Abstract
Docosahexaenoic acid (DHA) and arachidonic acid (AA), polyunsaturated fatty acids (PUFAs), are important for central nervous system function during development and in various pathological states. Astrocytes are involved in the biosynthesis of PUFAs in neuronal tissue. Here, we investigated the mechanism of DHA and AA release in cultured rat brain astrocytes.
Primary astrocytes were cultured under standard conditions and prelabeled with [14C]DHA or with [3H]AA. Adenosine 5′-triphosphate (ATP) (20 μM applied for 15 min), the P2Y receptor agonist, stimulates release of both DHA (289% of control) and AA (266% of control) from astrocytes. DHA release stimulated by ATP is mediated by Ca2+-independent phospholipase A2 (iPLA2), since it is blocked by the selective iPLA2 inhibitor 4-bromoenol lactone (BEL, 5 μM) and is not affected either by removal of Ca2+ from extracellular medium or by suppression of intracellular Ca2+ release through PLC inhibitor (U73122, 5 μM).
AA release, on the other hand, which is stimulated by ATP, is attributed to Ca2+-dependent cytosolic PLA2 (cPLA2). AA release is abolished by U73122 and, by removal of extracellular Ca2+, is insensitive to BEL and can be selectively suppressed by methyl arachidonyl fluorophosphonate (3 μM), a general inhibitor of intracellular PLA2 s.
Western blot analysis confirms the presence in rat brain astrocytes of 85 kDa cPLA2 and 40 kDa protein reactive to iPLA2 antibodies.
The influence of cAMP on regulation of PUFA release was investigated. Release of DHA is strongly amplified by the adenylyl cyclase activator forskolin (10 μM), and by the protein kinase A (PKA) activator dibutyryl-cAMP (1 mM). In contrast, release of AA is not affected by forskolin or dibutyryl-cAMP, but is almost completely blocked by 2,3-dideoxyadenosine (20 μM) and inhibited by 34% by H89 (10 μM), inhibitors of adenylyl cyclase and PKA, respectively.
Other neuromediators, such as bradykinin, glutamate and thrombin, stimulate release of DHA and AA, which is comparable to the release stimulated by ATP.
Different sensitivities of iPLA2 and cPLA2 to Ca2+ and cAMP reveal new pathways for the regulation of fatty acid release and reflect the significance of astrocytes in control of DHA and AA metabolism under normal and pathological conditions in brain.
Keywords: Protein kinase A, polyunsaturated fatty acids, lipid mediators, cyclic nucleotide, nucleotide receptor (P2Y receptor)
Introduction
Docosahexaenoic acid (DHA, C22:6n-3) and arachidonic acid (AA, eicosatetraenoic acid, C20:4n-6) are polyunsaturated fatty acids (PUFA) of great physiological significance (James et al., 2000; Moore, 2001). More than most other tissues, the brain is highly enriched in longchain PUFAs, first of all AA and DHA. While the properties of AA as a physiologically active substance or as a precursor of eicosanoids have been widely investigated, much less is known about the regulation of DHA metabolism. DHA is a substance of particular significance in mammalian brain. In this tissue, the content of DHA in the sn-2 position of cellular phospholipids reaches up to 50% of the total amount of unsaturated fatty acids (Garcia et al., 1998). Membrane composition concerning the proportions of n-3 and n-6 PUFAs in phospholipids has been shown to be crucial for normal functioning of the central nervous system (CNS) (summarized in Farooqui et al., 2000; Jump, 2002). The content of DHA and AA in brain phospholipids was found to be significantly reduced in patients with various neuronal disorders and neurodegenerative diseases, for example, Alzheimer's disease (Pettegrew et al., 1995; Markesbery, 1997). Free AA and DHA exhibit neuroprotective properties during ischemia and brain trauma (Lauritzen et al., 2000; Kim et al., 2001).
Astrocytes are the main source of AA and DHA in the brain since these cells, but not neurons, are able to synthesize DHA and AA from n-3 and n-6 precursor fatty acids with 18-, 20- and 22-carbon atoms (Moore, 1993, 2001; Williard et al., 2001). Astrocytes supply neurons with PUFAs (Moore, 2001). Moreover, astrocytes release AA after stimulation with different substances, which appear in the brain tissue during pathological conditions (Katsuki & Okuda, 1995). Astrocytes are able to release AA upon activation of muscarinic cholinergic receptors (Felder et al., 1989), adrenoceptors (Kanterman et al., 1990), metabotropic glutamate receptors (Stella et al., 1994), thrombin-activated receptors (Sergeeva et al., 2002) and nucleotide-activated P2Y purine receptors (Bruner & Murphy, 1990; Stella et al., 1997; Chen & Chen, 1998). Very little is known about agonist-stimulated DHA release. However, it is very important to understand the regulation of both of these PUFAs, since very often DHA has been found to exert effects opposite to those of AA, both on the cellular level (Calderaro et al., 1994) and in complex physiological systems (Whelan, 1996; Tashiro et al., 1998).
The main pathway leading to release of AA and DHA from phospholipids is hydrolysis by phospholipase A2 (PLA2). Therefore, pathophysiological states can be eventually controlled through modulation of activity of this enzyme. A family of different PLA2 isoforms has been described and classified (reviewed in Six & Dennis, 2000; Capper & Marshall, 2001). Of particular significance is the Ca2+-dependent cytosolic PLA2 (cPLA2) that is selective for arachidonate-containing phospholipids. This enzyme is considered to be a major producer of AA during acute stimulation of cells by diverse agents (for review, see Capper & Marshall, 2001). Only some studies reporting slow turnover and redistribution of DHA between phospholipid classes can be found in the literature (Capper & Marshall, 2001; Farooqui & Horrocks, 2001; Nagan & Zoeller, 2001). Very few data are available about acute agonist-stimulated release of DHA. Mobilization of DHA by stimulation of serotonin receptors in rat C6 glioma cells via PLA2 has been shown (Garcia & Kim, 1997).
Differentiation between PLA2 isoforms participating in the release of different PUFAs is important, because the PLA2 s are differently regulated through the intracellular signaling network. For cPLA2, there are well-described mechanisms of activation involving increase in concentration of intracellular Ca2+ ([Ca2+]i) and/or phosphorylation of this enzyme by various mitogen-activated protein (MAP) kinases (Lin et al., 1993; Kramer et al., 1996). Most recently, a regulatory role of PKC in cPLA2 phosphorylation and AA release in primary murine astrocytes has been described (Xu et al., 2002). Nevertheless, regulation of this enzyme through protein kinases is intricate. Some research groups reported that activation of the cAMP/PKA pathway stimulates (Kievit et al., 2001) and others published that cAMP/PKA inhibits cPLA2 (Murthy & Makhlouf, 1998; Xing et al., 1999). The mechanisms of acute activation of DHA release are still largely unclear.
The present study gives a comparative analysis of the regulation of DHA and AA release in rat astrocytes. As astrocytes play a key role in the supply of brain with PUFAs, we investigated the release of DHA and AA upon stimulation by ATP and compared it with the effects of other neuromediators and proinflammatory agents. We demonstrate that the ATP-induced release of DHA and AA, respectively, is mediated by different isoforms of PLA2. Furthermore, we detected pharmacological differences in the regulation of release of DHA and AA through PLC/InsP3 and cAMP/PKA pathways. These results will help to understand the processes controlling neurodegenerative diseases and pathological states of brain tissue.
