Abstract
Neuropeptide Y Y1 receptors are known to internalize following the binding of agonists. In the present study, a pseudopeptide Y1 receptor antagonist, homodimeric Ile-Glu-Pro-Dpr-Tyr-Arg-Leu-Arg-Tyr-CONH2 (GR231118), also induced Y1 receptor internalization in human embryonic kidney (HEK293) cells.
We demonstrated first that both specifically bound radiolabeled antagonist ([125I]GR231118) and agonist ([125I][Leu31, Pro34]PYY) underwent receptor-mediated sequestration/internalization in transfected HEK293 cells.
Agonist-induced Y1 receptor internalization was dependent on clathrin-coated pits and was regulated in part by Gi/o-protein activation as revealed by pertussin toxin sensitivity. In contrast, antagonist-induced sequestration of Y1 receptors was partly dependent on clathrin-coated pits, but independent from Gi/o-protein activation.
Exposure to high concentrations of agonist or antagonist caused a 50 and 75% loss of cell surface binding, respectively. The loss caused by the agonist rapidly recovered. This phenomenon was blocked by monensin, an inhibitor of endosome acidification, suggesting that cell surface receptor recovery is due to recycling. In contrast to the agonist, GR231118 induced a long-lasting sequestration of Y1 receptors in HEK293 cells.
Immunofluorescence labeling indicated that following 40 min of incubation with either the agonist or the antagonist, Y1 receptors followed markedly different intercellular trafficking pathways.
Taken together, these findings provided evidence that a pseudopeptide Y1 receptor antagonist can induce long-lasting disappearance of cell surface receptors through a pathway distinct from the classical endocytic/recycling pathway followed by stimulation with an agonist.
Keywords: GR231118, antagonist receptor internalization, neuropeptide YY1 receptor, fluorescent probe
Introduction
Neuropeptide Y (NPY) is one of the most abundant peptides found in the brain (Adrian et al., 1983). It has been implicated in many physiological functions such as food intake (Stanley & Leibowitz, 1984), the modulation of anxiety (Heilig et al., 1989), epileptic seizures and cerebral blood flow (Tuor et al., 1985; Dumont et al., 1992; Vezzani et al., 1999). To induce its effects, this peptide acts on at least five types of NPY receptors, namely Y1, Y2, Y4, Y5 and y6 (Michel et al., 1998). All of these receptors have been cloned in several species including man and shown to belong to the G-protein coupled receptor (GPCR) superfamily (Michel et al., 1998). The y6 receptor appears to be expressed as a functional protein in rabbit, dog and mouse, while its gene is truncated and not translated in human and is absent in the rat (Burkhoff et al., 1998). Among all subtypes of NPY receptors, the Y1 receptor was the first to be cloned (Eva et al., 1990). Many physiological actions of NPY and related peptides such as peptide YY (PYY) and the pancreatic polypeptide are likely mediated by Y1 receptors as suggested on the basis of knockout studies, and the use of selective agonists and antagonists (Dumont & Quirion, 2000; Thorsell & Heilig, 2002).
For many GPCRs, sustained stimulation by an agonist induces the loss of functional receptors at the cell surface, leading to a reduction in responsiveness to subsequent stimulation. This phenomenon is known as desensitization and is considered to be a physiological process required for the maintenance of cellular homeostasis (Hausdorff et al., 1990). Desensitization is often associated with receptor phosphorylation and internalization (Hausdorff et al., 1990). These processes are regulated by complex intracellular signaling cascades. The regulation of some GPCRs may be mediated by G-protein-independent mechanisms, since they may be desensitized and downregulated by antagonists (Roettger et al., 1997; Houle et al., 2000; Gray & Roth, 2001). However, it is generally believed that antagonists do not promote receptor internalization, as the internalization process normally requires the activation of the receptor. Some GPCRs, such as vasopressin V2, AT1 and bradykinin (BK) B2 receptor subtypes, were reported to internalize upon antagonist binding (Pfeiffer et al., 1998; Hunyady, 1999; Houle et al., 2000). However, this internalization was preferentially promoted by a pseudopeptide rather than nonpeptide antagonists (Pfeiffer et al., 1998; Hunyady, 1999; Houle et al., 2000). Other GPCRs, including cholecystokinin receptors, were equally efficiently internalized upon both peptide and nonpeptide antagonist stimulation (Roettger et al., 1997). As antagonist-mediated receptor downregulation may have important therapeutic implications, a better understanding of the mechanisms underlying this phenomenon is critical to establish potential atypical effects of some of these molecules.
Like most other GPCRs, Y1 receptors expressed at the cell surface are actively regulated by agonist occupancy (Fabry et al., 2000; Parker et al., 2001; 2002; Gicquiaux et al., 2002). It has been shown that upon exposure to agonists, Y1 receptors are rapidly endocytosed in transfected cells as well as in cells endogenously expressing Y1 receptors (Fabry et al., 2000). This process is apparently responsible for the normal regulation of Y1 receptors (Gicquiaux et al., 2002). Over the past decade, various antagonists have been synthesized and used to characterize Y1 receptors in different target tissues and cells (Doods et al., 1996; Pheng & Regoli, 1998). Homodimeric Ile-Glu-Pro-Dpr-Tyr-Arg-Leu-Arg-Tyr-CONH2 (GR231118) is one of them. It acts as a potent antagonist on Y1 receptors while exhibiting full agonistic effects at the Y4 receptors (Dumont & Quirion, 2000; Schober et al., 2000). Based on some of its chemical and pharmacological characteristics, GR231118 could be expected to induce Y1 receptor internalization as part of its antagonistic properties. First, it is a pseudopeptide that mimics the C-terminal portion of NPY, which is known to be responsible for receptor activation. Second, GR231118 does not possess residual agonistic activities on Y1 receptors (Lew et al., 1996). However, at very high concentrations, it behaves as an insurmountable antagonist on this receptor subtype (Hegde et al., 1995). This pharmacological property has been suggested to result from receptor sequestration (Houle et al., 2000). However, the regulation of Y1 receptors in response to a peptide antagonist such as GR231118 has not been investigated. In the present study, we used human embryonic kidney (HEK293) cells transfected with the rat Y1 receptor cDNA as a model to investigate the potential of GR231118 to induce Y1 receptor sequestration/internalization. Biochemical and imaging approaches were used to elucidate the fate of cell surface Y1 receptors following exposure to either a Y1 agonist or an antagonist. We observed that GR231118 binding induced a time-dependent sequestration of Y1 receptors in HEK293 cells. This process was mediated in part by clathrin-dependent and G-protein-independent mechanisms. In contrast, an agonist such as [Leu31, Pro34]PYY clearly exhibited clathrin-dependent internalization into early endosomes, which involved G-protein activation. Accordingly, antagonist-sequestered Y1 receptors were targeted to a nonrecycling pathway, whereas agonist-internalized receptors readily recycled to the cell membrane.
