Abstract
Endothelin-1 (ET-1) and tumor necrosis factor α (TNFα) by their action on adipocytes have been independently linked to the pathogenesis of insulino-resistance. In isolated adipocytes, TNFα induces the expression of the inducible nitric oxide synthase (iNOS). The purpose of the present work was, in the 3T3-F442A adipocyte cell line, to characterise TNFα-induced iNOS expression and to determine whether or not ET-1 could influence TNFα-induced iNOS expression and NO production.
In differentiated 3T3-F442A, treatment with TNFα (20 ng ml−1) induced the expression of a functional iNOS as demonstrated by nitrite assay, Western blot, reverse transcription–polymerase chain reaction and Northern blot analysis. TNFα-induced iNOS expression requires nuclear factor κB activation, but does not necessitate the activation of the PI-3 kinase/Akt and P38–MAP kinase pathways.
ET-1, but not ET-3, inhibited the TNFα-induced expression of iNOS protein and mRNA as well as nitrite production. The effects of ET-1 were blocked by a specific ETA (BQ123, pA2 7.4) but not by a specific ETB receptor antagonist (BQ788). 3T3-F442A adipocytes express the mRNAs for prepro-ET-1 and the ET-A receptor subtype, but not for the ET-B subtype.
The inhibitory effect of ET-1 was not affected by bisindolylmaleimide, SB 203580 or indomethacin, inhibitors of protein kinase C, p38-MAP kinase and cyclooxygenase, respectively, and was not associated with cAMP production. However, the effect of ET-1 was partially reversed by wortmannin, suggesting the involvement of PI3 kinase in the transduction signal of ET-1.
Differentiated 3T3-F442A adipocytes did not release ET-1 with or without exposure to TNFα, although the mRNA for preproET-1 was detected in both pre- and differentiated adipocytes.
Thus, these results confirm that adipocytes are a target for circulating ET-1 and demonstrate that the activation of the ETA receptor subtype can prevent TNFα-induced iNOS expression.
Keywords: Adipocyte, TNFα, iNOS expression, endothelin, ETA receptor subtype, insulin resistance
Introduction
Endothelin-1 (ET-1) is a 21-amino acids vasoactive peptide that was originally isolated from cultured porcine aortic endothelial cells (Yanagisawa et al., 1988). At least three closely related peptides termed ET-1, ET-2 and ET-3 have been identified. These peptides share sequence homology and arise through proteolytic processing (Russell & Davenport, 1999). In mammals, two ET receptor subtypes, coupled to heterotrimeric G proteins, have been cloned and characterised as ET-A and ET-B (Arai et al., 1990; Sakurai et al., 1990). ET-1 is a nonselective agonist of both ETA and ETB receptor subtypes, while ET3 is a preferential agonist of the ETB receptor subtype. ET-1 is one of the most potent vasoconstrictors identified to date (Rubanyi & Polokoff, 1994). However, besides its vascular effects, ET-1 exerts a wide variety of actions on many tissues including white adipose tissue. Indeed, ET-1 reduces lipoprotein lipase activity (Ishida et al., 1992), inhibits the differentiation of preadipocytes to adipocytes (Tanahashi et al., 1991; Hauner et al., 1994), stimulates glucose uptake (Imamura et al., 1999; Wu-Wong et al., 1999) and leptin production (Xiong et al., 2001). Elevated plasma ET-1 levels are associated with insulin resistance and have been observed in patients with type II diabetes (Takahashi et al., 1990; Kawamura et al., 1992), obesity (Ferri et al., 1995) or hypertension (Saito et al., 1990). Although ET-1 acutely stimulates glucose uptake, chronic treatment reduces insulin-dependent glucose uptake in both 3T3-L1 adipocyte and skeletal muscle (Ottosson-Seeberger et al., 1997; Ishibashi et al., 2000,2001). These observations suggest that ET-1 could be involved in the pathogenesis of insulin resistance.
Tumour necrosis factor-α (TNFα) by its action on adipocytes is also a key player in the generation of insulin resistance (Hotamisligil, 2000). In adipocyte cell lines such as the 3T3-F442A or 3T3-L1, TNFα, alone or in combination with other cytokines, induces the expression of the inducible isoform of nitric oxide synthase (iNOS) (Kapur et al., 1999; Merial et al., 2000). Targeted disruption of iNOS protects against obesity-linked insulin resistance (Perreault & Marette, 2001). Therefore, the production of TNFα and the induction of iNOS could also be one of the pathways involved in the establishment of syndrome X-linking obesity, type II diabetes and hypertension.
In various cell types (HUVEC, monocytes, Kupffer and ciliary epithelial cells), ET-1 is also able to induce iNOS expression (Stephenson et al., 1997; Schena et al., 1999; Prasanna et al., 2000). Furthermore, the induction of iNOS, by TNFα or other cytokines, could be positively or negatively regulated by ET-1 (glial, vascular smooth muscle or mesangial cells, Beck et al., 1995; Hirahashi et al., 1996; Ikeda et al., 1997; Oda et al., 1997; Murayama et al., 1998). However, in adipocytes, the effect of ET-1 on iNOS expression remains unknown.
In order to further substantiate a potential role of ET-1 in the pathogenesis of insulin resistance and syndrome X, the purpose of the present work was to determine, in an adipocyte cell line that does express iNOS in response to TNFα, the 3T3-F442A (Merial et al., 2000), whether or not this peptide could induce iNOS expression or modulate TNFα-induced iNOS expression and NO production and whether or not these adipocytes could produce ET-1.
Methods
Experiments were performed on 3T3-F442A cells. These cells are unipotent and fibroblast-like cells which undergo differentiation to mature fat cells filled with large lipid droplets when stimulated with 10% foetal bovine serum (FBS)+insulin. In this cell line, TNFα leads to a time- and concentration-dependent increase in iNOS induction (Mérial et al., 2000).
Cell culture
The 3T3-F442A preadipocyte cell line (passage 5–15) was cultured until confluence in a medium consisting of Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% donor calf serum and an antibiotic mixture (500 U ml−1 penicillin and 50 μg ml−1 streptomycin) in a 95% air–5% CO2 humidified atmosphere at 37°C. At confluence, cells were differentiated by incubation in DMEM supplemented with 10% foetal calf serum and 100 nM insulin. The medium was changed every other day. At 10 days after differentiation, cells were maintained overnight in a serum-deprived medium containing 0.1% bovine serum albumin (BSA) and treated as described in the Results section. After treatment, supernatants were collected for nitrite determination and cells were stored at −20°C until analysis.
Measurements of nitrite production
Nitrite concentration was assessed in cell supernatants using a kit based on the Griess reaction (Cayman Chemicals, Ann Arbor, MI, U.S.A.). After 10 min at room temperature, absorbance was read at a wavelength of 540 nm (iEMS, Labsystem, France). The nitrite concentrations were calculated from a standard curve obtained with increasing concentrations of NaNO2 (0–35 μM).
