Abstract
This study explored the role of the potassium ion in endothelium-derived hyperpolarizing factor (EDHF)-mediated vasodilatation in the bovine coronary artery.
Bradykinin-induced, EDHF-mediated vasodilatation was blocked by the Na+–K+ ATPase inhibitor, ouabain (1 μM), in a time-dependent manner, with maximal blockade seen after 90 min. In contrast, the KIR channel inhibitor, Ba2+ (30 μM), had no effect.
When the potassium content of the bathing solution was increased in a single step from 5.9 to 7–19 mM, powerful vasodilatation (max. 75.9±3.6%) was observed. Vasodilatation was transient and, consequently, cumulative addition of potassium produced little vasodilatation, with vasoconstriction predominating at the higher concentrations.
The magnitude of potassium-induced vasodilatation was similar in endothelium-containing and endothelium-denuded rings, and was unaffected by Ba2+ (30 μM), but abolished by ouabain (1 μM).
Ouabain (1 μM, 90 min) powerfully blocked bradykinin-induced, nitric oxide-mediated vasodilatation as well as that induced by the nitrovasodilator, glyceryl trinitrate, but that induced by the KATP channel opener, levcromakalim, was hardly affected.
Thus, activation of Na+–K+ ATPase is likely to be involved in the vasodilator responses of the bovine coronary artery to both nitric oxide and EDHF. These findings, together with the ability of potassium to induce powerful, ouabain- but not Ba2+-sensitive, endothelium-independent vasodilatation, are consistent with this ion contributing to the EDHF response in this tissue.
Keywords: Coronary artery, endothelium, endothelium-derived hyperpolarizing factor, EDHF, nitric oxide, ouabain, potassium, vasodilatation
Introduction
It is now generally accepted that an endothelium-derived hyperpolarizing factor (EDHF), distinct from nitric oxide and prostacyclin, regulates tone in the vasculature (for reviews see Félétou & Vanhoutte, 1999; Campbell & Harder, 2001). The nature of this EDHF remains unresolved, but studies using apamin and charybdotoxin show that the vasodilator mechanism involves the opening of small conductance (SKCa) and intermediate conductance (IKCa) calcium-activated potassium channels on the vascular endothelium (Waldron & Garland, 1994; Zygmunt & Högestätt, 1996; Edwards et al., 1998; Doughty et al., 1999). The ensuing endothelial hyperpolarization may then spread electrotonically via myoendothelial gap junctions, thereby hyperpolarizing and relaxing the underlying vascular smooth muscle (Chaytor et al., 1998, 2001). An alternative explanation, first proposed from findings on rat hepatic and mesenteric arteries, is that the potassium ion released from the activated endothelial cells diffuses to the smooth muscle and promotes hyperpolarization and relaxation by stimulating the Na+–K+ ATPase and the inward rectifier potassium channel (KIR) (Edwards et al., 1998). Some difficulties have, however, arisen with this particular proposal. For example, application of potassium failed to relax guinea-pig submucosal (Coleman et al., 2001), cerebral and mesenteric resistance vessels (Dong et al., 2000). Moreover, potassium-induced relaxation of rat mesenteric and renal resistance vessels was found to be endothelium-dependent (Doughty et al., 2001; Jiang & Dusting, 2001), a property incompatible with the cation acting as the EDHF. Subsequent work on rat mesenteric vessels suggests, however, that high levels of vasoconstrictor tone may be responsible for such anomalous findings, since at low levels of tone, potassium promotes reproducible, endothelium-independent smooth muscle hyperpolarization and relaxation (Dora & Garland, 2001; Richards et al., 2001; Dora et al., 2002). These authors suggest that high concentrations of vasoconstrictors lead to stimulation of calcium-activated potassium channels on the smooth muscle cells, and the ensuing potassium efflux so activates their own Na+–K+ ATPase and KIR, that additional activation by potassium released from the endothelium is no longer possible. They further propose that a high level of vasoconstrictor tone favours EDHF-mediated vasodilatation via the gap junctional mechanism, whereas at low tone the potassium-dependent pathway predominates.
Although the contribution of EDHF to vasodilatation generally increases with decreasing vessel diameter (Hwa et al., 1994; Shimokawa et al., 1996), this mechanism plays an important role in some large conduit vessels, such as the bovine and porcine coronary artery. In these vessels too, however, evidence both supporting (Bény & Schaad, 2000) and against (Quignard et al., 1999; Drummond et al., 2000; Pratt et al., 2001) a role for potassium as the EDHF has been obtained. In view of these inconsistent outcomes, the aim of this study was to re-evaluate the role of potassium as the EDHF in the bovine coronary artery. A preliminary account of these findings has already been published (Nelli et al., 2002).
