Abstract
In this study, we have used isolated brain mitochondria to investigate the effects of superoxide anions (O2−) on mitochondrial parameters related to apoptosis, such as swelling, potential, enzymatic activity, NAD(P)H, cytochrome c release, and caspase activity.
Addition of the reactive oxygen species (ROS) generator KO2 produced brain mitochondrial swelling, which was blocked by cyclosporin A (CSA), and which was Ca2+ independent.
Calcium induced mitochondrial swelling only at high concentrations and in the presence of succinate. This correlated with the increase in O2− production detected with hydroethidine in mitochondrial preparations exposed to Ca2+ and the fact that ROS were required for Ca2+-induced mitochondrial swelling.
Superoxide anions, but not Ca2+, decreased citrate synthase and dehydrogenase enzymatic activities and dropped total mitochondrial NAD(P)H levels.
Calcium, but not O2−, triggered a rapid loss of mitochondrial potential. Calcium-induced Δψm dissipation was inhibited by Ruthenium Red, but not by CSA.
Calcium- and superoxide-induced mitochondrial swelling released cytochrome c and increased caspase activity from isolated mitochondria in a CS A-sensitive manner.
In summary, superoxide potently triggers mitochondrial swelling and the release of proteins involved in activation of postmitochondrial apoptotic pathways in the absence of mitochondrial depolarization.
Keywords: Caspase, mitochondrion, excitotoxicity, apoptosis, ROS, brain
Introduction
Mitochondrial dysfunctions, because of DNA defects, protein mutations or alterations in electron transport (Koutnikova et al., 1997) have been linked to neurodegenerative diseases (Sheehan et al., 1997; Brown et al., 2000) and aging processes (Wallace, 2001). Mitochondria play a central role in different cell death pathways leading to either apoptosis or necrosis (Green & Reed, 1998; Kroemer & Reed, 2000). The first evidence that mitochondria play an important role in cellular death mechanisms came from the observation that ‘in vitro' models required an organelle fraction enriched in mitochondria to induce apoptosis (Newmeyer et al., 1994). Apoptosis might involve changes in respiration-dependent mitochondrial potential (Δψm) and, in some models, formation of a voltage-dependent high conductance multiproteic channel, referred to as mitochondrial permeability transitory pore (MPTP) (Bernardi, 1999). However, there is increasing evidence suggesting that MPTP activation is also important for necrosis activation (Kroemer & Reed, 2000).
The Δψm role in apoptosis is still an enigma, as either preservation (Prehn et al., 1996) or dissipation (Stout et al., 1998) has been shown to block neuronal death. Moreover, the relation between MPTP and Δψm remains unclear. There are reports suggesting that for some apoptotical stimuli, MPTP formation is independent of mitochondrial transmembrane depolarization (Madesh & Hajnoczky, 2001). As a result of MPTP opening, mitochondria might lose their membrane permeability control, releasing internal proteins (cytochrome c, apoptosis-inducing factor (AIF), SMAC/Diablo, caspase family members, etc.) to the cytoplasm. This release results in the activation of different pathways that lead to cell death. For example, cytochrome c participates in the apoptosome formation, and AIF results in chromatin condensation and DNA fragmentation (Susin et al., 1999). Although the physiological role of MPTP has not yet been determined, its role as mediator of cell injury and death is generally accepted.
Calcium influx and O2− production within the mitochondria prior to cell death are events shared by cells treated with neurotoxic agents like NMDA, veratridine and β-amyloid (Bindokas et al., 1996; Jordan et al., 2000, 2002; Longo et al., 2000). Overexpression of proteins that regulate Ca2+ and O2− levels protect neuronal cultures from neurotoxic stimuli (Jordan et al., 1995; Prehn et al., 1997). Evidence has been presented suggesting that intracellular O2− production during apoptosis injury is Ca2+ dependent and from mitochondrial origin (Ankarcrona et al., 1995; Bindokas et al., 1996; Jordan et al., 2000) and either stimuli result in MPTP formation (for review, see Bernardi, 1999). In the present study, we have analyzed the plausible effects of O2− and Ca2+ on mitochondrial swelling and Δψm dissipation using isolated rat brain mitochondria.
Methods
Mitochondria isolation
Mitochondria were isolated from the cortex of adult Sprague–Dawley rats by conventional centrifugation as previously described (Jordán et al., 2002). Briefly, tissue was manually homogenized with four strokes with a Teflon pestle in solution I (Sol I) containing 230 mM mannitol, 70 mM sucrose, 1 mM EGTA, and 5 mM HEPES, pH 7.4 on ice. After centrifugation at 4°C (106 × g; 80 s), supernatant was layered onto solution II (460 mM mannitol, 14 mM sucrose, 1 mM EGTA, and 10 mM HEPES, pH 7.4), and centrifuged (800 × g; 3 min). The top layer was then centrifuged (2000 × g; 5 min) and the mitocondrial pellet resuspended in solution III (215 mM mannitol, 71 mM sucrose, 10 mM succinate, and 10 mM HEPES, pH 7.4) and kept on ice until swelling determinations. To exclude that the observed effects were becuase of contaminating synaptosome, we isolated brain mitochondria using a Percoll gradient as previously described (Kristal & Dubinsky, 1997). No differences in KO2-induced swelling were observed between mitochondria isolated by both the methods.
Permeability transition pore activity
Permeability transition pore opening was assayed spectrophotometrically as previously described (Kristal et al., 2000; Galindo et al., 2003). Specifically, mitochondria were suspended to reach a protein concentration of 1 mg ml−1 in 200 μl of solution III. Changes in absorbance at 540 nm (A540), indicating mitochondrial swelling because of MPTP opening, were followed, after addition of different compounds, using a microplate reader (BioRad, Hercules, CA, U.S.A.). Initial A540 values were ≅0.8, and minor differences in loading were compensated by representing the data as the fraction of the initial absorbance measure remaining at a given time. Mitochondrial protein concentrations were quantified spectophotometrically (Micro BCA Protein Reagent Kit, Pierce, Rockford, IL, U.S.A.), with bovine serum albumin as standard.