Western blot analysis
Cells were washed twice with phosphate-buffered saline and collected. After brief centrifugation (1000 × g, 2 min, 4°C), cell pellets were resuspended in 200 μl of lysis buffer (RIPA buffer, Roche, Meylan Cedex, France). The lysate was then centrifuged at 13,000 × g for 15 min at 4°C and the protein concentration of the supernatant was determined using the method of Lowry. For iNOS detection, 70 μg proteins were resolved by electrophoresis on 3–8% Tris-acetate gels (NuPage, Invitrogen, Groningen, The Netherlands) under denaturing conditions. After transfer to nitrocellulose membranes (Amersham Pharmacia Biotech, Saint-Quentin en Yvelines, France) and Ponceau staining to verify equal loading of the lanes, membranes were blocked overnight in 10 mM Tris-HCl, 100 mM NaCl, 0.15% Tween-20 and 5% milk fat at 4°C and then incubated at room temperature with the primary antibody (polyclonal mouse anti-iNOS diluted 1 : 5000, BD Transduction Laboratories, Lexington, KY, U.S.A.) for 90 min. After three washes, nitrocellulose membranes were incubated with the secondary antibody (anti-mouse immunoglobulin conjugated with horse-radish peroxidase diluted 1 : 2000, Amersham Pharmacia Biotech) for 45 min. Immunoblots were washed three times and the immunocomplexes were detected using a chemiluminescence reagent kit (ECL plus, Amersham Pharmacia Biotech). A protein extract from murine macrophages (RAW 264.7) treated with a mixture of interferonγ (IFNγ, 10 ng ml−1) plus lipopolysaccharide (LPS 1 μg ml−1) was used as a positive control for iNOS.
Extraction of RNA and reverse transcription–polymerase chain reaction
Total RNAs were extracted with the RNAqueous-4 PCR protocol (Ambion, Austin, Texas, U.S.A.) and 2 μg were reverse-transcribed with oligo dT in accordance with the first-strand cDNA synthesis protocol from Amersham Pharmacia Biotech (Saint-Quentin, France). PCR reactions were performed in 100 μl of a solution containing 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 0.2 mM dNTP, 2 μl of single-strand cDNA preparation, 0.3 μM of each primer and 2 units of Amplitaq gold polymerase (Perkin-Elmer, Courtaboeuf, France). A 30-cycle program at 94°C for 1 min, 60°C for 2 min and 72°C for 2 min, a hot start at 94°C for 9 min and a final extension at 72°C for 8 min was performed. The forward and reverse PCR primers were specific to the positions 1819–1842 and 2347–2370 for mouse iNOS (Kone et al., 1995). The other used primers were localised to the positions 166–189 and 495–518 for mppET-1 (accession number: AB081657), to the positions 999–1020 and 1546–1570 for mETAR (accession number: BC008277) and to the positions 653–674 and 1219–1240 for mETBR (accession number: BC026553). The PCR fragments were analysed by electrophoresis on a 1.5% agarose gel, transferred onto a Hybond N+ membrane and hybridised with α32P-dCTP radiolabelled oligonucleotide using terminal transferase (Amersham Pharmacia Biotech). The probe containing the nucleotides 2065–2086 of the iNOS sequence was used. The specific probes of mppET-1, mETAR and mETBR correspond to the nucleotides 319–342 of the mppET-1 sequence, 1320–1342 of the mETAR sequence and 1069–1090 of the mETBR sequence, respectively. Membranes were hybridised for 16 h at 42°C in a buffer containing a five-fold concentrated standard saline citrate, five-fold concentrated Denhardt's solution, 100 μg ml−1 tRNA and 0.1% w v−1 SDS. After two washes at 50°C for 30 min in SSC 2X supplemented with 0.1% w v−1 SDS, membranes were exposed to BioMax MS film (Kodak, Châlon-sur-saône, France) for 1 h at −70°C with two intensifying screens. G3PDH primers and probes (Clontech) were used in order to verify equal loading on lanes.
Northern blot analysis
After extraction, total RNA was dissolved in a solution containing 50% (v v−1) formamide, 2 M formaldehyde, 40 mM 3-N-morpholinopropanesulphonic acid (MOPS, pH 7), 10 mM sodium acetate, 1 mM EDTA, 0.01% bromophenol blue and xylene cyanol. RNA samples were heated for 10 min at 65°C and then loaded onto 1% denaturated agarose gel. After electrophoresis, mRNAs were transferred to a Hybond N+ membrane in the presence of SSC 10 ×. The membrane was incubated for 16 h at 68°C in Express Hyb solution (Clonetech, Basingstoke, U.K.) containing 106 cpm ml−1 of specific radiolabelled probes (iNOS and β-actin cDNA). The membranes were then washed two times in SSC 2 × supplemented with 0.1% SDS for 5 min at 35°C and two times in SSC 0.1 × supplemented with 0.1% SDS for 30 min at 65°C. The radioactivity was measured with a fluorescent image analyser FLA-200 (FujiFilm Co., Tokyo, Japan). For each lane, the radioactivity was normalised with the corresponding signal obtained for β-actin.
ET-1 determination
Cells were treated or not in the presence of TNFα (20 ng ml−1, 20 h) and/or phosphoramidon (6 h, 100 μM; a nonselective inhibitor of the putative ET-1-converting enzyme) and the accumulation of ET-1 was measured in the supernatant. After treatment, the culture medium was separated in 100 μl aliquots and frozen at −20°C until assay. ET-1 levels were determined with an enzyme-linked immunosorbent assay kit (Amersham Pharmacia Biotech). At the same time, positive controls were performed with two cell lines: human umbilical vein endothelial cells and the human epithelial cell line (A549) which produce a phosphoramidon-sensitive and time-dependent accumulation of ET-1 in the culture medium (Deprez-Roy et al., 2000). The specificity of the assay was verified with ET-1, ET-2, ET-3, big-ET-1 (1–38), Big ET-1 C-terminal fragment (22–38), ET-1 C-terminal fragment (11–21) and a fragment which includes the cleavage site (19–26). The kit recognised both ET-1 and ET-2 but did not crossreact with the other peptides tested (<3%).
Cyclic AMP determination
For cyclic AMP assays, after treatment, cells were collected in HCl (0.1 N) and then frozen at −20°C. Samples were first acetylated by the addition of acetic anhydride/triethylamine (1 : 2) and cyclic AMP levels were assessed by immunoassay (R&D System, Abington, U.K.).
Cell viability
The cell viability after treatments was measured by detection of lactate dehydrogenase (LDH) activity in the culture medium by the use of a cytotoxicity detection kit (Cytotoxicity Detect kit, Roche, Meylan cedex). In the present work, none of the compounds, under similar experimental conditions at their highest concentrations, increased LDH activity in 3T3-F442A differentiated adipocytes.
Materials
All chemicals were purchased from Sigma (Saint-Quentin Fallavier, France). DMEM and donor calf serum were obtained from Life Technologies (Cergy Pontoise, France) and foetal calf serum came from AbCys (Paris, France). Proteins were assessed by the kit Bio-Rad DC Protein (Bio-Rad Laboratories, Ivry/Seine, France).
Statistical analysis
Values are expressed as means±standard error (s.e.m.) and were analysed by a one-way ANOVA followed by a Dunnett's test for multiple comparison. n indicates the number of independent experiments (different passage) performed in duplicate or quadruplicate. P was considered significant when <0.05. The ED50 (concentrations of endothelin causing half-maximal inhibition of TNFα-induced nitrite production) were calculated by using the Michaelis–Menten equation and nonlinear regression that included all data points. The pA2 value was calculated following Tallarida's method (Tallarida et al., 1979).
