Abstract
Chronic hypoxia (CH) increases lung tissue expression of all types of nitric oxide synthase (NOS) in the rat. However, it remains unknown whether CH-induced changes in functional and histological NOS distributions are correlated in rat small pulmonary arteries.
We measured the effects of NOS inhibitors on the internal diameters (ID) of muscular (MPA) and elastic (EPA) pulmonary arteries (100–700 μm ID) using an X-ray television system on anaesthetized rats. We also conducted NOS immunohistochemical localization on the same vessels.
Nonselective NOS inhibitors induced ID reductions in almost all MPA of CH rats (mean reduction, 36±3%), as compared to ∼60% of control rat MPA (mean, 10±2%). The inhibitors reduced the ID of almost all EPA with similar mean values (∼26%) in both CH and control rats. On the other hand, inducible NOS (iNOS)-selective inhibitors caused ID reductions in ∼60% of CH rat MPA (mean, 15±3%), but did so in only ∼20% of control rat MPA (mean, 2±2%). This inhibition caused only a small reduction (mean, ∼4%) in both CH and control rat EPA. A neuronal NOS-selective inhibitor had no effect.
The percentage of endothelial NOS (eNOS)-positive vessels was ∼96% in both MPA and EPA from CH rats, whereas it was 51 and 91% in control MPA and EPA, respectively. The percentage for iNOS was ∼60% in both MPA and EPA from CH rats, but was only ∼8% in both arteries from control rats.
The data indicate that in CH rats, both functional and histological upregulation of eNOS extensively occurs within MPA. iNOS protein increases sporadically among parallel-arranged branches in both MPA and EPA, but its vasodilatory effect is predominantly observed in MPA. Such NOS upregulation may serve to attenuate hypoxic vasoconstriction, which occurs primarily in MPA and inhibit the progress of pulmonary hypertension.
Keywords: Chronic hypoxia, pulmonary hypertension, small pulmonary arteries, endogenous NO, L-NAME, L-NMMA, L-canavanine, s-methylisothiourea, 7-nitro indazole, NOS immunohistochemistry
Introduction
The change in lung expression of nitric oxide synthase (NOS) in response to chronic hypoxia (CH) has extensively been studied in the rat. Northern and Western blot analyses in hypoxic rats have demonstrated increased lung tissue expression of mRNA and protein for endothelial NOS (eNOS) (Xue et al., 1994; Shaul et al., 1995; Le Cras et al., 1996; 1998; Xue & Johns, 1996; Tyler et al., 1999), inducible NOS (iNOS) (Xue et al., 1994; Le Cras et al., 1996; Xue & Johns, 1996), and neuronal NOS (nNOS) (Xue & Johns, 1996). The significant increases in gene and protein expression were observed as early as 24 h after hypoxia (Xue & Johns, 1996). NOS immunohistochemistry has shown that the hypoxic increase in eNOS staining intensity is induced in the pulmonary arteries (diameter 50–300 μm) but not in veins (60–300 μm) (Resta et al., 1997). On the other hand, iNOS increased in vascular smooth muscle at all levels of hypoxic pulmonary vessels, although it was very limited in normoxic pulmonary vessels (Xue et al., 1994; Xue & Johns, 1996). Moreover, the majority of nNOS immunoreactivity was distributed in bronchial epithelial cells, although it was found in the neurons surrounding the large bronchi (Xue & Johns, 1996).
The functional role of endogenous nitric oxide (NO) in modulating pulmonary vascular tone of the CH rat has been examined chiefly by use of isolated perfused lungs or isolated proximal elastic pulmonary arteries (main or extralobar pulmonary arteries). Most, but not all (Adnot et al., 1991; Eddahibi et al., 1992), pressure-flow studies of hypoxic lungs have suggested either normal or increased responsiveness to the nonselective NOS inhibitor (Barer et al., 1993; Russ & Walker, 1993; Isaacson et al., 1994; Roos et al., 1996; Muramatsu et al., 1997; Tyler et al., 1999) and to endothelium-dependent vasodilators (Barer et al., 1993; Russ & Walker, 1993; Isaacson et al., 1994; Muramatsu et al., 1996; Resta & Walker, 1996; Roos et al., 1996). In contrast, isolated proximal arteries have impaired responsiveness to endothelium-dependent vasodilators (Crawley et al., 1992; Rodman, 1992; Carville et al., 1993; Shaul et al., 1993; Maruyama & Maruyama, 1994). These data suggest the possibility that the effect of CH on endogenous NO-mediated vasodilatation differs between the muscular (resistance) and elastic (conduit) pulmonary vessels in the rat. Recently, using a selective iNOS inhibitor, a pressure-flow study has suggested that NO derived from iNOS does not modulate pulmonary vasoconstrictor responsiveness in isolated lungs from CH rats (Resta et al., 1999).
Despite such extensive structural and functional studies, the following problems have yet to be examined sufficiently in the CH rat. One issue is whether, and to what extent, the effect of hypoxia on the NO-mediated basal vascular tone regulation varies along the series-connected small pulmonary arteries, from elastic to muscular segment levels and, moreover, between the parallel-arranged vascular branches within each vascular segment level. Another is whether the increased iNOS and nNOS can contribute to the basal tone regulation in these small arteries. To resolve these issues, we applied a specially designed X-ray television system (Sada et al., 1985; Shirai et al., 1986) on anaesthetized rats and directly measured internal diameter (ID) changes due to NOS inhibition in the pulmonary arterial trees (100–700 μm ID), which contain both muscular and distal elastic segments (Kay, 1983; Sasaki et al., 1995). We used the in vivo pulmonary circulation associated with natural blood perfusate and flow pattern, because these are important factors for determining the expression of NO activity (Hakim, 1994; Sprague et al., 1995). The ID changes were compared between 4-week hypoxic rats and normoxic control rats. Nω-nitro-L-arginine methyl ester (L-NAME) or Nω-monomethyl-L-arginine (L-NMMA) was used for nonselective NOS inhibition, L-canavarine or S-methylisothiourea sulphate for selective inhibition of iNOS (Szabo et al., 1994; Teale & Atkinson, 1994; Liaudet et al., 1996) and 7-nitro indazole for selective inhibition of nNOS (Moore et al., 1993; Kalisch et al., 1996; Okamoto et al., 1997). We found that nonselective NOS inhibition and iNOS-selective inhibition cause significant ID changes, but nNOS-selective inhibition does not. Therefore, after these physiological studies, we further analysed eNOS and iNOS immunohistochemical localization in the pulmonary arteries to examine the correspondence between the structural and functional upregulation of NOS.
Methods
The study was conducted in accordance with the Guiding Principles for the Care and Use of Animals in the Field of Physiological Sciences, published by the Physiological Society of Japan. Male Sprague–Dawley rats were used for all experiments.
Chronic environment
Rats aged 6 weeks at the start of the experiment were placed in either a normobaric environmental chamber maintained at 10% O2 (hypoxic group, n=15) or a chamber open to room air (control normoxic group, n=11) for 4 weeks. Carbon dioxide was removed by self-indicating soda lime granules and excess humidity prevented by cooling of the recirculation circuit. The environment within the chamber was monitored with a mass spectrometer. All hypoxic and normoxic rats were kept in the same room, at the same light–dark cycle. Food and water were available ad libitum. The chamber was opened for ∼10 min daily to clean the cages and replenish food and water.
Experimental procedure and angiography
Rats were anaesthetized with pentobarbital sodium (50 mg kg−1 i.p.) and supplemental doses (10–20 mg kg−1 h−1 i.v.) were administered to maintain an appropriate level of anaesthesia. Each rat was intubated with an endotracheal tube and artificially ventilated with room air. A syrustic catheter (outer diameter, ∼500 μm) was introduced from the right jugular vein into the left main pulmonary artery. Another catheter was inserted into the right femoral artery. Thereafter, the left-side rib cage was partially excised to expose directly the left lung to the X-ray. The end-expiratory pressure was adjusted to 3.0 cm H2O to prevent lung collapse. Heparin sodium (500 IU kg−1) was administered to prevent blood coagulation.
The system and experimental set-up used in the angiography have been described previously in detail (Sada et al., 1985; Shirai et al., 1986). Briefly, the rat was placed inside an X-ray apparatus box (Hitex) and fixed in such a manner that the exposed left lobe automatically came into contact with a plate just above the beryllium faceplate of an X-ray-sensitive, 1-in. vidicon camera (Hamamatsu Photonics). During temporary cessation of ventilation for ∼3 s at end expiration, a contrast medium (0.2 ml, 60% Urografin) was injected into the main pulmonary artery at a constant speed (0.15 ml s−1), and its passage through the pulmonary vascular bed recorded serially at high speed (30 frames s−1) on a videotape recorder (PVW-2800, Sony). During the experiment, the temperature in the box was maintained at 25–28°C, and the surface of the exposed lung kept wet by warm (37°C) saline. Blood gases and pH were examined by a blood gas analyzer (ABL-2, Radiometer).
ID measurement
The serial angiograms recorded on the videotape recorder were then transferred to a digital image processor (DVS-5000, Hamamatsu). To obtain the arteriogram for measuring ID, two to three serial frames within a diastolic phase, in which vascular trees were extensively filled with contrast medium, were added up and averaged by the digital image processor. The processed image was electrically transferred to an image hard copy unit (model 4634, Sony Tektronix) and copied clearly onto paper. The ID of the pulmonary vessels on the copy was then measured manually using a digitizer (model 9874A, Hewlett-Packard) connected to a minicomputer. The readers of the angiograms were blinded to the treatment protocol.
Analysis of ID response
Following the method we described in a previous study (Shirai et al., 1986), we took a random selection of many vascular sites for the ID measurements. The ID percentage change in response to an NOS inhibitor was calculated at each measured vascular site. These sites were classified into three vascular groups, that is the muscular arteries (100–300 μm ID), smaller transitional elastic arteries (400–500 μm), and larger classical elastic arteries (600–700 μm), according to their vascular branching pattern and baseline ID sizes (Kay, 1983; Sasaki et al., 1995). By pooling all the data within a vascular group, the mean value of the ID percentage change was obtained in each of the three groups. The ID responses were separated into three types (constriction, dilatation, and no change) according to the following definition: an increase or decrease more than 5% in the percentage ID change is defined as dilatation or constriction, respectively, and a change below 5% as no change.
