Abstract
In this study, we determined a pharmacological profile of store-operated channels (SOCs) in smooth muscle cells of rabbit pial arterioles. Ca2+-indicator dyes, fura-PE3 or fluo-4, were used to track [Ca2+]i and 10 μM methoxyverapamil (D600) was present in all experiments on SOCs to prevent voltage-dependent Ca2+ entry. Store depletion was induced using thapsigargin or cyclopiazonic acid.
SOC-mediated Ca2+ entry was inhibited concentration dependently by Gd3+ (IC50 101 nM). It was also inhibited by 10 μM La3+ (70% inhibition, N=5), 100 μM Ni2+ (57% inhibition, N=5), 75 μM 2-aminoethoxydiphenylborate (66% inhibition, N=4), 100 μM capsaicin (12% inhibition, N=3) or preincubation with 10 μM wortmannin (76% inhibition, N=4). It was completely resistant to 1 μM nifedipine (N=5), 10 μM SKF96365 (N=6), 10 μM LOE908 (N=14), 10–100 μM ruthenium red (N=1+2), 100 μM sulindac (N=4), 0.5 mM streptomycin (N=3) or 1 : 10,000 dilution Grammostolla spatulata venom (N=4).
RT–PCR experiments on isolated arteriolar fragments showed expression of mRNA species for TRPC1, 3, 4, 5 and 6.
The pharmacological profile of SOC-mediated Ca2+ entry in arterioles supports the hypothesis that these SOCs are distinct from tonically active background channels and several store-operated and other nonselective cation channels described in other cells. Similarities with the pharmacology of TRPC1 support the hypothesis that TRPC1 is a subunit of the arteriolar smooth muscle SOC.>
Keywords: Calcium stores, calcium channels, store-operated channels, cerebral arteriole, smooth muscle
Introduction
Store-operated channels (SOCs) are Ca2+-permeable plasma membrane channels that open in response to depletion of sarco/endoplasmic reticular Ca2+ stores (‘store-depletion'). They are distinct from voltage-gated Ca2+ channels and open in the absence of receptor activation, and thus would appear not to be receptor-operated channels (ROCs). Although it remains plausible that store depletion and receptor activation are parallel signalling pathways to the same channel, in pial arterioles complete store depletion does not alter ROC activation, as if SOCs and ROCs are distinct (Guibert & Beech, 1999). Pharmacological distinctions between SOCs and ROCs have also been described in rat aorta smooth muscle and in the A7r5 smooth muscle cell line (Iwamuro et al., 1999; Zhang et al., 1999). Many cell types, including vascular smooth muscle cells, also express stretch-operated Ca2+-permeable channels (Welsh et al., 2000; Wu & Davis, 2001) and it is unclear if or how these are related to SOCs.
In several types of smooth muscle, SOC-mediated Ca2+ entry elicits contraction (Gibson et al., 1998). In others, however, there is a lack of contractile function, as if there is a tight association with the refilling of Ca2+ stores (Casteels & Droogmans, 1981; Flemming et al., 2002). Ca2+ entry through SOCs could also have other specific functions. For example, a role in smooth muscle proliferation has been proposed (Magnier-Gaubil et al., 1996; Golovina, 1999; Golovina et al., 2001; Yu et al., 2003) and it can be hypothesised that SOCs have a discrete function in relation to gene expression, as certain other Ca2+ channels do in neurones (Crabtree, 1999; Hunton et al., 2002). SOCs may also have general, housekeeping functions, for example, to regulate sarco/endoplasmic reticulum Ca2+ concentration. Thus, in many respects, the function of SOCs in smooth muscle is poorly understood and several possibilities remain to be explored.
Even within the vascular smooth muscle cell type, evidence for SOC diversity is emerging. For example, SOCs are outwardly rectifying nonselective cation channels in aortic smooth muscle cells, whereas they are inwardly rectifying with a marked selectivity for Ca2+ in portal vein smooth muscle cells (Trepakova et al., 2001; Albert & Large, 2002). SOCs in pulmonary artery smooth muscle cells are nifedipine-resistant, whereas those in retinal arterioles are nifedipine-sensitive (Curtis & Scholfield, 2001; Ng & Gurney, 2001). SOCs also exist in other cell types, a striking example of which is the highly Ca2+-selective calcium-release activated calcium (CRAC) channel of rat basophilic leukaemia (RBL) and Jurkat cells (Barritt, 1999; Lewis, 1999). SOC diversity may be explained by differential expression of channel subunits. The primary candidate subunits of mammalian SOCs are homologues of the Drosophila TRP protein. These mammalian TRP proteins are numerous (at least 19) and on the basis of amino-acid sequence can be divided into three subfamilies (Clapham et al., 2001; Montell et al., 2002). One of these (the TRPV subfamily) includes the well-characterised vanilloid or capsaicin receptor (or TRPV1; Caterina et al., 1997) and the CaT1 protein (TRPV6), which has been proposed as a subunit of the CRAC channel (Yue et al., 2001, but see Voets et al., 2001). Most studies, however, have focused on the hypothesis that the TRPC subfamily of TRP proteins is involved in SOC formation. For example, endothelial cells of TRPC4 knockout mice have reduced SOC-mediated signals (Freichel et al., 2001), and a TRPC1 blocking antibody and antisense DNA targeted to TRPC1 mRNAs inhibit native SOCs (Liu et al., 2000; Brough et al., 2001; Xu & Beech, 2001; Beech et al., 2003).
There is a paucity of data on the pharmacology of SOCs and related channels in smooth muscle (McFadzean & Gibson, 2002). In this study, we aimed to produce a pharmacological profile of SOCs in cerebral arteriolar smooth muscle cells based on the known pharmacology of a range of other Ca2+-permeable and nonselective cation channels. The data enable comparisons with related channels and heterologously expressed TRPC proteins, testing of the hypothesis that SOCs are diverse, and provide a foundation both for future improvements in selective SOC pharmacology and studies aimed at revealing SOC functions.
Methods
Male Dutch dwarf rabbits (1–1.5 kg) were killed by an intravenous overdose of 70 mg kg−1 sodium pentobarbitone in accordance with the Code of Practice, U.K. Animals Scientific Procedures Act 1986. The brain was placed in ice-cold oxygenated Hanks solution and fragments of pial membrane dissected from across the cortical surface and incubated in Hanks solution containing 0.032 mg ml−1 protease and 0.2 mg ml−1 collagenase (type 1A) for 10 min at 37°C. The mixture was placed at 4°C for 15 min and mechanically agitated to isolate fragments of arterioles. After centrifugation (1000 r.p.m.) for 5 min, the supernatant was replaced with fresh Hanks solution. Arterioles were resuspended and dropped onto polylysine-coated coverslips and stored at 4°C. Experiments were performed within 10 h. Arteriole fragments used in recordings had an external diameter of <45 μm, and lacked visible adventitia or endothelial cells (Cheong et al., 2001).
For Ca2+-imaging experiments, isolated arterioles were pre-incubated with 1–5 μM fura-PE3 AM (Vorndran et al., 1995) or 1 μM fluo-4 AM (Gee et al., 2000) at 30–37°C for 1 h in standard bath solution. This was followed by a 0.5–1 h wash period in standard bath solution at room temperature. The fura-PE3 AM and fluo-4 AM incubation and wash periods and all experiments were in the presence of 10 μM methoxyverapamil (D600), unless [Ca2+]i-elevation in response to raised [K+]o was studied. In elevated [K+]o experiments, wortmannin (1 μM) was included in the fura-PE3 incubation solution to prevent contraction without effect on voltage-gated Ca2+ channels (Unno et al., 1998). Preincubation with 1 μM thapsigargin (TG) occurred for 0.5–1.5 h, and during the fura-PE3 and fluo-4 loading and washing periods when applicable.
Fura-PE3 and fluo-4 fluorescence was observed with an inverted epifluorescence microscope (Nikon, Japan; or Zeiss, Germany). A xenon arc lamp provided excitation light, the wavelength of which was selected by a monochromator (Till Photonics, Germany). Emission was collected via 510 and 530 nm filters for fura-PE3 and fluo-4, respectively. A cooled CCD camera (Hamamatsu, Japan) sampled the collected light, producing images at 12-bit (Orca-ER) or 14-bit (H4880-82) resolution. Every 10 or 20 s, images were sampled in pairs for two fura-PE3 excitation wavelengths (340, 345 or 355 and 380 nm) or singularly for the fluo-4 excitation wavelength (494 nm). Images were analysed off-line using regions of interest (ROIs), which selected parts of the image frame corresponding to individual smooth muscle cells within arterioles. Three ROIs were also selected that were distant from the arteriole and used for background subtraction. [Ca2+]i is expressed as the ratio of the background-subtracted emission intensities for the two fura-PE3 excitation wavelengths ((340, 345 or 355 nm)/(380 nm)) or as the background-subtracted emission intensity for the single fluo-4 excitation wavelength (494 nm). With the Nikon imaging system, 345 or 355/380 nm excitation wavelengths were used while those used with the Zeiss imaging system were 340/380 nm. This was because of different light transmission and capture efficiencies. Thus, baseline R values varied between imaging systems. Imaging was controlled by Openlab 2 software (Image Processing & Vision Company Ltd, U.K.). Analysis and image presentation utilised Origin, Excel and Powerpoint software. Agents were applied to arterioles using a continuous bath perfusion system with a flow rate of 4 ml min−1. All experiments were at room temperature.
