Abstract
Systemic administration of phenethylamine-derived, 5-hydroxytryptamine2 (5-HT2) receptor agonists inhibits the firing of midbrain 5-HT neurones, but the 5-HT receptors involved are poorly defined, and the contribution of peripheral mechanisms is uncertain. This study addresses these issues using extracellular recordings of 5-HT neurones in the dorsal raphe nucleus of anaesthetised rats.
The 5-HT2 receptor agonists DOI ((±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride) and DOB ((±)-2,5-dimethoxy-4-bromoamphetamine hydrobromide), caused a dose-related (10–100 μg kg−1 i.v.) inhibition of 5-HT neuronal activity, with the highest dose reducing firing rates by >80%.
Pretreatment with the 5-HT2 receptor antagonist ritanserin (1 mg kg−1 i.v.) completely blocked the action of DOI. The 5-HT2A receptor antagonist MDL 100,907 (0.2 mg kg−1 i.v.) blocked the action of both DOI and DOB. In comparison, the 5-HT2B/C receptor antagonist SB 206553 (0.5 mg kg−1 i.v.) caused a small, but statistically significant, shift to the right in the dose response to DOI and DOB.
Pretreatment with the peripherally acting 5-HT2 receptor antagonist BW 501C67 (0.1 mg kg−1 i.v.) had no effect on the DOI-induced inhibition of 5-HT cell firing, but completely blocked the DOI-induced rise in mean arterial blood pressure.
These data indicate that the inhibition of 5-HT cell firing induced by systemic administration of DOI and DOB is mediated predominantly by the 5-HT2A receptor-subtype, but that 5-HT2B/C receptors also play a minor role. Moreover, central and not peripheral mechanisms are involved. Given evidence that 5-HT2 receptors are not located on 5-HT neurones, postsynaptic 5-HT feedback mechanisms are implicated.
Keywords: 5-HT; dorsal raphe nucleus; 5-HT2A receptors; 5-HT2C receptors; MDL 100,907; SB 206553; BW 501C67
Introduction
Feedback regulation is an essential aspect of the physiology of central 5-hydroxytryptamine (5-HT) neurones (Aghajanian, 1978). The role of presynaptic autoregulatory mechanisms in the control of 5-HT neurones is well recognised. Thus, somatodendritic 5-HT1A autoreceptors regulate the firing of 5-HT neurones in the dorsal raphe nucleus (DRN), while 5-HT1B autoreceptors regulate 5-HT release in terminal regions (Barnes & Sharp, 1999). Drug action at 5-HT autoreceptors has been linked to a range of behaviours including anxiolysis and changes in feeding and sexual behaviour (Barnes & Sharp, 1999). Moreover, knowledge of these autoreceptors has been fundamental to the development of novel antidepressant strategies. Specifically, evidence that 5-HT1A autoreceptors are desensitised by selective serotonin reuptake inhibitors (Blier et al., 1990), has led to the use of 5-HT1A receptor antagonists as antidepressant augmentation agents (Artigas et al., 2001).
In addition to 5-HT autoreceptors, recent reports indicate that 5-HT neurones are also regulated by postsynaptic 5-HT receptors. For example, experiments showing that cortical lesions attenuate the inhibitory effect of 5-HT1A agonists on 5-HT cell firing suggest that postsynaptic 5-HT1A receptors in cortical regions regulate the firing of DRN 5-HT neurones (Ceci et al., 1994; Hajós et al., 1999). A projection from the medial prefrontal cortex to the DRN has been proposed as the underlying anatomical substrate (Hajós et al., 1998; Varga et al., 2001).
5-HT2 receptors are located postsynaptically (Palacios et al., 1991; Cornea-Hebert et al., 1999; Verge & Calas, 2000) and there is evidence that these receptors may also regulate 5-HT neurotransmission. Early experiments indicated that the phenethylamine derivative, DOM (1-(2,5-di-methoxy-4-methylphenyl)-2-aminopropane), inhibits the firing of DRN 5-HT neurones in vivo (Aghajanian et al., 1970). Later work found that DOI ((±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride) has a similar effect and causes an associated decrease in 5-HT release (Wright et al., 1990; Garratt et al., 1991). Although DOM and DOI are 5-HT2 receptor agonists, the role of 5-HT2 receptors in the inhibition of 5-HT cell firing is uncertain. Thus, one detailed study found that the nonselective 5-HT2 receptor antagonists, ritanserin and ketanserin, did not block the effects of DOI (Garratt et al., 1991). On the other hand, a recent paper reported two instances in which the firing of a 5-HT neurone was inhibited by DOI and then completely restored by the 5-HT2A selective antagonist MDL 100,907 (Martin-Ruiz et al., 2001). The latter result is seemingly at odds with in vitro electrophysiological data that implicates roles for both 5-HT2A and 5-HT2C receptors in the regulation of 5-HT cell firing (Liu et al., 2000). A final complicating factor is the proposal that peripheral and not central 5-HT2 receptors are involved in the DOI-induced inhibition of 5-HT neuronal firing (Penington & Reiffenstein, 1986).
The present study tested the effect of certain phenethylamine-derived 5-HT2 agonists on the firing of 5-HT neurones in the rat DRN in vivo, and used recently available selective antagonists to characterise the 5-HT receptor subtypes involved. The peripheral antagonist, BW 501C67 (Mawson & Whittington, 1970; Fuller et al., 1986), was used to establish whether the effect of 5-HT2 agonists is mediated centrally or via peripheral mechanisms. Preliminary accounts of some of these experiments were presented to the British Pharmacological Society (Boothman et al., 2001,2003).