In the present study, the mean pulmonary arterial pressure (PAP) of the CH rat was ∼3 mmHg larger than that of the control rat under baseline conditions, suggesting that the baseline ID at the same serial segments of arteries may differ between these rats. Therefore, we compared the baseline ID of primary branches (400–600 μm) arising from the axial artery or peripheral branches (<300 μm) arising from the primary branch (Sasaki et al., 1995) between these rats. However, there was no significant difference in the mean baseline ID value at any of the serial segments.
Experimental protocols for angiography
In each of the hypoxic and normoxic groups, the baseline angiogram was recorded first, and then an injection of nonselective NOS inhibitor (n=4), L-NAME (50 mg kg−1 i.v., n=2) or L-NMMA (60 mg kg−1 i.v., n=2), an injection of iNOS selective inhibitor (n=4), L-canavanine (100 mg kg−1 i.v., n=2) or S-methylisothiourea sulphate (3 mg kg−1 i.v., n=2), or an injection of nNOS selective inhibitor, 7-nitro indazole (50 mg kg−1 i.p., n=3), was performed. The angiograms following the injection of a nonselective NOS inhibitor or an iNOS selective inhibitor were taken ∼20 min after the injection ended. Similarly, the angiogram was recorded 30–40 min after the injection of an nNOS selective inhibitor. In our preliminary dose–response data, the doses of NOS inhibitors were enough to cause maximal levels of ID reduction. Moreover, the magnitudes and distribution patterns of the ID reduction caused by the two chemically different nonselective NOS inhibitors, as well as the two iNOS inhibitors, were similar. Therefore, we considered that nonspecific effects other than those due to NOS inhibition are negligibly small in the doses used in the present study and that pooling the data from the two different inhibitors is reasonable.
We found significant ID reductions after the injections of a nonselective NOS inhibitor and an iNOS selective inhibitor, but not after nNOS selective inhibitor injection. To examine whether these ID constrictions primarily resulted from inhibiting the release of NO derived from L-arginine, the third angiogram following the addition of L-arginine (100–200 mg kg−1 i.v.) was further recorded in the rats given a nonselective NOS inhibitor or an iNOS selective inhibitor.
In the current study, PAP increased by ∼3 mmHg after the nonselective NOS inhibitor administration in the CH rat. It is thus possible that the pressure-sensing mechanism (see Discussion) influenced the ID change pattern in response to the inhibitor. To examine this possibility, we measured the effects of mechanically induced PAP increase on the ID of pulmonary arteries in CH rats (n=4). PAP was increased ∼4 mmHg above the baseline value by partially interrupting blood flow into the right side of the lungs. The angiogram of the left lung was recorded at the time when the PAP increase was maintained for 50–60 s periods.
There was an interval of ∼15 min between each angiography to eliminate any influence of the contrast medium. All experiments were finished within a few hours of removal from the chamber.
Immunohistochemistry and histology
Within the rats that had been used for recording angiograms with nonselective NOS inhibition and iNOS selective inhibition, immunolocalization of NOS proteins in the pulmonary arteries was performed. Four out of eight hypoxic rats and four out of eight normoxic rats were employed. After putting marks on the left lung regions, where the ID changes had been measured, the lungs were isolated from the rats under anaesthesia. Cannulas were inserted into the pulmonary artery and left atrium, and the lungs were perfused with heparinized phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in 0.1 M PBS (pH 7.3). Simultaneously, the lungs were inflated via the trachea to a pressure of 23 cm H2O with 4% paraformaldehyde and then placed in 10% buffered formaline for paraffin embedding. The left lung tissues that had been marked were cut into 7–9-mm sections. Paraffin sections 2 μm thick were mounted onto precleaned slides (Superfrost Plus; Fisher Scientific, Springfield, NJ, U.S.A.), dewaxed in 100% xylene, and then rehydrated in graded alcohol solutions. Throughout the protocol, slides were washed, as appropriate, in PBS. Antigen retrieval was performed by microwave treatment and proteinase K treatment for eNOS and iNOS immunostaining, respectively. Sections were treated with 0.3% H2O2 (30 min) to inhibit endogenous peroxidases and incubated with normal serum (30 min) to reduce nonspecific binding of secondary antibodies. Sections were then incubated at 4°C overnight with either of two anti-NOS antibodies: (1) a mouse monoclonal antibody raised against a peptide fragment of amino acids 1030–1209 of human eNOS (1:3000 dilution; Transduction Laboratories, Lexington, KY, U.S.A.) or (2) a rabbit polyclonal antibody raised against a synthetic peptide corresponding to amino-acid residues 1131–1144 (with one additional N-terminal cysteine) of mouse macrophage iNOS (1:3000 dilusion; Alexis Corporation, Lausen, Switzerland). The anti-eNOS antibody was characterized for use in Western blot analysis (identifying protein bands at 140 kDa for eNOS), and showed wide species reactivity, including reactivity with human and rat NOS (Transduction Laboratories). The anti-iNOS antibody was also characterized by Western blot (identifying a band at 130 kDa) and showed reactivity with human and rat NOS (Alexis Corporation). After washing off unbounded primary antibodies, sections were incubated with secondary biotinylated antibodies against mouse or rabbit (DAKO Co., Japan) (30 min), followed by incubation in avidin/biotin/horseradish peroxidase complex (DAKO Co., Japan) (30 min). Subsequently, peroxidase activity was visualized by incubation (3 min) with 0.05% 3,3′-diaminobenzidine (DAKO Co., Japan), which gives a brown reaction product. The reaction was stopped by washing with water. The slides were then counterstained with Mayer's haematoxylin, dehydrated, and mounted. For negative control studies, slides were incubated with mouse IgG (for monoclonal antibodies) or rabbit IgG (for polyclonal antibodies) instead of primary antibody. No staining was observed in these negative control sections. Serial sections were stained with elastic Van Gieson's method to distinguish arteries and veins by the presence of an internal elastic lamina (Resta et al., 1997).
According to the classification proposed by Sakai et al., the elastic and muscular arteries were distinguished by the structure of the media. Vessel diameters were calculated from their external circumference. Oblique sections in which the elastic laminae were indistinct at one or more points on the circumference were excluded from analysis. Slides were examined independently by three blinded reviewers and vessels counted for NOS-positive or NOS-negative staining.
Statistical methods
The significance of differences in ID values and haemodynamic data between the conditions of baseline and NOS inhibition (among the values of baseline, NOS inhibitor alone, and NOS inhibitor+L-arginine) was tested by a paired t-test (analysis of variance (ANOVA) and Scheffe's test). The differences in the ID and haemodynamic data between control and hypoxic groups were examined by an unpaired t-test. ID response differences among the different serial vascular segments were assessed by analysis of variance (ANOVA) and Scheffe's test. All data are expressed as mean±s.e.m., and P<0.05 was considered significant.
Results
Baseline values of body and right ventricle (RV) and left ventricle weight, and blood gases in control and CH rats
The mean values of body weight, (right ventricle (RV)/left ventricle (LV)+septum) ratio, and systemic arterial blood PO2(PaO2), PCO2 (PaCO2) and pH are compared between the control and CH rats (Table 1). Blood gases were measured during normoxic ventilation. Final body weight was ∼30 g smaller in the CH rat than in the control rat, while RV/(LV+septum) ratio ∼0.30 larger. There were no significant differences in blood gases between these rats.
Table 1.
Baseline values of body weight, RV/(LV+septum) weight ratio, and blood gases during normoxic ventilation
| Control rats | 4-wk hypoxia rats | |
|---|---|---|
| Parameters | (n=11) | (n=11) |
| Initial body weight (g) | 197±2 | 196±2 |
| Final body weight (g) | 327±7 | 299±5* |
| RV/(LV+septum) ratio | 0.33±0.03 | 0.62±0.05** |
| pH | 7.41±0.02 | 7.37±0.04 |
| PO2 (Torr) | 96±6 | 93±5 |
| PCO2 (Torr) | 33±2 | 35±2 |
Values are mean±s.e.m.
P<0.05
P<0.01 vs control rats.
Haemodynamic responses due to NOS inhibition
Mean PAP and mean systemic arterial pressure (SAP), before and after NOS inhibitor injection, are compared between the control and CH rats (Table 2). These parameters were measured just before injection of the contrast medium under normoxic ventilation. In the CH rat, the nonselective NOS inhibitors significantly increased PAP and SAP by 3.2 and 34 mmHg, respectively. In the normoxic rat, these inhibitors caused a significant increase (30 mmHg) only in SAP. In contrast, the iNOS and nNOS selective inhibitors significantly changed neither PAP nor SAP in both control and CH rats.
Table 2.
Mean pulmonary arterial pressure (PAP) and systemic arterial pressure (SAP) before and after NOS inhibitor injection
| Blood pressure (mmHg) | Control rats | 4-week hypoxia rats |
|---|---|---|
| (1) Nonselective NOS inhibition (NNI) | ||
| PAP (baseline) | 18.0±0.5 | 21.1±1.0* |
| PAP with NNI | 18.6±0.6 | 24.3±1.1*,** |
| PAP with NNI+L-arginine | 17.9±0.6 | 23.3±1.2* |
| SAP (baseline) | 93±6 | 101±7 |
| SAP with NNI | 123±7** | 135±9** |
| SAP with NNI+L-arginine | 98±7 | 108±9 |
| (2) iNOS selective inhibition (ISI) | ||
| PAP (baseline) | 17.2±0.6 | 22.1±1.1* |
| PAP with ISI | 17.4±0.7 | 22.9±1.1* |
| PAP with ISI+L-arginine | 17.1±0.7 | 22.2±1.1* |
| SAP (baseline) | 96±6 | 105±7 |
| SAP with ISI | 98±6 | 113±8 |
| SAP with ISI+L-arginine | 94±7 | 107±8 |
| (3) nNOS selective inhibition (NSI) | ||
| PAP (baseline) | 18.0±0.5 | 22.4±1.0* |
| PAP with NSI | 18.2±0.5 | 22.6±1.0* |
| SAP (baseline) | 94±6 | 102±7 |
| SAP with NSI | 96±6 | 105±7 |
Values are mean+s.e.m. The mean value in classes 1 and 2 was obtained by pooling all the data in response to two different nonselective NOS inhibitors and two iNOS-selective inhibitors, respectively.