Experimental procedures
Astrocyte preparation
Primary astrocyte-enriched cell cultures were obtained according to the method of Hamprecht & Löffler (1985). In brief, newborn rats were decapitated, and the brains were removed and collected in ice-cold Puck's-D1 solution (137.0 mM NaCl, 5.4 mM KCl, 0.44 mM KH2PO4, 0.3 mM Na2HPO4, 5.5 mM glucose, pH 7.4). The brains were gently passed through nylon meshes of 250 μm and 136 μm pore width, in consecutive order. The cell suspension was centrifuged at 4°C for 5 min at 500 × g. The cells were resuspended in 10 ml growth medium (DMEM supplemented with 10% (v v−1) fetal calf serum, 20 U ml−1 penicillin and 20 μg ml−1 streptomycin).
Cell culture
Astrocytes were seeded in 250 ml flasks at a starting density of 6 × 105 cells ml−1 (20 ml per flask) in DMEM supplemented with 10% (v v−1) fetal calf serum and incubated at 37°C with an atmosphere containing 10% CO2. The medium was changed after 5 days. After a further 2 days, the cells were washed with Hank's solution (Ca2+, Mg2+ free, 50 mM NaCl, 5 mM KCl, 0.2 mM KH2PO4, 0.17 mM Na2HPO4, 5.0 mM glucose, 58.4 mM sucrose, pH 7.4) and detached from the plastic with trypsin–EGTA (0.05–0.02% (w v−1)) solution. As we have shown previously, in these cultures more than 95% of the cells are positive for GFAP, an astrocyte-specific marker (Ubl & Reiser, 1997). Then DMEM containing 10% (v v−1) fetal calf serum was added to stop the trypsin action. After that the cells were centrifuged for 5 min at 500 × g, resuspended in DMEM with 10% (v v−1) fetal calf serum and plated for the radiolabeling experiment in 96-well plates at a starting density of 1.5 × 105 cells ml−1, 200 μl per well.
Radiolabeling and assessment of DHA and AA release
For the cells seeded in 96-well plates, after 24 h the culture medium was changed to serum-free DMEM containing [14C]docosahexaenoic acid (5 nCi per well) or [3 H]arachidonic acid (50 nCi per well) for 24 h. Then cells were washed twice with 200 μl of HEPES-buffered saline (HBS; buffer composition in mM: 145 NaCl, 5.4 KCl, 1 MgCl2, 1.8 CaCl2, 25 glucose, 20 HEPES, pH 7.4 adjusted with tris(hydroxymethyl)-aminomethane) and 160 μl of DMEM was added. After 20 min, substances were added to reach a final volume of 200 μl. Aliquots of 195 μl were taken out at the time points indicated and radioactivity was measured by liquid scintillating counting with 3 ml of ‘Ultima Gold' scintillation cocktail (Packard Instruments, U.S.A.) on a 1600TR Liquid Scintillation Analyzer (Packard Instruments, U.S.A.). Each sample was counted twice.
Cytosolic Ca2+
The cytosolic Ca2+ activity ([Ca2+]i) was measured using the Ca2+-sensitive fluorescent dye fura-2/AM. For dye loading, the cells grown on a coverslip in DMEM with 10% (v v−1) fetal calf serum were placed in 1 ml HBS for 30 min at 37°C, supplemented with 2 μM fura-2/AM. Loaded cells were transferred into a perfusion chamber with a bath volume of about 0.2 ml and mounted on an inverted microscope Axiovert 135 (Zeiss, Germany). During the experiments, the cells were continuously superfused with medium heated to 37°C. The perfusion system was combined with a six-port valve (Thomachrom, Type RH 0112) from Reichelt (Heidelberg, Germany) to allow the switch between solutions containing different agents to be tested.
Single-cell fluorescence measurements of [Ca2+]i were performed using an imaging system from TILL Photonics GmbH (Munich, Germany). Cells were excited alternately at 340 and 380 nm for 50–70 ms at each wavelength with a rate of 0.33 Hz and the resultant emission was collected above 510 nm. Images were stored on a computer and subsequently the changes in fluorescence ratio of (F340 to F380) were determined from selected regions of interest covering a single cell. Fluorescence ratios from the same day measurement were compared. In part of the experiments autofluorescence was determined at the end of each experiment and fluorescence ratios were calculated after subtraction of autofluorescence and calibrated to free [Ca2+]i according to standard protocol (Grynkiewicz et al., 1985). Each experimental protocol was performed in one experiment using at least two different coverslips with cells from the same culture. Then replica experiments were carried out using cells from another preparation.
Western blot analysis
Confluent cells were deprived of serum for 24 h before use. Monolayers were washed twice with ice-cold phosphate-buffered saline (PBS) and lysed in modified RIPA buffer (50 mM Tris, pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, 1 mM NaF), and one tablet of Protease Inhibitor Cocktail (Roche Molecular Biochemicals, Mannheim, Germany) per 50 ml. The cell lysate was gently shaken on a rocker for 15 min at 4°C. The lysate was centrifuged at 14,000 × g in a precooled centrifuge for 15 min, and the supernatant was immediately transferred to a fresh centrifuge tube, discarding the pellet. Protein concentration was determined by the Bradford method using bovine serum albumin as the standard. Samples containing equal amounts of protein were subjected to gradient (4–12%) NuPage™ (Invitrogen) SDS–polyacrylamide gel electrophoresis (20 μg per lane) and transferred to nitrocellulose membrane. Membranes were blocked with 5% nonfat dry milk for 1 h at room temperature and rinsed in PBS with 0.1% Tween 20 three times. Then followed an incubation for 90 min at room temperature with specific antibodies against group IV cPLA2 (Santa Cruz) and group VI iPLA2 (Santa Cruz). After three rinses, membranes were further incubated for 90 min at room temperature with peroxidase-conjugated anti-mouse or anti-goat IgG (1 : 10,000), respectively (Dianova, Hamburg, Germany). Membranes were washed three times, and proteins were visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech). The experiments were performed twice with cells from different preparations.
Data analysis and statistics
Data concerning DHA and AA release are presented in text and figures as means and standard error of samples from three experiments, each characterized in groups of six replica samples. Data were subjected to an ANOVA with Dunnett's posthoc comparison. Statistical significance was established at P<0.05.
Results
Stimulation of DHA and AA release from astrocytes by ATP
We studied the release of DHA and AA from rat astrocytes stimulated by ATP, the agonist of P2Y receptors (Bruner & Murphy, 1990; Stella et al., 1997; Chen & Chen, 1998). The astrocytes were prelabeled for 24 h with [14C]DHA and [3 H]AA. The cells incorporated in both cases about 70% of the radioactivity applied. The release of PUFAs from the cellular phospholipids was determined by the appearance of radioactivity in the culture medium. The nonstimulated, basal release corresponded to 0.5–0.6% of incorporated radioactivity for DHA and 0.7–0.8% for AA.
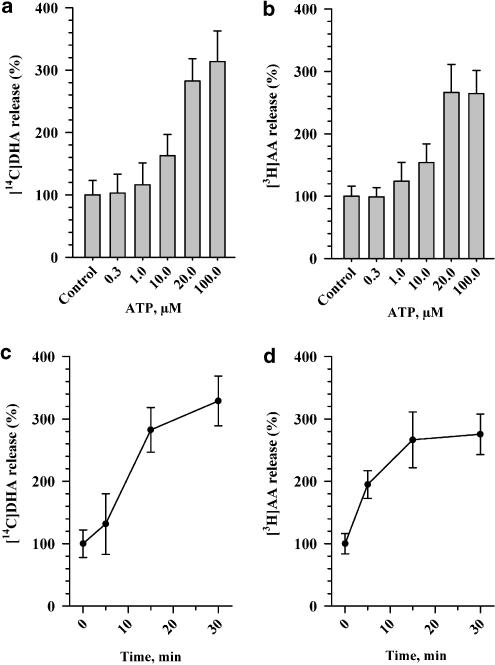
ATP stimulated acute release of both DHA and AA in a concentration- and time-dependent manner (Figure 1). After 15 min stimulation, release reached a plateau and at a concentration of ATP of approximately 20 μM release was maximal. For further detailed investigations of mechanisms underlying acute release of DHA and AA, we chose the concentration of ATP 20 μM for both DHA and AA with a stimulation time of 15 min.