Methods
Animals
All procedures were carried out in accordance with the U.K. Home Office Animals (Scientific Procedures) Act (1986) and associated Home Office guidelines. Male Sprague– Dawley rats (240–320 g; Harlan Olac, Bicester, U.K.) were housed in groups under conditions of constant temperature (21±1°C) and humidity under a 24 h light–dark cycle (lights on 08:00–20:00 h) with food and water freely available. In all experiments, rats were anaesthetised with chloral hydrate (460 mg kg−1 i.p.) with additional doses (60–120 mg kg−1 i.p.) administered as required, supplemented with a single dose of saffan (1.2 mg kg−1 i.v.) during surgery. A lateral tail vein was cannulated for drug administration. Body temperature was maintained at 36°C using a thermoregulated heating pad.
Electrophysiological recording of 5-HT neuronal activity
Extracellular single-unit recordings of 5-HT neurones were made essentially as described previously (Hajós et al., 1998). Single barrel glass electrodes (filled with 2 M NaCl containing 2% pontamine sky blue, in vitro resistance 6–20 MΩ) were lowered under stereotaxic control into the DRN (coordinates of A/P −7.5 mm, L/M 0.0 mm D/V −4.5 mm to −5.5 mm; Paxinos and Watson, 1986). Single-unit potentials were amplified and filtered (Gain 1 k; 500 Hz to 1.5 kHz band pass; Neurolog system, Digitimer Ltd., Welwyn Garden City, U.K.), captured using a 1401plus interface system and analysed off-line using Spike2 software (Version 4.01) (Cambridge Electronic Design, Cambridge, U.K.).
5-HT neurones were identified on the basis of their electrophysiological characteristics (Hajós et al., 1995). All cells included in this study fulfilled at least three of the following criteria: slow firing rate (<2 Hz), regular firing pattern (typical coefficient of variation <0.5), triphasic extracellular waveform with a wide action potential duration (>1.5 ms) and an inhibitory response to administration of the 5-HT1A receptor agonist 8-OH-DPAT (10 μg kg−1 i.v.). Most 5-HT neurones discharged action potentials in single spikes, but some 5-HT neurones discharging both single spikes and spikes in very short bursts (Hajós et al., 1995) were included.
Following a 5 min period of baseline recording, agonists were administered in increasing doses (10, 20, 40, 80, 100 μg kg−1 i.v. at 2 min intervals), either alone or in animals pretreated for 5 min with an antagonist. In one set of experiments, DOI was administered alone or in animals pretreated with 1 mg kg−1 i.v. ritanserin (5-HT2 receptor antagonist), 0.2 mg kg−1 i.v. MDL 100,907 (5-HT2A receptor antagonist) or 0.5 mg kg−1 i.v. SB 206553 (5-HT2B/C receptor antagonist). In a second set of experiments, (±)-2,5-dimethoxy-4-bromoamphetamine hydrobromide (DOB) was administered alone or in animals pretreated with 0.2 mg kg−1 i.v. MDL 100,907 or 0.5 mg kg−1 i.v. SB 206553. In a third set of experiments, DOI was administered alone or in animals pretreated with 0.1 mg kg−1 i.v. BW 501C67 (peripheral 5-HT2 receptor antagonist).
In animals pretreated with ritanserin and MDL 100,907, a vehicle (5% glucose solution with 20 μl 100% acetic acid) was injected 3 min prior to the antagonist. When neurones were ‘lost' during recording, an alternative neurone was sought if no more than the first dose of agonist had been given. At the end of every experiment, a small amount of dye was expelled by iontophoresis (−3.6 mA, pulses of 200 ms duration with 21 ms interpulse interval for 30 min) and the location of the electrode was confirmed histologically.
Firing rates were quantified in the final 1 min of each baseline and post-vehicle/drug interval. The regularity of cell firing was calculated over the same period using the coefficient of variation analysis (standard deviation of interspike interval/interspike interval mean). Neurones discharging spikes in short bursts were analysed using the first spike of each burst.
Recordings of arterial blood pressure
In additional experiments arterial blood pressure was measured in chloral hydrate-anaesthetised rats. A carotid artery was cannulated, connected to a pressure transducer and the signal was captured (1401plus C.E.D. interface) and analysed off-line (Spike2 version 4.01 software, Cambridge Electronic Design, U.K.). Following a 5 min baseline period of stable blood pressure, either saline (5 × 0.1 ml kg−1) or DOI (10, 20, 40, 80, 100 μg kg−1 i.v.) was injected at 2 min intervals. In some experiments, 0.1 mg kg−1 i.v. BW 501C67 was injected 5 min prior to DOI.
Mean arterial pressure (MAP) was measured over the final 1 min of each baseline and post-saline/drug interval. Systolic (S) and diastolic pressure (D) were discriminated and MAP was calculated using the formula, D+1/3(S−D), with values averaged over each 1 min period.
Statistical analysis
For electrophysiological experiments, the effect of agonist alone was tested by comparing the firing rate at each dose with pre-drug values using one-way ANOVA and Dunnett's post hoc test (Graph Pad Prism Software, San Diego, CA, USA). The effect of antagonist was tested by comparing the firing rate after each agonist dose in the presence and absence of antagonist using two-way ANOVA and Bonferroni's post hoc test across all drug conditions in each set of experiments. The effect of antagonist and vehicle treatments on 5-HT neurone firing rate, and of all drugs on the regularity of 5-HT neurone firing were tested by comparing pre- and post-drug values using paired two-tailed Student's t-test.
