoxic activity.
Specific CB2 receptor ligands could be useful anti-inflammatory agents, while avoiding the neurotoxic and psychoactive effects of CB1 receptor ligands such as Δ9-THC.
Keywords: Alzheimer's disease, cannabinoid receptors, cytokines, human microglia, inflammation, marijuana, multiple sclerosis, neuroprotection, SH-SY5Y neuroblastoma cells, THP-1 monocytic cells
Introduction
The medicinal use of cannabis can be traced back as far as ancient Chinese and Egyptian civilizations. It has continued into modern times with cannabis preparations being included in British and United States pharmacopoeias as late as the 1930s. This medicinal potential has since been overshadowed by the widespread ‘recreational' use of cannabis. Cannabis is now classified as an hallucinogenic substance and is banned in most jurisdictions. Mood, memory, perception and psychomotor activity are among the many neuronal functions that are affected by cannabis ingredients (Dewey, 1986). One consequence of its widespread recreational use, however, has been a rediscovery of its therapeutic potential, particularly in inflammatory conditions such as multiple sclerosis (Baker et al., 2000; Robson, 2001). A dilemma has been created by these conflicting effects. Discouraging the social use of cannabis results in withholding a potentially valuable treatment for some intractable diseases. Clearly, an important advance would result from identifying agents selective for the immune effects of cannabis while avoiding its psychoactivity. Such a possibility has been indicated by investigations into cannabinoid receptors. Two G-protein coupled receptors, designated as CB1 and CB2, have so far been identified (for reviews see Pertwee, 1997; Glass & Northup, 1999; Howlett et al., 2002). The CB1 receptor is highly concentrated in the central nervous system, while the CB2 receptor, which is also referred to as the peripheral cannabinoid receptor, is widely distributed and is particularly strongly expressed by immune system cells, including monocytes (Cabral & Dove Pettit, 1998; Klein et al., 1998). These data imply the existence of multiple endogenous ligands. Endogenous ligands that have so far been identified include anandamide (AEA) and 2-arachidonylglycerol (Felder & Glass, 1998; Hillard, 2000; Piomelli et al., 2000).
Cannabinoids have already been shown to suppress several macrophage functions, including phagocytosis, cytolysis and cytokine secretion (Cabral & Dove Pettit, 1998; Klein et al., 1998; Berdyshev, 2000). In this paper, we evaluated the ability of various cannabinoid receptor ligands to exert direct toxic effects on human SH-SY5Y neuroblastoma cells, but to suppress the neurotoxic activity of activated human microglia and microglia-like THP-1 cells. We tested two specific CB2 receptor ligands, JWH-015 and indomethacin morpholinylamide (BML-190); an endocannabinoid AEA; and two cannabis ingredients, Δ9- and Δ8-tetrahydrocannabinol (THC). Specificity of the ligands was evaluated by using the selective CB1 receptor antagonist SR141716A and the selective CB2 receptor antagonist SR144528.
We first determined that human microglia and human promyelocytic HL-60 cells selectively express CB2 receptors, neuronal SH-SY5Y cells selectively express CB1 receptors, while THP-1 cells express both receptors. We next found that direct neurotoxicity of cannabinoid ligands was mediated through the CB1 receptors, while amelioration of THP-1 cell neurotoxicity was mediated through the CB2 receptors. Our results suggest that ligands selective for the CB2 receptor might have anti-inflammatory properties, while ligands selective for the CB1 receptor might be responsible for the psychoactive and deleterious neuronal effects.
Methods
Reagents
The following cannabinoid receptor ligands were from Sigma (St Louis, MO, U.S.A.): Δ9-THC; Δ8-THC; the endocannabinoid AEA (arachidonylethanolamide) (Facci et al., 1995); the specific CB2 receptor ligands JWH-015 (1-propyl-2-methyl-3-(1-naphthoyl)indole) (Showalter et al., 1996; Griffin et al., 2000; Huffman, 2000) and indomethacin morpholinylamide (BML-190, Gallant et al., 1996; Chang et al., 2001). Cannabinoid receptor antagonists were obtained from the National Institute on Drug Abuse (NIDA, Bethesda, MD, U.S.A.) and included N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboximide hydrochloride (SR141716A), which is selective for the CB1 receptor (Pertwee, 1997) and N-[(1S)-endo-1,3,3-trimethylbi-cyclo[2.2.1]heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methyl-benzyl)-pyrazole-3-carboxamide (SR144528), which is selective for the CB2 receptor (Glass, 2001; Howlett et al., 2002). Two inhibitors of the enzymatic hydrolysis of AEA (De Petrocellis et al., 2001) phenylmethylsulfonyl fluoride (PMSF) and methylarachidonoyl fluorophosphonate (MAFP) were from Sigma and Cayman Chemical Company (Ann Arbor, MI, U.S.A.), respectively. The 5-lipoxygenase (5-LOX) inhibitor REV 5901 was also supplied by Cayman Chemical Company. Drugs were dissolved and diluted in dimethyl sulfoxide (DMSO) with the exception of Δ9- and Δ8-THC that were supplied in ethanol and subsequently diluted in this vehicle. The final concentration of vehicle solvents in tissue culture medium was 0.5%. At this concentration, neither of the vehicles had any effect on the parameters analyzed. Nevertheless, equal amounts of vehicle were added to all control samples against which the effects of various drugs were assessed. The following substances used in various assays were obtained from Sigma: bacterial lipopolysaccharide (LPS, from E.coli 055:B5), diaphorase (EC 1.8.1.4, from Clostridium kluyveri, 5.8 U mg−1 solid), dimethyl sulfoxide, p-iodonitrotetrazolium violet, NAD+, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide), phosphatase substrate Sigma 104. Human recombinant IFN-γ was purchased from Bachem California (Torrance, CA, U.S.A.) and PeproTech Canada (Ottawa, ON, Canada). Antibodies used for Western blotting (216407, rabbit polyclonal anti-CB2 receptor, 1 : 250 dilution) were from Calbiochem (San Diego, CA, U.S.A.), while peroxidase-labeled anti-rabbit antibodies (1 : 5000) were purchased from Sigma. Antibodies used for immunocytochemistry included rabbit polyclonal antiglial fibrillary acidic protein (GFAP) used at 1 : 20,000 dilution and mouse monoclonal anti-CD68 used at 1 : 400 (both from DAKO, Carpinteria, CA, U.S.A.), while rabbit polyclonal anti-CB2 receptor antibodies (from Calbiochem) were used at 1 : 1000. Antibodies used in enzyme-linked immunoabsorbent assays (ELISA) were as follows: for IL-1β capture, a rabbit polyclonal (1 : 1000, a gift from Dr H. Ziltener, The Biomedical Research Centre, Vancouver, BC, Canada); for IL-1β detection, mouse monoclonal (1 : 50, obtained from Dr A.E. Berger, The Upjohn Company, Kalamazoo, MI, U.S.A.); for tumor necrosis factor-α (TNF-α) capture, a mouse monoclonal (1 : 2000, Chemicon, Temecula, CA, U.S.A.); for TNF-α detection, biotinylated rabbit polyclonal (1 : 200, PeproTech Canada). The alkaline phosphatase-labeled anti-mouse antibodies (1 : 3000) were supplied by GIBCO BRL, Life Technologies (Burlington, ON, Canada), while ExtrAvidin-alkaline phosphatase (1 : 20,000) was from Sigma. Human recombinant IL-1β and TNF-α used for ELISA calibrations were from PeproTech Canada.
Cell culture
The human monocytic THP-1 and promyelocytic HL-60 cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA, U.S.A.). The human neuroblastoma SH-SY5Y cell line was a gift from Dr R. Ross, Fordham University, NY. These cells were grown in Dulbecco's modified Eagle's medium-nutrient mixture F12 ham (DMEM-F12) supplemented with 10% fetal bovine serum (FBS, GIBCO BRL, Life Technologies) and containing gentamicin (50 μg ml−1). All cell lines were used without initial differentiation.
Human microglial cells were isolated from surgically resected temporal lobe tissue. We thank Dr J. Maguire, Department of Pathology and Laboratory Medicine, Vancouver General Hospital for providing the surgical specimens. Protocols described by De Groot et al. (1997; 2000) were used with minor modifications. Tissues were placed in a sterile Petri dish, rinsed with Hank's balanced salt solution, and visible blood vessels were removed. After washing tissues two more times with Hank's balanced salt solution, tissues were chopped into small (<2 mm3) pieces by using a sterile scalpel. The fragments were transferred into a 50 ml centrifuge tube containing 10 ml of 0.25% trypsin solution, and incubated at 37°C for 20 min. Subsequently DNase I (from bovine pancreas, Pharmacia Biotech, Baie d'Urfé, PQ, Canada) was added to reach a final concentration of 50 μg ml−1. Tissues were incubated for an additional 10 min at 37°C. The cell suspension was diluted with 10 ml of DMEM-F12 with 10% FBS, and gently triturated by using a 10 ml pipette with a wide mouth. After centrifugation at 275 × g for 10 min, the cell pellet was resuspended in the serum-containing medium, triturated several times and passed through a 100 μm nylon cell strainer (Becton Dickinson, Franklin Lakes, NJ, U.S.A.). The cell suspension was then centrifuged once more (275 × g for 10 min), resuspended into 10 ml of DMEM-F12 with 10% FBS containing gentamicin (50 μg ml−1), and plated onto uncoated 10-cm tissue culture plates (Becton Dickinson). Plates were placed in a humidified 5% CO2, 95% air atmosphere at 37°C for 2 h in order to achieve adherence of microglial cells. Nonadherent cells with myelin debris were discarded by replacing the cell medium in the plates. Microglial cells were cultured for 5–7 days, then detached from the plates by trypsinization (0.25% trypsin EDTA solution from GIBCO BRL, Life Technologies), resuspended into DMEM-F12 medium containing 5% FBS and used for cytotoxicity experiments. Immunostaining (see below) with antibodies against CD68, which stains microglia as well as macrophages, and GFAP, which is a marker of astrocytes, showed that the isolated cultures contained 95.6±0.7% (N=4) microglial cells. The viability of monocytic cells and microglia in the presence of various inhibitors was monitored visually with a phase-contrast microscope and also by release of lactate dehydrogenase (LDH), and by MTT assay that detects live cells (see below). The latter two assays were performed after 24 h incubation with the drug.
