Abstract
Creatine (CR) supplementation augments muscle strength in skeletal muscle cells by increasing intracellular energy pools. However, the effect of CR supplementation on endothelial cells remains to be clarified.
In this study, we investigated whether CR supplementation had any anti-inflammatory activity against human pulmonary endothelial cells in culture.
We confirmed that supplementation with 0.5 mM CR significantly increased both intracellular CR and phosphocreatine (PC) through a CR transporter while keeping intracellular ATP levels constant independent of CR supplementation and a CR transporter antagonist.
In the assay system of endothelial permeability, supplementation with 5 mM CR significantly suppressed the endothelial permeability induced by serotonin and H2O2.
In cell adhesion experiments, supplementation with 5 mM CR significantly suppressed neutrophil adhesion to endothelial cells.
In the measurement of adhesion molecules, CR supplementation with more than 0.5 mM CR significantly inhibited the expressions of ICAM-1 and E-selectin on endothelial cells, and the inhibition was significantly suppressed by an adenosine A2A receptor antagonist.
The present study suggests that CR supplementation has anti-inflammatory activities against endothelial cells.
Keywords: Creatine, endothelial cell, H2O2, permeability, phosphocreatine, ATP, serotonin, adhesion, ICAM-1, E-selectin
Introduction
Creatine (CR) is tightly linked to the intracellular energy under the form of ATP (Wyss & Kaddurah-Daouk, 2000). Cells take in CR from the blood stream through a CR transporter and store it as CR and its phosphorylated form, phosphocreatine (PC) (Snow & Murphy, 2001). CR receives a phosphoryl group from ATP to generate PC by using the CR kinase inside cells. Intracellular ATP has such a short half-life that cells are equipped with two major channels of ATP supply. One is glycolysis and oxidative phosphorylation; the other is the PC energy system. While the former constantly provides ATP, the latter works on acute demand as an energy buffer to maintain an intracellular ATP level. Because PC transfers the phosphoryl group to ADP to synthesize ATP quickly, the total amount of ATP and PC represents an intracellular energy pool. The PC energy system is well characterized in the case of skeletal muscle cells because about 94% of CR is distributed therein (Wyss & Kaddurah-Daouk, 2000).
It is known that supplementation with high-dose CR further increases intracellular energy pools of muscle tissue as well as serum CR levels in vivo. Ingestion of high-dose CR up to 20 g increases CR serum levels to 2 mM in a dose-dependent manner (Schedel et al., 1999). Moreover, supplementation with more than 20 g of CR has been reported to increase the intracellular contents of both CR and PC (Vandenberghe et al., 1997; Snow et al., 1998). Furthermore, some reports suggest that the increase in intracellular energy pools enhances progress in muscle strength (Vandenberghe et al., 1997; Williams & Branch, 1998), although opposite results have also been reported (Snow et al., 1998; Vandenberghe et al., 1999). Therefore, many athletes are taking high-dose CR to build muscular strength. Recently, CR supplementation has also been applied to the treatment of various pathological conditions such as gyrate atrophy (Sipilä et al., 1981; Vannas-Sulonen et al., 1985), McArdle disease (Vorgerd et al., 2000), Duchenne muscular dystrophy (Felber et al., 2000), myasthenia gravis (Stout et al., 2001), and amyotrophic lateral sclerosis (Mazzini et al., 2001). Findings in these reports suggest that CR supplementation augments or restores cell functions by increasing intracellular energy pools.
The PC energy system and CR supplementation have been extensively investigated in muscle cells, but not in endothelial cells. To our knowledge, there have been only a few reports on the presence of PC and ATP in endothelial cells (Loike et al., 1992; Windischbauer et al., 1994). It remains to be elucidated whether CR supplementation may influence the cell functions of endothelial cells. In this study, we postulated that CR supplementation may inhibit inflammation in endothelial cells by increasing intracellular high-energy phosphate compounds.
Methods
Coating Millicell-CM with gelatin
Millicell-CM with a 0.4 μm pore-size (12 mm, 0.6 cm2) was briefly covered with 2% gelatin solution containing 5% sucrose, dried after removing the solution, and washed with sterilized 0.15 M phosphate-buffered saline (PBS), pH 7.4, for 10 min. The membrane was fixed with 1% glutaraldehyde solution for 30 min, washed with PBS 5 times, and incubated in 0.1 M glycine solution for 30 min. The coated membrane was used for the culture of endothelial cells after being washed with PBS and subsequent culture medium.