Data from blood pressure experiments, testing the effect of DOI against saline or pretreatment with BW 501C67, were analysed across all drug conditions using two-way ANOVA and Bonferroni's post hoc test. The effect of BW 501C67 alone was tested by comparing pre- and post-drug values using a two-tailed Student's t-test. For all experiments, mean±s.e.m. values are presented. P-values of 0.05 or less were considered statistically significant.
Drugs
The drugs used (from the sources indicated) were: DOI (Sigma-Aldrich, U.K.), DOB (Sigma-Aldrich, U.K.), (±)-8-hydroxy-2-(dipropylamino)-tetralin (8-OH-DPAT; Sigma-Aldrich, U.K.), n-3-pyridinyl-3,5-dihydro-5-methyl-benzo (1,2-b:4,5-b′)dipyrrole-1(2H)carboxamide (SB 206553; Sigma-Aldrich, U.K.), ritanserin (gift, Janssen Phamaceuticals, Beerse, Belgium), R-(+)-α-(2,3-dimethoxyphenil)-1-[2-(4-fluorophenylethyl)]-4-piperidine-methanol (MDL 100,907; gift, Eli Lilly & Co., Windlesham, U.K.) and α-anilino-N-2-m-chlorophenoxypropylacetamidine (BW 501C67; generous gift from Dr A. Ramage). All drugs were dissolved in 0.9% saline, except ritanserin and MDL 100,907 which were dissolved in 5% glucose solution with 20 μl 100% acetic acid and brought to pH 5 with 5 M NaOH.
Results
Electrophysiological characteristics of 5-HT neurones
A total of 83 presumed 5-HT neurones in the DRN were recorded. Of these, 9 were excluded from the analysis due to an atypical response to 5-HT2 agonist administration (see later). The 74 neurones analysed fired broad triphasic spikes (waveform length 3.29±0.10 ms) in a slow and regular firing pattern (baseline firing rate, 0.96±0.06 Hz; baseline coefficient of variation, 0.31±0.01).
Effect of DOI and DOB on 5-HT neuronal activity
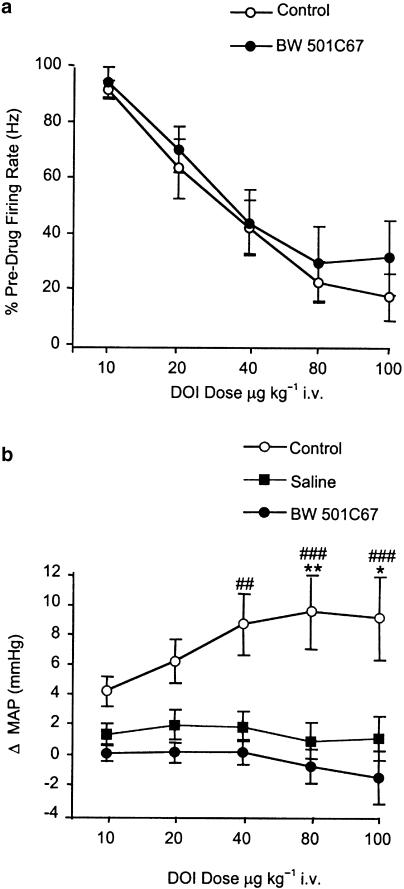
Systemic administration of DOI (10–100 μg kg−1 i.v.) caused a dose-related inhibition of 5-HT cell firing compared to predrug values (Figure 1 and Figure 2a). This effect was statistically significant at 20 μg kg−1, and the highest dose tested reduced firing by 83% (range 57–100%) of pre-drug values (one-way ANOVA: [F5,40=22.1] P<0.0001; Dunnett's post hoc test P<0.05 20 μg kg−1, P<0.001 40–80 μg kg−1) (Figure 3a). Similarly, DOB (10–100 μg kg−1 i.v.) caused a marked and dose-related inhibition in 5-HT cell firing to 84% (range 59–100%) of pre-drug values (one-way ANOVA: [F5,24=15.5] P<0.0001; Dunnett's post hoc test P<0.01 20–100 μg kg−1) (Figure 3b). Neither DOI nor DOB altered the regularity of 5-HT cell firing (Figure 5b).
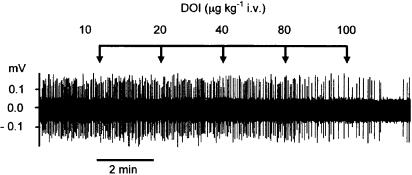
Figure 1.
Effect of the 5-HT2 agonist DOI on the firing of a 5-HT neurone in the DRN of an anaesthetised rat. Each vertical line in the spike train represents a single action potential. DOI was administered in increasing doses at 2 min intervals as indicated.
Figure 2.
Rate metre recordings demonstrating the effect of DOI on 5-HT cell firing either (a) alone or in rats pretreated with (b) the 5-HT2 receptor antagonist ritanserin (1 mg kg−1 i.v.), (c) the 5-HT2A receptor antagonist MDL 100,907 (0.2 mg kg−1 i.v.) or (d) the 5-HT2B/C receptor antagonist SB 206553 (0.5 mg kg−1 i.v.). Antagonists were administered 5 min prior to DOI, which was given in increasing doses at 2 min intervals. Note also the characteristic inhibitory response of the 5-HT neurones to the 5-HT1A receptor agonist 8-OH-DPAT (10 μg kg−1 i.v.).
