Abstract
In rat aortic smooth muscle cells (RASMCs), the putative nuclear factor kappa B (NFκB) inhibitor Pyrrolidine dithiocarbamate (PDTC) was found to inhibit lipopolysaccharide (LPS)-stimulated NFκB DNA-binding. However, further investigation identified the site of inhibition as being at, or upstream of, the inhibitory kappa B kinases (IKKs) as their kinase activity was substantially reduced.
In addition, PDTC potentiated LPS-stimulated c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAP kinase) and MAP kinase-activated protein kinase-2 activity (the downstream target of p38 MAP kinase).
Another inhibitor of NFκB signalling, the serine protease inhibitor Nαp-tosyl-L-lysine chloro-methylketone (TLCK), also inhibited LPS-stimulated IKK activity and potentiated JNK activity in response to LPS, suggesting that cross-talk may occur between the NFκB and stress-activated protein kinase pathways at the level of IKK or at a common point upstream.
Infection of RASMCs with an adenovirus encoding either inhibitory kappa Bα or a dominant-negative IKKβ potentiated LPS-stimulated JNK activity.
These studies therefore suggest that the loss of NFκB DNA-binding and resultant transcriptional activity, rather than the loss of IKK activity, is sufficient to cause an increase in JNK activity. This shows that either pharmacological or molecular inhibition of NFκB DNA-binding enhances JNK activation in vascular smooth muscle cells, an effect that may contribute to the pathophysiological effects of LPS.
Keywords: Aortic smooth muscle cells, inhibitory kappa B kinase, inhibitory kappa B, nuclear factor kappa B, c-Jun N-terminal kinase, p38 MAP kinase, pyrrolidine dithiocarbamate, Nα-p-tosyl-L-lysine-chloro-methylketone
Introduction
Lipopolysaccharide (LPS), is a cell wall component of Gram-negative bacteria, a complex glycolipid, composed of a hydrophilic polysaccharide and a hydrophobic Lipid A moiety (Seydel et al., 2000). The lipid A moiety binds CD14, a 55 kDa glycoprotein present as either a soluble factor or a glycosylphosphatidylinositol (GPI)-anchored membrane protein. Members of the Toll-like receptor (TLR) family are transmembrane spanning proteins that have been identified as part of the LPS receptor complex. TLR-4 is favoured as the LPS receptor, although there is evidence that TLR-2 is responsive to purified isolates of LPS. The LPS-responsive receptor complex also contains LPS-binding protein (LBP) and MD-2 (Zhang & Ghosh, 2000). The TLRs in mediating their intracellular effects utilise various interleukin-1 (IL-1) signalling pathway proteins such as MyD88, IL-1 receptor-associated kinase (IRAK), tumour necrosis factor receptor-associated factor (TRAF)6, TRAF2 and transforming growth factor-β-activated kinase (TAK1) (Takeuchi & Akira, 2001).
In a number of cell types including macrophages, smooth muscle and endothelial cells, LPS strongly activates the nuclear transcription factor nuclear factor kappa B (NFκB) (Zhang & Ghosh, 2000). This event is regulated by the phosphorylation-dependent degradation of an inhibitory protein, inhibitory kappa B (IκB) which is mediated by the IκB kinases (IKKs) (Rothwarf & Karin, 1999). Recently, we have shown that both NFκB -inducing kinase (NIK) and IKKs are essential for LPS-induced NFκB-mediated transcription in rat aortic smooth muscle cells (RASMCs) (Torrie et al., 2001). LPS also stimulates the activation of all three main classes of mitogen-activated protein kinase (MAP kinase) homologue; p42/44 MAP kinase, and c-Jun N-terminal kinase (JNK) and p38 MAP kinase (the stress-activated protein (SAP) kinases) (Zhang & Ghosh, 2000). How these pathways contribute to the actions of LPS in RASMCs is unknown.
Recent evidence indicates that cross-talk between the NFκB and SAP kinase cascades takes place. It has been shown that for TNFα-stimulation, NFκB and the SAP kinase cascades can share common regulatory components such as TRAF2 (Liu et al., 1996; Natoli et al., 1997) and TAK1 (Su et al., 1997). TAK1 has been classified as an MEKK that acts upstream of JNK, p38 MAP kinase and IKK activation (Craig et al., 2000), while another kinase of this class, MEK kinase 1 (MEKK1), has been shown to activate IKK (Mercurio et al., 1997) and JNK in certain cell types (Lu et al., 1997). Thus, inhibition of one of these upstream intermediates is likely to have profound effects on both SAP kinase and NFκB pathways. Recently it has been shown that activation of p38 MAP kinase results in the phosphorylation-dependent inhibition of IKK activity in some cell types (Bowie & O'Neill, 2000). However, a similar model of cross-regulation is not observed for RASMCs (Torrie et al., 2001).