Methods
Drugs
[Leu31, Pro34]PYY and radioiodinated probes were synthesized in our laboratory (Dumont et al., 1995). GR231118 was a gift from Glaxo Welcome (Research Triangle Park, NC, USA) and Bodipy-[Leu31, Pro34]NPY was supplied by PerkinElmer Life Sciences (Montreal, Quebec, Canada). R-N2-(diphenylacetyl)-N-(4-hydroxyphenyl)-methylargininamide, code name BIBP3226 and [3H]BIBP3226 were generously provided by Boerhinger Ingelheim (Germany). Iodine-125 was obtained from ICN Pharma Canada Ltd. (Montreal, Quebec, Canada). The rat Y1 cDNA was generously provided by Dr H. Herzog (Garvan Institute, Sydney, Australia). The expression vector pTR5-DC-GFP/TK/hygro was obtained from Dr D.D. Mosser (Biotechnology Institute, Montreal, Quebec, Canada). Phenylarsine oxide (PAO), filipin III, methyl-β-cyclodextrin (MβCD) and pertussis toxin (PTX) were purchased from Sigma Co. (St Louis, MO, U.S.A.). Texas Red-conjugated anti-rabbit IgG was purchased from Invitrogen (Carlsbad, CA, U.S.A.). A polyclonal rabbit antibody directed against a C-terminal sequence (LKQASPVAFKKISMNDNE) of the Y1 receptor was generously given by Dr A.G. Beck-Sickinger (Leipzig, Germany). All other reagents were of the highest grade commercially available.
Cell cultures
HEK293 cells were transfected with the rat Y1 cDNA inserted in expressing pTR5-DC-GFP/TK/hygro vector using calcium phosphate as described previously (Tong et al., 1995). Stable HEK293 cells expressing Y1 receptors were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum and antibiotics in 5% CO2/95% air. Binding studies were performed on living cells grown to confluence in polylysine-coated 24-well plates. Binding of fluo-ligands and immunofluorescence labeling were performed on cells plated on glass removable polylysine-coated coverslips and in eight-chamber Lab-Tek slides, respectively.
Binding and sequestration/internalization of the ligands
For the time course association experiments, cells were washed with cold Krebs buffer and incubated at 37°C in Krebs buffer containing 0.1%, bovine serum albumin (BSA), 0.05% bacitracin and 50 pM [125I] [Leu31, Pro34]PYY or [125I] GR231118 for different times (2–60 min). To determine the specific nature of the binding, 1 μM of unlabeled ligand was added to the incubation medium. To quantify the ratio of internalized/total bound ligand, surface-bound radioactivity was washed out or not with cold hypertonic acid solution (0.2 M acetic acid, 0.5 M NaCl, pH 3.5) for 3 min and twice with cold Krebs. Cells were then dissolved in sodium hydroxide (1 N) and radioactivity was counted using a gamma counter (Packard Instruments).
Mechanism of sequestration/internalization processes
To determine whether agonist- and antagonist-induced endocytosis was mediated by clathrin-coated pits or caveolae, cells were preincubated for 30 min at 37°C with sucrose (0.4 M) or PAO (20 μM) (two inhibitors of clathrin lattice formation), or with filipin III (1 μg ml−1) or MβCD (1 mM) (two caveolae disruptors). To investigate the involvement of Gi/o protein in endocytosis, cells were treated for 24 h at 37°C with PTX (200 ng ml−1 of culture medium) (an inhibitor of Gi/o-protein activation) and washed in Krebs buffer (containing 0.1% BSA). Cells were then incubated as above with radiolabeled ligands for 40 min at 37°C in Krebs buffer containing each inhibitor. Thereafter, cells were either washed with cold Krebs buffer or with the hypertonic acid solution. They were then solubilized and assayed for radioactivity content.
Receptor recycling
Transfected cells were incubated in Krebs buffer with or without 50 μM monensin (an inhibitor of endosome acidification) for 40 min at 37°C. Cells were then incubated for another 40 min at 37°C with or without 1 μM of the unlabeled agonist [Leu31, Pro34]PYY, or antagonist, GR231118, and washed with cold Krebs buffer. They were then incubated in Krebs buffer, in the absence or presence of monensin (50 μM) for different times at 37°C to allow for recovery of cell surface binding sites. The density of cell surface receptors was evaluated with binding assays at 4°C using [125I] GR231118 as tracer. Results were expressed as a percentage of binding to nonstimulated control cells not treated with monensin.
Fluorescence labeling
To visualize agonist binding and internalization, cells were incubated with 5 nM of the fluorescent probe, bodipy-[Leu31, Pro34]NPY, in Krebs buffer (0.1% BSA) for 5, 10 and 40 min at 37°C. Cells were then washed with either cold Krebs buffer or acidic solution, air-dried and mounted on glass slide with Aquamount. Labeled cells were examined under a Nikon confocal microscope system (PCM200) equipped with argon and HeNe lasers.
In some cases, cells were preincubated with either hypertonic sucrose (0.4 M) solution or PAO (20 μM) for 30 min at 37°C prior to the addition of the fluorescent ligand. After 40 min of incubation with 5 nM bodipy-[Leu31, Pro34]NPY was carried out in the presence of the drugs, cells were acid-washed and processed for confocal microscopic analysis.
Pulse-chase experiments were also carried out in which cells were treated or not with monensin (50 μM) for 30 min prior to the addition of bodipy-[Leu31, Pro34]NPY (5 nM) to the medium. After 40 min of incubation at 37°C, cells were washed with cold Krebs buffer and incubated again in Krebs buffer containing or not monensin (50 μM) at 37°C for different times. Labeled cells were examined by confocal microscopy.
Immunofluorescence labeling of Y1 receptors in transfected HEK293 cells
To monitor receptor distribution of immunoreactive Y1 receptors following exposure to agonist versus antagonist, Y1-transfected HEK293 cells, grown in eight-chamber Lab-Tek slide to 60% confluence, were treated with cycloheximide (70 μM) for 4 h prior to incubation in Krebs buffer containing 0.1% BSA, 0.05% bacitracin and 70 μM cycloheximide with or without 1 μM GR231118 or [Leu31, Pro34]PYY at 37°C for 30 min. Cells were washed with PBS (1 mM CaCl2) and fixed for 20 min in 4% PFA in PBS. Cells were then permeabilized for 5 min in PBS containing 1% NGS and 0.1% Triton X-100 and incubated for 1 h at room temperature with rabbit anti-Y1 receptor antibody (1 : 100). Cells were washed three times with PBS and exposed to 1 : 100 dilution of goat anti-rabbit IgG-Texas Red conjugated antibody for 1 h at room temperature, washed and then mounted in Vectashield. Immunofluorescence labeling was examined under confocal microscopy as described above (St-Pierre et al., 2000).