Culture of endothelial cells
We first determined the CR concentration of endothelial cell basal medium (EBM)-2 containing 5% fetal calf serum (FCS), and found it 2.85±0.62 μM (n=6). We used 5% FCS-containing EBM-2 supplemented with 50 μM CR as a basal culture medium in following experiments because endothelial cells are constantly exposed to 50 μM CR in vivo. Human endothelial cells from pulmonary peripheral vessels were seeded at 2.5 × 104 cells cm−2 on six-well plastic plates and on gelatin-coated Millicell-CM membranes for quantification of CR, PC, and ATP, and for assay of permeability, respectively.
Cells were cultured in a CO2 incubator until a confluent monolayer formed, and were then subjected to the following experiments. In the case of the Millicell-CM membranes, 400 and 600 μl of the medium were added to the inner and outer chambers, respectively, to balance the liquid levels.
Characterization of cultured endothelial cells
To characterize the cultured endothelial cells, we examined the uptake of low-density lipoprotein (LDL) and the expression of Factor VIII, which parameters can demonstrate the characteristics of endothelial cells.
Uptake of LDL was examined histochemically by incubating cells in media containing 10 μg ml−1 of LDL labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine perchlorate (Dil-Ac-LDL) for 4 h at 37°C. Labeled cells were washed with media free of Dil-Ac-LDL several times then observed under a fluorescence microscope IX70 (Olympus, Japan) at 514 nm for excitation and at 550 nm for emission.
Expression of Factor VIII on cultured cells was examined immunohistochemically with a rabbit anti-human Factor VIII polyclonal antibody using Histofine Simple Stain MAX PO (MULTI). In brief, cells were fixed in 4% ice-cold paraformaldehyde solution for 10 min at 4°C, and endogenous peroxidase activity was inactivated in 100% methanol containing 0.3% H2O2 for 30 min at room temperature. After nonspecific binding was blocked by incubation of specimens with 2% goat sera for 30 min, specimens were incubated with a primary antibody (10 μg ml−1) for 30 min. Then they were incubated with Universal Immuno-peroxidase Polymer conjugated with anti-rabbit antibodies and peroxidases for 10 min. Color development was conducted with 3, 3′-diaminobenzidine tetrahydrochloride for 10 min. Specimens were washed three times with 0.5 M PBS containing 0.1% Tween-20 between steps.
Measurement of CR
CR was quantified according to the method in a previous report (Yasuhara et al., 1982). Two enzymatic solutions, Reagents I and II, were prepared and kept on ice before assay of creatine. Reagent I was 0.1 M PBS (pH 7.8) containing 3 U ml−1 sarcosine oxidase, 0.3 U ml−1 formaldehyde dehydrogenase, and 0.1 mM β-nicotinamide adenine dinucleotide (β-NAD+). Reagent II was 20 U ml−1 creatine amidinohydrolase dissolved in 0.1 M PBS. In brief, 20 μl of standard CR solution or of the sample was added to 50 μl of 0.1 M PBS containing 3 U ml−1 of sarcosine oxidase, 0.3 U ml−1 of formaldehyde dehydrogenase, and 0.1 mM of β-NAD+, and incubated for 5 min at 37°C. Then 50 μl of 20 U ml−1 creatine amidinohydrolase in 0.1 M PBS was added to the reaction mixture. The absorbance of the reaction mixture was read at 340 nm with an ultraviolet analyzer Ultrospec 3000 (Nippon Bio-Rad Laboratories, Japan).
Measurement of PC
Assay of PC consisted of two processes (Loike et al., 1979). First, ATP was eliminated from the samples by incubation of the reaction mixture at 37°C for 5 min. The reaction mixture contained 30 μl of sample and 350 μl of buffer made with 36 μl of 10 mM AMP, 9 μl of 100 μM ADP, 36 μl of firefly extract, 23 μl of distilled water, and 246 μl of 10 mM phosphate in 100 mM sodium arsenite buffer (pH 7.4) containing 4 mM MgSO4. Second, PC was converted to ATP by incubation of the reaction mixture with 50 μl of 5 mg ml−1 creatinephosphokinase, and its chemiluminescence was measured at 4°C for 10 min by a luminometer BLR-301 (Aloka, Japan).
Measurement of ATP
The level of ATP was determined by the luciferin–luciferase method (Strehler & McElroy, 1957; Beutler & Baluda, 1964) with an ATP Assay Kit. In brief, 200 μl of sample or ATP standard was mixed with 200 μl of 25 mM HEPES (pH 7.75) containing 10 mM MgCl2 and 0.02% NaN3 along with 100 μl of luciferase solution. Chemiluminescence of the mixture was measured at 4°C for 1 min.
