Abstract
The present study evaluated the ability of the administration of platelet activating factor (PAF) to induce the upregulation of B1 receptors in the rat paw.
Local treatment with PAF resulted in a time-dependent increase of oedema formation induced by the B1 receptor agonist des-Arg9-BK (des-Arg9-bradykinin), but not by the B2 receptor agonist tyrosine8-bradykinin. Functional upregulation of B1 receptors was accompanied by a prominent increase of B1 receptor mRNA expression in the rat paw.
In PAF-treated paws, des-Arg9-BK-induced oedema formation was significantly inhibited by the B1 receptor antagonists des-Arg9-[Leu8]-BK and R-715. The effects of PAF pretreatment were receptor operated, as assessed by the effects of the PAF receptor antagonist WEB2086 or by desensitisation of PAF receptors.
The protein synthesis inhibitor cycloheximide, the anti-inflammatory steroid dexamethasone or the nuclear factor (NF-κB) blockers pyrrolidine-dithiocarbamate (PDTC) and Nα-tosyl-L-chloromethylketone significantly blocked the functional upregulation of B1 receptors.
The selectin inhibitor fucoidin, an anti-CD18 antibody or an anti-rat neutrophil antiserum, also significantly prevented des-Arg9-BK-induced paw oedema in rats pretreated with PAF.
Intradermal injection of PAF induced a 25-fold increase of myeloperoxidase activity in the rat paw, a response that was significantly inhibited by fucoidin, anti-CD-18, anti-rat neutrophil antiserum or PDTC.
Local treatment with PAF also resulted in a marked increase of NF-κB activation, an effect largely prevented by PDTC or by the anti-rat neutrophil antiserum.
Collectively, the present results indicate that the induction of B1 receptors following treatment with the chemotatic mediator PAF is dependent on the recruitment of neutrophils, an event that is under the control of adhesion molecules, protein synthesis and NF-κB activation. These findings provide new insights into the role played by cell migration and chemotatic factors on B1 receptor upregulation in vivo.
Keywords: B1 receptor expression, platelet activating factor, oedema formation, neutrophil influx, myeloperoxidase activity, NF-κB binding activation
Introduction
Platelet activating factor (PAF) is a potent phospholipid mediator involved in several physiological events (Ishii et al., 1998) and, potentially, in many diseases, such as asthma, anaphylaxis, acute pancretatitis, psoriasis, endotoxic shock, dermatitis and inflammatory bowel disease (Evangelou, 1994; Ishii & Shimizu, 2000). PAF is produced by various tissues and cell types, including platelets, neutrophils, macrophages, leucocytes, eosinophils and endothelial cells (Chao & Olson, 1993; Korth et al., 1995). Pharmacological actions of PAF are mediated by a unique seven transmembrane G protein-coupled receptor (Ishii et al., 1998; Ishii & Shimizu, 2000). Among its many effects, PAF induces profound modifications on neutrophils, including chemoattraction and activation (Zhou et al., 1994; Franciose et al., 1996). Stimulation of PAF receptor leads to an increase of diacylglycerol, inositoltriphosphate and calcium (Ca2+). Furthermore, PAF is able to activate several types of phospholipases (PLA2, PLCβ) and protein kinases, such as protein kinase C, tyrosine kinase and mitogen-activated protein (MAP)-kinases (Richardson et al., 1996; Ishii & Shimizu, 2000). More recently, it has been reported that PAF is capable of activating the nuclear factor-κB (NF-κB), mainly the p50 subunit, and enhancing its DNA-binding activity in the rat intestine (De Plaen et al., 1998).
Kinins are important mediators involved in several biological events and inflammatory conditions (Bhoola et al., 1992). Their effects are amplified by the release of and/or interaction with other inflammatory mediators (Sharma & Buchanan, 1994; Campos & Calixto, 1995; Böckmann and Paegelow, 2000). The actions of kinins are mediated by the activation of two distinct G protein-coupled receptors, B1 and B2 receptors (Marceau & Bachvarov, 1998; Calixto et al., 2001; Couture et al., 2001). B2 receptors are generally constitutive and responsible for most of the physiological actions of kinins (Calixto et al., 2000, 2001; Newton et al., 2002), whereas B1 receptors are rarely expressed under normal conditions. However, B1 receptors may be upregulated during inflammatory processes, after tissue injury or by some inflammatory cytokines and bacterial products (reviewed by Marceau et al., 1998; Calixto et al., 2000, 2001).
It has been demonstrated that the modulation of B1 receptor expression may be secondary to the stimulation of distinct groups of protein kinases and the activation of the transcriptional NF-κB (Larrivé et al., 1998; Schanstra et al., 1998; Campos et al., 1999; Cabrini et al., 2001). More recently, we showed that IL-1β-induced B1 receptor upregulation in the rat paw constitutes an event linked to the recruitment of neutrophils by a mechanism dependent on adhesion molecules, activation of PAF receptors and release of TNFα (Campos et al., 2002). The latter evidence allowed us to suggest that chemotatic factors, such as PAF, and the signalling pathways related to this inflammatory mediator, might exert a pivotal role in kinin B1 receptor functional upregulation. In the present study, we sought to investigate the effect of the local treatment with PAF on the modulation of B1 receptor-mediated inflammatory responses in the rat paw. Additionally, we have attempted to analyse by means of pharmacological and molecular approaches some of the mechanisms underlying PAF-induced B1 receptor upregulation in the rat paw.
Methods
Measurement of rat paw oedema
Non-fasted male Wistar rats (140–180 g) kept in controlled room temperature (22±2°C) under a 12 : 12 h light–dark cycle (lights on 06:00 h) were used. The experiments were conducted according to the procedures described previously (Campos & Calixto, 1995). Animals received a 0.1 ml intradermal (i.d.) injection in one hind paw (right paw) of phosphate-buffered saline (PBS, composition mmol l−1: NaCl 137, KCl 2.7, phosphate buffer 10) containing the selective kinin B1 des-Arg9-bradykinin (des-Arg9-BK, 100 nmol paw−1) or B2 receptor agonist tyrosine8-bradykinin (Tyr8-BK, nmol paw−1). The contralateral paw (left paw) received 0.1 ml of PBS and was used as control. Oedema was measured by use of a plethysmometer (Ugo Basile) at several time points (10, 20, 30, 60 and 120 min) after injection of des-Arg9-BK or Tyr8-BK. Oedema is expressed in milliliters as the difference between the right and left paws.
In most cases, animals received an injection of PAF (10 nmol paw−1) at different intervals of time (6, 12 and 24 h prior) prior to and at the same site of injection of kinins. In all, 6 h was the earliest time point chosen because the paw oedema evoked by PAF is completely resolved only 6 h after the administration of this lipid mediator (Cordeiro et al., 1986). In a separate group of experiments, in order to assess the possible systemic effects of i.d. treatment with PAF, rats received PAF (10 nmol paw−1) in the right paw and the B1 receptor agonist des-Arg9-BK (100 nmol paw−1) was injected in the left paw. Oedema was evaluated as described above.