P<0.05 vs control rats.
P<0.05 vs baseline.
Regional changes in NOS-inhibitor induced responses in parallel- and series-arranged pulmonary arteries following 4-week hypoxia
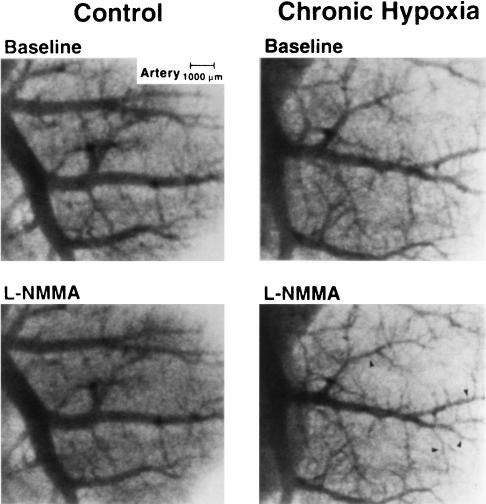
Figure 1 shows typical ID changes of small pulmonary arteries in response to L-NMMA injection in the control and CH rats. On injection, slight ID decreases were induced in the control rat. In the CH rat, in contrast, clear ID decreases occurred in many branches, particularly smaller branches.
Figure 1.
Typical angiograms of small pulmonary arteries obtained before (top panels) and after (bottom panels) nonselective NOS inhibitor (L-NMMA) injection in control (left panels) and chronically hypoxic (right panels) rats. Clear vasoconstrictions are chiefly observed in more distal side vessels ≲300 μm (arrowheads).
The mean value of the NOS inhibitor-induced ID change at each level of the muscular, transitional elastic, and classical elastic arteries is shown in the control and CH rats (Figure 2). During nonselective NOS inhibition (left), the ID of all these arteries significantly constricted in both control and CH rats. The ID constriction of the muscular arteries was significantly (P<0.05) smaller than those of the transitional and classical elastic arteries in the control rat. Comparing the ID constriction due to nonselective NOS inhibition between the control and CH rats, there was a large difference in the muscular arteries and a small but significant difference in the transitional elastic arteries, whereas no significant difference was present in the classical elastic arteries. On the other hand, during iNOS selective inhibition (right), no significant ID response was induced in any of the arteries in the control rat. However, in the CH rat, a significant ID constriction occurred locally in the muscular arteries. Therefore, a significant difference between the control and CH rats was restricted in these arteries. During nNOS selective inhibition, no significant ID response was observed at any of the muscular and elastic arteries in the control and CH rats. The mean values of ID changes for the muscular, transitional elastic, and classical elastic arteries of the control rat were 0±2, 2±2, and 1±2%, respectively, and those of the CH rat were 3±2, 1±2, and 3±2%, respectively.
Figure 2.
Mean values of NOS inhibitor-induced ID changes for muscular, transitional elastic, and classical elastic pulmonary arteries are shown in control (open column) and chronically hypoxic (solid column) rats. CH greatly enhanced ID reductions due to nonselective NOS inhibition (left) and iNOS-selective inhibition (right) primarily in muscular pulmonary arteries. *P<0.05, **P<0.01 vs control.
After making an L-arginine injection, the ID decreases due to nonselective NOS inhibition and iNOS selective inhibition were completely abolished in both control and CH rats. The mean values of ID percentage changes in response to nonselective NOS inhibitor+L-arginine in the muscular, transitional elastic, and classical elastic arteries were −2±4, 2±4, and 4±3%, respectively, in the control rat, and were −3±4, −1±4, and 2±4% for the CH rat, respectively. The mean values of ID percentage changes in response to iNOS selective inhibitor+L-arginine in the three levels of vessels were 3±4, 4±4, and 1±4%, respectively, in the control rat, and were 1±4, 3±4, and −2±4% for the CH rat, respectively.
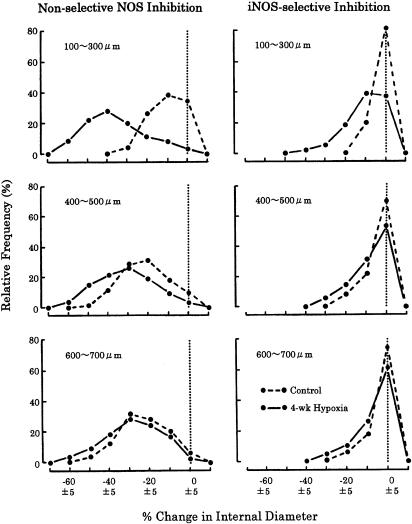
Relative frequency distribution of ID response due to NOS inhibition is compared between the control and CH rats (Figure 3). During nonselective NOS inhibition (left panels), the frequency curves of the control and CH rats were very different in the muscular arteries (100–300 μm), but not in the transitional (400–500 μm) and classical (600–700 μm) elastic arteries. In the muscular arteries, the peaks of the control and CH curves were observed at 5–15 and 35–45% constriction, respectively. Moreover, ∼40% of ID responses were no change in the control vessels, but almost all ID responses were significant constriction in the CH vessels. In the transitional and classical elastic arteries, almost all ID responses were significant constriction in both control and CH vessels. The peaks of the control and CH distributions in the transitional arteries were at 15–25 and 25–35% constriction, respectively, but those for classical arteries were about the same level (25–35% constriction).
Figure 3.
Relative frequency distributions of ID changes due to nonselective NOS inhibition (left) and iNOS-selective inhibition (right) are shown in control (broken line) and chronically hypoxic (solid line) rats. Dotted line indicates no ID change as defined in Methods section. Clear difference in distribution pattern between control and hypoxic rats is seen primarily in muscular pulmonary arteries (100–300 μm) in both types of NOS inhibition.
During iNOS selective inhibition (right panels), a clear difference between the control and CH response distributions existed only in the muscular arteries. In these arteries, the peaks of the control and CH curves were observed at no change and 5–15% constriction, respectively. Significant constriction was only ∼20% of the ID responses in the control distribution, but ∼60% in the CH. However, it is noteworthy that, in contrast to the case of nonselective NOS inhibition, ∼40% of muscular artery responses were still no change in the CH rat. In the elastic arteries, just as in the muscular arteries, ∼80% of the ID responses in the control distribution curve were no change, and the curves of the control and CH rats displayed similar patterns.
During nNOS selective inhibition, more than 95% of the ID responses were no change and the remaining responses, if any, were small constrictions in both the control and CH rats.
Effects of PAP increases due to nonselective NOS inhibition on ID
The mechanically induced ∼4 mmHg PAP increase had no significant effect on the ID of the muscular and elastic arteries in the CH rat. The mean value of percentage ID change due to the PAP increase for all these arteries was 3±3%.
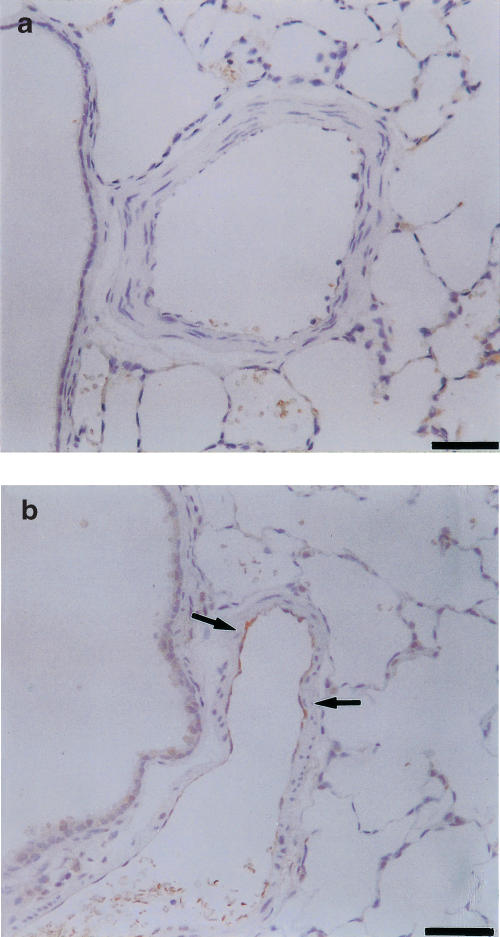
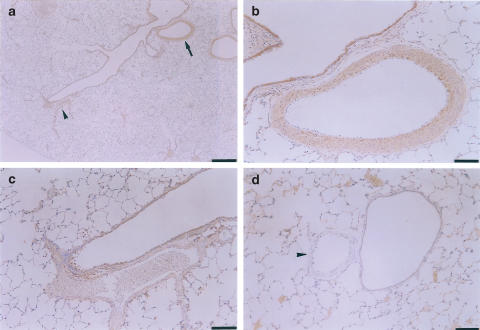
NOS immunoreactivity changes
Figure 4 shows representative photographs of eNOS immunostaining in the small muscular pulmonary arteries from the control (a) and CH (b) rats. eNOS immunoreactivity was detected in the endothelium of hypoxic arteries (Figure 4b) but not in the control arteries (Figure 4a). Figure 5 shows typical examples of iNOS immunostaining of CH and control rat lungs. The low-magnification view of the hypoxic lung (Figure 5a) showed that an iNOS immunopositive muscular pulmonary artery (arrow) and an iNOS-immunonegative muscular artery (arrowhead) are simultaneously observed in the same section. In the higher-magnification view, the former artery displayed strong iNOS immunostaining primarily in the smooth muscle layers (Figure 5b). However, the latter showed no immunostaining even with higher magnification (Figure 5c). iNOS immunoreactivity was not detected in the control arteries (arrowhead in Figure 5d).