Monitoring of the mitochondrial membrane potential
Changes in mitochondrial membrane potential were measured in the presence of 0.1 μg ml−1. rhodamine 123 (Rh 123) as described by Emaus et al. (1996). The excitation and emission wavelengths for Rh 123 were 503 and 525 nm (slit 3 nm), respectively, using a Perkin-Elmer (luminiscence-spectrophotometer LS50B) fluorimeter.
Assay for mitochondrial NAD(P)H levels
NAD(P)H fluorescence in intact mitochondria (1 mg ml−1 at 25°C) was measured spectrofluorimetrically with excitation and emission wavelengths of 340 nm (slit 3 nm) and 460 nm (slit 5 nm), respectively, as previously described (Rover Junior et al., 1998). We therefore refer to NAD(P)H, indicating the signal derived from either NADH or NADPH, or both. Under these conditions, an increase in autofluorescence signal indicates an increase in the reduced state of the pyridine nucleotide, NAD(P)H, and a decrease in autofluorescence signal indicates an increased oxidation to NAD(P)+.
Mitochondrial dehydrogenase activity
Mitochondrial dehydrogenase activity was estimated by the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide thiazolyl) blue (MTT) to its corresponding blue formazan product according to a procedure described elsewhere (Mosmann, 1983). After 30 min incubation of mitochondrial suspension with Ca2+ or O2−, mitochondria were further incubated for 15 min with 0.5 mg ml−1 MTT. At the end of the incubation period, absorbances were read on a microplate reader (BioRad, Hercules, CA, U.S.A.) with a test wavelength of 540 nm and a reference wavelength of 630 nm.
Citrate synthase activity
Citrate synthase (EC 4.1. 3.7) was measured at 410 nm using a microplate reader (BioRad, Hercules, CA, U.S.A.) at room temperature as described by Shepherd & Garland (1969). The reaction buffer contains 100 mM Tris-HCl, pH 8, 0.1 mM acetyl-CoA, 0.2 mM DTNB, 0.1 (V V−1) Triton X-100, and 500 μg protein of mitochondrial suspension in a final volume of 0.2 ml. The assay is initiated by the addition of 10 μl of 20 mM oxalacetate and 10 min later absorbance was read on a microplate reader (BioRad, Hercules, CA, U.S.A.).
Immunoblot analysis
Immunoblot analysis was performed on extramitochondrial extracts from control and treated mitochondrial suspensions. In all cases, mitochondria suspensions were treated with resuspension (control), CaCl2, or KO2 for 30 min and then centrifuged at 15,000 rpm for 15 min. The supernatants (S100), extramitochondrial fractions, were precipitated by trichloroacetic acid (TCA) (10%, 4°C) overnight and centrifuged (15,000 × g;15 min). Pellets were resuspended in 40 μl loading buffer and boiled for 15 min. Samples were loaded onto a 15% SDS-polyacrylamide gel, separated and transferred to a PVDF membrane, which was incubated with mouse monoclonal anticytochrome c antibody (Santa Cruz Biotechnology Inc., U.S.A.; 1 : 1000 dilution). The secondary antibody used (1 : 5000 dilution) was a peroxidase-labeled anti-mouse (Promega, Madison, WI., U.S.A.). The signal was detected using an enhanced chemiluminescence detection kit (Amersham ECL RPN 2106 Kit). Ruthenium Red (5 μM) or cyclosporin A (CsA) (10 μM) was added 15 min before and maintained during CaCl2 or KO2 treatment.
Assay of caspase enzymatic activity
Mitochondrial suspension aliquots were exposed for 30 min to CaCl2 or KO2 and then centrifuged at 15,000 × g for 15 min. Supernatants, lacking mitochondria, were collected and incubated at 37°C in a buffer containing 25 mM HEPES (pH 7.5), 10% sucrose, 0.1 CHAPS and 10 mM DTT with the fluorogenic substrate N-acetyl-Asp-Glu-Val-Asp-7-amido-4- trifluoromethylcoumarin (DEVD-AFC) (15 μM in DMSO, Calbiochem System Products) (Jordan et al., 1997). Substrate cleavage emitted a fluorescent signal that was quantified in a fluorometer Perkin-Elmer (luminiscence-spectrophotometer LS50B) (excitation 400 nm, emission 505 nm). Enzymatic activity is expressed as arbitrary fluorescent units (AFU). Ruthenium Red (5 μM) or CsA (10 μM) was added 15 min before and maintained during CaCl2 or KO2 treatment.
Statistical analysis
Quantitative data are expressed as means±s.e.m. and significance was determined by Student's t-test. Statistical significance was considered at the P<0.05 level.
Results
Superoxide induces brain mitochondrial swelling in a Ca2+-independent manner
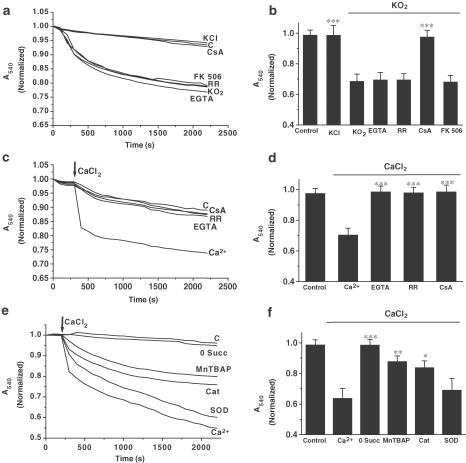
Induction of mitochondrial swelling in isolated brain mitochondria was monitored by following 540 nm absorbance (A540) decrease (Bernardi et al., 1999). We used KO2 as a source of O2− anions and corresponding degraded ROS products. KO2 addition (5 μmol mg−1 mit) resulted in an extensive mitochondrial swelling (Figure 1a) that was greatly inhibited by the pretreatment and presence of 10 μM CsA, a MPTP blocker, but not by the calcineurin inhibitor FK506 (200 nM), (Figure 1b). Addition of KCl (5 mol mg−1 mit) did not result in mitochondrial swelling, confirming that KO2-induced mitochondrial swelling was because of reactive reactive oxygen species (ROS) and not to potassium (Figure 1a).