Figure 1.
Release of DHA (a,c) and AA (b,d) from rat astrocytes stimulated with ATP (a,b), concentration dependence, (c,d) time dependence. Cells were labeled with [14C]docosahexaenoic acid or [3H]arachidonic acid in DMEM containing 10% fetal calf serum for 24 h, washed twice with HBS and fresh DMEM was added. Then 20 min later, ATP was added at 37°C and radioactivity in the medium was measured over time, as described in Experimental procedures. Release without any addition (control) was taken as 100%. Each point represents the mean±s.e.m. This experiment was carried out three times with similar results.
Involvement of different isoforms of PLA2 in DHA and AA release
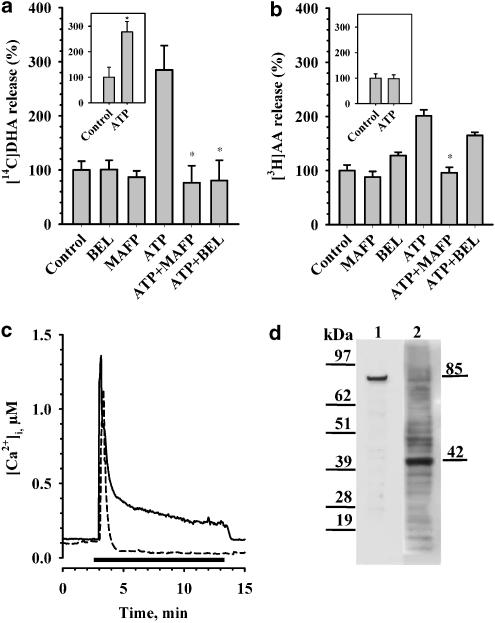
The possible involvement of different isoforms of PLA2 in ATP-induced release of AA and DHA was tested using enzyme inhibitors: methyl arachidonyl fluorophosphonate (MAFP), a general inhibitor of all types of cytosolic PLA2 (Riendeau et al., 1994; Lio et al., 1996), and 4-bromoenol lactone (BEL), a specific irreversible inhibitor of Ca2+-independent PLA2 (iPLA2) (Ackermann et al., 1995). In a first series of experiments, the concentrations of the inhibitors were varied and the lowest values producing stable maximal inhibitory effect after 15 min of stimulation were determined. These were chosen for our further experiments. The inhibitors were applied to the radioactively labeled astrocytes 10 min prior to stimulation with 20 μM ATP for 15 min. The inhibitors did not affect the basal release. Both inhibitors of PLA2, MAFP (3 μM) and BEL (5 μM), completely blocked the ATP-stimulated release of DHA (Figure 2a). However, AA release stimulated by ATP was blocked only by MAFP, whereas BEL produced a negligible, statistically nonsignificant attenuation (Figure 2b). These pharmacological data led to the conclusion that iPLA2 is involved in release of DHA and cPLA2 is involved in release of AA.
Figure 2.
Determination of different isoforms of PLA2 involved in the release of DHA (a) and AA (b). In the main diagram data for ATP-stimulated release in Ca2+-containing medium are shown; in the insets data for Ca2+-free medium are shown. Cells were labeled as described in the legend for Figure 1. For the release in Ca2+-free medium (experiments shown in insets), the cells after two washes with HBS were washed by HBS without Ca2+ and fresh Ca2+-free HSB was added. When applicable, 30 min later, inhibitors of iPLA2 (BEL, 5 μM) and cPLA2 (MAFP, 3μM) were added. After another 10 min, 20 μM ATP was added at 37°C for 15 min and then the radioactivity in the medium was measured as described in Experimental procedures. Release without any addition (control) was taken as 100%. ATP-stimulated release under the influence of inhibitors of PLA2 was evaluated for statistical difference with release stimulated by ATP alone. Release stimulated by ATP in Ca2+-free medium was evaluated for statistical difference with release observed without any addition (control). *The value is statistically (P<0.05) different from the reference value. (c) Profile of [Ca2+]i in astrocytes, stimulated by 20 μM ATP in Ca2+- containing (solid line) or Ca2+-free medium (dashed line). The time period of exposure of cell to ATP is represented by black bar. Typical traces are shown. (d) Expression of PLA2 s in astrocytes analyzed by Western blot. An aliquot of cell lysate subjected to SDS/PAGE (4–12%) followed by Western blot analysis using specific antibodies against group IV cPLA2 (1) and group VI iPLA2 (2). Two different blots are aligned.
To clarify the isoform of PLA2 involved, we tested ATP-stimulated release of DHA and AA in Ca2+-free medium. The cells were washed twice with buffer without Ca2+ and remained in this buffer for 30 min before the ATP stimulus was applied for 15 min. Under these conditions, ATP still stimulated DHA release (Figure 2a, inset), but failed to stimulate AA release (Figure 2b, inset). These results confirm our notion that release of DHA is mediated by activity of iPLA2, whereas the release of AA is due to calcium-dependent cPLA2.
It is known that iPLA2 does not need Ca2+ for enzymatic activity and cPLA2 demands a continuous rise of [Ca2+]i to be translocated from cytosol to substrate, which is the cellular membrane (Hirabayashi et al., 1999). Therefore, we studied changes of [Ca2+]i in ATP-stimulated cells in Ca2+-containing or Ca2+-free medium. As shown in Figure 2c, in the presence of extracellular Ca2+, after the initial peak response, in astrocytes [Ca2+]i stays on an elevated level during the whole time period of exposure to agonist (solid line). In the absence of extracellular Ca2+, only an initial peak was observed, and then [Ca2+]i rapidly fell to a level slightly lower than that seen before stimulation (dashed line).
The expression of PLA2 s in astrocytes was investigated using Western blot analysis. We could show expression of the 85 kDa group IV cPLA2. In addition, we found a protein band with a molecular mass of approximately 42 kDa, reactive to antibodies specific for group VI iPLA2 (Figure 2d).
Signal transduction pathways mediating release of PUFA
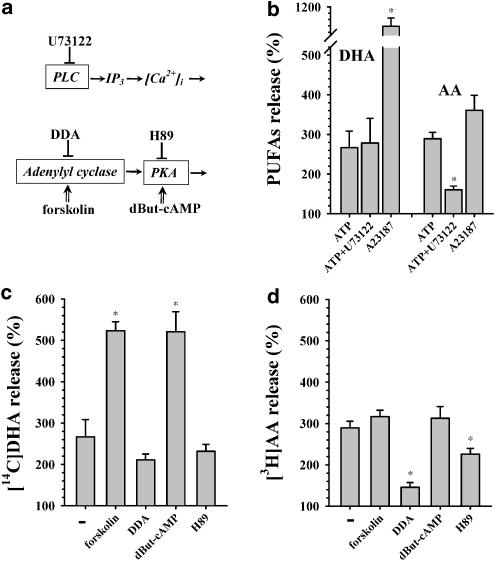
Activity of PLA2 s can be regulated by intracellular Ca2+ level and/or phosphorylation of PLA2 by different protein kinases (Capper & Marshall, 2001). Thus, we studied the influence of the following agents on ATP-stimulated release of DHA and AA: U73122, inhibitor of phospholipase C (PLC); calcium ionophore A23187; forskolin, activator of adenylyl cyclase; 2,3-dideoxyadenosine (DDA), inhibitor of adenylyl cyclase; N6, O2-dibutyryl-cAMP (dBut-cAMP), activator of protein kinase A (PKA); and H89, inhibitor of PKA. All substances, which are presented schematically in Figure 3a, were added 10 min prior to stimulation with ATP. The values are compared to the respective ATP-mediated release of the PUFAs.