Figure 4.
eNOS immunostaining of small muscular pulmonary arteries of control (a) and chronically hypoxic (b) rats. eNOS protein was detected in endothelium of hypoxic arteries (arrows), but not in control arteries. Scale bars=50 μm.
Figure 5.
iNOS immunostaining of muscular pulmonary arteries of chronically hypoxic (a–c) and control (d) rats. (a) Low magnification view. iNOS-positive (arrow) and iNOS-negative (arrowhead) muscular pulmonary arteries are seen in the same section. (b, c) Higher magnification views of iNOS-positive and iNOS-negative arteries, respectively. (d) iNOS-negative muscular artery (arrowhead). Scale bars: (a) 500 μm, (b, c) 50 μm, (d) 100 μm.
The quantitative data on the distribution of NOS immunoreactivity in the pulmonary arteries of control vs CH lungs are shown in Table 3. In control lungs, eNOS immunoreactivity was distributed only in 9–51% of the muscular arteries, although 91% of the elastic arteries were positive for eNOS. However, the percentage of eNOS-positive vessels greatly increased up to 89–94% in the muscular arteries of CH lungs, while the increase was slight (7%) in the elastic arteries. On the other hand, the percentage of iNOS-positive vessels was small (<10%) in both muscular and elastic arteries of control lungs and increased only to 44–55 and to 68% in these arteries of CH lungs.
Table 3.
Pulmonary arteries positive and negative for NOS
| Elastic (400–700 μm) | Muscular (100–300 μm) | Muscular (<100 μm) | ||||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | |
| eNOS | ||||||
| C | 39 (90.7) | 4 (9.3) | 56 (50.9) | 54 (49.1) | 23 (8.9) | 236 (91.1) |
| CH | 43 (97.7) | 1 (2.3) | 97 (94.2) | 6 (5.8) | 210 (88.6) | 27 (11.4) |
| iNOS | ||||||
| C | 4 (9.8) | 37 (90.2) | 7 (6.0) | 109 (94.0) | 4 (1.6) | 252 (98.4) |
| CH | 30 (68.2) | 14 (31.8) | 56 (55.4) | 45 (44.6) | 104 (43.7) | 134 (56.3) |
Values are presented as number (%). Number of NOS-positive and NOS-negative vessels was counted in control (C; n=4) and 4-week hypoxic (CH; n=4) rats.
Discussion
We quantitatively measured the ID changes of the muscular (resistance) and elastic (conduit) pulmonary arteries in response to different types of NOS inhibitors using an X-ray television system on the control and CH rats under anaesthesia. Moreover, we conducted immunohistochemical analyses to localize NOS proteins in the pulmonary arteries within the control and CH lungs where the ID changes were examined. This was performed to investigate the possible contributions of the hypoxia-induced increase in NOS expression to regulating tone at individual pulmonary arterial branches with natural blood circulation.
RV hypertrophy
The presence of pulmonary hypertension in rats breathing hypoxic air was reflected in an increased ratio of RV/LV+S, which averaged 0.62±0.05 vs 0.33±0.02 in normoxic controls. These ratios in the CH and control rats agree with those of others (Abraham et al., 1971; Rabinovitch et al., 1979; Muramatsu et al., 1997).
ID constriction mechanisms in pulmonary arteries in response to NOS inhibitor injection
We showed that L-arginine injection completely abolishes the ID reductions in response to nonselective NOS inhibitors and iNOS selective NOS inhibitors in the pulmonary arteries. This suggests that inhibiting the basal release of NO derived from L-arginine is primarily responsible for the ID constrictions.
In the present study, L-NAME (50 mg kg−1 mg kg−1 i.v.) or L-NMMA (60 mg kg−1 i.v.) was used for nonselective NOS inhibition and L-canavanine (100 mg kg−1 i.v.) or S-methylisothiourea sulphate (3 mg kg−1 i.v.) for selective iNOS inhibition. We determined the doses of NOS inhibitors based on the previous data (Loeb & Longnecker, 1992; McCormack & Paterson, 1993; Oka et al., 1993; Huang et al., 1994; Szabo et al., 1994; Teale & Atkinson, 1994; Liaudet et al., 1996) and our preliminary dose–response data (see Methods). Previous studies (Teale & Atkinson, 1994; Liaudet et al., 1996) have shown that L-canavanine (100 mg kg−1 i.v.) almost completely suppresses the lipopolysaccharide-induced hypotension in anaesthetized rats, although it does not significantly affect blood pressure in the absence of lipopolysaccharide. S-methylisothiourea sulphate (3 mg kg−1 i.v.) caused a potent pressor response in lipopolysaccharide-treated rats as compared with control rats and a restoration of blood pressure to prelipopolysaccharide levels (Szabo et al., 1994). The present study has shown a significant ID reduction in the CH rat, but no significant response in the control rat in response to L-canavanine and S-methylisothiourea sulphate. From these previous and present data, it seems reasonable to assume that the doses of iNOS inhibitors we employed were adequate to inhibit iNOS, but with minor effects on the other types of NOS in vivo.
For nNOS selective inhibition, we used 7-nitro indazole (50 mg kg−1 i.p.). These doses of the inhibitor were able to cause 60–80% reduction of nNOS activity in the rat (Kalisch et al., 1996; Okamoto et al., 1997), suggesting that the doses of nNOS inhibitor were also adequate to inhibit nNOS in the current study. Therefore, our finding that 7-nitro indazole had no significant effect on any pulmonary arteries (100–700 μm) of the control and CH rats is likely to mean that the role of nNOS in controlling the vascular tone of these vessels is minimal, if any at all. This is consistent with the immunohistochemical data that nNOS immunoreactivity was not detected in the small pulmonary vessels of normoxic and CH rats (Xue & Johns, 1996) and with the physiological data of no significant contribution of nNOS to modulating pulmonary vascular tone in the mouse (Fagan et al., 1999b) and cat (Shirai et al., 1999). Our result also suggests that sympathetic nerve activity increase caused by nNOS inhibition in the brain stem (Umans, 1995) contributed little to the nonselective NOS inhibitor-induced ID reduction.
The contribution of pressure- and flow-sensing mechanisms (Bevan & Laher, 1991) to the NOS inhibitor-induced ID response has been discussed. There was a PAP elevation of ∼3 mmHg in response to nonselective NOS inhibitors in the CH rat (Table 2), suggesting the possibility that the pressure-sensing mechanism influenced the ID change pattern caused by the local action of nonselective NOS inhibitor on the small pulmonary arteries. To examine this possibility, we measured ID changes of the pulmonary vessels in response to mechanically induced increases in PAP. No significant ID change was found in response to ∼4 mmHg PAP rise, suggesting that this possibility is small.
Pulmonary blood flow was not measured in this study, but it was reported that cardiac output decreases by 15–40% in response to nonselective NOS inhibitor in normoxic (Loeb & Longnecker, 1992; McCormack & Paterson, 1993; Oka et al., 1993; Huang et al., 1994) and chronically hypoxic rats (Oka et al., 1993) and did not significantly change with iNOS selective inhibitors in these rats (Liaudet et al., 1996; Resta et al., 1999). Therefore, another possibility is that the flow-sensing mechanism affected the ID change pattern in response to nonselective NOS inhibitor administration. However, in preliminary experiments, we found no significant ID change in response to ∼40% decrease in pulmonary blood flow caused by partially occluding the circumference of inferior vena cava with a 4-0 silk ligature. This suggested that this possibility is also small.
However, these results cannot completely exclude these possibilities, since we did not determine the local pressure- and flow-mediated vasomotor responses at different serial segments of the pulmonary vessels. To determine these responses, it would be necessary to measure directly the local pressure and flow velocity at the same site where vascular dimensions are being assessed.
Contribution of endogenous NO to controlling basal pulmonary vascular tone in normoxic rats
Previous pressure-flow studies have shown that nonselective NOS inhibitors increase basal pulmonary vascular resistance in intact and isolated lungs of the lamb, pig, rabbit, cat, and man (Persson et al., 1990; Fineman et al., 1991; McMahon et al., 1991; Gordon & Tod, 1993; Nelin & Dawson, 1993; Cremona et al., 1994; Stamler et al., 1994; Albertini et al., 1996), although no effect in the dog (Nishiwaki et al., 1992; Cremona et al., 1994; Leeman et al., 1994). This suggests that endogenous NO plays a contributory role in regulating basal pulmonary vascular tone for all these animals except the dog (Barnes & Liu, 1995). On the other hand, in the rat, this problem has mainly been studied using isolated perfused lungs, but with inconsistent results. Many investigators have shown no or little effect of nonselective NOS inhibitors on the basal pulmonary vascular resistance (Robertson et al., 1990; Hasunuma et al., 1991; Barer et al., 1993; Hampl et al., 1993; McCormack & Paterson, 1993; Russ & Walker, 1993; Isaacson et al., 1994; Resta et al., 1999), although others have shown a significant effect (Barnard et al., 1993; Roos et al., 1996; Cadogan et al., 1999). In the present study, we considered the change in ID to be better explained as an index of local vasomotor response in a given vessel than the change in calculated resistance estimated from the pressure–flow relation. Therefore, we directly measured ID changes of the rat pulmonary arteries using an X-ray TV system (Sada et al., 1985; Shirai et al., 1986). Our data showed that nonselective NOS inhibitor injection causes a significant ID reduction in both muscular (100–300 μm) and elastic (400–700 μm) segment levels in normoxic control rats (Figure 2). Relative frequency distribution of ID response showed that the peak of the frequency curve of the elastic arteries is located on the more constrictor side than that of the muscular arteries, and that the constrictor response occurs in most of the elastic arteries but only in about half of the muscular arteries (Figure 3). This suggests greater effects of NO on basal tone of the elastic pulmonary arteries and lesser effects for the muscular arteries in the normoxic rat. Moreover, there was the coexistence of relatively small magnitudes of constrictions and no change with similar frequencies among parallel-arranged muscular arteries (Figure 3). This ID response pattern may partly explain the previous data of no or little change in the pressure–flow relation of the entire lung (Robertson et al., 1990; Hasunuma et al., 1991; Barer et al., 1993; Hampl et al., 1993; McCormack & Paterson, 1993; Russ & Walker, 1993; Isaacson et al., 1994; Resta et al., 1999) and the present data of no significant PAP rise in response to nonselective NOS inhibition, if we assume that local redistributions of blood flow from constricted to nonconstricted muscular arteries occurred and then recruited new peripheral channels such as capillaries, maintaining entire pulmonary vascular resistance within nearly baseline levels. In addition, because the previous pressure–flow studies were mostly performed in constant perfused lungs, a decrease in NO production due to the artificial perfusate composition (Sprague et al., 1995) and/or mechanical flow pattern (Hakim, 1994) may have occurred in these studies, making it more difficult to detect the small magnitude of vasoconstriction due to NOS inhibition in the muscular arteries.