Results
TNFα-induced iNOS expression in 3T3-F442A
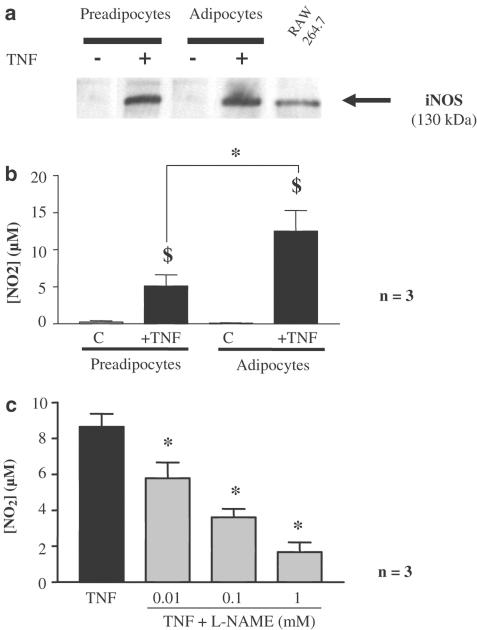
In response to TNFα (20 ng ml−1, 20 h) both preadipocytes and differentiated adipocytes expressed iNOS protein as observed by Western blot (Figure 1a). The iNOS expression was associated with nitrite production in the cell supernatant. A significant increase in nitrite concentration was observed after TNFα treatment in both preadipocytes and differentiated adipocytes, but the nitrite accumulation was significantly larger in differentiated cells than in preadipocytes (P<0.01, Figure 1b). This accumulation of nitrites was significantly inhibited in a concentration-dependent manner by L-nitroarginine methyl ester (L-NAME: 10 μM–1 mM, Figure 1c) and abolished by aminoguanidine (1 mM) or S-methyl-isothiourea (1 mM) (data not shown).
Figure 1.
TNFα-induced iNOS expression in 3T3-F442A. (a) Preadipocytes and 10 days-differentiated 3T3-F442A cells were treated or not for 20 h with TNFα (20 ng ml−1). Protein extracts were analysed by Western blot using specific NOS antibodies. Representative blots from three independent experiments are shown. A protein extract from murine macrophages (RAW 264.7) treated with a mixture of IFNγ (10 ng ml−1) plus LPS (1 μg ml−1) was used as a positive control for iNOS. (b) TNFα-induced nitrite production on preadipocytes and differentiated adipocytes. Results are expressed as mean±s.e.m. from three independent experiments performed in duplicate. $ indicates a statistically significant difference between control and TNFα-treated cells, while * indicates a statistically significant difference between preadipocytes and differentiated adipocytes (one-way ANOVA followed by a Dunnett's post hoc test, P<0.05). (c) TNFα-induced nitrite production in differentiated adipocytes and NO-synthase inhibition. Results are expressed as mean±s.e.m. from three independent experiments performed in duplicate. * indicates a statistically significant difference induced by the treatment with L-NAME (one-way ANOVA followed by a Dunnett's post hoc test, P<0.05). Complete inhibition of TNFα-induced nitrite production was also obtained with aminoguanidine (1 mM) or S-methyl-isothiourea (1 mM; data not shown).
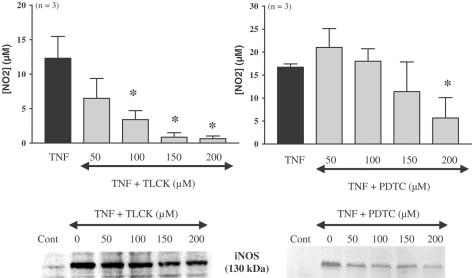
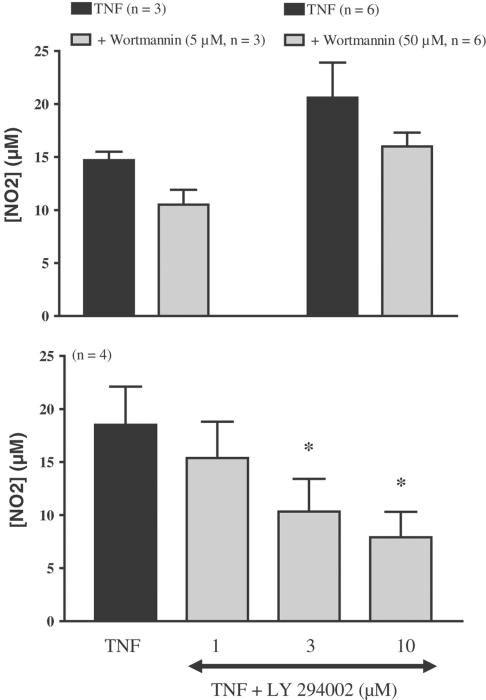
In differentiated adipocytes pretreated 1 h before the induction of iNOS by TNFα (10 ng ml−1, 20 h) with either tosyllysine chloromethylketone (TLCK, 50–200 μM) or pyrrolidine dithiocarbamate (PDTC, 50–200 μM), two inhibitors of nuclear factor κB (NF-κB), the nitrite accumulation and the iNOS protein expression were inhibited in a concentration-dependent manner. This inhibition was statistically significant for concentrations of 100 μM and above for TLCK, but only at the highest concentration tested for PDTC (Figure 2). Under the same experimental conditions, an inhibitor of p38 MAP kinase, SB 2033580 (1 and 10 μM) and a PI-3 kinase inhibitor, wortmannin (5 and 50 μM) did not affect TNFα-induced NO production (Figure 3) and iNOS expression (data not shown). In contrast, a structurally different PI-3 kinase inhibitor, LY 294002 (1, 3 and 10 μM) produced a concentration-dependent inhibition of TNFα-induced nitrite production that was statistically significant at the two highest concentrations tested (Figure 3), but this compound did not affect the iNOS expression (data not shown).
Figure 2.
TNFα-induced iNOS expression and involvement of NF-κB in differentiated 3T3-F442A adipocytes. Differentiated 3T3-F442A adipocytes were treated or not for 20 h with TNFα in the presence of increasing concentrations of two NF-κB inhibitors, TLCK (50–200 μM) or PDTC (50–200 μM). Nitrites were assessed in cell supernatants. Results are expressed as mean±s.e.m. from three independent experiments performed in duplicate. * indicates a statistically significant difference versus TNFα-treated cells (one-way ANOVA followed by Dunnett's post hoc test, P<0.05). iNOS protein was detected by Western blot. A representative autoradiography from three independent experiments performed in duplicate is shown.
Figure 3.
TNFα-induced iNOS expression and involvement of PI3-kinase in differentiated 3T3-F442A adipocytes. Differentiated 3T3-F442A adipocytes were treated or not for 20 h with TNFα in the presence of increasing concentrations of two PI3-kinase inhibitors, wortmannin (5 and 50 μM, top panel) and LY 294002 (1–10 μM, bottom panel). Nitrites were assessed in cell supernatants. Results are expressed as mean±s.e.m. from three to six independent experiments performed in duplicate. * indicates a statistically significant difference versus TNFα-treated cells (one-way ANOVA followed by Dunnett's post hoc test, P<0.05).