Figure 3.
Different regulation of DHA and AA release from astrocytes via PLC/InsP3/Ca2+ and cAMP-PKA pathways. (a) Targets of the modulators used are shown for inhibitors by hammerheads (⊥) and for activators by arrows (⇑). (b) U73122 (5 μM) as inhibitor of PLC and A23187 (10 μM) as potent activator of [Ca2+]i were used to study involvement of the PLC/InsP3/Ca2+ pathway in DHA and AA release. Involvement of the cAMP-PKA pathway in DHA (c) and AA (d) release was estimated with activator (forskolin, 10 μM) and inhibitor (2,3-dideoxyadenosine (DDA), 20 μM) of adenylyl cyclase, and activator (dBut-cAMP, 1 mM) and inhibitor (H89, 10 μM) of PKA. Cells were labeled as described in the legend for Figure 1. Then 20 min later, modulators were added. After another 10 min, 20 μM ATP was added at 37°C for 15 min and then the radioactivity in the medium was measured, as described in Experimental procedures. Each point represents the mean ± s.e.m. Release without any addition was assumed as 100%. This experiment was carried out three times with similar results. Release was compared with release stimulated by ATP alone (‘-' in c and d). *The value is statistically (P<0.05) different from the control value for unaffected response to ATP.
Activity of cPLA2 depends upon intracellular Ca2+ rise. Activation of PLC and subsequent formation of inositol 1,4,5-trisphosphate (InsP3) lead to efflux of Ca2+ from the stores of endoplasmic reticulum and to a rise in [Ca2+]i. Inhibition of the PLC/InsP3 pathway by U73122 produced no change in DHA release, whereas at the same time AA release was almost completely suppressed by U73122 (Figure 3b). This result confirms our conclusion that the stimulated release of DHA is a calcium-independent process, while the release of AA is a calcium-dependent event.
Ca2+ ionophore activates influx of extracellular Ca2+ and, therefore, stimulates rise in [Ca2+]i. Upon exposure to A23187, astrocytes released large amounts of both DHA and AA (Figure 3b). Release of AA stimulated by ionophore was comparable with that stimulated by ATP. Remarkably, release of DHA was 11-fold of the control value (1130±30%) and more than three-fold higher than receptor-activated release (Figure 3b). Lower concentrations of A23187 stimulated release of DHA, which was still much higher than receptor-activated release (450±40% for 1 μM of A23187 and 770±50 % for 3 μM of A23187). We tested the effects of inhibitors of different protein kinases, PKA inhibitor H89 (10 μM), PKC inhibitor GF-109203X (20 μM) and p42/44 MAPK inhibitor U0126 (10 μM), on A23187-stimulated release of DHA. We found that GF-109203X reduced A23187-stimulated release of DHA by 35% and U0126 by 54%, but H89 did not inhibit DHA release.
cPLA2 exhibits multiple phosphorylation sites (Capper & Marshall, 2001). Phosphorylation sites for some of the kinases are not yet identified. The cAMP/PKA signal transduction pathway actively participates in the regulation of different types of kinase. Application of ATP together with forskolin, which leads to stimulation of adenylyl cyclase, induced a significant increase (up to 520±20%) in DHA release (Figure 3c). However, there was clearly no influence of forskolin on AA release (Figure 3d). Activation of PKA by dBut-cAMP significantly stimulated release of DHA (285±20% from nonstimulated release, data not shown) and resulted in a prominent increase in ATP-stimulated DHA release, from 270±40 to 520±50% (Figure 3c). Stimulation of astrocytes with dBut-cAMP also produced AA release (249±20% of nonstimulated release, data not shown), but did not affect the value of ATP-stimulated AA release (Figure 3d). The latter is consistent with the results obtained with forskolin, since this activator of adenylyl cyclase did not affect release of AA.
Inhibition of adenylyl cyclase by DDA produced only a minor decrease in ATP-stimulated DHA release (Figure 3c). Remarkably, DDA almost totally blocked ATP-induced release of AA, and inhibition of PKA by H89 also resulted in a significant decrease (34%) in AA release (Figure 3d).
Stimulation of DHA and AA release from astrocytes by various neuromediators
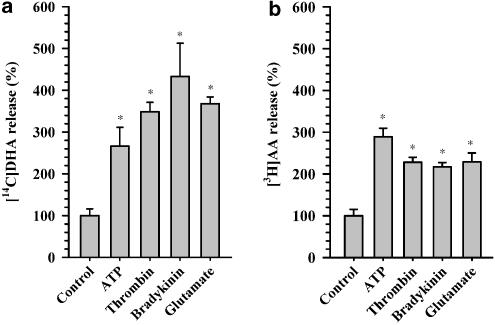
We compared the release of DHA and AA from rat astrocytes, which was stimulated by ATP with the release induced by thrombin, bradykinin and glutamate. These substances are chemically and structurally completely different. ATP is a nucleoside triphosphate, thrombin is a protease enzyme, bradykinin is a peptide, and glutamate is an amino acid. Nevertheless, all these substances are agonists of G protein-coupled receptors, whose activation in astrocytes leads to an increase in [Ca2+]i. In exploratory experiments, the concentrations of the agonists were varied and the lowest values producing stable maximal stimulatory effect after 15 min of exposure were identified. Those concentrations were determined as follows: thrombin (0.1 U ml−1), bradykinin (1 μM) and glutamate (100 μM).
All substances tested are able to induce significant DHA and AA release. The values for release of DHA were (in %±s.e.m., relative to unstimulated release): ATP, 270±40; thrombin, 350±20; bradykinin, 430±80; glutamate, 370±20 (Figure 4a). For release of AA, we obtained (in percentage, relative to unstimulated release): ATP, 290±20; thrombin, 230±10; bradykinin, 220±10; glutamate, 230±20, as depicted in Figure 4b. The potency of the stimuli differed between DHA and AA, as ATP was the most potent activator of AA release, and bradykinin and glutamate stimulated a large release of DHA.
Figure 4.
Release of DHA (a) and AA (b) from rat astrocytes stimulated with different agents. Cells were labeled with [14C]docosahexaenoic acid or [3 H]arachidonic acid in DMEM containing 10% fetal calf serum for 24 h, washed twice with HBS, and fresh DMEM was added. Then 20 min later, agents (ATP, 20 μM; thrombin, 0.1 U ml−1; bradykinin, 1 μM; and glutamate, 100 μM) were added at 37°C for 15 min and then radioactivity in the medium was measured as described in Experimental procedures. Stimulated release is compared with release without any addition (control), which is taken as 100%. Each point represents the mean±s.e.m. This experiment was carried out three times with similar results. *The value is statistically (P<0.05) different from the control value.