The present study also demonstrated that selective inhibitors of iNOS had no significant effects on any levels of the arteries observed in control rats (Figure 2). Relative frequency distribution of ID response showed that iNOS selective inhibition causes no ID changes in ∼80% of the muscular and elastic arteries, and small magnitudes of ID constriction in the remaining arteries (Figure 3). The data suggest a minor contribution of iNOS-derived NO to basal tone regulation in both muscular and elastic arteries, and probably explain the findings that selective iNOS inhibition had no effect on the baseline pulmonary vascular resistance in the rat (Resta et al., 1999). Moreover, considering in connection with the above-mentioned suggestion of minor role of nNOS in regulating vascular tone of the muscular and elastic arteries, the present study suggests that, during normoxia, the predominant isoform of NOS regulating basal tone of these arteries is eNOS. This is consistent with the findings in the mouse that baseline pulmonary arterial pressure in eNOS null lungs significantly increased compared with wild type (Steudel et al., 1997; Fagan et al., 1999b), but the pressure in iNOS null lungs and nNOS null lungs did not (Fagan et al., 1999b). We have also suggested the primary role of eNOS in regulating basal ID of the 100–1700 μm cat pulmonary arteries including muscular and elastic segments (Shirai et al., 1999).
The present immunohistochemical analyses in the control rat have shown that eNOS-positive vessels were found in 91% of the elastic arteries >400 μm, but only in 9–51% of muscular arteries <300 μm (Table 3). This is consistent with the previous rat data that eNOS immunoreactivity is distributed among 96% of the pulmonary vessels (>150 μm ID), but only in 2–20% of the vessels smaller than this (Xue & Johns, 1996). The iNOS distribution data (Table 3) also agree with the previous data of very limited iNOS expression in vascular smooth muscle of all normoxic rat pulmonary arteries (Xue et al., 1994; Xue & Johns, 1996; Cadogan et al., 1999). We have further shown that in normoxic lungs, such eNOS and iNOS immunoreactivity distributions are almost in accord with the frequency distributions of ID constriction due to nonselective and iNOS selective inhibition, respectively (Figure 3).
Regional differences in vasodilator effect of endogenous NO in chronically hypoxic pulmonary arteries
It is generally agreed that main and extralobar pulmonary arteries isolated from CH rats have blunted endothelium-dependent relaxation to various receptor-dependent vasodilators (Crawley et al., 1992; Rodman, 1992; Carville et al., 1993; Shaul et al., 1993; Maruyama & Maruyama, 1994) and to receptor-independent Ca2+ ionophore A-23187 (Carville et al., 1993; Shaul et al., 1993). However, different results have been obtained concerning whether CH alters the NO-mediated basal tone regulation in these proximal elastic pulmonary arteries. Some researchers have suggested that the effect of nonselective NOS inhibitor on basal tone is not significantly changed by CH (Rodman, 1992; Maruyama & Maruyama, 1994), whereas others have suggested its enhancement (Oka et al., 1993). Moreover, basal NO production assessed by measuring cyclic guanosine-3′,5′-monophosphate (GMP) synthesis has been shown to decrease in main pulmonary arteries during chronic hypoxia (Shaul et al., 1993). The present study showed that, in the transitional elastic arteries (400–500 μm), the nonselective NOS inhibitor-induced ID reduction is slightly but significantly larger in the CH rat than in the control rat, although no significant difference is present in the classical elastic arteries (600–700 μm) (Figure 2). In addition, the frequency distribution of ID response showed that the CH distribution curve is located slightly on the more constrictor side than the control curve in the transitional arteries, whereas no clear difference exists between these curves in the classical arteries (Figure 3). These results suggest that within the intralobar elastic arteries, small but significant degree of an enhancement of the NO-mediated basal vascular tone regulation is caused locally in the most distal vascular segment.
In the muscular pulmonary arteries (100–300 μm ID), there has been no direct observation on the change in NO-mediated control of basal vascular tone in response to CH. The present study has for the first time supplied information on the muscular arteries that the ID reduction due to nonselective NOS inhibition is significantly larger in the CH rat than in the control rat (Figure 2). Frequency distribution of ID response due to nonselective NOS inhibition showed that the CH distribution curve is located extremely on the more constrictor side than the control curve. Moreover, significant constriction was almost all ID responses in the CH vessels, but about half in the control vessels (Figure 3). These ID response patterns suggest that an increase in NO-mediated suppression of basal vascular tone occurs in most branches of the muscular arteries during CH, and probably explain the previous findings (Barer et al., 1993; Oka et al., 1993; Isaacson et al., 1994; Roos et al., 1996) that nonselective NOS inhibitor increased calculated resistance more greatly in perfused lungs obtained from CH rats than in those from normoxic rats.
It has recently been shown that iNOS inhibition with L-N6-(1-iminoethyl)lysine dihydrochloride does not alter the dose–response change in calculated pulmonary arterial and venous resistance due to U-46619 in isolated, saline-perfused lungs from CH rats (Resta et al., 1999). Moreover, this inhibition had no significant effect on PAP, SAP, and cardiac output in conscious hypoxic rats. From these data, they concluded that NO derived from iNOS does not modulate pulmonary vasoconstrictor responsiveness during CH (Resta et al., 1999). The present study similarly showed that during iNOS inhibition (with L-canavanine or S-methylisothiourea), PAP and SAP did not significantly change in the CH rat (Table 2). However, we simultaneously found that there were significant ID reductions mainly in the muscular pulmonary arteries (Figure 2). Moreover, the frequency distribution of the ID response during iNOS inhibition showed that the CH distribution curve is located on the more constrictor side than the control curve in the muscular arteries, but, about half of the CH muscular arteries still exhibited no change, in contrast to the case of nonselective NOS inhibition (Figure 3). Therefore, for the same possible reason (local redistributions of flow from constricted to nonconstricted arteries) as mentioned above in the heterogenous vasoconstrictor effect of nonselective NOS inhibition on normoxic rats, we suppose that such a coexistence of ID constriction and no change in the parallel-arranged arteries during iNOS inhibition is also difficult to detect from changes in the pulmonary pressure–flow relation.
Previous studies have shown that in 2–4-week hypoxic rats, NOS immunoreactivity became markedly positive in the endothelial cells of most 80–150 μm pulmonary arteries compared with normoxic rats (Xue et al., 1994) and that the percentages (∼95%) of eNOS-positive pulmonary vessels >150 μm ID are similar in 0–7-day hypoxic rats (Xue & Johns, 1996). Our findings that the percentages of eNOS-positive muscular arteries greatly rose up to 89–94% in the 4-week hypoxic lung, but those for elastic arteries displayed a slight increase (Table 3), support the previous data. However, we also provided new information that the eNOS distribution change corresponds well to the hypoxic change in the distribution of nonselective NOS inhibitor-induced ID reduction (Figure 3). On the other hand, iNOS protein has been shown to increase in the vascular smooth muscle of both muscular and elastic arteries obtained from CH rats (Xue et al., 1994; Xue & Johns, 1996). For the first time, we found that 4-week hypoxia increases iNOS immunoreactivity scatteringly among the muscular and elastic arteries (Figure 5 and Table 3). The iNOS distribution in the muscular arteries was in accord with the distribution of iNOS inhibitor-induced ID reduction in these arteries (Figure 3). However, the increase in iNOS expression in the elastic arteries seems to be inconsistent with the data showing no significant effect of iNOS selective inhibition and no enhancement of nonselective NOS inhibitor effect in these arteries (Figure 2 and Figure 3). The reason for this discrepancy remains to be elucidated. However, it has been shown that CH increases cyclic GMP phosphodiesterase activity in the larger intrapulmonary elastic arteries in the rat, but does not change it in the muscular arteries (MacLean et al., 1997). It is therefore possible that in the elastic arteries, increased production of NO from iNOS cannot rise cyclic GMP level due to an increase in activity of cyclic GMP phosphodiesterase enzymes which catalyse cyclic GMP hydrolysis, while it can increase cyclic GMP level in the muscular arteries.
Together with the present finding of no significant ID response to nNOS inhibition, we conclude that in CH rats, both vasodilator effects of eNOS- and iNOS-derived NO are enhanced primarily in the muscular pulmonary arteries (100–300 μm); the enhancement of eNOS effects is induced extensively in these arteries, while that for iNOS sporadically in them. This is consistent with the previous data (Steudel et al., 1998; Fagan et al., 1999a,1999b) of marked pulmonary hypertension in eNOS-deficient mice raised in chronic hypoxia when compared with wild type, and suggests that NO production by eNOS is vital to counterbalance hypoxic pulmonary vasoconstriction, which is localized in muscular arteries (Shirai et al., 1986,1997; Barnes & Liu, 1995; Weir & Archer, 1995) and serves to inhibit the progress of pulmonary hypertension.