Hanks solution contained (mM): NaCl 137, NaH2PO4 0.34, KCl 5.4, K2HPO4 0.44, D-glucose 8, N-[2-hydroxyethyl]piperazine-N′-2-ethanesulphonic acid (HEPES) 5, CaCl2 0.01. Standard bath solution contained (mM): NaCl 130, KCl 5, D-glucose 8, HEPES 10, MgCl2 1.2, CaCl2 1.5. Ca2+-free solution was standard bath solution in which 0.4 mM EGTA replaced the CaCl2. When the extracellular K+ concentration was raised, the NaCl concentration in the bath solution was reduced by the equimolar amount. All solutions were titrated to pH 7.4 with NaOH.
Two protocols were used to deplete Ca2+ stores and activate SOCs. In the first protocol, arterioles were pretreated with 1 μM TG in standard (1.5 mM Ca2+) bath solution. In the second protocol, 10 μM cyclopiazonic acid (CPA) was applied in Ca2+-free solution and store-operated Ca2+ entry was observed on the subsequent readdition of 1.5 mM Ca2+.
For arteriolar diameter measurements, a video edge-detection system (Living Systems Instrumentation, Inc., Burlington, U.S.A.) was used. Arterioles were placed on a bath on the stage of an inverted trinocular microscope (Nikon TMS, Japan) with a CCD camera (Sony, Japan). Signals were captured by an A-D converter (Picolog software, Pico Technology, Cambridge, U.K.) and stored on a computer.
Messenger RNA was isolated from arteriole fragments with Dynabeads® Oligo (dT)25 according to the manufacturer's instructions (Dynal). Bead complexes were washed and transferred to a 20 μl SuperScript™ reverse transcriptase (Gibco-BRL) reaction at 42°C for 60 min. Forward and reverse PCR primer sequences (accession numbers are in parentheses) were β-actin (V01217) TTGTAACCAACTGGGACGATATG and GATCTTGATCTTCATGGTGCTGG, TRPC1 (U31110) TGGTATGAAGGGTTGGAAGAC and GGTATCATTGCTTTGCTGTTC, TRPC3 (U47050) TGACTTCCGTTGTGCTCAAATATG and CCTT-CTGAAGTCTTCTCCTTCTGC, TRPC4 (AF175406) TCTGCAGATATCTCTGGGAAGGATGC and AAGCTT-TGTTCGAGCAAATTTCCATTC, TRPC5 (AF029983) TGAGAATGA-GAACTTGGAG and TACTCAGCCTTGAACTCATTC, and TRPC6 (AF057748) AGTTTTAAGACACTGTTCTGG and TTCTGATATTGTCTTGGAGG. Thermal cycling was 95°C (5 min), 40 cycles at 94°C (30 s), 53–60°C (1 min), 72°C (1 min), and 72°C (7 min). Products were detected on a 1.5% agarose gel. Product identities of TRPC1, TRPC4 and β-actin were also confirmed by direct sequencing using an ABI PRISM dye terminator.
Data sets are expressed as mean±s.e.m. for the number of cells (ROIs) from which measurements were made. This involved up to five cells from each arteriole and so numbers of arterioles are also given: n/N, where n is the number of cells and N the number of arterioles. Statistical comparisons between two groups were made using unpaired Student's t-tests in which a significant difference was assumed if P<0.05. Some data sets were fit with the Hill equation, {(Dmax−Dmin)xs/(xs+EC50s)}+Dmin, where x is the concentration, s the slope, EC50 the concentration giving half-maximal response and Dmax and Dmin are the maximum and minimum values.
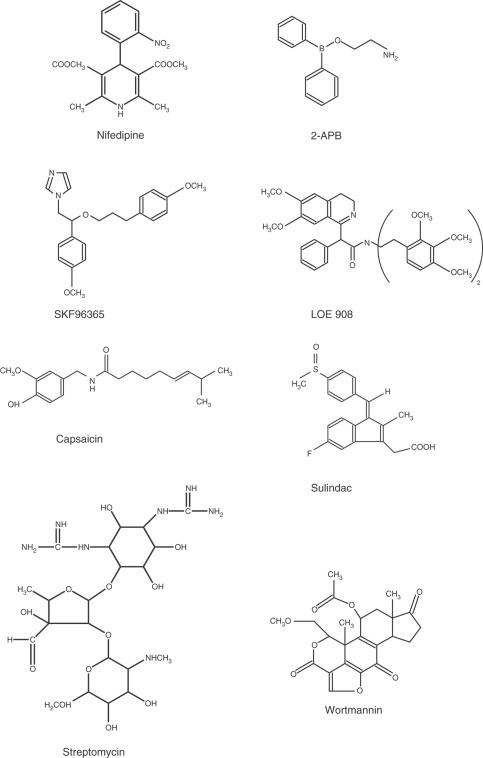
General salts were from BDH (U.K.) or Sigma (U.K.). Protease, collagenase, TG, D600, Gd3+, La3+, Ni2+, capsaicin, nifedipine, streptomycin and sulindac were from Sigma. 2-Aminoethoxydiphenylborate (2-APB) was from Tocris Cookson Limited (U.K.) or Sigma. Fura-PE3 AM, endothelin-1 (ET-1) and SKF96365 were from Calbiochem (U.K.). Fluo-4 was from Molecular Probes (U.K.). CPA was from Affiniti Research Products Limited (U.K.) or Tocris Cookson Limited. Ruthenium red was from Calbiochem or Sigma. Wortmannin (WT) was from Tocris Cookson Limited. Grammostolla spatulata venom was from Spider Pharm Inc. (Yarnell, AZ, U.S.A.). LOE 908 was a kind gift from Boehringer Ingelheim (Germany). Chemical structures of some of these agents are shown in Figure 1. Ruthenium red is [(NH3)5RuORu(NH3)4ORu(NH3)5]Cl6. LOE 908 is (RS)-(3,4-dihydro-6,7-dimethoxyisoquinoline-1-γl)-2-phenyl-N,N-di-[2-(2,3,4-trimethoxyphenyl)ethyl]acetamide. SKF96365 is 1-{β-[3-(4-methoxyphenyl)propoxy]-4-methoxyphenethyl}-1H-imidazole hydrochloride.
Figure 1.
Chemical structures of agents tested for activity against cerebral arteriolar SOCs.
Fura-PE3 AM, fluo-4 AM, capsaicin, TG, 2-APB, LOE 908, sulindac and WT were made up as stock solutions in 100% dimethyl sulphoxide (DMSO) and, with the exception of experiments involving capsaicin, the final DMSO concentration did not exceed 0.2%. To avoid precipitation of capsaicin (100 μM), bath solutions contained a total of 1% DMSO. In these experiments, 1% DMSO was present throughout (i.e. including the period before application of capsaicin). Nifedipine was made up as a stock solution in 100% ethanol. Other chemicals were prepared in water/salt solution.
Results
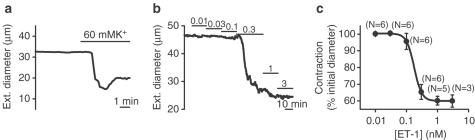
Viability of the enzymatically isolated rabbit arteriolar fragments was confirmed by contraction studies. Arterioles contracted to 60 mM K+ or endothelin-1 in the extracellular bath solution (Figure 2a–c). The concentration dependence of the response to endothelin-1 was similar to our previous findings and to that observed in human pial arteries (Guibert & Beech, 1999; Pierre & Davenport, 1999).
Figure 2.
Contractile response of isolated rabbit cerebral arterioles to elevated external K+ concentration and endothelin-1 (ET-1). (a, b) Plots of external diameter of arterioles against time. (a) An isolated arteriole contracted to 60 mM K+ and then partially relaxed in the continued presence of 60 mM K+. (b) ET-1 caused concentration-dependent contraction of arterioles. Concentrations are in nM. (c) Concentration–response curve for ET-1, N=number of arterioles. EC50=0.18 nM. Data shown are mean±s.e.m. and the smooth curve is the fitted Hill equation.
