Abstract
Rheumatoid arthritis reduces verapamil oral clearance thereby increases plasma concentration of the drug. This coincides with reduced drug effects through an unknown mechanism.
The effect of interferon-induced acute inflammation on the pharmacokinetics and electrocardiogram of verapamil (20 mg kg−1, p.o.) and nifedipine (0.1 mg kg−1, i.v.) was studied in Sprague–Dawley rats.
The effect of both acute and chronic inflammation on radioligand binding to cardiac L-type calcium channels was also investigated.
Acute inflammation resulted in increased plasma concentration of verapamil but had no effect on that of nifedipine. Verapamil binding to plasma proteins was unaffected.
As has been reported for humans, the increased verapamil concentration coincided with a reduction in the degree to which PR interval is prolonged by the drug. The effect of nifedipine on PR interval was also reduced by inflammation.
Maximum binding of 3H-nitrendipine to cardiac cell membrane was significantly reduced from 63.2±2.5 fmol mg−1 protein in controls to 46.4±2.0 in acute inflammation and from 66.8±2.2 fmol mg−1 protein in controls to 42.2±2.0 in chronic inflammation.
Incubation of the normal cardiac cell membranes with 100 and 1000 pg ml−1 of rat tissue necrosis factor-α did not influence the binding indices to the calcium channels.
Our data suggest that the reduced calcium channel responsiveness is because of altered binding to channels.
Keywords: Inflammation, cytokines, TNF, NO, calcium channels, PR interval, ECG, receptor downregulation
Introduction
Inflammatory conditions and proinflammatory cytokines have been shown to depress cytochrome P450 (CYP450) isozyme activities (Cawthorne et al., 1976; Descotes, 1985; Peterson & Renton, 1986; Moreno et al., 1987). As a result clearance, particularly of drugs that are efficiently cleared via hepatic metabolism, is reduced and consequently plasma concentrations are elevated (Belpaire et al., 1986; Parent et al., 1992; Sakai et al., 1992; Abdel-Razzak et al., 1993; Ferrari et al., 1993a, 1993b; Piquette-Miller & Jamali, 1995). Proinflammatory mediators such as inducible nitric oxide (NO) and tissue necrosis factor-α (TNF) are believed (1) to elevate plasma α1-acid glycoproteins responsible for drug – protein binding, hence reduce the unbound fraction, and (2) to diminish hepatic clearance (CL) of drugs (Piafsky, 1978; Khatsenko et al., 1993; Cribb et al., 1994; Monshouwer et al., 1996). Both of the above effects can result in increased drug concentrations in plasma (Kulmatycki & Jamali, 2001).
Therapeutic consequences of inflammation-induced pharmacokinetic alterations are, however, not well understood. In vitro, alteration of endothelial function and reduced endothelium-dependent relaxation (Fang et al., 1991), calcium movement (i.e. increased intracellular calcium) and reduced responsiveness to verapamil relaxation (Fontaine et al., 1986) in isolated aortae from adjuvant arthritic rats have been noticed previously. In vivo, plasma concentrations of verapamil and its active metabolite norverapamil are drastically increased in the presence of rheumatoid arthritis in humans (Mayo et al., 2000). This was expected to increase a patient's response to the drug's ability to prolong PR interval. PR interval is a sensitive measure of calcium channel inhibitory activity. Quite to the contrary, however, the inflammatory disease significantly reduced the drug effect (Mayo et al., 2000). The mechanism behind this reduced responsiveness is not understood, although in vitro evidence suggested that increased expression of proinflammatory cytokines may cause downregulation of receptors involved (Shore et al., 1997; Laporte et al., 1998). We have tested this possibility using two animal models of inflammation. We first tested if acute inflammation in the rat influences pharmacokinetics and pharmacodynamics of verapamil in a fashion similar to that reported in human rheumatoid arthritis. In addition, we included nifedipine in the study since, as well as being a calcium channel antagonist, our preliminary data had revealed that inflammation had no effect on i.v. doses of the drug. Hence, the effect of inflammation on pharmacodynamics could be investigated in the absence of pharmacokinetic changes. Subsequently, we examined whether binding of a specific calcium channel blocker is altered when the animal is afflicted by either acute or chronic inflammation.
Methods
The study protocol was approved by the University of Alberta Health Sciences Animal Welfare Committee.
Materials
Verapamil hydrochloride and norverapamil, manufactured by Knoll Pharmaceuticals (Stuttgart, Germany), were gifts from G.D. Searle (Skoki, II, U.S.A.). The internal standard (+) glaucine and heptafluorobutanol were purchased from Aldrich (Milwaukee, WI, U.S.A.). High-performance liquid chromatography (HPLC) grade hexane was purchased from Caledon Laboratories (Georgetown, Canada). HPLC grade isopropanol was purchased from BDH Inc. (Toronto, Canada). Heptane was purchased from Mallinckrodt (Paris, KT, France) and 98% ethanol was purchased from Stanley (Vancouver, Canada). Nifedipine, triethylamine (TEA), 10 U ml−1 Asperigillus nitrate reductase, 0.1 M FAD, 1 mM NADPH, 1500 U ml−1 LDH, 100 mM pyruvic acid, sulfanilamide, naphthylethylenediamine dihydrochloride, sucrose, benzamidine, dithiothreitol, ZnSO4, MgSO4, ethylene glycol bis-aminoethyl ether tetra-acetic acid, phenylmethylsulfonyl floride, pepstatin, aprotinin and leupeptin were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.). Human interferon α2a (IFN) × 106 U ml−1 (F. Hoffmann-La Roche, Basel, Switzerland) was a generous gift from the pharmacy department at the Cross Cancer Institute, Edmonton, Alberta, Canada. Rat TNF ELISA kit was purchased from Endogen (Woburn, MA, U.S.A.) 3H-nitrendipine (87 Ci mmol−1) was purchased from Amersham Pharmacia Biotech Inc. (Quebec, Canada). Killed, dried Mycobacterium butyricum was purchased from Difco Laboratories (Detroit, MI, U.S.A.).
Inflammation and animal preparation for pharmacokinetic and pharmacodynamic experiments
Adult, male, Sprague–Dawley rats (333±25 g) were acclimated to a 12-h day–night cycle, housed in rodent cages, and fed standard rodent chow. The animals' right jugular vein was cannulated under methoxyflurane anesthesia. Rats were returned to their cages and allowed to recover overnight. Two doses of 5 × 104 IU INF were administered to the IFN group and the control group received two 0.2 ml of sterile normal saline solution s.c. approximately 12 and 2 h prior to the drug (verapamil, or nifedipine) or placebo (drug-free vehicle) administration.
Electrocardiogram (ECG) leads were placed on the chest at the time of the jugular cannulation and brought around to each animal's back. The ECG was monitored for a minimum of 1 h or until a stable baseline was established before drug or placebo administration. For the determination of drug concentrations, serial blood samples (approximately 250 μl) were subsequently drawn at 0, 15, 30, 45 min and 1, 1.5, 2, 3, 4, 6 and 8 h after verapamil and at 0, 15, 45, 60 and 80 min for nifedipine. For pharmacodynamic analysis, PR interval and heart rate (HR) were recorded for 3 s approximately 1 min prior to each blood sampling.