Measurement of protein
Protein was quantified according to Lowry's method with the use of a DC protein assay kit (Lowry et al., 1951). A volume of 200 μl of the sample or bovine serum albumin as a standard protein was mixed with 100 μl of mixture containing reagents A and S at a 50 : 1 ratio, to which was added 800 μl of reagent B, then the absorbance of the mixture was measured at 750 nm.
Effect of exogenously added CR on intracellular CR, PC, and ATP
Endothelial cells were cultured up to confluence using EBM-2 containing 5% FCS and 0.5 mM CR with or without 5 mM β-guanidinopropionic acid (GPA) on six-well plastic plates. Cells were washed three times with ice-cold PBS containing 4.1 mM Mg2+ and 2.5 mM Ca2+, and harvested with a cell scraper after the addition of 500 μl of ice-cold 0.04 M Tris-borate buffer (pH 9.2). Cell suspensions were boiled for 5 min to inactivate ATPase, then centrifuged at 1200 × g for 5 min at 4°C. Supernatants were subjected to measurement of CR, PC, and ATP, and pellets were resuspended in 1% sodium dodecylsulfate for measurement of protein. Samples were stored at −80°C until assay.
Effect of CR supplementation on endothelial permeability induced by serotonin and H2O2
Endothelial permeability was measured under stimulation with serotonin or H2O2 using fluorescein isothiocyanate-dextran (FITC-dex, MW 70 kD) as a tracer according to the method described in previous reports (Simionescu & Palade, 1971; Hashida et al., 1986). In brief, FITC-dex (1.4 mg) was added to the inner chamber to reach a final concentration of 50 μM, and serotonin or H2O2 was added to the outer chamber to reach a final concentration of 0.1 μM. Fluorescence in 20 μl of medium removed from an outer chamber was measured at 0, 30, 90, 180, and 240 min by Biolumin 960TM fluorometer (Molecular Dynamics Japan, Japan). The content of FITC-dex in samples was calculated using a standard curve of FITC-dex. Endothelial permeability was expressed as the content of FITC-dex (μg) permeating into the outer chamber.
Endothelial cells were cultured in a culture medium containing 0.05, 0.5, and 5 mM of CR for 24 h before assay of endothelial permeability. After incubation with exogenously added CR, cultured cells were equilibrated in Hank's balanced saline solution (HBSS) then subjected to the endothelial permeability assay. To examine the role of a CR transporter in the effect of extracellular CR, GPA was added to the medium at 5 mM.
Labeling of neutrophils with 51Cr
Human neutrophils were separated using Histopaque-1077 and Histopaque-1119 according to the method of English (English & Andersen, 1974), and were suspended in PBS free of Mg2+ and Ca2+. Neutrophils were dispensed at 6 × 105 cells per tube, then labeled with 51Cr by reacting with 3.7 MBq of Na2 51CrO4 in the presence of 1 μM n-formyl-Met-Leu-Phe (FMLP) for 40 min in a CO2 incubator (Balazovich et al., 1996; Chen & Christou, 1996). Labeled neutrophils were washed twice with HBSS containing 0.9 mM Mg2+ and 1.26 mM Ca2+, then resuspended in EBM-2 for the following experiment.
Effect of CR supplementation on neutrophil adhesion to endothelial cells
Neutrophil adhesion to endothelial cells was assessed as reported previously (Ishii et al., 2002). Endothelial cells were preincubated in 0.05, 0.5, and 5 mM CR for 24 h in a CO2 incubator. After washing out the CR, endothelial cells were stimulated with 25 ng ml−1 of TNF-α for 3 h. A volume of 100 μl of 51Cr-labeled neutrophils (corresponding to 8.5 × 104 cells) were reacted to endothelial cells for 30 min in a CO2 incubator, then washed with HBSS free of Mg2+ and Ca2+ to remove nonadherent neutrophils. Adherent cells were lysed by 200 μl of 1 N NaOH to measure radioactivity. Neutrophil adhesion was calculated by dividing the radioactivity of adherent neutrophils by that of total neutrophils.