Figure 3.
Effect of DOI (a) and DOB (b) in the presence of the 5-HT2 receptor antagonist ritanserin (1 mg kg−1 i.v.), the 5-HT2A receptor antagonist MDL 100,907 (0.2 mg kg−1 i.v.) or the 5-HT2B/C receptor antagonist SB 206553 (0.5 mg kg−1 i.v.). Antagonists were administered 5 min prior to either DOI or DOB, which was given in increasing doses at 2 min intervals. Controls received DOI or DOB alone. Data points are mean±s.e.m. of nobservations at agonist doses of 10, 20, 40, 80, 100 μg kg−1 respectively: (a) control n=8,8,8,8,6; ritanserin n=6,6,5,4,4; MDL 100,907 n=7,6,4,4,4; SB 206553 n=7,6,6,5,5; (b) control n=5,5,5,5,5; MDL 100907 n=3,3,3,3,3; SB 205663 n=8,8,8,6,6. **P<0.01, ***P<0.001 for control versus ritanserin, ##P<0.01, ###P<0.001 for control versus MDL 100907, +P<0.05, ++P<0.01 for control versus SB 206553 (two-way ANOVA with Bonferroni's post hoc test).
Figure 5.
Effect of 5-HT receptor antagonists and vehicle alone on the firing rate (a), and firing regularity (b) of 5-HT neurones. Data for 5-HT2 receptor agonists are also included in (b). Measurements were made during the final 1 min of a 5 min pretreatment for antagonists or a 2 min period for agonist or vehicle. Doses were ritanserin (1.0 mg kg−1 i.v.), MDL 100,907 (0.2 mg kg−1 i.v.), SB 206553 (0.5 mg kg−1 i.v.), BW 501C67 (0.1 mg kg−1 i.v.), DOI (40 μg kg−1 i.v.), DOB (40 μg kg−1 i.v.). Data are mean±s.e.m. from groups of 3–10 rats. P>0.05 versus predrug values (Student's two-tailed paired t-tests).
Effect of DOI and DOB on 5-HT neuronal activity in the presence of 5-HT2 receptor antagonists
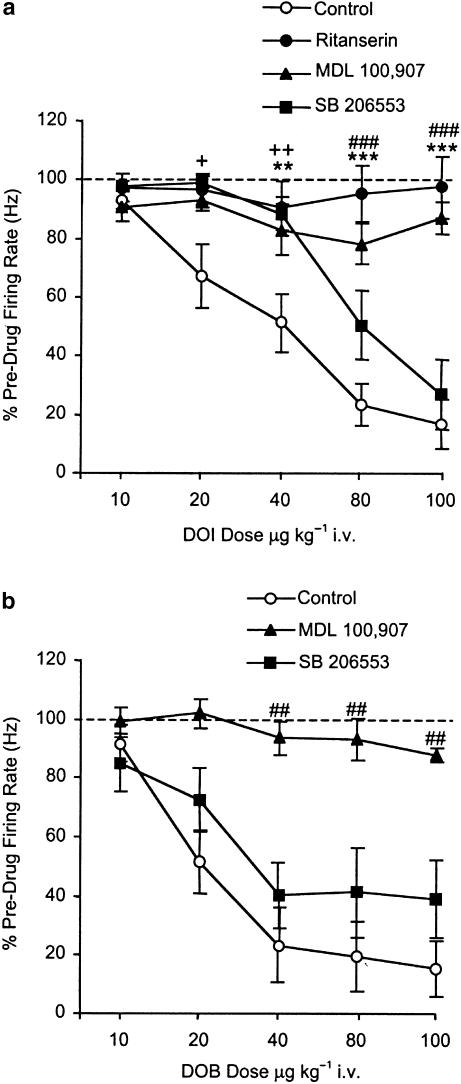
Pretreatment with the nonselective 5-HT2 receptor antagonist ritanserin (1 mg kg−1 i.v.) completely blocked the inhibition of 5-HT cell firing induced by DOI (10–100 μg kg−1 i.v.) (Figure 2b and Figure 3a). Pretreatment with the selective 5-HT2A antagonist MDL 100,907 (0.2 mg kg−1 i.v.) also blocked the inhibition of 5-HT cell firing induced by DOI (10–100 μg kg−1 i.v.) (Figure 2c and Figure 3a). Pretreatment with the 5-HT2B/C antagonist SB 206553 (0.5 mg kg−1 i.v.) caused a moderate attenuation of the effect of DOI as indicated by a rightward shift in the dose response (two-way ANOVA: interaction [F12,97=5.0] P<0.0001, treatment [F3,97=32.9] P<0.0001, dose [F4,97=17.3] P<0.0001 (Figure 2d and Figure 3a).
Pretreatment with MDL 100,907 (0.2 mg kg−1 i.v.) also blocked the inhibitory effect of DOB (10–100 μg kg−1 i.v.). In comparison, SB 206553 caused a small but statistically significant shift to the right in the dose response to DOB although this was not as clearcut as with DOI (two-way ANOVA: interaction [F8,61=1.2] P>0.05, treatment [F2,61=21.4] P<0.0001, dose [F4,61=7.3] P<0.0001; subsequent two-way ANOVA for control versus SB 206553 revealed a significant effect of treatment [F1,51=4.3] P<0.05) (Figure 3b).