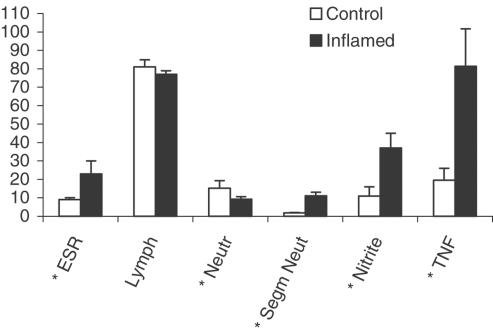
The volume of blood collected at time zero was 500 μl to allow for the measurement of erythrocyte sedimentation rate, white cell count and proinflammatory mediators. Data presented in Figure 1 are those generated for the verapamil study. For the nifedipine study, only the segmented neutrophil and plasma nitrites were measured. The results were very close to those depicted in Figure 1.
Figure 1.
Effect of IFN-induced acute inflammation on selected hematologic and proinflammatory markers. Key: ESR, erythrocyte sedimentation rate (mm h−1); Lymph, lymphocytes (% white blood cells); neutr., neutrophil (% white blood cells); segm. neut., segmented neutrophil (% white blood cells); nitrite (μM); TNF, tissue necrosis factor (pg ml−1); mean±s.d. (n=6–8); Bars are mean±s.d.; *, significantly different from control (P<0.05).
Rats were divided into test (verapamil and nifedipine) and their respective control groups (n=8/group). Verapamil was administered orally as single 20 mg kg−1 doses. A dose of 20 mg kg−1 verapamil was chosen to achieve blood levels in the rats similar to human after an 80 mg oral dose. For nifedipine, single i.v. doses of 0.1 mg kg−1 were administered by slow infusion. We chose the i.v. route since our previous data had shown that at this dose, nifedipine produced sufficient submaximal cardiovascular effect (Eliot & Jamali, 1999). The drugs were dissolved in polyethyleneglycol (PEG-400).
The ECG was measured using braided stainless-steel, Teflon-coated electrodes placed subcutaneously in the left and right axilla and over the xyphoid process. The ECG amplifier was a Honeywell for Medicine ECG amplifier (Honeywell Electronics for Medicine, Edmonton, Canada) and the data recorded using Acknowledge® Software (World Precision Instruments, Miami, FL, U.S.A.) on a personal computer. The mean of five cycles was taken for the measurements of PR intervals and HR. Maximum change from baseline and the area under the percent change in effect versus time curve (AUEC) were used for statistical analysis. AUEC from zero to 8 h was calculated using the trapezoidal rule.
Verapamil pharmacokinetic indices were calculated based on the model-independent analysis approach using WinNonLin for Windows (v 1.0) (Scientific Consulting, Inc., Apex, NC, U.S.A.). Area under the concentration–time curve (AUC) of norverapamil was measured using the trapezoidal rule. Nifedipine pharmacokientic data are presented as plasma concentration–time curves.
Stereospecific verapamil and norverapamil assay
A stereospecific verapamil assay was used as previously published by Shibukawa & Wainer (1992). Briefly, to 100 μl of plasma, 75 μl of 400 ng ml−1 (+) glaucine as internal standard, 100 μl of 2 M NaOH, 0.4 ml sodium phosphate buffer (pH 7.0, ionic strength 0.1), and 6 ml heptane : heptafluorobutanol (99 : 1) were added. The sample was vortexed for 1.0 min, then centrifuged at 2000 × g for 10 min. The organic layer was transferred to glass tubes and evaporated to dryness in a vacuum centrifuge at 60°. The resulting residue was reconstituted in 200 μl of mobile phase (hexane–isopropanol–ethanol–TEA (85 : 7.5 : 7.5:1.0 v−1 v−1 v−1 v−1, at flow rate of 1 ml min−1)), and 100 μl injected into the HPLC. The achiral column (5 cm × 4.6 mM ID supelcosil) and chiral column (250 mM × 4.6 ID Chiralpak AD column) were kept at 31°. The fluorescence detector was set at an excitation of 272 nM and emission of 317 nM. A Hewlett-Packard 3390A reporting integrator (Hewlett-Packard, Avondale, PA, U.S.A.) was used to record and integrate the chromatograms. Standard curves were linear over the range of 2.5–200 ng ml−1 (r2⩾0.966). The sensitivity for R- and S-verapamil was 2.5 ng ml−1 (CV⩽5%) and 7.5 ng ml−1 for R- and S-norverapamil (CV⩽13%), respectively.
Nifedipine assay
Plasma nifedipine concentrations were measured by a previously reported HPLC method (Grundy et al., 1994). The procedure involves extraction of nifedipine from plasma under alkaline conditions (pH 12), separation via reversed-phase HPLC and ultraviolet detection (350 nM). Calibration curves were linear (r2> 0.99) from 10 to 400 ng ml−1 using 150 μl of rat plasma. Intra and interday variability was less than 10%.
Hematological analysis
White blood cell counts were performed after mixing the whole blood with glacial acetic acid to lyse red cells and staining with gentian violet. Counts were then performed manually with a Neubauer cytometer (Neubaur, Buffalo, NY, U.S.A.). Blood smears were differentially stained with Jenner's stain. A total of 100 white cells were counted to determine the percentage of lymphocytes, neutrophils, segmented neutrophils, eosinophils and monocytes. Erythrocyte sedimentation rate was determined using the Wintrobe method.
Serum nitrite analysis
Since nitrate anion is the final and stable product of both NO and ONOO, it can be used as an indirect measure of both of the latter compounds (Beckman & Koppenol, 1996). Serum NO2− was measured using the method of Grisham et al. (1990). Briefly, 100 μl of standard solution or plasma, in duplicate, was incubated with Asperigillus nitrate reductase in the presence of FAD, NADPH, and HEPES buffer in a 37° water bath for 30 min to reduce all nitrate to nitrite. The reaction was quenched by adding LDH and pyruvic acid and incubation in a 37° water bath for 10 min. This was then treated with the freshly premixed Griess reagent, mixture of 0.2% napthylethylenediamine (in distilled water) and 2% sulfanilamide (in 5% ortho-phosphoric acid), and incubated at room temperature for 10 min. Absorbance on a microplate was measured at 543 nM using a Vmax Molecular Devices plate reader (Bio-Tek Instruments Inc., Winooski, VT, U.S.A.). The assay was linear from 5 to 200 μM (r2⩾0.966 and CV⩽5%).
Verapamil protein binding study
Protein binding was measured in two separate cohorts of animals, control (n=8) and IFN treated (n=8). The animals were prepared as described under the section inflammation and animal preparation for pharmacokinetic and pharmacodynamic experiments. Rats were anesthetized with methoxyflurane and bled. The blood (1–2 ml/rat) was centrifuged at 2000 × g for 15 min. The resulting serum (0.5–1 ml/rat) was adjusted to pH 7.4 with 0.1 N HCl. The serum was spiked with 100 ng ml−1 of R-verapamil and 200 ng ml−1 S-verapamil to approximate the enantiomeric ratio observed in vivo. Aliquots of 0.5 ml serum were incubated at 37°C for a predetermined period of 1 h, then transferred to Amico® micropartition chambers for ultrafiltration (Amicon Division of W.R. Grace & Co, Danvers, MA, U.S.A.). The chambers were centrifuged at 2000 × g for 1 h. Both filtrate and nonfiltrate concentrations were measured by HPLC. The fraction unbound fu was determined as the concentration unbound (Cu) divided by total concentration (Ct). To ensure that the concentrations were above the minimum quantifiable limit for the HPLC assay, the content of two micropartition chambers were pooled allowing for a total of four measurements per group.