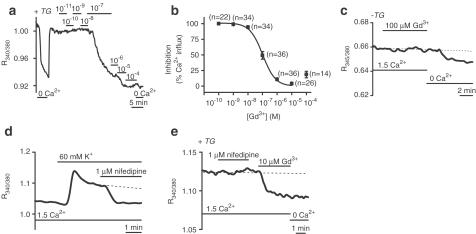
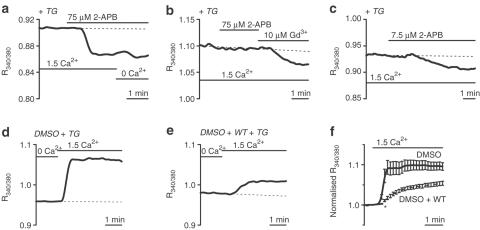
SOCs were investigated in the presence of 10 μM D600, which completely blocks 60 mM K+ evoked Ca2+ entry in these arterioles (Guibert & Beech, 1999) and avoids complications due to Ca2+ entry through voltage-gated Ca2+ channels. SOCs activated by store depletion with TG were inhibited concentration dependently by Gd3+ with an IC50 of 101 nM (Figure 3a, b). Acute store depletion with CPA in standard (1.5 mM Ca2+) bath solution similarly induced Gd3+ sensitivity (n/N=32/8) (data not shown). Gd3+ (100 μM) had no effect in the absence of store depletion (Figure 3c) (n/N=43/10).
Figure 3.
Store depletion activates Gd3+-sensitive, nifedipine-resistant SOCs in smooth muscle cells of isolated cerebral arterioles. [Ca2+]i is given as the ratio of fura-PE3 signals. Arterioles were in standard (1.5 mM Ca2+) bath solution unless indicated. Ca2+-free bath solution (‘0 Ca2+) contained 0.4 mM EGTA. (a–c) All solutions contained 10 μM D600. (a) Inhibition of SOC-mediated [Ca2+]i rise by increasing Gd3+ concentrations in a TG-pretreated arteriole. (b) Concentration–inhibition curve for the effect of Gd3+ on arteriolar SOCs. n=number of individual smooth muscles. N (number of arterioles) was ⩾4 for each Gd3+ concentration. IC50=101 nM, slope=1.00. Data shown are mean±s.e.m. and the smooth curve is the fitted Hill equation. (c) No effect of 100 μM Gd3+ without TG pretreatment. Ca2+-free solution reduced [Ca2+]i. (d) In the absence of D600, the [Ca2+]i rise induced by 60 mM K+ was inhibited by nifedipine. (e) All solutions contained 10 μM D600. There was no effect of nifedipine following TG pretreatment. Gd3+ lowered [Ca2+]i. (c–e; and in Figure 4-6). The broken line shows a prediction of the trend in [Ca2+]i if the blocker had not been applied.
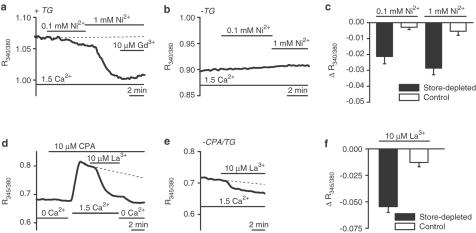
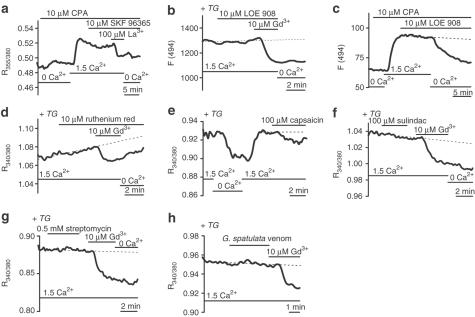
In the absence of D600, activation of voltage-gated Ca2+ channels by 60 mM K+ solution evoked a [Ca2+]i rise that was inhibited by 1 μM nifedipine (Figure 3d). There was 91.5±4.1% inhibition compared with the baseline prior to application of 60 mM K+ (n/N=19/4). SOCs, however, were not sensitive to 1 μM nifedipine (Figure 3e) (n/N=24/5). In the same arterioles, SOC-mediated Ca2+ entry was inhibited by 97.2±2.1% by 10 μM Gd3+ (Figure 3e). Store depletion conferred sensitivity to 0.1–1 mM Ni2+ or 10 μM La3+ (Figure 4a–f). Thus, store depletion induced Gd3+, Ni2+ and La3+ sensitivity, but not nifedipine sensitivity.
Figure 4.
Cerebral arteriolar SOCs are Ni2+- and La3+-sensitive. Arterioles were in standard (1.5 mM Ca2+) or Ca2+-free (0.4 mM EGTA) bath solution. All solutions contained 10 μM D600. (a–c) Ni2+ reduced [Ca2+]i following TG pretreatment (a), but had no effect in control conditions (b). (c) Mean±s.e.m. Ni2+-induced reduction of fura-PE3 ratio in the TG-pretreated ‘store-depleted' (as in a; 0.1 and1 mM Ni2+, n/N=19/5) and control (as in b; 0.1 and 1 mM Ni2+, n/N=27/6) groups. (d–f) La3+ caused a large reduction in [Ca2+]i after store depletion with CPA in Ca2+-free solution (d), and had a smaller effect in control conditions (e). (f) Mean ±s.e.m. La3+-induced reduction of fura-PE3 ratio in CPA/0 Ca2+ ‘store-depleted' (as in D, n/N=15/3) and control (as in e, n/N=58/12) groups.
2-APB inhibits SOCs or CRACs in other cell types (Dobrydneva & Blackmore, 2001; Gregory et al., 2001; Kukkonen et al., 2001; Prakriya & Lewis, 2001). In four experiments on store-depleted arterioles 75 μM 2-APB strongly reduced [Ca2+]i (Figure 5a). There was 66.5±2.7% inhibition compared with [Ca2+]i in the absence of extracellular Ca2+ (n/N=20/4). In two other store-depleted arterioles, there was no effect of 75 μM 2-APB even though Gd3+ was effective (Figure 5b). There are stimulatory effects of low micromolar concentrations of 2-APB on CRAC channels (Prakriya & Lewis, 2001). However, 7.5 μM 2-APB had no effect on four store-depleted arterioles (data not shown) and reduced [Ca2+]i in two arterioles (Figure 5c). There were no effects of 75 μM 2-APB on [Ca2+]i in fura-PE3-loaded arterioles without store depletion (n/N=35/7) or on the autoflourescence of arterioles without fura-PE3 loading (n/N=15/3) (data not shown). Preincubation with micromolar concentrations of Wortmanin (WT)–a kinase inhibitor (Ui et al., 1995)–inhibits store operated Ca2+ entry in various cell types (Jenner et al., 1996; Broad et al., 2001; Tran et al., 2001). Arterioles were pretreated with or without 10 μM WT for 1 h prior to incubation with TG in Ca2+-free solution. The increase in [Ca2+]i upon re-addition of 1.5 mM Ca2+ was significantly inhibited by WT (Figure 5d–f).
Figure 5.
2-APB and WT inhibit cerebral arteriolar SOCs. Arterioles were in standard (1.5 mM Ca2+) or Ca2+-free (0.4 mM EGTA) bath solution. All solutions contained 10 μM D600. (a–c) Effects of 2-APB in TG-pretreated arterioles. (a) 2-APB (75 μM) rapidly reduced [Ca2+]i. (b) In another arteriole there was no effect of 75 μM 2-APB but Gd3+ reduced [Ca2+]i, confirming SOC activation. (c) 2-APB (7.5 μM) slowly reduced [Ca2+]i. (d–f) Preincubation with WT inhibited Ca2+ entry in TG-pretreated arterioles. Arterioles were first incubated with either 0.1% DMSO (d) or 0.1% DMSO and 10 μM WT (e) in standard (1.5 mM Ca2+) bath solution. After this period, arterioles were incubated with TG in Ca2+-free (0.4 mM EGTA) bath solution. The increase in [Ca2+]i associated with the readdition of 1.5 mM Ca2+ was reduced in WT-pretreated arterioles. (f) Mean±s.e.m. data for experiments as shown in (d) and (e), normalised to the value of R340/380 at the point of addition of 1.5 mM Ca2+. DMSO group, n/N=18/4. WT group, n/N=20/4. P<0.001 for the data point marked * and all subsequent data points.
SKF96365 or LOE 908 inhibits SOCs and ROCs in several cell types and may distinguish between different subtypes of these channels (Merritt et al., 1990; Krautwurst et al., 1993,1994; Iwamuro et al., 1999; Zhang et al., 1999). Using the CPA store-depletion protocol, 10 μM SKF96365 had no effect on SOC-mediated Ca2+ entry (Figure 6a; n/N=16/6). At 30 μM, SKF96365 had no inhibitory effect (n=3/2) or produced a 17.7±0.8% transient inhibition (n/N=3/1) (data not shown). Using the TG depletion protocol, 30 μM SKF96365 caused a slow increase in [Ca2+]i in all cells, which was preceded by a transient reduction in 21% of cells (n/N=19/4). There was no effect of 30 μM SKF96365 in arterioles without fura-PE3 loading (n/N=10/2).