Effects of DOI on 5-HT neuronal activity and blood pressure in the presence of peripheral 5-HT2 antagonists
Pretreatment with the peripheral 5-HT2 antagonist BW 501C67 (0.1 mg kg−1 i.v.) had no effect on the DOI-induced inhibition of 5-HT cell firing (two-way ANOVA: interaction [F4,60=0.1] P>0.05, treatment [F1,60=1.2] P>0.05, dose [F4,60=20.0] P<0.0001) (Figure 4a).
Figure 4.
Effect of DOI on 5-HT neuronal activity (a) and mean arterial blood pressure (b) in the presence and absence of the peripheral 5-HT2 receptor antagonist BW 501C67 (0.1 mg kg−1 i.v.). DOI was given in increasing doses at 2 min intervals. When tested, BW 501C67 was administered 5 min prior to DOI. Controls received DOI alone and the saline condition received five sequential injections of saline. Data points are mean±s.e.m. of n observations at agonist doses of 10, 20, 40, 80,100 μg kg−1 respectively: (a) control n=8,8,8,8,6; BW 501C67 n=8,7,6,6,5; (b) control n=6,6,6,6,6; saline n=4,4,4,4,4; BW 501C67 n=6,6,6,6,6. *P<0.05, **P<0.01 for control versus saline, ##P<0.01, ###P<0.001 for control versus BW 501C67 (two-way ANOVA with Bonferroni's post hoc test).
In separate experiments, DOI induced a dose-related (10–100 μg kg−1 i.v.) increase in MAP compared to saline controls. BW 501C67 (0.1 mg kg−1 i.v.) abolished this effect of DOI (two-way ANOVA: interaction [F8,65=1.0] P>0.05, treatment [F2,65=38.2] P<0.0001, dose [F4,65=0.5] P>0.05) (Figure 4b). BW 501C67 (0.1 mg kg−1 i.v.) alone did not alter MAP (two-tailed Student's t-test, P>0.05).
Effect of 5-HT2 antagonists and vehicles alone on 5-HT neuronal activity
None of the 5-HT receptor antagonists tested (1 mg kg−1 i.v. ritanserin, 0.2 mg kg−1 i.v. MDL 100,907, 0.5 mg kg−1 i.v. SB 206553 or 0.1 mg kg−1 i.v. BW 501C67) altered either the rate or regularity of 5-HT cell firing (paired two-tailed Student's t-tests P>0.05). Similarly, these parameters were not altered by saline or 5% glucose solution with acetic acid vehicles (paired two-tailed Student's t-tests, P>0.05) (Figure 5).
Excitation of 5-HT neurones by DOI and DOB
A small number of 5-HT neurones (9/83) were found to increase in firing rate in response to DOI or DOB administration (10–100 μg kg−1 i.v.). This increase ranged between 112 and 195% of predrug levels, and was detected both in the presence and absence of antagonist pretreatment (DOI alone n=1 cell, ritanserin/DOI n=1, MDL 100,907/DOI n=1, MDL 100,907/DOB n=1, SB 206553/DOB n=2, BW 501C67/DOI n=3).
Discussion
Systemic administration of phenethylamine-derived 5-HT2 agonists inhibits the firing of 5-HT neurones, but the role of specific 5-HT2 receptor subtypes is uncertain, and a peripheral site of action has been suggested (see Introduction). The present study addresses these issues using extracellular recordings of 5-HT neurones in the DRN of anaesthetised rats.
It was found that the phenethylamine-derived 5-HT2 agonists DOI or DOB inhibit the firing of 5-HT neurones, in confirmation of earlier studies testing DOI and DOM (Aghajanian et al., 1970; Wright et al., 1990). Pretreatment with the 5-HT2 receptor-selective antagonist ritanserin completely blocked the inhibitory action of DOI, implicating the involvement of 5-HT2 receptors in this effect. Although an earlier study found that ritanserin did not block this effect of DOI (Garratt et al., 1991), the dose of ritanserin was lower than that used here (0.5 versus 1 mg kg−1 in current experiments), and the pretreatment period was longer (20 versus 5 min).
Pretreatment with the 5-HT2A receptor-selective antagonist MDL 100,907 (Kehne et al., 1996), at a dose (0.5 mg kg−1) that causes full occupancy of 5-HT2 receptors in animal PET studies (Hirani et al., 2003), also blocked the inhibition of 5-HT cell firing induced by DOI and DOB. These findings are in accordance with a recent paper describing two instances in which the firing of a DRN 5-HT neurone was inhibited by DOI and then restored by MDL 100,907 (Martin-Ruiz et al., 2001). These observations suggest that 5-HT2A receptors play a major role in the 5-HT2 agonist-induced inhibition of 5-HT cell firing; however, other data in the present study also suggest the involvement, albeit minor, of 5-HT2B/C receptors. Thus, the 5-HT2B/C receptor-selective antagonist SB 206553 (0.5 mg kg−1 i.v.) caused a small, but statistically significant, shift to the right in the dose response to both DOI and DOB.
SB 206553 has over a 100-fold higher affinity for 5-HT2B/C binding sites versus 5-HT2A and other 5-HT receptor subtypes in vitro (pKI 5-HT2A=5.8; pA2 5-HT2B=8.9; pKI 5-HT2C=7.9; Kennett et al., 1996). Moreover, SB 206553 acts as a potent antagonist in in vivo models of 5-HT2C function with an ID50 of 0.3 mg kg−1 i.v. (Kennett et al., 1996; Millan et al., 1997) and completely reverses 5-HT2 receptor agonist-induced decreases in dopamine neurone firing at the dose used in the present study (Gobert et al., 2000). The low levels of 5-HT2B receptors in the rat brain (Barnes & Sharp, 1999) make it more likely that 5-HT2C receptors are involved. It is possible that the role of 5-HT2C receptors may be underestimated in the present experiments, as both DOI and DOB have a marginally higher affinity for 5-HT2A compared to 5-HT2C receptor subtypes (Ki values–DOI: 5-HT2A=19 nM, 5-HT2C=30 nM; DOB: 5-HT2A=41 nM, 5-HT2C=70 nM; Glennon et al., 1992).