In vascular smooth muscle cells, the upstream components of either the NFκB or the SAP kinase pathways stimulated in response to LPS have not, as yet, been clearly defined. Furthermore, it is unclear whether these pathways exhibit any form of cross-talk regulation. Therefore, we sought to examine the effect of inhibiting the NFκB pathway upon the SAP kinase cascade by using the NFκB inhibitors pyrrolidine dithiocarbamate (PDTC) and Nα-p-tosyl-L-lysine-chloro-methylketone (TLCK), and also by using adenoviral constructs encoding wild-type IκBα (Ad.IκBα) and a dominant-negative IKKβ (Ad.IKKβ+/−) to block components of the NFκB pathway specifically.
In this study we found that in RASMCs, PDTC abolished not only LPS-stimulated NFκB DNA-binding activity, but also the cellular depletion of IκBα and -β isoforms and LPS-stimulated IKK activity, suggesting a site of inhibition at the level of IKK or upstream. However, to our surprise we found that PDTC also caused a marked synergy in LPS-mediated activation of JNK and p38 MAP kinase and the downstream target of p38 MAP kinase, MAP kinase-activated protein kinase-2 (MAPKAP kinase-2). The proteosome inhibitor TLCK, previously described as an inhibitor of NFκB activation (Henkel et al., 1993; Mellits et al., 1993), also caused enhanced activation of the SAP kinases and inhibited LPS-mediated IKK activity. The specific inhibition of the NFκB pathway by infection of RASMCs with Ad.IKKβ+/− or Ad.IκBα caused an increase in basal JNK activity and potentiated LPS-stimulated JNK activation. Infection of cells with Ad.IκBα had no effect on IKK activity, but very effectively inhibited NFκB DNA-binding. These studies show cell type-specific cross-talk regulation between the IKK/IκB/NFκB and SAP kinase cascades and identify the effect as being dependent on NFκB DNA-binding and not a direct cross-talk event at the level of IKK or above.
Experimental procedures
Materials
The plasmid containing the cDNA encoding the glutathione S-transferase-tagged truncated N-terminus of c-Jun (GST-c-Jun5–89) was donated by J.R. Woodgett (Ontario Cancer Institute, Princess Margaret Hospital, Toronto, Canada). The cDNA encoding the GST-tagged truncated N-terminus of IκB (GST-IκBN) was provided by R. Hay (School of Biomedical Sciences, University of St Andrews, Scotland, U.K.). The KKLNRTLSVA peptide substrate was a gift from P. Cohen (MRC Protein Phosphorylation Unit, University of Dundee, Scotland, U.K.). Antibodies against p42/44 MAP kinase and inducible nitric oxide synthase (iNOS) were obtained from Affiniti Research Products (Exeter, U.K.). IκBα and -β and IKKα and -β antibodies were obtained from Santa Cruz (Santa Cruz, U.K.). Reporter antibodies and the ECL detection system were from Amersham (Buckinghamshire, U.K.). [γ-32P] ATP (3000 Ci mmol−1) was from NEN.
Cell culture
Smooth muscle cells were isolated from the thoracic aortae of 180–200 g male Sprague–Dawley rats by digestion with collagenase and elastase as previously described (Paul et al., 1997; Oitzinger et al., 2001). RASMCs were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% foetal calf serum (FCS) and used as previously outlined (Plevin et al., 1996; Paul et al., 1997).
Adenovirus-mediated expression of a dominant-negative IKKβ or wild-type IκBα
A recombinant replication-deficient adenoviral vector encoding a kinase-deficient IKKβ gene (Ad.IKKβ+/−), which acts in a dominant-negative manner, or wild-type porcine IκBα gene (Ad.IκBα) were used. These constructs were previously described by Oitzinger et al. (2001) and Wrighton et al. (1996), respectively. The virus was propagated in 293 human embryonic kidney cells, then purified by ultracentrifugation in a caesium chloride gradient. The titre of the viral stock was determined by the end point dilution method (Nicklin & Baker, 1999). RASMCs when approximately 70% confluent were incubated with adenovirus at a multiplicity of infection (m.o.i.). of 100 for 16 h in normal growth medium after which the medium was replaced. The cells were stimulated 40 h post-infection and quiesced in serum-free medium for 16 h prior to stimulation. Infection with a control adenoviral vector encoding green fluorescent protein (Ad.GFP) was also performed; fluorescence microscopy confirmed that effective infection took place.