Figure 6.
Effects of SOC and other nonselective cation-channel inhibitors on cerebral arteriolar SOCs. Arterioles were in standard (1.5 mM Ca2+) or Ca2+-free bath solution (0.4 mM EGTA). All solutions contained 10 μM D600. (a) No effect of 10 μM SKF 96365 in a zero Ca2+/CPA-treated arteriole, but La3+ did reduce [Ca2+]i. (b) In a TG-pretreated arteriole, 10 μM LOE 908 had no effect although Gd3+ reduced [Ca2+]i. (c) In an arteriole treated with zero Ca2+/CPA, LOE 908 had no effect on [Ca2+]i. (d–h) Effects of 10 μM ruthenium red (d), 100 μM capsaicin (e), 100 μM sulindac (f), 0.5 mM streptomycin (g) and 1:10,000 G. spatulata venom (h) on [Ca2+]i in TG-pretreated arterioles. Only capsaicin (e) had any effect on [Ca2+]i. For agents having no effect, SOC activation was confirmed by Gd3+-induced reduction of [Ca2+]i.
To study the effect of LOE 908, we used fluo-4 instead of fura-PE3 because there are LOE 908-mediated artefacts associated with fura-2 (Iwamuro et al., 1999) and in our fura-PE3 experiments LOE 908 apparently strongly reduced arteriolar [Ca2+]i even in the absence of extracellular Ca2+ (n/N=6/2) (data not shown). No such reductions in [Ca2+]i occurred when using fluo-4. In store-depleted arterioles (TG protocol) in the presence of extracellular Ca2+ LOE 908 (10 μM) had no effect, even though Gd3+-sensitivity was evident in all cases (Figure 6b; mean inhibition by 10 μM Gd3+ was 81.2±6.6%, n/N=13/3). Similarly, there was no effect of LOE 908 on [Ca2+]i in arterioles treated with CPA (Figure 6c; n/N=43/11).
Ruthenium red (0.03–10 μM) inhibits a number of Ca2+-permeable channels including VR1 (TRPV1; Caterina et al., 1997), VRL-1 (TRPV2; Caterina et al., 1999), OTRPC4 (TRPV4; Strotmann et al., 2000), ECaC1 (TRPV5; Nilius et al., 2001) and CaT1 (TRPV6; Hoenderop et al., 2001). [Ca2+]i in store-depleted arterioles (TG protocol) was, however, resistant to 10 μM (n/N=5/1) (Figure 6d) or 100 μM (n/N=10/2) ruthenium red. Subsequent application of Gd3+ inhibited SOC-mediated Ca2+ entry (n/N=15/3). At submicromolar concentrations capsaicin is a vanilloid receptor (VR1) agonist. However, 10–100 μM capsaicin also inhibits SOC-mediated Ca2+ entry in PC12 and HL-60 cells (Choi & Kim, 1999; Choi et al., 2000) and CRAC-mediated Ca2+-entry in Jurkat T cells (Fischer et al., 2001). SOC-mediated Ca2+-entry in arterioles was nevertheless relatively resistant. Capsaicin (100 μM) reduced [Ca2+]i by 12.3±3.7% (n/N=10/3) in store-depleted arterioles (Figure 6e). The nonsteroidal anti-inflammatory sulindac (60 μM) inhibits SOC-mediated Ca2+ entry in HRT-18 cells (Weiss et al., 2001). However, SOC-mediated Ca2+ entry was resistant to 100 μM sulindac (Figure 6f; n/N=19/4). In the same arterioles, 10 μM Gd3+ reduced [Ca2+]i by 84.6±5.2% (n/N=19/4).
Streptomycin at low micromolar concentrations inhibits stretch-activated cation channels in vestibular hair cells and ventricular myocytes (Ohmori, 1985; Gannier et al., 1994; Belus & White, 2001). SOC-mediated Ca2+ entry in arterioles was, in contrast, resistant to even 0.5 mM streptomycin (Figure 6g; n/N=15/3). In the same arterioles, 10 μM Gd3+ caused 96.6±3.4% reduction of the SOC-mediated Ca2+ signal. Grammostolla spatulata venom (1 : 100,000 dilution) inhibits stretch-activated cation channels in coronary arteriolar smooth muscle cells (Wu & Davies, 2001), but at 1 : 10,000 dilution it had no effect on SOC-mediated Ca2+ entry (Figure 6h; n/N=20/4).
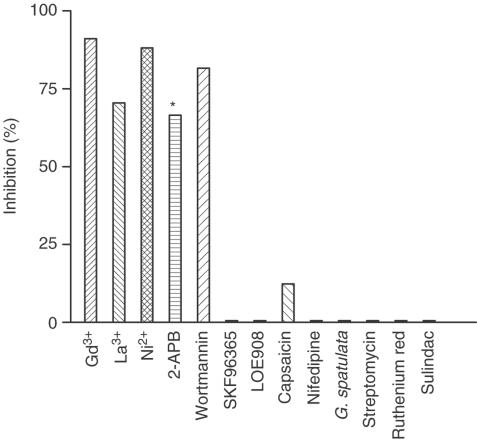
In summary, SOC-mediated Ca2+ entry in arterioles is inhibited by Gd3+, La3+, Ni2+, 2-APB and WT, and is largely resistant to nifedipine, SKF96365, LOE 908, ruthenium red, capsaicin, sulindac, streptomycin or G. spatulata venom (Figure 7).
Figure 7.
Pharmacological profile of SOCs in cerebral arteriolar smooth muscle cells. Data are the mean inhibition of Ca2+ entry in store-depleted arterioles. Concentrations (μM): Gd3+ (10), La3+ (10), Ni2+ (1000), 2-APB (75), WT (10), SKF 96365 (10), LOE 908 (10), capsaicin (100), nifedipine (1), streptomycin (500), ruthenium red (100) and sulindac (100). G. spatuluta venom (1 : 10,000 dilution). All agents were applied topically except WT, which was preincubated with arterioles before SOC activation. Store depletion was achieved using the TG protocol for all agents except La3+ and SKF 96365 for which the CPA store-depletion protocol was used. Both protocols were used for experiments with LOE 908. *The mean inhibition given for 2-APB is from experiments in which only clear inhibitory effects occurred.
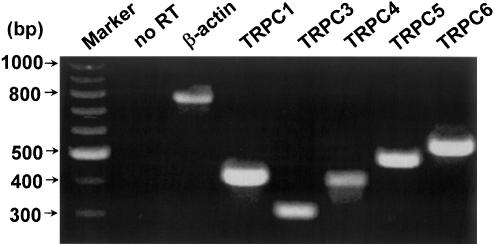
The determination of a pharmacological profile of SOCs in arterioles enables a comparison with the known pharmacology of heterologously expressed TRPC genes, which are suggested to encode SOC subunits. TRPC1 is already known to contribute to the SOCs of cerebral arterioles (Xu & Beech, 2001), but it may not account for all of the SOC signal and it may form heteromultimers with other TRPCs. Therefore, we used RT–PCR analysis of isolated arteriolar fragments to explore if additional TRPC genes are expressed. TRPC3, 4, 5 and 6 were detected (Figure 8). Genomic DNA was absent from the samples as indicated by the absence of a β-actin PCR product when the reverse transcriptase was excluded.
Figure 8.
Detection of multiple TRPC mRNAs in isolated rabbit pial arterioles. Agarose gel electrophoresis of DNA amplicons produced from RT–PCR experiments. The predicted sizes of the amplicons are 763 bp (β-actin), 423 bp (TRPC1), 318 bp (TRPC3), 415 bp (TRPC4), 489 bp (TRPC5) and 551 bp (TRPC6). Positions of DNA ladder markers and a β-actin ‘no RT' control are shown on the left.
Discussion
Through these experiments we have shown that SOC-mediated Ca2+-entry in cerebral arteriolar smooth muscle is inhibited by Gd3+, La3+, Ni2+, 2-APB and WT, and slightly by high concentrations of capsaicin. Importantly, sensitivity to Gd3+, Ni2+ or 2-APB did not exist in control arterioles that were not store-depleted. This is compelling evidence for the activation of a distinct set of ion channels in response to store depletion that are pharmacologically distinct from tonically active background channels. The SOCs were resistant to a range of other agents (Figure 7), which have been shown to inhibit SOCs and Ca2+-permeable cation channels activated by other mechanisms in different cell types.