Cytotoxicity of THP-1 cells and microglia towards SH-SY5Y neuroblastoma
The cytotoxicity experiments were performed as described previously (Klegeris et al., 1999; Klegeris & McGeer, 2000a). Briefly, human monocytic THP-1 cells were seeded into 24-well plates at a concentration of 4 × 105 cells per well in 0.8 ml of DMEM-F12 medium containing 5% FBS, while human microglial cells were used at a five times lower concentration. The cells were incubated in the presence of various drugs or corresponding vehicle solutions for 30 min prior to the addition of an activating stimulus (0.5 μg ml−1 LPS with 150 U ml−1 IFN-γ). Cannabinoid receptor antagonists were added 10 min before JWH-015. After 24 h incubation, 0.4 ml of cell-free supernatant was transferred to each well containing SH-SY5Y cells. The cells had been plated 24 h earlier at a concentration of 2 × 105 ml−1 in 0.5 ml of DMEM-F12 medium containing 5% FBS. After 72 h of incubation, the neuronal culture media were sampled for LDH to determine release from dead cells, while evaluation of surviving cells was performed by the MTT assay. Ligands were added to THP-1 cell cultures 30 min before their stimulation.
Cell viability assays: LDH release
Cell death was evaluated by LDH release. LDH activity in cell culture supernatants was measured by an enzymatic test as described by Decker & Lohmann-Matthes (1988), in which formation of the formazan product of iodonitrotetrazolium dye was followed colorimetrically. Briefly, 100 μl of cell culture supernatants were pipetted into the wells of 96-well plates, followed by addition of 15 μl lactate solution (36 mg ml−1 in phosphate-buffered saline (PBS)) and 15 μl p-iodonitrotetrazolium violet solution (2 mg ml−1 in PBS). The enzymatic reaction was started by addition of 15 μl of NAD+/diaphorase solution (3 mg ml−1 NAD+; 2.3 mg solid ml−1 diaphorase). After 15 min incubation, the reaction was terminated by addition of 15 μl oxamate (16.6 mg ml−1). Optical densities (ODs) were measured by a microplate reader with a 490-nm filter, and the amount of LDH that had been released was expressed as a percentage of the value obtained in comparative wells, where remaining cells were 100% lysed by 1% Triton X-100. Subsequently, values obtained in the presence of various inhibitors were normalized against control values obtained in the presence of corresponding vehicle solution.
Cell viability assays: reduction of formazan dye (MTT)
The MTT assay was performed as described by Mosmann (1983) and by Hansen et al. (1989). This method is based on the ability of viable, but not dead, cells to convert the tetrazolium salt (MTT) to colored formazan. The viability of SH-SY5Y cells was determined by adding MTT to the SH-SY5Y cell cultures to reach a final concentration of 1 mg ml−1. Following a 1-h incubation at 37°C, the dark crystals formed were dissolved by adding to the wells an equal volume of SDS/DMF extraction buffer (20% sodium dodecyl sulfate, 50% N,N-dimethyl formamide, pH 4.7). Subsequently, plates were placed overnight at 37°C and ODs at 570 nm were measured by transferring 100 μl aliquots to 96-well plates and using the microplate reader with a corresponding filter to record values. The viable cell value was calculated as a percentage of the value obtained from cells incubated with fresh medium only. Subsequently, values obtained in the presence of various inhibitors were normalized against control values obtained in the presence of corresponding vehicle solution.
Measurement of IL-1β and TNF-α
Cytokine levels were measured in cell-free supernatants following 48 h incubation of cells. ELISA methods were employed as described previously for IL-6 (Klegeris et al., 1999) with some modifications. Briefly, cells were seeded into 24-well culture plates (0.6 ml, 5 × 105 cells ml−1). They were exposed to a combination of 0.5 μg ml−1 LPS and 150 U ml−1 IFN-γ and, after 48 h incubation, the concentration of cytokines in cell-free supernatants was measured. Capture antibodies were diluted in 0.1 M bicarbonate coating buffer, pH 8.2. Aliquots (50 μl) were added to each well of Corning (Acton, MA, U.S.A.) Easy Wash 96-well plates. The plates were incubated overnight at 4°C. Nonspecific binding sites were blocked by incubation of the wells for 2 h at room temperature with 200 μl of 3% BSA in PBS. Samples and recombinant cytokine standards diluted in PBS/3% BSA were added at 100 μl well−1, and plates were incubated for 3 h at room temperature. Detection antibodies were diluted in PBS/3% BSA and added at 100 μl to each well. Plates were incubated for 1 h at room temperature. Alkaline phosphatase-labeled antibodies (for IL-1β measurement) and ExtrAvidin-alkaline phosphatase (for TNF-α measurement) were added in PBS/3% BSA at 100 μl well−1, followed by 45 min of incubation at room temperature. After each of the above experimental steps, plates were washed two to eight times with 0.5% Tween in PBS, pH 7.0. OD at 405 nm was read by a microplate reader after a 120 min incubation of wells with substrate buffer containing 1 mg ml−1 Sigma 104 phosphate substrate in 0.1 M diethanolamine buffer, pH 9.8. Concentrations of cytokines in the experimental samples were calculated according to the optical densities obtained from wells containing standards of recombinant cytokine. For each set of experiments standard curves were run, where concentrations of cytokines were reduced to levels that were indistinguishable from readings obtained with media alone. These blank values were subtracted from readings of experimental samples. The detection limits, which correspond to media alone +2 s.d.'s were 0.17±0.08 U ml−1 for IL-1β and 0.3±0.1 U ml−1 for TNF-α.
Reverse transcription polymerase chain reaction (RT–PCR)
Total RNA from THP-1, HL-60, human microglial and SH-SY5Y neuronal cell cultures was isolated by utilization of the Trizol reagent (GIBCO BRL, Life Technologies), according to the manufacturers' instructions. Reverse transcription and PCR analyses were performed essentially as described before (Klegeris & McGeer, 2000b). The PCR primers used to detect the CB1 receptor (Genbank accession NM_001840) were: forward, 5′ ATACCACCTTCCGCACCATCACCAC3 ′; and reverse, 5′ GCTGGGGTTCAGGACCATGAAACAC 3′. The PCR primers used to detect the CB2 receptor (Genbank accession NM_001841) were: forward, 5′ TGAAGATTGGCAGCGTGACTATGAC 3′; and reverse, 5′ AAAAGAGGAAGGCGATGAACAGGAG 3′. The primers were designed to produce specific fragments of 314 bp (CB1 receptor) and 285 bp (CB2 receptor). PCR amplification was carried out using AmpliTaq Gold DNA polymerase (Perkin-Elmer, Foster City, CA, U.S.A.). The amplification program consisted of an initial denaturation step at 94°C, which was extended to 9 min in order to activate AmpliTaq Gold enzyme. This was followed by an annealing step at 55°C for 30 s, and an initial synthesis step at 72°C for 3 min. The remaining cycles were 1 min at 94°C, 30 s at 55°C, and 1 min at 72°C. The number of cycles performed for both the CB1 and the CB2 receptor was 35. It was extended to 42 cycles for those samples where no product could be observed after 35 cycles. After amplification, PCR products were separated in a 6% polyacrylamide gel and visualized by ethidium bromide staining. Polaroid photographs of the gels were taken.
Immunoblot analyses
Immunoblot analysis for the CB2 receptor was carried out according to standard protocols. Equivalent amounts of protein (45 μg per lane) were separated through 7.5% polyacrylamide gels and transferred to Immobilon-P membranes (Millipore, Bedford, MA, U.S.A.). Membranes were dried, and immunostaining performed after 2 h pretreatment with 5% skimmed milk in Tris-buffered saline. Membranes were incubated with primary antibodies overnight at 4°C, followed by incubation with secondary peroxidase-labeled antibodies for 1.5 h at room temperature. After an extensive (2 h) wash with Tris-buffered saline containing 0.05% Tween-20, the specific bands were visualized by a chemiluminescence assay (Pierce Chemical Co., Rockford, IL, U.S.A.).