Possible factors responsible for nonuniform iNOS increase in hypoxic pulmonary arteries
iNOS was expressed in about half of the muscular pulmonary arteries of the chronic hypoxic rat, while eNOS in almost all these vessels (Table 3). It has been suggested that in the rat, the increase in eNOS expression after chronic hypoxia is due to the direct effect of hypoxia or hypoxia-induced factors independently of changes in haemodynamics (Everett et al., 1998; Le Cras et al., 1998). Therefore, it is possible that during chronic hypoxia, almost all muscular pulmonary arteries were exposed to low oxygen tension and, in turn, the upregulation of eNOS was induced in these arteries. On the other hand, the increase in iNOS expression may be caused by flow/wall shear stress (Rairigh et al., 1999; Gosgnach et al., 2000). We have previously shown that during acute inhalation of 5% O2, ∼60% of branches constrict in 100–200 μm feline muscular pulmonary arteries, but others display no significant response (Shirai et al., 1986), meaning that hypoxia does not result in uniformly distributed pulmonary vasoconstriction. Acute hypoxia has also been shown to increase the heterogeneity of pulmonary vascular transit times throughout dog lung lobe (Dawson et al., 1983). Moreover, chronic hypoxia at high altitude has been suggested to induce inhomogenous pulmonary vasoconstriction and regional overperfusion (Richalet, 1995; Hultgren, 1996; Bartsch, 1997). Such heterogenous distribution of vasoconstriction and blood flow may regionally increase shear stress and damage vascular endothelium (Richalet, 1995; West et al., 1995; Bartsch, 1997; Botney, 1999). Indeed, endothelial injury with swelling in small peripheral arteries was observed in chronically hypoxic rats (Meyrick & Reid, 1978). Thus, we suggest a possibility that the heterogenous shear force distribution induced the vascular smooth muscle iNOS expression sporadically among the muscular pulmonary arteries (Figure 5 and Table 3) through the release of inflammatory mediators such as cytokines and eicosanoids and adhesion molecules (Richalet, 1995; Bartsch, 1997; Schoene et al., 1988; Hansson et al., 1994; Kubo et al., 1996; Rabinovitch, 1997) and/or oxidative stress-induced NF- κ B activation after endothelial damage (Gosgnach et al., 2000).
In conclusion, we suggest that, in 4-week hypoxia rats, functional and histological upregulation of eNOS and iNOS occurs chiefly in the muscular segments of pulmonary arteries. The eNOS upregulation is induced extensively in the muscular arteries, but the iNOS upregulation sporadically among these arteries. nNOS has no significant vasodilator effect. Such eNOS and iNOS upregulation may contribute to attenuating hypoxic pulmonary vasoconstriction which is localized in the muscular segments and, in turn, inhibit the progress of pulmonary hypertension.
Acknowledgments
This study was supported, in part, by a grant for the ‘Ground Research for Space Utilization' promoted by NASDA and the Japan Space Forum; by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology; by the Promotion of Fundamental studies in Health Science of the Organization for Pharmacological Safety and Research (OPSR); and by Health Sciences Grants (Funds for Human Genome Research and Regeneration Medicine Research, Japan.
Abbreviations
- CH
chronic hypoxia
- GMP
guanosine-3′,5′-monophosphate
- ID
internal diameter
- LV
left ventricle
- L-NAME
Nω-nitro-L-arginine methyl ester
- L-NMMA
Nω-monomethyl-L-arginine
- NO
nitric oxide
- eNOS
endothelial NO synthase
- iNOS
inducible NO synthase
- nNOS
neuronal NO synthase
- PAP
pulmonary arterial pressure
- RV
right ventricle
- SAP
systemic arterial pressure
References
- ABRAHAM A.S., KAY J.M., COLE R.B., PINCOCK A.C. Haemodynamic and pathological study of the effect of chronic hypoxia and subsequent recovery of the heart and pulmonary vasculature of the rat. Cardiovasc. Res. 1971;5:95–102. doi: 10.1093/cvr/5.1.95. [DOI] [PubMed] [Google Scholar]
- ADNOT S., RAFFESTIN B., EDDAHIBI S., BRAQUET P., CHABRIER P.-E. Loss of endothelium-dependent relaxant activity in the pulmonary circulation of rats exposed to chronic hypoxia. J. Clin. Invest. 1991;87:155–162. doi: 10.1172/JCI114965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALBERTINI M., VANELLI G., CLEMENT G. PGI2 and nitric oxide involvement in the regulation of systemic and pulmonary basal vascular tone in the pig. Prostaglandins Leukotr. Essent. Fatty Acids. 1996;54:273–278. doi: 10.1016/s0952-3278(96)90058-7. [DOI] [PubMed] [Google Scholar]
- BARER G., EMERY C., STEWART A., BEE D., HOWARD P. Endothelial control of the pulmonary circulation in normal and chronically hypoxic rats. J. Physiol. 1993;463:1–16. doi: 10.1113/jphysiol.1993.sp019581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNARD J.W., WILSON P.S., MOORE T.M., THOMPSON W.J., TAYLOR A.E. Effect of nitric oxide and cyclooxygenase products on vascular resistance in dog and rat lungs. J. Appl. Physiol. 1993;74:2940–2948. doi: 10.1152/jappl.1993.74.6.2940. [DOI] [PubMed] [Google Scholar]
- BARNES P.J., LIU S.F. Regulation of pulmonary vascular tone. Pharmacol. Rev. 1995;47:87–131. [PubMed] [Google Scholar]
- BARTSCH P. High altitude pulmonary edema. Respiration. 1997;64:435–443. doi: 10.1159/000196720. [DOI] [PubMed] [Google Scholar]
- BEVAN J.A., LAHER I. Pressure and flow-dependent vascular tone. FASEB J. 1991;5:2267–2273. doi: 10.1096/fasebj.5.9.1860618. [DOI] [PubMed] [Google Scholar]
- BOTNEY M.D. Role of hemodynamics in pulmonary vascular remodeling. Am. J. Respir. Crit. Care Med. 1999;159:361–364. doi: 10.1164/ajrccm.159.2.9805075. [DOI] [PubMed] [Google Scholar]
- CADOGAN E., HOPKINS N., GILES S., BANNIGAN J.G., MOYNIHAN J., MCLOUGHLIN P. Enhanced expression of inducible nitric oxide synthase without vasodilator effect in chronically infected lungs. Am. J. Physiol. 1999;277:L616–L627. doi: 10.1152/ajplung.1999.277.3.L616. [DOI] [PubMed] [Google Scholar]
- CARVILLE C., RAFFESTIN B., EDDAHIBI S., BLOUQUIT Y., ADNOT S. Loss of endothelium-dependent relaxation in proximal pulmonary arteries from rats exposed to chronic hypoxia: effects of in vivo and in vitro supplementation with L-arginine. J. Cardiovasc. Pharmacol. 1993;22:889–896. doi: 10.1097/00005344-199312000-00018. [DOI] [PubMed] [Google Scholar]
- CRAWLEY D.E., ZHAO L., GIEMBYCZ M.A., LIU S. Chronic hypoxia impairs soluble guanylyl cyclase-mediated pulmonary arterial relaxation in the rat. Am. J. Physiol. 1992;263:L325–L332. doi: 10.1152/ajplung.1992.263.3.L325. [DOI] [PubMed] [Google Scholar]
- CREMONA G., WOOD A.M., HALL L.W., BOWER E.A., HIGENBOTTAM T. Effect of inhibitors of nitric oxide release and action on vascular tone in isolated lungs of pig, sheep, dog and man. J. Physiol. 1994;481:185–195. doi: 10.1113/jphysiol.1994.sp020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON C.A., BRONIKOWSKI T.A., LINEHAN J.H., HAKIM T.S. Influence of pulmonary vasoconstriction on ling water and perfusion heterogeneity. J. Appl. Physiol. 1983;54:654–660. doi: 10.1152/jappl.1983.54.3.654. [DOI] [PubMed] [Google Scholar]
- EDDAHIBI S., ADNOT S., CARVILLE C., BLOUQUIT Y., RAFFESTIN B. L-Arginine restores endothelium-dependent relaxation in pulmonary circulation of chronically hypoxic rats. Am. J. Physiol. 1992;263:L194–L200. doi: 10.1152/ajplung.1992.263.2.L194. [DOI] [PubMed] [Google Scholar]
- EVERETT A.D., LE CRAS T.D., XUE C., JOHNS R.A. eNOS expression is not altered in pulmonary vascular remodeling due to increased pulmonary blood flow. Am. J. Physiol. 1998;274:L1058–L1065. doi: 10.1152/ajplung.1998.274.6.L1058. [DOI] [PubMed] [Google Scholar]
- FAGAN K.A., FOUTY B.W., TYLER R.C., MORRIS K.G., JR, HEPLER L.K., SATO K., LE CRAS T.D., ABMAN S.H., WEINBERGER H.D., HUANG P.L., MCMURTRY I.F., RODMAN D.M. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J. Clin. Invest. 1999a;103:291–299. doi: 10.1172/JCI3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAGAN K.A., TYLER R.C., SATO K, FOUTY B.W., Morris K.G., JR, HUANG P.L., MCMURTRY I.F., RODMAN D.M. Relative contributions of endothelial, inducible, and neuronal NOS to tone in the murine pulmonary circulation. Am. J. Physiol. 1999b;277:L472–L478. doi: 10.1152/ajplung.1999.277.3.L472. [DOI] [PubMed] [Google Scholar]