All experimental procedures were performed under slight anaesthesia with 2,2,2 tribromoethanol (0.125 g kg−1, i.p.). The reported experiments were carried out in accordance with current guidelines for the care of laboratory animals and ethical guidelines for investigations of experiments in conscious animals (Zimmermann, 1983).
Analysis of some mechanisms involved in B1-receptor-mediated paw oedema in rats pretreated with PAF
To confirm the involvement of kinin B1 receptors on des-Arg9-BK-induced rat paw oedema, animals pretreated with PAF (10 nmol paw−1, 6 h before) received an i.d. injection of the selective B1 receptor agonist des-Arg9-BK (100 nmol paw−1) in association with the selective B1 des-Arg9-[Leu8]-bradykinin (des-Arg9-[Leu8]-BK, 100 nmol paw−1) or R-715 (60 nmol paw−1) receptor antagonists (Campos & Calixto, 1995; Pinheiro et al., 2001).
To confirm the contribution of PAF receptors for the effects of the intraplantar administration of PAF, animals treated with PAF received a co-injection of the selective PAF receptor antagonist WEB2086 (15 μg paw−1) (Campos et al., 2002). Involvement of PAF receptors was further assessed using a desensitisation protocol, in which animals received single daily i.d. injections of PAF (10 nmol paw−1) for seven successive days prior to the experiments (Cordeiro et al., 1986).
The possible involvement of protein synthesis in the upregulation of B1 receptors by PAF treatment was evaluated by treating different groups of animals with the anti-inflammatory steroid dexamethasone (0.5 mg kg−1, s.c.) or with the protein synthesis inhibitor cycloheximide (1.5 mg kg−1, s.c.), both administered 6 h before PAF injection. In a similar manner, to investigate the participation of NF-κB activation, other groups of animals received the inhibitors of the NF-κB pyrrolidine-dithiocarbamate (PDTC) (100 mg kg−1, i.p.) or Nα-tosyl-L-chloromethylketone (TLCK) (2 mg kg−1, i.p.) 30 min before PAF treatment (Campos et al., 1998; 1999).
In another set of experiments, to examine the contribution of adhesion molecules for the capacity of PAF to induce B1 receptor upregulation, animals received the nonspecific selectin inhibitor fucoidin (10 mg kg−1, i.v., 15 min) or the anti-CD18 (integrin β2 chain) monoclonal antibody (WT3, 1 mg kg−1, i.v., 15 min) (Teixeira & Hellewell, 1997; Campos et al., 2002). To confirm that the migrating leucocytes involved in the effects of PAF on B1 receptor upregulation were indeed neutrophils, rats were treated systemically with the anti-rat neutrophil antiserum (34 μg kg−1, i.p.) 30 min prior to PAF administration (Adams & Tepperman, 2001).
Expression of B1 receptor mRNA in the rat paw
Functional studies were extended by analysing B1 receptor mRNA expression after treatment with PAF. The methodology used was similar to that described previously (Campos et al., 2002). Rats received an i.d. injection of PAF (10 nmol paw−1, 3–12 h) and were killed. The subcutaneous tissue of the paws was removed and immediately frozen in liquid nitrogen. The samples were homogenised and the total RNA was extracted using the Trizol Reagent (Gibco BRL® Rockville, MD, U.S.A.). Of total RNA, 1 μg was reverse transcribed (RT) using oligo dT as primer (25 μg m l−1) and 200 U of reverse transcriptase (Gibco BRL®), in 20 μl of PCR buffer containing (mM): dNTP 0.5, DTT 10, MgCl2 2.5, KCl 50 and Tris-HCl pH 8.4 20. The samples were incubated for 50 min at 42°C, heated for 15 min at 70°C and cooled in ice. After treatment with 2 U of RNAse H (20 min, 37°C), cDNA amplification of a specific sequence of rat B1 receptor and β-actin was performed by PCR using the following primers: sense TGAAGCTGTGAGCTC-TTTG and antisense GCCAGTTGAAACGGTTCCC for B1 receptor, and sense GTTCCGATGCCCCGAGGATCT and antisense GCATTTGCGGTGCACGATGGA for rat β-actin, respectively. β-Actin cDNA was used for standardisation of the amount of RNA. A measure of 5 μl of RT aliquots were mixed in a Tris-HCl buffer 20 mM (pH 8.4) containing: MgCl2 1.5 mM, dNTP 300 μM, 2 μg ml−1 of each primer and 50 U ml−1 of Taq polymerase (Gibco BRL®), in a final volume of 100 μl. The cycling protocol used was the following: 4 min at 94°C, 36 cycles of 35 s at 94°C/45 s at 60°C/45 s at 72°C and finally, 7 min at 72°C. Aliquots of 25 μl were analysed on a 20% Tris/borate/EDTA (TBE) polyacrylamide gel and stained by ethidium bromide. The size of products is 450 bp for B1 receptor and 600 bp for β-actin.
Measurement of myeloperoxidase activity
Neutrophil recruitment to the rat paw was assessed indirectly by means of tissue myeloperoxidase (MPO) activity according to the method described before (Souza et al., 2000; Campos et al., 2002). For this purpose, animals received an i.d. injection of PAF (10 nmol paw−1, 1–24 h) in the right paw. PBS-injected paws were used as control. In different sets of experiments, MPO activity was assessed in animals treated with fucoidin (10 mg kg−1, i.v., 15 min), anti-CD18 (1 mg kg−1, i.v., 15 min), anti-rat neutrophil antiserum (34 μg kg−1, i.p., 30 min) or PDTC (100 mg kg−1, i.p., 30 min).
At the time of sacrifice, the subcutaneous tissue of the paws was removed, homogenised at 5% (w v−1) in EDTA/NaCl buffer (pH 4.7) and centrifuged at 10 000 r.p.m. for 15 min, at 4°C. The pellet was resuspended in hexadecyltrimethyl ammo-nium bromide 0.5% buffer (pH 5.4) and the samples were frozen and thawed for three times in liquid nitrogen. Upon thawing, the samples were recentrifuged (10 000 r.p.m., 15 min, at 4°C) and 25 μl of the supernatant was used for the MPO assay. The enzymatic reaction was assessed with 1.6 mM tetramethylbenzidine, 80 mM NaPO4 and 0.3 mM hydrogen peroxide. The absorbance was measured at 690 nm and the results are expressed in optical density (OD) per milligram of tissue.
Assessing of NF-κB activity
NF-κB activation following local treatment with PAF was evaluated by eletrophoretic mobility shift assay. Animals received an i.d. injection of PAF (10 nmol paw−1, 1–12 h) in the right paw. PBS-injected paws were used as control. In another series of experiments, animals were pretreated with PDTC (100 mg kg−1, i.p., 30 min) or with the anti-rat neutrophil antiserum (34 μg kg−1, i.p., 30 min) and tissues processed for NF-κB activation at 3 h.