Results
Time course of the association and sequestration/internalization of the radioligands
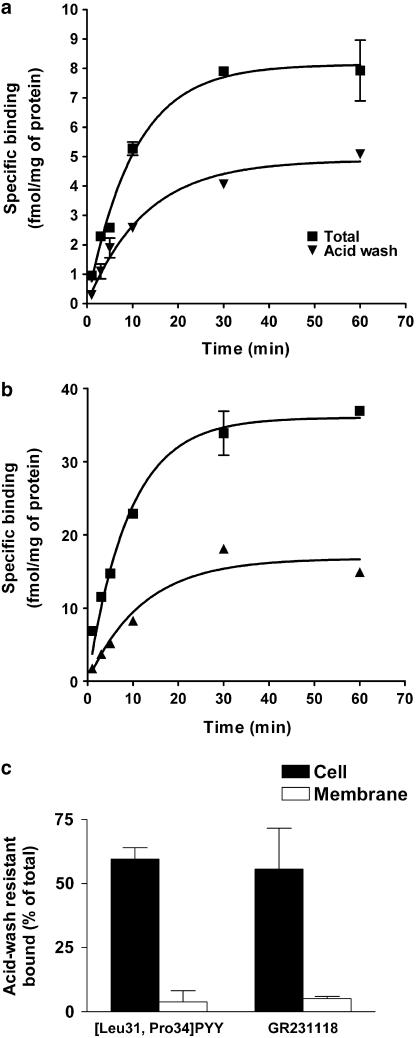
As previously demonstrated [125I][Leu31, Pro34]PYY (agonist) and [125I]GR231118 (antagonist) specifically bound to rat Y1 receptors expressed in HEK293 cells with KD values of 0. 8 and 0.2 nM, respectively (Dumont & Quirion, 2000). Specific binding of these ligands at 37°C on HEK293 cells increased with time (t1/2=6.3–7.1 min) and reached equilibrium within 30 min (Figure 1a, b). The amount of sequestered/internalized radioligand was assessed after removal of cell surface-bound ligand with hypertonic acid solution. Acid-wash-resistant binding of both ligands increased with time but was slightly delayed compared to total binding (t1/2=8.4–8.9 min) and reached a maximum of 50 and 45% of total binding for the agonist and the antagonist, respectively (Figure 1a, b). When performed on membrane homogenates (Figure 1c), the binding of either ligand was no longer resistant to acid wash, confirming that the acid-resistant fraction corresponded to radioactivity sequestrated and/or internalized into the cells. No specific binding was detected for either ligand in nontransfected HEK293 cells (data not shown).
Figure 1.
Binding and sequestration/internalization of radiolabeled ligands in Y1 transfected HEK293 cells. (a, b) HEK293 cells transfected with rat Y1 receptor cDNA were incubated with either [125I] [Leu31, Pro34] PYY (a) or [125I] GR231118 (b) for different times at 37°C. Cells were then washed with cold Krebs (total binding) or hypertonic acid solution (acid wash). To determine nonspecific binding, cells were incubated with radiolabeled probes in the presence of 1 μM unlabeled ligand. (c) Whole cells (filled bars) or rat brain homogenates (open bars) were incubated with [125I] [Leu31, Pro34] PYY ([LP]PYY) or [125I] GR231118 for 40 min at 37°C. Cells/membranes were then washed or not with hypertonic acid solution. Acid-wash-resistant binding expressed as percentage of total. Data are mean±s.e.m. of four replicates.
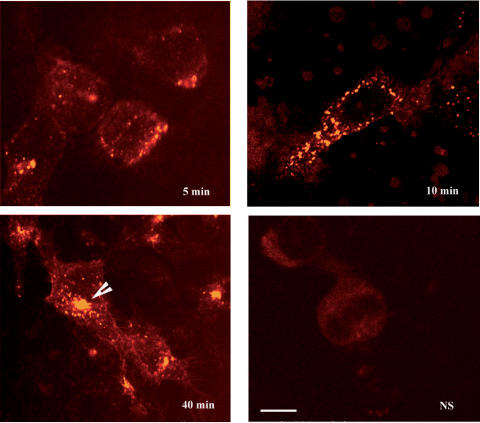
To confirm that receptor-bound agonist underwent time-dependent internalization, cells were incubated with the fluorescent agonist, bodipy-[Leu31, Pro34]NPY. This ligand binds specifically and with high affinity to rat Y1 receptors transfected and expressed in HEK293 cells (Dumont et al., 2001). The incorporation of a bodipy molecule did not affect the agonistic properties of [Leu31, Pro34]NPY, as verified by measuring its capacity to inhibit forskolin-induced cAMP production (data not shown). Confocal microscopic images showed that bodipy-[Leu31, Pro34]NPY labeling of Y1-transfected cells increased with time. Following 5 min of exposure to the ligand, weak, punctate labeling was seen at the periphery of the cells (Figure 2). After 10 min of exposure, these acid-wash-resistant fluorescent puncta were more numerous and distributed throughout the cytoplasm. At 40 min, they were clustered in the perinuclear region (Figure 2).
Figure 2.
Internalization of bodipy-[Leu31, Pro34]NPY in Y1-transfected HEK293 cells. Cells were incubated with 5 nM bodipy-[Leu31, Pro34]NPY for 5, 10 and 40 min. They were then washed with hypertonic acid solution and processed for confocal microscopic analysis as described in Methods. To determine nonspecific (NS) binding, 1 μM [Leu31, Pro34]PYY was added to the medium. Following 5 and 10 min incubations, the internalized ligand is detected in the form of small fluorescent puncta distributed throughout the cytoplasm. At 40 min, these puncta have coalesced in a small juxta-nuclear compartment reminiscent of the pericentriolar recycling endosome. No fluorescent labeling is evident in cells incubated with an excess of nonfluorescent ligand. Images are representative of triplicate experiments. Scale bar: 8 μm.
Mechanisms of sequestration/internalization of the agonist and the antagonist
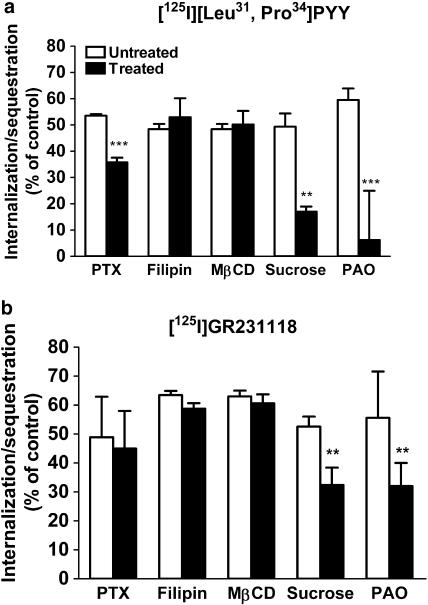
To investigate the mechanisms involved in the sequestration/internalization of the agonist and antagonist used in our study, the amount of radioligand sequestered following a 40 min incubation was determined in the presence of different inhibitors of endocytosis. Figure 3a shows that specific [125I] [Leu31, Pro34]PYY sequestration/internalization was reduced by 20% in cells treated with PXT, suggesting a mechanism partly dependent upon Gi/o-protein. In contrast, sequestration of [125I] GR231118 was not altered by PTX (Figure 3b). Treatment with caveolae disruptors, such as filipin and MβCD, had no effect on the sequestration/internalization profile of either agonist or antagonist (Figure 3a, b). In contrast, the endocytosis inhibitors sucrose and PAO almost completely inhibited acid-wash-resistant binding of [125I][Leu31, Pro34]PYY (Figure 3a), as well as intracellular fluorescent labeling following a 40 min incubation with bodipy-[Leu31, Pro34]NPY (Figure 4a, b). Internalization of [125I]GR231118 was much less dramatically, albeit still significantly, reduced by sucrose and PAO, suggesting that it was only partly dependent on clathrin-coated pit formation.