An important new observation has been that SOCs in smooth muscle cells of retinal arterioles are nifedipine-sensitive (Curtis & Scholfield, 2001). Although a defining characteristic of many SOCs is the opposite of this (i.e. resistance to classical Ca2+ antagonists) there are other examples of SOCs with sensitivity to Ca2+ antagonists – particularly some dihydropyridines (Hopf et al., 1996; Willmott et al., 1996; Auld et al., 2000; Young et al., 2001). Nevertheless, consistent with many other studies we found SOCs that were resistant to dihydropyridine and phenylalkylamine Ca2+ antagonists. One of several differences between our study and that of Curtis & Scholfield (2001) is that we included 10 μM D600 throughout the experiments. Although this ensured that we did not study voltage-dependent Ca2+ entry, we cannot exclude that D600 prevented us from observing nifedipine-sensitive SOCs (assuming they are also D600-sensitive). What is clear is that the SOC pharmacology we describe is for nifedipine-resistant SOCs, and we have no evidence for nifedipine-sensitive SOCs in pial arterioles.
A comparison of our SOC pharmacological profile with that of SOCs described in other types of smooth muscle suggests there is SOC diversity. Sensitivity to block by Ni2+ may be a common feature (McDaniel et al., 2001; Ng & Gurney, 2001; Trepakova et al., 2001; Wilson et al., 2002). However, in marked contrast to SOCs in arterioles, SOCs in anococcygeus smooth muscle cells are resistant to Gd3+ or La3+ at concentrations up to 400 μM (Wayman et al., 1996). SOCs in smooth muscle of renal arteries show some sensitivity to 100 μM Gd3+, while those in pulmonary arterial smooth muscle do not (Wilson et al., 2002). In intrapulmonary arterial smooth muscle, SOCs are sensitive to 1 μM La3+ yet those in main pulmonary artery are blocked only by La3+ concentrations ⩾100 μM (Robertson et al., 2000; Ng & Gurney, 2001).
Experiments with the highest concentration of SKF96365 (30 rather than 10 μM) were complicated by a stimulatory effect–a slowly developing but pronounced elevation of [Ca2+]i. A similar effect occurs in human left internal mammary artery smooth muscle cells in primary culture (T.J.P. Bachelor & D.J. Beech, unpublished obervations). We have not explored the mechanism, but it may be related to inhibition of Na+/Ca2+ exchange and/or induction of Ca2+ release from endoplasmic reticulum (Leung et al., 1996; Jan et al., 1999; Arakawa et al., 2000). Activation of Ca2+-permeable channels has also been observed in response to 15–200 μM SKF 96365 (Dietl & Volkl, 1994; Schwarz et al., 1994; Leung et al., 1996; Jan et al., 1999). A stimulatory effect of 30 μM SKF96365 could have masked an inhibitory action on arteriolar SOCs, but in this regard it is significant that 10 μM SKF96365 had neither inhibitory nor stimulatory effects. This contrasts with the marked inhibitory effect of 10 μM SKF96365 on SOCs in smooth muscle cells of the anococcygeus or pulmonary artery and on ROCs in ileal smooth muscle cells (Wayman et al., 1996; Zhang et al., 1999; Zholos et al., 2000; Ng & Gurney, 2001).
2-APB has emerged as a potential basis for small molecule inhibitors of SOCs, although as with many of the other SOC inhibitors it lacks specificity and has effects on the IP3 receptor (Maruyama et al., 1997), SERCA (Bilmen et al., 2002) and voltage-gated K+ channels (Wang et al., 2002). In common with SOCs in other cell types, including the CRAC channel, we found 2-APB had a blocking effect in the mid-to-high micromolar concentration range. However, the effects of this compound are not simple. In some instances (albeit with a low sample number), we found SOCs were resistant to 2-APB. This may be explained by the existence of multiple types of SOCs, if regulation of SOCs alters sensitivity to 2-APB, or by the existence of a counter-balancing stimulatory effect of 2-APB. SOCs with poor or no sensitivity to 2-APB have been observed, as have stimulatory effects of 2-APB (Kukkonen et al., 2001; Prakriya & Lewis, 2001). Our experiments were complicated by a slowly developing 2-APB (75 μM)-induced elevation of [Ca2+]i in about 50% of cells (R. Flemming & D.J. Beech, unpublished).
Inhibition of store-operated Ca2+ entry with the fungal metabolite WT has variously indicated roles for tyrosine kinases, myosin light chain kinase and cellular polyphosphoinositides in the coupling mechanism between store depletion and activation of SOCs (Jenner et al., 1996; Broad et al., 2001; Tran et al., 2001). Pretreatment with 10 μM WT inhibited store-operated Ca2+ entry in arterioles. Various cellular kinases are inhibited by this concentration of WT and so a role for one or more kinase in the activation of arteriolar SOCs is indicated. Further investigation is required. In contrast, TG induced store-operated Ca2+ entry in porcine endothelial cells is resistant to preincubation with WT at concentrations up to 100 μM (Kuroiwa-Matsumoto et al., 2000).
Further evidence for SOC diversity comes from comparisons with SOCs in other cell types. Insensitivity of pial arteriolar SOCs to LOE 908 distinguishes these channels from SOCs in endothelial cells (Encabo et al., 1996). Sulindac-sensitive SOCs in HRT-18 (Weiss et al., 2001) contrast with pial arteriolar SOCs that are completely resistant to this drug. The poor sensitivity of pial arteriolar SOCs to capsaicin distinguishes them from SOCs in PC12, HL-60 and Jurkat T cells (Choi & Kim 1999; Choi et al., 2000; Fischer et al., 2001).
Stretch-activated cation channels mediate the depolarisation response to stretch in various cell types. These nonselective cation channels are inhibited by low micromolar concentrations of Gd3+ (Hamill & McBride, 1996) including the channel in cerebral artery smooth muscle cells (Welsh et al., 2000). The antibiotic streptomycin and venom of G. spatula inhibit stretch-activated cation channels (Ohmori, 1985; Gannier et al., 1994; Belus & White, 2001; Wu & Davis, 2001), but have no effect on pial arteriolar SOCs.
The suggestion that some TRPCs are subunits of SOCs (reviewed by Zitt et al., 2002) and the detection of mRNAs for several TRPCs in the isolated arterioles (Figure 8) helps justify a comparison of the pharmacology of arteriolar SOCs with that available for heterologously expressed TRPCs. TRPC1 has been found to be sensitive to block by 0.02–1 mM Gd3+ or 0.1–1 mM La3+ (Zitt et al., 1996; Sinkins et al., 1998; Liu et al., 2000). Thus, sensitivity of arteriolar SOCs to similar concentrations of Gd3+ and La3+ is consistent with our earlier suggestion that TRPC1 is a subunit of these SOCs (Xu & Beech, 2001). Intriguingly, there was a slight stimulation of Ca2+ entry with a higher (100 μM) Gd3+ concentration (Figure 3b). We have also noticed that following sustained application of 10–100 μM La3+ an increase in [Ca2+]i is sometimes seen subsequent to its strong blocking effect (R. Flemming, S.-Z. Xu & D. J. Beech, unpublished observations). TRPC4 and TRPC5 are stimulated by La3+ and Gd3+ (Schaefer et al., 2000; Strübing et al., 2001; Jung et al., 2003). Both were detected in arterioles and hence may be candidates for subunits of the pial arteriolar SOC. TRPC7, however, is insensitive to 0.1 mM Gd3+ (Okada et al., 1999) and thus would seem an unlikely candidate. Although TRPC6 is inhibited by La3+ or Gd3+, it is also inhibited by 4 μM SKF96365 (Inoue et al., 2001), suggesting that TRPC6 is also not a subunit of the pial arteriolar SOC. Ruthenium red inhibits many types of channel: ryanodine receptors, some types of K+ channel and members of the TRPV family of TRP proteins including VR1, VRL-1, OTRPC4, ECaC1 and CaT1 (Caterina et al., 1997; 1999; Tominaga et al., 1998; Strotmann et al., 2000; Hoenderop et al., 2001; Nilius et al., 2001). The resistance of pial arteriolar SOCs to a high concentration of ruthenium red indicates that TRPVs do not comprise subunits of the arteriolar SOC. A potential weakness in making these pharmacological comparisons is that TRPs are known to be able to form hetermultimers (Strübing et al., 2001; Hofmann et al., 2002). It will be important to determine not only the expression of TRP proteins in vascular smooth muscle, but also their tendency to heteromultimerise in native cells.
Thus, SOCs in arterioles have a distinct pharmacological profile. Knowledge of this profile provides support for the hypothesis that there are multiple types of SOC in smooth muscle and will facilitate comparisons with heterologously expressed genes that encode putative subunits of SOCs. The results presented in this study support the proposal that TRPC1, possibly along with TRPC4 and/or TRPC5, comprises the arteriolar SOC. The existence of distinct SOCs in smooth muscle may aid the targeting of novel therapeutic drugs to specific vascular beds.
Acknowledgments
We thank the British Heart Foundation and Wellcome Trust for support and Boehringer Ingelheim for kindly providing LOE 908.