Figure 1.
KO2 and high Ca2+ induce mitochondrial swelling. A–B. KO2 addition (5 μmol mg−1 protein; arrow), induces mitochondrial swelling in brain-isolated mitochondria in an MPTP sensitive manner, where Ca2+ cycling is not involved. Mitochondrial suspensions were incubated during 15 min with CsA (10 μM), FK 506 (200 nM), Ruthenium Red (RR; 5 μM), or EGTA (1 mM), prior to addition of KO2. Control (c) and the effect of KCl (5 mM) addition on non-treated mitochondria are also shown. C–D. Calcium addition (15 μmol mg−1 mitochondria) induces mitochondrial swelling in brain-isolated mitochondria. Mitochondrial suspensions were incubated during 15 min with CsA (10 μM), Ruthenium Red (RR;5 μM), or EGTA (1 mM) before Ca2+ addition. Control non-treated mitochondria (c) are also shown. E–F. Ca2+-induced swelling is ROS and succinate dependent. In absence of succinate (0 Succ), Ca2+ failed to induce mitochondrial swelling. Mitochondria were preincubated for 15 min with MnTBAP (10 nM), catalase (Cat;1750 U) or SOD (450 U), prior to Ca2+ addition (15 μmol mg−1 mit). Control nontreated mitochondria (c) are also shown. Lines represent mean values of one experiment performed by triplicate. Histograms (panels b, d, f) represent mean values±s.e.m. of normalized A540 at 2200 s from at least five different mitochondrial preparations. ***P<0.001;**P<0.01;*P<0.05 as compared with KO2 or Ca2+-treated mitochondria in absence of the drug.
Since a variety of chemically different pro-oxidants have been shown to induce Ca2+ release from mitochondria and the fact that Ca2+ induces mitochondrial swelling in liver mitochondria, we next investigated the existence of a positive feedback between O2− anions and Ca2+ in mitochondrial swelling. Blockade of Ca2+ influx, by the presence of the mitochondrial Ca2+ uptake blocker, Ruthenium Red, or by addition of the Ca2+ chelator EGTA (1 mM) did not affect KO2-induced mitochondrial swelling, suggesting that Ca2+ does not seem to play a significant role in KO2-induced mitochondrial swelling (Figure 1c). In contrast to noncerebral tissue, only relatively high Ca2+ concentrations induced brain mitochondrial swelling and required the presence of succinate in the mitochondrial resuspension medium, because Ca2+ failed to induce mitochondrial swelling in its absence (0 Succ, Figure 1c), while KO2 did not (data not show). Even at these high Ca2+ concentrations, the Ca2+ Ca2+-induced mitochondrial swelling was blocked by Ruthenium Red, EGTA, and CsA (Figure 1d). Lower Ca2+ concentrations did not produce significant swelling (data not shown), although we cannot exclude that mitochondrial swelling may even be underestimated, as some heavy microsomal membranes could not be entirely separated from mitochondria.
The succinate requirement for Ca2+ to induce mitochondrial swelling suggested a role for coenzyme Q as a significant generator of O2− (Kowaltowski et al., 1995). To test if O2− production takes place, in isolated mitochondria, during Ca2+ exposures, selective oxidation of hydroethidine, by O2−, to ethidium was measured. Brain mitochondria showed a basal O2− production rate of 0.0426±0.0072 AFU min−1 (n=16). Calcium (15 μmol mg−1 mit) caused an increase in the rate of O2− production reaching a value of 0.0675±0.0153 AFU min−1 (P<0.05; n=16). To check whether this O2−, or its derived ROS, produced by Ca2+ in mitochondria, participated in Ca2+-induced mitochondrial swelling, we tested the effects of different free radical scavengers. As shown in Figure 1e, MnTBAP, a manganese porphyrin with SOD mimic activity, or catalase addition (1750 U ml−1) significantly attenuated Ca2+-induced mitochondrial swelling, while addition of SOD (450 U ml−1) did not block significantly Ca2+-induced swelling (Figure 1f). Consistently with the utilization of KO2 as a ROS source, the addition of antioxidant drugs decreased significantly KO2-induced mitochondrial swelling (data not shown).
KO2-induced mitochondrial swelling occurs in absence of Δψm depolarization
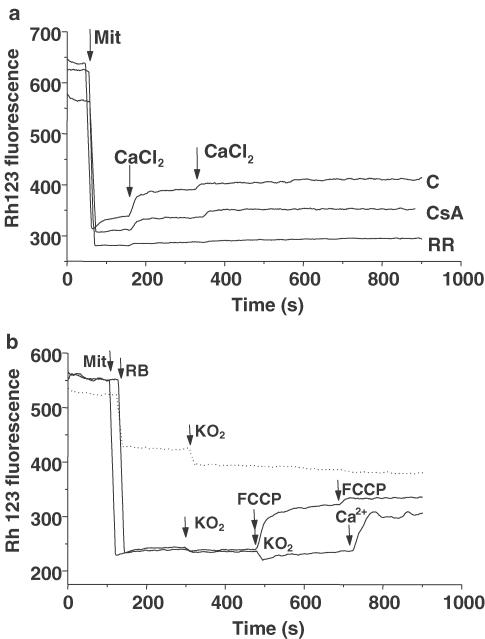
One of the most intriguing questions that remains unanswered is whether mitochondrial swelling produces Δψm depolarization or if Δψm dissipation precedes mitochondrial swelling. In the next set of experiments, we studied whether Ca2+ or O2− was able to modify Δψm. We monitored Δψm changes by measuring the release of the cationic membrane-permeant fluorescent probe Rh 123 preloaded into mitochondria. Under these conditions, total fluorescence of mitochondrial suspension will increase if the organelles depolarize. As shown in Figure 2, Ca2+, but not KO2, induced mitochondrial depolarization. Calcium concentrations required to evoke Δψm depolarization were 20-fold lower than those necessary to induce mitochondrial swelling. Mitochondrial depolarization was dependent of Ca2+ influx, but independent of MPTP opening, since it was blocked by 15 min pretreatment with Ruthenium Red (5 μM) but not by CsA (10 μM) (Figure 2a). Nevertheless, KO2 additions failed to influence Δψm within the same concentration range that caused mitochondrial swelling (Figure 2b).