Immunocytochemistry
Human microglial cell cultures were plated on glass coverslips and fixed by air drying followed by incubation for 10 min in 4% paraformaldehyde in PBS. Subsequently, cell membranes were permeabilized with an 0.2% solution of Triton X-100 in PBS (5 min). Endogenous peroxidase was inactivated by incubation for 30 min with 0.5% hydrogen peroxide in PBS. Nonspecific binding sites were blocked by incubation for 1.5 h with 5% skim milk powder in PBS containing 1% of normal serum from the animal in which the secondary antibody was raised. Staining was performed by incubating cells overnight at room temperature with a primary antibody (see Reagents section) diluted in 2% skim milk powder in PBS. After several washes with PBS, the corresponding secondary biotinylated antibody was added at 1 : 1000, and cells incubated for 1 h at room temperature. This was followed by PBS washes and 30 min incubation with avidin-biotinylated horseradish peroxidase complex (1 : 1000, ABC Elite from Vector Laboratories, Burlingame, CA, U.S.A.). After several PBS washes, peroxidase labeling was visualized by incubation in 0.3% 3.3-diaminobenzidine containing 1% nickel ammonium sulfate, 50 mM imidazole and 0.001% hydrogen peroxide in 0.05 M Tris-HCl buffer, pH 7.6. When a dark-blue color developed, sections were washed, mounted on glass slides and coverslipped with Entellan (BDH, Toronto, ON, Canada). Controls without the primary antibody showed no significant staining.
Statistical analysis
Data are presented as means±s.e.m. The concentration-dependent effects of various drugs were evaluated statistically by the randomized blocks design analysis of variance (ANOVA). The effect of REV 5901 on the dose-dependent inhibition by JWH-015 was evaluated by two-factor ANOVA, while the paired Student's t-test was used to assess the effects of the antagonists of cannabinoid receptors and inhibitors of AEA hydrolysis. Holm's step-down method was used to correct for multiple comparisons (Holm, 1979).
Results
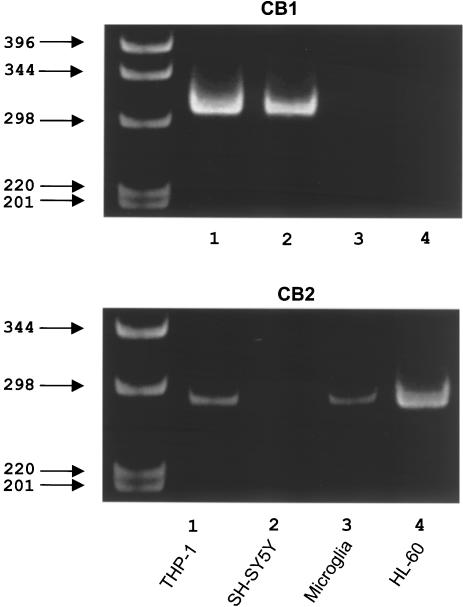
Figure 1 shows the results of RT–PCR analysis of the various cell lines for expression of the CB1 and CB2 receptor genes. PCR products yielded single bands of the predicted sizes of 314 and 285 bp, respectively. The CB1 receptor was expressed by THP-1 and SH-SY5Y cells, but not by microglia or HL-60 cells. The CB2 receptor was expressed by THP-1, microglia and HL-60 cells, but not by the neuronal SH-SY5Y cells. Thus, neuronal SH-SY5Y cells selectively express the CB1 receptor, while human microglia selectively express the CB2 receptor. THP-1 cells express both receptors.
Figure 1.
Polaroid photographs of a typical ethidium bromide-stained gel demonstrating PCR products for CB1 and CB2 receptors after 35 amplification cycles. Amplification products are shown for human THP-1 monocytic cells, human SH-SY5Y neuroblastoma cells, human postmortem microglia and human promyelocytic HL-60 cells. Location of molecular size markers in base pairs is shown in the left lane. Notice that CB1 receptor mRNA bands are observed in human monocytic THP-1 and SH-SY5Y neuronal cell extracts, while CB2 receptor bands are present in THP-1, HL-60 and microglial cell extracts.
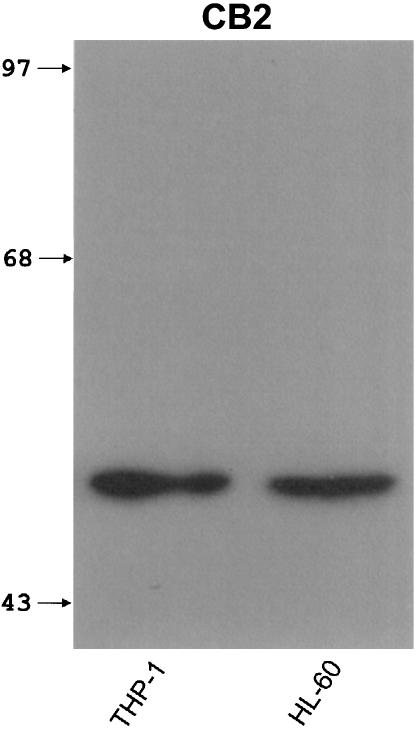
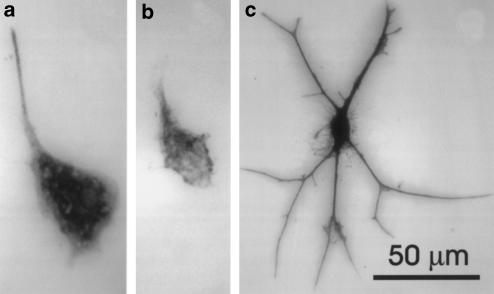
Figure 2 shows the results of immunoblot experiments for the CB2 receptor protein. A single band of approximately 50 kDa was obtained for THP-1 and HL-60 cells, which corresponds well with previously reported values for the CB2 receptor (Munro et al., 1993; Nowell et al., 1998). The human promyelocytic HL-60 cell line was used as a positive control since the CB2 receptor was initially identified in these cells (Munro et al., 1993; Galiegue et al., 1995). Insufficient protein was available from cultures of human microglia to permit immunoblotting analysis of these cells. Therefore, immunocytochemistry was carried out on microglial cell cultures obtained from surgical specimens. Staining of such cultures with antibodies against CD68 showed that the majority of cells (95.6±0.7%, N=4) were of microglial lineage (Figure 3a). When cell cultures were stained by antibodies recognizing the CB2 receptor (Figure 3b), cells with the same morphology were positive. The few remaining cells were astrocytes as evidenced by their astrocytic morphology and positive staining for GFAP (Figure 3c).
Figure 2.
An example of immunoblot showing CB2 receptor-specific bands in extracts of human monocytic THP-1 and promyelocytic HL-60 cells. Equivalent amounts of protein (45 μg/lane) were separated through 15% polyacrylamide gels and visualized by a chemiluminescence assay. Location of molecular size markers (kDa) is shown in the left lane.
Figure 3.
Immunostaining of cultures from surgically resected human temporal lobe tissue. (a) microglial cell stained with an antibody to CD68; (b) staining of microglia with an antibody to the CB2 receptor; (c) staining of one of a small number of astrocytes that appeared in the cultures with an antisera to GFAP. Calibration bar in (c) is for all three panels.
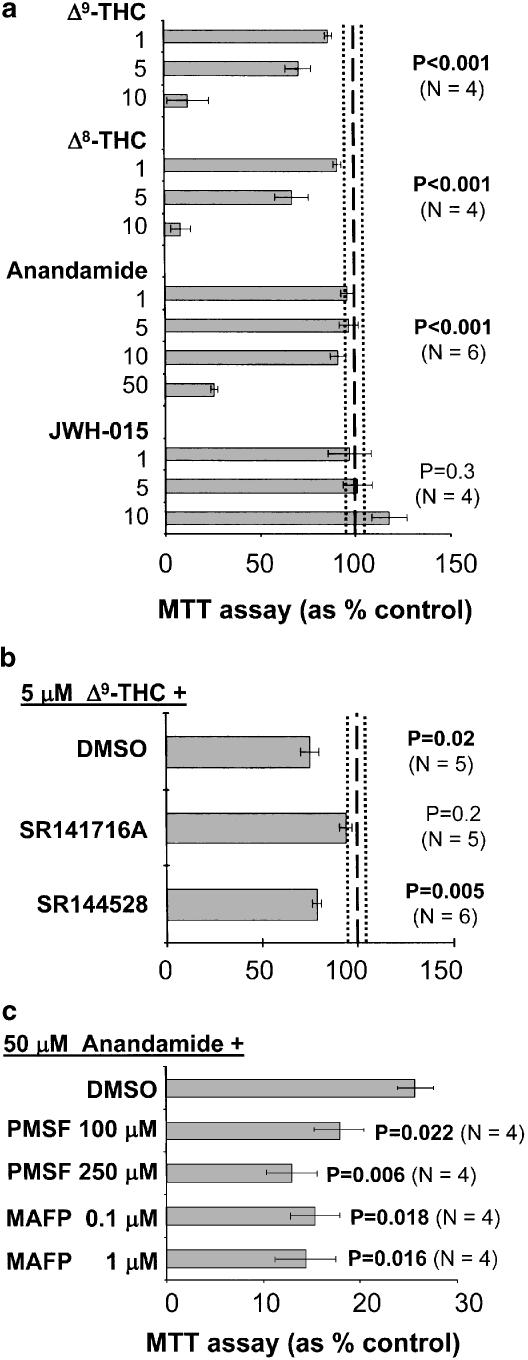
Figure 4 illustrates the effects of various ligands and CB receptor antagonists when applied directly to SH-SY5Y cells. Concentrations shown to be effective in inhibiting THP-1 cell toxicity (see Figure 5) were used with the exception of AEA that exhibited toxicity only at a high dose of 50 μM (Figure 4a). Δ9- and Δ8-THC had a prominent and dose-dependent toxic effect on the cells as demonstrated by the MTT assay while JWH-015 had no effect. The toxic effect of Δ9-THC was abolished by blockade of the CB1 receptors by pretreatment of SH-SY5Y cells for 10 min with the selective antagonist SR141716A. Similar treatment with the selective CB2 receptor antagonist SR144528 provided no protection (Figure 4b). These data indicate that the direct neurotoxic action of cannabis derivatives on SH-SY5Y neuroblastoma cells was mediated through their CB1 receptor. Figure 4c shows that the toxic action of AEA was enhanced by pretreatment of SH-SY5Y cells for 10 min with PMSF and MAFP at two different concentrations. These drugs inhibit the enzymatic hydrolysis of AEA (De Petrocellis et al., 2001).