ET-1 and TNFα-induced iNOS expression in differentiated 3T3-F442A
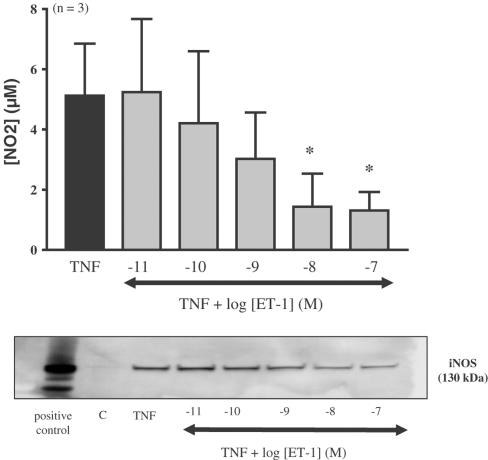
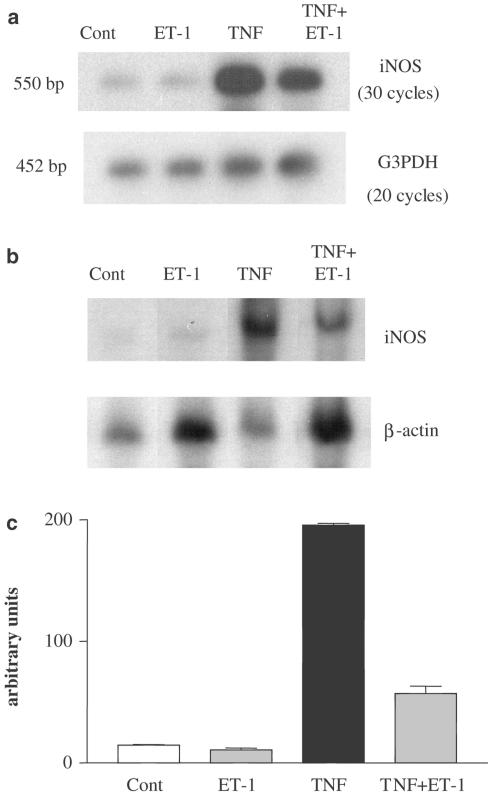
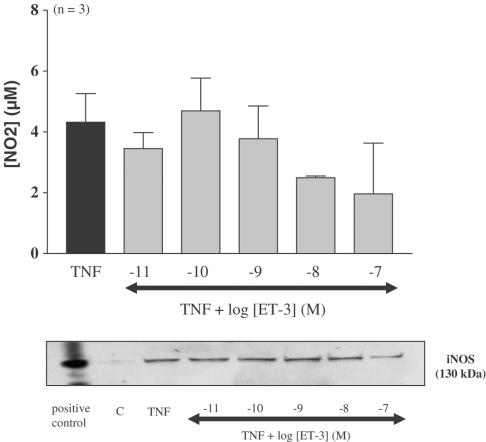
Differentiated adipocytes were submitted to increasing concentrations of ET-1 (10 pM–100 nM) for 20 h in control or in TNFα-treated adipocytes (ET-1 was added 15 min before TNFα). Nitrite determination and Western blot analysis showed that ET-1 alone did not induce any significant changes in nitrite accumulation or increase in iNOS expression (data not shown). However, the addition of ET-1 to cells treated with TNFα led to a concentration-dependent decrease in iNOS mRNA expression, iNOS expression and nitrite accumulation (ED50 4.2 nM) that was significant at 10 and 100 nM (Figures 4, 5). In contrast, ET-3 (10 pM–100 nM) did not significantly decrease TNFα-induced nitrite production (Figure 6).
Figure 4.
Effect of ET-1 on TNFα-induced iNOS expression in differentiated 3T3-F442A adipocytes. Differentiated 3T3-F442A adipocytes were treated for 20 h with TNFα in the presence or absence of increasing concentrations of ET-1. Nitrite levels were determined in the cell supernatant and protein extracts were analysed by Western blot using specific NOS antibodies. A representative autoradiograph taken from three independent experiments is shown. Results are expressed as mean±s.e.m. from three independent experiments. * indicates a statistically significant difference induced by ET-1 when compared to TNFα alone (one-way ANOVA followed by a Dunnett's post hoc test for multiple comparison, P<0.05). A representative autoradiography of three independent experiments is shown. A protein extract from murine macrophages (RAW 264.7) treated with a mixture of IFNγ (10 ng ml−1) plus LPS (1 μg ml−1) was used as a positive control for iNOS.
Figure 5.
Effect of ET-1 on TNFα-induced iNOS mRNA expression in differentiated 3T3-F442A adipocytes. Differentiated 3T3-F442A cells were treated for 20 h with or without TNFα in the presence or absence of ET-1 (10 nM). (a) mRNA extracts were analysed by RT–PCR. A representative autoradiography taken from two independent experiments is shown. (b) mRNA were also analysed by Northern blot. A representative autoradiography is shown. (c) Results of densitometric analysis are presented and expressed as mean±s.e.m. from two independent experiments (the radioactivity was normalised with the corresponding signal obtained with β-actin).
Figure 6.
Effect of ET-3 on TNFα-induced iNOS expression in differentiated 3T3-F442A adipocytes. Differentiated 3T3-F442A adipocytes were treated for 20 h with TNFα in the presence or absence of increasing concentrations of ET-3. Nitrite levels were determined in the cell supernatant and protein extracts were analysed by Western blot. Results are expressed as mean±s.e.m. from three independent experiments performed in duplicate. ET-3 did not produce any statistically significant inhibition (one-way ANOVA followed by a Dunnett's post hoc test for multiple comparison, P>0.05). A representative autoradiography from three independent experiments is shown. A protein extract from murine macrophages (RAW 264.7) treated with a mixture of IFNγ (10 ng ml−1) plus LPS (1 μg ml−1) was used as a positive control for iNOS.
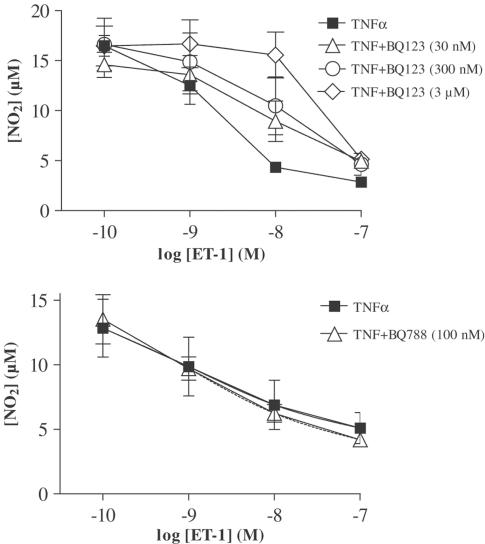
Cells were treated with increasing concentrations of the specific antagonist of the ET-A receptor subtype (BQ123, 30, 300 and 3000 nM) or with the specific antagonist of the ET-B receptor subtype (BQ788, 100 nM). Then cells were treated or not with increasing concentrations of ET-1 (0.1–100 nM) and TNFα (20 ng ml−1, 20 h). BQ123 or BQ788 alone did not affect TNFα-induced nitrite production. However, BQ123 provoked a concentration-dependent shift to the right of the ET-1 concentration–response curve (pA2 7.4; slope of the Schild plot: 0.8±0.2), while BQ788 was ineffective (Figure 7).
Figure 7.
Endothelin receptor subtype involved in the regulation of iNOS expression in differentiated 3T3-F442A adipocytes. Differentiated 3T3-F442A cells were treated for 20 h with TNFα and increasing concentrations of ET-1 in the presence or not of the specific ET-A antagonist (BQ123, top panel) or the specific ET-B antagonist (BQ788, lower panel). Nitrite accumulation was assayed in cell supernatant by the use of Griess reagents. Results are expressed as mean±s.e.m. from three independent experiments performed in duplicate.
Expression of ET-1 and mRNA for ET-1 receptor subtypes in 3T3-F442A
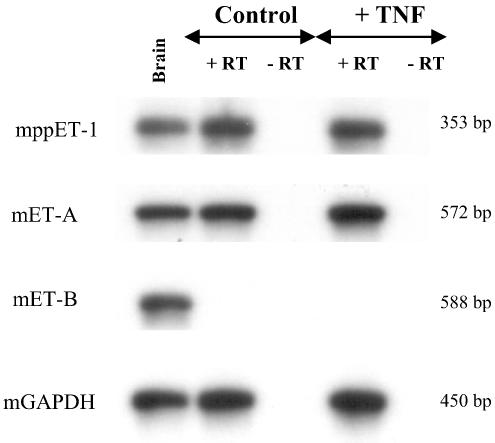
In preadipocytes (data not shown) and differentiated 3T3-F442A adipocytes, the mRNA encoding for preproET-1 and the ET-A receptor subtype was detected by RT–PCR. However, those cells did not express the mRNA for the ET-B receptor subtype. The presence of TNFα (20 ng ml−1, 20 h) did not affect the expression of those mRNAs either in preadipocytes or differentiated 3T3-F442A adipocytes (Figure 8).