Figure 2.
Changes in mitochondrial membrane potential as assessed by Rh 123. Fluorescence excited at 503 nm and emitted at 527 nm was measured in isolated mitochondria. (a) Addition of mitochondria (Mit, 500 μg) caused a decrease in fluorescence intensity because of quenching of Rh 123. CaCl2 (0.75 μmol mg−1 mit) was added under control conditions (trace c). In traces RR and CsA, mitochondria were incubated with Ruthenium Red (5 μM, trace RR) or CsA (10 μM, trace CsA) for 15 min and then added to Rh 123 solution. (b) The protocol was similar, but two KO2 (2.5 and 5 μmol mg−1 mit, continuous line) sequential pulses were added. Calcium was added to the incubation buffer to ensure that the mitochondria could be depolarized. Data are expressed as mean values obtained from one experiment performed in triplicate. Similar data were found in at least four different experiments. FCCP (0.1 μM) was added to induce mitochondrial depolarization. Dashed line represents control experiments performed by adding mitochondrial resuspension buffer (RB) and KO2 solutions in absence of brain mitochondria.
Effects of high Ca2+ and O2− on mitochondrial enzymatic activity and NAD(P)H levels
The next set of experiments were performed to analyze the possible effects of Ca2+ and O2− on mitochondrial metabolic enzymatic activity.
Mitochondrial dehydrogenase activity was measured by determining the formation of MTT into formazan. Under our experimental conditions, control mitochondrial suspension (500 μg protein) had an enzymatic activity of 0.165±0.036 (n=4) and was not affected by the treatment with Ca2+ (15 μmol mg−1 mit) for 30 min (0.145 × 0.034; n=4). However, 30 min incubation of intact mitochondria with KO2 (5 μmol mg−1 mit) produced a marked decrease in dehydrogenase activity (0.052±0.016; P<0.001; n=4).
Furthermore, the Krebs cycle enzyme citrate synthase was also affected by the presence of O2−. Mitochondrial citrate synthase was measured by determining the reaction of free CoA with DTNB. Incubation of brain mitochondria (500 μg protein) with KO2 (5 μmol mg−1 mit) for 30 min, did result in a loss in enzymatic activity (0.175±0.015 (P<0.001; n=5)) compared to control mitochondrial suspension (0.356±0.026; n=5), while Ca2+ (15 μmol mg−1 mit; 30 min) did not alter citrate synthase activity (0.3494±0.037; n=5).
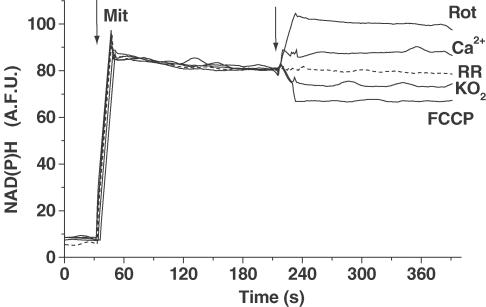
Recently, NAD(P)H has been proposed to act as a direct antioxidant agent (Kirsch & De Groot, 2001). Therefore, we were interested in whether the NAD(P)H levels were modified by these treatments when using either O2− or Ca2+. As Figure 3 illustrates, Ca2+ (15 μmol mg−1 mit) increases NAD(P)H levels in a Ruthenium Red (5 μM, 15 min)-sensitive manner, while, following KO2 addition (5 μmol mg−1 mit), an initial rapid decline in mitochondrial NAD(P)H concentration was observed. Rotenone, a mitochondrial respiratory chain complex I inhibitor (10 μM, Rot) and the mitocondrial uncoupler, FCCP (1 μM, FCCP) were added to estimate the maximal and the minimum nucleotide signal, respectively (Figure 3).
Figure 3.
Effect of KO2 and Ca2+ on mitochondrial NAD(P)H levels. NAD(P)H levels were monitored spectrofluorimetrically as described in methods. After 60 s of base line, intact mitochondria (500 μg) were added. Arrow indicates CaCl2 (15 μmol mg−1 mit; Ca2+), KO2 (5 μmol mg−1 mit; KO2), rotenone (10 μM, Rot), and FCCP (1 μM, FCCP) additions. Dashed line represents mitochondria preparation preincubated for 15 min in the presence of Ruthenium Red (5 μM; RR) before CaCl2 addition. One single experiment is shown, but similar data were found in four to six different mitochondrial preparations.
O2− induces release of cytochrome c and caspase activity from mitochondria
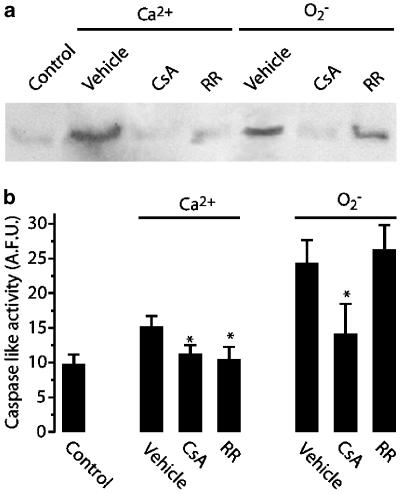
A consequence of mitochondrial swelling is the release of intramitochondrial proteins like cytochrome c to the cytoplasm (Maestre et al., 2003). In order to confirm the occurrence of this intramitochondrial protein release in our model, we exposed isolated brain mitochondria for 30 min to CaCl2 (15 μmol mg−1 mit) or KO2 (5 μmol mg−1 mit) and tested for cytochrome c presence and DEVD-like caspase activity in the extramitochondrial medium. As shown in Figure 4a, direct evidence for cytochrome c release from rat brain mitochondria was provided by Western blotting technique of cytochrome c in the S100 supernatant, after treating mitochondria with either Ca2+ or O2−. We next examined whether DEVD caspase activity was released from isolated mitochondria following exposure to Ca2+ or KO2. After these treatments, we performed measurements of the cleavage of a fluorometric caspase substrate, DEVD-AFC (Figure 4b). Increases in DEVD-caspase activity were found in S100 after Ca2+ and O2− additions (Figure 4b). It is worthy noting that in both assays, O2− effect was higher than Ca2+, perhaps because of the the fact that Ca2+ might inhibit caspase activity, as seems to happen in Ca2+-mediated neuronal death (Reimertz et al., 2001). Consistently with the mitochondrial swelling results, this effect was abolished by CsA, whereas Ruthenium Red was only effective against Ca2+ treatments (Figure 4b).