Effect of CR supplementation on adhesion expression on endothelial cells
Endothelial cells supplemented with 0.05, 0.5, and 5 mM CR for 24 h were stimulated with TNF-α for 3 h in the presence and the absence of 1 μM ZM241385. The expressions of ICAM-1 and E-selectin were measured semiquantitatively as reported by Mulligan et al. (1993). The cells were fixed with ice-cold 1% paraformaldehyde solution for 10 min, then incubated in 10% skimmed milk to inhibit the nonspecific adsorption of antibodies. Fixed cells were incubated with a mouse anti-human E-selectin IgG, a mouse anti-human ICAM-1 IgG1, or a mouse control IgG1 at 5 μg ml−1 for 45 min at room temperature. The cells were washed with PBS three times, and incubated with a peroxidase-labeled rabbit anti-mouse IgG at 1 : 500 dilution for 10 min. After brief washes, cells were incubated in 0.4 mg ml−1 of o-phenylenediamine solution for 30 min. Color development was stopped by 3 M H2SO4, and the absorbance (OD) was read at 492 nm and taken to represent the relative expression of ICAM-1 and E-selectin.
Materials
Materials used were: Dil-Ac-LDL (Biomedical Technology, U.S.A.), human endothelial cells and EBM-2 (Bio-Whittaker, U.S.A.), ATP Assay Kit (Calbiochem-Novabiochem, U.S.A.), 24-well cell culture plate (Corning Costar, U.S.A.), mouse control IgG1 (Dako Cytomation, Japan), porcine gelatin (Difco Laboratories, U.S.A.), TNF-α (Gibco-BRL Life Technologies, U.S.A.), Na2 51CrO4 (NEN Life Science Products, U.S.A.), Histofine Simple Stain MAX PO (MULTI) (Nichirei, Japan), DC protein assay kit (Nippon Bio-Rad Laboratories, Japan), Millicell-CM membrane and six-well plastic plate (Nihon Millipore, Japan), β-NAD+ (Oriental Yeast, Japan), mouse anti-human E-selectin IgG, mouse anti-human ICAM-1 IgG1, and peroxidase-labeled rabbit anti-mouse IgG (Serotec, U.S.A.), ADP, AMP, CR, creatine amidinohydrolase, creatinephosphokinase, firefly extract, FITC-dex, FMLP, formaldehyde dehydrogenase, GPA, Histopaque-1077, Histopaque-1119, PC, sarcosine oxidase, and serotonin (Sigma, U.S.A.), ZM241385 (Tocris Cookson, U.S.A.), and rabbit anti-human Factor VIII polyclonal antibody (YLEM, Italy). All other materials were obtained from Wako Pure Chemicals (Japan).
Statistical analysis
All data are given as the mean±one s.e.m. Statistical comparisons were performed by one-way analysis of variance. If a significant variance was detected, individual group differences were determined by Bonferroni post-test. A probability value less than 0.05 was regarded as statistically significant.
Results
Characteristics of cultured endothelial cells
Cultured cells assembled in a confluent monolayer with a cobblestone-like appearance in a week. All the cells were positive for LDL uptake and the immunoreactivity of Factor VIII, indicating that cultured cells were pure endothelial cells.
Intracellular contents of CR, PC, and ATP
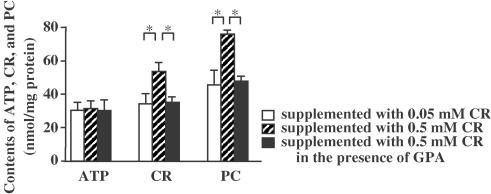
Culturing endothelial cells in 0.5 mM CR for 24 h significantly increased intracellular contents of CR and PC, and the effects of CR supplementation were significantly suppressed by the CR transporter antagonist GPA (Figure 1). In contrast, the intracellular content of ATP was not affected by CR supplementation and GPA.
Figure 1.
Effect of CR supplementation on intracellular contents of CR, PC, and ATP. Asterisks indicate P<0.05 (n=5).
Effect of CR supplementation on endothelial permeability induced by serotonin and H2O2
Basal endothelial permeability was increased up to 240 min in a time-dependent manner. Stimulation with serotonin and H2O2 significantly increased endothelial permeability up to 180 min. In this system, agonist-induced increases in endothelial permeability were not significant at 240 min, because basal permeability was found to reach submaximal permeability. Therefore, we assessed endothelial permeability at 180 min in the following experiments.
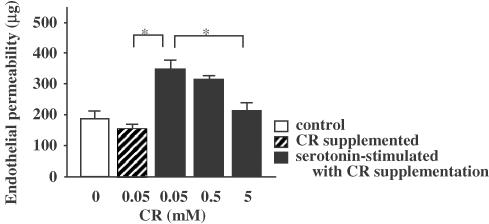
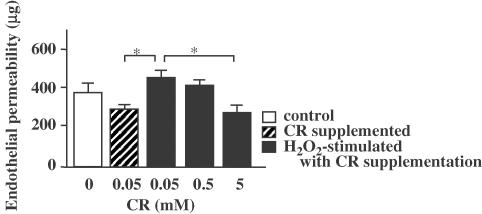
It was observed that 5 mM CR significantly inhibited the endothelial permeability induced by 0.1 μM serotonin (Figure 2). A measure of 5 mM CR was also observed to significantly inhibit the endothelial permeability induced by 0.1 μM H2O2 (Figure 3). Although not significantly, 0.5 mM CR slightly inhibited the permeability induced by both stimuli. Even 5 mM of CR did not decrease permeability below basal permeability.