Plasma TNF measurement
The concentration of TNF in rat plasma samples was measured in the animals used for radioligand binding study. A commercially available ELISA kit based on the manufacturer's guideline was used. In brief, 50 μl of standards or plasma, in duplicates, were added to the anti-TNF precoated strip well plate (96-well microplate) and incubated at room temperature for 1 h. Then 50 μl of biotinylated antibody was added and incubated for 2 h. Streptavidin–horseradish peroxidase solution (100 μl) was added and incubated for 30 min. Tetramethylbenzidine substrate solution (100 μl) was added and microplate developed in the dark for 30 min. In the last step, 100 μl of the stop solution was added and the absorbance was read at 450–550 nM in a Vmax Molecular Devices plate reader (Molecular Devices Corp., U.S.A.). The sensitivity of the assay was 10 pg ml−1 with a CV⩽10%.
Equilibrium radioligand binding study in the presence of acute and chronic inflammation
Acute inflammation was induced as described under the section inflammation and animal preparation for pharmacokinetic and pharmacodynamic experiments. The chronic inflammation (adjuvant arthritis) was introduced by intralymphatical injection of 0.2 ml of a 50 mg ml−1 of Mycobacterium butyricum, suspended in squalene, into the base of the tail. Control rats received 0.2 ml of sterile normal saline intralymphatically into the tail base. Animals were under close observation and when the first sign of arthritis, that is, increased paw thickness (10–14 days), was observed, they were killed using a CO2 chamber. For each inflamed rat a control one was included. Hearts were treated as described below. Plasma was harvested from blood immediately and stored at –70° until analyzed for TNF and NO levels. In addition, blood smears were prepared to count the segmented neutrophils.
The cardiac cell membrane samples were prepared using a conventional method (Chien et al., 1995; Mann et al., 1995). Hearts from control and inflamed rats were cut into small pieces and placed in freshly prepared ice-cold buffer (10 ml g−1 wet tissue) and homogenized using an Ultra Turrex T25 homogenizer at a speed of 20,000 r.p.m. for 30 s, while the tube was immersed in ice and water. The crude homogenate was placed in clean plastic tubes and centrifuged at 5000 × g at 4° for 10 min to disrupt nuclei and cytoskeletal particles. The supernatant was then centrifuged at 100,000 × g for 1 h and the resulting pellet suspended in 10 ml of the original buffer before division into small centrifuge tubes. Samples were kept at –70° until used for radioligand equilibrium binding study. An aliquot was used to determine the protein concentration by the method of Bradford (1976) using Bio-Rad protein assay solution.
Nitrendipine equilibrium binding was carried out based on the method of Mann et al. (1995). 3H-nitrendipine was used at increasing concentration (0, 0.025, 0.05, 0.1, 0.2, 0.4, 0.8 μM, n=6) in the presence and absence of unlabelled nifedipine (2 × 10−5 M) in a final volume of 5 ml Tris buffer pH 7.4. Binding was initiated by the addition of 2 mg of cell membrane protein to each tube followed by incubation for 90 min at room temperature in the dark. The incubation was terminated by rapid filtration of the media through Whatman Gf/B filters that were washed three times with 5 ml ice-cold Tris buffer. The filters were placed in counting vials and immersed in 4 ml of CytoScint scintillation liquid cocktail (ICN Biochemicals, Costa Mesa, CA, U.S.A.). The tubes were shaken vigorously for 30 s. The radioactivity of each filter was determined using a Beckman LS 6500 multi-purpose counter (Fullerton, CA, U.S.A.). Total and nonspecific binding were determined in the presence and absence of nifedipine, respectively. The specific binding was determined by subtraction of nonspecific binding from the total binding. The data were fitted to a rectangular hyperbola using Prism software (GraphPad Software, Inc., San Diego, CA, U.S.A.). The goodness of fit was estimated based on the standard deviation of the vertical distance from the line (standard deviation of residuals). The dissociation constant (KD) and the maximum number of binding sites (Bmax) were determined from the program's curve fitting analysis.
The binding behavior of normal cell membranes was compared with that of membranes from INF-inflamed and adjuvant arthritic rats, using separate control animals for each condition. In addition, the direct effect of TNF exposure on binding in normal membrane preparations was studied by incubating 100 and 1000 pg ml−1 of TNF for 15 min prior to ligand binding.
Statistical analysis
The pharmacokinetic and electrophysiological data are shown as the arithmetic mean±s.d. Student's t-test for independent samples was used to compare treatment groups at α=0.05 for all experiments.
Results
As depicted in Figure 1, INF treatment resulted, on average, in about a six-fold increase in segmented neutrophils and a more than two-fold rise in erythrocyte sedimentation rate. In addition, INF-induced inflammation caused about three- and four-fold increases in serum nitrite and TNF concentrations, respectively.
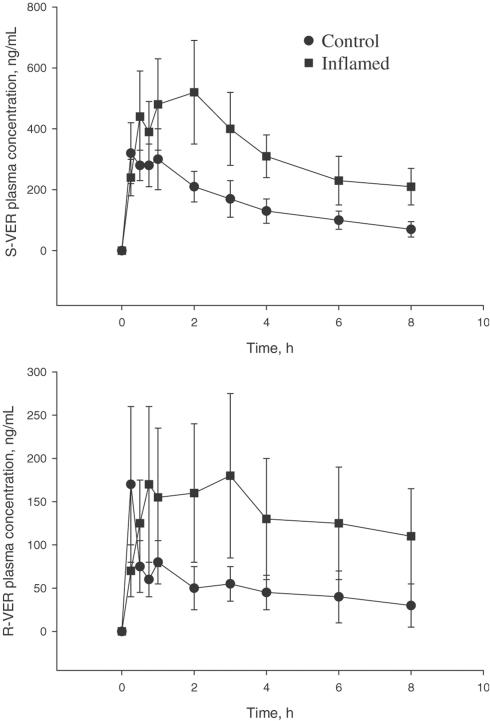
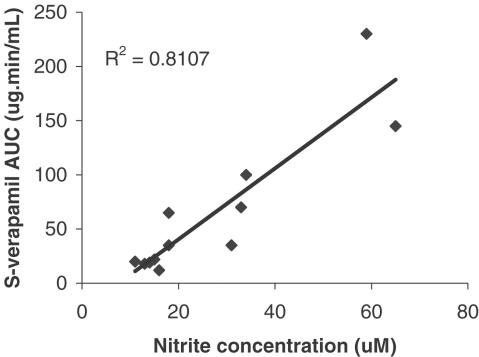
Pretreatment with INF significantly altered pharmacokinetics of verapamil (Figure 2 and Table 1). It resulted in substantial increases in AUC0→∞ of both S- and R-verapamil. A corresponding significant decrease in oral CL/F was also observed for both enantiomers. Interanimal variability was much greater in the inflamed rats. Nevertheless, the effect was so pronounced for each enantiomer that the differences between two groups were highly significant (P<0.01). The time to peak concentration (Tmax) was also altered with a 2.5-fold increase for R-verapamil and a 3.3-fold increase for S-verapamil. A 68% decrease in the terminal elimination rate constant λz) was observed for R-verapamil, but the effect on S-verapamil was insignificant. Protein binding, expressed as fu, was not significantly altered by the treatment with INF. There was a significant correlation between serum nitrate and AUC of both verapamil enantiomers as shown in Figure 3 for S-verapamil.