Figure 4.
Toxicity of cannabinoids and synthetic ligands of cannabinoid receptors towards SH-SY5Y neuroblastoma cells. (a) SH-SY5Y cells were incubated with various concentrations of drugs (μM, shown on the ordinate) alone, and cell viability was assessed 72 h later by the MTT assay. (b) Toxic effect of Δ9-THC was abolished by the CB1 receptor antagonist SR141716A, but not by the CB2 receptor antagonist SR144528. Receptor antagonists (1μM) or their corresponding vehicle solutions were added 10 min before exposure of SH-SY5Y cells to 5 μM Δ9-THC. (c) Toxicity of anandamide was enhanced by inhibitors of the enzymatic hydrolysis of anandamide (PMSF and MAFP). Inhibitors or their corresponding vehicle solutions were added 10 min before exposure of SH-SY5Y cells to 50 μM anandamide. Data (means±s.e.m.) are expressed as percent control, where 100% (shown as a dashed line) is the value obtained in the presence of vehicle solution only. The dotted lines in (a) and (b) represent s.e.m. intervals. The number of independent experiments as well as P-values obtained for each of the drug treatments are presented on the figure. The concentration-dependent effects of various drugs in (a) were assessed by randomized blocks design ANOVA, while the effects of receptor antagonists in (b) and (c) were estimated by the paired Student's t-test and corrected for multiple comparisons by Holm's step-down method.
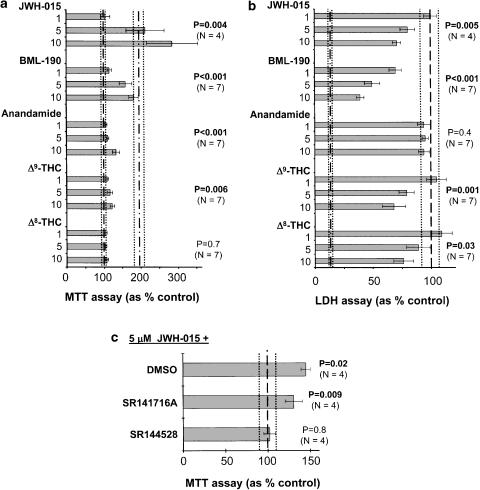
Figure 5.
Decreased toxicity of THP-1 monocytic cell secretions towards SH-SY5Y cells by cannabinoids and synthetic ligands of cannabinoid receptors. THP-1 cells were pretreated with various concentrations of drugs (μM, shown on the ordinate) for 30 min before stimulation with LPS (0.5 μg ml−1) and IFN-γ (150 U ml−1). After 24 h incubation, the cell-free supernatants of THP-1 cells were transferred to the wells containing SH-SY5Y cells. The viability of SH-SY5Y cells was assessed after 72 h by the MTT assay (a) and by measuring the LDH activity in the supernatants (b). Data (means±s.e.m.) are expressed as % control, where 100% (shown as a dashed line) is the value obtained from supernatants of stimulated THP-1 cells in the presence of corresponding vehicle solution. The dash-dotted line represents the mean value obtained from supernatants of unstimulated THP-1 cells, while the dotted lines represent s.e.m. intervals. The number of independent experiments for each set of data is also shown. The concentration-dependent effects of various drugs were assessed by randomized blocks design ANOVA and P-values obtained for each of the drug treatments are presented on the figure. (c) The antineurotoxic effect of JWH-015 was abolished by the CB2 receptor antagonist SR144528 but not by the CB1 receptor antagonist SR141716A. Receptor antagonists (1 μM) or their corresponding vehicle solutions were added 10 min before exposure of THP-1 cells to 5 μM JWH-015. The number of independent experiments for each set of data is shown together with P-values that were obtained by the paired Student's t-test (versus THP-1 cells stimulated in the presence of vehicle solutions only) and corrected for multiple comparisons by Holm's step-down method.
Figure 5 shows the effects of cannabinoids and CB2 receptor ligands on THP-1 cell secretions that were toxic to neuronal SH-SY5Y cells. The agents were added to THP-1 cells 30 min before addition of the stimulant combination of LPS and IFN-γ. The reduction of toxicity was assessed in two ways: (1) through retention of the ability to reduce formazan by cells surviving in culture, that is, the MTT assay (Figure 5a); and (2) through measurement of LDH released into the culture medium by cells dying after being exposed to the neurotoxic secretions from stimulated THP-1 cells (Figure 5b). THP-1 cells generate neurotoxic secretions only when they are stimulated. The effects of supernatants from unstimulated THP-1 cells are indistinguishable from medium alone (Klegeris & McGeer, 2000a). None of the drugs had any effect on viability of THP-1 cells as measured by LDH and MTT assays after the 24 h incubation period (data not shown).
The MTT values (Figure 5a) were normalized to zero inhibitor concentration that was set at 100%. Agents reducing neurotoxicity enhanced survival so that reductions in neurotoxicity were measured as a percent increase over the base 100% value. The mean absolute value of live cells at zero inhibitor concentration was 50.2±1.7% (N=39) of total cells after 72 h.
Figure 5b shows the relative values of LDH released into the medium during 72 h of SH-SY5Y culture in THP-1 cell supernatants that had been exposed or not exposed to inhibitors. The scale is also in percent, where 100% represents zero concentration of the inhibitor (i.e. medium from stimulated THP-1 cells only). The reduction of LDH released in the presence of inhibitors therefore represents the degree of sparing achieved by each agent. In these experiments, each set of data was normalized to the zero drug concentration value. The mean absolute value of dead cells at zero inhibitor concentration was 41.6±2.4% (N=39) of total cells after 72 h. Culture with unstimulated THP-1 cell supernatants resulted in death of 5.3±0.6% (N=7) at 72 h, similar to the death occurring in SH-SY5Y cells cultured in media alone.
Figure 5 shows that the two specific CB2 receptor ligands, JWH-015 and BML-190, reduced the neurotoxicity of THP-1 cell supernatants in a dose-dependent fashion. At a concentration of 10 μM, cell death declined by 25–50% according to the LDH assay and cell survival increased by 200–300% in the MTT assay. The endogenous ligand AEA had much weaker effects. It failed to demonstrate any reduction in LDH release and showed only a slight increase at high concentration in the MTT assay. Δ9- and Δ8-THC gave mixed results. When added to THP-1 cells, these agents reduced release of LDH from SH-SY5Y cells indicating a reduction in THP-1 cell neurotoxicity. However, Δ8-THC showed no effect on the MTT assay and Δ9-THC showed only marginal improvement in cell viability. Moreover, they were the only ligands to reduce the viability of SH-SY5Y cell when added to these neuronal cells directly. Since Δ9- and Δ8-THC would be expected to persist in the supernatants transferred from THP-1 cell cultures, a mixed effect of these two ligands would be anticipated, with protection being generated from interaction with the THP-1 cell CB2 receptors, but direct neurotoxicity occurring because of interaction with the CB1 receptors on SH-SY5Y cells.
Figure 5c shows that the antineurotoxic action of JWH-015 was inhibited by pretreatment of cells for 10 min with the CB2 receptor antagonist SR144528. Similar treatment with the selective CB1 receptor antagonist SR141716A was without an effect. These data indicate that the antineurotoxic action of JWH-015 on THP-1 cells was mediated through their CB2 receptor.
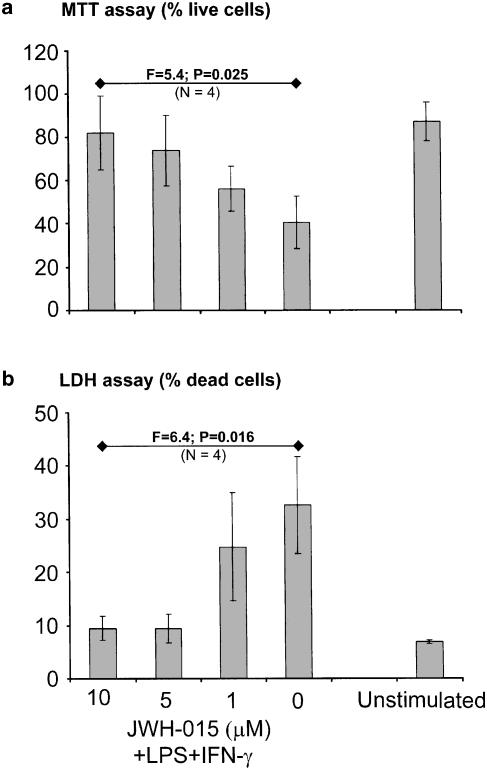
The inhibitory effect of the specific CB2 receptor ligand JWH-015 on THP-1 cells was not confined to this particular cell line. Figure 6 shows data obtained by using human microglial cells isolated from tissues of four cases that had undergone surgical temporal lobe resection. These cells, similar to THP-1 and human post-mortem microglial cells (Klegeris & McGeer, 2000a), became toxic towards neuronal SH-SY5Y cells after LPS and IFN-γ stimulation. As was the case with THP-1 cells, JWH-015 effectively inhibited this cytotoxic activity of microglial cells. The effects on microglia were even more potent since JWH-015 in the 5–10 μM range was able to reduce the toxicity to the levels comparable to unstimulated microglia in both assays. Measurements of the viability of microglia after the 24-h incubation showed no decrease in cell viability by JWH-015 treatment.