Figure 2.
Inhibitory effect of CR supplementation on serotonin-induced endothelial permeability. Asterisks indicate P<0.05 (n=5).
Figure 3.
Inhibitory effect of CR supplementation on H2O2-induced endothelial permeability. Asterisks indicate P<0.05 (n=5).
Effect of CR supplementation on neutrophil adhesion to endothelial cells
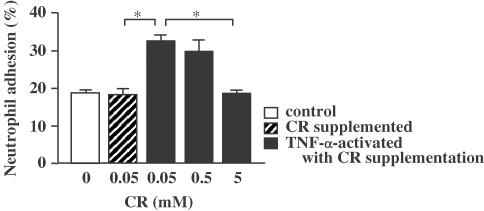
Adhesion of neutrophils to endothelial cells was increased from 18.3 to 32.7% by TNF-α (Figure 4). TNF-α-induced adhesion of neutrophils was significantly inhibited to 18.6% by 5 mM of CR supplementation.
Figure 4.
Inhibitory effect of CR supplementation on neutrophil adhesion to endothelial cells. Asterisks indicate P<0.05 (n=5).
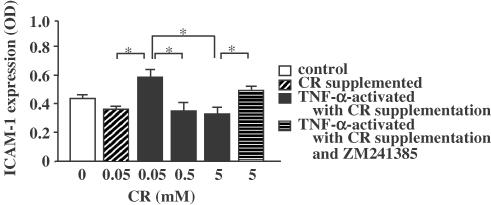
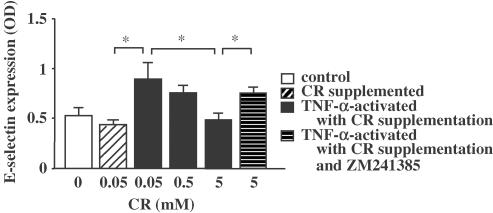
Effect of CR supplementation on the expressions of ICAM-1 and E-selection on endothelial cells
Control antibody-treated endothelial cells showed no significant absorbance. The relative expressions of ICAM-1 and E-selectin were significantly increased from 0.36 OD to 0.58 OD and from 0.43 OD to 0.89 OD by TNF-α, respectively (Figure 5 and Figure 6). More than 0.5 mM of CR supplementation significantly suppressed the expressions of ICAM-1 and E-selectin in a dose-dependent manner. The inhibition of both expressions by 5 mM CR was significantly suppressed by ZM241385.
Figure 5.
Inhibitory effect of CR supplementation on TNF-α-induced ICAM-1 expression on endothelial cells. Asterisks indicate P<0.05 (n=5).
Figure 6.
Inhibitory effect of CR supplementation on TNF-α-induced E-selectin expression on endothelial cells. Asterisks indicate P<0.05 (n=5).
Discussion
In this study, we examined the effect of CR supplementation on endothelial cell parameters in vitro, and found that it inhibited endothelial permeability, neutrophil adhesion to endothelial cells, and adhesion molecule expression. It is suggested that CR supplementation may serve as a suppressor of inflammation at the endothelial cell level.
It is highly possible that the PC energy system is active in endothelial cells, based on the fact that a few reports demonstrated that endothelial cells of umbilical veins contained PC (Loike et al., 1992; Windischbauer et al., 1994). However, those previous reports did not show intracellular CR levels or the role of CR in endothelial cells. We first measured the intracellular CR to assess whether CR might exist inside cultured endothelial cells. We demonstrated a significant level of intracellular CR, and found that CR supplementation further increased it through a CR transporter.
Endothelial cells were also found to contain a sufficient amount of high-energy phosphate compounds. The contents of PC and ATP were 44 and 30 nmol mg protein−1, respectively, which are three to five times higher than their contents in umbilical endothelial cells (Loike et al., 1992). The reason for the difference is unknown, but it might depend upon localization or functions of endothelial cells. On the other hand, muscle cells have been reported to contain more high-energy phosphate compounds than endothelial cells do. ATP is a requisite for muscle cells to constrict. In addition, muscle cells are equipped with the PC energy system to prevent ATP depletion. Such evidence suggests that the PC energy system is active in endothelial cells, although its role is unclear.