Figure 2.
Plasma concentration–time profile of verapamil in control and IFN-induced inflamed rats following single oral doses of 20 mg kg−1 racemic drug. AUC of both S and R-verapamil are significantly higher in inflamed than control rats. Points are the mean ± s.d. (n=8/group).
Table 1.
Pharmacokinetic indices of verapamil in control and INF-induced inflamed rats (n=8/group)
| Parameter | Control | Inflamed |
|---|---|---|
| Tmax (min) | ||
| S | 36.4±8.6 | 118.6±25.1*** |
| R | 28.1±7.1 | 69.4±12.3* |
| Cmax (ng ml−1) | ||
| S | 42.4±9.4 | 68.9±15.4 |
| R | 16.1±8.7 | 19.0±8.5 |
| λz (h−1) | ||
| S | 0.46±0.19 | 0.37±0.48 |
| R | 0.57±0.13 | 0.18±0.04* |
| AUC0→8 (μg min−1 ml−1) | ||
| S | 49.6±4.7 | 163.3±33.5** |
| R | 9.7±2.4 | 69.7±19.6** |
| AUC0→∞ (μg min−1 ml−1) | ||
| S | 53.4±6.7 | 178.4±48.2** |
| R | 11.2±2.8 | 74.4±26.6* |
| CL/F (l min−1 kg−1) | ||
| S | 0.22±0.03 | 0.075±0.01* |
| R | 1.5±0.5 | 0.27±0.05* |
| fu | ||
| S | 0.067±0.011 | 0.077±0.0057 |
| R | 0.178±0.017 | 0.184±0.0096 |
Significantly different from control. P<0.05
P<0.01
P<0.001.
Figure 3.
The correlation between serum NO2 - and S-verapamil area under plasma concentration–time curves after 20 mg kg−1 oral dose to control and INF-treated inflamed rats.
INF approximately doubled the AUC0→∞ of both S and R-norverapamil (Table 2). No statistically significant changes were observed in λz of the metabolites. The ratio of norverapamil/verapamil AUC was significantly altered for S-nor-verapamil demonstrating a 2.7-fold decrease from control values.
Table 2.
Pharmacokinetic parameters of norverapamil in control and IFN-induced inflamed rats (n=8/group)
| Parameter | Control | INα2a |
|---|---|---|
| AUC0→8 (μg min−1 ml−1) | ||
| S | 62.0±12.4 | 108.9±15.7* |
| R | 14.2±3.5 | 36.0±8.5* |
| Norverapamil : verapamil AUC ratio | ||
| S | 1.6±0.3 | 0.6±0.2* |
| R | 2.5±0.9 | 1.3±0.4 |
| λz (h−1) | ||
| S | 0.21±0.06 | 0.18±0.1 |
| R | 0.20±0.1 | 0.31±0.1 |
Different from control, P<0.05.
As expected, verapamil exhibited significant stereoselectivity in its pharmacokinetics. The extent of this stereoselectivity was in close agreement with that reported previously for healthy rats (Laethem et al., 1994). Interestingly, however, the difference between the two enantiomers was substantially smaller in the inflamed (S : R AUC ratio, 2.40) as compared with controls (S : R AUC ratio, 4.77). Inflammation had a similar effect on the AUC of norverapamil, but the differences did not reach statistical significance (S : R ratio 4.36 in control and 3.25 in inflamed rats).
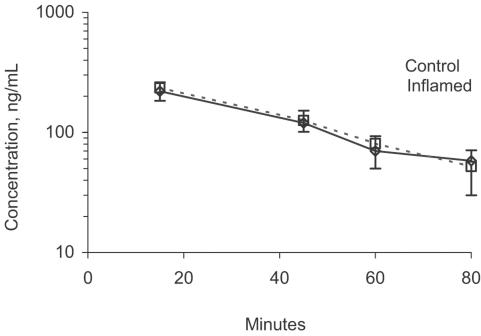
Plasma nifedipine concentrations were not influenced by inflammation (Figure 4).
Figure 4.
Nifedipine plasma concentration–time profile in control and INF-induced inflamed rats following administration of single i.v. doses of 0.1 mg kg−1 (n=8/group).
Pharmacodynamic changes
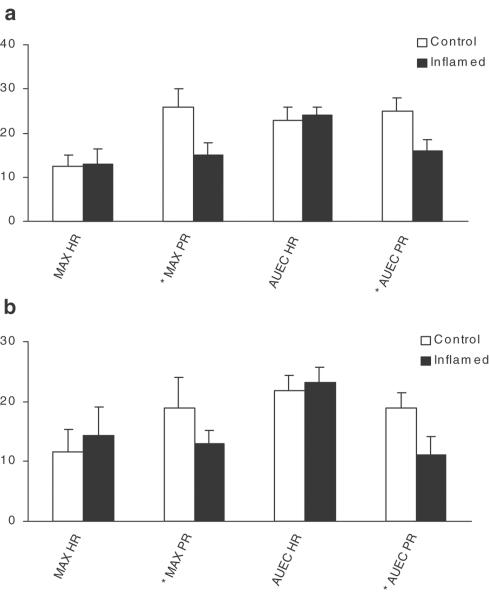
As depicted in Figure 5, verapamil and nifedipine administration resulted in a significant dromotropic effect, measured as PR interval prolongation, as well as a chronotropic effect expressed as reduced HR. Compared with the control group, the INF-treated rats demonstrated a significantly shorter prolongation of PR interval, but showed the same effect on the HR despite the increased plasma concentration of verapamil in the latter group. The prolonging effect of nifedipine on PR interval was also reduced by inflammation (Figure 5). With regard to the PR prolongation, both the maximum effect and AUEC were significantly different in the inflamed group compared with the control rats (Figure 5).
Figure 5.
Effect on inflammation on the potency of verapamil (a) nifedipine (b) to influence PR interval prolongation (PR) and (HR). Columns present maximum percent change from baseline (MAX) or the area under the percent effect change from the baseline versus time curve (AUEC, % h−1) during the 0–8 h period postdose; *denoted a significant difference between control and inflamed group; n=8/group.
Equilibrium radioligand binding
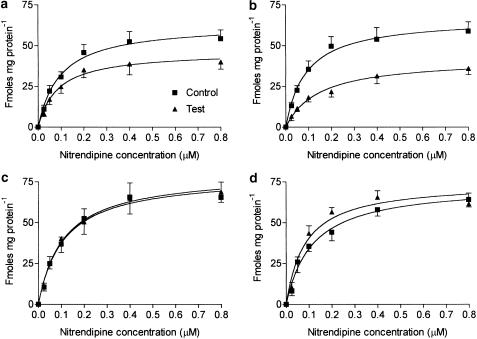
As depicted in Figure 6, both acute and chronic inflammation significantly reduced Bmax compared with normal rats (acute: controls, 63.2±2.5; INF treated, 46.4±2.0; chronic: controls, 66.8±2.2, adjuvant treated, 42.2±2.0 fmol mg−1 protein). In addition, adjuvant arthritis caused a statistically significant increase in KD compared with normal rats (normal, 0.09±0.01; adjuvant arthritis, 0.14±0.02 μM). The constants were not significantly different in acute arthritis (0.09±0.01 versus 0.08±0.01 μM, respectively). Direct addition of 100 or 1000 pg ml−1 of rat TNF to normal cardiac cell membrane preparations did not influence the binding indices (Bmax: 100 pg ml−1; TNF: controls, 78.9±4.0; tests, 80.4±3.2 fmol mg−1 protein; 1000 pg ml−1, TNF: controls, 73.4±4.0; tests, 74.6±5.6 fmol mg−1 protein; KD: 100 pg ml−1 TNF: controls, 0.11±0.02; tests 0.11±0.01 μM; 1000 pg ml−1 TNF: controls, 0.11±0.02; tests, 0.08±0.02 μM). Curve fitting analysis indicated that, in every case, a single binding site model conformed most closely to the observed data.