At the time of sacrifice, the subcutaneous tissue of the paws was removed and frozen under liquid nitrogen. Nuclear extracts were prepared as described by D'Acquisto et al. (1999) with some modifications. Tissues were suspended in 400 μl of ice-cold hypotonic lysis buffer (HEPES 10 mM, MgCl2 1.5 mM, KCl 10 mM, PMSF 0.5 mM, soybean trypsin inhibitor 1.5 μg m l−1, pepstatin A 7 μg m l−1, leupeptin 5 μg m l−1, benzamidine 0.1 mM, DTT 0.5 mM) and were homogenised in Ultra-turrax for 1 min. The homogenate was chilled on ice for 15 min and then vigorously shaken for 15 min in the presence of 25 μl of Nonidet P-40 10%. The nuclear fraction was precipitated by centrifugation at 1500 × g for 5 min. The nuclear pellet was resuspended in 200 μl of high salt extraction buffer (HEPES 20 mM pH 7.9, NaCl 420 mM, MgCl2 1.5 mM, EDTA 0.2 mM, glycerol 25% v v−1, PMSF 0.5 mM, soybean trypsin inhibitor 1.5 μg m l−1, pepstatin A 7 μg m l−1, leupeptin 5 μg m l−1, benzamidine 0.1 mM, DTT 0.5 mM), incubated under continuous shaking at 4°C for 30 min and then centrifuged for 15 min at 13,000 × g. The supernatant was aliquoted and stored at −80°C. Protein concentration was determined by the Biorad protein assay kit (BioRad®).
Eletrophoretic mobility shift assay was performed by use of the Gel Shift Assay System kit from Promega, according to the manufacturer's instructions. Briefly, NF-κB double-stranded consensus oligonucleotide probe (5′-AGTTGAGGGGACTTTCCCAGGC-3′) was end-labelled with [γ-32P] ATP (DuPont, New England) in the presence of T4 polynucleotide kinase for 10 min at 37°C. Unincorporated nucleotides were removed by passing the reaction mixture over a Sephadex G-25 spin column (Pharmacia). In a total volume of 20 μl, nuclear extracts (10 μg) were incubated with gel shift binding buffer (mM: Tris-HCl pH 7.5 10, MgCl2 1, NaCl 50, DTT 0.5, EDTA 0.5, glycerol 4% and 1 μg of poly(dIdC)) for 20 min at room temperature. Further, each sample was incubated for 30 min at room temperature with 25,000 c.p.m. of 32P-labelled NF-κB consensus oligonucleotide. Protein–DNA complexes were resolved by nondenaturing 6% acrylamide : bisacrylamide (37.5 : 1) in 0.25 × Tris-borate/EDTA buffer at 150 V for 2 h. The gel was vacuum-dried and analysed using a FUJIX BAS 2000 (Düsseldorf, Germany) Phosphor-Imager system. For competition studies, NF-κB or TFIID (5′-5′-GCAGAGCATATAAGGTGAGGTAGGA-3′) unlabelled double-stranded oligonucleotide was included in molar excess over the amount of radiolabelled probe in order to detect specific and nonspecific DNA/protein interactions, respectively.
Drugs and reagents
The following drugs and reagents were used: BSA, cycloheximide, dexamethasone, DTT, EDTA, fucoidin, hexadecyltrimethyl ammonium bromide (HTAB), PBS tablets, PDTC, PMSF, TLCK, 2,2,2 tribromoethanol, TMB (tetramethylbenzidine), Tyr8-BK (all from Sigma Chemical Company, St Louis, MO, U.S.A.); Des-Arg9-BK, des-Arg9-Leu8-BK and PAF (all from Bachem Bioscience Inc., King of Prussia, PA, U.S.A.); NaPO4, hydrogen peroxide, MgCl2, KCl, Tris-HCl, NaCl (all from Merck, Germany). Anti-rat neutrophil antiserum was obtained from Accurate Chemicals, San Diego, CA, U.S.A. WEB2086 was a gift of Boehringer-Manhein, Germany. Anti-CD18 mAB was from Professor Paul Hellewell, University of Sheffield, U.K. R-715 was kindly donated by Dr Domenico Regoli, University of Sherbrooke, CA, U.S.A.
Stock solutions of des-Arg9-BK, des-Arg9-Leu8-BK, Tyr8-BK and R-715 were prepared in PBS. PAF was prepared in a BSA 0.1% solution. All were stocked in siliconised plastic tubes, maintained at −18°C and diluted to the desired concentration just before using. Other drugs were prepared daily in 0.9% w v−1 NaCl solution, except dexamethasone which was diluted in ethanol 5%.
Statistical analysis
Results are presented as the mean±s.e.m. of four to six animals. The percentages of inhibition are reported as the mean±s.e.m. of inhibitions obtained for each individual experiment at the peak of oedema (20 min after injection of des-Arg9-BK), at 6 h (MPO experiments) or at 3 h after PAF injection (NF-κB activation). Statistical comparison of the data was performed by analysis of variance (ANOVA) followed by Dunnett's test or Newman–Keuls test or by use of Student's unpaired t-test. P-values less than 0.05 were considered significant.
Results
Upregulation of B1 receptors after treatment with PAF
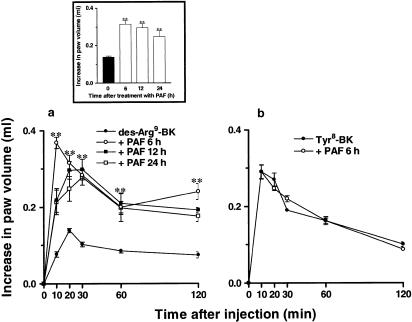
As reported previously (Campos et al., 1998), i.d. injection of the selective kinin B1 receptor agonist des-Arg9-BK (100 nmol paw−1) in naive animals produced a slight increase in rat paw volume (0.14±0.006 ml). In contrast, i.d. injection of des-Arg9-BK caused a marked increase in rat paw oedema (0.31±0.01 ml) in rats that had been pretreated with PAF (10 nmol paw−1, 6–24 h prior) (Figure 1a). Des-Arg9-BK-induced oedema formation was maximal 6 h following PAF treatment, remaining elevated up to 24 h (inset box, Figure 1). The same treatment with PAF failed to cause the upregulation of B1 receptor-mediated paw oedema when des-Arg9-BK (100 nmol paw−1) was injected in the contralateral paw (data not shown). Conversely, i.d. injection of the selective kinin B2 receptor agonist Tyr8-BK (3 nmol paw−1) produced significant oedema formation (0.27±0.017 ml) in naïve paws, a response that remained unaltered (0.25±0.01 ml) following local treatment with PAF (Figure 1b).
Figure 1.
(Panel a) Paw oedema induced by des-Arg9-BK (100 nmol paw−1) in control or in rats pretreated with PAF (10 nmol paw−1; 6, 12 or 24 h beforehand). Inset box indicates time-related increase of des-Arg9-BK (100 nmol paw−1; 20 min)-caused paw oedema following local treatment with PAF. (Panel b) Oedema formation induced by Tyr8-BK (3 nmol paw−1) in control or in PAF (10 nmol paw−1, 6 h prior)-treated animals. Values represent the mean±s.e.m. of four to six animals. In some cases, error bars are hidden within the symbols. Significantly different from control values **P<0.01.