Figure 6.
The specific CB2 receptor ligand JWH-015 in a concentration-dependent manner inhibits neurotoxicity of human microglia derived from surgical specimens. Human microglial cells were pretreated with various concentrations of JWH-015 ( μM, shown on the abscissa) for 30 min before stimulation with LPS (0.5 μg ml−1) and IFN-γ (150 U ml−1). After 24 h incubation, the cell-free supernatants of microglial cells were transferred to the wells containing SH-SY5Y cells. The viability of SH-SY5Y cells was assessed after 72 h by the MTT assay (a) and by measuring the LDH activity in the supernatants (b). Data (means±s.e.m.) are expressed as % live (a) or dead (b) cells. The concentration-dependent effects of JWH-015 were assessed by randomized blocks design ANOVA. Data were obtained from four independent experiments, F and P-values obtained for both assays are presented in the figure. Note that human microglial cells were used at a five times lower concentration than THP-1 cells.
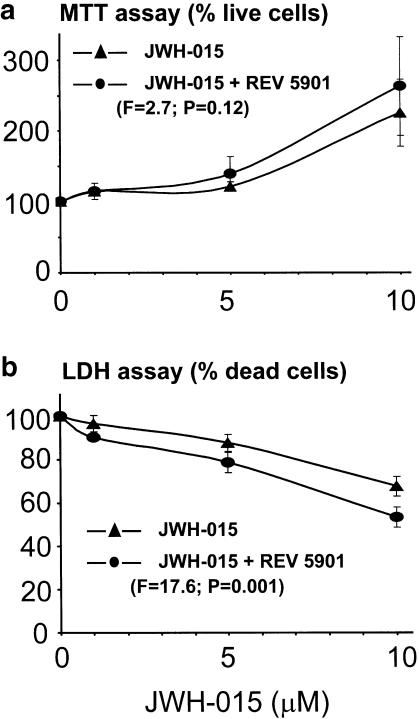
Figure 7 demonstrates that the antineurotoxic action of JWH-015 on THP-1 cells can be enhanced further by a specific 5-LOX inhibitor REV 5901. Our previous study showed that at 1 μM or less REV 5901 had no significant effect in this assay when added alone, but was able to enhance the effects of cyclooxygenase inhibitors (Klegeris & McGeer, 2002). Here, we demonstrate that similar synergism occurs between this 5-LOX inhibitor and the CB2 receptor ligand JWH-015.
Figure 7.
The antineurotoxic effects of the specific CB2 receptor ligand JWH-015 on THP-1 cell toxicity towards SH-SY5Y neuronal cells are enhanced by REV 5901, when this specific inhibitor of 5-LOX is added at a suboptimal dose of 1 μM. Experiments were performed as described in Figure 5. The abscissa represents the concentration of JWH-015 in μM and the ordinate the percent survival of cells where 100% represents the survival in the presence of vehicle solution. Note that at 1 μM or less REV 5901 by itself had no significant effect in this assay (Klegeris & McGeer, 2002). Data (means±s.e.m.) are expressed as % live (a) or dead (b) cells, N=4, F and P-values indicate the level of significance, two-factor ANOVA.
Ligands for cannabinoid receptors have been reported to influence inflammatory cytokine secretion (Shivers et al., 1994; Gallily et al., 1997; Molina-Holgado et al., 1999; Puffenbarger et al., 2000). To determine whether the neuroprotective effects of cannabinoid receptor ligands could be explained by alterations in inflammatory cytokine secretion, we tested five ligands for their influence on IL-1β and TNF-α secretion by stimulated THP-1 cells (Table 1). The cytokines were measured in cell-free supernatants of THP-1 cells that had been stimulated with LPS (0.5 μg ml−1) and IFN-γ (150 U ml−1) 48 h earlier. Mixed results were obtained indicating that the reduction in neurotoxicity could not be correlated with changes in cytokine secretion. BML-190 caused a significant increase in TNF-α secretion, but had no effect on IL-1β secretion. JWH-015 caused a significant decrease in both IL-1β and TNF-α. AEA, Δ9-THC and Δ8-THC all caused significant decreases in IL-1β secretion, and trends toward reductions in TNF-α secretion.
Table 1.
Effects of various cannabinoid receptor ligands on IL-1β and TNF-α secretion by stimulated THP-1 cells
| Ligand | Concentration (μM) | IL-1β secretion (% control) | P | TNF-α secretion (% control) | P |
|---|---|---|---|---|---|
| Δ9-THC | 10 | 55±13 (5) | <0.01 | 77±2 (3) | 0.07 |
| 5 | 64±7 (5) | 90±3 (3) | |||
| 1 | 92±5 (5) | 105±15 (3) | |||
| Δ8-THC | 10 | 53±11 (5) | 0.03 | 79±26 (3) | 0.07 |
| 5 | 69±7 (5) | 89±20 (3) | |||
| 1 | 92±5 (5) | 107±22 (3) | |||
| Anandamide | 10 | 64±11 (5) | <0.01 | 87±20 (3) | 0.2 |
| 5 | 87±4 (5) | 112±5 (3) | |||
| 1 | 90±7 (5) | 106±12 (3) | |||
| BML-190 | 10 | 101±7 (5) | 0.48 | 147±28 (4) | 0.04 |
| 5 | 101±5 (5) | 146±20 (4) | |||
| 1 | 100±3 (5) | 140±13 (4) | |||
| JWH-015 | 10 | 69±11 (4) | <0.01 | 84±8 (4) | 0.03 |
| 5 | 81±11 (4) | 81±9 (4) | |||
| 1 | 89±8 (4) | 105±6 (4) |
Drugs were administered to THP-1 cells 30 min before stimulation with a mixture of LPS (0.5 μg ml−1) and IFN-γ (150 U ml−1). IL-1β and TNF-α concentrations in cell-free supernatants were measured 48 h later. Data (means±s.e.m.) are expressed as percent change in cytokine concentrations, with mean absolute values obtained from stimulated cells in the presence of corresponding vehicle solutions being 1.3±0.5 U ml−1 for IL-1β and 6.8±2.6 ng ml−1 for TNF-α. The number of independent experiments for each set of data is shown in parentheses. The concentration-dependent effects of various drugs were assessed by randomized blocks design ANOVA and P-values obtained for each of the drug treatments are presented. Significant P-values are indicated in bold type.
Discussion
The data reported here show that CB2 receptor ligands have an antineurotoxic action on stimulated THP-1 cells and on human microglia. These cells express strongly the CB2 receptor, which is in keeping with the conclusion that CB2 is the predominant receptor on cells of leukocyte lineage (Galiegue et al., 1995). CB1 receptors have also been reported to be present on leukocytes and leukocyte cell lines (Daaka et al., 1995), which is consistent with our identification of CB1 receptors on THP-1 cells. However, it is unlikely that CB1 receptors were making a significant contribution to the antineurotoxic effect of cannabinoids for the following reasons. Firstly, the mRNA for the CB1 receptor was not detected in human microglia extracts. Secondly, the selective CB2 receptor ligands BML-190 and JWH-015 were the most active of the compounds tested, and thirdly the antineurotoxic effect of JWH-015 was blocked by the CB2 receptor antagonist SR144528 but not by the CB1 receptor antagonist SR141716A.
Although there was a good correspondence between the LDH and MTT assays in their measurements of the effects of JWH-015, BML-190 and AEA, the protective effects of Δ9- and Δ8-THC were much stronger according to the LDH assay than the MTT assay (Figure 5a versus b). This discrepancy could be because of toxic effects of residual THCs that were transferred to SH-SY5Y cells with supernatants.
The CB1 receptor is overwhelmingly dominant in the central nervous system. It has even been reported that the CB2 receptor is not expressed in brain (Griffin et al., 1999) despite the presence of resident microglia. Neurons therefore must express the CB1 receptor predominantly, as we have found for SH-SY5Y cells, and as others have found for neurons both in vivo and in vitro (Hirst & Lambert, 1995; Lew, 1996; Sanchez et al., 1998; Hohmann & Herkenham, 2000; Glass, 2001). As far as microglial cells are concerned, there have not been studies published on human cells thus far, although the presence of cannabinoid receptors has been shown on invertebrate and rodent microglia (Stefano et al., 1996; McCoy et al., 1999; Waksman et al., 1999; Carlisle et al., 2002). Both CB1- and CB2-receptor-mediated effects on these cells have been recorded. Thus Waksman et al. (1999) demonstrated the presence of functionally active CB1 receptors on cultured rat microglial cells, and showed that these receptors mediated inhibition of NO production. Our data showing the mRNA and protein for the CB2 receptor in human microglial cell extracts, combined with data on the pharmacological action of ligands selective for the CB2 receptor, indicate that microglia preferentially express functional CB2 receptors. These observations are consistent with previous reports that the CB2 receptor is expressed by cultured rat microglial cells (see Glass, 2001), and with a study showing that CB2 receptors mediate the effects of Δ9-THC on murine macrophage antigen processing (McCoy et al., 1999).