Methods
Preparation of bovine left anterior descending coronary artery rings
Sections of myocardium containing the left anterior descending coronary artery were cut from bovine hearts at a local abattoir and transported to the laboratory in Krebs solution. The coronary artery was then dissected out, cut into 2.5 mm transverse ring segments, which were suspended between two stainless-steel hooks within 10 ml organ baths and maintained at 37°C in Krebs solution gassed with O2 containing 5% CO2. Tension was recorded isometrically with Grass FTO3C transducers and displayed on a MacLab (A.D. Instruments, U.K.) data acquisition system. Resting tension was adjusted to 20 mN and tissues were allowed to equilibrate for 60 min before experiments were carried out, during which time the tension was readjusted to 20 mN, if required. In some experiments, the endothelium was removed by gentle abrasion and the success of this procedure was confirmed by the inability of bradykinin (0.3 μM) to induce vasodilatation.
Experimental protocols
In order to observe vasodilator responses, rings of bovine coronary artery were contracted to about 60% (150±20 mN; estimated from full concentration–response curves) of the maximal (U46619) 9,11-dideoxy-11α,9α-epoxy-methanoprostaglandin F2α-induced tone using a concentration of 10–100 nM. Some blocking agents, that is, NG-nitro-L-arginine methyl ester (L-NAME), charybdotoxin and ouabain enhanced U46619-induced tone in this preparation, so when these were employed, the concentration of the vasoconstrictor was reduced to ensure that the level of tone achieved was similar to that of control experiments.
Bradykinin-induced vasodilatation in bovine coronary artery involves the combined actions of nitric oxide and EDHF (Drummond et al., 2000). When we wished to study the EDHF-mediated component of vasodilatation to bradykinin (0.1–300 nM) in isolation, the nitric oxide synthase inhibitor, L-NAME (100 μM), and the cyclooxygenase inhibitor, indomethacin (3 μM), were present throughout. In contrast, when the nitric oxide-mediated component of vasodilatation to bradykinin was studied in isolation, indomethacin (3 μM) and the EDHF blockers, apamin and charybdotoxin (both 100 nM), were present throughout. In each case, bradykinin was added cumulatively, with subsequent additions being made once the maximum response to the previous concentration had been obtained. Cumulative concentration–response curves were also constructed for the vasodilator actions of the nitric oxide donor, glyceryl trinitrate (1 nM to 10 μM), and the KATP channel opener, levcromakalim (0.01–3 μM).
Vasodilator responses to potassium, added as KCl, were also studied. The potassium content of the Krebs solution was 5.9 mM and increasing this to a final bath concentration of 7–19 mM induced vasodilator responses. In the majority of experiments, these were elicited by giving a single concentration of potassium and leaving it in contact with the tissue until the maximum response had been obtained, although in other experiments (see below) the time course of onset and decay was studied. In a further set of experiments (see Results), concentration–response curves to potassium were studied following cumulative addition. Moreover, as will be seen in the Results, the duration of the relaxation induced by potassium, given as a first single addition, declined to a uniform level during the first 120 min of an experiment; there was also a tendency for the magnitude to decline, but this did not reach statistical significance. Thus, for consistency, unless otherwise stated, all experiments with potassium were conducted on tissues that had been equilibrated for at least 120 min.
In some experiments, the effects of ouabain (1 μM), an inhibitor of Na+–K+ ATPase, and of Ba2+ (as BaCl2, 30 μM), an inhibitor of KIR (Quayle et al., 1996), were examined on vasodilator responses. In these experiments, ouabain was present for a minimum of 90 min in view of its slow onset of blockade (see Results and Bény & Schaad, 2000), whereas Ba2+ was present for 30 min before vasodilator responses were elicited.
Drugs and chemicals
Apamin, bradykinin acetate, indomethacin, L-NAME, ouabain and U46619 were all obtained from Sigma (Poole, U.K.). Charybdotoxin was obtained from Latoxan (Valence, France). Glyceryl trinitrate (10% w w−1 in lactose) was a gift from NAPP Laboratories (Cambridge, U.K.). Levcromakalim was a gift from GlaxoSmithKline (Harlow, U.K.). All drugs were dissolved and diluted in 0.9% saline except indomethacin (0.01 M stock), which was dissolved in Na2CO3 (1 M), levcromakalim (0.1 M stock), which was dissolved in 70% ethanol and U46619 (1 mM), which was dissolved in 50% ethanol.
Statistical analysis
Results are expressed as the mean±s.e.m. of n observations, each from a separate coronary arterial ring. For all experiments, rings from a minimum of five animals was used. Vasodilator responses are expressed as percentage reduction of U46619-induced tone. Graphs were drawn and statistical comparisons made using one-way analysis of variance and Bonferroni's post-test, with the aid of a computer program, Prism (GraphPad, San Diego, CA, U.S.A.). A probability (P) less than or equal to 0.05 was considered significant.