Abbreviations
- 2-APB
2-aminoethoxydiphenylborate
- CPA
cyclopiazonic acid
- D600
methoxyverapamil
- DMSO
dimethyl sulphoxide
- Gd3+
gadolinium
- La3+
lanthanum
- Ni2+
nickel
- RBL cells
rat basophilic leukaemia cells
- ROCs
receptor-operated channels
- RT–PCR
reverse transcriptase polymerase chain reaction
- SOCs
store-operated channels
- TG
thapsigargin
- WT
wortmannin
References
- ALBERT A.P., LARGE W.A. A Ca2+-permeable non-selective cation channel activated by depletion of internal Ca(2+) stores in single rabbit portal vein myocytes. J. Physiol. 2002;538:717–728. doi: 10.1113/jphysiol.2001.013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARAKAWA N., SAKAUE M., YOKOYAMA I., HASHIMOTO H., KOYAMA Y., BABA A., MATSUDA T. KB-R7943 inhibits store-operated Ca2+ entry in cultured neurons and astrocytes. Biochem. Biophys. Res. Commun. 2000;279:354–357. doi: 10.1006/bbrc.2000.3968. [DOI] [PubMed] [Google Scholar]
- AULD A., CHEN J., BRERETON H.M., WANG Y.J., GREGORY R.B., BARRITT G.J. Store-operated Ca2+ inflow in Reuber hepatoma cells is inhibited by voltage-operated Ca2+ channel antagonists and, in contrast to freshly isolated hepatocytes, does not require a pertussis toxin-sensitive trimeric GTP-binding protein. Biochim. Biophys. Acta. 2000;1497:11–26. doi: 10.1016/s0167-4889(00)00045-8. [DOI] [PubMed] [Google Scholar]
- BARRITT G.J. Receptor-activated Ca2+ inflow in animal cells: a variety of pathways tailored to meet different intracellular Ca2+ signalling requirements. Biochem. J. 1999;337:153–169. [PMC free article] [PubMed] [Google Scholar]
- BEECH D.J., XU S.-Z., McHUGH D., FLEMMING R.TRPC1 store-operated cationic channel subunit Cell Calcium 2003(in press) [DOI] [PubMed]
- BELUS A., WHITE E. Effects of antibiotics on the contractility and Ca2+ transients of rat cardiac myocytes. Eur. J. Pharmacol. 2001;412:121–126. doi: 10.1016/s0014-2999(01)00717-8. [DOI] [PubMed] [Google Scholar]
- BILMEN J.G., WOOTTON L.L., GODFREY R.E., SMART O.S., MICHELANGELI F. Inhibition of SERCA Ca2+ pumps by 2-aminoethoxydiphenyl borate (2-APB). 2-APB reduces both Ca2+ binding and phosphoryl transfer from ATP, by interfering with the pathway leading to the Ca2+-binding sites. Eur. J. Biochem. 2002;269:3678–3687. doi: 10.1046/j.1432-1033.2002.03060.x. [DOI] [PubMed] [Google Scholar]
- BROAD L.M., BRAUN F.J., LIEVREMONT J.P., BIRD G.S., KUROSAKI T., PUTNEY J.W., JR Role of the phospholipase C-inositol 1,4,5-trisphosphate pathway in calcium release-activated calcium current and capacitative calcium entry. J. Biol. Chem. 2001;276:15945–15952. doi: 10.1074/jbc.M011571200. [DOI] [PubMed] [Google Scholar]
- BROUGH G.H., WU S., CIOFFI D., MOORE T.M., LI M., DEAN N., STEVENS T. Contribution of endogenously expressed Trp1 to a Ca2+-selective, store-operated Ca2+ entry pathway. FASEB J. 2001;15:1727–1738. [PubMed] [Google Scholar]
- CASTEELS R., DROOGMANS G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells or rabbit ear artery. J. Physiol. 1981;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATERINA M.J., ROSEN T.A., TOMINAGA M., BRAKE A.J., JULIUS D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CHEONG A., DEDMAN A.M., BEECH D.J. Expression and function of native potassium channel (Kvα1) subunits in terminal arterioles of rabbit. J. Physiol. 2001;534:691–700. doi: 10.1111/j.1469-7793.2001.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOI S.Y., HA H., KIM K.T. Capsaicin inhibits platelet-activating factor-induced cytosolic Ca2+ rise and superoxide production. J. Immunol. 2000;165:3992–3998. doi: 10.4049/jimmunol.165.7.3992. [DOI] [PubMed] [Google Scholar]
- CHOI S.Y., KIM K.T. Capsaicin inhibits phospholipase C-mediated Ca2+ increase by blocking thapsigargin-sensitive store-operated Ca2+ entry in PC12 cells. J. Pharmacol. Exp. Ther. 1999;291:107–114. [PubMed] [Google Scholar]
- CLAPHAM D.E., RUNNELS L.W., STRUBING C. The TRP ion channel family. Nat. Rev. Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- CRABTREE G.R. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell. 1999;96:611–614. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- CURTIS T.M., SCHOLFIELD C.N. Nifedipine blocks Ca2+ store refilling through a pathway not involving L-type Ca2+ channels in rabbit arteriolar smooth muscle. J. Physiol. 2001;532:609–623. doi: 10.1111/j.1469-7793.2001.0609e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIETL P., VOLKL H. Maitotoxin activates a nonselective cation channel and stimulates Ca2+ entry in MDCK renal epithelial cells. Mol. Pharmacol. 1994;45:300–305. [PubMed] [Google Scholar]
- DOBRYDNEVA Y., BLACKMORE P. 2-Aminoethoxydiphenyl borate directly inhibits store-operated calcium entry channels in human platelets. Mol. Pharmacol. 2001;60:541–552. [PubMed] [Google Scholar]
- ENCABO A., ROMANIN C., BIRKE F.W., KUKOVETZ W.R., GROSCHNER K. Inhibition of a store-operated Ca2+ entry pathway in human endothelial cells by the isoquinoline derivative LOE 908. Br. J. Pharmacol. 1996;119:702–706. doi: 10.1111/j.1476-5381.1996.tb15729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISCHER B.S., QIN D., KIM K., McDONALD T.V. Capsaicin inhibits Jurkat T-cell activation by blocking calcium entry current ICRAC. J. Pharmacol. Exp. Ther. 2001;299:238–246. [PubMed] [Google Scholar]
- FLEMMING R., CHEONG A., DEDMAN A.M., BEECH D.J. Discrete store-operated calcium influx into an intracellular compartment in rabbit arteriolar smooth muscle. J. Physiol. 2002;543:455–464. doi: 10.1113/jphysiol.2002.023366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREICHEL M., SUH S.H., PFEIFER A., SCHWEIG U., TROST C., WEISSGERBER P., BIEL M., PHILIPP S., FREISE D., DROOGMANS G., HOFMANN F., FLOCKERZI V., NILIUS B. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/−mice. Nat. Cell Biol. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- GANNIER F., WHITE E., LACAMPAGNE A., GARNIER D., LE GUENNEC J.Y. Streptomycin reverses a large stretch induced increases in [Ca2+]i in isolated guinea pig ventricular myocytes. Cardiovasc. Res. 1994;28:1193–1198. doi: 10.1093/cvr/28.8.1193. [DOI] [PubMed] [Google Scholar]
- GEE K.R., BROWN K.A., CHEN W.N., BISHOP-STEWART J., GRAY D., JOHNSON I. Chemical and physiological characterization of fluo-4 Ca2+-indicator dyes. Cell Calcium. 2000;27:97–106. doi: 10.1054/ceca.1999.0095. [DOI] [PubMed] [Google Scholar]
- GIBSON A., McFADZEAN I., WALLACE P., WAYMAN C.P. Capacitative Ca2+ entry and the regulation of smooth muscle tone. Trends Pharmacol. Sci. 1998;19:266–269. doi: 10.1016/s0165-6147(98)01222-x. [DOI] [PubMed] [Google Scholar]
- GOLOVINA V.A. Cell proliferation is associated with enhanced capacitative Ca2+ entry in human arterial myocytes. Am. J. Physiol. 1999;277:C343–C349. doi: 10.1152/ajpcell.1999.277.2.C343. [DOI] [PubMed] [Google Scholar]
- GOLOVINA V.A., PLATOSHYN O., BAILEY C.L., WANG J., LIMSUWAN A., SWEENEY M., RUBIN L.J., YUAN J.X. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H746–H755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- GREGORY R.B., RYCHKOV G., BARRITT G.J. Evidence that 2-aminoethyl diphenylborate is a novel inhibitor of store-operated Ca2+ channels in liver cells, and acts through a mechanism which does not involve inositol trisphosphate receptors. Biochem. J. 2001;354:285–290. doi: 10.1042/0264-6021:3540285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUIBERT C., BEECH D.J. Positive and negative coupling of the endothelin ETA receptor to Ca2+-permeable channels in rabbit cerebral cortex arterioles. J. Physiol. 1999;514:843–856. doi: 10.1111/j.1469-7793.1999.843ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILL O.P., McBRIDE D.W., JR The pharmacology of mechanogated membrane ion channels. Pharmacol. Rev. 1996;48:231–252. [PubMed] [Google Scholar]