The limited availability of human microglial cells makes it difficult to use them for extensive pharmacological investigations. In this study we confirmed the activity of the CB2 receptor ligand JWH-015 on human microglia after initial studies on THP-1 cells had shown it to be the most effective of the agents tested. Its activity was more powerful on microglia than on the THP-1 cells.
THP-1 cells appear generally to be a good model for human macrophages. They have a range of properties similar to microglia and other mononuclear phagocytes, including release of such products as superoxide anion, TNF-α, IL-1β, prostaglandin E2 and other as yet unidentified neurotoxins (Giulian et al., 1990; Lorton et al., 1996; Schwende et al., 1996; Combs et al., 1999; Klegeris & McGeer, 2000a; Yates et al., 2000). In keeping with human microglia, but unlike rodent microglia, these cells do not readily express inducible NO synthase (Denis, 1994; Colton et al., 2000; Walker et al., 2001). Various standard macrophage stimulants such as LPS, IFN-γ and β-amyloid protein readily induce rodent macrophages/microglia to generate NO synthase, which has been shown to be a mediator of rodent microglia-induced neuronal cell death in vitro (McMillian et al., 1995; Ii et al., 1996) and in vivo (Weldon et al., 1998).
We found a direct neurotoxic action on SH-SY5Y cells of the cannabinoids, but not the selective CB2 receptor ligands BML-190 and JWH-015. AEA had to be used at concentrations higher than those of Δ9-THC and Δ8-THC, even though it is a CB1 receptor agonist as well. This discrepancy could be due to the rapid metabolism of AEA by SH-SY5Y cells. Thus it has been reported before that inhibitors of the enzymatic hydrolysis of AEA enhance its receptor-mediated effects (Wiley et al., 2000; De Petrocellis et al., 2001). Here we demonstrate that the same is true in the case of AEA neurotoxicity (see Figure 4c). The above data indicate that SH-SY5Y neuron-like cells are adversely affected by Δ9-THC and Δ8-THC. This toxicity appears to be mediated by the CB1 receptors since it was blocked by the CB1 and not by the CB2 receptor antagonist. Such results are consistent with data showing that Δ9-THC and other cannabinoids cause neuronal death after prolonged exposure both in vivo and in vitro (Scallet, 1991; Chan et al., 1998).
Toxic effects of Δ9-THC on SH-SY5Y and other neuroblastoma cell types have previously been noted at concentrations equal to or lower than those employed in these experiments (Blevins & Regan, 1976; Lew, 1996). While such neurotoxic effects may be mediated primarily by the CB1 receptor (Chan et al., 1998), in some cases cannabinoid receptor-independent mechanisms could also be involved (e.g. Howlett, 1995; Di Marzo et al., 2000). Heavy use of cannabis is also known to have deleterious effects on cognition and memory (Pope & Yurgelun-Todd, 1996; Solowij et al., 2002) despite some reports of the neuroprotective effects of cannabinoids (for a review see Guzman et al., 2001).
In our hands, the effects of the various cannabinoid ligands on inflammatory cytokine secretion by stimulated THP-1 cells were variable. They did not correlate with the antineurotoxic effects of these ligands suggesting that neither IL-1β nor TNF-α are the principal mediators of THP-1 cell toxicity. For example, BML-190 significantly increased, and JWH-015 significantly decreased, TNF-α secretion. BML-190 had no effect on IL-1β secretion, while JWH-015 caused a significant decrease. These were the ligands that showed the greatest antineurotoxic action. The mixed ligands Δ9- and Δ8-THC and AEA all decreased secretion of both inflammatory cytokines. These latter results are consistent with reports in the literature that ligands for cannabinoid receptors reduce the production of inflammatory cytokines by rat microglia (Puffenbarger et al., 2000), as well as mouse and human macrophages (Zheng et al., 1992), and macrophage cell lines (Fischer-Stenger et al., 1993; Shivers et al., 1994; McCoy et al., 1999; Chang et al., 2001). However, results on cytokine secretion can be variable, since opposing effects on IL-6 and TNF-α production by human monocytes have been reported, depending on the ligands and concentration (Berdyshev et al., 1997). Furthermore, both cannabinoid receptor-dependent (McCoy et al., 1999) and independent (Puffenbarger et al., 2000) effects on cytokines secretion have been reported before. Therefore, the complexity of the effects observed in this study is likely because of a mixture of cannabinoid receptor-specific and receptor-independent mechanisms and will require additional studies.
In addition to BML-190 and JWH-015, several synthetic agents that have powerful and selective action against CB2 receptors have been described (Huffman, 2000). They may represent a new and powerful class of anti-inflammatory compounds. The CB2 receptor acts through selective G-proteins, which in turn affect adenylate cyclase (Glass & Northup, 1999). The mechanism of action of CB2 receptor ligands could therefore be different from other classes of anti-inflammatory agents so they might be suitable for combination therapy. An important advantage of using combination therapies might be use of lower, safer doses of drugs. Here we demonstrate in our in vitro model an enhanced benefit from combining a selective CB2 receptor ligand (JWH-015) with a specific 5-LOX inhibitor (REV 5901, see Figure 7). Similar synergism was observed before between REV 5901 and several nonsteroidal anti-inflammatory drugs that inhibit cyclooxygenases (Klegeris & McGeer, 2002). Since cannabinoid receptor ligands are structurally similar to arachidonic acid and some of them may serve as substrates for cyclooxygenases and lipoxygenases (Kozak et al., 2000; Chang et al., 2001), a possibility exists that these drugs may affect the enzymatic activity of cyclooxygenases by substrate competition. Alternatively, signaling pathways downstream of the CB2 receptor that are independent of 5-LOX (e.g. adenylate cyclase) may be responsible for the synergism observed in this study.
The CB2 receptor appears on all classes of leukocytes. Cannabinoids are known to suppress the actions of B cells, T cells and NK cells, as well as macrophages. The net effect is to reduce resistance to infection (for reviews see Cabral & Dove Pettit, 1998; Klein et al., 1998; Porter & Felder, 2001). This could be a negative consequence of CB2 receptor ligand therapy. Nevertheless, mice deficient in the CB2 receptor appear to be healthy (Buckley et al., 2000), which points towards the potential use of specific CB2 receptor ligands as anti-inflammatory agents, especially in diseases such as Alzheimer's disease and multiple sclerosis, where overactive microglia are believed to promote neuronal damage (reviewed by Neuroinflammation Working Group, 2000).
Acknowledgments
This work was supported by a grant from the Jack Brown and Family Alzheimer's disease Research Fund, and by a grant from the Alzheimer Society of Canada/CIHR/Astra Zeneca Canada.