Results
Effects of ouabain and barium on EDHF-mediated vasodilatation
Following induction of submaximal U46619-induced tone and in the presence of the nitric oxide synthase inhibitor, L-NAME (100 μM), and the cyclooxygenase inhibitor, indomethacin (3 μM), bradykinin (0.1–300 nM) induced con-centration-dependent vasodilatation of endothelium-containing rings of bovine coronary artery (max. 90.9±2.0%, n=12, Figure 1). This vasodilatation was mediated by EDHF since it was almost abolished in the presence of apamin and charybdotoxin (both 100 nM). The vasodilatation was also powerfully blocked in the presence of the Na+–K+ ATPase inhibitor, ouabain (1 μM), although the onset of the blockade was slow: no block was seen at 30 min, some was seen at 60 min, but 90 min was required for it to become fully established (max. relaxation 14.2±2.7%, n=7, P<0.001). Treatment with the KIR inhibitor, Ba2+ (30 μM), failed, however, to affect bradykinin-induced, EDHF-mediated vasodilatation and did not influence the blockade produced by ouabain (Figure 1b).
Figure 1.
(a) Following treatment of bovine coronary artery rings with the nitric oxide synthase inhibitor, L-NAME (100 μM), and the cyclooxygenase inhibitor, indomethacin (3 μM), bradykinin induced concentration-dependent vasodilatation that was entirely mediated by EDHF, since it was blocked by the combination of apamin and charybdotoxin (both 100 nM). This relaxation was also blocked in a time-dependent manner by ouabain (1 μM), given for 30, 60 and 90 min. (b) The KIR inhibitor, Ba2+ (30 μM), failed to affect either bradykinin-induced, EDHF-mediated vasodilatation, or the blockade of this induced by ouabain (1 μM). Data represent the mean±s.e.m of seven to 12 observations. ***P<0.001 indicates a significant difference from control (for clarity, asterisks are shown only for the highest concentration of bradykinin).
Vasodilatation induced by potassium
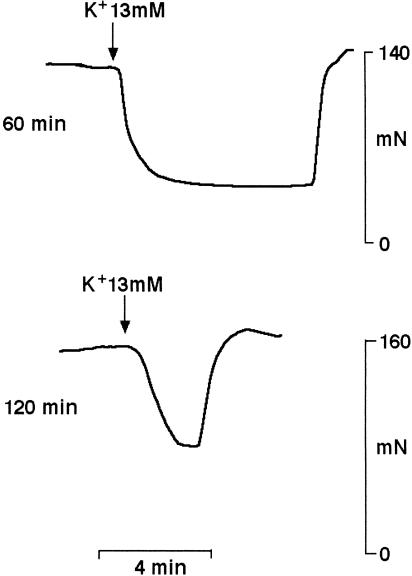
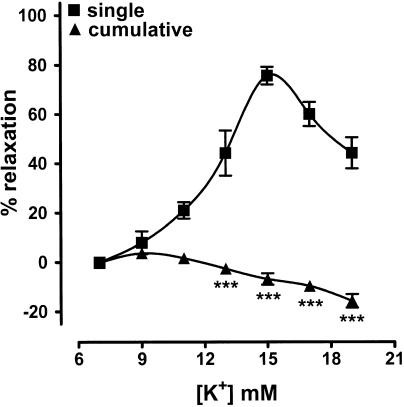
The potassium content of the Krebs solution was 5.9 mM, and vasodilatation was observed when this was increased to 7–19 mM in a single step on submaximally contracted, endothelium-denuded rings of coronary artery that had equilibrated for at least 120 min (Figure 2 and 3). This vasodilatation was concentration-dependent, with a maximum of 75.9±3.6%, n=8, occurring at 15 mM. The magnitude of the vasodilatation induced by a submaximal concentration of potassium (13 mM) was similar at the standard level of preconstriction of 152±21 mN (44.4±9.2%, n=6) and at 67±3 mN (46.8±10.7%, n=5). The duration of the vasodilatation did, however, vary according to the length of equilibration period before potassium was first added, with time from addition to half-decay of the response declining from 246.0±37.6 s, n=6, after 60 min to a uniform level of 109.0±7.5 s, n=6, P<0.001, at 180 min (Figure 2). There was also a tendency for the magnitude to decline (61.6±8.2%, n=7, and 44.4±9.2%, n=6, after 60 and 120 min, respectively), but this was not statistically significant. Furthermore, following washout, a second addition of potassium (13 mM) to tissues that had been equilibrated for 120 min produced a similar degree of relaxation (39.5±5.1%, n=6).
Figure 2.
Raising the bath concentration of potassium from 5.9 to 13 mM in a single step produced vasodilatation of submaximally contracted, endothelium-denuded rings of bovine coronary artery. The experimental traces show the vasodilatations seen when potassium was first given following equilibration for 60 or 120 min.