Figure 4.
Ca2+ and O2− addition induced release of cytochrome c and caspase activity from mitochondria. (a) Mitochondria were incubated during 30 min with resuspension (control), CaCl2 (15 μmol mg−1 mit), or KO2 (5 μmol mg−1 mit) and then centrifuged for 15 min at 15,000 rpm at 4°C (S100; see Methods section). For pharmacological studies, mitochondrial suspensions were pretreated for 15 min with Ruthenium Red (RR, 5 μM) or CsA (10 μM) before and during exposures. (a) Representative gel of S100 protein precipitated overnight with 10% TCA is shown. Pellets were subjected to polyacrylamide gel electrophoresis and immunoblot analysis using an antibody that recognizes cytochrome c. (b) S100 supernatant was assayed for caspase activity for 30 min. Data are represented as mean s.d. (n=4). *P<0.05 as compared with respective controls (vehicle).
Discussion
Changes in Ca2+ influx and O2− production within the mitochondria prior to cell death are events shared by cells treated with neurotoxic agents such as NMDA, veratridine and β-amyloid (Bindokas et al., 1996; Jordan et al., 2000; Longo et al., 2000). Evidence has been presented that intracellular O2− production during apoptosis is Ca2+ dependent and of mitochondrial origin (Ankarcrona et al., 1995; Bindokas et al., 1996; Jordan et al., 2000). Herein, we used brain-isolated mitochondria to study the effects of Ca2+ and O2− on organelle parameters such as swelling, Δψm, enzymatic activity, NAD(P)H and protein release.
The present data show that superoxide anions and Ca2+ produce brain mitochondrial swelling. Moreover, when we analyzed the implication of these two second messengers in mitochondrial swelling, we found that while O2−-induced swelling was Ca2+-independent, ROS participated significantly in Ca2+-induced mitochondrial swelling. Neither, Ca2+ free solution nor the presence of the Ca2+ uniporter blocker, Ruthenium Red, modified O2−-induced mitochondrial swelling. In contrast to noncerebral tissue, and in line with previous reports, only high Ca2+ concentration induced mitochondrial swelling (Berman et al., 2000; Schild et al., 2001; Brustovetsky et al., 2002). Moreover, Ca2+, even at these concentrations, required succinate in the resuspension solution to induce brain mitochondrial swelling, suggesting either its requirement for mitochondrial calcium uptake (Votyakova & Reynolds, 2001) or a role for coenzyme Q as a generator of O2− (Kowaltowski et al., 1995). This correlates well with the increase in superoxide production detected with the specific oxidation sensitive probe, hydroethidine, in mitochondrial preparations exposed to Ca2+. Indeed, Ca2+- induced swelling involved a ROS-dependent component, since the superoxide dismutase mimetic, MnTBAP, and catalase markedly decreased Ca2+-induced swelling.
The membrane permeable SOD mimetic MnTBAP seemed to be more efficient than the poorly membrane permeable SOD to block Ca2+-induced mitochondrial swelling. This difference can be explained, in terms of product compartmentalization by either of the following reasons: (i) O2− formed inside the mitochondria does not pass through mitochondrial membranes and SOD was less efficient, since it was added to the extramitochondrial compartment; (ii) SOD poorly penetrates into the mitochondria being unable to intercept the generated O2−; and (iii) excess O2− was degraded to H2O2 by MnSOD and exceeded mitochondrial catalase capacity to detoxify it. Consistent with the latter reason, mitochondria treatment with catalase significantly reduced Ca2+-induced swelling (Figure 1c).
We have also measured the effects of O2− and supraphysiological Ca2+ on mitochondrial metabolic activity. Superoxide anions, or its related ROS, compromised citrate synthase and dehydrogenase enzymatic activities. These effects seem to be more related to oxidation of the enzymes than to mitochondrial swelling, since Ca2+ did not modify them. Our observations might indicate that ROS are the second messengers involved in the decrease of these enzymatic activities showed in several neurodegenerative models, like mice deficient in MnSOD (Melov et al., 1999). Moreover, KO2 additions dropped total mitochondrial NAD(P)H levels. This is interesting because NAD(P)H, besides acting indirectly as an antioxidant in the reduction of GSSG to GSH, operates as an antioxidant in the matrix space, both by scavenging toxic free radicals and repairing biomolecule-derived radicals (Kirsch & De Groot, 2001). Interestingly, Ca2+ increased NADPH autofluorescence. This is consistent with the enhanced activity of Ca2+-dependent intramitochondrial enzymes, described in dissociated mouse sensory neurons (Duchen, 1992). This increase was blocked by the addition of Ruthenium Red, suggesting the dependence of the response on mitochondrial Ca2+ uptake (Duchen, 1992).
Under our experimental conditions Ca2+, but not O2−, produced a rapid loss of Δψm that was inhibited by Ruthenium Red, suggesting that Ca2+ mediates this mitochondrial effect after its uptake into the mitochondria. Indeed, our study reveals that Ca2+-triggered Δψm depolarization appeared to be MPTP independent, since CsA did not block this depolarization. These results are in agreement with observations on mitochondrial swelling that postulate that Ca2+ acts through a voltage sensor, while ROS-induced swelling results from an oxidative action (Petronilli et al., 1993a, 1993b). It has also been shown that CsA has no effect on Δψm depolarization after strong depolarizations (Brustovetsky & Dubinsky, 2000).