Figure 3.
Effect of PTX and endocytosis inhibitors on the sequestration/internalization of radiolabeled ligands in Y1-transfected HEK293 cells. Cells were treated with filipin (1 μg ml−1), MβCD (1 mM), hyperosmolar sucrose (0.4 M) or PAO (20 μM) for 30 min at 37°C or with PTX (200 ng ml−1) overnight at 37°C. Cells were then incubated with either 50 pM [125I] [Leu31, Pro34]PYY (a) or [125I] GR231118 (b) for 40 min. Radioactivity was counted after washing the cells with a hypertonic acid solution. Data are mean±s.e.m. of four to nine replicates and were analyzed by Student's impaired t-test, *P<0.01, **P<0.001.
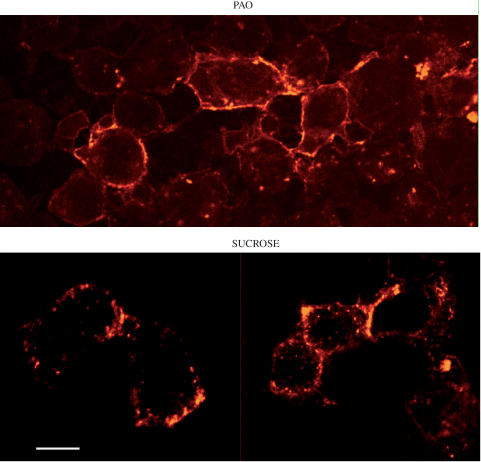
Figure 4.
Inhibition of bodipy-[Leu31, Pro34]NPY internalization in Y1-transfected HEK293 cells. Cells were incubated with PAO (20 μM) or sucrose (0.4 M) for 30 min at 37°C prior to the addition of 5 nM bodipy-[Leu31, Pro34]PPY for a further 40 min. Fluorescent labeling was examined by confocal microscopy. Following incubation with either drug, the bulk of the fluorescent ligand remains associated with the cell surface. In cells pretreated with sucrose, a few intracytoplasmic endosomes are visible, but considerably less than in untreated cells (compared with Figure 2 at the same time point). Scale bar: 8 μm.
Receptor recycling
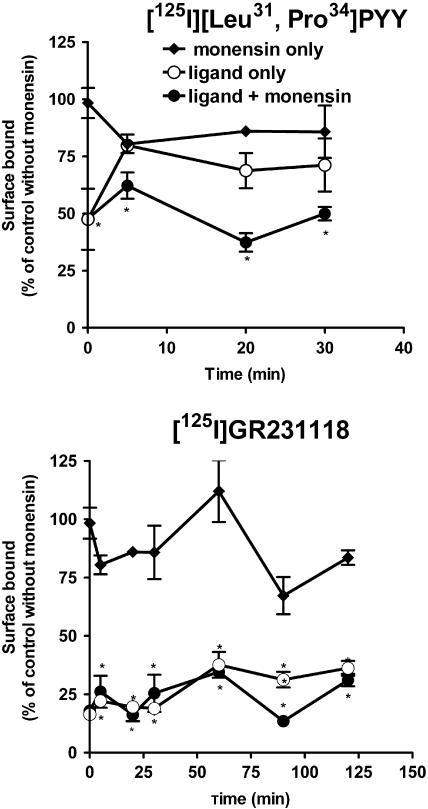
To determine whether sequestered/internalized Y1 receptors were recycled to the cell surface following agonist and/or antagonist stimulation, cells were incubated with 1 μM [Leu31, Pro34]PYY or GR231118 for 40 min at 37°C in the absence or presence of monensin, a toxin known to inhibit receptor recycling through the inhibition of endosome acidification. The level of Y1 receptors present at the cell surface after different recovery periods was then assessed by performing binding assays at 4°C using [125I]GR231118 as radioligand. The total number of cell surface binding sites in cells incubated with monensin was 80–100% (n=4) of that of untreated cells (control), indicating that monensin by itself slightly but nonsignificantly altered the number of surface receptors. Exposure to an unlabeled agonist or antagonist induced a loss of 50 and 75% of cell surface [125I]GR231118 binding sites, respectively (Figure 5a, b). The loss induced by [Leu31, Pro34]PYY recovered rapidly to 80% of control value following the removal of the agonist from the medium. By contrast, no recovery was observed in cells pretreated with unlabeled GR231118 even up to 2 h after washout, suggesting that receptors sequestered/internalized by the antagonist were not recycled to the cell surface. Treatment of the cells with monensin prevented recovery of agonist-induced losses of cell surface receptor binding sites (Figure 5a).
Figure 5.
Receptor internalization and recycling in Y1-transfected HEK293 cells. Surface receptors in cells treated or untreated with monensin (50 μM) were depleted by incubation with 1 μM unlabeled [Leu31, Pro34]PYY or GR231118 for 40 min at 37°C. Cell surface binding was then measured with binding assays at 4°C using [125I] GR231118 as tracer. Data expressed as percentage of total surface binding in control cells untreated with monensin. Data are the mean±s.e.m. of four replicates and were analyzed by Student's impaired t-test, *P<0.01.
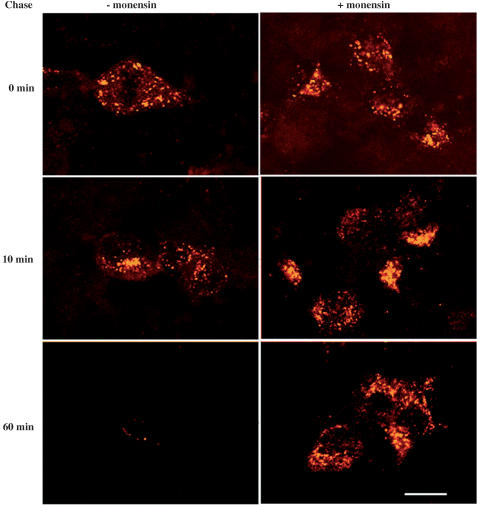
To verify whether the trafficking of internalized [Leu31, Pro34]NPY was endosome dependent, the binding and internalization of the fluorescent agonist were carried out in the absence or the presence of monensin and the cells were imaged after different recovery periods. Following 30 min of incubation with bodipy-[Leu31, Pro34]NPY, the intensity and intracellular distribution of fluorescent hot spots were similar in monensin-treated and nontreated cells (Figure 6). In cells untreated with monesin, the intensity of the fluorescent staining was markedly decreased after 10 min of fluorescent washout and was no longer visible in the cytoplasm after 60 min (Figure 6). In contrast, in cells treated with monensin, intracellular fluorescent staining remained practically unchanged between 0 and 60 min of agonist washout (Figure 6).