SDS–PAGE and immunoblotting
Western blotting of proteins was performed as previously described (Plevin et al., 1996; Paul et al., 1997). Rabbit polyclonal antibodies were employed for the detection of iNOS, IκBα and IκBβ, while a goat polyclonal antibody was utilised for the detection of p42/44 MAP kinase. All antibodies were titred to give optimum detection conditions.
Assay of NFκB activity: electrophoretic mobility shift assay (EMSA)
Preparation of nuclear extracts
Cells were grown on 10 cm dishes (RASMCs), exposed to vehicle, agents or LPS as appropriate, and reactions terminated by washing cells twice with ice-cold phosphate-buffered saline (PBS). Cells were then removed by scraping and transferred to Eppendorf tubes. Nuclear extracts were prepared as previously described (Schreiber et al., 1989) and the protein content of the recovered samples then determined by means of the Bradford assay.
DNA-binding reaction
Nuclear extracts (5 μg) were incubated in binding buffer (10 mM Tris-HCl pH 7.5, 4% (v v−1) glycerol, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM dithiothreitol (DTT), 50 mM NaCl, 50 μg ml−1 poly(dI-dC).poly(dI-dC)) for 15 min prior to addition of 1 μl (50,000 c.p.m.) of 32P-labelled double-stranded NFκB consensus oligonucleotide (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) (Promega, U.K.) for 20–30 min. Following incubation, 1 μl of gel loading buffer (10 × ; 250 mM Tris-HCl pH 7.5, 0.2% (w v−1) bromophenol blue, 40% (v v−1) glycerol) was added to samples and protein–DNA complexes resolved by non-denaturing electrophoresis on 5% (w v−1) acrylamide slab gels. Gels were initially pre-run in (0.5 ×) Tris-borate-EDTA buffer (TBE) for 30 min at 100 V and subsequent to loading of samples electrophoresis was maintained at 100 V for 45–60 min. Gels were dried and NFκB-probe complexes visualised by autoradiography.
IKK immunocomplex-kinase assay
Cells were incubated with vehicle or LPS as appropriate, washed twice in ice-cold PBS and then lysed with solubilisation buffer (20 mM Tris-HCl pH 7.6, 1 mM EDTA, 0.5 mM EGTA, 150 mM NaCl, 0.1% (w v−1) Brij 35, 1% (w v−1) Triton X-100, 20 mM sodium fluoride, 0.5 mM sodium orthovanadate, 20 mM β-glycerophosphate, 10 μg ml−1 aprotinin, 10 μg ml−1 pepstatin A, 10 μg ml−1 leupeptin and 1 mM PMSF). Solubilised extracts were clarified by centrifugation and then incubated with 1.5 μg of either IKKα- or -β-specific antisera (Santa Cruz, U.S.A.), precoupled to protein G-agarose, for 2 h at 4°C with rotation. Immunocomplexes were collected by centrifugation (13,000 × g, 1 min), washed once with solubilisation buffer and once with 25 mM HEPES buffer pH 7.6 containing 25 mM β-glycerophosphate, 20 mM NaF, 15 mM MgCl2 and 1 mM DTT before incubation in the same buffer containing 25 mM/5 μCi ATP/[γ-32P] ATP and 1 μg of a recombinant GST-fusion protein of the N-terminus of IκBα (containing serine residues S32 and S36) in a final volume of 30 μl for 30 min at 30°C. The reaction was terminated by the addition of 4 × sample buffer and boiled for 5 min. Aliquots of each sample were then subjected to electrophoresis on 10% SDS–PAGE gels and phosphorylated IκB visualised by autoradiography.
JNK kinase activity assay
Control or stimulated cells were solubilised in 20 mM HEPES buffer pH 7.7, containing 50 mM NaCl, 0.1 mM EDTA, 0.1 mM Na3VO4, 0.1 mM PMSF, 10 μg ml−1 aprotinin, 10 μg ml−1 leupeptin and 1% (v v−1) Triton X-100. Lysates were clarified and incubated with 20 μg GST-c-Jun5–89 immobilised on GSH–sepharose at 4°C for 3 h. Beads were then washed three times in solubilisation buffer and twice in 25 mM HEPES buffer pH 7.6 containing 20 mM β-glycerophosphate, 0.1 mM Na3VO4, 2 mM DTT. Precipitates were then incubated in the same buffer containing 25 μM/1 μCi ATP/[γ-32P]ATP in a final volume of 30 μl at 30°C for 30 min. The reaction was terminated by the addition of SDS sample buffer and the samples analysed by SDS–PAGE followed by radiography.