Figure 8.
mRNA expression of preproET-1, ET-A and ET-B receptor subtypes in differentiated 3T3-F442A adipocytes. Differentiated 3T3-F442A cells were treated for 20 h with or without TNFα. The total RNA extracts were analysed by RT–PCR from four independent experiments. The PCR products were identified by autoradiography in the presence of specific probes. Murine brain (200 ng mRNA, Clontech) were used as positive control for RT–PCR experiments. Identical results were obtained in preadipocytes (data not shown). +RT and −RT: with and without reverse transcription, respectively.
In the culture medium from 3T3-F442A, treated or not in the presence of TNFα (20 ng ml−1, 20 h) and/or phosphoramidon (6 h, 100 μM; a nonselective inhibitor of the putative ET-1-converting enzyme), the level of ET-1 was below the threshold of the detection value of the kit (12.5 pg ml−1).
ET-1 transduction pathway
Cells were treated or not for 30 min, 2 or 4 h with TNFα (20 ng ml−1) in the presence or absence of ET-1 (10 nM). The intracellular cAMP content was determined. No significant changes in cAMP levels could be observed after these treatments, when compared to controls (<2 pmol ml−1).
Cells were treated for 20 h with TNFα (20 ng ml−1) in the presence or not of ET-1 (10 nM) and/or various inhibitors: bisindolylmaleimide (a nonselective inhibitor of PKC, 10 μM), indomethacin (a nonselective inhibitor of cyclooxygenase, 10 μM), SB 203580 (10 μM) and wortmannin (50 μM). These inhibitors did not per se alter the basal levels of nitrite or the production of nitrite after TNFα stimulation (data not shown). Indomethacin, bisindolylmaleimide and SB 203580 did not reverse the inhibition by ET-1 of TNFα-induced nitrite accumulation, but wortmannin produced a partial but significant inhibition of the ET-1 effect (Table 1). In contrast, LY 294002 (1, 3 and 10 μM) amplified the inhibitory effect of ET-1 (Table 1).
Table 1.
Effects of various inhibitors on the inhibition produced by ET-1-on TNFα-induced nitrite accumulation in differentiated 3T3-F442A
| Inhibition of TNFα-induced nitrite accumulation (%) | |
|---|---|
| ET-1 (10 nM) | 74±17 |
| ET-1+indomethacin (10 μM) | 84±5 |
| ET-1 (10 nM) | 78±8 |
| ET-1+bisindolylmaleimide (10 μM) | 72±9 |
| ET-1 (10 nM) | 64±1 |
| ET-1+SB 203580 (10 μM) | 67±5 |
| ET-1+wortmannin (50 μM) | 39±6* |
| ET-1 (10 nM) | 64±7 |
| ET-1+LY 294002 (1 μM) | 63±11 |
| ET-1+LY 294002 (3 μM) | 74±9 |
| ET-1+LY 294002 (10 μM) | 84±6* |
Data are shown as mean±s.e.m. of three to four experiments performed with cells studied at three different passages (in duplicate for indomethacin and bisindolylmaleimide or quadruplicate for SB 203580, wortmannin and LY 294002). *indicates a statistically significant difference (ANOVA followed by a Dunnett's test for multiple comparison test, P<0.05).
Discussion
This study confirms that TNFα induces iNOS expression in 3T3-F442A adipocytes (Mérial et al., 2000) and further shows that ET-1 produces a concentration-dependent inhibition of the mRNA and protein expressions of iNOS through the activation of the ETA receptor subtype.
TNFα stimulation produced a significant expression of the iNOS protein in both preadipocytes and differentiated adipocytes. The iNOS protein was functional as the nitrite accumulation, an index of NO production (Ignarro et al., 1993), was quantifiable and inhibited by known inhibitors of NOS. The nitrite production was markedly more pronounced in differentiated adipocytes than in preadipocytes, confirming earlier results in the same cell line and in 3T3-L1 adipocytes (Kapur et al., 1999; Mérial et al., 2000).
The expression of iNOS is regulated in a cell-specific manner (Paul et al., 1997). In 3T3-F442A, the effects of TNFα on nitrite production and iNOS protein expression were inhibited by both TLCK and PDTC, two structurally different inhibitors of NF-κB activation, suggesting the involvement of this nuclear factor. These observations are in line with earlier works showing that several NF-κB-binding motifs are present in the promoter of the murine iNOS gene and that NF-κB activation is critical for its expression (Lowenstein et al., 1993). In numerous cell types, the induction of iNOS, by TNFα, cytokines or LPS, requires MAP kinase-mediated NF-κB activation (Kan et al., 1999; Pahan et al., 1999). In the present study, the activation of p38 MAP kinase has been ruled out since the expression of iNOS protein and the nitrite accumulation was not affected by a specific inhibitor of P38–MAP kinase, SB203580, studied at a concentration which is effective in 3T3 adipocytes (Konrad et al., 2001). In differentiated 3T3-L1, in response to TNFα, the p44/42 kinase pathway appears to be involved in the nuclear translocation of NF-κB (Jain et al., 1999). Depending on the cell type, TNFα-induced NF-κB activation could require the PI-3 kinase/Akt (protein kinase B) stimulation (Ozes et al., 1999) or, in contrast, inhibition of this pathway can enhance the expression of NO synthase (Pahan et al., 1999). In the present study, two structurally distinct inhibitors of PI3 kinase, wortmannin and LY 294002 did not affect iNOS protein expression as observed with Western blots. However, if the former was ineffective, even at a high concentration, in inhibiting TNFα-induced nitrite production, the latter produced a concentration-dependent inhibition. These inhibitory effects of LY 294002 were not associated with any apparent toxicity of this compound (or of wortmannin) as measured by LDH activity. However, LY 294002 is a less specific inhibitor of PI3 kinase than wortmannin (Davies et al., 2000). A direct inhibitory effect of LY 294002 on iNOS activity in murine astrocytes has previously been reported (Jung et al., 1999). Alternatively, LY 294002 can inhibit other proteins, such as FK506-binding protein 12-rapamycin-associated protein, involved in iNOS phosphorylation and activity (Sahl et al., 1998). Altogether, these results suggest that TNFα-induced iNOS expression requires NF-κB activation, but does not necessitate the activation of the PI-3 kinase/Akt and P38–MAP kinase pathways.
In the present study, ET-1 produced a concentration-dependent inhibition of the TNFα-induced iNOS mRNA expression, protein expression and nitrite production. The inhibitory effect of ET-1, a nonspecific agonist of endothelin receptor subtypes, was not mimicked by ET-3, a preferential agonist of the ETB receptor subtype. Furthermore, the specific antagonist of the ETA receptor subtype, BQ123 (Ihara et al., 1992), produced a concentration-dependent inhibition of the effects of ET-1, with a pA2 value consistent with its previously reported affinity. In contrast, the selective ETB antagonist, BQ788 (Ishikawa et al., 1994), was without effect. The concentrations of BQ123 chosen for the present study are within the range of the specificity of this compound for the ETA receptor subtype. However, the concentration of BQ788 chosen is well over the IC50 for this compound towards ETB receptor (≈100-fold) and slightly less than its IC50 for the ETA receptor (≈10-fold) (Bax & Saxena, 1994). Altogether, these results indicate that the inhibition of TNFα-induced expression of iNOS by endothelin involves exclusively the ETA receptor subtype. These results were further confirmed by RT–PCR experiments showing that the 3T3-F442A adipocytes expressed the mRNA for the ETA but not for the ETB receptor subtype. A similar mRNA expression was observed in the 3T3-L1 adipose cells (Idris et al., 2001; Xiong et al., 2001), while human adipocytes and murine fat pads express the mRNA for the two receptor subtypes (Engeli et al., 2001, Xiong et al., 2001). ET-1-induced inhibition of iNOS induction by stimulation of an ETA receptor subtype has also been observed in mesangial, vascular smooth muscle or lung epithelial cells (Beck et al., 1995; Hirahashi et al., 1996; Ikeda et al., 1997; Markewitz et al., 1997). Whether or not the inhibition of iNOS expression by ET-1 in 3T3-F442A cells is at the level of gene transcription or due to a decrease in the stability of iNOS mRNA is unknown at present.