Figure 6.
Distribution of bodipy-[Leu31, Pro34]NPY after pulse-chase labeling in Y1-transfected HEK293 cells. Cells untreated (left) and treated (right) with monensin were pulse labeled for 40 min with 2 nM bodipy-[Leu31, Pro34]NPY and chased for 0, 10 and 60 min at 37°C in the absence or presence of monensin. In the absence of monensin, there is a progressive disappearance of the fluorescent ligand from intracellular stores, whereas in the presence of the drug, the ligand remains trapped in endosomes due to their inability to dissociate from the receptor. Images are representative of duplicate experiments. Scale bar: 8 μm.
Immunohistochemical studies of receptor redistribution following exposure to Y1 agonist and antagonist
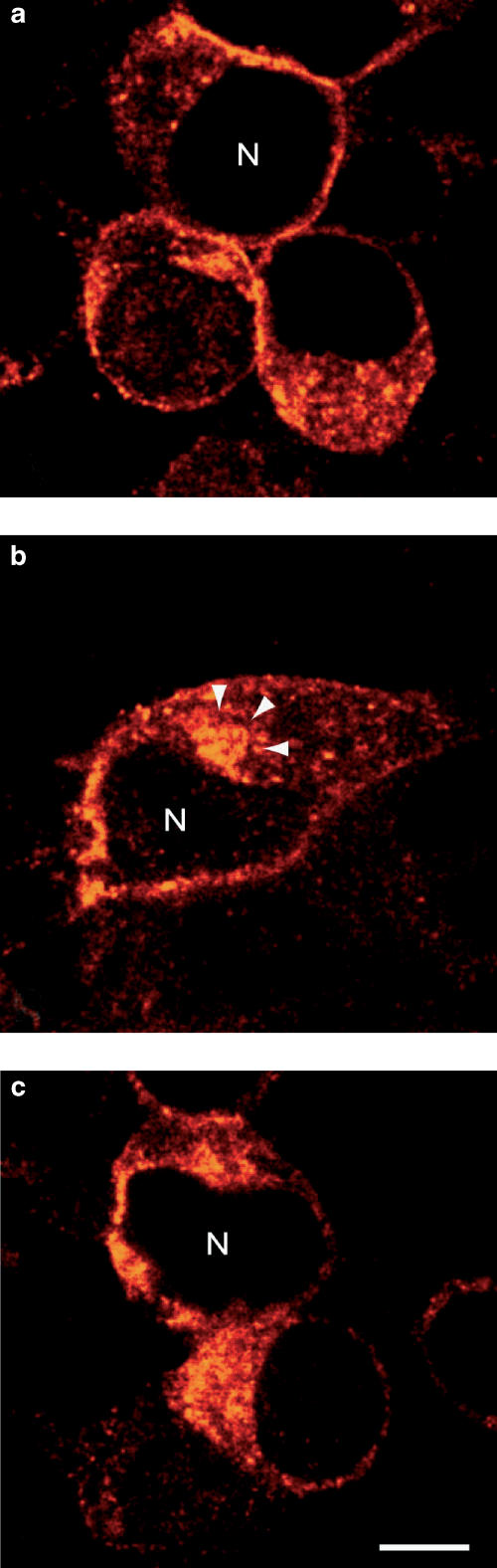
To investigate the redistribution of Y1 receptors following exposure to agonist versus antagonist, transfected cells were preincubated with unlabeled ligands for 40 min and immunostained using an anti-Y1 receptor antibody. In nonstimulated cells, Y1 receptor immunoreactivity was detected in the form of small fluorescent puncta distributed throughout the cytoplasm as well as on the cell surface (Figure 7). Following treatment with 1 μM [Leu31, Pro34]PYY for 40 min, fluorescent hot spots were mainly concentrated within the perinuclear region, in an area previously characterized as the pericentriolar recycling endosome (Vandenbulcke et al., 2000). By contrast, in cells stimulated with GR231118, the distributional pattern of immunoreactive Y1 receptors was virtually undistinguishable from that seen in unstimulated cells (Figure 7).
Figure 7.
Immunolabeling of Y1 receptors in Y1-transfected HEK293 cells. Cells were pretreated with cycloheximide (70 μM) and either fixed with paraformaldehyde and immunostained using a Y1 antibody or incubated for 4 h with the agonist [Leu31, Pro34]PYY (b) or the antagonist GR231118 (c) for 40 min at 37°C, before fixation and Y1 immunolabeling. All images were taken through the trans-nuclear (N) plane and are representative of duplicate experiments. Note that after incubation with the agonist, the immunoreactive receptor clusters in a juxta-nuclear zone akin to the pericentriolar recycling endosome (arrowheads), whereas after incubation with the antagonist, they remain haphazardly distributed throughout the cytoplasm. Scale bar: 8 μm.
We also verified whether a nonpeptidic antagonist could induce Y1 sequestration in HEK293 cells by examining the acid-wash-resistant binding of [3H]BIBP3226 (10 mM) on whole cells. Unlike GR231118, the nonspecific binding of [3H]BIBP3226 on living cells was as high as total binding. Thus, specific binding and internalization process cannot be investigated using such a nonpeptidic radioligand (data not shown). This is most likely due to the fact that BIBP2336, like all other nonpeptide Y1 receptor antagonists developed thus far, is very lipophilic and most likely can even directly cross the cell plasma membrane via a nonreceptor pathway. Furthermore, cells treated with 1 μM BIBP3226 displayed very low and diffuse Y1-like immunofluorescence following a 40 min to BIBP3226, suggesting that this compound may induce some cytotoxicity in HEK293 cells. In fact, in vivo studies have already reported toxic effect of BIBP3226 following i.c.v. injections (Rudolf et al., 1997).
Discussion
The present study demonstrates that in HEK293 cells transfected with Y1 receptors, the binding of the agonist [Leu31, Pro34]PYY as well as the antagonist GR231118 induces a time-dependent sequestration/internalization of ligand–receptor complexes. Receptor-mediated internalization/sequestration was first analyzed by quantifying the uptake of radioactive ligands into the cells after removal of surface-bound ligand by hypertonic acid wash. This approach has been widely used to investigate ligand-dependent GPCR/internalization (Nouel et al., 1997; Lee et al., 2002). As expected from earlier studies using Y1-transfected cells, binding of the agonist [Leu31, Pro34]PYY resulted in the internalization of over 60% of specifically bound radioactivity into the cells. Agonist endocytosis was confirmed by the use of a newly synthesized fluorescent probe, bodipy-[Leu31, Pro34]NPY (Pheng et al., 2001), which allowed the direct, confocal microscopic tracking of the intracellular trafficking of bound fluorescent ligand molecules.