Results
In preliminary studies (not shown), we found that preincubation of RASMCs and RAW 264.7 macrophages with the putative NFκB DNA-binding inhibitor PDTC (Brennan & O'Neill, 1996; Bowie et al., 1997) (100 μM, 60 min) abolished the induction of the 130 kDa isoform of iNOS in response to LPS (100 μg ml−1). This effect was observed at concentrations between 10 and 100 μM PDTC and is consistent with a number of other studies (Mulsch et al., 1993; Sherman et al., 1993; Xie et al., 1994; Flodstrom et al., 1996). We also observed the same effect by pretreating cells with another NFκB pathway inhibitor, TLCK, a proteosome inhibitor that prevents the degradation of IκB by inhibition of cellular serine protease activity (not shown) (Henkel et al., 1993; Mellits et al., 1993).
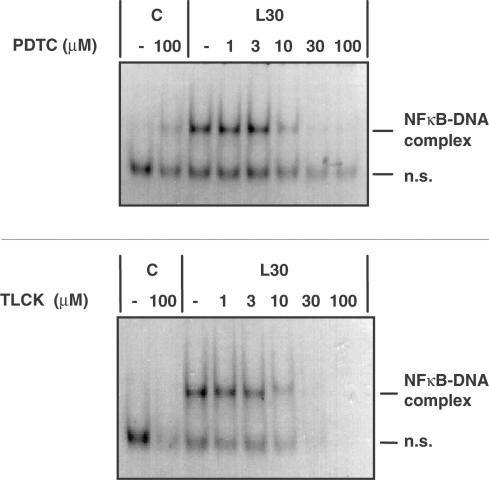
Initial studies were designed to confirm the site of action of these compounds. Initially, we examined the effect of PDTC upon LPS-stimulated NFκB activation in RASMCs, the kinetics of which have been described previously (Torrie et al., 2001). Preincubation with PDTC substantially reduced LPS-mediated NFκB DNA-binding at concentrations of 10 μM and above with complete inhibition being observed at between 30 and 100 μM (Figure 1). The effect of TLCK on LPS-stimulated NFκB activation in RASMCs was also examined and this paralleled the results obtained with PDTC in this cell type (Figure 1).
Figure 1.
The effect of PDTC and TLCK pretreatment on LPS- stimulated NFκB DNA-binding activity in RASMCs. RASMCs were incubated in the absence or presence of increasing concentrations (1–100 μM) of PDTC or TLCK for 60 min then exposed to vehicle (C) or 100 μg ml−1 LPS (L) for 30 min. Nuclear cell extracts were assayed for EMSA activity as outlined in Experimental procedures. Each autoradiogram is representative of at least four experiments. The position of the NFκB protein–DNA complex and non-specific DNA complexes (n.s.) are indicated.
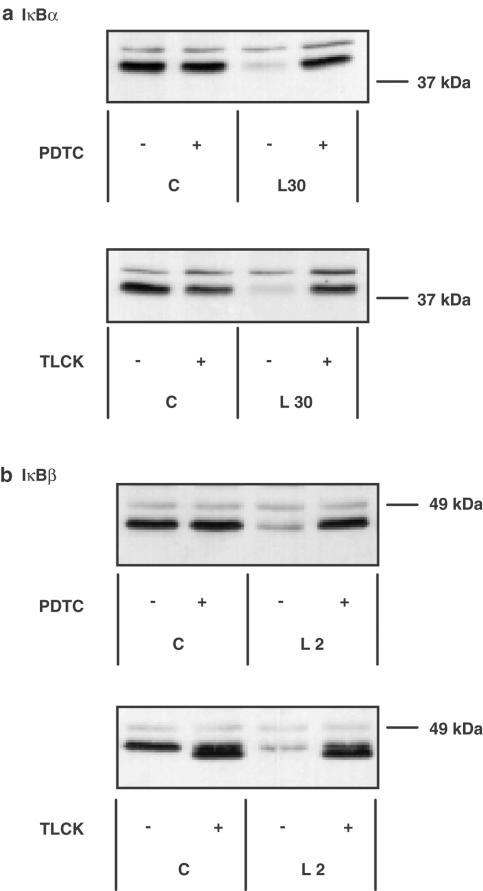
To further investigate the site and mechanism of action of these compounds, we looked upstream of NFκB DNA-binding at LPS-mediated degradation of IκB (Figure 2). In RASMCs, LPS stimulated a rapid loss of cellular IκBα, which was maximal by 15–30 min before returning to control values within 60 min. LPS also stimulated the degradation of IκBβ; however, the degradation was delayed relative to IκBα, became maximal by 2 h, and remained below control levels for at least 8 h (Torrie et al., 2001). Surprisingly, both PDTC and TLCK effectively reversed LPS-stimulated degradation of both IκBα and IκBβ at the time points of maximal degradation (Figure 2). For both inhibitors the effect was maximal at 30–100 μM, the concentration range observed to inhibit NFκB electrophoretic mobility shift assay (EMSA) activity. In contrast, no reversal of LPS-induced IκBα or -β loss was observed in RAW 264.7 macrophages confirming the lack of effect of PDTC upstream of NFκB in this cell type (data not shown).