Figure 3.
Concentration–response curves showing the effects of potassium (7–19 mM) on endothelium-denuded rings of bovine coronary artery. The Krebs solution contained 5.9 mM potassium and the values indicated are the final concentrations attained in the tissue baths. Concentration-dependent vasodilatation was seen when a single addition of potassium was made to a tissue, but cumulative addition produced little vasodilatation, with vasoconstriction predominating at the higher concentrations. Data represent the mean±s.e.m. of eight to 12 observations. ***P<0.001 indicates where cumulative addition produced a different response to that seen upon addition of a single concentration of potassium.
In contrast to the effects seen upon addition of single concentrations, potassium (7–19 mM) was a poor relaxant when applied in cumulative fashion: a maximum relaxation of 3.7±1.5%, n=8, was obtained at 9 mM, but higher concentrations produced a constrictor response (Figure 3).
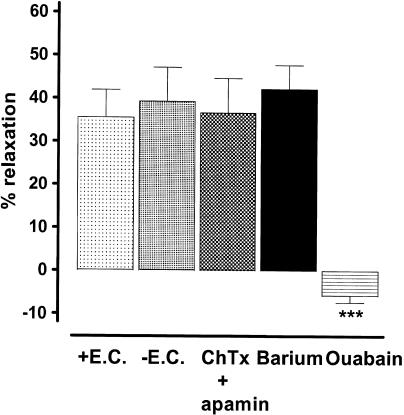
The vasodilator response to potassium (13 mM) was similar in endothelium-containing and endothelium-denuded rings, and those obtained on the latter were unaffected following treatment with apamin and charybdotoxin (both 100 nM) in combination, or Ba2+ (30 μM), but were reversed to a small vasoconstrictor response by ouabain (1 μM, 90 min, Figure 4).
Figure 4.
Histogram showing the vasodilatation of bovine coronary artery rings induced by increasing the bath concentration of potassium from 5.9 to 13 mM. The vasodilatation was similar on endothelium-denuded (−E.C.) and endothelium-intact (+E.C.) rings. Moreover, that produced on endothelium-denuded rings was unaffected by apamin and charybdotoxin (both 100 nM) in combination or by Ba2+ (30 μM), but was reversed to a small constrictor response by ouabain (1 μM). Data represent the mean±s.e.m. of six to eight observations. ***P<0.001 indicates a significant difference from the control response on endothelium-denuded rings.
Effects of ouabain on vasodilatation induced by endothelium-derived nitric oxide, glyceryl trinitrate and levcromakalim
In the presence of the EDHF blockers, apamin and charybdotoxin (both 100 nM), and the cyclooxygenase inhibitor, indomethacin (3 μM), bradykinin (0.1–300 nM) induced concentration-dependent vasodilatation of endothelium-containing rings of bovine coronary artery (max. 74.5±6.1%, n=7). This vasodilatation was mediated by nitric oxide, since it was abolished by L-NAME (100 μM, Figure 5a). Treatment with ouabain (1 μM, 90 min) also powerfully inhibited this bradykinin-induced, nitric oxide-mediated vasodilatation (max. relaxation 21.2±5.0%, n=8, P<0.001). The vasodilatation induced by the nitric oxide donor, glyceryl trinitrate (1 nM to 10 μM, Figure 5b), was also blocked, whereas that induced by the KATP channel opener, levcromakalim (0.01–3 μM, Figure 5c), was affected only slightly.
Figure 5.
Concentration–response curves showing the effects of ouabain (1 μM, >90 min) on vasodilatation of endothelium-containing rings of bovine coronary artery induced by: (a) bradykinin-stimulated release of nitric oxide, conducted in the presence of the EDHF blockers, apamin and charybdotoxin (both 100 nM), and the cyclooxygenase inhibitor, indomethacin (3 μM) (the abolition of this response by the nitric oxide synthase inhibitor, L-NAME (100 μM) is also shown, to indicate that it is entirely due to nitric oxide); (b) the nitric oxide donor, glyceryl trinitrate (GTN); and (c) the KATP channel opener, levcromakalim. Data represent the mean±s.e.m. of six to 10 observations. **P<0.005 and ***P<0.001 indicate a significant difference from control.
Discussion
In bovine coronary artery, endothelium-dependent vasodilatation to bradykinin involves the combined actions of nitric oxide and EDHF (Drummond et al., 2000; this study). The interaction between these two vasodilators is complex, but if any one component is blocked by the use of appropriate inhibitors (nitric oxide inhibitor or ODQ; apamin plus charybdotoxin), the other is still able to produce near maximal vasodilatation, although the concentration–response curve is shifted to the right by around a half log unit.