Y1 receptor internalization induced by the antagonist GR231118 was more unexpected, as antagonists are usually believed not to induce receptor endocytosis (Gray & Roth, 2001). Recent data, however, have indicated that serotonin 5-HT2A, cholecystokinin A (CCK-A), bradykinin B2 and vasopressin V2 receptors may similarly undergo antagonist-induced internalization (Roettger et al., 1997; Pfeiffer et al., 1998; Houle et al., 2000; Gray & Roth, 2001). Our data suggest that this may also be the case for NPY Y1 receptors. Indeed, GR231118 promoted Y1 receptor internalization in HEK293 cells and yet has clear antagonistic properties at Y1 receptors in vitro as well in vivo (Parker et al., 1998; Serone et al., 2000).
We observed several differences, however, between [Leu31, Pro34]PYY-and GR231118-induced Y1 receptor endocytosis, suggesting that distinct mechanisms underlie agonist versus antagonist induced internalization. [Leu31, Pro34]PYY-mediated Y1 endocytosis is clearly a clathrin-dependent process, since both acid-wash-resistant bound radioligand and intracellular fluorescent labeling were strongly inhibited by sucrose and PAO. Furthermore, the fluorescent agonist bodipy-[Leu31, Pro34]NPY was targeted to endosome-like intracellular compartments, as would be expected for material endocytosed through a clathrin-dependent pathway (Delaney et al., 2002). These results confirm and extend previous reports demonstrating that in transfected HEK293 cells, agonist-induced Y1 receptor internalization is largely mediated by a clathrin-dependent mechanism (Gicquiaux et al., 2002). This process appears to underlie agonist-induced internalization of most GPCRs including, for example, somatostatin 1 (sst1), substance P-neurokinin 1 (NK1), mu opioid (μ), β2 adrenergic, neurotensin 1 (NT1) and gastrin-releasing peptide receptors (Bohm et al., 1997). In contrast, GR231118-induced Y1 receptor endocytosis was only marginally affected by sucrose or PAO, suggesting that its internalization/sequestration largely proceeded through a clathrin-independent endocytic pathway. Other receptors such as endothelin-1 and cholescystokinin A subtypes were shown to internalize via an alternate, clathrin-independent but caveolae-dependent pathway (Dupree et al., 1993; Chun et al., 1994; Roettger et al., 1995). Furthermore, recent studies have suggested that Y1 receptors can undergo caveolae-dependent sequestration in SK-N-MC and transfected CHO cells (Fabry et al., 2000; Parker et al., 2002). However, this does not appear to be the case for Y1 receptors in HEK293 cells since [Leu31, Pro34]PYY and GR231118 internalization/sequestration was not affected by treatment of the cells with filipin, this may suggest that the mechanism involved in NPY Y1 receptor internalization may be cell-type specific.
Another key difference between agonist- and antagonist-induced Y1 receptor internalization was that endocytosis of the agonist, but not antagonist receptor complex, was linked to G protein activation. All cloned NPY receptors, including the Y1 subtype, are coupled to PTX-sensitive Gi/o-proteins (Gerald et al., 1996). Accordingly, blockade of Gi/o activation by treatment with PTX significantly eliminated a component of [Leu31, Pro34]PYY-induced Y1 receptor sequestration/internalization in transfected HEK293 cells. These observations are in agreement with those of Gicquiaux et al. (2002) who demonstrated that EGFP-conjugated human Y1 receptor internalization in HEK293 cells was partially mediated by Gi/o-protein activation. The fact that PTX only partially inhibited [Leu31, Pro34]PYY suggests that other intracellular messengers, including Ca2+ (Jacques et al., 2000), may play a role in agonist-induced Y1 receptor endocytosis. In contrast, PTX treatment was without effect on GR231118 internalization/sequestration, suggesting that the endocytosis induced by this antagonist is independent from Gi/o activation. This observation is consistent with a recent report showing that antagonist-induced GPCR internalization was unaffected by intracellular mediators such as receptor phosphorylation, cAMP production, intracellular Ca2+ mobilization, or trans-membrane proton efflux (Roettger et al., 1997).
An earlier study suggested that following agonist stimulation, internalized EGFP-coupled Y1 receptors were targeted to the recycling pathway in HEK293 cells (Gicquiaux et al., 2002). Our immunofluorescence results showing targeting of immunoreactive Y1 receptors to a juxta-nuclear, pericentriolar recycling endosome-like compartment suggest that the same phenomenon occurs in HEK293 cells. Accordingly [Leu31, Pro34]PYY cell surface binding rapidly returned to basal levels following agonist stimulation, suggesting that internalized receptors were efficiently recycled. The finding that return to baseline was inhibited by treatment with the ionophore monensin confirmed that it was indeed due to receptor recycling and not to targeting of intracellular reserve receptors to the plasma membrane. In contrast, the loss of cell surface radioligand binding induced by treatment with GR231118 failed to rapidly recover and was not altered by monensin, indicating that receptors internalized upon antagonist binding were not recycled. Indeed, immunocytochemical data indicated that GR231118-stimulated Y1 receptors did not cluster in the region of the pericentriolar recycling endosome, but rather remained haphazardly distributed throughout the cytoplasm, suggesting that antagonist-induced sequestration proceeded through a different pathway.
In summary, Y1 receptors can undergo ligand-induced internalization/sequestration following stimulation with both agonists and antagonists in HEK293 cells. However, the mechanisms involved in agonist versus antagonists-induced internalization were clearly distinct in that the sequestration induced by GR231118, in contrast to that produced by [Leu31, Pro34]PYY, was independent of G-protein activation and that the receptor was not targeted to the recycling pathway. If these results are applicable in vivo, they could suggest that chronic treatment with Y1 antagonists such as GR231118 may induce cell surface Y1 receptor losses, leading to apparent conditional knockout of Y1 receptor activity, this possibly being of clinical significance. Further studies will be required using water-soluble nonpeptide Y1 receptor antagonists in order to determine if Y1 receptor internalization observed with GR231118 applies to such molecules, those are not available at this time.
Acknowledgments
The authors wish to thank Dr A.G. Beck-Sickinger for providing us anti-Y1 antibodies. This work was supported by Heart and Stroke Foundation of Canada (HSFC) and Canadian Institute of Health Research (CIHR).