Figure 2.
The effect of PDTC and TLCK on LPS-stimulated IκBα and IκBβ degradation in RASMCs. RASMCs were incubated in the absence or presence of 100 μM PDTC or TLCK for 60 min then exposed to vehicle (C) or 100 μg ml−1 LPS (L) for 30 min for measuring effects on IκBα degradation (panel a) or 2 h for measuring effects on IκBβ degradation (panel b). Samples were assayed for IκBα or IκBβ content as outlined in Experimental procedures. Each blot is representative of at least three independent experiments.
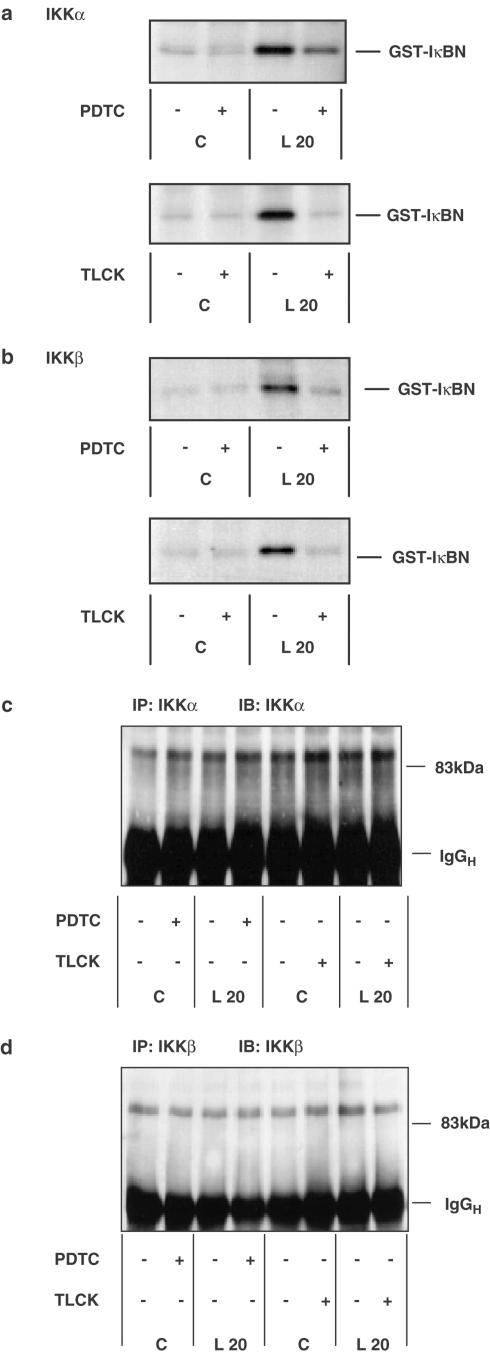
We further examined the potential site of action of PDTC and TLCK in the LPS-stimulated NFκB cascade by looking upstream of IκB degradation at LPS-induced activation of IKK (Figure 3). We have previously described LPS-stimulated IKK activity in RASMCs (Torrie et al., 2001) and similarly in these studies LPS stimulated an eight- to 10-fold increase in both IKKα and IKKβ activity in RASMCs as measured by an immunocomplex kinase assay in vitro. Preincubation with PDTC (100 μM) or TLCK (100 μM), strongly inhibited LPS-stimulated IKKα (Figure 3a) and IKKβ (Figure 3b) activity. Figures 3c and d show that recovery of IKKα or -β is not affected by inhibitor treatment. Our studies demonstrate that contrary to what has been described previously (Henkel et al., 1993; Mellits et al., 1993; Brennan & O'Neill, 1996; Bowie et al., 1997), treatment of cells with PDTC or TLCK inhibits LPS-stimulated NFκB DNA-binding, IκB degradation, and IKKα and -β activity in RASMCs.
Figure 3.
The effect of PDTC and TLCK on LPS-stimulated IKKα and IKKβ activity in RASMCs. RASMCs were incubated in the absence or presence of vehicle (−) or 100 μM PDTC or TLCK (+) for 60 min then exposed to vehicle (C) or 100 μg ml−1 LPS (L) for a further 20 min. Samples were assayed for IKKα (panel a) or IKKβ (panel b) activity or IKKα (panel c) or IKKβ (panel d) protein levels as outlined in Experimental procedures. Each autoradiogram/blot represents at least three individual experiments.