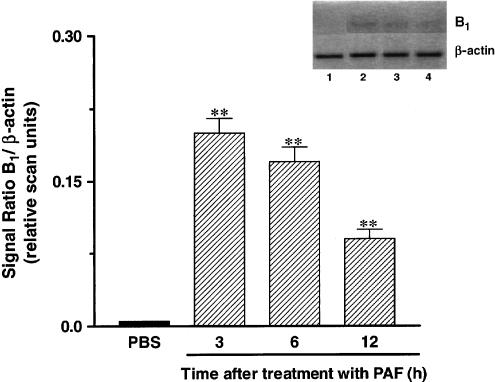
Results show that the functional increase of B1 receptors following PAF treatment (10 nmol paw−1, 3–12 h) was accompanied by a marked and time-related increase in kinin B1 receptor mRNA expression in the rat paw, as assessed by RT–PCR (Figure 2). B1 receptor mRNA expression peaked at 3 h and significant expression was detected even 12 h after PAF injection (Figure 2).
Figure 2.
Time-dependent effect of PAF treatment (10 nmol paw−1, 3–12 h) on kinin B1 receptor mRNA expression. Bottom: graphic representation of B1/β-actin signal ratio. (1) PBS (2) PAF 3 h (3) PAF 6 h (4) PAF 12 h. Number of replicates: three.
Mechanisms underlying the functional upregulation of B1 receptors in rats pretreated with PAF
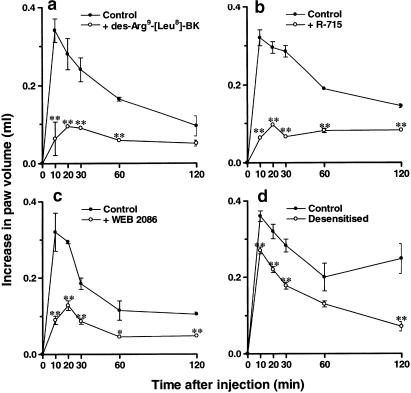
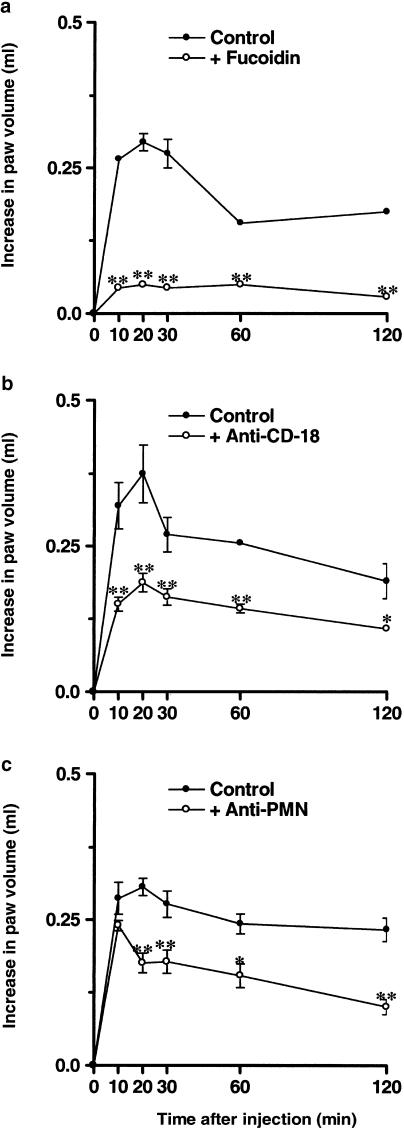
Paw oedema formation induced by des-Arg9-BK in rats pretreated with PAF (10 nmol paw−1, 6 h before) was significantly inhibited by the coinjection of the selective B1 receptor antagonists des-Arg9[Leu8]-BK (100 nmol paw−1) or R-715 (60 nmol paw−1). Percentages of inhibition obtained were 50±2 and 68±1%, respectively (Figure 3a and b). When the selective PAF receptor antagonist WEB2086 (15 μg paw−1) was coadministered with PAF, there was a marked inhibition of oedema formation in response to des-Arg9-BK (57±4%) (Figure 3c). In a similar manner, the increase of B1 receptor-mediated rat paw oedema was significantly prevented by the desensitisation of PAF receptors (32±3%) (Figure 3d). Next, we examined the role of the recruitment of leucocytes for the ability of PAF to induce function B1 receptor upregulation. The treatment of animals with the nonspecific selectin inhibitor fucoidin (10 mg kg−1, i.v., 15 min) or with a monoclonal anti-CD18 (anti-integrin β2 chain) antibody (WT3, 1 mg kg−1, i.v., 15 min) prior to PAF injection reduced des-Arg9-BK-induced paw oedema by 75±1 and 50±4%, respectively (Figure 4a and b). Moreover, depletion of neutrophils with an anti-rat neutrophil antiserum (34 μg kg−1, i.p., 30 min) prior to PAF injection also resulted in a significant inhibition (43±6%) of the increase in paw volume in response to des-Arg9-BK administration (Figure 4c).
Figure 3.
Effects of the coinjection of the selective kinin B1 receptor antagonists des-Arg9-[Leu8]-BK (panel a, 100 nmol paw−1) and R715 (panel b, 60 nmol paw−1) on des-Arg9-BK-induced paw oedema (100 nmol paw−1) in animals pretreated with PAF (10 nmol paw−1). Effects of the selective PAF receptor antagonist WEB2086 (panel c, 15 μg paw−1) or of PAF receptor desensitisation (panel d) on des- Arg9-BK-induced paw oedema (100 nmol paw−1) in animals pretreated with PAF (10 nmol paw−1). Each point represents the mean±s.e.m. of four to six animals. In some cases, error bars are hidden within the symbols. Significantly different from control values *P<0.05, **P<0.01.
Figure 4.
Effects of the treatment with the nonselective selectin inhibitor fucoidin (panel a, 10 mg kg−1, i.v., 15 min), an anti-CD-18 monoclonal antibody (panel b, 1 mg kg−1, i.v., 15 min) or an anti-rat neutrophil antiserum (panel c, 34 μg kg−1, i.p., 30 min) on des- Arg9-BK-induced paw oedema (100 nmol paw−1) in animals pretreated with PAF (10 nmol paw−1). Each point represents the mean±s.e.m. of four to six animals. In some cases, error bars are hidden within the symbols. Significantly different from control values *P<0.05, **P<0.01.
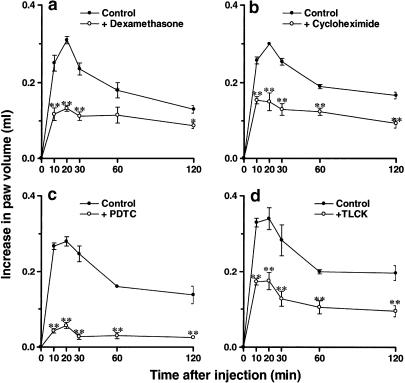
Figure 5 (a and b) demonstrates that treatment with the anti-inflammatory steroid dexamethasone (0.5 mg kg−1, s.c.) or with the protein synthesis inhibitor cycloheximide (1.5 mg kg−1, s.c.), both administered 6 h before PAF injection, significantly prevented the increase of rat paw oedema induced by des-Arg9-BK (57±2 and 50±8%, respectively). Similarly, des-Arg9-BK-induced paw oedema in animals pretreated with PAF was also reduced by the systemic treatment with the NF-κB activation blockers, PDTC (100 mg kg−1, i.p., 30 min) or TLCK (2 mg kg−1, i.p., 30 min)–79±3 and 48±7% inhibition, respectively (Figure 5c and d).