In this study, a small number of 5-HT neurones increased in activity in response to administration of DOI or DOB. An excitatory response of a subpopulation of 5-HT neurones to 5-HT2 agonists has been reported in previous studies both in vivo (Trulson et al., 1981; Martin-Ruiz et al., 2001) and in vitro (Liu et al., 2000), and has been likened to the excitatory action of the unsubstituted phenethylamine, amphetamine, on DRN 5-HT neurones (Aghajanian et al., 1970). In this study, DOI or DOB excited cells even in animals pretreated with ritanserin, MDL 100,907 or SB 206553 indicating that this effect is not 5-HT2 receptor mediated.
On the basis of observations that the decrease in 5-HT cell firing induced by DOI occurs together with a rise in blood pressure, it has been suggested that these effects are linked and that the site of action of DOI is not central but peripheral vascular 5-HT2 receptors (Penington & Reiffenstein, 1986). Importantly, in the present study it was found that pretreatment with the peripherally acting 5-HT2 antagonist BW 501C67 (Mawson & Whittington, 1970; Knowles & Ramage, 1999) had no effect on the DOI-induced inhibition of 5-HT cell firing, even although the drug completely blocked the peripheral 5-HT2 receptor-mediated pressor response as reported previously (Fuller et al., 1986). These results not only implicate a central site of action of DOI, but provide further evidence that 5-HT cell firing in the DRN can change independently of alterations in blood pressure (Foote et al., 1969; Fornal et al., 1990).
The present data suggest that the inhibition of 5-HT neuronal activity by phenethylamine 5-HT2 agonists is mediated centrally, and there are a number of putative neuroanatomical substrates that might be involved. Although 5-HT2 receptors are not located on 5-HT neurones (Cornea-Hebert et al., 1999), a direct action of phenethylamines within the DRN is supported by the presence of 5-HT2A and 5-HT2C receptor mRNA in this region (Wright et al., 1995), and a report that DOI inhibits 5-HT cell firing when locally applied (Garratt et al., 1991). Also, a recent in vitro electrophysiological study found evidence that 5-HT2A and 5-HT2C receptors located on GABA interneurones within the DRN mediate a local inhibitory feedback onto the adjacent 5-HT neurones (Liu et al., 2000). Alternative or additional sites of action include the 5-HT2 receptor-mediated regulation of DRN afferents such as inputs from the medial prefrontal cortex (Hajós et al., 1998; Martin-Ruiz et al., 2001), lateral habenula (Aghajanian & Wang, 1977) and locus coeruleus (Peyron et al., 1996).
The putative 5-HT2 receptor feedback system does not appear to be tonically active, as the 5-HT2 receptor antagonists alone had no effect on 5-HT cell firing rate, and previous studies indicate that these drugs do not alter brain extracellular 5-HT (Ichikawa et al., 1998). Endogenous 5-HT might, however, activate 5-HT2 receptor-mediated feedback pathways under conditions of elevated 5-HT such as in the presence of a 5-HT uptake inhibitor. It is noteworthy that although the inhibition of 5-HT cell firing by 5-HT uptake inhibitors can be reversed by WAY 100635, this effect is often short lasting and is not detected in all neurones (Gartside et al., 1995).
In conclusion, the present data suggest that the inhibition of DRN 5-HT neurones by the phenethylamine 5-HT2 agonists, DOI and DOB, involves activation of central 5-HT2 receptors of the 5-HT2A subtype, with the 5-HT2B/C subtype also playing a minor role. As evidence suggests that 5-HT2 receptors are not located on 5-HT neurones, a postsynaptic 5-HT2 receptor-mediated feedback loop is implicated. Since antagonists of other 5-HT feedback pathways (5-HT autoreceptors) have SSRI augmentation properties (see Introduction), 5-HT2 receptor antagonists may also have this potential.
Acknowledgments
This work was supported by a MRC programme grant (T.S.) and a MRC Industrial Collaborative Studentship with Eli Lilly & Co. (L.J.B.). We express thanks for the gifts of ritanserin (Janssen Pharmaceuticals), MDL 100,907 (Eli Lilly & Co.) and BW 501C67 (Dr A. Ramage).