Discussion
The current investigation is the first one to give a detailed comparative analysis of release of DHA and AA from astrocytes. The possibility that DHA might play a role in the action of agonists in astrocytes is still largely ignored. So far only very few data are available concerning agonist-stimulated acute DHA release (Garcia & Kim, 1997; Birbes et al., 1998), besides the numerous studies of AA release (Bruner & Murphy, 1990; Stella et al., 1994; Tence et al., 1995; Chen & Chen, 1998; Lal et al., 1998; Sergeeva et al., 2002). We have also shown that the neurotransmitters bradykinin, glutamate and ATP, and the mediator in inflammation, thrombin, are capable of stimulating DHA and AA release. Thus, an important finding of the present study is the fact that release of both acids can be stimulated similarly by agents, which play a role in regulation of CNS function.
The main site of storage for PUFAs in mammalia is the sn-2 position of membrane phospholipids, and the enzymes responsible for their release are intracellular phospholipases of the A2 family (Capper & Marshall, 2001). Literature data concerning the mechanism of transfer of free PUFAs across plasma membrane are still controversial (for review see Berk, 1996; Zakim, 1996). Nevertheless, this transfer is very fast (within a time range of seconds) and thus this transient process has not to be considered in our study.
Genes coding for iPLA2 and cPLA2 have been reported to be expressed in different brain regions and in astrocytes (Molloy et al., 1998; Zanassi et al., 1998; Yang et al., 1999). Using Western blot analysis we have shown expression of group IV cPLA2 in astrocytes. Importantly, we have also found a protein with a molecular mass of approximately 42 kDa that was reactive to antibodies specific for the internal region of 88 kDa group VI iPLA2. Group VI iPLA2 has multiple splice variants (Winstead et al., 2000), and the protein we detected might be one of them. Such a protein with a molecular mass of about 40 kDa and reactive to antibodies to full-length iPLA2 has also been detected by two other research groups (Liu & McHowat, 1998; Larsson Forsell et al., 1999).
Another possible candidate protein with a molecular mass of 40 kDa could be plasmalogen-selective iPLA2 (Hirashima et al., 1992), which has been detected in the brain. This enzyme preferentially hydrolyzes plasmalogens, phospholipids containing vinyl-ether chain at the sn-1 position. This DHA-rich type of phospholipids is particularly abundant in brain tissue and is considered to be a main acceptor for incorporated or newly synthesized DHA. Plasmalogens from neuronal membranes contain up to 70% of DHA at the sn-2 position (Farooqui & Horrocks, 2001; Nagan & Zoeller, 2001).
There is considerable interest in determining the role of each PLA2 in mediating cellular functions. cPLA2 is considered as signaling PLA2, regulating stimulus-induced AA metabolism (Capper & Marshall, 2001). The Ca2+-independent cytosolic PLA2 (iPLA2) has generally been assumed to play the role of house-keeping enzyme, participating generally in slow lipid remodeling (Winstead et al., 2000).
In the present investigation we have demonstrated that acute release of DHA from stimulated astrocytes is mediated by iPLA2, which can be selectively inhibited by suicide substrate BEL (Ackermann et al., 1995). This substance had only a minor inhibitory effect on AA release. AA release, on the other hand, can be selectively and completely blocked by MAFP, a general inhibitor of all cytosolic PLA2 s (Riendeau et al., 1994; Lio et al., 1996). This pharmacological evidence leads us to the conclusion that AA is released by Ca2+-dependent group IV cPLA2. Therefore, in astrocytes different enzymes mediate the release of different acids; group VI iPLA2 is responsible for DHA release and group IV cPLA2 for AA release. Further data support this conclusion. Group IV cPLA2 requires prolonged rise in cytosolic Ca2+ concentration to be stably translocated to membranes (Hirabayashi et al., 1999). Removal of Ca2+ from the extracellular medium, as we show here, dramatically decreases the time-span of Ca2+ increase after stimulation of astrocytes by ATP. Thus, the suppression of AA release under Ca2+-free conditions strongly supports the notion that group IV cPLA2 has a main role in acute AA release. Disruption of Ca2+ responses, however, did not affect the release of DHA. The dependence of AA release on Ca2+ is confirmed by inhibition of AA release by U73122. U73122 abolishes release of Ca2+ from the stores in endoplasmic reticulum because it inhibits PLC and subsequent production of InsP3 (Jin et al., 1994; Jeremic et al., 2001). Expression of mRNA for secretory group II PLA2 has been shown in brain and in astrocytes (Molloy et al., 1998; Zanassi et al., 1998; Thomas et al., 2000). However, this enzyme does not play a role in the processes that we observed in the current study.
Interesting results were obtained with Ca2+ ionophore A23187, which bypasses the receptor pathway and directly causes influx of extracellular Ca2+. Despite the fact that DHA release is not dependent on extracellular Ca2+, the A23187-induced release of DHA was more than two times higher than that caused by the other stimuli. There are data that Ca2+ ionophore A23187, besides increasing [Ca2+]i, has some other specific effects. These could be activation of kinases, such as cGMP-PK (Pryzwansky et al., 1995) and PKC (Chakraborti et al., 1992), which in turn might additionally evoke release of DHA. The addition of lower than 10 μM concentrations of A23187 (1 and 3 μM) which are reported as nonstimulating for PKC (Chakraborti et al., 1992), resulted in reduced release of DHA, resulted in reduced release of DHA, but still higher than that observed for receptor agonists. In our study, we have found that release stimulated by 10 μM of ionophore can be blocked in part by inhibitors of PKC and p42/44 MAPK. Nevertheless, calcium ionophore A23187 is a very potent, but rather nonphysiological, stimulus and therefore it is often difficult to relate its effects to signaling pathways found in cells.
Most importantly, our results demonstrate that release of PUFAs in astrocytes, besides involving different isoforms of PLA2 s, is differently regulated by the messenger cAMP. We observed that release of DHA is weakly dependent on inhibition of PKA or reduction of cAMP level. DHA release, however, can be dramatically amplified by forskolin, a stimulator of cAMP synthesis. Interestingly, the level of DHA release from astrocytes that is achieved by stimulation with A23187 is almost as high as the DHA release evoked by simultaneous stimulation of the cAMP/PKA pathway and of ATP receptors. The release of AA can be blocked by adenylyl cyclase inhibitor DDA and significantly reduced by PKA inhibitor H89. Our finding that both forskolin and dBut-cAMP did not potentiate the AA release, which was evoked by ATP, could be due to the fact that ATP-stimulated AA release is close to the maximally possible level, which is also observed with ionophore A23187, and thus cannot be further enhanced. On the other hand, it cannot be excluded that in astrocytes the basal activity of PKA is relatively high during activation of cPLA2 and hence further stimulation of the cAMP/PKA pathway does not result in increased cPLA2 activity.
Knowledge about regulation of intracellular PLA2 activity through the cAMP/PKA pathway is still rather vague. Some researchers report that activation of PKA downregulates synthesis of lipid mediators, including PAF and AA, and release of its metabolites (Xing et al., 1999; Flamand et al., 2002). Nevertheless, PKA, activated after stimulation of adenylyl cyclase, can in turn activate MAPK cascade (Aimond et al., 2000; Kievit et al., 2001). MAPK can phosphorylate cPLA2 (Aimond et al., 2000) and iPLA2 thereby increasing PLA2 activity (Kievit et al., 2001). As yet it is still largely unknown how phosphorylation affects PLA2 activity (Dessen et al., 1999; Song et al., 1999). Our data demonstrate that blockage or activation of adenylyl cyclase differently modulate release of DHA and AA through PKA.