On the basis of the combined actions on rat hepatic and mesenteric arteries of ouabain and Ba2+, which respectively block Na+–K+ ATPase and KIR, Edwards et al. (1998) proposed that potassium is the EDHF. Following this initial report, many workers employed ouabain and Ba2+, as part of a strategy to investigate the role of potassium in the EDHF response, but with variable results, even from separate studies of the same vessel. For example, one study on the porcine coronary artery reported that ouabain had no effect on the EDHF-mediated response induced by bradykinin (Quignard et al., 1999), while another described its abolition (Bény & Schaad, 2000). This latter study reported that blockade by ouabain was slow in onset, although no time course data were given. Bény & Schaad (2000) were also critical of the previous study for failing to ensure that blockade by ouabain was fully established before effects on EDHF were examined. As a consequence, we decided to study formally the time course of the onset of blockade by ouabain and, like Bény & Schaad (2000), found it to be slow. Indeed, we found on bovine coronary artery that ouabain (1 μM) produced no block of bradykinin-induced, EDHF-mediated vasodilatation at 30 min, some was apparent at 60 min, but at 90 min the response was powerfully inhibited. Thus, as with the study on porcine coronary artery (Quignard et al., 1999), inappropriately short exposure times to ouabain may explain why previous studies on bovine coronary artery reported either no blockade (Drummond et al., 2000) or modest blockade (∼30%; Pratt et al., 2001) of bradykinin-induced, EDHF-mediated vasodilatation.
The powerful inhibitory action of prolonged (>90 min) exposure to ouabain is supportive of the involvement of Na+–K+ ATPase, and perhaps therefore of potassium, in the EDHF response in the bovine or porcine coronary artery. Nevertheless, the selectivity of the action of ouabain requires consideration. Consequently, we examined its effects on nitric oxide-mediated vasodilatation induced by bradykinin in the bovine coronary artery, under conditions where EDHF was blocked by apamin and charybdotoxin, and cyclooxygenase was blocked by indomethacin. Although others have suggested that a nitric oxide synthase inhibitor may not be fully effective (Cohen et al., 1997), we found that L-NAME abolished this relaxation, thus giving confidence that it was mediated entirely by nitric oxide. We, in fact, found that this nitric oxide-mediated vasodilatation too was powerfully inhibited by ouabain (1 μM, >90 min). Thus, in this tissue ouabain inhibits equally well nitric oxide- and EDHF-mediated vasodilator responses. Our findings should not, perhaps, be surprising since previous studies have reported blockade of acetylcholine-induced, nitric oxide-mediated vasodilatation with ouabain in canine femoral artery (De Mey & Vanhoutte, 1980), rat aorta (Rapoport et al., 1985a) and rabbit aorta (Ferrer et al., 1999). We also found that ouabain blocked the effects of the nitric oxide donor, glyceryl trinitrate, although the degree of blockade was less than with acetylcholine, probably because the endothelium can release only a finite amount of nitric oxide, whereas the nitric oxide donor can be freely added to overcome the blockade. Indeed, previous work had already established this pattern of blockade by ouabain on vasodilatation induced by the nitric oxide donor, sodium nitroprusside, or the membrane permeant analogue 8-bromo cyclic GMP (Rapoport & Murad, 1983; Rapoport et al., 1985b; Tamaoki et al., 1997; Ferrer et al., 1999). These authors proposed that relaxation induced by nitric oxide and nitric oxide donors arises in part through cyclic GMP-dependent activation of Na+–K+ ATPase, a conclusion supported by studies measuring ouabain-sensitive 86Rb uptake by vascular smooth muscle cells (Tamaoki et al., 1997). Others have, however, suggested that nitric oxide can activate Na+–K+ ATPase independently of cyclic GMP (Gupta et al., 1995). Regardless of the precise explanation, all of these studies demonstrate that activation of Na+–K+ ATPase forms an important component of the relaxant mechanism utilised by nitric oxide.
Ouabain is not, however, a general inhibitor of smooth muscle relaxation, since we found that vasodilatation of the bovine coronary artery induced by the KATP channel opener, levcromakalim, was hardly affected by ouabain. Our findings are consistent with previous reports showing a lack of effect of ouabain on vasodilatation induced with KATP channel openers in the bovine (Drummond et al., 2000; Pratt et al., 2001) and porcine (Bény & Schaad, 2000) coronary artery. Thus, in view of the blockade of nitric oxide, ouabain can be regarded as a selective inhibitor, not strictly of EDHF, but of vasodilatation involving activation of Na+–K+ ATPase.
In keeping with previous reports on bovine coronary artery (Drummond et al., 2000; Pratt et al., 2001), we found that Ba2+, which inhibits KIR (Quayle et al., 1996), had no effect on bradykinin-induced, EDHF-mediated vasodilatation; it also failed to modify the blockade induced by ouabain. A similar lack of effect was seen on porcine coronary artery (Bény & Schaad, 2000), suggesting that activation of KIR is not involved in the EDHF response in either vessel.