Figure 5.
Effects of the treatment with the protein synthesis inhibitors dexamethasone (panel a, 0.5 mg kg−1, s.c., 6 h) and cycloheximide (panel b, 1.5 mg kg−1, s.c., 6 h) or NF-κB activation inhibitors PDTC (panel c, 100 mg kg−1, i.p., 30 min) and TLCK (panel d, 2 mg kg−1, i.p., 30 min) on des-Arg9-BK-induced paw oedema (100 nmol paw−1) in animals pre-treated with PAF (10 nmol paw−1). Each point represents the mean±s.e.m. of four to six animals. In some cases, error bars are hidden within the symbols. Significantly different from control values *P<0.05, **P<0.01.
Measurement of MPO activity
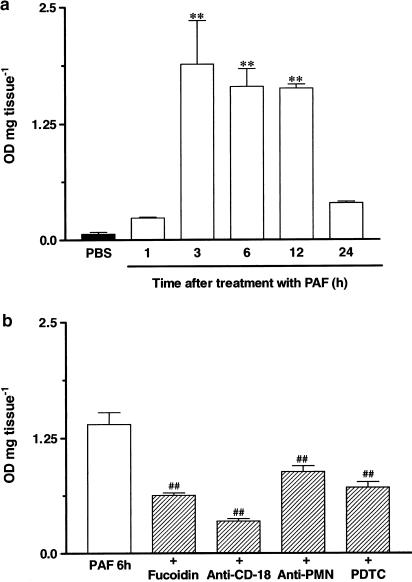
Results depicted in Figure 6a demonstrate that the i.d. injection of PAF (10 nmol paw−1) induced a marked time-dependent neutrophil accumulation in the rat paw, as assessed by MPO activity measurement. The increase of MPO levels following local treatment with PAF reached maximal values at 3–12 h (about 25-fold increase), returning to basal values after 24 h.
Figure 6.
(Panel a) Time-dependent effect of PAF injection (10 nmol paw−1, 1–24 h) on MPO activity in the rat paw. (Panel b) Effects of the inhibitors of cell migration fucoidin (10 mg kg−1, i.v., 15 min) and anti-CD18 (1 mg kg−1, i.v., 15 min), an anti-PMN antibody (34 μg kg−1, i.p., 30 min) and the NF-κB activation inhibitor PDTC (100 mg kg−1, i.p., 30 min) on PAF-induced neutrophil influx in the rat paw. Each column represents the mean±s.e.m. of four to six animals. Significantly different from PBS (**) or PAF-injected paws (♯♯) values **P<0.01.
Next, we evaluated whether the strategies used above to prevent leucocyte accumulation were indeed effective in modulating PAF-induced (10 nmol paw−1, 6 h) neutrophil influx in the rat paw. The treatment of animals with fucoidin (10 mg kg−1, i.v., 15 min), the anti-CD18 (integrin β2 chain) monoclonal antibody (WT3, 1 mg kg−1, i.v., 15 min) or the anti-rat neutrophil antiserum (34 μg kg−1, i.p., 30 min) significantly reduced MPO activity induced by PAF injection (38±5, 79±2 and 46±4%, respectively). Interestingly, PAF-induced elevation in MPO activity in the rat paw was also reduced by the pretreatment with the NF-κB inhibitor PDTC (100 mg kg−1, i.p., 30 min) (51±2%) (Figure 6b).
Effect of the intraplantar injection of PAF on the induction of NF-κB activation
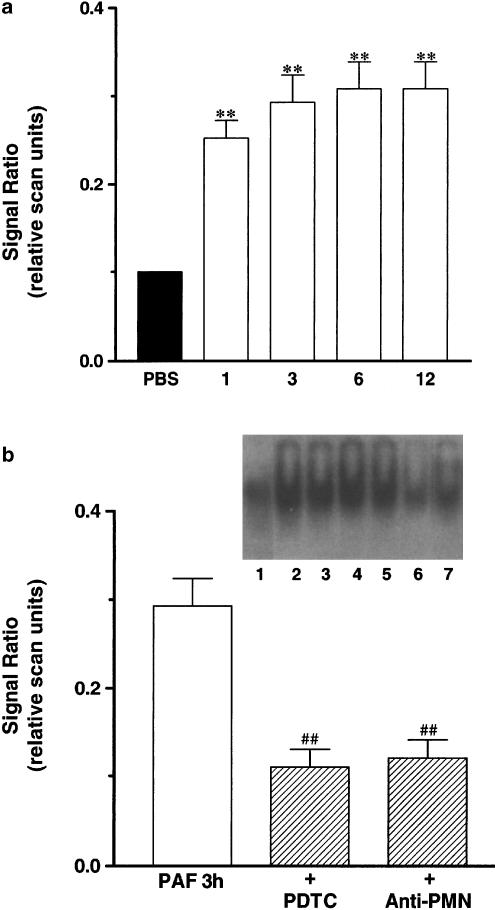
NF-κB activation was analysed by means of eletrophoretic mobility shift assay. Results of Figure 7a show that NF-κB activity was markedly enhanced (about three-fold) in nuclear extracts obtained from PAF (10 nmol paw−1, 1–12 h)-treated paws. Significant activation of NF-κB was found as early as 1 h and persisted until 12 h after PAF injection (Figure 7a). To examine the contribution of neutrophils for PAF-induced NF-κB activation, animals were pretreated with an anti-rat neutrophil antiserum (34 μg kg−1, i.p., 30 min). As seen in Figure 7b, the antiserum significantly diminished by 53±6% the PAF-induced increase of NF-κB binding activity. For comparison, in animals treated with the NF-κB inhibitor PDTC (100 mg kg−1, i.p., 30 min), there was 57±8% inhibition of NF-κB activation (Figure 7b).
Figure 7.
(Panel a) Time-dependent effect of PAF treatment (10 nmol paw−1, 3–12 h) on NF-κB activation. (Panel b) Effect of the NF-κB activation inhibitor PDTC (100 mg kg−1, i.p., 30 min) or an anti-rat neutrophil antiserum (34 μg kg−1, i.p., 30 min) on NF-κB activation induced 3 h after PAF injection. Bottom: graphic representation of relative scan units. (1) PBS (2) PAF 1 h (3) PAF 3 h (4) PAF 6 h (5) PAF 12 h (6) PDTC (7) anti-PMN. Number of replicates: three.