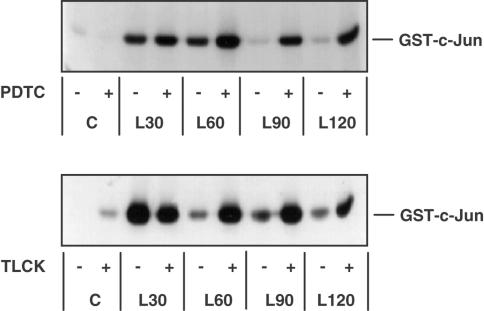
It is clear that PDTC and TLCK are effective inhibitors of the NFκB cascade, active at the level of, or upstream of, IKK, in RASMCs. We therefore used PDTC and TLCK as a means of investigating the influence of the NFκB cascade on SAP kinase activity and the possible involvement of a regulatory element common to both the SAP kinase and NFκB cascades. We tested the effects of PDTC and TLCK on LPS-stimulated JNK and p38 MAP kinase activity. Initial experiments showed that, in RASMCs, LPS-stimulated JNK activity between 15 and 30 min before it returned to basal levels within 60–90 min. (data not shown). Surprisingly, we found that both PDTC and TLCK acted synergistically with LPS in activating JNK at the later time points (Figure 4). While 100 μM PDTC alone did not appear to activate either kinase, it potentiated LPS stimulation of JNK (Figure 4) at the time points tested. TLCK (100 μM) caused a small stimulation of JNK activity in the absence of LPS and potentiated the LPS-stimulated response (Figure 4). At the later time points, when LPS-stimulated JNK returned to basal levels, a marked enhanced activation of LPS-stimulated JNK activity was observed following PDTC and TLCK pretreatment, similar to the activity induced by 0.5 M sorbitol (data not shown). At a higher concentration (100 μM), TLCK alone was able to stimulate JNK in a time-dependent manner. The level of activation was approximately 50% of that observed for sorbitol. PDTC and TLCK also potentiated LPS-stimulated p38 MAP kinase activity and associated MAPKAP kinase-2 activity in these cells with kinetics similar to that observed for JNK activation (data not shown).
Figure 4.
The effect of PDTC and TLCK on LPS-stimulated JNK activity in RASMCs. RASMCs were incubated in the absence or presence of vehicle (−) or 100 μM PDTC or TLCK (+) for 60 min then stimulated with 100 μg ml−1 LPS for the times indicated. Samples were then assayed for JNK activity as outlined in Experimental procedures. Each autoradiogram is representative of at least three others.
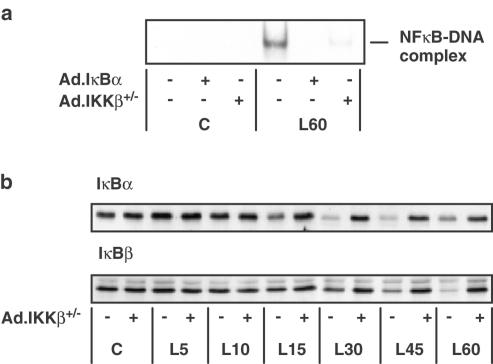
These results strongly indicate that inhibition of the NFκB pathway at some level caused an increase in SAP kinase activity in this cell type. However, the use of pharmacological inhibitors did not allow us to differentiate between a crosstalk effect mediated by a component of the NFκB pathway such as IKK and an effect attributable to the inhibition of NFκB-dependent transcription. In order to understand the role of IKK in the activation of SAP kinase activity, it was necessary for us to have the tools that would allow us to inhibit IKK directly and also inhibit the NFκB cascade downstream of IKK in a specific and defined manner. We therefore used recombinant adenoviral vectors encoding dominant-negative IKKβ (Ad.IKKβ+/−) or wild-type IκBα (Ad.IκBα). Infection with Ad.IκBα causes an inhibition of NFκB translocation to the nucleus without influencing IKK activation, whereas Ad.IKKβ+/− inhibits the kinase activity of the IKK complex, thereby preventing IκB degradation and blocking NFκB translocation (Stehlik et al., 1998; Mechtcheriakova et al., 2001).