Not much is known so far about regulation of DHA release. The present study is the first one to address the comparison of the regulation of hydrolysis of phospholipids containing DHA and AA within the brain. DHA and AA are important for various nervous system functions and, moreover, represent precursors of numerous physiologically active substances. Oxidized metabolites of DHA often have activity opposite to that of products from AA (eicosanoids) made by the same enzyme (O'Keefe et al., 1990; Arntzen et al., 1998; Akiba et al., 2000). Moreover, DHA at high concentrations can suppress prostanoid formation, because it binds to cyclooxygenase but is not converted by this enzyme (Marshall & Kulmacz, 1988; Akiba, 2000). Therefore, the balance between DHA and AA in the brain can control especially those processes that are related to inflammation. Importantly, we could separate the regulation of release of these two PUFAs. We here identify different isoforms of enzymes of the PLA2 family to be involved in release of DHA and AA. Furthermore, we reveal differences in signal transduction which controls activation of these enzymes. Our results provide possibilities for strategies to suppress the development of pathological states in brain tissue, where release of PUFAs and their metabolic conversion into inflammatory mediators are involved.
Acknowledgments
This work was supported by BMBF (01ZZ0170), Land Sachsen-Anhalt (Grant 2923A/0028H), and Fonds der Chemischen Industrie.
Abbreviations
- AA
arachidonic acid
- cAMP
cyclic adenosine monophosphate
- dBut-cAMP, N6
O2-dibutyryl-cAMP
- ATP
adenosine 5′-triphosphate
- BEL
4-bromoenol lactone
- [Ca2+]i
concentration of free intracellular Ca2+
- CNS
central nervous system
- DDA
2,3-dideoxyadenosine
- DHA
docosahexaenoic acid
- EGTA
ethylenglycol-bis (β-aminoethylether)N,N,N′,N′-tetraacetic acid
- HBS
HEPES-buffered saline
- InsP3
inositol 1,4,5-trisphosphate
- MAFP
methyl arachidonyl fluorophosphonate
- PLA2
phospholipase A2
- iPLA2
calcium-independent PLA2
- cPLA2
cytosolic PLA2, Pls-PLA2, plasmalogen-selective PLA2
- PLC
phospholipase C
- PUFA
polyunsaturated fatty acid
- PKA
protein kinase A
References
- ACKERMANN E.J., CONDE-FRIEBOES K., DENNIS E.A. Inhibition of macrophage Ca2+-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J. Biol.Chem. 1995;270:445–450. doi: 10.1074/jbc.270.1.445. [DOI] [PubMed] [Google Scholar]
- AIMOND F., RAUZIER J.M., BONY C., VASSORT G. Simultaneous activation of p38 MAPK and p42/44 MAPK by ATP stimulates the K+ current ITREK in cardiomyocytes. J. Biol. Chem. 2000;275:39110–39116. doi: 10.1074/jbc.M008192200. [DOI] [PubMed] [Google Scholar]
- AKIBA S., MURATA T., KITATANI K., SATO T. Involvement of lipoxygenase pathway in docosapentaenoic acid-induced inhibition of platelet aggregation. Biol. Pharm. Bull. 2000;23:1293–1297. doi: 10.1248/bpb.23.1293. [DOI] [PubMed] [Google Scholar]
- ARNTZEN K.J., BREKKE O.L., VATTEN L., AUSTGULEN R. Reduced production of PGE2 and PGF2 alpha from decidual cell cultures supplemented with N-3 polyunsaturated fatty acids. Prostaglandins Other Lipid Mediat. 1998;56:183–195. doi: 10.1016/s0090-6980(98)00048-3. [DOI] [PubMed] [Google Scholar]
- BERK P.D. How do long-chain free fatty acids cross cell membranes. Proc. Soc. Exp. Biol. Med. 1996;212:1–4. doi: 10.3181/00379727-212-43985. [DOI] [PubMed] [Google Scholar]
- BIRBES H., PAGEAUX J.F., FAYARD J.M., LAGARDE M., LAUGIER C. Protein kinase C inhibitors stimulate arachidonic and docosahexaenoic acids release from uterine stromal cells through a Ca2+-independent pathway. FEBS Lett. 1998;432:219–224. doi: 10.1016/s0014-5793(98)00869-2. [DOI] [PubMed] [Google Scholar]
- BRUNER G., MURPHY S. ATP-evoked arachidonic acid mobilization in astrocytes is via a P2Y-purinergic receptor. J. Neurochem. 1990;55:1569–1575. doi: 10.1111/j.1471-4159.1990.tb04940.x. [DOI] [PubMed] [Google Scholar]
- CALDERARO V., PARRILLO C., BALESTRIERI M.L., GIOVANE A., FILIPPELLI A., ROSSI F. Docosahexaenoic acid and signaling pathways in rabbit colon. Mol. Pharmacol. 1994;45:737–746. [PubMed] [Google Scholar]
- CAPPER E.A., MARSHALL L.A. Mammalian phospholipases A2: mediators of inflammation, proliferation and apoptosis. Prog. Lipid Res. 2001;40:167–197. doi: 10.1016/s0163-7827(01)00002-9. [DOI] [PubMed] [Google Scholar]
- CHAKRABORTI S., MICHAEL J.R., SANYAL T. Defining the role of protein kinase C in calcium-ionophore-(A23187)-mediated activation of phospholipase A2 in pulmonary endothelium. Eur. J. Biochem. 1992;206:965–972. doi: 10.1111/j.1432-1033.1992.tb17007.x. [DOI] [PubMed] [Google Scholar]
- CHEN W.C., CHEN C.C. ATP-induced arachidonic acid release in cultured astrocytes is mediated by Gi protein coupled P2Y1 and P2Y2 receptors. Glia. 1998;22:360–370. doi: 10.1002/(sici)1098-1136(199804)22:4<360::aid-glia5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- DESSEN A., TANG J., SCHMIDT H., STAHL M., CLARK J.D., SEEHRA J., SOMERS W.S. Crystal structure of human cytosolic phospholipase A2 reveals a novel topology and catalytic mechanism. Cell. 1999;97:349–360. doi: 10.1016/s0092-8674(00)80744-8. [DOI] [PubMed] [Google Scholar]
- FAROOQUI A.A., HORROCKS L.A. Plasmalogens, phospholipase A2, and docosahexaenoic acid turnover in brain tissue. J. Mol. Neurosci. 2001;16:263–272. doi: 10.1385/jmn:16:2-3:263. [DOI] [PubMed] [Google Scholar]
- FAROOQUI A.A., HORROCKS L.A., FAROOQUI T. Glycerophospholipids in brain: their metabolism, incorporation into membranes, functions, and involvement in neurological disorders. Chem. Phys. Lipids. 2000;106:1–29. doi: 10.1016/s0009-3084(00)00128-6. [DOI] [PubMed] [Google Scholar]
- FELDER C.C., KANTERMAN R.Y., MA A.L., AXELROD J. A transfected m1 muscarinic acetylcholine receptor stimulates adenylate cyclase via phosphatidylinositol hydrolysis. J. Biol. Chem. 1989;264:20356–20362. [PubMed] [Google Scholar]