Abbreviations
- DRN
dorsal raphe nucleus
- GABA
γ-aminobutyric acid
- 5-HT
5-hydroxytryptamine
- ISI
inter-spike interval
- MAP
mean arterial pressure
- SSRI
selective serotonin reuptake inhibitor
References
- AGHAJANIAN G.K.Feedback regulation of central monoaminergic neurones: evidence from single cell recording studies Essays in Neurochemistry and Neuropharmacology 1978Chichester: J. Wiley and Sons Ltd; 1–32.ed. M Youdim, D.S., Lovenberg, W., Lagnado, J. pp [PubMed] [Google Scholar]
- AGHAJANIAN G.K., FOOTE W.E., SHEARD M.H. Action of psychotogenic drugs on single midbrain raphe neurons. J. Pharmacol. Exp. Ther. 1970;171:178–187. [PubMed] [Google Scholar]
- AGHAJANIAN G.K., WANG R.Y. Habenular and other midbrain raphe afferents demonstrated by a modified retrograde tracing technique. Brain Res. 1977;122:229–242. doi: 10.1016/0006-8993(77)90291-8. [DOI] [PubMed] [Google Scholar]
- ARTIGAS F., CELADA P., LARUELLE M., ADELL A. How does pindolol improve antidepressant action. Trends Pharmacol. Sci. 2001;22:224–228. doi: 10.1016/s0165-6147(00)01682-5. [DOI] [PubMed] [Google Scholar]
- BARNES N.M., SHARP T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- BLIER P., DE MONTIGNY C., CHAPUT Y.A role for the serotonin system in the mechanism of action of antidepressant treatments: preclinical evidence J. Clin. Psychiatry 199051Suppl14–20.discussion 21 [PubMed] [Google Scholar]
- BOOTHMAN L.J., ALLERS K.A., RASMUSSEN K., SHARP T. Electrophysiological evidence for 5HT2 receptor-mediated control of 5-HT cell firing in the dorsal raphe nucleus of the anaesthetised rat. Br. J. Pharmacol. 2001;134:134P. doi: 10.1038/sj.bjp.0705328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOOTHMAN L., ALLERS K., RASMUSSEN K., SHARP T. Evidence that 5-HT2 receptor agonist induced inhibition of 5-HT cell firing is mediated by central and not peripheral 5-HT2 receptors. Br. J. Pharmacol. 2003;138:3P. doi: 10.1038/sj.bjp.0705328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CECI A., BASCHIROTTO A., BORSINI F. The inhibitory effect of 8-OH-DPAT on the firing activity of dorsal raphe serotoninergic neurons in rats is attenuated by lesion of the frontal cortex. Neuropharmacology. 1994;33:709–713. doi: 10.1016/0028-3908(94)90177-5. [DOI] [PubMed] [Google Scholar]
- CORNEA-HEBERT V., RIAD M., WU C., SINGH S.K., DESCARRIES L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J. Comp. Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- FOOTE W.E., SHEARD M.H., AGHAJANIAN G.K. Comparison of effects of LSD and amphetamine on midbrain raphe units. Nature. 1969;222:567–569. doi: 10.1038/222567a0. [DOI] [PubMed] [Google Scholar]
- FORNAL C.A., LITTO W.J., MORILAK D.A., JACOBS B.L. Single-unit responses of serotonergic neurons to vasoactive drug administration in behaving cats. Am. J. Physiol. 1990;259:R963–R972. doi: 10.1152/ajpregu.1990.259.5.R963. [DOI] [PubMed] [Google Scholar]
- FULLER R.W., KURZ K.D., MASON N.R., COHEN M.L. Antagonism of a peripheral vascular but not an apparently central serotonergic response by xylamidine and BW 501C67. Eur. J. Pharmacol. 1986;125:71–77. doi: 10.1016/0014-2999(86)90084-1. [DOI] [PubMed] [Google Scholar]
- GARRATT J.C., KIDD E.J., WRIGHT I.K., MARSDEN C.A. Inhibition of 5-hydroxytryptamine neuronal activity by the 5-HT agonist, DOI. Eur. J. Pharmacol. 1991;199:349–355. doi: 10.1016/0014-2999(91)90499-g. [DOI] [PubMed] [Google Scholar]
- GARTSIDE S.E., UMBERS V., HAJOS M., SHARP T. Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. Br. J. Pharmacol. 1995;115:1064–1070. doi: 10.1111/j.1476-5381.1995.tb15919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLENNON R.A., RAGHUPATHI R., BARTYZEL P., TEITLER M., LEONHARDT S. Binding of phenylalkylamine derivatives at 5-HT1C and 5-HT2 serotonin receptors: evidence for a lack of selectivity. J. Med. Chem. 1992;35:734–740. doi: 10.1021/jm00082a014. [DOI] [PubMed] [Google Scholar]
- GOBERT A., RIVET J.M., LEJEUNE F., NEWMAN-TANCREDI A., ADHUMEAU-AUCLAIR A., NICOLAS J.P., CISTARELLI L., MELON C., MILLAN M.J. Serotonin(2C) receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic, pathways: a combined dialysis and electrophysiological analysis in the rat. Synapse. 2000;36:205–221. doi: 10.1002/(SICI)1098-2396(20000601)36:3<205::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- HAJÓS M., GARTSIDE S.E., VILLA A.E., SHARP T. Evidence for a repetitive (burst) firing pattern in a sub-population of 5-hydroxytryptamine neurons in the dorsal and median raphe nuclei of the rat. Neuroscience. 1995;69:189–197. doi: 10.1016/0306-4522(95)00227-a. [DOI] [PubMed] [Google Scholar]
- HAJÓS M., HAJÓS-KORCSOK E., SHARP T. Role of the medial prefrontal cortex in 5-HT1A receptor-induced inhibition of 5-HT neuronal activity in the rat. Br. J. Pharmacol. 1999;126:1741–1750. doi: 10.1038/sj.bjp.0702510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAJÓS M., RICHARDS C.D., SZEKELY A.D., SHARP T. An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience. 1998;87:95–108. doi: 10.1016/s0306-4522(98)00157-2. [DOI] [PubMed] [Google Scholar]