Discussion
Inflammation is characterised by the production of cellular and humoral mediators acting in concert to amplify and extend this process. Temporal changes observed during the inflammatory reaction are linked to the regulation of many inflammatory genes (McIntyre et al., 1997). Among these, kinin B1 receptors have been described as molecular entities highly upregulated following different stimuli, such as tissue trauma, bacterial products and proinflammatory cytokines. Several lines of investigation have shown that B1 receptor upregulation is largely dependent on the activation of transcription factors and de novo protein synthesis (Marceau, 1995; Marceau et al., 1998; Calixto et al., 2001; Couture et al., 2001). Although many recent studies have addressed the mechanisms of B1 receptor expression under inflammatory conditions, the exact sequence of events implicated in this process remains undefined. In the present study, we have evaluated some of the possible mechanisms involved in the up-regulation of kinin B1 receptors after local treatment with the chemotatic factor PAF in the rat paw. Attempts were also made in order to determine the contribution of PAF-induced neutrophil migration and NF-κB activation for the functional and molecular upregulation of B1 receptors. This was achieved by means of functional, biochemical and molecular approaches in combination with various pharmacological tools.
PAF is a proinflammatory lipid mediator known to promote an increase of vascular permeability and oedema formation when given into diverse tissues (Ishii et al., 1998; Ishii & Shimizu, 2000). To avoid an interference of PAF-induced oedema formation on the effects of des-Arg9-BK, all experiments evaluating functional oedematous response were conducted 6 h after PAF injection, as by this time PAF-induced oedema is resolved (Cordeiro et al., 1986). Our results showed that the paw oedema elicited by des-Arg9-BK was significant between 6 and 24 h following PAF treatment. This was a PAF receptor-dependent event, as it was markedly attenuated by concomitant injection of the PAF receptor antagonist WEB-2086 or by PAF receptor desensitisation. Moreover, the event was restricted to the site of injection, as i.d. injection of PAF failed to induce the increase of des-Arg9-BK-mediated oedema in the contralateral paw. The effects of PAF appeared to be specific for B1 receptors, as the same treatment with PAF failed to modify paw oedema formation induced by the selective B2 receptor agonist Tyr8-BK. Furthermore, oedema induced by des-Arg9-BK was almost completely blocked by the coinjection of two selective B1 receptor antagonists des-Arg9 [Leu8]-BK and R-715. Upregulation of B1, without a significant change of B2 receptor-mediated oedematogenic responses, has also been demonstrated after treatment with attenuated Mycobacterium bovis bacillum (BCG) or with the proinflammatory cytokines IL-1β and TNFα (Campos et al., 1997; 1998). In contrast, a significant reduction of Tyr8-BK-caused paw oedema has been observed in other conditions, such as following the systemic treatment with LPS or in streptozotocin-diabetic rats (Campos et al., 1996; 2001), demonstrating the plasticity of B1 receptor expression. Altogether, the results above provide strong pharmacological evidence that PAF, by acting on PAF receptors, induce the functional upregulation of B1.
Not only was a B1 receptor-dependent functional response upregulated after PAF injection, but we could also demonstrate a time-dependent increase of B1 receptor mRNA expression. These data show for the first time that treatment with PAF is able to evoke de novo synthesis of B1 receptors in the rat paw. A similar result has been demonstrated following the local treatment with the proinflammatory cytokine IL-1β (Campos et al., 2002). The importance of protein synthesis involvement in this model was reinforced by the results showing that PAF-induced upregulation of a functional response to des-Arg9-BK injection was markedly inhibited by the anti-inflammatory steroid dexamethasone and by the protein synthesis inhibitor cycloheximide. The same schedule of treatment with these two drugs was effective in blocking B1 receptor functional upregulation induced by other stimuli (Campos et al., 1996, 1997, 1998). Thus, our results suggest that the functional upregulation of B1 receptors relies heavily on local protein synthesis.
Several studies have now reported on the ability of PAF to stimulate neutrophil influx to sites of inflammation (Zhou et al., 1994; Franciose et al., 1996; Ishii & Shimizu, 2000). In addition, it has been shown that some proinflammatory effects of LPS or IL-1β are mediated by the endogenous release of PAF (Han et al., 2002). One could speculate that PAF, along with other chemotatic factors, might be involved in the upregulation of B1 receptors during the inflammatory response. In fact, we have previously demonstrated that IL-1β-induced B1 receptors upregulation in the rat paw is a process dependent on adhesion molecules, PAF receptor activation and neutrophil migration (Campos et al., 2002). In order to examine the possible involvement of the influx of neutrophils for the ability of PAF to induce the molecular and functional upregulation of B1 receptors, initial experiments evaluated the ability of PAF to induce neutrophil recruitment in the rat paw. The injection of PAF induced a striking neutrophil accumulation, as assessed by an increase of up to 25-fold in MPO activity in the rat paw. Neutrophil influx and functional B1 receptor expression were markedly prevented by fucoidin and by an anti-CD18 monoclonal antibody, demonstrating the importance of cell adhesion molecules for both processes. More importantly, data using anti-rat neutrophil anti-serum suggested that the leucocytes responsible for the upregulation of B1 receptors were neutrophils. Thus, it is clear that drugs that prevented neutrophil accumulation also interfered with the functional upregulation of B1 receptors. Together with other data from the literature (Ahluwalia & Perretti, 1996; McLean et al., 2000; Campos et al., 2002), the present results suggest that the recruitment of neutrophils is one of the prerequisites for the upregulation of B1 receptors in inflamed tissues.
It has been reported that PAF is capable of activating the NF-κB and enhancing DNA-binding activity in vivo or in vitro (De Plaen et al., 1998, 2000; Choi et al., 2000), and PAF-induced angiogenic response is markedly inhibited by antisense oligonucleotides to NF-κB subunits (Ko et al., 2002). In addition, LPS-induced NF-κB activation is partially dependent on PAF receptor activation (De Plaen et al., 2000). As the transcription factor NF-κB has been shown to be involved in the upregulation of B1 receptors under various experimental conditions, a series of experiments were conducted to evaluate its participation in our system. Our results clearly show that PAF treatment promoted a marked increase of NF-κB translocation in the rat paw, as assessed by the eletrophoretic mobility shift assay. PAF-induced NF-κB activation was prevented by the treatment with two NF-κB blockers, PDTC and TLCK. More importantly, the injection of PDTC prior to PAF prevented the functional upregulation of B1 receptors. Together, these results demonstrate a role for NF-κB activation in PAF-induced functional upregulation of B1 receptors in the rat paw.
Interestingly, the treatment with the anti-rat neutrophil antiserum also attenuated the activation of NF-κB 3 h after injection of PAF, implicating the influx of neutrophils in the activation of NF-κB at later time points. On the other hand, NF-κB activation was already present before the first wave of neutrophil recruitment (compare Figures 6 and 7) and PAF-mediated neutrophil influx was markedly inhibited by PDTC. The latter results suggest that activation of NF-κB was part of a series of events leading to neutrophil migration into the rat paw. Thus, it appears that the reciprocal activation of NF-κB and neutrophil influx amplify each other and, together, promote the molecular and functional upregulation of B1 receptors.
In conclusion, our results indicate that the local treatment with PAF induced kinin B1 receptor upregulation in the rat paw by a mechanism that involved an amplification circuit between the transcription factor NF-κB and the recruitment of neutrophils. It appears that the recruitment of leucocytes is a pivotal signal for the regulation of B1 receptor-mediated inflammatory responses.