Although the effects of exogenous application of potassium support a role for this ion in the EDHF response in rat hepatic and mesenteric arteries (Edwards et al., 1998), not all reports are consistent with this. For example, in bovine coronary artery, potassium either failed to relax (Pratt et al., 2001) or relaxed only slightly (∼18%; Drummond et al., 2000). We found, however, that potassium could indeed relax this tissue in a powerful (max. 75.9±3.6% at 15 mM) and concentration-dependent manner over the range 7–19 mM, but only if it was applied in noncumulative fashion. If applied cumulatively, as by Pratt et al. (2001), we obtained only a modest relaxation (max. 3.7±1.5% at 9 mM), with contraction predominating at the higher concentrations, probably because of depolarization occurring as a result of blockade of Na+–K+ ATPase (Bény & Schaad, 2000; Drummond et al., 2000; Pratt et al., 2001). Others too have reported that the mode of delivery of potassium is an important determinant of whether the ion will or will not mimic the EDHF response. Specifically, on porcine coronary artery, application of potassium in short pulses, which was regarded as more accurately reflecting the profile of EDHF release, was more effective in producing smooth muscle hyperpolarization than long exposures (Bény & Schaad, 2000). We found on bovine coronary artery that the response to a single application of potassium was an immediate transient vasodilatation, which resolved into a small, sustained contraction. Such transience therefore explains why cumulative application of potassium fails to evoke convincing relaxation of bovine coronary artery. In addition, unlike rat mesenteric resistance vessels where the magnitude of the vasodilatation to potassium declined as the level of tone was increased (Dora & Garland, 2001; Dora et al., 2002), we found on bovine coronary artery that relaxation in percentage terms was similar at ∼30 and ∼60% of maximal U46619-induced tone. We did, however, find that the vasodilatation to potassium was more prolonged and there was a tendency, although not significant, for the magnitude to be greater if the cation was first given after equilibration for 60 min as opposed to 120 min. Following 120 min, however, responses to potassium were quite reproducible. It is therefore possible that the more pronounced vasodilatation seen at earlier time points arose because the smooth muscle Na+–K+ ATPase had not yet fully equilibrated and was more sensitive to stimulation by added potassium.
We found on bovine coronary artery that the relaxation induced by potassium was endothelium-independent, consistent perhaps with it acting as the EDHF in this artery. Moreover, in contrast to EDHF-mediated vasodilatation induced by bradykinin, that induced by potassium was not blocked by the combination of apamin and charybdotoxin. This too would be expected if potassium were the EDHF, since although apamin and charybdotoxin should block release of potassium from the endothelium (Waldron & Garland, 1994; Zygmunt & Högestätt, 1996; Edwards et al., 1998; Doughty et al., 1999), it should not block the effects of potassium added exogenously. Other characteristics consistent with such a role for potassium in the bovine coronary artery are our findings that potassium-induced vasodilatation is blocked by ouabain but not by Ba2+. Findings in agreement with these have previously been obtained on bovine (Drummond et al., 2000) and porcine (Bény & Schaad, 2000) coronary artery. Thus, potassium-induced responses, like those induced following stimulation of EDHF by bradykinin, occur as a consequence of activation of Na+–K+ ATPase but not of KIR. In rat coronary resistance vessels, however, Ba2+, but not ouabain, abolished potassium-induced vasodilatation (Knot et al., 1996), probably reflecting the greater density of KIR channels in vessels of small diameter (Quayle et al., 1996).
In conclusion, on the basis of the actions of ouabain, activation of Na+–K+ ATPase is likely to be involved in the vasodilator responses of the bovine coronary artery induced by both nitric oxide and EDHF. In contrast, the lack of effect of Ba2+ precludes involvement of KIR channels in the EDHF response. These findings, together with the ability of potassium to induce powerful, ouabain- but not Ba2+-sensitive, endothelium-independent vasodilatation are consistent with this ion contributing, at least in part, to the EDHF response in this tissue.
Acknowledgments
This work was supported by the Wellcome Trust. We gratefully acknowledge the help of the MHS inspectors at Sandyford Abattoir, Paisley, in providing bovine tissues.