Extracellular CR is tightly linked to intracellular energy pools in myocardial and skeletal muscle cells (Seraydarian and Artaza, 1976). On the other hand, we demonstrated that CR supplementation stimulates endothelial cells to generate intracellular PC by CR uptake through a CR transporter. These findings, taken together, suggest that most cells, including muscle and endothelial cells, might be equipped with the mechanism by which CR supplementation induces intracellular energy pools. However, the capacity of PC biosynthesis is likely to differ among cell types. CR supplementation was found to increase the PC concentration in endothelial cells to 74 nmol mg protein−1, which value was comparable to that in skeletal muscle cells (Seraydarian and Artaza, 1976). However, PC concentration in myocardial cells has been reported to be 107 nmol mg protein−1 after CR supplementation (Seraydarian and Artaza, 1976). In contrast with the level of PC, that of intracellular ATP was found to be unsusceptible to CR supplementation or a CR transporter antagonist. Given that PC can provide ATP immediately on demand, these findings suggest that CR supplementation increases intracellular energy pools in endothelial cells through the PC energy system.
We hypothesized that CR supplementation might influence endothelial functions by augmenting intracellular energy pools, and examined the effect of CR supplementation on endothelial permeability, neutrophil adhesion to endothelial cells, and the expression of adhesion molecules. Supplementation with 5 mM CR significantly inhibited endothelial permeability independent of the type of stimulus used. In addition, CR supplementation was found to inhibit the adhesion of neutrophils to endothelial cells. This inhibition can be partly explained by adhesion molecule suppression; CR supplementation suppressed both ICAM-1 and E-selectin expressions on endothelial cells in a dose-dependent manner.
There is a possibility that ATP released from endothelial cells may be involved in the CR supplementation-induced inhibition of endothelial permeability, neutrophil adhesion to endothelial cells, and the expression of adhesion molecules. It has been reported that endothelial cells release ATP during acute inflammation or shear stress (Bodin & Burnstock, 1998;2001). Like endothelial cells, epithelial cells have also been reported to release ATP (Knight et al., 2002).
Furthermore, adenosine has been shown to have an inhibitory activity against endothelial permeability and adhesion molecule expression through the adenosine A2A receptor (Haselton et al., 1993; Okusa et al., 2000; McPherson et al., 2001). We also demonstrated that the adenosine A2A receptor antagonist suppressed the CR supplementation-mediated inhibition of adhesion molecule expressions. Taken together, CR supplementation may enhance ATP release from endothelial cells by increasing PC storage, resulting in anti-inflammatory activities through the adenosine A2A receptor against endothelial cells.
In summary, CR supplementation inhibited endothelial permeability, neutrophil adhesion, and adhesion molecule expression on cultured endothelial cells. Findings in the present study suggest that CR supplementation might inhibit endothelial cell-mediated inflammation by increasing intracellular PC contents.
Abbreviations
- CR
creatine
- Dil-Ac-LDL
LDL labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine perchlorate
- EBM
endothelial cell basal medium
- FITC-dex
fluorescein isothiocyanate-dextran
- FMLP
n-formyl-Met-Leu-Phe
- GPA
β-guanidinopropionic acid
- HBSS
Hank's balanced saline solution
- LDL
low-density lipoprotein
- β-NAD+
β-nicotinamide adenine dinucleotide
- PC
phosphocreatine
References
- BALAZOVICH K.J., SUCHARD S.J., REMICK D.G., BOXER L.A. Tumor necrosis factor-α and FMLP receptors are functionally linked during FMLP-stimulated activation of adherent human neutrophils. Blood. 1996;88:690–696. [PubMed] [Google Scholar]