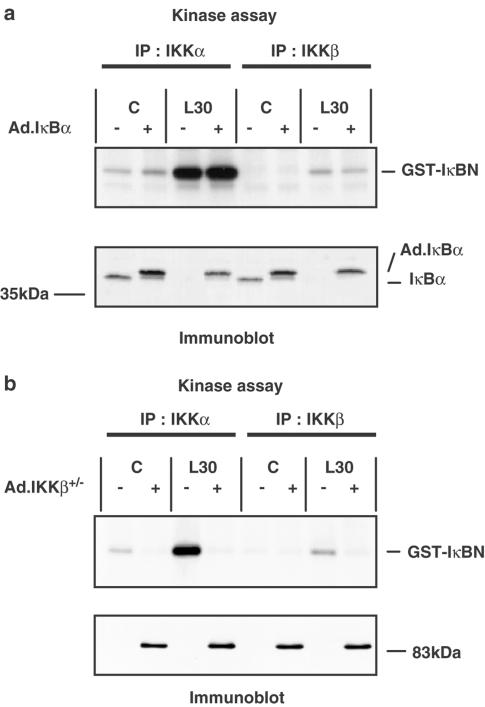
RASMCs were infected with an Ad.GFP construct in order to determine the efficiency of infection. Fluorescence microscopy at an m.o.i. of 100 revealed that 96±1% of the cells were found to have β-galactosidase activity (n=4); this was reduced to 85±7% at an m.o.i. of 30 (n=4). Infection with this construct had no effect on basal or LPS-stimulated NFκB DNA-binding, IKK activity or IκB degradation (data not shown). No visible toxicity to the cells was observed as a result of adenovirus-mediated gene over-expression. Infection of RASMCs with either Ad.IKKβ+/− or Ad.IκBα caused a concentration-dependent inhibition of LPS-stimulated NFκB DNA-binding activity with maximal effect at an m.o.i. of 100 (Figure 5a). Infection with Ad.IKKβ+/− very effectively reversed LPS-stimulated IκBα and –β degradation without affecting basal IκB levels, as shown by the time-course data represented in Figure 5b. Figure 6 shows the effect of Ad.IκBα and Ad.IKKβ+/− on IKK activity. Infection of RASMCs with Ad.IκBα had no effect on IKKα or -β activity at an m.o.i. of up to 300 despite high expression levels (Figure 6a, lower panel). However Ad.IKKβ+/− at an m.o.i. of 100 caused a complete inhibition of LPS-stimulated IKKα and -β activity at each time point studied. Basal IKKα activity was also reduced (Figure 6b). As shown here, we consistently observed IKKβ activity to be lower than IKKα activity, this may reflect a difference in the efficiency of the antibodies used to immunoprecipitate the respective kinases. It should be noted that the endogenous IKKβ (87 kDa) can be detected by Western blotting, but is not visible in this figure because the level of expression is so low in comparison to the overexpressed dominant-negative construct. The ability of the dominant-negative IKKβ construct to inhibit the kinase activity of IKKα reflects the importance of IKKβ in the activation of the IKK complex. IKKβ is recognised as the dominant kinase in the IKK complex (Delhase et al., 1999), in mice deficient in IKKα, full cytokine-stimulated kinase activity was still observed, whereas the absence of IKKβ prevented all IKK kinase activity (Hu et al., 1999; Li Q. et al., 1999; Li Z.-W. et al., 1999; Takeda et al., 1999; Tanaka et al., 1999).
Figure 5.
The effect of Ad.IKKβ+/− and Ad.IκBα upon LPS-stimulated NFκB DNA- binding activity and Ad.IKKβ+/− on LPS-stimulated IκBα and IκBβ degradation in RASMCs. RASMCs were infected with Ad.IKKβ+/− or Ad.IκBα at an m.o.i. of 100. At 40 h postinfection, the cells were exposed to 100 μg ml−1 LPS for the times indicated (min). In panel a, RASMCs were infected with adenovirus as indicated and then exposed to vehicle (C) or LPS (L) for 60 min. NFκB DNA binding was measured in nuclear cell extracts by EMSA as outlined in Experimental procedures. Each autoradiogram is representative of at least four experiments. The position of the NFκB protein–DNA complex is indicated. In panel b, RASMCs were infected with Ad.IKKβ+/− and exposed to vehicle (C) or LPS (L) for the times (min) indicated. Samples were assayed for IκBα or IκBβ content as outlined in Experimental procedures. Each blot represents at least three experiments.
Figure 6.
The effect of Ad.IκBα and Ad.IKKβ+/− upon LPS-stimulated IKKα and IKKβ activity in RASMCs. RASMCs were infected with Ad.IκBα (panel a) or Ad.IKKβ+/− (panel b) at an m.o.i. of 100. At 40 h postinfection, the cells were exposed to vehicle (C) or 100 μg ml−1 LPS (L) for 30 min. Samples were assayed for IKKα and IKKβ activity as outlined in Experimental procedures. Each sample was assayed for IκBα (panel a, lower section) or IKKβ expression (panel b, lower section) as outlined in Experimental procedures. Each blot represents at least three individual experiments.