Acknowledgments
This work was supported by grants from CNPq, CAPES, FINEP e PRONEX (Brazil). G.F.P. and E.S.S. are undergraduate and PhD students receiving a grant from CNPq and CAPES, respectively. M.M. Campos holds a Post-doctoral Fellowship from CAPES.
Abbreviations
- des-Arg9-BK
des-Arg9-bradykinin
- MPO
myeloperoxidase
- NF-κB
nuclear factor κB
- PAF
platelet activating factor
- PBS
phosphate-buffered saline
- PDTC
pyrrolidine-dithiocarbamate
- TLCK
Nα-tosyl-L-chloromethylketone
- Tyr8-BK
tyrosine8-bradykinin
References
- ADAMS J.K., TEPPERMAN B.L. Colonic production and expression of IL-4, IL-6, and IL-10 in neonatal suckling rats after LPS challenge. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G755–G762. doi: 10.1152/ajpgi.2001.280.4.G755. [DOI] [PubMed] [Google Scholar]
- AHLUWALIA A., PERRETTI M. Involvement of bradykinin B1 receptors in the polymorphonuclear leukocyte accumulation induced by IL-1β in vivo in the mouse. J. Immunol. 1996;156:269–274. [PubMed] [Google Scholar]
- BHOOLA K.D., FIGUEROA C.D., WORTHY K. Bioregulation of kinins: kallikreins, kininogens and kininases. Pharmacol. Rev. 1992;44:1–80. [PubMed] [Google Scholar]
- BÖCKMANN S., PAEGELOW I. Kinins and kinin receptors: importance for the activation of leukocytes. J. Leukoc. Biol. 2000;68:587–592. [PubMed] [Google Scholar]
- CABRINI D.A., CAMPOS M.M., TRATSK K.S., MERINO V.F., SILVA J.A., JR, SOUZA G.E., AVELLAR M.C., PESQUERO J.B., CALIXTO J.B. Molecular and pharmacological evidence for modulation of kinin B(1) receptor expression by endogenous glucocorticoids hormones in rats. Br. J. Pharmacol. 2001;132:567–577. doi: 10.1038/sj.bjp.0703846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALIXTO J.B., CABRINI D.A., FERREIRA J., CAMPOS M.M. Kinins in pain and inflammation. Pain. 2000;87:1–5. doi: 10.1016/S0304-3959(00)00335-3. [DOI] [PubMed] [Google Scholar]
- CALIXTO J.B., CABRINI D.A., FERREIRA J., CAMPOS M.M. Inflammatory pain: kinins and antagonists. Curr. Opin. Anaesthesiol. 2001;14:519–526. doi: 10.1097/00001503-200110000-00010. [DOI] [PubMed] [Google Scholar]
- CAMPOS M.M., CABRINI D.A., CARDOZO A.H.M., RAE G.A., HUIDOBRO TORO J.-P., CALIXTO J.B. Changes in paw oedema triggered by bradykinin B1 and B2 receptors in streptozotocin-diabetic rats. Eur. J. Pharmacol. 2001;416:169–177. doi: 10.1016/s0014-2999(01)00883-4. [DOI] [PubMed] [Google Scholar]
- CAMPOS M.M., CALIXTO J.B. Involvement of B1 and B2 receptors in bradykinin-induced rat paw oedema. Br. J. Pharmacol. 1995;114:1005–1013. doi: 10.1111/j.1476-5381.1995.tb13305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPOS M.M., HENRIQUES M.G.M.O., CALIXTO J.B. The role of B1 and B2 receptors in oedema formation after long-term treatment with Mycobacterium bovis bacillus Calmette-Guérin (BCG) Br. J. Pharmacol. 1997;120:502–508. doi: 10.1038/sj.bjp.0700914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPOS M.M., SOUZA G.E.P., CALIXTO J.B. Up-regulation of B1 mediating des-Arg9-BK-induced rat paw oedema by systemic treatment with bacterial endotoxin. Br. J. Pharmacol. 1996;117:793–798. doi: 10.1111/j.1476-5381.1996.tb15262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPOS M.M., SOUZA G.E.P., CALIXTO J.B. Modulation of kinin B1 receptor-mediated rat paw oedema by IL-1β and TNFα. Peptides. 1998;19:1269–1276. doi: 10.1016/s0196-9781(98)00087-4. [DOI] [PubMed] [Google Scholar]
- CAMPOS M.M., SOUZA G.E.P., CALIXTO J.B. In vivo B1 kinin-receptor up-regulation. Evidence for involvement of protein kinases and nuclear factor-κB pathways. Br. J. Pharmacol. 1999;127:1851–1859. doi: 10.1038/sj.bjp.0702715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPOS M.M., SOUZA G.E.P., RICCI N.D., PESQUERO J.L., TEIXEIRA M.M., CALIXTO J.B. The role of migrating leukocytes in IL-1β-induced up- regulation of kinin B1 receptors in rats. Br. J. Pharmacol. 2002;135:1107–1114. doi: 10.1038/sj.bjp.0704488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAO W., OLSON M.S. Platelet-activating factor: receptors and signal transduction. Biochem. J. 1993;292:617–629. doi: 10.1042/bj2920617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOI J.H., CHUNG W.J., HAN S.J., LEE H.B., CHOI I.W., LEE H.K., JANG K.Y., LEE D.G., HAN S.S., PARK K.H., IM S.Y. Selective involvement of reactive oxygen intermediates in platelet-activating factor-mediated activation of NF-kappaB. Inflammation. 2000;24:385–398. doi: 10.1023/a:1007068010645. [DOI] [PubMed] [Google Scholar]
- CORDEIRO R.S., MARTINS M.A., SILVA P.M., FARIA NETO H.C., CASTANHEIRA J.R., VARGAFTIG B.B. Desensitization to PAF-induced rat paw oedema by repeated intraplantar injections. Life Sci. 1986;39:1871–1878. doi: 10.1016/0024-3205(86)90297-3. [DOI] [PubMed] [Google Scholar]
- COUTURE R., HARRISSON M., VIANNA R.M., CLOUTIER F. Kinin receptors in pain and inflammation. Eur. J. Pharmacol. 2001;429:161–176. doi: 10.1016/s0014-2999(01)01318-8. [DOI] [PubMed] [Google Scholar]
- D'ACQUISTO F., ALENTI A., IANARO A., CARNUCCIO R. Nuclear factor-kappaB activation mediates inducible nitric oxide synthase expression in carrageenin-induced rat pleurisy. Naunyn Schmiedebergs Arch. Pharmacol. 1999;360:670–675. doi: 10.1007/s002109900149. [DOI] [PubMed] [Google Scholar]
- DE PLAEN I.G., TAN X.D., CHANG H., QU X.W., LIU Q.P., HSUEH W. Intestinal NF-κB is activated, mainly as p50 homodimers, by platelet-activating factor. Biochim. Biophys. Acta. 1998;1392:185–192. doi: 10.1016/s0005-2760(98)00024-1. [DOI] [PubMed] [Google Scholar]