Figure 6.
3H-nitrendipine binding to L-type calcium channels in rat cardiac cell membrane preparations. Keys: (a) IFN-induced acute inflammation rats; (b) adjuvant arthritis rats; preparation from healthy rats incubated with 100 (c) and 1000 pg ml−1 (D) of rat-TNFα. Bmax is significantly decreased in both inflamed groups compared with their respective control groups; n=6–8/group.
Discussion
The effect of inflammation on the pharmacokinetics of many drugs including verapamil has mostly been reported in experimental animals such as rats, rabbits and dogs (Bishop et al., 1981; Piquette-Miller & Jamali, 1993; Laethem et al., 1994, 1995). Only a few studies have presented human data (Schneider et al., 1979, 1981, 1982; Mayo et al., 2000). Inflammation inhibits CL of drugs. The effect is mainly seen after oral doses of drugs with efficient hepatic CL such as propranolol and verapamil. Drugs with low or intermediate hepatic extraction ratio seems to be less sensitive to this effect (Emami et al., 1998). Even for drugs that are highly extracted by the liver through the first pass, the inhibitory effect of inflammation is less evident following i.v. doses (Piquette-Miller & Jamali, 1993). This is because i.v. doses escape the first-pass metabolism. Hence, as we expected, pharmacokinetics of nifedipine were not influenced by inflammation (Figure 4). This, we attribute to the drug's efficient CL in the gut rather than the liver (Grundy et al., 1977) and the i.v. route of administration. Indeed, control and inflamed rats exhibited almost superimposible plasma concentration–time curves (Figure 4). Our data generated using control rats were in close agreement with those reported earlier (Grundy et al., 1977; Eliot & Jamali, 1999).
Nevertheless, therapeutic consequences of the acknowledged effect of inflammation on pharmacokinetics of drugs have remained mainly speculative. It is generally believed that higher drug concentrations, particularly that of plasma protein unbound fractions, should result in higher effect and/or toxicity. Our laboratory has recently presented a different view (Mayo et al., 2000). It was reported that, as expected, in patients afflicted with rheumatoid arthritis, plasma concentrations of verapamil enantiomers are markedly elevated. A reduced fraction of free verapamil concentration was also observed, perhaps because of the acknowledged association between inflammation and increased α1-acid glycoprotein expression (Piafsky, 1978). However, because of the substantial increase in total verapamil concentration in plasma, the decreased fraction did not amount to a reduced concentration of the unbound drug (Table 1). A lower unbound drug concentration (Yasuhara et al., 1985; Belpaire et al., 1986; Walker et al., 1986), therefore, could not be the mechanism behind the observed reduction in verapamil-induced prolongation of the PR interval in rheumatoid arthritis. For the β-adrenoceptor antagonist, sotalol, which is negligibly bound to plasma proteins, we have also observed reduced response caused by inflammation (Kulmatycki et al., 2001). Hence, as a potential mechanism for our observation, we offered a reduced calcium channel responsiveness (Mayo et al., 2000). The present data lend support to this notion.
The rat appears to be a suitable model for the study of the influence of inflammation on the pharmacokinetics and pharmacodynamics of verapamil. The data depicted in Figure 1 clearly indicate that the administration of INF according to the protocol followed in our study results in a significant inflammatory condition. The hematological observations coupled with increased TNF and nitrite, a metabolic product of NO, are all indicative of inflammation (Tilg, 1997). Equally important was our observation that plasma concentrations of verapamil (Table 1) and its metabolite (Table 2) were significantly increased by inflammation. These observations are similar to those we have made with patients suffering from rheumatoid arthritis (Mayo et al., 2000). Indeed, the greater interanimal variability observed in inflamed rats, as compared with controls, also resembles the results that we have reported for rheumatoid arthritis patients (Mayo et al., 2000). This may be attributed to the variability in the severity of inflammation. A significant negative association has been observed between propranolol CL and severity of inflammation in adjuvant arthritis (Piquette-Miller & Jamali, 1995). We have also observed a longer peak plasma time for inflamed rats compared with the control group (Table 1). This, however, may be because of more fluctuation in plasma concentrations in inflamed rats as depicted in Figure 2, and the inherent difficulties in pinpointing the exact Tmax.
It is also worth noting that the ratio of AUC of S over R, a reflection of the extent of stereoselectivity of verapamil pharmacokinetics, decreased from 4.77 in controls to 2.40 in the inflamed rats. This suggests that the effect of inflammation on the CL of verapamil is stereoselective, that is, the pharmacologically active S enantiomer is affected less than that its antipode. However, the impact of inflammation on the stereoselectivity of verapamil pharmacokinetics seems to be dependent upon the species and type of inflammation. The inhibitory effect of endotoxin on verapamil CL, although stereoselective, appears to be in the opposite direction of that observed after administration of INF (Laethem et al., 1994). Human rheumatoid arthritis also results in the same extent of inhibition for the two enantiomers (Mayo et al., 2000). Nevertheless, all these inflammatory conditions result in reduced CL of verapamil.
Despite increases in the plasma concentration of verapamil enantiomers, the potency of drug in prolonging PR interval was reduced in inflamed rats. This is another similarity between the observations made in humans (Mayo et al., 2000) and rats, and further confirms that the rat with acute inflammation is, indeed, a suitable model to study the mechanism of diminished L-type calcium channels responsiveness secondary to inflammation. Following i.v. doses of nifedipine, we also observed reduced response secondary to acute inflammation (Figure 5) but in the absence of pharmacokinetic changes (Figure 4). This is important since the reduced response is confirmed in the absence of potential confounding factors.
In our human study, we used PR interval prolongation as a reproducible and sensitive marker of the calcium channel response. Single doses of antagonists prolong PR interval quickly and in a dose-dependent fashion without a need for cardiac stimulation (Johnston et al., 1981; Abernethy et al., 1993). Nevertheless, the marker does not provide insight into the mechanism of reduced receptor responsiveness. The rat model presented here, therefore, was used to measure directly the effect of inflammation on the characteristics of the cardiac L-type calcium channels.
To investigate the possibility that L-type calcium channels are downregulated in the presence of acute and chronic inflammation, we performed equilibrium radioligand binding studies using cardiac cell membranes from normal and inflamed rats. We used 3H-nitrendipine, a specific L-type calcium channel ligand. It has been reported that verapamil blocks not only L-type calcium channel, but is also a potent blocker of HERG K-channel in the heart and alveolar epithelial cells (DeCoursey, 1995; Zhang et al., 1999). Dihydropyridines, such as nitrendipine, do not block the rapid delayed rectifier current (Zhang et al., 1999). Our in vivo data demonstrate that, similar to what has been observed for verapamil, response to nifedipine is also reduced by inflammation (Figure 5).