Abbreviations
- AEA
anandamide
- DMEM-F12
Dulbecco's modified Eagle's medium-nutrient mixture F12 ham
- ELISA
enzyme-linked immunoabsorbent assays
- FBS
fetal bovine serum
- GFAP
glial fibrillary acidic protein
- LDH
lactate dehydrogenase
- LPS
lipopolysaccharide
- MAFP
methylarachidonoyl fluorophosphonate
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- PBS
phosphate-buffered saline
- PMSF
phenylmethylsulfonyl fluoride
- THC
tetrahydrocannabinol
- TNF-α
tumor necrosis factor-α
References
- BAKER D., PRYCE G., CROXFORD J.L., BROWN P., PERTWEE R.G., HUFFMAN J.W., LAYWARD L. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature. 2000;404:84–87. doi: 10.1038/35003583. [DOI] [PubMed] [Google Scholar]
- BERDYSHEV E.V. Cannabinoid receptors and the regulation of immune response. Chem. Phys. Lipids. 2000;108:169–190. doi: 10.1016/s0009-3084(00)00195-x. [DOI] [PubMed] [Google Scholar]
- BERDYSHEV E.V., BOICHOT E., GERMAIN N., ALLAIN N., ANGER J.P., LAGENTE V. Influence of fatty acid ethanolamides and Δ9-tetrahydrocannabinol on cytokine and arachidonate release by mononuclear cells. Eur. J. Pharmacol. 1997;330:231–240. doi: 10.1016/s0014-2999(97)01007-8. [DOI] [PubMed] [Google Scholar]
- BLEVINS R.D., REGAN J.D. Delta-9-tetrahydrocannabinol: effect on macromolecular synthesis in human and other mammalian cells. Arch. Toxicol. 1976;35:127–135. doi: 10.1007/BF00372766. [DOI] [PubMed] [Google Scholar]
- BUCKLEY N.E., MCCOY K.L., MEZEY E., BONNER T., ZIMMER A., FELDER C.C., GLASS M., ZIMMER* A. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB2 receptor. Eur. J. Pharmacol. 2000;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- CABRAL G.A., DOVE PETTIT D.A. Drugs and immunity: cannabinoids and their role in decreased resistance to infectious disease. J. Neuroimmunol. 1998;83:116–123. doi: 10.1016/s0165-5728(97)00227-0. [DOI] [PubMed] [Google Scholar]
- CARLISLE S.J., MARCIANO-CABRAL F., STAAB A., LUDWICK C., CABRAL G.A. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int. Immunopharmacol. 2002;2:69–82. doi: 10.1016/s1567-5769(01)00147-3. [DOI] [PubMed] [Google Scholar]
- CHAN G.C., HINDS T.R., IMPEY S., STORM D.R. Hippocampal neurotoxicity of Δ9-tetrahydrocannabional. J. Neurosci. 1998;18:5322–5332. doi: 10.1523/JNEUROSCI.18-14-05322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG Y.H., LEE S.T., LIN W.W. Effects of cannabinoids on LPS-stimulated inflammatory mediator release from macrophages: involvement of eicosanoids. J. Cell. Biochem. 2001;81:715–723. doi: 10.1002/jcb.1103. [DOI] [PubMed] [Google Scholar]
- COLTON C.A., CHERNYSHEV O.N., GILBERT D.L., VITEK M.P. Microglial contribution to oxidative stress in Alzheimer's disease. Ann. NY Acad. Sci. 2000;899:292–307. doi: 10.1111/j.1749-6632.2000.tb06195.x. [DOI] [PubMed] [Google Scholar]
- COMBS C.K., JOHNSON D.E., CANNADY S.B., LEHMAN T.M., LANDRETH G.E. Identification of microglial signal transduction pathways mediating a neurotoxic response to amyloidogenic fragments of β-amyloid and prion proteins. J. Neurosci. 1999;19:928–939. doi: 10.1523/JNEUROSCI.19-03-00928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAAKA Y., KLEIN T.W., FRIEDMAN H. Expression of cannabinoid receptor mRNA in murine and human leukocytes. Adv. Exp. Med. Biol. 1995;373:91–96. doi: 10.1007/978-1-4615-1951-5_13. [DOI] [PubMed] [Google Scholar]
- DE GROOT C.J.A., LANGEVELD C.H., JONGENELEN C.A.M., MONTAGNE L., VAN DER VALK P., DIJKSTRA C. Establishment of human adult astrocyte cultures derived from postmortem multiple sclerosis and control brain and spinal cord regions: immunophenotypical and functional characterization. J. Neurosci. Res. 1997;49:342–354. doi: 10.1002/(sici)1097-4547(19970801)49:3<342::aid-jnr9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- DE GROOT C.J.A., MONTAGNE L., JANSSEN I., RAVID R., VAN DER VALK P., VEERHUIS R. Isolation and characterization of adult microglial cells and oligodendrocytes derived from postmortem human brain tissue. Brain Res. Protocols. 2000;5:85–94. doi: 10.1016/s1385-299x(99)00059-8. [DOI] [PubMed] [Google Scholar]
- DE PETROCELLIS L., BISOGNO T., MACCARRONE M., DAVIS J.B., FINAZZI-AGRO A., DI MARZO V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J. Biol. Chem. 2001;276:12856–12863. doi: 10.1074/jbc.M008555200. [DOI] [PubMed] [Google Scholar]
- DECKER T., LOHMANN-MATTHES M.L. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J. Immunol. Methods. 1988;15:61–69. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- DENIS M. Human monocytes/macrophages: NO or no NO. J. Leukocyte Biol. 1994;55:682–684. doi: 10.1002/jlb.55.5.682. [DOI] [PubMed] [Google Scholar]
- DEWEY W.L. Cannabinoid pharmacology. Pharmacol. Rev. 1986;38:151–178. [PubMed] [Google Scholar]
- DI MARZO V., BREIVOGEL C.S., TAO Q., BRIDGEN D.T., RAZDAN R.K., ZIMMER A.M., ZIMMER A., MARTIN B.R. Levels, metabolism, and pharmacological activity of anandamide in CB1 cannabinoid receptor knockout mice: evidence for non-CB1, non-CB2 receptor-mediated actions of anandamide in mouse brain. J. Neurochem. 2000;75:2434–2444. doi: 10.1046/j.1471-4159.2000.0752434.x. [DOI] [PubMed] [Google Scholar]
- FACCI L., DAL TOSO R., ROMANELLO S., BURIANI A., SKAPER S.D., LEON A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc. Natl. Acad. Sci. U.S.A. 1995;92:3376–3380. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDER C.C., GLASS M. Cannabinoid receptors and their endogenous agonists. Annu. Rev. Pharmacol. Toxicol. 1998;38:179–200. doi: 10.1146/annurev.pharmtox.38.1.179. [DOI] [PubMed] [Google Scholar]
- FISCHER-STENGER K., DOVE PETTIT D.A., CABRAL G.A. Δ9-tetrahydrocannabinol inhibition of tumor necrosis factor-α: suppression of post-translational events. J. Pharmacol. Exp. Ther. 1993;267:1558–1565. [PubMed] [Google Scholar]
- GALIEGUE S., MARY S., MARCHAND J., DUSSOSSOY D., CARRIERE D., CARAYON P., BOUABOULA M., SHIRE D., LE FUR G., CASELLAS P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- GALLANT M., DUFRESNE C., GAREAU Y., GUAY D., LEBLANC Y., PRASIT P., ROCHETTE C., SAWYER N., SLIPETZ D.M., TREMBLAY N., METTERS K.M., LABELLE M. New class of potent ligands for the human peripheral cannabinoid receptor. Bioorg. Med. Chem. Lett. 1996;6:2263–2268. [Google Scholar]
- GALLILY R., YAMIN A., WAKSMANN Y., OVADIA H., WEIDENFELD J., BAR-JOSEPH A., BIEGON A., MECHOULAM R., SHOHAMI E. Protection against septic shock and suppression of tumor necrosis factor α and nitric oxide production by dexanabinol (HU-211), a nonpsychotropic cannabinoid. J. Pharmacol. Exp. Ther. 1997;283:918–924. [PubMed] [Google Scholar]
- GIULIAN D., VACA K., NOONAN C.A. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990;250:1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- GLASS M. The role of cannabinoids in neurodegenerative diseases. Prog. Neuro-Psychopharmacol. Biol. Psychiat. 2001;25:743–765. doi: 10.1016/s0278-5846(01)00162-2. [DOI] [PubMed] [Google Scholar]
- GLASS M., NORTHUP J.K. Agonist selective regulation of G proteins by cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1999;56:1362–1369. doi: 10.1124/mol.56.6.1362. [DOI] [PubMed] [Google Scholar]
- GRIFFIN G., TAO Q., ABOOD M.E. Cloning and pharmacological characterization of the rat CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 2000;292:886–894. [PubMed] [Google Scholar]
- GRIFFIN G., WRAY E.J., TAO Q., MCALLISTER S.D., RORRER W.K., AUNG M.M., MARTIN B.R., ABOOD M.E. Evaluation of the cannabinoid CB2 receptor-selective antagonist, SR144528: further evidence for cannabinoid CB2 receptor absence in the rat central nervous system. Eur. J. Pharmacol. 1999;377:117–125. doi: 10.1016/s0014-2999(99)00402-1. [DOI] [PubMed] [Google Scholar]
- GUZMAN M., SANCHEZ C., GALVE-ROPERH I. Control of the cell survival/death decision by cannabinoids. J. Mol. Med. 2001;78:613–625. doi: 10.1007/s001090000177. [DOI] [PubMed] [Google Scholar]
- HANSEN M.B., NIELSEN S.E., BERG K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- HILLARD C.J. Biochemistry and pharmacology of the endocannabinoids arachidonylethanolamide and 2-arachidonylglycerol. Prostaglandins. Other Lipid Mediators. 2000;61:3–18. doi: 10.1016/s0090-6980(00)00051-4. [DOI] [PubMed] [Google Scholar]
- HIRST R.A., LAMBERT D.G. Do SH-SY5Y human neuroblastoma cells express cannabinoid receptors. Biochem. Soc. Trans. 1995;23:418S. doi: 10.1042/bst023418s. [DOI] [PubMed] [Google Scholar]