- HOENDEROP J.G., VENNEKENS R., MULLER D., PRENEN J., DROOGMANS G., BINDELS R.J., NILIUS B. Function and expression of the epithelial Ca2+ channel family: comparison of mammalian ECaC1 and 2. J. Physiol. 2001;537:747–761. doi: 10.1111/j.1469-7793.2001.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFMANN T., SCHAEFER M., SCHULTZ G., GUDERMANN T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. USA. 2002;99:7461–7466. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPF F.W., REDDY P., HONG J., STEINHARDT R.A. A capacitative calcium current in cultured skeletal muscle cells is mediated by the calcium-specific leak channel and inhibited by dihydropyridine compounds. J. Biol. Chem. 1996;271:22358–22367. doi: 10.1074/jbc.271.37.22358. [DOI] [PubMed] [Google Scholar]
- HUNTON D.L., LUCCHESI P.A., PANG Y., CHENG X., DELL'ITALIA L.J., MARCHASE R.B. Capacitative calcium entry contributes to nuclear factor of activated T-cells nuclear translocation and hypertrophy in cardiomyocytes. J. Biol. Chem. 2002;277:14266–14273. doi: 10.1074/jbc.M107167200. [DOI] [PubMed] [Google Scholar]
- INOUE R., OKADA T., ONOUE H., HARA Y., SHIMIZU S., NAITOH S., ITO Y., MORI Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular α1-adrenoceptor-activated Ca2+-permeable cation channel. Circ. Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- IWAMURO Y., MIWA S., ZHANG X.F., MINOWA T., ENOKI T., OKAMOTO Y., HASEGAWA H., FURUTANI H., OKAZAWA M., ISHIKAWA M., HASHIMOTO N., MASAKI T. Activation of three types of voltage-independent Ca2+ channel in A7r5 cells by endothelin-1 as revealed by a novel Ca2+ channel blocker LOE 908. Br. J. Pharmacol. 1999;126:1107–1114. doi: 10.1038/sj.bjp.0702416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAN C.R., HO C.M., WU S.N., TSENG C.J. Multiple effects of 1-[β-[3-(4-methoxyphenyl)propoxy]-4-methoxyphenethyl]-1H-imidazole hydrochloride (SKF 96365) on Ca2+ signaling in MDCK cells: depletion of thapsigargin-sensitive Ca2+ store followed by capacitative Ca2+ entry, activation of a direct Ca2+ entry, and inhibition of thapsigargin-induced capacitative Ca2+ entry. Naunyn Schmiedebergs Arch. Pharmacol. 1999;359:92–101. doi: 10.1007/pl00005336. [DOI] [PubMed] [Google Scholar]
- JENNER S., FARNDALE R.W., SAGE S.O. Wortmannin inhibits store-mediated calcium entry and protein tyrosine phosphorylation in human platelets. FEBS Lett. 1996;381:249–251. doi: 10.1016/0014-5793(96)00130-5. [DOI] [PubMed] [Google Scholar]
- JUNG S., MUHLE A., SCHAEFER M., STROTMANN R., SCHULTZ G., PLANT T.D. Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J. Biol. Chem. 2003;278:3562–3571. doi: 10.1074/jbc.M211484200. [DOI] [PubMed] [Google Scholar]
- KRAUTWURST D., DEGTIAR V.E., SCHULTZ G., HESCHELER J. The isoquinoline derivative LOE 908 selectively blocks vasopressin-activated nonselective cation currents in A7r5 aortic smooth muscle cells. Naunyn Schmiedebergs Arch. Pharmacol. 1994;349:301–307. doi: 10.1007/BF00169297. [DOI] [PubMed] [Google Scholar]
- KRAUTWURST D., HESCHELER J., ARNDTS D., LOSEL W., HAMMER R., SCHULTZ G. Novel potent inhibitor of receptor-activated nonselective cation currents in HL-60 cells. Mol. Pharmacol. 1993;43:655–659. [PubMed] [Google Scholar]
- KUKKONEN J.P., LUND P.E., AKERMAN K.E. 2-aminoethoxydiphenyl borate reveals heterogeneity in receptor-activated Ca2+ discharge and store-operated Ca2+ influx. Cell Calcium. 2001;30:117–129. doi: 10.1054/ceca.2001.0219. [DOI] [PubMed] [Google Scholar]
- KUROIWA-MATSUMOTO M., HIRANO K., AHMED A., KAWASAKI J., NISHIMURA J., KANAIDE H. Mechanisms of the thapsigargin-induced Ca2+ entry in in situ endothelial cells of the porcine aortic valve and the endothelium-dependent relaxation in the porcine coronary artery. Br. J. Pharmacol. 2000;131:115–123. doi: 10.1038/sj.bjp.0703548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEUNG Y.M., KWAN C.Y., LOH T.T. Dual effects of SK&F 96365 in human leukemic HL-60 cells. Inhibition of calcium entry and activation of a novel cation influx pathway. Biochem. Pharmacol. 1996;51:605–612. doi: 10.1016/s0006-2952(95)02181-7. [DOI] [PubMed] [Google Scholar]
- LEWIS R.S. Store-operated calcium channels. Adv. Second Messenger Phosphoprotein Res. 1999;33:279–307. doi: 10.1016/s1040-7952(99)80014-7. [DOI] [PubMed] [Google Scholar]
- LIU X., WANG W., SINGH B.B., LOCKWICH T., JADLOWIEC J., O'CONNELL B., WELLNER R., ZHU M.X., AMBUDKAR I.S. Trp1, a candidate protein for the store-operated Ca2+ influx mechanism in salivary gland cells. J. Biol. Chem. 2000;275:3403–3411. doi: 10.1074/jbc.275.5.3403. [DOI] [PubMed] [Google Scholar]
- MAGNIER-GAUBIL C., HERBERT J.M., QUARCK R., PAPP B., CORVAZIER E., WUYTACK F., LEVY-TOLEDANO S., ENOUF J. Smooth muscle cell cycle and proliferation. Relationship between calcium influx and sarco-endoplasmic reticulum Ca2+ ATPase regulation. J. Biol. Chem. 1996;271:27788–27794. doi: 10.1074/jbc.271.44.27788. [DOI] [PubMed] [Google Scholar]
- MARUYAMA T., KANAJI T., NAKADE S., KANNO T., MIKOSHIBA K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem. (Tokyo) 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- McDANIEL S.S., PLATOSHYN O., WANG J., YU Y., SWEENEY M., KRICK S., RUBIN L.J., YUAN J.X. Capacitative Ca2+ entry in agonist-induced pulmonary vasoconstriction. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280:L870–L880. doi: 10.1152/ajplung.2001.280.5.L870. [DOI] [PubMed] [Google Scholar]
- McFADZEAN I., GIBSON A. The developing relationship between receptor-operated and store-operated calcium channels in smooth muscle. Br. J. Pharmacol. 2002;135:1–13. doi: 10.1038/sj.bjp.0704468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERRITT J.E., ARMSTRONG W.P., BENHAM C.D., HALLAM T.J., JACOB R., JAXA-CHAMIEC A., LEIGH B.K., McCARTHY S.A., MOORES K.E., RINK T.J. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem. J. 1990;271:515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONTELL C., BIRNBAUMER L., FLOCKERZI V., BINDELS R.J., BRUFORD E.A., CATERINA M.J., CLAPHAM D.E., HARTENECK C., HELLER S., JULIUS D., KOJIMA I., MORI Y., PENNER R., PRAWITT D., SCHARENBERG A.M., SCHULTZ G., SHIMIZU N., ZHU M.X. A unified nomenclature for the superfamily of TRP cation channels. Mol. Cell. 2002;9:229–231. doi: 10.1016/s1097-2765(02)00448-3. [DOI] [PubMed] [Google Scholar]
- NG L.C., GURNEY A.M. Store-operated channels mediate Ca2+ influx and contraction in rat pulmonary artery. Circ. Res. 2001;89:923–929. doi: 10.1161/hh2201.100315. [DOI] [PubMed] [Google Scholar]
- NILIUS B., PRENEN J., VENNEKENS R., HOENDEROP J.G., BINDELS R.J., DROOGMANS G. Pharmacological modulation of monovalent cation currents through the epithelial Ca2+ channel ECaC1. Br. J. Pharmacol. 2001;134:453–462. doi: 10.1038/sj.bjp.0704272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHMORI H. Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J. Physiol. 1985;359:189–217. doi: 10.1113/jphysiol.1985.sp015581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA T., INOUE R., YAMAZAKI K., MAEDA A., KUROSAKI T., YAMAKUNI T., TANAKA I., SHIMIZU S., IKENAKA K., IMOTO K., MORI Y. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca2+-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J. Biol. Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- PIERRE L.N., DAVENPORT A.P. Blockade and reversal of endothelin-induced constriction in pial arteries from human brain. Stroke. 1999;30:638–643. doi: 10.1161/01.str.30.3.638. [DOI] [PubMed] [Google Scholar]