The present data (Figure 6) indicate that inflammation causes a significant reduction in the maximum capacity (Bmax) of the L-type calcium channel receptor to bind ligands. The effect was more pronounced in adjuvant arthritic rats. The reduction in the number of binding sites could be because of factors influencing transcription, for example, gene downregulation and reduced mRNA synthesis. The effect may also be at the posttranscription level. A direct exposure of the receptor to the proinflammatory mediators may also alter receptor response. Inflammation has also been reported to weaken the potency of β-adrenergic and potassium channel antagonists in the rat (Kulmatycki et al., 2001). Interestingly, this effect is reversed when TNF concentration is lowered by the administration of an anti-TNF antibody suggestive of a link between the elevated cytokine concentration and the receptor downregulation. However, under our experimental conditions, direct exposure of the heart membrane preparations to TNF did not alter the binding capacity of the cardiac cell membrane preparations for nitrendipine (Figure 6c and d).
Our in vivo observation of receptor downregulation is in close agreement with several in vitro reports dealing with different receptors or conditions. Downregulation of cardiac and pulmonary receptors by proinflammatory cytokines or inflammatory lung and heart diseases has been reported. β2-adrenoceptors in cultured human airway smooth muscle cell showed reduced responsiveness in the presence of IL-1 (Shore et al., 1997; Laporte et al., 1998) and mRNA expression for L-type calcium channels subunits was reduced in human atrial fibrillation (Grammer et al., 2001). Brundle et al. (2001) noted a downregulation of L-type calcium channels and several K-channels, including HERG, in paroxysmal and persistent atrial fibrillation in humans.
The observed reduced cardiac receptor responsiveness may have important therapeutic consequences. Hinz & Phol (1975) found that about 40% of rheumatoid arthritis patients showed some evidence of heart failure. Rheumatoid arthritis patients have an increased mortality rate (Prior et al., 1984; Mutru et al., 1985) and die on average 2.5 years earlier in community-based studies (Myllykangas-Luosujarvi et al., 1995) and up to 18 years in hospital-based cohorts (Wolfe et al., 1994). Approximately 50% of these deaths are because of cardiovascular diseases (Wolfe et al., 1994). The reason for this increased mortality in rheumatoid arthritis is not clear. However, in addition to the generally acknowledged risk factors (i.e. hypertension, smoking, high cholesterol and obesity), elevated baseline diastolic blood pressure and thrombotic variables have been suggested for arthritic patients (McEntegart et al., 2001). Very recently, in myocardial infarction, another form of inflammation, the presence of proinflammatory mediators such as C-reactive protein (CRP), TNF and interleukin-6 (IL-6), have been identified as additional risk factors (Pietila et al., 1996; Griselli et al., 1999; Biasucci et al., 2000; Torre-Amione et al., 2000; Chew et al., 2001; Koukkunen et al., 2001; Lindmark et al., 2001). Indeed, there appears to be a close association between death and elevated CRP a proinflammatory marker, after myocardial infarction (Pietila et al., 1996). Therefore, it is reasonable to suggest that in both RA and cardiovascular patients, elevated proinflammatory mediators may be involved in their high mortality rate. The mechanism of action of these pathophysiological changes, however, remains to be discovered. The present rat data, coupled with our recently reported observation in RA, suggest that the presence of proinflammatory mediators may reduce response to cardiac therapies in patients with concomitant inflammatory conditions.
Acknowledgments
This work was supported by the Canadian Institute of Health Research grant # 983587. We thank Dr Reza Mehvar for his initial help with the HPLC assay, Ted Germaine of the Surgical-Medical Research Institute for his help and donation of equipment, and the support of the Cross Cancer Institute Pharmacy Department.
Abbreviations
- AUC
area under concentration – time curve
- AUC0–8
area under concentration – time (0–8 h) curve
- AUEC
area under effect–time curve
- Bmax
maximum number of binding sites
- CL
clearance
- Cu
unbound concentration
- Ct
total concentration
- CRP
C-reactive protein
- fu
fraction unbound
- HPLC
high-performance liquid chromatography
- HR
heart rate
- INF
interferon
- IL-6
interleukin-6
- KD
dissociation constant
- λz
elimination rate constant
- NO
nitric oxide
- PEG
polyethyleneglycol
- Tmax
time to maximum plasma concentration
- TNF
tissue necrosis factor-α
References
- ABDEL-RASSAK Z., LOYER P., FAUTREL A., GAUTIER J.C., CORCOS L., TURLIN P.D., GUILLOUZO A. Cytokines down-regulate expression of major cytochrome P450 enzymes in adult human hepatocytes in primary culture. Mol. Pharmacol. 1993;44:707–715. [PubMed] [Google Scholar]
- ABERNETHY D., WAINER I., LONGSTRETH. J., ADRAWIS. N. Stereoselective verapamil disposition & dynamics in aging during racemic verapamil administration. J. Pharmacol Exp. Ther. 1993;266:904–911. [PubMed] [Google Scholar]
- BECKMAN J., KOPPENOL W. Nitric oxide, superoxide, and peroxynitrite: the good the bad and the ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- BELPAIRE F.M., BOGAERT M.G., MUGABO P., ROSSEEL M.T. Binding to serum alpha 1-acid glycoprotein and effect of beta-adrenoceptor antagonists in rats with inflammation. Br. J. Pharmacol. 1986;88:697–705. doi: 10.1111/j.1476-5381.1986.tb10253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIASUCCI L.M., LIUZZO G., ANGIOLILLO D.J., SPERTI G., MASERI A. Inflammation and acute coronary syndromes. Herz. 2000;25:108–112. doi: 10.1007/pl00001947. [DOI] [PubMed] [Google Scholar]
- BISHOP H., SCHNEIDER R.E., WELLING P.G. Plasma propranolol concentrations in rats with adjuvant-induced arthritis. Biopharm. Drug Dispos. 1981;2:291–297. doi: 10.1002/bdd.2510020310. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CAWTHORNE M.A., PALMER E.D., GREEN J. Adjuvant induced arthritis and drug-metabolizing enzymes. Biochem. Pharmacol. 1976;25:2683–2688. doi: 10.1016/0006-2952(76)90257-4. [DOI] [PubMed] [Google Scholar]