Abbreviations
- BIBP3226
R-N2-(diphenylacetyl)-N-(4-hydroxyphenyl)-methylargininamide
- BSA
bovine serum albumin
- GPCR
G-protein coupled receptor
- GR231118
homodimeric Ile-Glu-Pro-Dpr-Tyr-Arg-Leu-Arg-Tyr-CONH2
- HEK293
human embryonic kidney cells
- NPY
neuropeptide Y
- MβCD
methyl-β-cyclodextrin
- PAO
phenylarsine oxide
- PTX
pertussis toxin
- PYY
peptide YY
References
- ADRIAN T.E., ALLEN J.M., BLOOM S.R., GHATEI M.A., ROSSOR M.N., ROBERTS G.W., CROW T.J., TATEMOTO K., POLAK J.M. Neuropeptide Y distribution in human brain. Nature. 1983;306:584–586. doi: 10.1038/306584a0. [DOI] [PubMed] [Google Scholar]
- BOHM S.K., GRADY E.F., BUNNETT N.W.Regulatory mechanisms that modulate signalling by G-protein-coupled receptors Biochem. J. 19973221–18.(Part 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURKHOFF A., LINEMEYER D.L., SALON J.A. Distribution of a novel hypothalamic neuropeptide Y receptor gene and it's absence in rat. Brain Res. Mol. Brain Res. 1998;53:311–316. doi: 10.1016/s0169-328x(97)00302-1. [DOI] [PubMed] [Google Scholar]
- CHUN M., LIYANAGE U.K., LISANTI M.P., LODISH H.F. Signal transduction of a G protein-coupled receptor in caveolae: colocalization of endothelin and its receptor with caveolin. Proc. Natl. Acad. Sci. U.S.A. 1994;91:11728–11732. doi: 10.1073/pnas.91.24.11728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELANEY K.A., MURPH M.M., BROWN L.M., RADHAKRISHNA H. Transfer of M2 muscarinic acetylcholine receptors to clathrin-derived early endosomes following clathrin-independent endocytosis. J. Biol. Chem. 2002;277:33439–33446. doi: 10.1074/jbc.M205293200. [DOI] [PubMed] [Google Scholar]
- DOODS H.N., WIELAND H.A., ENGEL W., EBERLEIN W., WILLIM K.D., ENTZEROTH M., WIENEN W., RUDOLF K. BIBP 3226, the first selective neuropeptide Y1 receptor antagonist: a review of its pharmacological properties. Regul. Pept. 1996;65:71–77. doi: 10.1016/0167-0115(96)00074-2. [DOI] [PubMed] [Google Scholar]
- DUMONT Y., FOURNIER A., ST-PIERRE S., QUIRION R. Characterization of neuropeptide Y binding sites in rat brain membrane preparations using [125I][Leu31,Pro34]peptide YY and [125I]peptide YY3-36 as selective Y1 and Y2 radioligands. J. Pharmacol. Exp. Ther. 1995;272:673–680. [PubMed] [Google Scholar]
- DUMONT Y., MARTEL J.C., FOURNIER A., ST-PIERRE S., QUIRION R. Neuropeptide Y and neuropeptide Y receptor subtypes in brain and peripheral tissues. Prog. Neurobiol. 1992;38:125–167. doi: 10.1016/0301-0082(92)90038-g. [DOI] [PubMed] [Google Scholar]
- DUMONT Y., QUIRION R. [125I]- GR231118: a high affinity radioligand to investigate neuropeptide Y Y1 and Y4 receptors. Br. J. Pharmacol. 2000;129:37–46. doi: 10.1038/sj.bjp.0702983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUMONT Y., ST-PIERRE J.A., THAKUR M., PHENG L.H., LANGLOIS D., SLON-USAKIEWICZ J., WENHAM D., GAUDREAU P., QUIRION R.Characterisation of Bodipy-conjugated fluo-neuropeptide Y analogs in Y1, Y2, Y4 and Y5 receptor binding assays 2001. Society for Neuroscience, Abstract Viewer/Itinarary Planner. Washington, DC., Program No. 30.15
- DUPREE P., PARTON R.G., RAPOSO G., KURZCHALIA T.V., SIMONS K. Caveolae and sorting in the trans-Golgi network of epithelial cells. EMBO J. 1993;12:1597–1605. doi: 10.1002/j.1460-2075.1993.tb05804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVA C., KEINANEN K., MONYER H., SEEBURG P., SPRENGEL R. Molecular cloning of a novel G protein-coupled receptor that may belong to the neuropeptide receptor family. FEBS Lett. 1990;271:81–84. doi: 10.1016/0014-5793(90)80377-u. [DOI] [PubMed] [Google Scholar]
- FABRY M., LANGER M., ROTHEN-RUTISHAUSER B., WUNDERLI-ALLENSPACH H., HOCKER H., BECK-SICKINGER A.G. Monitoring of the internalization of neuropeptide Y on neuroblastoma cell line SK-N-MC. Eur. J. Biochem. 2000;267:5631–5637. doi: 10.1046/j.1432-1327.2000.01631.x. [DOI] [PubMed] [Google Scholar]
- GERALD C., WALKER M.W., CRISCIONE L., GUSTAFSON E.L., BATZL-HARTMANN C., SMITH K.E., VAYSSE P., DURKIN M.M., LAZ T.M., LINEMEYER D.L., SCHAFFHAUSER A.O., WHITEBREAD S., HOFBAUER K.G., TABER R.I., BRANCHEK T.A., WEINSHANK R.L. A receptor subtype involved in neuropeptide-Y-induced food intake. Nature. 1996;382:168–171. doi: 10.1038/382168a0. [DOI] [PubMed] [Google Scholar]
- GICQUIAUX H., LECAT S., GAIRE M., DIETERLEN A., MELY Y., TAKEDA K., BUCHER B., GALZI J.L. Rapid internalization and recycling of the human neuropeptide Y Y(1) receptor. J. Biol. Chem. 2002;277:6645–6655. doi: 10.1074/jbc.M107224200. [DOI] [PubMed] [Google Scholar]
- GRAY J.A., ROTH B.L. Paradoxical trafficking and regulation of 5-HT(2A) receptors by agonists and antagonists. Brain Res. Bull. 2001;56:441–451. doi: 10.1016/s0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- HAUSDORFF W.P., CARON M.G., LEFKOWITZ R.J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- HEGDE S.S., BONHAUS D.W., STANLEY W., EGLEN R.M., MOY T.M., LOEB M., SHETTY S.G., DESOUZA A., KRSTENANSKY J. Pharmacological evaluation of 1229U91, a novel high-affinity and selective neuropeptide Y-Y1 receptor antagonist. J. Pharmacol. Exp. Ther. 1995;275:1261–1266. [PubMed] [Google Scholar]
- HEILIG M., SODERPALM B., ENGEL J.A., WIDERLOV E. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacology (Berl) 1989;98:524–529. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- HOULE S., LARRIV INVERTED QUESTION MARKEE J.F., BACHVAROVA M., BOUTHILLIER J., BACHVAROV D.R., MARCEAU F. Antagonist-induced intracellular sequestration of rabbit bradykinin B(2) receptor. Hypertension. 2000;35:1319–1325. doi: 10.1161/01.hyp.35.6.1319. [DOI] [PubMed] [Google Scholar]
- HUNYADY L. Molecular mechanisms of angiotensin II receptor internalization. J. Am. Soc. Nephrol. 1999;10 Suppl 11:S47–S56. [PubMed] [Google Scholar]