- PRAKRIYA M., LEWIS R.S. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J. Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON T.P., HAGUE D., AARONSON P.I., WARD J.P. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J. Physiol. 2000;525:669–680. doi: 10.1111/j.1469-7793.2000.t01-1-00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAEFER M., PLANT T.D., OBUKHOV A.G., HOFMANN T., GUDERMANN T., SCHULTZ G. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J. Biol. Chem. 2000;275:17517–17526. doi: 10.1074/jbc.275.23.17517. [DOI] [PubMed] [Google Scholar]
- SCHWARZ G., DROOGMANS G., NILIUS B. Multiple effects of SK&F 96365 on ionic currents and intracellular calcium in human endothelial cells. Cell Calcium. 1994;15:45–54. doi: 10.1016/0143-4160(94)90103-1. [DOI] [PubMed] [Google Scholar]
- SINKINS W.G., ESTACION M., SCHILLING W.P. Functional expression of TrpC1: a human homologue of the Drosophila Trp channel. Biochem. J. 1998;331:331–339. doi: 10.1042/bj3310331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STROTMANN R., HARTENECK C., NUNNENMACHER K., SCHULTZ G., PLANT T.D. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- STRÜBING C., KRAPIVINSKY G., KRAPIVINSKY L., CLAPHAM D.E. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- TOMINAGA M., CATERINA M.J., MALMBERG A.B., ROSEN T.A., GILBERT H., SKINNER K., RAUMANN B.E., BASBAUM A.I., JULIUS D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- TRAN Q.K., WATANABE H., LE H.Y., PAN L., SETO M., TAKEUCHI K., OHASHI K. Myosin light chain kinase regulates capacitative Ca2+ entry in human monocytes/macrophages. Arterioscler. Thromb. Vasc. Biol. 2001;21:509–515. doi: 10.1161/01.atv.21.4.509. [DOI] [PubMed] [Google Scholar]
- TREPAKOVA E.S., GERICKE M., HIRAKAWA Y., WEISBROD R.M., COHEN R.A., BOLOTINA V.M. Properties of a native cation channel activated by Ca2+ store depletion in vascular smooth muscle cells. J. Biol. Chem. 2001;276:7782–7790. doi: 10.1074/jbc.M010104200. [DOI] [PubMed] [Google Scholar]
- UI M., OKADA T., HAZEKI K., HAZEKI O. Wortmannin as a unique probe for an intracellular signalling protein, phosphoinositide 3-kinase. Trends Biochem. Sci. 1995;20:303–307. doi: 10.1016/s0968-0004(00)89056-8. [DOI] [PubMed] [Google Scholar]
- UNNO T., BEECH D.J., KOMORI S., OHASHI H. Inhibitors of spasmogen-induced Ca2+ channel suppression in smooth muscle cells from small intestine. Br. J. Pharmacol. 1998;125:667–674. doi: 10.1038/sj.bjp.0702112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOETS T., PRENEN J., FLEIG A., VENNEKENS R., WATANABE H., HOENDEROP J.G., BINDELS R.J., DROOGMANS G., PENNER R., NILIUS B. CaT1 and the calcium release-activated calcium channel manifest distinct pore properties. J. Biol. Chem. 2001;276:47767–47770. doi: 10.1074/jbc.C100607200. [DOI] [PubMed] [Google Scholar]
- VORNDRAN C., MINTA A., POENIE M. New fluorescent calcium indicators designed for cytosolic retention or measuring calcium near membranes. Biophys. J. 1995;69:2112–2124. doi: 10.1016/S0006-3495(95)80082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Y., DESHPANDE M., PAYNE R. 2-Aminoethoxydiphenyl borate inhibits phototransduction and blocks voltage-gated potassium channels in Limulus ventral photoreceptors. Cell Calcium. 2002;32:209–216. doi: 10.1016/s0143416002001562. [DOI] [PubMed] [Google Scholar]
- WAYMAN C.P., McFADZEAN I., GIBSON A., TUCKER J.F. Two distinct membrane currents activated by cyclopiazonic acid-induced calcium store depletion in single smooth muscle cells of the mouse anococcygeus. Br. J. Pharmacol. 1996;117:566–572. doi: 10.1111/j.1476-5381.1996.tb15228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS H., AMBERGER A., WIDSCHWENDTER M., MARGREITER R., OFNER D., DIETL P. Inhibition of store-operated calcium entry contributes to the anti-proliferative effect of non-steroidal anti-inflammatory drugs in human colon cancer cells. Int. J. Cancer. 2001;92:877–882. doi: 10.1002/ijc.1280. [DOI] [PubMed] [Google Scholar]
- WELSH D.G., NELSON M.T., ECKMAN D.M., BRAYDEN J.E. Swelling-activated cation channels mediate depolarization of rat cerebrovascular smooth muscle by hyposmolarity and intravascular pressure. J. Physiol. 2000;527:139–148. doi: 10.1111/j.1469-7793.2000.t01-1-00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLMOTT N.J., CHOUDHURY Q., FLOWER R.J. Functional importance of the dihydropyridine-sensitive, yet voltage-insensitive store-operated Ca2+ influx of U937 cells. FEBS Lett. 1996;394:159–164. doi: 10.1016/0014-5793(96)00939-8. [DOI] [PubMed] [Google Scholar]
- WILSON S.M., MASON H.S., SMITH G.D., NICHOLSON N., JOHNSTON L., JANIAK R., HUME J.R. Comparative capacitative calcium entry mechanisms in canine pulmonary and renal arterial smooth muscle cells. J. Physiol. 2002;543:917–931. doi: 10.1113/jphysiol.2002.021998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU X., DAVIS M.J. Characterization of stretch-activated cation current in coronary smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H1751–H1761. doi: 10.1152/ajpheart.2001.280.4.H1751. [DOI] [PubMed] [Google Scholar]
- XU S.Z., BEECH D.J. TrpC1 is a membrane-spanning subunit of store-operated Ca2+ channels in native vascular smooth muscle cells. Circ. Res. 2001;88:84–87. doi: 10.1161/01.res.88.1.84. [DOI] [PubMed] [Google Scholar]
- YOUNG R.C., SCHUMANN R., ZHANG P. Nifedipine block of capacitative calcium entry in cultured human uterine smooth-muscle cells. J. Soc. Gynecol. Investig. 2001;8:210–215. doi: 10.1016/s1071-5576(01)00109-5. [DOI] [PubMed] [Google Scholar]
- YU Y., SWEENEY M., ZHANG S., PLATOSHYN O., LANDSBERG J., ROTHMAN A., YUAN J.X. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am. J. Physiol. 2003;284:C316–330. doi: 10.1152/ajpcell.00125.2002. [DOI] [PubMed] [Google Scholar]
- YUE L., PENG J.B., HEDIGER M.A., CLAPHAM D.E. CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature. 2001;410:705–709. doi: 10.1038/35070596. [DOI] [PubMed] [Google Scholar]
- ZHANG X.F., IWAMURO Y., ENOKI T., OKAZAWA M., LEE K., KOMURO T., MINOWA T., OKAMOTO Y., HASEGAWA H., FURUTANI H., MIWA S., MASAKI T. Pharmacological characterization of Ca2+ entry channels in endothelin-1-induced contraction of rat aorta using LOE 908 and SK&F 96365. Br. J. Pharmacol. 1999;127:1388–1398. doi: 10.1038/sj.bjp.0702661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOLOS A.V., TSYTSYURA Y.D., PHILYPPOV I.B., SHUBA M.F., BOLTON TB. Voltage-dependent inhibition of the muscarinic cationic current in guinea-pig ileal cells by SK&F 96365. Br. J. Pharmacol. 2000;129:695–702. doi: 10.1038/sj.bjp.0703115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZITT C., HALASZOVICH C.R., LUCKHOFF A. The TRP family of cation channels: probing and advancing the concepts on receptor-activated calcium entry. Prog. Neurobiol. 2002;66:243–264. doi: 10.1016/s0301-0082(02)00002-3. [DOI] [PubMed] [Google Scholar]
- ZITT C., ZOBEL A., OBUKHOV A.G., HARTENECK C., KALKBRENNER F., LUCKHOFF A., SCHULTZ G. Cloning and functional expression of a human Ca2+-permeable cation channel activated by calcium store depletion. Neuron. 1996;16:1189–1196. doi: 10.1016/s0896-6273(00)80145-2. [DOI] [PubMed] [Google Scholar]