- CHEW D.P., BHATT D.L., ROBBINS M.A., PENN M.S., SCHNEIDER J.P., LAUER M.S., TOPOL E.J., ELLIS S.G. Incremental prognostic value of elevated baseline C-reactive protein among established markers of risk in percutaneous coronary intervention. Circulation. 2001;104:992–997. doi: 10.1161/hc3401.095074. [DOI] [PubMed] [Google Scholar]
- CHIEN A.J., ZHAO X., SHIROKOV R.E., PURI T.S., CHANG C.F., SUN D., RIOS E., HOSEY M.M. Roles of a membrane-localized beta subunit in the formation and targeting of functional L-type Ca2+ channels. J. Biol. Chem. 1995;270:30036–30044. doi: 10.1074/jbc.270.50.30036. [DOI] [PubMed] [Google Scholar]
- CRIBB A., DELAPORTE E., KIM S., NOVAK R., RENTON K. Regulation of CYP1A and CYP2E induction in the rat during production of IFα/β. J. Pharmacol. Exp. Ther. 1994;268:487–494. [PubMed] [Google Scholar]
- DECOURSEY T.E. Mechanism of K+ channel block by verapamil and related compounds in the rat alveolar epithelial cells. J. Gen. Physiol. 1995;106:745–779. doi: 10.1085/jgp.106.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESCOTES J. Immunomodulating agents and hepetic drug metabolizing enzymges. Drug Metab. Rev. 1985;16:175–185. doi: 10.3109/03602538508991434. [DOI] [PubMed] [Google Scholar]
- ELIOT L.A., JAMALI F. Pharmacokinetics and pharmacodynamics of nifedipine in untreated and atorvastatin-treated hyperlipidemic rats. J. Pharmacol. Exp. Ther. 1999;291:188–193. [PubMed] [Google Scholar]
- EMAMI J., PASUTTO F.M., JAMALI F. Effect of experimental diabetes mellitus and arthritis on the pharmacokinetics of hydroxychloroquine enantiomers in rats. Pharm. Res. 1998;15:897–903. doi: 10.1023/a:1011928732588. [DOI] [PubMed] [Google Scholar]
- FANG Z.Y., FONTAINE J., UNGER P., BERKENBOOM G. Alterations of the endothelial function of isolated aortae in rats with adjuvant arthritis. Arch. Int. Pharmacodyn. 1991;311:122–130. [PubMed] [Google Scholar]
- FERRARI L., HERBER R., BATT A.M., SIEST G. Differential effects of human recombinant IL-1β and dexamethasone on hepatic drug-metabolizing enzymes in male and female rats. Biochem. Pharmacol. 1993a;45:2269–2277. doi: 10.1016/0006-2952(93)90198-6. [DOI] [PubMed] [Google Scholar]
- FERRARI L., JOUZEAU J.Y., GILLET P., HERBER R., FENER P., BATT A.M., NETTER P. IL-1β differentially repressess drug-metabolizing enzymes in arthritic female rats. J. Pharmacol. Exp. Ther. 1993b;264:1012–1020. [PubMed] [Google Scholar]
- FONTAINE J., HERCHUELZ A., DEBERG E., POCHET R., FAMAEY J.P. Calcium movements in aorta from arthritic rats. Int. Tissue React. 1986;8:295–301. [PubMed] [Google Scholar]
- GRAMMER J.B., ZENG X., BOSCH R.F., KUHLKAMP V. Atrial L-type Ca2+ channel, Beta-adrenoreceptor, and 5-hydroxytryptamine type 4 receptor mRNA in human atrial fibrilation. Basic Res. Cardiol. 2001;96:82–90. doi: 10.1007/s003950170081. [DOI] [PubMed] [Google Scholar]
- GRISELLI M., HERBERT J., HUTCHINSON W.L., TAYLOR K.M., SOHAIL M., KRAUSZ T., PEPYS M.B. C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J. Exp. Med. 1999;190:1733–1740. doi: 10.1084/jem.190.12.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRISHAM M., JOHNSON G., GAUTREAUX M., BERG R. Measurement of nitrate and nitrite in extracellular fluids: a window to systemic nitric oxide metabolism. Methods: Companion Methods Enzymol. 1990;7:84–90. [Google Scholar]
- GRUNDY J.S., ELIOT L.A., FOSTER R.T. Extrahepatic first-pass metabolism of nifedipine in the rat. Biopharm. Drug Dispos. 1997;18:509–522. doi: 10.1002/(sici)1099-081x(199708)18:6<509::aid-bdd38>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- GRUNDY J.S., KHERANI R., FOSTER R.T. Sensitive high-performance liquid chromatographic assay for nifedipine in human plasma utilizing ultraviolet detection. J. Chromatogr. 1994;654:146–151. doi: 10.1016/0378-4347(93)e0449-z. [DOI] [PubMed] [Google Scholar]
- HINZ G., PHOL W. Diagnostic-therapeutic problems on heart involvement in chronic polyarthritis. Z. Rheumatol. 1975;34:39–48. [PubMed] [Google Scholar]
- JOHNSTON A., BURGESS C., HAMER J. Systemic availability of oral verapamil and effect on PR interval in man. Br. J. Clin. Pharmacol. 1981;12:397–400. doi: 10.1111/j.1365-2125.1981.tb01233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHATSENKO O., GROSS S., RIFKIND A., VANE J. Nitric oxide is a mediator of the decrease in CYP450-dependent metabolism caused by immunostimulants. Proc. Natl. Acad. Sci. U.S.A. 1993;90:11147–11151. doi: 10.1073/pnas.90.23.11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOUKKUNEN H., PENTTILA K., KEMPPAINEN A., HALINEN M., PENTTILA I., RANTANEN T., PYORALA K. C-reactive protein, fibrinogen, interleukin-6 and tumor necrosis factor-alpha in the prognostic classification of unstable angina pectoris. Ann. Med. 2001;33:37–47. doi: 10.3109/07853890109002058. [DOI] [PubMed] [Google Scholar]
- KULMATYCKI KM., ABOUCHEHADE K., SATTARI S., JAMALI F. Drug-disease interaction: reduced beta-adrenergic and potassium channel antagonist activities of sotalol in the presence of acute and chronic inflammatory conditions in the rat. Br. J. Pharmacol. 2001;133:286–294. doi: 10.1038/sj.bjp.0704067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KULMATYCLI K.M., JAMALI F. Therapeutic relevance of altered cytokine expression. Cytokines. 2001;14:1–10. doi: 10.1006/cyto.2000.0827. [DOI] [PubMed] [Google Scholar]
- LAETHEM M.E., BELPAIRE F.M., WIJNANT P., ROSSEEL M.T., BOGAERT M.G. Influence of endotoxin on the stereoselective pharmacokinetics of oxprenolol, propranolol, and verapamil in the rat. Chirality. 1994;6:405–410. doi: 10.1002/chir.530060508. [DOI] [PubMed] [Google Scholar]
- LAETHEM M.E., BELPAIRE F.M., WIJNANT P., BOGAERT M.G. Stereoselective pharmacokinetics of oxprenolol, propranolol, and verapamil: species differences and influence of endotoxin. Chirality. 1995;7:616–622. doi: 10.1002/chir.530070811. [DOI] [PubMed] [Google Scholar]
- LAPORTE J.D., MOORE P.E., PANTTIERI R.A., WINFRIED M., HEYDER J., SHORE S.A. Prostanoids mediate IL-1β induced β-adrenergic hyporesponsiveness in human airway smooth muscle cells. Am. J. Physiol. 1998;275:L491–L501. doi: 10.1152/ajplung.1998.275.3.L491. [DOI] [PubMed] [Google Scholar]
- LINDMARK E., DIDERHOLM E., WALLENTIN L., SIEGBAHN A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: effects of an early invasive or noninvasive strategy. J. Am. Med. Assoc. 2001;286:2154–2156. doi: 10.1001/jama.286.17.2107. [DOI] [PubMed] [Google Scholar]