Abbreviations
- BKCa
large conductance calcium-activated potassium channel
- EDHF
endothelium-derived hyperpolarizing factor
- IKCa
intermediate conductance calcium-activated potassium channel
- KIR
inward rectifier potassium channel
- KATP
ATP-sensitive potassium channel
- L-NAME
NG-nitro-L-arginine methyl ester
- SKCa
small conductance calcium-activated potassium channel
References
- BÉNY J.L., SCHAAD O. An evaluation of potassium ions as endothelium-derived hyperpolarizing factor in porcine coronary arteries. Br. J. Pharmacol. 2000;131:965–973. doi: 10.1038/sj.bjp.0703658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL W.B., HARDER D.R. Prologue: EDHF–what is it. Am. J. Physiol. 2001;280:H2413–H2416. doi: 10.1152/ajpheart.2001.280.6.H2413. [DOI] [PubMed] [Google Scholar]
- CHAYTOR A.T., EVANS W.H., GRIFFITH T.M. Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries. J. Physiol. 1998;508:561–573. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAYTOR A.T., MARTIN P.E.M., EDWARDS D.H., GRIFFITH T.M. Gap junctional communication underpins EDHF-type relaxations evoked by ACh in the rat hepatic artery. Am. J. Physiol. 2001;280:H2441–H2450. doi: 10.1152/ajpheart.2001.280.6.H2441. [DOI] [PubMed] [Google Scholar]
- COHEN R.A., PLANE F., NAJIBI S., HUK I., MALINSKI T., GARLAND C.J. Nitric oxide is the mediator of both endothelium-dependent relaxation and hyperpolarization of the rabbit carotid artery. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4193–4198. doi: 10.1073/pnas.94.8.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLEMAN H.A., TARE M., PARKINGTON H.C. EDHF is not K+ but may be due to spread of current from the endothelium in guinea pig arterioles. Am. J. Physiol. 2001;280:H2478–H2483. doi: 10.1152/ajpheart.2001.280.6.H2478. [DOI] [PubMed] [Google Scholar]
- DE MEY J.G., VANHOUTTE P.M. Interaction between Na+, K+ exchanges and the direct inhibitory effect of acetylcholine on canine femoral arteries. Circ. Res. 1980;46:826–835. doi: 10.1161/01.res.46.6.826. [DOI] [PubMed] [Google Scholar]
- DONG H., JIANG Y., COLE W.C., TRIGGLE C.R. Comparison of the pharmacological properties of EDHF-mediated vasorelaxation in guinea-pig cerebral and mesenteric resistance vessels. Br. J. Pharmacol. 2000;130:1983–1991. doi: 10.1038/sj.bjp.0703474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DORA K.A., GARLAND C.J. Properties of smooth muscle hyperpolarization and relaxation to K+ in the rat isolated mesenteric artery. Am. J. Physiol. 2001;280:H2424–H2429. doi: 10.1152/ajpheart.2001.280.6.H2424. [DOI] [PubMed] [Google Scholar]
- DORA K.A., INGS N.T., GARLAND C.J. KCa channel blockers reveal hyperpolarization and relaxation to K+ in rat isolated mesenteric artery. Am. J. Physiol. 2002;283:H606–H614. doi: 10.1152/ajpheart.01016.2001. [DOI] [PubMed] [Google Scholar]
- DOUGHTY J.M., BOYLE J.P., LANGTON P.D. Blockade of chloride channels reveals relaxations of rat small mesenteric arteries to raised potassium. Br. J. Pharmacol. 2001;132:293–301. doi: 10.1038/sj.bjp.0703769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGHTY J.M., PLANE F., LANGTON P.D. Charybdotoxin and apamin block EDHF in rat mesenteric artery if selectively applied to the endothelium. Am. J. Physiol. 1999;276:H1107–H1112. doi: 10.1152/ajpheart.1999.276.3.H1107. [DOI] [PubMed] [Google Scholar]
- DRUMMOND G.R., SELEMIDIS S., COCKS T.M. Apamin-sensitive, nonnitric oxide (NO) endothelium-dependent relaxations to bradykinin in the bovine isolated coronary artery: no role for cytochrome P-450 and K+ Br. J. Pharmacol. 2000;129:811–819. doi: 10.1038/sj.bjp.0703107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G., DORA K.A., GARDENER M.J., GARLAND C.J., WESTON A.H. K+ is an endothelium-derived hyperpolarising factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- FÉLÉTOU M., VANHOUTTE P.M. The third pathway: endothelium-dependent hyperpolarization. J. Physiol. Pharmacol. 1999;50:525–534. [PubMed] [Google Scholar]
- FERRER M., MARIN J., ENCABO A., ALONSO M.J., BALFAGON G. Role of K+ channels and sodium pump in the vasodilation induced by acetylcholine, nitric oxide, and cyclic GMP in the rabbit aorta. Gen. Pharmacol. 1999;33:35–41. doi: 10.1016/s0306-3623(98)00259-6. [DOI] [PubMed] [Google Scholar]