- FINEMAN J.R., HEYMANN M.A., SOIFER S.J. Nω-nitro-L-arginine attenuates endothelium-dependent pulmonary vasodilation in lambs. Am. J. Physiol. 1991;260:H1299–H1306. doi: 10.1152/ajpheart.1991.260.4.H1299. [DOI] [PubMed] [Google Scholar]
- GORDON J.B., TOD M.L. Effects of Nω-nitro-L-arginine on total and segmental vascular resistances in developing lamb lungs. J. Appl. Physiol. 1993;75:76–85. doi: 10.1152/jappl.1993.75.1.76. [DOI] [PubMed] [Google Scholar]
- GOSGNACH W., MESSIKA-ZEITOUN D., GONZALEZ W., PHILIPE M., MICHEL J.-B. Shear stress induces iNOS expression in cultured smooth muscle cells: roles of oxidative stress. Am. J. Physiol. 2000;279:C1880–C1888. doi: 10.1152/ajpcell.2000.279.6.C1880. [DOI] [PubMed] [Google Scholar]
- HAKIM T.S. Flow-induced release of EDRF in the pulmonary vasculature: site of release and action. Am. J. Physiol. 1994;267:H363–H369. doi: 10.1152/ajpheart.1994.267.1.H363. [DOI] [PubMed] [Google Scholar]
- HAMPL V., ARCHER S.L., NELSON D.P., WEIR E.K. Chronic EDRF inhibition and hypoxia: effects on pulmonary circulation and systemic blood pressure. J. Appl. Physiol. 1993;75:1748–1757. doi: 10.1152/jappl.1993.75.4.1748. [DOI] [PubMed] [Google Scholar]
- HANSSON G.K., GENG Y.-J., HOLM J., HARDHAMMAR P., WENNMALM A., JENNISCHE E. Arterial smooth muscle cells express nitric oxide synthase in response to endothelial injury. J. Exp. Med. 1994;180:733–738. doi: 10.1084/jem.180.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASUNUMA K., YAMAGUCHI T., RODMAN D.M., O'BRIEN R.F., MCMURTRY I.F. Effects of inhibitors of EDRF and EDHF on vasoreactivity of perfused rat lungs. Am. J. Physiol. 1991;260:L97–L104. doi: 10.1152/ajplung.1991.260.2.L97. [DOI] [PubMed] [Google Scholar]
- HUANG M., LEBLANC M.L., HESTER R.L. Systemic and regional hemodynamics after nitric oxide synthase inhibition: role of a neurogenic mechanism. Am. J. Physiol. 1994;267:R84–R88. doi: 10.1152/ajpregu.1994.267.1.R84. [DOI] [PubMed] [Google Scholar]
- HULTGREN H.N. High-altitude pulmonary edema: current concepts. Annu. Rev. Med. 1996;47:267–284. doi: 10.1146/annurev.med.47.1.267. [DOI] [PubMed] [Google Scholar]
- ISAACSON T.C., HAMPL V., WEIR E.K., NELSON D.P., ARCHER S.L. Increased endothelium-derived NO in hypertensive pulmonary circulation of chronically hypoxic rats. J. Appl. Physiol. 1994;76:933–940. doi: 10.1152/jappl.1994.76.2.933. [DOI] [PubMed] [Google Scholar]
- KALISCH B.E., CONNOP B.P., JHAMANDAS K., BENINGER R.J., BOEGMAN R.J. Differential action of 7-nitro indazole on rat brain nitric oxide synthase. Neurosci. Lett. 1996;219:75–78. doi: 10.1016/s0304-3940(96)13194-3. [DOI] [PubMed] [Google Scholar]
- KAY J.M. Pulmonary vasculature and nerves. Comparative morphologic features of the pulmonary vasculature in mammals. Am. Rev. Respir. Dis. 1983;128:S53–S57. doi: 10.1164/arrd.1983.128.2P2.S53. [DOI] [PubMed] [Google Scholar]
- KUBO K, HANAOKA M., YAMAGUCHI S., HAYANO T., HAYASAKA M., KOIZUMI T., FUJIMOTO K., KOBAYASHI T., HONDA T. Cytokines in bronchoalveolar lavage fluid in patients with high altitude pulmonary oedema at moderate altitude in Japan. Thorax. 1996;51:739–742. doi: 10.1136/thx.51.7.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LE CRAS T.D., TYLER R.C., HORAN M.P., MORRIS K.G., TUDER R.M., MCMURTRY I.F. Effects of chronic hypoxia and altered hemodynamics on endothelial nitric oxide synthase expression in the adult rat lung. J. Clin. Invest. 1998;101:795–801. doi: 10.1172/JCI786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LE CRAS T.D., XUE C., RENGASAMY A., JOHNS R.A. Chronic hypoxia upregulates endothelial and inducible NO synthase gene and protein expression in rat lung. Am. J. Physiol. 1996;270:L164–L170. doi: 10.1152/ajplung.1996.270.1.L164. [DOI] [PubMed] [Google Scholar]
- LEEMAN M., ZEGERS DE BEYL V., DELCROIX M., NAEIJE R. Effects of endogenous nitric oxide on pulmonary vascular tone in intact dogs. Am. J. Physiol. 1994;266:H2343–H2347. doi: 10.1152/ajpheart.1994.266.6.H2343. [DOI] [PubMed] [Google Scholar]
- LIAUDET L., FEIHL F., ROSSELET A., MARKERT M., HURNI J.-M., PERRET C. Beneficial effects of L-canavanine, a selective inhibitor of inducible nitric oxide synthase, during rodent endotoxaemia. Clin. Sci. 1996;90:369–377. doi: 10.1042/cs0900369. [DOI] [PubMed] [Google Scholar]
- LOEB A.L., LONGNECKER D.E. Inhibition of endothelium-derived relaxing factor-dependent circulatory control in intact rats. Am. J. Physiol. 1992;262:H1494–H1500. doi: 10.1152/ajpheart.1992.262.5.H1494. [DOI] [PubMed] [Google Scholar]
- MACLEAN M.R., JOHNSTON E.D., MCCULLOCH K.M., POOLEY L., HOUSLAY M.D., SWEENEY G. Phosphodiesterase isoforms in the pulmonary arterial circulation of the rat: changes in pulmonary hypertension. J. Pharmacol. Exp. Ther. 1997;283:619–624. [PubMed] [Google Scholar]
- MARUYAMA J., MARUYAMA K. Impaired nitric oxide-dependent responses and their recovery in hypertensive pulmonary arteries of rats. Am. J. Physiol. 1994;266:H2476–H2488. doi: 10.1152/ajpheart.1994.266.6.H2476. [DOI] [PubMed] [Google Scholar]
- MCCORMACK D.G., PATERSON N.A.M. Loss of hypoxic pulmonary vasoconstriction in chronic pneumonia is not mediated by nitric oxide. Am. J. Physiol. 1993;265:H1523–H1528. doi: 10.1152/ajpheart.1993.265.5.H1523. [DOI] [PubMed] [Google Scholar]
- MCMAHON T.J., HOOD J.S., BELLAN J.A., KADOWITZ P.J. Nω-nitro-L-arginine methyl ester selectively inhibits pulmonary vasodilator responses to acetylcholine and bradykinin. J. Appl. Physiol. 1991;71:2026–2031. doi: 10.1152/jappl.1991.71.5.2026. [DOI] [PubMed] [Google Scholar]
- MEYRICK B., REID L. The effect of continued hypoxia on rat pulmonary arterial circulation. An ultrastructural study. Lab. Invest. 1978;38:188–200. [PubMed] [Google Scholar]
- MOORE P.K., WALLACE P., GAFFEN Z., HART S.L., BABBEDGE R.C. Characterization of the novel nitric oxide synthase inhibitor 7-nitro indazole and related indazoles: antinociceptive and cardiovascular effects. Br. J. Pharmacol. 1993;110:219–224. doi: 10.1111/j.1476-5381.1993.tb13795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURAMATSU M., RODMAN D.M., OKA M., MCMURTRY I.F. Thapsigargin stimulates NO activity in hypoxic hypertensive rat lungs and pulmonary arteries. J. Appl. Physiol. 1996;80:1336–1344. doi: 10.1152/jappl.1996.80.4.1336. [DOI] [PubMed] [Google Scholar]
- MURAMATSU M., RODMAN D.M., OKA M., MCMURTRY I.F. Endothelin-1 mediates nitro-L-arginine vasoconstriction of hypertensive rat lungs. Am. J. Physiol. 1997;272:L807–L812. doi: 10.1152/ajplung.1997.272.5.L807. [DOI] [PubMed] [Google Scholar]
- NELIN L.D., DAWSON C.A. The effect of Nω-nitro-L-arginine methyl ester on hypoxic vasoconstriction in the neonatal pig lung. Pediatr. Res. 1993;34:349–353. doi: 10.1203/00006450-199309000-00022. [DOI] [PubMed] [Google Scholar]
- NISHIWAKI K., NYHAN D.P., ROCK P., DESAI P.M., PETERSON W.P., PRIBBLE C.G., MURRAY A. Nω-nitro-L-arginine and pulmonary vascular pressure–flow relationship in conscious dogs. Am. J. Physiol. 1992;262:H1331–H1337. doi: 10.1152/ajpheart.1992.262.5.H1331. [DOI] [PubMed] [Google Scholar]
- OKA M., HASUNUMA K., WEBB S.A., STELZNER T.J., RODMAN D.M., MCMURTRY I.F. EDRF suppresses an unidentified vasoconstrictor mechanism in hypertensive rat lungs. Am. J. Physiol. 1993;264:L587–L597. doi: 10.1152/ajplung.1993.264.6.L587. [DOI] [PubMed] [Google Scholar]
- OKAMOTO H., HUDETZ A.G., ROMAN R.J., BOSNJAK Z.J., KAMPINE J.P. Neuronal NOS-derived NO plays permissive role in cerebral blood flow response to hypercapnia. Am. J. Physiol. 1997;272:H559–H566. doi: 10.1152/ajpheart.1997.272.1.H559. [DOI] [PubMed] [Google Scholar]
- PERSSON M.G., GUSTAFSSON L.E., WIKLUND N.P., MONCADA S., HEDQVIST P. Endogenous nitric oxide as a probable modulator of pulmonary circulation and hypoxic pressor response in vivo. Acta Physiol. Scand. 1990;140:449–457. doi: 10.1111/j.1748-1716.1990.tb09021.x. [DOI] [PubMed] [Google Scholar]