- JACQUES D., SADER S., EL-BIZRI N., CHOUFFANI S., HASSAN G., SHBAKLO H. Neuropeptide Y induced increase of cytosolic and nuclear Ca2+ in heart and vascular smooth muscle cells. Can. J. Physiol. Pharmacol. 2000;78:162–172. [PubMed] [Google Scholar]
- LEE M.C., CAHILL C.M., VINCENT J.P., BEAUDET A. Internalization and trafficking of opioid receptor ligands in rat cortical neurons. Synapse. 2002;43:102–111. doi: 10.1002/syn.10014. [DOI] [PubMed] [Google Scholar]
- LEW M.J., MURPHY R., ANGUS J.A. Synthesis and characterization of a selective peptide antagonist of neuropeptide Y vascular postsynaptic receptors. Br. J. Pharmacol. 1996;117:1768–1772. doi: 10.1111/j.1476-5381.1996.tb15352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHEL M.C., BECK-SICKINGER A., COX H., DOODS H.N., HERZOG H., LARHAMMAR D., QUIRION R., SCHWARTZ T., WESTFALL T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol. Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- NOUEL D., GAUDRIAULT G., HOULE M., REISINE T., VINCENT J.P., MAZELLA J., BEAUDET A. Differential internalization of somatostatin in COS-7 cells transfected with SST1 and SST2 receptor subtypes: a confocal microscopic study using novel fluorescent somatostatin derivatives. Endocrinology. 1997;138:296–306. doi: 10.1210/endo.138.1.4834. [DOI] [PubMed] [Google Scholar]
- PARKER E.M., BABIJ C.K., BALASUBRAMANIAM A., BURRIER R.E., GUZZI M., HAMUD F., MUKHOPADHYAY G., RUDINSKI M.S., TAO Z., TICE M., XIA L., MULLINS D.E., SALISBURY B.G. GR231118 (1229U91) and other analogues of the C-terminus of neuropeptide Y are potent neuropeptide Y Y1 receptor antagonists and neuropeptide Y Y4 receptor agonists. Eur. J. Pharmacol. 1998;349:97–105. doi: 10.1016/s0014-2999(98)00171-x. [DOI] [PubMed] [Google Scholar]
- PARKER S.L., KANE J.K., PARKER M.S., BERGLUND M.M., LUNDELL I.A., LI M.D. Cloned neuropeptide Y (NPY) Y1 and pancreatic polypeptide Y4 receptors expressed in Chinese hamster ovary cells show considerable agonist-driven internalization, in contrast to the NPY Y2 receptor. Eur. J. Biochem. 2001;268:877–886. doi: 10.1046/j.1432-1327.2001.01966.x. [DOI] [PubMed] [Google Scholar]
- PARKER S.L., PARKER M.S., LUNDELL I., BALASUBRAMANIAM A., BUSCHAUER A., KANE J.K., YALCIN A., BERGLUND M.M. Agonist internalization by cloned Y1 neuropeptide Y (NPY) receptor in Chinese hamster ovary cells shows strong preference for NPY, endosome-linked entry and fast receptor recycling. Regul. Pept. 2002;107:49–62. doi: 10.1016/s0167-0115(02)00094-0. [DOI] [PubMed] [Google Scholar]
- PFEIFFER R., KIRSCH J., FAHRENHOLZ F. Agonist and antagonist-dependent internalization of the human vasopressin V2 receptor. Exp. Cell Res. 1998;244:327–339. doi: 10.1006/excr.1998.4159. [DOI] [PubMed] [Google Scholar]
- PHENG L.H., DUMONT Y., FOURNIER A., BEAUDET A., QUIORION R.Regulation of cell surface neuropeptide Y Y1 receptor in HEK293 cell by agonist and antagonist Society for Neuroscience 2001. Abstract Viewer/Itinarary Planner. Washington, DC., Program No. 461.9
- PHENG L.H., REGOLI D. Bioassays for NPY receptors: old and new. Regul. Pept. 1998;75–76:79–87. doi: 10.1016/s0167-0115(98)00055-x. [DOI] [PubMed] [Google Scholar]
- ROETTGER B.F., GHANEKAR D., RAO R., TOLEDO C., YINGLING J., PINON D., MILLER L.J. Antagonist-stimulated internalization of the G protein-coupled cholecystokinin receptor. Mol. Pharmacol. 1997;51:357–362. [PubMed] [Google Scholar]
- ROETTGER B.F., RENTSCH R.U., PINON D., HOLICKY E., HADAC E., LARKIN J.M., MILLER L.J. Dual pathways of internalization of the cholecystokinin receptor. J. Cell Biol. 1995;128:1029–1041. doi: 10.1083/jcb.128.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUDOLF K., EBERLEIN W., ENGEL W., WIELAND H.A., WILLIM K.D., ENTZEROTH M., WIENEN W., BECK-SICKINGER A., DOODS H.N.BIBP3226, a potent and selective neuropeptide Y Y1-receptor antagonist. Structure–activity studies and localization of the human Y1 receptor binding site Neuropeptide Y and Drug Development 1997London: Academic Press; 175–190.ed. Grundemar, L. & Bloom, S.R., pp [Google Scholar]
- SCHOBER D.A., GACKENHEIMER S.L., HEIMAN M.L., GEHLERT D.R. Pharmacological characterization of 125I-1229U91 binding to Y1 and Y4 neuropeptide Y/peptide YY receptors. J. Pharmacol. Exp. Ther. 2000;293:275–280. [PubMed] [Google Scholar]
- SERONE A.P., WRIGHT C.E., ANGUS J.A. Role of NPY Y1 receptors in cardiovascular control in the conscious rabbit. J. Cardiovasc. Pharmacol. 2000;35:315–321. doi: 10.1097/00005344-200002000-00021. [DOI] [PubMed] [Google Scholar]
- STANLEY B.G., LEIBOWITZ S.F. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984;35:2635–2642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- ST-PIERRE J.A., NOUEL D., DUMONT Y., BEAUDET A., QUIRION R. Sub-population of cultured hippocampal astrocytes expresses neuropeptide Y Y1 receptors. Glia. 2000;30:82–91. [PubMed] [Google Scholar]
- THORSELL A., HEILIG M. Diverse functions of neuropeptide Y revealed using genetically modified animals. Neuropeptides. 2002;36:182–193. doi: 10.1054/npep.2002.0897. [DOI] [PubMed] [Google Scholar]
- TONG Y., DUMONT Y., SHEN S.H., HERZOG H., SHINE J., QUIRION R. Expression of the neuropeptide Y Y1 receptor in human embryonic kidney 293 cells: ligand binding characteristics, in situ hybridization and receptor autoradiography. Brain Res. Mol. Brain Res. 1995;34:303–308. doi: 10.1016/0169-328x(95)00176-s. [DOI] [PubMed] [Google Scholar]
- TUOR U.I., EDVINSSON L., MCCULLOCH J. Neuropeptide Y and cerebral blood flow. Lancet. 1985;1:1271. doi: 10.1016/s0140-6736(85)92336-0. [DOI] [PubMed] [Google Scholar]
- VANDENBULCKE F., NOUEL D., VINCENT J.P., MAZELLA J., BEAUDET A.Ligand-induced internalization of neurotensin in transfected COS-7 cells: differential intracellular trafficking of ligand and receptor J. Cell Sci. 20001132963–2975.(Part 17) [DOI] [PubMed] [Google Scholar]
- VEZZANI A., SPERK G., COLMERS W.F. Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]