In 3T3-F442A, as in many other cells, agents that increase cAMP levels inhibit the expression of iNOS (Galea & Feinstein, 1999; Merial et al., unpublished observations). Furthermore, in glial cells, the inhibition of iNOS expression by an acute treatment with ET-1 is attributed to cAMP elevation (Oda et al., 1997) linked to cyclooxygenase activation (Sokolovsky, 1995). However, in the present study, the inhibitory effect of ET-1 was not affected by an effective concentration of indomethacin (Yu et al., 1995), a nonspecific cyclooxygenase inhibitor, and was not associated with a rise in cAMP, thus ruling out this pathway. Alternatively, following the activation of the ETA receptor, the major pathways linked to the transduction signal are the three kinases: protein kinase C, PI-3-kinase and MAP kinase (Douglas & Ohlstein, 1997). However, bisindolylmaleimide, the nonselective inhibitor of protein kinase C and SB 203580, an inhibitor of the p38-MAP kinase, were ineffective, at concentrations which have been proved to be active in 3T3 cell lines (Feve et al., 1995; Konrad et al., 2001), ruling out these pathways. In contrast, wortmannin, though at an elevated concentration, partially but significantly inhibited the effect of ET-1, suggesting the involvement of PI3-kinase. The effects of the structurally different inhibitor of PI3-kinase, LY 294002, were difficult to interpret because of its intrinsic inhibitory effect on TNFα-induced nitrite production. In 3T3-L1 adipocytes, the ET-1-induced acute and chronic stimulation of glucose transport as well as heterologous insulin desensitisation are mediated by ETA receptor activation. The latter involves PI3-kinase activation (Ishibashi et al., 2001) while the involvement of this pathway in ET-1-induced glucose transport is more controversial (Wu-Wong et al., 1999; Imamura et al., 1999; Ishibashi et al., 2000; Park et al., 2001). In 3T3-F442A adipocytes, the precise signalling pathway of the inhibition by ET-1 of TNFα-induced iNOS expression remains to be established.
In endothelial and epithelial cells, ET-1 production is inhibited by a nonspecific inhibitor of the putative endothelin-converting enzyme, phosphoramidon, and is increased by TNFα (Kanse et al., 1991; Marsden & Brenner, 1992; Corder et al., 1995; Deprez-Roy et al., 2000). In human adipose tissue and cultured preadipocytes, the expression of the ET-1 gene has been detected by RT–PCR and the secretion of big-ET-1 but not ET-1 has been measured in the culture medium, suggesting that adipocytes lack the necessary converting enzyme (Engeli et al., 2001). The present study partially confirms these findings as 3T3-F442A cells express the mRNA for prepro-ET1 but do not release the mature peptide, ET-1, in the culture medium either in control conditions or after TNFα treatment.
The results of the present study show that in contrast to its effects on other cell types, ET-1 does not induce iNOS expression in 3T3F442A adipocytes and inhibits TNFα-induced iNOS expression. As iNOS itself is involved in obesity-linked insulin resistance (Perreault & Marette, 2001), this indicates a complex role for ET-1 in the adipocytes since some of these effects, such as heterologous desensitisation of insulin signalling in adipocytes, are in favour of the generation of insulin resistance (Ishibashi et al., 2001), while others (inhibition of iNOS expression, present study) would have the opposite effect. Nevertheless, adipocytes are a target for circulating ET-1 and could increase circulating ET-1 by synthesising pre-proET-1. Whether or not a crosstalk between adipocytes and endothelial cells, via endothelin and iNOs expression, is involved in the establishment of syndrome X deserves further attention.
Abbreviations
- DMEM
Dulbecco's modified Eagle's medium
- ET-1
endothelin-1
- FBS
foetal bovine serum
- iNOS
Inducible nitric oxide synthase
- IFNγ
interferon γ
- L-NAME
L-nitroarginine methyl ester
- LPS
lipopolysaccharide
- MAP kinase
mitogen-activated protein kinase
- NF-κB
nuclear factor κB
- PDTC
pyrrolidine dithiocarbamate
- RT–PCR
reverse transcription–polymerase chain reaction
- TLCK
tosyllysine chloromethylketone
- TNFα
tumour necrosis factor α
References
- ARAI H., HORI S., ARAMORI I., OHKUBO H., NAKANISHI S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- BAX W.A., SAXENA P.R. The current endothelin receptor classification: time for consideration. Trends Pharmacol. Sci. 1994;15:379–386. doi: 10.1016/0165-6147(94)90159-7. [DOI] [PubMed] [Google Scholar]
- BECK K.F., MOHAUPT M.G., STERZEL R.B. Endothelin-1 inhibits cytokine-stimulated transcription of inducible nitric oxide synthase in glomerular mesangial cells. Kidney Int. 1995;48:1893–1899. doi: 10.1038/ki.1995.488. [DOI] [PubMed] [Google Scholar]
- CORDER R., CARRIR M., KHAN N., KLEMM P., VANE J.R. Cytokine regulation of endothelin-1 release from bovine aortic endothelial cells. J. Cardiovasc. Pharmacol. 1995;26:S56–S58. [PubMed] [Google Scholar]
- DAVIES S.P., REDDY H., CAIVANO M., COHEN P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEPREZ-ROY I., COGE F., BERTRY L., GALIZZI J.P., FELETOU M., VANHOUTTE P.M., CANET E. Endothelin-1 pathway in human alveolar epithelial cell line A549 and human umbilical vein endothelial cells. Acta Pharmacol. Sin. 2000;21:499–506. [PubMed] [Google Scholar]
- DOUGLAS S.A., OHLSTEIN E.H. Signal transduction mechanisms mediating the vascular actions of endothelin. J. Vasc. Res. 1997;34:152–164. doi: 10.1159/000159219. [DOI] [PubMed] [Google Scholar]
- ENGELI S., JANKE J., GORZELNIAK K., LUFT F.C., SHARMA A.M., KLINIK F.-V., DELBRUCK M. Expression of endothelin-system genes and secretion of endothelin-1 by human adipocytes. Int. J. Obes. 2001;25:P158. [Google Scholar]
- FERRI C., BELLINI C., DESIDERI G., DI FRANCESCO L., BALDONCINI R., SANTUCCI A., DE MATTIA G. Plasma endothelin-1 levels in obese hypertensive and normotensive men. Diabetes. 1995;44:431–436. doi: 10.2337/diab.44.4.431. [DOI] [PubMed] [Google Scholar]