- FLAMAND N., SURETTE M.E., PICARD S., BOURGOIN S., BORGEAT P. Cyclic AMP-mediated inhibition of 5-lipoxygenase translocation and leukotriene biosynthesis in human neutrophils. Mol. Pharmacol. 2002;62:250–256. doi: 10.1124/mol.62.2.250. [DOI] [PubMed] [Google Scholar]
- GARCIA M.C., KIM H.Y. Mobilization of arachidonate and docosahexaenoate by stimulation of the 5-HT2A receptor in rat C6 glioma cells. Brain Res. 1997;768:43–48. doi: 10.1016/s0006-8993(97)00583-0. [DOI] [PubMed] [Google Scholar]
- GARCIA M.C., WARD G., MA Y.C., SALEM N., KIM H.Y. Effect of docosahexaenoic acid on the synthesis of phosphatidylserine in rat brain in microsomes and C6 glioma cells. J. Neurochem. 1998;70:24–30. doi: 10.1046/j.1471-4159.1998.70010024.x. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- HAMPRECHT B., LOFFLER F. Primary glial cultures as a model for studying hormone action. Methods Enzymol. 1985;109:341–345. doi: 10.1016/0076-6879(85)09097-8. [DOI] [PubMed] [Google Scholar]
- HIRABAYASHI T., KUME K., HIROSE K., YOKOMIZO T., IINO M., ITOH H., SHIMIZU T. Critical duration of intracellular Ca2+ response required for continuous translocation and activation of cytosolic phospholipase A2. J. Biol. Chem. 1999;274:5163–5169. doi: 10.1074/jbc.274.8.5163. [DOI] [PubMed] [Google Scholar]
- HIRASHIMA Y., FAROOQUI A.A., MILLS J.S., HORROCKS L.A. Identification and purification of calcium-independent phospholipase A2 from bovine brain cytosol. J. Neurochem. 1992;59:708–714. doi: 10.1111/j.1471-4159.1992.tb09426.x. [DOI] [PubMed] [Google Scholar]
- JAMES M.J., GIBSON R.A., CLELAND L.G. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am. J. Clin Nutr. 2000;71:343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- JEREMIC A., JEFTINIJA K., STEVANOVIC J., GLAVASKI A., JEFTINIJA S. ATP stimulates calcium-dependent glutamate release from cultured astrocytes. J. Neurochem. 2001;77:664–675. doi: 10.1046/j.1471-4159.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- JIN W., LO T.M., LOH H.H., THAYER S.A. U73122 inhibits phospholipase C-dependent calcium mobilization in neuronal cells. Brain Res. 1994;642:237–243. doi: 10.1016/0006-8993(94)90927-x. [DOI] [PubMed] [Google Scholar]
- JUMP D.B. The biochemistry of n-3 polyunsaturated fatty acids. J. Biol. Chem. 2002;277:8755–8758. doi: 10.1074/jbc.R100062200. [DOI] [PubMed] [Google Scholar]
- KANTERMAN R.Y., FELDER C.C., BRENNEMAN D.E., MA A.L., FITZGERALD S., AXELROD J. Alpha 1-adrenergic receptor mediates arachidonic acid release in spinal cord neurons independent of inositol phospholipid turnover. J. Neurochem. 1990;54:1225–1232. doi: 10.1111/j.1471-4159.1990.tb01952.x. [DOI] [PubMed] [Google Scholar]
- KATSUKI H., OKUDA S. Arachidonic acid as a neurotoxic and neurotrophic substance. Prog. Neurobiol. 1995;46:607–636. doi: 10.1016/0301-0082(95)00016-o. [DOI] [PubMed] [Google Scholar]
- KIEVIT P., LAUTEN J.D., MAURER R.A. Analysis of the role of the mitogen-activated protein kinase in mediating cyclic-adenosine 3′,5′-monophosphate effects on prolactin promoter activity. Mol. Endocrinol. 2001;15:614–624. doi: 10.1210/mend.15.4.0614. [DOI] [PubMed] [Google Scholar]
- KIM H.Y., AKBAR M., KIM K.Y. Inhibition of neuronal apoptosis by polyunsaturated fatty acids. J. Mol. Neurosci. 2001;16:223–227. doi: 10.1385/JMN:16:2-3:223. [DOI] [PubMed] [Google Scholar]
- KRAMER R.M., ROBERTS E.F., UM S.L., BORSCH-HAUBOLD A.G., WATSON S.P., FISHER M.J., JAKUBOWSKI J.A. p38 mitogen-activated protein kinase phosphorylates cytosolic phospholipase A2 (cPLA2) in thrombin-stimulated platelets. Evidence that proline-directed phosphorylation is not required for mobilization of arachidonic acid by cPLA2. J. Biol. Chem. 1996;271:27723–27729. doi: 10.1074/jbc.271.44.27723. [DOI] [PubMed] [Google Scholar]
- LAL M.A., PROULX P.R., HEBERT R.L. A role for PKC epsilon and MAP kinase in bradykinin-induced arachidonic acid release in rabbit CCD cells. Am. J. Physiol. 1998;274:F728–F735. doi: 10.1152/ajprenal.1998.274.4.F728. [DOI] [PubMed] [Google Scholar]
- LARSSON FORSELL P.K., KENNEDY B.P., CLAESSON H.E. The human calcium-independent phospholipase A2 gene multiple enzymes with distinct properties from a single gene. Eur. J. Biochem. 1999;262:575–585. doi: 10.1046/j.1432-1327.1999.00418.x. [DOI] [PubMed] [Google Scholar]
- LAURITZEN I., BLONDEAU N., HEURTEAUX C., WIDMANN C., ROMEY G., LAZDUNSKI M. Polyunsaturated fatty acids are potent neuroprotectors. EMBO J. 2000;19:1784–1793. doi: 10.1093/emboj/19.8.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN L.L., WARTMANN M., LIN A.Y., KNOPF J.L., SETH A., DAVIS R.J. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- LIO Y.C., REYNOLDS L.J., BALSINDE J., DENNIS E.A. Irreversible inhibition of Ca2+-independent phospholipase A2 by methyl arachidonyl fluorophosphonate. Biochim. Biophys. Acta. 1996;1302:55–60. doi: 10.1016/0005-2760(96)00002-1. [DOI] [PubMed] [Google Scholar]
- LIU S.J., MCHOWAT J. Stimulation of different phospholipase A2 isoforms by TNF-alpha and IL-1beta in adult rat ventricular myocytes. Am. J. Physiol. 1998;275:H1462–H1472. doi: 10.1152/ajpheart.1998.275.4.H1462. [DOI] [PubMed] [Google Scholar]
- MARKESBERY W.R. Oxidative stress hypothesis in Alzheimer's disease. Free Radic. Biol. Med. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- MARSHALL P.J., KULMACZ R.J. Prostaglandin H synthase: distinct binding sites for cyclooxygenase and peroxidase substrates. Arch. Biochem. Biophys. 1988;266:162–170. doi: 10.1016/0003-9861(88)90246-9. [DOI] [PubMed] [Google Scholar]
- MOLLOY G.Y., RATTRAY M., WILLIAMS R.J. Genes encoding multiple forms of phospholipase A2 are expressed in rat brain. Neurosci. Lett. 1998;258:139–142. doi: 10.1016/s0304-3940(98)00838-6. [DOI] [PubMed] [Google Scholar]
- MOORE S.A. Cerebral endothelium and astrocytes cooperate in supplying docosahexaenoic acid to neurons. Adv. Exp. Med. Biol. 1993;331:229–233. doi: 10.1007/978-1-4615-2920-0_36. [DOI] [PubMed] [Google Scholar]
- MOORE S.A.Polyunsaturated fatty acid synthesis and release by brain-derived cells in vitro J. Mol. Neurosci. 200116195–200.discussion 215–121 [DOI] [PubMed] [Google Scholar]