- BEUTLER E., BALUDA M.C. Simplified determination of blood adenosine triphosphate using the firefly system. Blood. 1964;23:688–698. [PubMed] [Google Scholar]
- BODIN P., BURNSTOCK G. Increased release of ATP from endothelial cells during acute inflammation. Inflamm. Res. 1998;47:351–354. doi: 10.1007/s000110050341. [DOI] [PubMed] [Google Scholar]
- BODIN P., BURNSTOCK G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J. Cardiovasc. Pharmacol. 2001;38:900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- CHEN X., CHRISTOU N.V. Relative contribution of endothelial cell and polymorphonuclear neutrophil activation in their interactions in systemic inflammatory response syndrome. Arch. Surg. 1996;131:1148–1154. doi: 10.1001/archsurg.1996.01430230030006. [DOI] [PubMed] [Google Scholar]
- ENGLISH D., ANDERSEN B.R. Single-step separation of red blood cells, granulocytes and mononuclear leukocytes on discontinuous density gradients of ficoll–hypaque. J. Immunol. Methods. 1974;5:249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- FELBER S., SKLADAL D., WYSS M., KREMSER C., KOLLER A., SPERL W. Oral creatine supplementation in Duchenne muscular dystrophy: a clinical and 31P magnetic resonance spectroscopy study. Neurol. Res. 2000;22:145–150. doi: 10.1080/01616412.2000.11741051. [DOI] [PubMed] [Google Scholar]
- HASELTON F.R., ALEXANDER J.S., MUELLER S.N. Adenosine decreases permeability of in vitro endothelial monolayers. J. Appl. Physiol. 1993;74:1581–1590. doi: 10.1152/jappl.1993.74.4.1581. [DOI] [PubMed] [Google Scholar]
- HASHIDA R., ANAMIZU C., YAGYU-MIZUNO Y., OHKUMA S., TANAKA T. Transcellular transport of fluorescein dextran through an arterial endothelial cell monolayer. Cell Struct. Funct. 1986;11:343–349. doi: 10.1247/csf.11.343. [DOI] [PubMed] [Google Scholar]
- ISHII Y., KIMURA T., MORISHIMA Y., MOCHIZUKI M., NOMURA A., SAKAMOTO T., UCHIDA Y., SEKIZAWA K. S-carboxymethylcysteine inhibits neutrophil activation mediated by N-formyl-methionyl-leucyl-phenylalanine. Eur. J. Pharmacol. 2002;449:183–189. doi: 10.1016/s0014-2999(02)01981-7. [DOI] [PubMed] [Google Scholar]
- KNIGHT G.E., BODIN P., DE GROAT W.C., BURNSTOCK G. ATP is released from guinea pig ureter epithelium on distension. Am. J. Physiol. 2002;282:F281–F288. doi: 10.1152/ajprenal.00293.2000. [DOI] [PubMed] [Google Scholar]
- LOIKE J.D., CAO L., BRETT J., OGAWA S., SILBERSTEIN S.C., STERN D. Hypoxia induced glucose transporter expression in endothelial cells. Am. J. Physiol. 1992;263:C326–C333. doi: 10.1152/ajpcell.1992.263.2.C326. [DOI] [PubMed] [Google Scholar]
- LOIKE J.D., LOZLER V.F., SILVERSTEIN S.C. Increased ATP and creatine phosphate turnover in phagocytosing mouse peritoneal macrophages. J. Biol. Chem. 1979;254:9558–9564. [PubMed] [Google Scholar]
- LOWRY O.H., ROSENBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MAZZINI L., BALZARINI C., COLOMBO R., MORA G., PASTORE I., DE AMBROGIO R., CALIGARI M. Effects of creatine supplementation on exercise performance and muscular strength in amyotrophic lateral sclerosis: preliminary results. J. Neurol. Sci. 2001;191:139–144. doi: 10.1016/s0022-510x(01)00611-6. [DOI] [PubMed] [Google Scholar]
- MCPHERSON J.A., BARRINGHAUS K.G., BISHOP G.G., SANDERS J.M., RIEGER J.M., HESSELBACHER S.E., GIMPLE L.W., POWERS E.R., MACDONALD T., SULLIVAN G., LINDEN J., SAREMBOCK I.J. Adenosine2A receptor stimulation reduces inflammation and neointimal growth in a murine carotid ligation model. Arterioscler. Thromb. Vasc. Biol. 2001;21:791–796. doi: 10.1161/01.atv.21.5.791. [DOI] [PubMed] [Google Scholar]
- MULLIGAN M.S., VAPORCIYAN A.A., MIYASAKA M., TAMATANI T., WARD P.A. Tumor necrosis factor-α regulates in vivo intrapulmonary expression of ICAM-1. Am. J. Pathol. 1993;142:1739–1749. [PMC free article] [PubMed] [Google Scholar]