- RABINOVITCH M. Pulmonary hypertension: updating a mysterious disease. Cardiovasc. Res. 1997;34:268–272. doi: 10.1016/s0008-6363(97)00053-9. [DOI] [PubMed] [Google Scholar]
- RABINOVITCH M., GAMBLE W., NADAS A.S., MIETTINEN O.S., REID L. Rat pulmonary circulation after chronic hypoxia: hemodynamic and structural features. Am. J. Physiol. 1979;236:H818–H827. doi: 10.1152/ajpheart.1979.236.6.H818. [DOI] [PubMed] [Google Scholar]
- RAIRIGH R.L., STORME L., PARKER T.A., LE CRAS T.D., KINSELLA J.P., JAKKULA M., ABMAN S.H. Inducible NO synthase inhibition attenuates shear stress-induced pulmonary vasodilation in the ovine fetus. Am. J. Physiol. 1999;276:L513–L521. doi: 10.1152/ajplung.1999.276.3.L513. [DOI] [PubMed] [Google Scholar]
- RESTA T.C., GONZALES R.J., DAIL W.G., SANDERS T.C., WALKER B.R. Selective upregulation of arterial endothelial nitric oxide synthase in pulmonary hypertension. Am. J. Physiol. 1997;272:H806–H813. doi: 10.1152/ajpheart.1997.272.2.H806. [DOI] [PubMed] [Google Scholar]
- RESTA T.C., O'DONAUGHY T.L., EARLEY S., CHICOINE L.G., WALKER B.R. Unaltered vasoconstrictor responsiveness after iNOS inhibition in lungs from chronically hypoxic rats. Am. J. Physiol. 1999;276:L122–L130. doi: 10.1152/ajplung.1999.276.1.L122. [DOI] [PubMed] [Google Scholar]
- RESTA T.C., WALKER B.R. Chronic hypoxia selectively augments endothelium-dependent pulmonary arterial vasodilation. Am. J. Physiol. 1996;270:H888–H896. doi: 10.1152/ajpheart.1996.270.3.H888. [DOI] [PubMed] [Google Scholar]
- RICHALET J.-P. High altitude pulmonary oedema: still a place for controversy. Thorax. 1995;50:923–929. doi: 10.1136/thx.50.9.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON B.E., WARREN J.B., NYE P.C.G. Inhibition of nitric oxide synthesis potentiates hypoxic vasoconstriction in isolated rat lungs. Exp. Physiol. 1990;75:255–257. doi: 10.1113/expphysiol.1990.sp003399. [DOI] [PubMed] [Google Scholar]
- RODMAN D.M. Chronic hypoxia selectively augments rat pulmonary artery Ca2+ and K+ channel-mediated relaxation. Am. J. Physiol. 1992;263:L88–L94. doi: 10.1152/ajplung.1992.263.1.L88. [DOI] [PubMed] [Google Scholar]
- ROOS C.M., FRANK D.U., XUE C., JOHNS R.A., RICH G.F. Chronic inhaled nitric oxide: effects on pulmonary vascular endothelial function and pathology in rats. J. Appl. Physiol. 1996;80:252–260. doi: 10.1152/jappl.1996.80.1.252. [DOI] [PubMed] [Google Scholar]
- RUSS R.D., WALKER B.R. Maintained endothelium-dependent pulmonary vasodilation following chronic hypoxia in the rat. J. Appl. Physiol. 1993;74:339–344. doi: 10.1152/jappl.1993.74.1.339. [DOI] [PubMed] [Google Scholar]
- SADA K., SHIRAI M., NINOMIYA I. X-ray TV system for measuring microcirculation in small pulmonary vessels. J. Appl. Physiol. 1985;59:1013–1018. doi: 10.1152/jappl.1985.59.3.1013. [DOI] [PubMed] [Google Scholar]
- SASAKI S.-I., KOBAYASHI N., DAMBARA T., KIRA S., SAKAI T. Structural organization of pulmonary arteries in the rat lung. Anat. Embryol. 1995;191:477–489. doi: 10.1007/BF00186738. [DOI] [PubMed] [Google Scholar]
- SCHOENE R.B., SWENSON E.R., PIZZO C.J., HACKETT P.H., ROACH R.C., MILLS W.J., JR, HENDERSON W.R., JR, MARTIN T.R. The lung at high altitude: bronchoalveolar lavage in acute mountain sickness and pulmonary edema. J. Appl. Physiol. 1988;64:2605–2613. doi: 10.1152/jappl.1988.64.6.2605. [DOI] [PubMed] [Google Scholar]
- SHAUL P.W., NORTH A.J., BRANNON T.S., UJIIE K., WELLS L.B., NISEN P.A., LOWENSTEIN C.J., SNYDER S.H., STAR R.A. Prolonged in vivo hypoxia enhances nitric oxide synthase type I and type III gene expression in adult rat lung. Am. J. Respir. Cell Mol. Biol. 1995;13:167–174. doi: 10.1165/ajrcmb.13.2.7542896. [DOI] [PubMed] [Google Scholar]
- SHAUL P.W., WELLS L.B., HORNING K.M. Acute and prolonged hypoxia attenuates endothelial nitric oxide production in rats pulmonary arteries by different mechanisms. J. Cardiovasc. Pharmacol. 1993;22:819–827. doi: 10.1097/00005344-199312000-00007. [DOI] [PubMed] [Google Scholar]
- SHIRAI M., IKEDA S., MIN K.-Y., SHIMOUCHI A., KAWAGUCHI A.T., NINOMIYA I. Segmental differences in vasodilatation due to basal NO release in in vivo cat pulmonary vessels. Respir. Physiol. 1999;116:159–169. doi: 10.1016/s0034-5687(99)00053-5. [DOI] [PubMed] [Google Scholar]
- SHIRAI M., SADA K., NINOMIYA I. Effects of regional alveolar hypoxia and hypercapnia on small pulmonary vessels in cats. J. Appl. Physiol. 1986;61:440–448. doi: 10.1152/jappl.1986.61.2.440. [DOI] [PubMed] [Google Scholar]
- SHIRAI M., SHIMOUCHI A., KAWAGUCHI A.T., IKEDA S., SUNAGAWA K., NINOMIYA I. Endogenous nitric oxide attenuates hypoxic pulmonary vasoconstriction of small pulmonary arteries and veins in anaesthetized cats. Acta Physiol. Scand. 1997;159:263–264. doi: 10.1046/j.1365-201X.1997.106357000.x. [DOI] [PubMed] [Google Scholar]
- SPRAGUE R.S., STEPHENSON A.H., DIMMITT R.A., WEINTRAUB N.A., BRANCH L., MCMURDO L., LONIGRO A.J. Effect of L-NAME on pressure–flow relationships in isolated rabbit lungs: role of red blood cells. Am. J. Physiol. 1995;269:H1941–H1948. doi: 10.1152/ajpheart.1995.269.6.H1941. [DOI] [PubMed] [Google Scholar]
- STAMLER J.S., LOH E., RODDY M.-A., CURRIE K.E., CREAGER M.A. Nitric oxide regulates basal systemic and pulmonary vascular resistance in healthy humans. Circulation. 1994;89:2035–2040. doi: 10.1161/01.cir.89.5.2035. [DOI] [PubMed] [Google Scholar]
- STEUDEL W., ICHINOSE F., HUANG P.L., HURFORD W.E., JONES R.C., BEVAN J.A., FISHMAN M.C., ZAPOL W.M. Pulmonary vasoconstriction and hypertension in mice with targeted disruption of the endothelial nitric oxide synthase (NOS 3) gene. Circ. Res. 1997;81:34–41. doi: 10.1161/01.res.81.1.34. [DOI] [PubMed] [Google Scholar]
- STEUDEL W., SCHERRER-CROSBIE M., BLOCH K.D., WEIMANN J., HUANG P.L., JONES R.C., PICARD M.H., ZAPOL W.M. Sustained pulmonary hypertension and right ventricular hypertrophy after chronic hypoxia in mice with congenital deficiency of nitric oxide synthase 3. J. Clin. Invest. 1998;101:2468–2477. doi: 10.1172/JCI2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABO C., SOUTHAN G.J., THIEMERMANN C. Beneficial effects and improved survival in rodent models of septic shock with S-methylisothiourea sulfate, a potent and selective inhibitor of inducible nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12472–12476. doi: 10.1073/pnas.91.26.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEALE D.M., ATKINSON A.M. L-Canavanine restores blood pressure in a rat model of endotoxin shock. Eur. J. Pharmacol. 1994;271:87–92. doi: 10.1016/0014-2999(94)90268-2. [DOI] [PubMed] [Google Scholar]
- TYLER R.C., MURAMATSU M., ABMAN S.H., STELZNER T.J., RODMAN D.M., BLOCH K.D., MCMURTRY I.F. Variable expression of endothelial NO synthase in three forms of rat pulmonary hypertension. Am. J. Physiol. 1999;276:L297–L303. doi: 10.1152/ajplung.1999.276.2.L297. [DOI] [PubMed] [Google Scholar]
- UMANS J.G. Nitric oxide in the regulation of blood flow and arterial pressure. Annu. Rev. Physiol. 1995;57:771–790. doi: 10.1146/annurev.ph.57.030195.004011. [DOI] [PubMed] [Google Scholar]
- WEIR E.K., ARCHER S. The mechanism of acute hypoxic pulmonary vasoconstriction: the tale of two channels. FASEB J. 1995;9:183–189. doi: 10.1096/fasebj.9.2.7781921. [DOI] [PubMed] [Google Scholar]
- WEST J.B., COLICE G.L., LEE Y.-J., NAMBA Y., KURDAK S.S., FU Z., OU L.C., MATHIEU-COSTELLO O. Pathogenesis of high-altitude pulmonary oedema: direct evidence of stress failure of pulmonary capillaries. Eur. Respir. J. 1995;8:523–529. [PubMed] [Google Scholar]
- XUE C., JOHNS R.A. Upregulation of nitric oxide synthase correlates temporally with onset of pulmonary vascular remodeling in the hypoxic rat. Hypertension. 1996;28:743–753. doi: 10.1161/01.hyp.28.5.743. [DOI] [PubMed] [Google Scholar]
- XUE C., RENGASAMY A., LE CRAS T.D., KOBERNA P.A., DAILEY G.C., JOHNS R.A. Distribution of NOS in normoxic vs hypoxic rat lung: upregulation of NOS by chronic hypoxia. Am. J. Physiol. 1994;267:L667–L678. doi: 10.1152/ajplung.1994.267.6.L667. [DOI] [PubMed] [Google Scholar]