The lack of O2− effects on Δψm observed here are consistent with the fact that mitochondrial depolarization is not required for the induction of apoptosis in models where an overproduction of O2− or the release of cytochrome c takes place (Petronilli et al., 1993a, 1993b; Kluck et al., 1997; Vander Heiden et al., 1997; Krohn et al., 1998; Chiu & Oleinick, 2001) like hippocampal neurons treated with staurosporine (Krohn et al., 1999) or neurons subjected to growth factor withdrawal (Kluck et al., 1997). In other models, protein release happens before Δψm depolarization, indicating that disruption of the outer membrane, but not the inner mitochondrial membrane, is the primary structural alteration (Vander Heiden et al., 1997), and that the electron-transport chain can maintain the Δψm even after cytochrome c has been released (Goldstein et al., 2000). Moreover, it has been described that O2−-induced cytochrome c release does not produce Δψm loss (Madesh & Hajnoczky, 2001) and in other models as RAW 264.7 cells, ROS-induced cytochrome c release is accomplished without relevant changes of Δψm (Hortelano et al., 1999).
In agreement with the above data, both Ca2+-and O2−-induced swelling resulted in the release of mitochondrial intermembrane proteins. We found that, after mitochondrial swelling, the release of a cytochrome c and the activation of DEVD-caspase took place in isolated mitochondria. This release was sensitive to the presence of CsA suggesting that it was mediated by MPTP. This protein release has been observed in neurodegenerative models, such as focal ischemia and Parkinson's disease (Namura et al., 2001; Viswanath et al., 2001) and has been correlated with the point-of-no-return for cell death pathways (Hirsch et al., 1998). Although the synaptosome contamination can overestimate DVED-like caspase activity, our observations are consistent with previous results from Yuan et al. (2001) which showed that mitochondrial caspases are regulated independently of the cytosolic pool of caspases, and that staurosporine treatment led to preferential autoprocessing of caspase-9 associated with mitochondria. On the other hand, Ca2+-induced smaller cytochrome c and DVED-like caspase activity release from mitochondria than KO2, perhaps throughout calpain-I activation that has been shown to inhibit cytochrome c release and caspase activation in a cell-free system (Lankiewicz et al., 2000).
In summary, our data provide direct information and detailed evidence on how superoxide acts on mitochondria to initiate apoptotic pathways, and that these changes are initiated in the absence of mitochondrial depolarization. However, more work is necessary to identify the mechanism that could explain how MPTP might be activated without loss of mitochondrial potential.
Acknowledgments
This work has been supported, in part, by Grants SAF99-0060 from CICYT, BFI2001- 1565 from Ministerio de Ciencia y Tecnología;G03/167 from Ministerio de Sanidad, GC-02- 019 and PAI-02-031 from Consejería de Ciencia y Tecnología, JCCM and from Fundación Campollano-Banco Santander Central Hispano to V.C., BFI2001-1058 from Ministerio de Ciencia y Tecnología and GC02-029 from Consejería de Sanidad JCCM to C.G.G and SAF2002-04721 from CICYT to J.J. M.F.G. is a fellow from JCCM. We are grateful to the excellent technical work of Juana Rozalén.
Abbreviations
- AFU
Arbitrary fluorescent units
- CsA
cyclosporin A
- DEVD-AFC
N-acetyl-Asp-Glu-Val-Asp-7-amido-4-trifluoromethylcoumarin
- MPTP
mitochondrial permeability transitory pore
- MnTBAP
Mn(IIItetrakis(4-benzoic acid)porphyrin chloride)
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide thiazolyl blue
- Rh 123
rhodamine 123
- TCA
trichloroacetic acid
- Δψm
mitochondrial membrane potential
References
- ANKARCRONA M., DYPBUKT J.M., BONFOCO E., ZHIVOTOVSKY B., ORRENIUS S., LIPTON S.A., NICOTERA P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- BERMAN S.B., WATKINS S.C., HASTINGS T.G. Quantitative biochemical and ultrastructural comparison of mitochondrial permeability transition in isolated brain and liver mitochondria: evidence for reduced sensitivity of brain mitochondria. Exp. Neurol. 2000;164:415–425. doi: 10.1006/exnr.2000.7438. [DOI] [PubMed] [Google Scholar]
- BERNARDI P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol. Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- BINDOKAS V.P., JORDAN J., LEE C.C., MILLER R.J. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J. Neurosci. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN M.D., TROUNCE I.A., JUN A.S., ALLEN J.C., WALLACE D.C. Functional analysis of lymphoblast and cybrid mitochondria containing the 3460, 11778, or 14484 Leber's hereditary optic neuropathy mitochondrial DNA mutation. J. Biol. Chem. 2000;275:39831–39836. doi: 10.1074/jbc.M006476200. [DOI] [PubMed] [Google Scholar]
- BRUSTOVETSKY N., BRUSTOVETSKY T., JEMMERSON R., DUBINSKY J.M. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J. Neurochem. 2002;80:207–218. doi: 10.1046/j.0022-3042.2001.00671.x. [DOI] [PubMed] [Google Scholar]