- MURTHY K.S., MAKHLOUF G.M. Differential regulation of phospholipase A2 (PLA2)-dependent Ca2+ signaling in smooth muscle by cAMP- and cGMP-dependent protein kinases. Inhibitory phosphorylation of PLA2 by cyclic nucleotide-dependent protein kinases. J. Biol. Chem. 1998;273:34519–34526. doi: 10.1074/jbc.273.51.34519. [DOI] [PubMed] [Google Scholar]
- NAGAN N., ZOELLER R.A. Plasmalogens: biosynthesis and functions. Prog. Lipid Res. 2001;40:199–229. doi: 10.1016/s0163-7827(01)00003-0. [DOI] [PubMed] [Google Scholar]
- O'KEEFE S.F., LAGARDE M., GRANDGIRARD A., SEBEDIO J.L. Trans n-3 eicosapentaenoic and docosahexaenoic acid isomers exhibit different inhibitory effects on arachidonic acid metabolism in human platelets compared to the respective cis fatty acids. J. Lipid Res. 1990;31:1241–1246. [PubMed] [Google Scholar]
- PETTEGREW J.W., KLUNK W.E., KANAL E., PANCHALINGAM K., MCCLURE R.J. Changes in brain membrane phospholipid and high-energy phosphate metabolism precede dementia. Neurobiol. Aging. 1995;16:973–975. doi: 10.1016/0197-4580(95)02017-9. [DOI] [PubMed] [Google Scholar]
- PRYZWANSKY K.B., WYATT T.A., LINCOLN T.M. Cyclic guanosine monophosphate-dependent protein kinase is targeted to intermediate filaments and phosphorylates vimentin in A23187-stimulated human neutrophils. Blood. 1995;85:222–230. [PubMed] [Google Scholar]
- RIENDEAU D., GUAY J., WEECH P.K., LALIBERTE F., YERGEY J., LI C., DESMARAIS S., PERRIER H., LIU S., NICOLL-GRIFFITH D.Arachidonyl trifluoromethyl ketone, a potent inhibitor of 85-kDa phospholipase A2, blocks production of arachidonate and 12-hydroxyeicosatetraenoic acid by calcium ionophore-challenged platelets J. Biol. Chem. 199426915619–15624.et al [PubMed] [Google Scholar]
- SERGEEVA M., STROKIN M., WANG H., UBL J.J., REISER G. Arachidonic acid and docosahexaenoic acid suppress thrombin-evoked Ca2+ response in rat astrocytes by endogenous arachidonic acid liberation. J. Neurochem. 2002;82:1252–1261. doi: 10.1046/j.1471-4159.2002.01052.x. [DOI] [PubMed] [Google Scholar]
- SIX D.A., DENNIS E.A. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim. Biophys. Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- SONG C., CHANG X.J., BEAN K.M., PROIA M.S., KNOPF J.L., KRIZ R.W. Molecular characterization of cytosolic phospholipase A2-beta. J. Biol. Chem. 1999;274:17063–17067. doi: 10.1074/jbc.274.24.17063. [DOI] [PubMed] [Google Scholar]
- STELLA N., ESTELLES A., SICILIANO J., TENCE M., DESAGHER S., PIOMELLI D., GLOWINSKI J., PREMONT J. Interleukin-1 enhances the ATP-evoked release of arachidonic acid from mouse astrocytes. J. Neurosci. 1997;17:2939–2946. doi: 10.1523/JNEUROSCI.17-09-02939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STELLA N., TENCE M., GLOWINSKI J., PREMONT J. Glutamate-evoked release of arachidonic acid from mouse brain astrocytes. J. Neurosci. 1994;174:568–575. doi: 10.1523/JNEUROSCI.14-02-00568.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TASHIRO T., YAMAMORI H., TAKAGI K., HAYASHI N., FURUKAWA K., NAKAJIMA N. n-3 versus n-6 polyunsaturated fatty acids in critical illness. Nutrition. 1998;14:551–553. doi: 10.1016/s0899-9007(98)00048-3. [DOI] [PubMed] [Google Scholar]
- TENCE M., MURPHY N., CORDIER J., PREMONT J., GLOWINSKI J. Synergistic effects of acetylcholine and glutamate on the release of arachidonic acid from cultured striatal neurons. J. Neurochem. 1995;64:1605–1613. doi: 10.1046/j.1471-4159.1995.64041605.x. [DOI] [PubMed] [Google Scholar]
- THOMAS G., BERTRAND F., SAUNIER B. The differential regulation of group II(A) and group V low molecular weight phospholipases A2 in cultured rat astrocytes. J. Biol. Chem. 2000;275:10876–10886. doi: 10.1074/jbc.275.15.10876. [DOI] [PubMed] [Google Scholar]
- UBL J.J., REISER G. Characteristics of thrombin-induced calcium signals in rat astrocytes. Glia. 1997;21:361–369. doi: 10.1002/(sici)1098-1136(199712)21:4<361::aid-glia3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- WHELAN J. Antagonistic effects of dietary arachidonic acid and n-3 polyunsaturated fatty acids. J. Nutr. 1996;126:1086S–1091S. doi: 10.1093/jn/126.suppl_4.1086S. [DOI] [PubMed] [Google Scholar]
- WILLIARD D.E., HARMON S.D., KADUCE T.L., PREUSS M., MOORE S.A., ROBBINS M.E., SPECTOR A.A. Docosahexaenoic acid synthesis from n-3 polyunsaturated fatty acids in differentiated rat brain astrocytes. J. Lipid Res. 2001;42:1368–1376. [PubMed] [Google Scholar]
- WINSTEAD M.V., BALSINDE J., DENNIS E.A. Calcium-independent phospholipase A2: structure and function. Biochim. Biophys. Acta. 2000;1488:28–39. doi: 10.1016/s1388-1981(00)00107-4. [DOI] [PubMed] [Google Scholar]
- XING M., POST S., OSTROM R.S., SAMARDZIJA M., INSEL P.A. Inhibition of phospholipase A2-mediated arachidonic acid release by cyclic AMP defines a negative feedback loop for P2Y receptor activation in Madin-Darby canine kidney D1 cells. J. Biol. Chem. 1999;274:10035–10038. doi: 10.1074/jbc.274.15.10035. [DOI] [PubMed] [Google Scholar]
- XU J., WENG Y.I., SIMONYI A., KRUGH B.W., LIAO Z., WEISMAN G.A., SUN G.Y., SIMONI A. Role of PKC and MAPK in cytosolic PLA2 phosphorylation and arachidonic acid release in primary murine astrocytes. J. Neurochem. 2002;83:259–270. doi: 10.1046/j.1471-4159.2002.01145.x. [DOI] [PubMed] [Google Scholar]
- YANG H.C., MOSIOR M., NI B., DENNIS E.A. Regional distribution, ontogeny, purification, and characterization of the Ca2+-independent phospholipase A2 from rat brain. J. Neurochem. 1999;73:1278–1287. doi: 10.1046/j.1471-4159.1999.0731278.x. [DOI] [PubMed] [Google Scholar]
- ZAKIM D. Fatty acids enter cells by simple diffusion. Proc. Soc. Exp. Biol. Med. 1996;212:5–14. doi: 10.3181/00379727-212-43986. [DOI] [PubMed] [Google Scholar]
- ZANASSI P., PAOLILLO M., SCHINELLI S. Coexpression of phospholipase A2 isoforms in rat striatal astrocytes. Neurosci. Lett. 1998;247:83–86. doi: 10.1016/s0304-3940(98)00262-6. [DOI] [PubMed] [Google Scholar]