- DE PLAEN I.G., TAN X.D., CHANG H., WANG L., REMICK D.G., HSUEH W. Lipopolysaccharide activates nuclear factor kappaB in rat intestine: role of endogenous platelet-activating factor and tumor necrosis factor. Br. J. Pharmacol. 2000;129:307–314. doi: 10.1038/sj.bjp.0703055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANGELOU A.M. Platelet-activating factor (PAF): implications for coronary heart and vascular diseases. Prostaglandins Leukot. Essent. Fatty Acids. 1994;50:1–28. doi: 10.1016/0952-3278(94)90101-5. [DOI] [PubMed] [Google Scholar]
- FRANCIOSE R.J., MOORE F.A., READ R.A., CARL V.S., BANERJEE A. Hypoxia/reoxygenation of human endothelium activates PMNs to detach endothelial cells via a PAF mechanism. J. Surg. Res. 1996;61:459–462. doi: 10.1006/jsre.1996.0146. [DOI] [PubMed] [Google Scholar]
- HAN S.J., KO H.M., CHOI J.H., SEO K.H., LEE H.S., CHOI E.K., CHOI I.W., LEE H.K., IM S.Y. Molecular mechanisms for lipopolysaccharide-induced biphasic activation of nuclear factor-kappa B (NF-kappa B) J. Biol. Chem. 2002;277:44715–44721. doi: 10.1074/jbc.M202524200. [DOI] [PubMed] [Google Scholar]
- ISHII I., SAITO E., TAKASHI I., UI M., SHIMIZU T. Agonist-induced sequestration, recycling and resensitization of platelet-activating factor: role of citoplasmic tail phosphorylation in each process. J. Biol. Chem. 1998;273:9878–9885. doi: 10.1074/jbc.273.16.9878. [DOI] [PubMed] [Google Scholar]
- ISHII S., SHIMIZU T. Platelet-activating factor (PAF) receptor and genetically engineered PAF receptor mutant mice. Prog. Lip. Res. 2000;39:41–82. doi: 10.1016/s0163-7827(99)00016-8. [DOI] [PubMed] [Google Scholar]
- KO H.M., SEO K.H., HAN S.J., AHN K.Y., CHOI I.H., KOH G.Y., LEE H.K., RA M.S., IM S.Y. Nuclear factor kappaB dependency of platelet-activating factor-induced angiogenesis. Cancer Res. 2002;62:1809–1814. [PubMed] [Google Scholar]
- KORTH R.M., HIRAFUJI M., BEVENISTE J., RUSSO-MARIE F. Human umbilical vein endothelial cells: specific binding of platelet-activating factor and cytosolic calcium flux. Biochem. Pharmacol. 1995;49:1793–1799. doi: 10.1016/0006-2952(95)00025-u. [DOI] [PubMed] [Google Scholar]
- LARRIVÉ J.F., BACHVAROV D.R., HOULE F., LANDRY J., HUOT J., MARCEAU F. Role of mitogen-activated protein kinases in the expression of the B1 kinin receptors induced by tissue injury. J. Immunol. 1998;160:1419–1426. [PubMed] [Google Scholar]
- MARCEAU F. Kinin B1 receptors: a review. Immunopharmacology. 1995;30:1–26. doi: 10.1016/0162-3109(95)00011-h. [DOI] [PubMed] [Google Scholar]
- MARCEAU F., BACHVAROV D.R. Kinin receptors. Clin. Rev. Allergy Inflamm. 1998;16:385–401. doi: 10.1007/BF02737658. [DOI] [PubMed] [Google Scholar]
- MARCEAU F., HESS J.F., BACHVAROV D.R. The B1 receptors for kinins. Pharmacol. Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- MCINTYRE T.M., MODUR V., PRESCOTT S.M., ZIMMERMANN G.A. Molecular mechanisms of early inflammation. Thromb. Haem. 1997;78:302–305. [PubMed] [Google Scholar]
- MCLEAN P.G., AHLUWALIA A., PERRETTI M. Association between kinin B1 receptor expression and leukocyte trafficking across mouse mesenteric 7postcapillary venules. J. Exp. Med. 2000;192:367–380. doi: 10.1084/jem.192.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON R., EDDLESTON J., HADDAD E.B., HAWISA S., MAK J., LIM S., FOX A.J., DONELLY L.E., CHUNG K.F. Regulation of kinin receptors in airway epithelial cells by inflammatory cytokines and dexamethasone. Eur. J. Pharmacol. 2002;441:193–202. doi: 10.1016/s0014-2999(01)01624-7. [DOI] [PubMed] [Google Scholar]
- PINHEIRO R.M., CAMPOS M.M., CALIXTO J.B. Analysis of the mechanisms involved in the inflammatory response induced by des-Arg9-bradykinin in the rat pleural cavity. Inflamm. Res. 2001;50:570–576. doi: 10.1007/PL00000235. [DOI] [PubMed] [Google Scholar]
- RICHARDSON R.M., HARIBABU B., ALI H., SNYDERMAN R. Cross-desensitization among receptors for platelet-activating factor and peptide chemoattractants: evidence for independent regulatory pathways. J. Biol. Chem. 1996;271:28717–28724. doi: 10.1074/jbc.271.45.28717. [DOI] [PubMed] [Google Scholar]
- SCHANSTRA J.P., BATAILLÉ E., CASTANÕ M.E.M., BARASCUD Y., HIRTZ C., PESQUERO J.B., PECHER C., GAUTHIER F., GIROLAMI J.P., BASCANDS J.L. The B1-agonist [des-Arg10]-kallidin activates transcription factors NF-κB and induces homologous up-regulation of the bradykinin B1-receptor in cultured human lung fibroblasts. J. Clin. Invest. 1998;101:2080–2091. doi: 10.1172/JCI1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARMA J.N., BUCHANAN W.W. Pathogenic responses of bradykinin system in chronic inflammatory rheumatoid disease. Exp. Toxicol. Pathol. 1994;46:421–433. doi: 10.1016/S0940-2993(11)80053-9. [DOI] [PubMed] [Google Scholar]
- SOARES A.C, PINHO V.S., SOUZA D.G., SHIMIZU T., ISHII S., NICOLI J.R., TEIXEIRA M.M. Role of the platelet-activating factor (PAF) receptor during pulmonary infection with gram negative bacteria. Br. J. Pharmacol. 2002;137:621–628. doi: 10.1038/sj.bjp.0704918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUZA D.G., COUTINHO S.F., SILVEIRA M.R., CARA D.C., TEIXEIRA M.M. Treatment with a leukotriene B4 receptor antagonist inhibitis local and remote reperfusion injury after transient ischemia of the superior mesenteric artery in the rat. Eur. J. Pharmacol. 2000;403:121–314. doi: 10.1016/s0014-2999(00)00574-4. [DOI] [PubMed] [Google Scholar]
- TEIXEIRA M.M., HELLEWELL P.G. The effect of the selectin binding polysaccharide fucoidin on eosinophil recruitment in vivo. Br. J. Pharmacol. 1997;120:1059–1066. doi: 10.1038/sj.bjp.0701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU W., JAVORS M.A., OLSON M.S. Impaired surface expression of PAF receptors on human neutrophils is dependent upon cell activation. Arch. Biochem. Biophys. 1994;308:439–445. doi: 10.1006/abbi.1994.1062. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;6:109. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]