- BRUSTOVETSKY N., DUBINSKY J.M. Limitations of cyclosporin A inhibition of the permeability transition in CNS mitochondria. J. Neurosci. 2000;20:8229–8237. doi: 10.1523/JNEUROSCI.20-22-08229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIU S.M., OLEINICK N.L. Dissociation of mitochondrial depolarization from cytochrome c release during apoptosis induced by photodynamic therapy. Br. J. Cancer. 2001;84:1099–1106. doi: 10.1054/bjoc.2000.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUCHEN M.R. Ca2+-dependent changes in the mitochondrial energetics in single dissociated mouse sensory neurons. Biochem. J. 1992;283:41–50. doi: 10.1042/bj2830041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMAUS R.K., GRUNWALD R., LEMASTERS J.J. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochim. Biophys. Acta. 1996;850:436–448. doi: 10.1016/0005-2728(86)90112-x. [DOI] [PubMed] [Google Scholar]
- GALINDO M.F., JORDÁN J., GONZÁLEZ-GARCÍA C., CEÑA V. Chromaffin cell death induced by 6-hydroxydopamine is independent of mitochondrial swelling and caspase activation. J. Neurochem. 2003;84:1066–1073. doi: 10.1046/j.1471-4159.2003.01592.x. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN J.C., WATERHOUSE N.J., JUIN P., EVAN G.I., GREEN D.R. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat. Cell. Biol. 2000;2:156–162. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]
- GREEN D.R., REED J.C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- HIRSCH T., SUSIN S.A., MARZO I., MARCHETTI P., ZAMZAMI N., KROEMER G. Mitochondrial permeability transition in apoptosis and necrosis. Cell. Biol. Toxicol. 1998;14:141–145. doi: 10.1023/a:1007486022411. [DOI] [PubMed] [Google Scholar]
- HORTELANO S., ALVAREZ A.M., BOSCA L. Nitric oxide induces tyrosine nitration release of cytochrome c preceding an increase of mitochondrial transmembrane potential in macrophages. FASEB. J. 1999;13:2311–2317. doi: 10.1096/fasebj.13.15.2311. [DOI] [PubMed] [Google Scholar]
- JORDAN J., GALINDO M.F., CALVO S., GONZÁLEZ-GARCÍA C., CEÑA V. Veratridine induces apoptotic death in bovine chromaffin cells through superoxide production. Br. J. Pharmacol. 2000;130:1496–1504. doi: 10.1038/sj.bjp.0703451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDAN J., GALINDO M.F., MILLER R.J. Role of calpain- and interleukin-1 beta converting enzyme-like proteases in the beta-amyloid-induced death of rat hippocampal neurons in culture. J. Neurochem. 1997;68:1612–1621. doi: 10.1046/j.1471-4159.1997.68041612.x. [DOI] [PubMed] [Google Scholar]
- JORDAN J., GALINDO M.F., TORNERO D., BENAVIDES A., GONZALEZ C., AGAPITO M.T., GONZALEZ-GARCIA C., CENA V. Superoxide anions mediate veratridine-induced cytochrome c release and caspase activity in bovine chromaffin cells. Br. J. Pharmacol. 2002;137:993–1000. doi: 10.1038/sj.bjp.0704953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDAN J., GHADGE G.D., PREHN J.H., TOTH P.T., ROOS R.P., MILLER R.J. Expression of human copper/zinc-superoxide dismutase inhibits the death of rat sympathetic neurons caused by withdrawal of nerve growth factor. Mol. Pharmacol. 1995;47:1095–1100. [PubMed] [Google Scholar]
- KIRSCH M., DE GROOT H. NAD(P)H, a directly operating antioxidant. FASEB. J. 2001;15:1569–1574. doi: 10.1096/fj.00-0823hyp. [DOI] [PubMed] [Google Scholar]
- KLUCK R.M., BOSSY-WETZEL E., GREEN D.R., NEWMEYER D.D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- KOUTNIKOVA H., CAMPUZANO V., FOURY F., DOLLE P., CAZZALINI O., KOENIG M. Studies of human, mouse and yeast homologues indicate a mitochondrial function for frataxin. Nat. Genet. 1997;16:345–351. doi: 10.1038/ng0897-345. [DOI] [PubMed] [Google Scholar]
- KOWALTOWSKI A.J., CASTILHO R.F., VERCESI A.E. Ca2+-induced mitochondrial membrane permeabilization: role of coenzyme Q redox state. Am. J. Physiol. 1995;269:C141. doi: 10.1152/ajpcell.1995.269.1.C141. [DOI] [PubMed] [Google Scholar]
- KRISTAL B.S., STAATS P.N., SHESTOPALOV A.I. Biochemical characterization of the mitochondrial permeability transition in isolated forebrain mitochondria. Dev. Neurosci. 2000;22:376–383. doi: 10.1159/000017463. [DOI] [PubMed] [Google Scholar]
- KRISTAL B.S., DUBINSKY J.M. Mitochondrial permeability transition in the central nervous system: induction by calcium cycling-dependent and - independent pathways. J. Neurochem. 1997;69:524–538. doi: 10.1046/j.1471-4159.1997.69020524.x. [DOI] [PubMed] [Google Scholar]
- KROEMER G., REED J.C. Mitochondrial control of cell death. Nat. Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- KROHN A.J., PREIS E., PREHN J.H. Staurosporine-induced apoptosis of cultured rat hippocampal neurons involves caspase-1-like proteases as upstream initiators and increased production of superoxide as a main downstream effector. J. Neurosci. 1998;18:8186–8197. doi: 10.1523/JNEUROSCI.18-20-08186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROHN A.J., WAHLBRINK T., PREHN J.H. Mitochondrial depolarization is not required for neuronal apoptosis. J. Neurosci. 1999;19:7394–7404. doi: 10.1523/JNEUROSCI.19-17-07394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANKIEWICZ S., MARC LUETJENS C., TRUC BUI N., KROHN AJ POPPE M., COLE G.M., SAIDO T.C., PREHN J.H. Activation of calpain I converts excitotoxic neuron death into a caspase-independent cell death. J. Biol. Chem. 2000;275:17064–17071. doi: 10.1074/jbc.275.22.17064. [DOI] [PubMed] [Google Scholar]
- LONGO V.D., VIOLA K.L., KLEIN W.L., FINCH C.E. Reversible inactivation of superoxide-sensitive aconitase in Abeta1-42-treated neuronal cell lines. J. Neurochem. 2000;75:1977–1985. doi: 10.1046/j.1471-4159.2000.0751977.x. [DOI] [PubMed] [Google Scholar]