- MANN C.D., VU T.B., HRDINA P.D. Protein kinase C in rat brain cortex and hippocampus: effect of repeated administration of fluoxetine and desipramine. Br. J. Pharmacol. 1995;115:595–600. doi: 10.1111/j.1476-5381.1995.tb14973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAYO PR., SKEITH K., RUSSELL AS., JAMALI F. Decreased dromotropic response to verapamil despite pronounced increase drug concentration in rheumatoid arthritis. Br. J. Clin. Pharmacol. 2000;50:605–613. doi: 10.1046/j.1365-2125.2000.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCENTEGART A., CAPELL H.A., CRERAN D., RUMLEY A., WOODWARD M., LOWE G.D. Cardiovascular risk factors, including thrombotic variables, in a population with rheumatoid arthritis. Rheumatology. 2001;40:640–644. doi: 10.1093/rheumatology/40.6.640. [DOI] [PubMed] [Google Scholar]
- MONSHOUWER M., WITKAMP R.F., NIJMEIJER S.M., VAN AMSTERDAM J.G., VAN MIERT A.S.J.P.A.M. Suppression of cytochrome P450- and UDP-glucuronosyl transferase-dependent enzyme activities by proinflammatory cytokines and possible role of nitric oxide in primary cultures of pig hepatocytes. Toxicol. Appl. Pharmacol. 1996;137:237–244. doi: 10.1006/taap.1996.0077. [DOI] [PubMed] [Google Scholar]
- MORENO J.J., ESCOFET A., CASTELL M., CASTELLOTE C., QUERALT J. Hepatic cytochrome P-450 activities and serum biochemical changes in adjuvant arthritis. Med. Sci. Res. 1987;15:1469–1470. [Google Scholar]
- MUTRU O., LAAKSO M., ISOMAKI H., KOOTA K. Ten years mortality and causes of death in patients with rheumatoid arthritis. BMJ. 1985;15:1797–1799. doi: 10.1136/bmj.290.6484.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYLLYKANGAS-LUOSUJARVI R., AHO K., KAUTIAINEN H., ISOMAKI H. Cardiovascular mortality in women with rheumatoid arthritis. J. Rheumatol. 1995;22:1065–1067. [PubMed] [Google Scholar]
- PARENT C., BELANGER PM., JUTRAS L., DU SOUICH P. Effect of inflammation on the rabbit hepatic cytochrome P450 isoenzymes: alterations in the kinetics and dynamics of tolbutamide. J. Pharmacol. Exp. Ther. 1992;261:780–787. [PubMed] [Google Scholar]
- PETERSON T.C., RENTON K.W. Kupfer cell factor mediated depression of hepatic parenchymal cell cytochrome P-450. Biochem. Pharmacol. 1986;35:1491–1497. doi: 10.1016/0006-2952(86)90114-0. [DOI] [PubMed] [Google Scholar]
- PIAFSKY K. Increased plasma protein binding of pro-pranolol and chlorpromazine mediated by disease-induced elevations of plasma 1-acid glycoprotein. N. Engl. J. Med. 1978;299:1435–1445. doi: 10.1056/NEJM197812282992604. [DOI] [PubMed] [Google Scholar]
- PIETILA KO., HARMOINEN AP., JOKINITTY J., PASTERNACK A.L. Serum C-reactive protein in acute myocardial infarction and its relationship to mortality during 24 months of follow-up in patients under thrombolytic treatment. Eur. Heart J. 1996;18:1834–1836. doi: 10.1093/oxfordjournals.eurheartj.a015068. [DOI] [PubMed] [Google Scholar]
- PIQUETTE-MILLER M., JAMALI F. Selective effect of adjuvant arthritis on the disposition of propranolol enantiomers in rats detected using a stereospecific HPLC assay. Pharm. Res. 1993;10:294–299. doi: 10.1023/a:1018907431893. [DOI] [PubMed] [Google Scholar]
- PIQUETTE-MILLER M., JAMALI F. Influence of severity of inflammation on the disposition of propranolol enantiomers in ketoprofen-treated and untreated adjuvant arthrititic rats. Drug Metab. Dispos. 1995;23:240–245. [PubMed] [Google Scholar]
- PRIOR P., SYMMONS D.P., SCOTT D.L., BROWN R., HAWKINS C.F. Cause of death in rheumatoid arthritis. Br. J. Rheumatol. 1984;23:92–99. doi: 10.1093/rheumatology/23.2.92. [DOI] [PubMed] [Google Scholar]
- SAKAI H., OKAMOTO T., KIKKAWA Y. Suppression of hepatic drug metabolism by the interferon inducer Poly, I.C. J. Pharmacol. Exp. Ther. 1992;263:381–386. [PubMed] [Google Scholar]
- SCHNEIDER R.E., BISHOP H. Beta-blocker plasma concentrations and inflammatory disease: clinical implications. Clin. Pharmacokinet. 1982;7:281–284. doi: 10.2165/00003088-198207040-00001. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER R.E., BISHOP H., HAWKINS C.F. Plasma propranolol concentrations and the erythrocyte sedimentation rate. Br. J. Clin. Pharmacol. 1979;8:43–47. doi: 10.1111/j.1365-2125.1979.tb05907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER R.E., BISHOP H., KENDALL M.J., QUARTERMAN C.P. Effect of inflammatory disease on plasma concentrations of three beta-adrenoceptor blocking agents. Int. J. Clin. Pharmacol. Ther. Toxicol. 1981;19:158–162. [PubMed] [Google Scholar]
- SHIBUKAWA A., WAINER I.W. Simultaneous direct determination of the enantiomers of verapamil and norverapamil in plasma using a derivatized amylose high-performance liquid chromatographic chiral stationary phase. J. Chromatogr. 1992;574:85–92. doi: 10.1016/0378-4347(92)80101-u. [DOI] [PubMed] [Google Scholar]
- SHORE SA., LAPORTE J., HALL I.P., HARDY E., PANETTIERI R.A. Effect of IL-1β on responses of cultured human airway smooth muscle cells to bronchodilator agents. Am. J. Respir. Cell Mol. Biol. 1997;16:702–712. doi: 10.1165/ajrcmb.16.6.9191472. [DOI] [PubMed] [Google Scholar]
- TILG H. New insights into the mechanisms of interferon alfa (sic): an immunoregulatroy and anti-inflammatory cytokine. Gastroenterology. 1997;112:1017–1021. doi: 10.1053/gast.1997.v112.pm9041265. [DOI] [PubMed] [Google Scholar]
- TORRE-AMIONE G., VOOLETICH M.T., FARMER J.A. Role of tumor necrosis factor-alpha in the progression of heart failure: therapeutic implications. Drugs. 2000;59:745–751. doi: 10.2165/00003495-200059040-00002. [DOI] [PubMed] [Google Scholar]
- WALKER K.A., BARBER H.E., HAWKSWORTH G.M. Mechanism responsible for altered propranolol disposition in adjuvant-induced arthritis in the rat. Drug Metab. Dispos. 1986;14:482–486. [PubMed] [Google Scholar]
- WOLFE F., MITCHEL D.M., SIBLEY J.T., FRIES J.F., BLOCH D.A., WILLIAMS C.A., SPITZ P.W., HAGA M., KLEINHEKSEL S.M., CATHY M.A. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481–494. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- YASUHARA M., FUJIWARA J., KITADE S., KATAYAMA H., OKUMURA K., HORI R. Effect of altered plasma protein binding on pharmacokinetics and pharmacodynamics of propranolol in rats after surgery: role of alpha-1-acid glycoprotein. J. Pharmacol. Exp. Ther. 1985;235:513–520. [PubMed] [Google Scholar]
- ZHANG S., ZHOU Z., GONG Q., MAKIELSKI J.C., JANUARY C.T. Mechanism of block and identification of the verapamil binding domain to HERG potassium channels. Circ. Res. 1999;84:989–998. doi: 10.1161/01.res.84.9.989. [DOI] [PubMed] [Google Scholar]