- FEVE B., PIETRI-ROUXEL F., EL HADRI K., DRUMARE M.F., STROBERG A.D. Long term phorbol ester treatment down-regulates the beta 3-adrenergic receptor in 3T3-F442A adipocytes. J. Biol. Chem. 1995;270:10952–10959. doi: 10.1074/jbc.270.18.10952. [DOI] [PubMed] [Google Scholar]
- GALEA E., FEINSTEIN D.L. Regulation of the expression of the inflammatory nitric oxide synthase (NOS2) by cyclic AMP. FASEB J. 1999;13:21–25. doi: 10.1096/fasebj.13.15.2125. [DOI] [PubMed] [Google Scholar]
- HAUNER H., PETRUSCHKE T., GRIES F.A. Endothelin-1 inhibits the adipose differentiation of cultured human adipocyte precursor cells. Metabolism. 1994;43:227–232. doi: 10.1016/0026-0495(94)90250-x. [DOI] [PubMed] [Google Scholar]
- HIRAHASHI J., NAKAKI T., HISHIKAWA K., MARUMO T., YASUMORI T., HAYASHI M., SUZUKI H., SARUTA T. Endothelin-1 inhibits induction of nitric oxide synthase and GTP cyclohydrolase I in rat mesangial cells. Pharmacology. 1996;53:241–249. doi: 10.1159/000139436. [DOI] [PubMed] [Google Scholar]
- HOTAMISLIGIL G.S. Molecular mechanisms of insulin resistance and the role of the adipocyte. Int. J. Obes. Relat. Metab. Disord. 2000;24 Suppl 4:S23–S27. doi: 10.1038/sj.ijo.0801497. [DOI] [PubMed] [Google Scholar]
- IDRIS I., PATIAG D., GRAY S., DONELLY R. Tissue and time-dependent effects of endothelin-1 on insulin-stimulated glucose uptake. Biochem. Pharmacol. 2001;62:1705–1708. doi: 10.1016/s0006-2952(01)00815-2. [DOI] [PubMed] [Google Scholar]
- IGNARRO L.J., FUKUTO J.M., GRIESCAVAGE J.M., ROGERS N.E., BYRNS R.E. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from L-arginine. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8103–8107. doi: 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IHARA M., ISHIKAWA K., FUKURODA T., SAEKI T., FUNABASHI K., FUKAMI T., SUDA H., YANO M. In vitro biological profile of a highly potent novel endothelin (ET) antagonists BQ-123 selective for the ETA receptor. J. Cardiovasc. Pharmacol. 1992;20:S11–S14. doi: 10.1097/00005344-199204002-00005. [DOI] [PubMed] [Google Scholar]
- IKEDA U., YAMAMOTO K., MAEDA Y., SHIMPO M., KANBE T., SHIMADA K. Endothelin-1 inhibits nitric oxide synthesis in vascular smooth muscle cells. Hypertension. 1997;29:65–69. doi: 10.1161/01.hyp.29.1.65. [DOI] [PubMed] [Google Scholar]
- IMAMURA T., ISHIBASHI K., DALLE S., UGI S., OLEFSKY J.M. Endothelin-1-induced GLUT-4 translocation is mediated via Gαq/11 protein and phosphatidylinositol 3-kinase in 3T3-L1 adipocytes. J. Biol. Chem. 1999;274:33691–33695. doi: 10.1074/jbc.274.47.33691. [DOI] [PubMed] [Google Scholar]
- ISHIBASHI K.I., IMAMURA T., SHARMA P.M., HUANG J., UGI S., OLEFSKY J.M. Chronic endothelin-1 treatment leads to heterologous desensitization of insulin signaling in 3T3-L1 adipocytes. J. Clin. Invest. 2001;107:1193–1202. doi: 10.1172/JCI11753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIBASHI K., IMAMURA T., SHARMA P.M., UGI S., OLEFSKY J.M. The acute and chronic stimulatory effects of endothelin-1 on glucose transport are mediated by distinct pathways in 3T3-L1 adipocytes. Endocrinology. 2000;141:4623–4628. doi: 10.1210/endo.141.12.7820. [DOI] [PubMed] [Google Scholar]
- ISHIDA F., SAEKI K., SAEKI T., ISHIKAWA K., IHARA M., KAMEI T., YANO M. Suppressive effects of the endothelin receptor (ET-A) antagonist BQ-123 on ET-1-induced reduction of lipoprotein lipase activity in 3T3-L1 adipocytes. Biochem. Pharmacol. 1992;44:1431–1436. doi: 10.1016/0006-2952(92)90545-t. [DOI] [PubMed] [Google Scholar]
- ISHIKAWA K., IHARA M, NOGUCHI K., MASE T., MINO N., SAEKI T., FUKURODA T., FUKAMI T., OZAKI S., NAGASE T., NISHIKIBE M., YANO M. Biochemical and pharmacological profile of a potent and selective endothelin B-receptor antagonist, BQ-788. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4892–4896. doi: 10.1073/pnas.91.11.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAIN R.G., PHELPS K.D, PEKALA P.H. Tumor necrosis factor-alpha initiated signal transduction in 3T3-L1 adipocytes. J. Cell. Physiol. 1999;179:58–66. doi: 10.1002/(SICI)1097-4652(199904)179:1<58::AID-JCP8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- JUNG Y.D., KIM M.S., LEE K.S., KANG I.C., NAH A.S., SONG D.U., YANG S.Y., KIM J.K., AHN B.W. 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY 294002) inhibits nitric oxide production in cultured murine astrocytes. Pharmacol. Res. 1999;40:423–427. doi: 10.1006/phrs.1999.0533. [DOI] [PubMed] [Google Scholar]
- KAN H., XIE Z., FINKEL M.S. TNF-alpha enhances cardiac myocyte NO production through MAP kinase-mediated NF-kappaB activation. Am. J. Physiol. 1999;277:H1641–H1646. doi: 10.1152/ajpheart.1999.277.4.H1641. [DOI] [PubMed] [Google Scholar]
- KANSE S.M., TAKAHASHI K., LAM H.C., REES A., WARREN J.B., PORTA M., MOLINATTI P., GHATEI M., BLOOM S.R. Cytokine stimulated endothelin release from endothelial cells. Life Sci. 1991;48:1379–1384. doi: 10.1016/0024-3205(91)90434-d. [DOI] [PubMed] [Google Scholar]
- KAPUR S., MARCOTTE B., MARETTE A. Mechanism of adipose tissue iNOS induction in endotoxemia. Am. J. Physiol. 1999;276:E635–E641. doi: 10.1152/ajpendo.1999.276.4.E635. [DOI] [PubMed] [Google Scholar]
- KAWAMURA M., OHGAWARA H., NARUSE M., SUZUKI N., IWASAKI N., NARUSE K., HORI S., DEMURA H., OMORI Y. Increased plasma endothelin in NIDDM patients with retinopathy. Diabetes Care. 1992;15:1396–1397. doi: 10.2337/diacare.15.10.1396. [DOI] [PubMed] [Google Scholar]
- KONE B.C., SCHOWOBEL J., TURNER P., MOHAUPT M.G., CANGRO C.B. Role of NF-κB in the regulation of inducible nitric oxide synthase in an MTAL cell line. Am. J. Physiol. 1995;269:F718–F729. doi: 10.1152/ajprenal.1995.269.5.F718. [DOI] [PubMed] [Google Scholar]
- KONRAD D., SOMWAR R., SWEENEY G., YAWORSKI K., HAYASHI M., RAMLAL T., KLIP A. The antihyperglycemic drug α-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation. Diabetes. 2001;50:1464–1471. doi: 10.2337/diabetes.50.6.1464. [DOI] [PubMed] [Google Scholar]
- LOWENSTEIN C.J., ALLEY E.W., RAVAL P., SNOWMAN A.M., SNYDER S.H., RUSSELL S.W., MURPHY W.J. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc. Natl. Acad. Sci. U.S.A. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKEWITZ B.A., MICHAEL J.R., KOHAN D.E. Endothelin-1 inhibits the expression of inducible nitric oxide synthase. Am. J. Physiol. 1997;272:L1078–L1083. doi: 10.1152/ajplung.1997.272.6.L1078. [DOI] [PubMed] [Google Scholar]