- HIRANI E., SHARP T., SPRAKES M., GRASBY P., HUME S. Effect of endogenous 5-HT on [11C]MDL 100907 binding investigated in rat brain using PET and in situ immediate early gene expression. Br. J. Pharmacol. 2003;138:181P. [Google Scholar]
- ICHIKAWA J., KUROKI T., DAI J., MELTZER H.Y. Effect of antipsychotic drugs on extracellular serotonin levels in rat medial prefrontal cortex and nucleus accumbens. Eur. J. Pharmacol. 1998;351:163–171. doi: 10.1016/s0014-2999(98)00308-2. [DOI] [PubMed] [Google Scholar]
- KEHNE J.H., BARON B.M., CARR A.A., CHANEY S.F., ELANDS J., FELDMAN D.J., FRANK R.A., VAN GIERSBERGEN P.L., MCCLOSKEY T.C., JOHNSON M.P., MCCARTY D.R., POIROT M., SENYAH Y., SIEGEL B.W., WIDMAIER C. Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J. Pharmacol. Exp. Ther. 1996;277:968–981. [PubMed] [Google Scholar]
- KENNETT G.A., WOOD M.D., BRIGHT F., CILIA J., PIPER D.C., GAGER T., THOMAS D., BAXTER G.S., FORBES I.T., HAM P., BLACKBURN T.P. In vitro and in vivo profile of SB 206553, a potent 5-HT2C/5-HT2B receptor antagonist with anxiolytic-like properties. Br. J. Pharmacol. 1996;117:427–434. doi: 10.1111/j.1476-5381.1996.tb15208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOWLES I.D., RAMAGE A.G. Evidence for a role for central 5-HT2B as well as 5-HT2A receptors in cardiovascular regulation in anaesthetized rats. Br. J. Pharmacol. 1999;128:530–542. doi: 10.1038/sj.bjp.0702822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU R., JOLAS T., AGHAJANIAN G. Serotonin 5-HT2 receptors activate local GABA inhibitory inputs to serotonergic neurons of the dorsal raphe nucleus. Brain Res. 2000;873:34–45. doi: 10.1016/s0006-8993(00)02468-9. [DOI] [PubMed] [Google Scholar]
- MARTIN-RUIZ R., PUIG M.V., CELADA P., SHAPIRO D.A., ROTH B.L., MENGOD G., ARTIGAS F. Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J. Neurosci. 2001;21:9856–9866. doi: 10.1523/JNEUROSCI.21-24-09856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAWSON C., WHITTINGTON H. Evaluation of the peripheral and central antagonistic activities against 5-hydroxytryptamine of some new agents. Br. J. Pharmacol. 1970;39:223P–224P. [PMC free article] [PubMed] [Google Scholar]
- MILLAN M.J., GIRARDON S., BERVOETS K. 8-OH-DPAT-induced spontaneous tailflicks in the rat are facilitated by the selective serotonin 5-HT2C agonist, RO 60-0175: blockade of its actions by the novel 5-HT2C receptor antagonist SB 206,553. Neuropharmacology. 1997;36:743–745. doi: 10.1016/s0028-3908(97)00071-3. [DOI] [PubMed] [Google Scholar]
- PALACIOS J.M., MENGOD G., SARASA M., VILARO M.T., POMPEIANO M., MARTINEZ-MIR M.I. The use of in situ hybridization histochemistry for the analysis of neurotransmitter receptor expression at the microscopic level. J. Recept. Res. 1991;11:459–472. doi: 10.3109/10799899109066421. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C.The rat brain in stereotoxic co-ordinates 1986London: Academic Press Ltd; 2nd Edition [Google Scholar]
- PENINGTON N.J., REIFFENSTEIN R.J. Direct comparison of hallucinogenic phenethylamines and D-amphetamine on dorsal raphe neurons. Eur. J. Pharmacol. 1986;122:373–377. doi: 10.1016/0014-2999(86)90420-6. [DOI] [PubMed] [Google Scholar]
- PEYRON C., LUPPI P.H., FORT P., RAMPON C., JOUVET M. Lower brainstem catecholamine afferents to the rat dorsal raphe nucleus. J. Comp. Neurol. 1996;364:402–413. doi: 10.1002/(SICI)1096-9861(19960115)364:3<402::AID-CNE2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- TRULSON M.E., HEYM J., JACOBS B.L. Dissociations between the effects of hallucinogenic drugs on behavior and raphe unit activity in freely moving cats. Brain Res. 1981;215:275–293. doi: 10.1016/0006-8993(81)90507-2. [DOI] [PubMed] [Google Scholar]
- VARGA V., SZEKELY A.D., CSILLAG A., SHARP T., HAJOS M. Evidence for a role of GABA interneurones in the cortical modulation of midbrain 5-hydroxytryptamine neurones. Neuroscience. 2001;106:783–792. doi: 10.1016/s0306-4522(01)00294-9. [DOI] [PubMed] [Google Scholar]
- VERGE D., CALAS A. Serotoninergic neurons and serotonin receptors: gains from cytochemical approaches. J. Chem. Neuroanat. 2000;18:41–56. doi: 10.1016/s0891-0618(99)00050-2. [DOI] [PubMed] [Google Scholar]
- WRIGHT D.E., SEROOGY K.B., LUNDGREN K.H., DAVIS B.M., JENNES L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J. Comp. Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- WRIGHT I.K., GARRATT J.C., MARSDEN C.A. Effects of a selective 5-HT2 agonist, DOI, on 5-HT neuronal firing in the dorsal raphe nucleus and 5-HT release and metabolism in the frontal cortex. Br. J. Pharmacol. 1990;99:221–222. doi: 10.1111/j.1476-5381.1990.tb14683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]