- OKUSA M.D., LINDEN J., HUANG L., RIEGER J.M., MACDONALD T.L., HUYNH L.P. A2A adenosine receptor-mediated inhibition of renal injury and neutrophil adhesion. Am. J. Physiol. 2000;279:F809–F818. doi: 10.1152/ajprenal.2000.279.5.F809. [DOI] [PubMed] [Google Scholar]
- SCHEDEL J.-M., TANAKA H., KIYONAGA A., SHINDO M., SCHUTZ Y. Acute creatine ingestion in human: consequences on serum creatine and creatinine concentrations. Life Sci. 1999;65:2463–2470. doi: 10.1016/s0024-3205(99)00512-3. [DOI] [PubMed] [Google Scholar]
- SERAYDARIAN M.W., ARTAZA L. Regulation of energy metabolism by creatine in cardiac and skeletal muscle cells in culture. J. Mol. Cell. Cardiol. 1976;8:669–678. doi: 10.1016/0022-2828(76)90009-2. [DOI] [PubMed] [Google Scholar]
- SIMIONESCU N., PALADE G.E. Dextran and glycogens as particulate tracers for studying capillary permeability. J. Cell Biol. 1971;50:616–624. doi: 10.1083/jcb.50.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIPILÄ I., RAPOLA J., SIMELL O., VANNAS A. Supplementary creatine as a treatment for gyrate atrophy of the choroids and retina. N. Engl. J. Med. 1981;304:867–870. doi: 10.1056/NEJM198104093041503. [DOI] [PubMed] [Google Scholar]
- SNOW R.J., MCKENNA M.J., SELIG S.E., KEMP J., STATHIS C.G., ZHAO S. Effect of creatine supplementation on sprint exercise performance and muscle metabolism. J. Appl. Physiol. 1998;84:1667–1673. doi: 10.1152/jappl.1998.84.5.1667. [DOI] [PubMed] [Google Scholar]
- SNOW R.J., MURPHY R.M. Creatine and the creatine transporter: a review. Mol. Cell. Biochem. 2001;224:169–181. doi: 10.1023/a:1011908606819. [DOI] [PubMed] [Google Scholar]
- STOUT J.R., ECKERSON J.M., MAY E., COULTER C., BRADLEY-POPOVICH E.J. Effects of resistance exercise and creatine supplementation on myasthenia gravis: a case study. Med. Sci. Sports Exerc. 2001;33:869–872. doi: 10.1097/00005768-200106000-00003. [DOI] [PubMed] [Google Scholar]
- STREHLER B.L., MCELROY W.D. Assay of adenosine triphosphate. Methods Enzymol. 1957;3:871–873. [Google Scholar]
- VANDENBERGHE K., GORIS M., VAN HECKE P., VAN LEEMPUTTE M., VANGERVEN L., HESPEL P. Long-term CR intake is beneficial to muscle performance during resistance training. J. Appl. Physiol. 1997;83:2055–2063. doi: 10.1152/jappl.1997.83.6.2055. [DOI] [PubMed] [Google Scholar]
- VANDENBERGHE K., VAN HECKE P., VAN LEEMPUTTE M., VANGERVEN F., HESPEL P. Phosphocreatine resynthesis is not affected by CR loading. Med. Sci. Sports Exerc. 1999;31:236–242. doi: 10.1097/00005768-199902000-00006. [DOI] [PubMed] [Google Scholar]
- VANNAS-SULONEN K., SIPILÄ I., VANNA A., SIMELL O., RAPOLA J. Gyrate atrophy of choroids and retina. A five-year follow-up of creatine supplementation. Ophthalmology. 1985;92:1719–1727. doi: 10.1016/s0161-6420(85)34098-8. [DOI] [PubMed] [Google Scholar]
- VORGERD M., GREHL T., JAGER M., MULLER K., FREITAG G., PATZOLD T., BRUNS N., FABIAN K., TEGENTHOFF M., MORTIER W., LUTTMANN A., ZANGE J., MALIN J.P. Creatine therapy in myophosphorylase deficiency (McArdle disease): a placebo-controlled crossover trial. Arch. Neurol. 2000;57:956–963. doi: 10.1001/archneur.57.7.956. [DOI] [PubMed] [Google Scholar]
- WILLIAMS M.H., BRANCH J.D. CR supplementation and exercise performance: an update. J. Am. Coll. Nutr. 1998;17:216–234. doi: 10.1080/07315724.1998.10718751. [DOI] [PubMed] [Google Scholar]
- WINDISCHBAUER A., GRIESMACHER A., MULLER M.M. In vitroeffects of hypoxia and reoxygenation on human umbilical endothelial cells. Eur. J. Clin.Chem. Clin. Biochem. 1994;32:279–284. doi: 10.1515/cclm.1994.32.4.279. [DOI] [PubMed] [Google Scholar]
- WYSS M., KADDURAH-DAOUK R. Creatine and creatine metabolism. Physiol. Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- YASUHARA M., FUJITA S., ARIUE K., KOHDA K., HAYASHI C. A new enzymatic method to determine creatine. Clin. Chim. Acta. 1982;112:181–188. doi: 10.1016/0009-8981(82)90277-7. [DOI] [PubMed] [Google Scholar]