- MADESH M., HAJNOCZKY G. VdAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J. Cell Biol. 2001;155:1003–1016. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAESTRE I., JORDÁN J., CALVO S., REIG J.A., CEÑA V., SORIA B., PRENTKI M., ROCHE E. Mitochondrial dysfunction is involved in apoptosis induced by serum withdrawal fatty acids in the β-cell line INS-1. Endocrinology. 2003;144:335–345. doi: 10.1210/en.2001-211282. [DOI] [PubMed] [Google Scholar]
- MELOV S., COSKUN P., PATEL M., TUINSTRA R., COTTRELL B., JUN A.S., ZASTAWNY T.H., DIZDAROGLU M., GOODMAN S.I., HUANG T.T., MIZIORKO H., EPSTEIN C.J., WALLACE D.C. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:846–851. doi: 10.1073/pnas.96.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSMANN T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- NAMURA S., NAGATA I., TAKAMI S., MASAYASU H., KIKUCHI H. Ebselen reduces cytochrome c release from mitochondria subsequent DNA fragmentation after transient focal cerebral ischemia in mice. Stroke. 2001;32:1906–1911. doi: 10.1161/01.str.32.8.1906. [DOI] [PubMed] [Google Scholar]
- NEWMEYER D.D., FARSCHON D.M., REED J.C. Cell-free apoptosis in Xenopus egg extracts: inhibition by Bcl-2 and requirement for an organelle fraction enriched in mitochondria. Cell. 1994;79:353. doi: 10.1016/0092-8674(94)90203-8. [DOI] [PubMed] [Google Scholar]
- PETRONILLI V., COLA C., BERNARDI P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore. II. The minimal requirements for pore induction underscore a key role for transmembrane electrical potential, matrix pH, and matrix Ca2+ J. Biol. Chem. 1993a;268:1011–1016. [PubMed] [Google Scholar]
- PETRONILLI V., COLA C., MASSARI S., COLONNA R., BERNARDI P. Physiological effectors modify voltage sensing by the cyclosporin A-sensitive permeability transition pore of mitochondria. J. Biol. Chem. 1993b;268:21939–21945. [PubMed] [Google Scholar]
- PREHN J.H., BINDOKAS V.P., JORDAN J., GALINDO M.F., GHADGE G.D., ROOS R.P., BOISE L.H., THOMPSON C.B., KRAJEWSKI S., REED J.C., MILLER R.J. Protective effect of transforming growth factor-beta 1 on beta-amyloid neurotoxicity in rat hippocampal neurons. Mol. Pharmacol. 1996;49:319–328. [PubMed] [Google Scholar]
- PREHN J.H.M., JORDÁN J., GHADGE G.G., PREIS E., GALINDO M.F., ROOS R.P., KRIEGLSTEIN J., MILLER R.J. Ca2+ and reactive species in staurosporine-induced neuronal apoptosis. J. Neurochemi. 1997;68:1679–1685. doi: 10.1046/j.1471-4159.1997.68041679.x. [DOI] [PubMed] [Google Scholar]
- REIMERTZ C., KOGEL D., LANKIEWICZ S., POPPE M., PREHN J.H. Ca2+-induced inhibition of apoptosis in human SH-SY5Y neuroblastoma cells: degradation of apoptotic protease activating factor-1 (APAF-1) J. Neurochem. 2001;78:1256–1266. doi: 10.1046/j.1471-4159.2001.00503.x. [DOI] [PubMed] [Google Scholar]
- ROVER JUNIOR L., FERNANDES J.C., DE OLIVEIRA NETO G., KUBOTA L.T., KATEKAWA E., SERRANO S.H. Study of NADH stability using ultraviolet–visible spectrophotometric analysis and factorial design. Anal. Biochem. 1998;260:50–55. doi: 10.1006/abio.1998.2656. [DOI] [PubMed] [Google Scholar]
- SCHILD L., KEILHOFF G., AUGUSTIN W., REISER G., STRIGGOW F. Distinct Ca2+ thresholds determine cytochrome c release or permeability transition pore opening in brain mitochondria. FASEB. J. 2001;15:565–567. doi: 10.1096/fj.00-0551fje. [DOI] [PubMed] [Google Scholar]
- SHEEHAN J.P., SWERDLOW R.H., PARKER W.D., MILLER S.W., DAVIS R.E., TUTTLE J.B. Altered calcium homeostasis in cells transformed by mitochondria from individuals with Parkinson's disease. J. Neurochem. 1997;68:1221–1233. doi: 10.1046/j.1471-4159.1997.68031221.x. [DOI] [PubMed] [Google Scholar]
- SHEPHERD D., GARLAND P.B. The kinetic properties of citrate synthase from rat liver mitochondria. Biochem. J. 1969;114:597–610. doi: 10.1042/bj1140597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOUT A.K., RAPHAEL H.M., KANTEREWICZ B.I., KLANN E., REYNOLDS I.J. Glutamate-induced neuron death requires mitochondrial calcium uptake. NatNeurosci. 1998;1:366–373. doi: 10.1038/1577. [DOI] [PubMed] [Google Scholar]
- SUSIN S.A., Lorenzo H.K., ZAMZAMI N., MARZO I., SNOW B.E., BROTHERS G.M., MANGION J., JACOTOT E., COSTANTINI P., LOEFFLER M., LAROCHETTE N., GOODLETT D.R., AEBERSOLD R., SIDEROVSKI D.P., PENNINGER J.M., KROEMER G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- VANDER HEIDEN M.G., CHANDEL N.S., WILLIAMSON E.K., SCHUMACKER P.T., THOMPSON C.B. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- VISWANATH V., WU Y., BOONPLUEANG R., CHEN S., STEVENSON F.F., YANTIRI F., YANG L., BEAL M.F., ANDERSEN J.K. Caspase-9 activation results in downstream caspase-8 activation and bid cleavage in 1-methyl-4-phenyl- 1,2,3,6-tetrahydropyridine-induced Parkinson's disease. J. Neurosci. 2001;21:9519–9528. doi: 10.1523/JNEUROSCI.21-24-09519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOTYAKOVA T.V., REYNOLDS I.J. DeltaPsi(m)-dependent and -independent production of reactive oxygen species by rat brain mitochondria. J. Neurochem. 2001;79:266–277. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- YUAN H., MUTOMBA M., PRINZ I., GOTTLIEB R.A. Differential processing of cytosolic and mitochondrial caspases. Mitochondrion. 2001;1:61–69. doi: 10.1016/s1567-7249(01)00002-2. [DOI] [PubMed] [Google Scholar]
- WALLACE D.C. A mitochondrial paradigm for degenerative diseases and ageing. Novartis Found Symp. 2001;235:247–263. doi: 10.1002/0470868694.ch20. [DOI] [PubMed] [Google Scholar]