- GUPTA S., MORELAND R.B., MUNARRIZ R., DALEY J., GOLDSTEIN I., DE TEJADA I.S. Possible role of Na+–K+ ATPase in the regulation of human corpus cavernosum smooth muscle contractility by nitric oxide. Br. J. Pharmacol. 1995;116:2201–2206. doi: 10.1111/j.1476-5381.1995.tb15054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HWA J.J., GHIBAUDI L., WILLIAMS P., CHATTERJEE M. Comparison of acetylcholine-induced relaxation in large and small arteries of rat mesenteric vascular bed. Am. J. Physiol. 1994;266:H952–H958. doi: 10.1152/ajpheart.1994.266.3.H952. [DOI] [PubMed] [Google Scholar]
- JIANG F., DUSTING G.J. Endothelium-dependent vasorelaxation independent of nitric oxide and K+ release in isolated renal arteries of rats. Br. J. Pharmacol. 2001;132:1558–1564. doi: 10.1038/sj.bjp.0703965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOT H.J., ZIMMERMANN P.A., NELSON M.T. Extracellular K+-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K+ channels. J Physiol. 1996;492:419–430. doi: 10.1113/jphysiol.1996.sp021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELLI S., WILSON W.S., LAIDLAW H, LLANO A., MIDDLETON S., PRICE A.G., MARTIN W. Potassium ion as the EDHF in bovine coronary artery. Br. J. Pharmacol. 2002;137:14P. doi: 10.1038/sj.bjp.0705329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRATT P.F., LI P.L., HILLARD C.J., KURIAN J., CAMPBELL W.B. Endothelium-independent, ouabain-sensitive relaxation of bovine coronary arteries by EETs. Am. J. Physiol. 2001;280:H1113–H1121. doi: 10.1152/ajpheart.2001.280.3.H1113. [DOI] [PubMed] [Google Scholar]
- QUAYLE J.M., DART C., STANDEN N.B. The properties and distribution of inward rectifier potassium currents in pig coronary arterial smooth muscle. J. Physiol. 1996;494:715–726. doi: 10.1113/jphysiol.1996.sp021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUIGNARD J.-F., FÉLÉTOU M., THOLLON C., VILAINE J.-P., DUHAULT J., VANHOUTTE P.M. Potassium ions and endothelium-derived hyperpolarizing factor in guinea-pig carotid and porcine coronary arteries. Br. J. Pharmacol. 1999;127:27–34. doi: 10.1038/sj.bjp.0702493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPOPORT R.M., MURAD F. Effect of ouabain and alterations in potassium concentration on relaxation induced by sodium nitroprusside. Blood Vessels. 1983;20:255–264. doi: 10.1159/000158478. [DOI] [PubMed] [Google Scholar]
- RAPOPORT R.M., SCHWARTZ K., MURAD F. Effects of Na+, K+-pump inhibitors and membrane depolarizing agents on acetylcholine-induced endothelium-dependent relaxation and cyclic GMP accumulation in rat aorta. Eur. J. Pharmacol. 1985a;110:203–209. doi: 10.1016/0014-2999(85)90212-2. [DOI] [PubMed] [Google Scholar]
- RAPOPORT R.M., SCHWARTZ K., MURAD F. Effect of sodium–potassium pump inhibitors and membrane-depolarizing agents on sodium nitroprusside-induced relaxation and cyclic guanosine monophosphate accumulation in rat aorta. Circ. Res. 1985b;57:164–170. doi: 10.1161/01.res.57.1.164. [DOI] [PubMed] [Google Scholar]
- RICHARDS G.R., WESTON A.H., BURNHAM M.P., FÉLÉTOU M., VANHOUTTE P.M., EDWARDS G. Suppression of K+-induced hyperpolarization by phenylephrine in rat mesenteric artery: relevance to studies of endothelium-derived hyperpolarizing factor. Br. J. Pharmacol. 2001;134:1–5. doi: 10.1038/sj.bjp.0704256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMOKAWA H., YASUTAKE H., FUJII K., OWADA M.K., NAKAIKE R., FUKOMOTO Y., TAKAYANAGI T., NAGAO T., EGASHIRA K., FUJISHIMA M., TAKESHITA A. The importance of the hyperpolarising mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J. Cardiovasc. Pharmacol. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- TAMAOKI J., TAGAYA E., NISHIMURA K., ISONO K., NAGAI A. Role of Na+–K+ ATPase in cyclic GMP-mediated relaxation of canine pulmonary artery smooth muscle cells. Br. J. Pharmacol. 1997;122:112–116. doi: 10.1038/sj.bjp.0701351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALDRON G.J., GARLAND C.J. Effect of potassium channel blockers on L-NAME insensitive relaxations in rat small mesenteric artey. Can. J. Physiol. Pharmacol. 1994;72:11. [Google Scholar]
- ZYGMUNT P.M., HÖGESTÄTT E.D. Role of potassium channels in endothelium-dependent relaxation resistant to nitroarginine in the rat hepatic artery. Br. J. Pharmacol. 1996;117:1600–1606. doi: 10.1111/j.1476-5381.1996.tb15327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]