- MARSDEN P.A., BRENNER B.M. Transcriptional regulation of the endothelin-1 gene by TNF alpha. Am. J. Physiol. 1992;262:C854–C861. doi: 10.1152/ajpcell.1992.262.4.C854. [DOI] [PubMed] [Google Scholar]
- MÉRIAL C., BOULOUMIE A., TROCHERIS V., LAFONTAN M., GALITZKY J. Nitric oxide-dependent downregulation of adipocyte UCP-2 expression by tumor necrosis factor α. Am. J. Physiol. 2000;279:C1100–C1106. doi: 10.1152/ajpcell.2000.279.4.C1100. [DOI] [PubMed] [Google Scholar]
- MURAYAMA T., ODA H., SASAKI Y., OKADA T., NOMURA Y. Regulation of inducible NO synthase expression by endothelin in primary cultured glial cells. Life Sci. 1998;62:1491–1495. doi: 10.1016/s0024-3205(98)00095-2. [DOI] [PubMed] [Google Scholar]
- ODA H., MURAYAMA T., SASAKI Y., OKADA T., NOMURA Y. Endothelin enhances lipopolysaccharide-induced expression of inducible nitric oxide synthase in rat glial cells. Eur. J. Pharmacol. 1997;339:253–260. doi: 10.1016/s0014-2999(97)01369-1. [DOI] [PubMed] [Google Scholar]
- OTTOSSON-SEEBERGER A., LUNDBERG J.M., ALVESTRAND A., AHLBORG G. Exogenous endothelin-1 causes peripheral insulin resistance in healthy humans. Acta Physiol. Scand. 1997;161:211–220. doi: 10.1046/j.1365-201X.1997.00212.x. [DOI] [PubMed] [Google Scholar]
- OZES O.N., MAYO L.D., GUSTIN J.A., PFEFFER S.R., PFEFFER L.M., DONNER D.B. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- PAHAN K., RAYMOND J.R., SINGH I. Inhibition of phosphatidylinositol 3-kinase induces nitric-oxide synthase in lipopolysaccharide- or cytokine-stimulated C6 glial cells. J. Biol. Chem. 1999;274:7528–7536. doi: 10.1074/jbc.274.11.7528. [DOI] [PubMed] [Google Scholar]
- PARK J.G., BOSE A., LESZYK J., CZECH M.P. PYK2 as a mediator of endothelin-1/Gα11 signalling to GLUT4 glucose transporters. J. Biol. Chem. 2001;276:47751–47754. doi: 10.1074/jbc.C100524200. [DOI] [PubMed] [Google Scholar]
- PAUL A., BRYANT C., LAWSON M.F., CHILVERS E.R., PLEVIN R. Dissociation of lipopolysaccharide-mediated induction of nitric oxide synthase and inhibition of DNA synthesis in RAW 264.7 macrophages and rat aortic smooth muscle cells. Br. J. Pharmacol. 1997;120:1439–1444. doi: 10.1038/sj.bjp.0701070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERREAULT M., MARETTE A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat. Med. 2001;7:1138–1143. doi: 10.1038/nm1001-1138. [DOI] [PubMed] [Google Scholar]
- PRASANNA G., KRISHNAMOOTHY R., HULET C., ZHANG H., ZHANG X., YORIO T. Endothelin-1 induces nitric oxide synthase-2 expression in human non-pigmented ciliary epithelial cells. Am. J. Respir. Crit. Care Med. 2000;161:982–989. doi: 10.1006/exer.2000.0908. [DOI] [PubMed] [Google Scholar]
- RUBANYI G.M., POLOKOFF M.A. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol. Rev. 1994;46:325–415. [PubMed] [Google Scholar]
- RUSSELL F.D., DAVENPORT A.P. Secretory pathways in endothelin synthesis. Br. J. Pharmacol. 1999;126:391–398. doi: 10.1038/sj.bjp.0702315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO Y., NAKAO K., MUKOYAMA M., IMURA H. Increased plasma endothelin level in patients with essential hypertension. N. Engl. J. Med. 1990;322:205. doi: 10.1056/nejm199001183220315. [DOI] [PubMed] [Google Scholar]
- SAKURAI T., YANAGISAWA M., TAKUWA Y., MIYAZAKI H., KIMURA S., GOTO K., MASAKI T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- SAHL B., WAGEY R., MAROTTA A., TAO J.S., PELECH S. Activation of phosphatidylinositol 3-kinase, protein kinase B and P70 S6 kinases in lipopolysaccharides-stimulated raw 264.7 cells: differential effects of rapamycin, LY294002 and wortmannin on nitrite oxide production. J. Immunol. 1998;161:6947–6954. [PubMed] [Google Scholar]
- SCHENA M., MULATERO P., SCHIAVONE D., MENGOZZI G., TESIO L., CHIANDUSSI L., VEGLIO F. Vasoactive hormones induce nitric oxide synthase mRNA expression and nitric oxide production in human endothelial cells and monocytes. Am. J. Hypertens. 1999;12:388–397. [PubMed] [Google Scholar]
- SOKOLOVSKY M. Endothelin receptor subtypes and their role in transmembrane signaling mechanisms. Pharmacol. Ther. 1995;68:435–471. doi: 10.1016/0163-7258(95)02015-2. [DOI] [PubMed] [Google Scholar]
- STEPHENSON K., GUPTA A., MUSTAFA S.B., HALFF G.A. Endothelin-stimulated nitric oxide production in the isolated Kupffer cell. J. Surg. Res. 1997;73:149–154. doi: 10.1006/jsre.1997.5191. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI K., GHATEI M.A., LAM H.C., O'HALLORAN D.J., BLOOM S.R. Elevated plasma endothelin in patients with diabetes mellitus. Diabetologia. 1990;33:306–310. doi: 10.1007/BF00403325. [DOI] [PubMed] [Google Scholar]
- TALLARIDA R.J., COWAN A., ADLER M.W. pA2 and receptor differentiation: a statistical analysis of competitive antagonism. Life Sci. 1979;25:637–654. doi: 10.1016/0024-3205(79)90505-8. [DOI] [PubMed] [Google Scholar]
- TANAHASHI T., YAMAGUSHI K., ISHIKAWA S., KUSUHARA M., ADACHI I., ABE O. Endothelin-1 inhibits adipogenic differentiation of 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 1991;177:854–860. doi: 10.1016/0006-291x(91)91868-d. [DOI] [PubMed] [Google Scholar]
- WU-WONG J.R., BERG C.E., WANG J., CHIOU W.J., FISSEL B. Endothelin stimulates glucose uptake and GLUT-4 translocation via activation of endothelin ET-A receptor in 3T3-L1 adipocytes. J. Biol. Chem. 1999;274:8103–8110. doi: 10.1074/jbc.274.12.8103. [DOI] [PubMed] [Google Scholar]
- XIONG Y., TANAKA H., RICHARDSON J.A., WILLIAMS S.C., SLAUGHTER C.A., NAKAMURA M., CHEN J.U.L., YANAGISAWA M. Endothelin-1 stimulates leptin production in adipocytes. J. Biol. Chem. 2001;276:28471–28477. doi: 10.1074/jbc.M103478200. [DOI] [PubMed] [Google Scholar]
- YANAGISAWA M., KURIHARA H., KIMURA S., TOMOBE Y., KOBAYASHI M., MITSUI Y., YAZAKI Y., GOTO K., MASAKI T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- YU K., BAYONA W., KALLEN C.B., HARDING H.P., RAVERA C.P., MCMAHON G., BROWN M., LAZAR M.A. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J. Biol. Chem. 1995;41:23975–23983. doi: 10.1074/jbc.270.41.23975. [DOI] [PubMed] [Google Scholar]