- HOHMANN A.G., HERKENHAM M. Localization of cannabinoid CB1 receptor mRNA in neuronal subpopulations of rat striatum: a double-label in situ hybridization study. Synapse. 2000;37:71–80. doi: 10.1002/(SICI)1098-2396(200007)37:1<71::AID-SYN8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- HOLM S. A simple sequentially repetitive multiple test procedure. Scand. J. Statist. 1979;6:65–70. [Google Scholar]
- HOWLETT A.C. Pharmacology of cannabinoid receptors. Annu. Rev. Pharmacol. Toxicol. 1995;35:607–634. doi: 10.1146/annurev.pa.35.040195.003135. [DOI] [PubMed] [Google Scholar]
- HOWLETT A.C., BARTH F., BONNER T.I., CABRAL G., CASELLAS P., DEVANE W.A., FELDER C.C., HERKENHAM M., MACKIE K., MARTIN B.R., MECHOULAM R., PERTWEE R.G. International Union of Pharmacology XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- HUFFMAN J.W. The search for selective ligands for the CB2 receptor. Curr. Pharm. Design. 2000;6:1323–1337. doi: 10.2174/1381612003399347. [DOI] [PubMed] [Google Scholar]
- II M., SUNAMOTO M., OHNISHI K., ICHIMORI Y. β-Amyloid protein-dependent nitric oxide production from microglial cells and neurotoxicity. Brain Res. 1996;720:93–100. doi: 10.1016/0006-8993(96)00156-4. [DOI] [PubMed] [Google Scholar]
- KLEGERIS A., MCGEER P.L. Cyclooxygenase and 5-lipoxygenase inhibitors protect against mononuclear phagocyte neurotoxicity. Neurobiol. Aging. 2002;23:789–796. doi: 10.1016/s0197-4580(02)00021-0. [DOI] [PubMed] [Google Scholar]
- KLEGERIS A., MCGEER P.L. Interaction of various intracellular signaling mechanisms involved in mononuclear phagocyte toxicity toward neuronal cells. J. Leukocyte. Biol. 2000a;67:127–133. doi: 10.1002/jlb.67.1.127. [DOI] [PubMed] [Google Scholar]
- KLEGERIS A., MCGEER P.L. R-(-)-deprenyl inhibits monocytic THP-1 cell neurotoxicity independently of monoamine oxidase inhibition. Exp. Neurol. 2000b;166:458–464. doi: 10.1006/exnr.2000.7517. [DOI] [PubMed] [Google Scholar]
- KLEGERIS A., WALKER D.G., MCGEER P.L. Toxicity of human THP-1 monocytic cells towards neuron-like cells is reduced by non-steroidal anti-inflammatory drugs (NSAIDs) Neuropharmacology. 1999;38:1017–1025. doi: 10.1016/s0028-3908(99)00014-3. [DOI] [PubMed] [Google Scholar]
- KLEIN T.W., NEWTON C., FRIEDMAN H. Cannabinoid receptors and immunity. Immunol. Today. 1998;19:373–381. doi: 10.1016/s0167-5699(98)01300-0. [DOI] [PubMed] [Google Scholar]
- KOZAK K.R., ROWLINSON S.W., MARNETT L.J. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J. Biol. Chem. 2000;275:33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- LEW G.M. Tau protein after delta-9-tetrahydrocannabinol in a human neuroblastoma cell line. Gen. Pharmacol. 1996;27:1141–1143. doi: 10.1016/0306-3623(95)02150-7. [DOI] [PubMed] [Google Scholar]
- LORTON D., KOCSIS J.-M., KING L., MADDEN K., BRUNDEN K.R. β-Amyloid induces increased release of interleukin-1β from lipopolysaccharide-activated human monocytes. J. Neuroimmunol. 1996;67:21–29. doi: 10.1016/0165-5728(96)00030-6. [DOI] [PubMed] [Google Scholar]
- MCCOY K.L., MATVEYEVA M., CARLISLE S.J., CABRAL G.A. Cannabinoid inhibition of the processing of intact lysozyme by macrophages: evidence for CB2 receptor participation. J. Pharmacol. Exp. Ther. 1999;289:1620–1625. [PubMed] [Google Scholar]
- MCMILLIAN M., KONG L.-Y., MULLIS-SAVIN S., WILSON B., DAS K., HUDSON P., HONG J.-S., BING G. Selective killing of cholinergic neurons by microglial activation in basal forebrain mixed neuronal/glial cultures. Biochem. Biophys. Res. Commun. 1995;215:572–577. doi: 10.1006/bbrc.1995.2503. [DOI] [PubMed] [Google Scholar]
- MOLINA-HOLGADO E., GUAZA C., BORRELL J., MOLINA-HOLGADO F. Effects of cannabinoids on the immune system and central nervous system. Therapeutic implications. BioDrugs. 1999;12:317–326. doi: 10.2165/00063030-199912050-00001. [DOI] [PubMed] [Google Scholar]
- MOSMANN T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- MUNRO S., THOMAS K.L., ABU-SHAAR M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Neuroinflammation Working Group Inflammation and Alzheimer's disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOWELL K.W., PETTIT D.A., CABRAL W.A., ZIMMERMAN H.W., JR, ABOOD M.E., CABRAL G.A. High-level expression of the human CB2 cannabinoid receptor using a baculovirus system. Biochem. Pharmacol. 1998;55:1893–1905. doi: 10.1016/s0006-2952(98)00081-1. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- PIOMELLI D., GIUFFRIDA A., CALIGNANO A., RODRIGUEZ DE FONSECA F. The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol. Sci. 2000;21:218–224. doi: 10.1016/s0165-6147(00)01482-6. [DOI] [PubMed] [Google Scholar]
- POPE H.G., YURGELUN-TODD D. The residual cognitive effects of heavy marijuana use in college students. JAMA. 1996;275:521–527. [PubMed] [Google Scholar]
- PORTER A.C., FELDER C.C. The endocannabinoid nervous system: unique opportunities for therapeutic intervention. Pharmacol. Therapeut. 2001;90:45–60. doi: 10.1016/s0163-7258(01)00130-9. [DOI] [PubMed] [Google Scholar]
- PUFFENBARGER R.A., BOOTHE A.C., CABRAL G.A. Cannabinoids inhibit LPS-inducible cytokine mRNA expression in rat microglial cells. Glia. 2000;29:58–69. [PubMed] [Google Scholar]
- ROBSON P. Therapeutic aspects of cannabis and cannabinoids. Br. J. Psychiat. 2001;178:107–115. doi: 10.1192/bjp.178.2.107. [DOI] [PubMed] [Google Scholar]
- SANCHEZ C., GALVE-ROPERH I., CANOVA C., BRACHET P., GUZMAN M. Δ9-Tetrahydrocannabinol induces apoptosis in C6 glioma cells. FEBS Lett. 1998;436:6–10. doi: 10.1016/s0014-5793(98)01085-0. [DOI] [PubMed] [Google Scholar]
- SCALLET A.C. Neurotoxicology of cannabis and THC: a review of chronic exposure studies in animals. Pharmacol. Biochem. Behav. 1991;40:671–676. doi: 10.1016/0091-3057(91)90380-k. [DOI] [PubMed] [Google Scholar]
- SCHWENDE H., FITZKE E., AMBS P., DIETER P. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J. Leukoc. Biol. 1996;59:555–561. [PubMed] [Google Scholar]
- SHIVERS S.C., NEWTON C., FRIEDMAN H., KLEIN T.W. Δ9-Tetrahydrocannabinol (THC) modulates IL-1 bioactivity in human monocyte/macrophage cell lines. Life Sci. 1994;54:1281–1289. doi: 10.1016/0024-3205(94)00856-6. [DOI] [PubMed] [Google Scholar]
- SHOWALTER V.M., COMPTON D.R., MARTIN B.R., ABOOD M.E. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J. Pharmacol. Exp. Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- SOLOWIJ N., STEPHENS R.S., ROFFMAN R.A., BABOR T., KADDEN R., MILLER M., CHRISTIANSEN K., MCREE B., VENDETTI J., THE MARIJUANA TREATMENT PROJECT RESEARCH GROUP Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- STEFANO G.B., LIU Y., GOLIGORSKY M.S. Cannabinoid receptors are coupled to nitric oxide release in invertebrate immunocytes, microglia, and human monocytes. J. Biol. Chem. 1996;271:19238–19242. doi: 10.1074/jbc.271.32.19238. [DOI] [PubMed] [Google Scholar]
- WAKSMAN Y., OLSON J.M., CARLISLE S.J., CABRAL G.A. The central cannabinoid receptor (CB1) mediates inhibition of nitric oxide production by rat microglial cells. J. Pharmacol. Exp. Ther. 1999;288:1357–1366. [PubMed] [Google Scholar]
- WALKER D.G., LUE L.-F., BEACH T.G. Gene expression profiling of amyloid beta peptide-stimulated human post-mortem brain microglia. Neurobiol. Aging. 2001;22:957–966. doi: 10.1016/s0197-4580(01)00306-2. [DOI] [PubMed] [Google Scholar]
- WELDON D.T., ROGERS S.D., GHILARDI J.R., FINKE M.P., CLEARY J.P., O'HARE E., ESLER W.P., MAGGIO J.E., MANTYH P.W. Fibrillar β-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo. J. Neurosci. 1998;18:2161–2173. doi: 10.1523/JNEUROSCI.18-06-02161.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILEY J.L., DEWEY M.A., JEFFERSON R.G., WINCKLER R.L., BRIDGEN D.T., WILLOUGHBY K.A., MARTIN B.R. Influence of phenylmethylsulfonyl fluoride on anandamide brain levels and pharmacological effects. Life Sci. 2000;67:1573–1583. doi: 10.1016/s0024-3205(00)00749-9. [DOI] [PubMed] [Google Scholar]
- YATES S.L., BURGESS L.H., KOCSIS-ANGLE J., ANTAL J.M., DORITY M.D., EMBURY P.B., PIOTRKOWSKI A.M., BRUNDEN K.R. Amyloid β and amylin fibrils induce increases in proinflammatory cytokine and chemokine production by THP-1 cells and murine microglia. J. Neurochem. 2000;74:1017–1025. doi: 10.1046/j.1471-4159.2000.0741017.x. [DOI] [PubMed] [Google Scholar]
- ZHENG Z.M., SPECTER S., FRIEDMAN H. Inhibition by delta-9-tetrahydrocannabinol of tumor necrosis factor alpha production by mouse and human macrophages. Int. J. Immunopharmacol. 1992;14:1445–1452. doi: 10.1016/0192-0561(92)90017-f. [DOI] [PubMed] [Google Scholar]