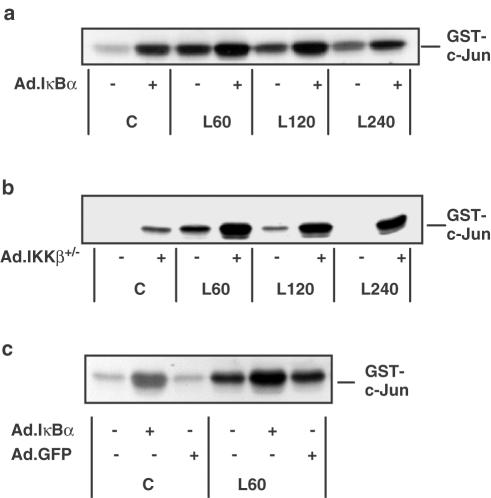
Having confirmed the ability of Ad.IκBα and Ad.IKKβ+/− to inhibit the NFκB cascade in the specific manner expected, we examined the effect of the adenoviral constructs on JNK activity in RASMCs. In Figure 7, infection of RASMCs with Ad.IκBα (panels a and c) or Ad.IKKβ+/− (panel b) stimulated JNK activity in the absence of LPS and potentiated LPS-stimulated JNK activity, both the degree of activation and its duration were increased by adenovirus-mediated NFκB pathway blockade. In order to exclude the possibility of these effects being due to a nonspecific stress response caused by adenoviral infection of the cells the RASMCs were infected with Ad.GFP (Figure 7, panel c). No effect on basal or LPS-stimulated JNK activity was observed. The effects of Ad.IκBα or Ad.IKKβ+/− were very similar to those observed after PDTC and TLCK treatment. This indicates that the effect of the pharmacological inhibitors PDTC and TLCK on JNK activity is related to their influence on the NFκB pathway and not a ‘general' effect. Infection of cells with Ad.IκBα also inhibited NFκB DNA-binding (Figure 5) without inhibiting IKK activity (Figure 6a) and was shown to stimulate JNK activity and potentiate LPS-stimulated JNK activity. Therefore, we conclude that inhibition of NFκB DNA-binding and the resultant loss of transcription of certain NFκB-dependent genes is responsible for the effects of NFκB pathway inhibitors on SAP kinase activity.
Figure 7.
The effect of Ad.IκBα, Ad.IKKβ+/− and Ad.GFP on LPS-stimulated JNK activity in RASMCs. RASMCs were infected with Ad.IκBα (panels a & c) at an m.o.i. of 100, Ad.IKKβ+/− (panel b) at an m.o.i. of 100 or Ad.GFP (panel c) at an m.o.i. of 300. At 40 h postinfection, the cells were exposed to vehicle (C) or 100 μg ml−1 LPS (L) for the times indicated (min). Samples were assayed for JNK activity as outlined in Experimental procedures. Each autoradiogram is representative of at least three individual experiments.
Discussion
In this study, we have presented the first evidence to show that in vascular smooth muscle cells, inhibition of the endotoxin-stimulated IKK/IκB/NFκB signalling pathway results in the enhanced activation of both JNK and p38 MAP kinase. This was initially demonstrated by utilising two structurally distinct compounds, PDTC and TLCK, that are believed to inhibit NFκB activation by intervening at different sites in the IKK/IκB/NFκB pathway, and then by using recombinant adenovirus encoding either dominant-negative IKKβ, to facilitate a direct and specific inhibition of the NFκB pathway at the level of IKK, or wild-type IκBα, to inhibit the pathway downstream of IKK at the level of the NFκB/IκBα complex.
PDTC is believed to directly inhibit NFκB DNA-binding activity, whereas TLCK is thought to prevent protease-mediated degradation of IκB isoforms (Henkel et al., 1993; Mellits et al., 1993). Initially, we found that neither PDTC nor TLCK acted specifically at the sites previously identified (Brennan & O'Neill, 1996; Bowie et al., 1997). This study has determined that, in rat aortic smooth muscle cells, PDTC is active at, or upstream of, IKK in the NFκB cascade. This is similar to observations in J774 macrophages (Chen & Lin, 2001) and isolated gastric parietal cells (Todisco et al., 1999), where PDTC was observed to inhibit IKK activity; however, these studies were not designed to determine whether PDTC could influence other signalling cascades as its mechanism of action, for example, the direct effects on JNK suggested by several recent studies (Liao et al., 2000; Chung et al., 2000; Chen et al., 2001). By using two structurally distinct pharmacological inhibitors of the NFκB pathway in addition to specific molecular inhibitors of the NFκB pathway and measuring their effects on the activity of components of the SAP kinase and the NFκB cascades, we were able to draw conclusions on the interactions between the SAP kinases and the NFκB pathway and the likely targets for the inhibitory actions of PDTC and TLCK. Several additional proteins have been identified upstream of IKK including NIK (Malinin et al., 1997; Natoli et al., 1997), plenty of SH3 (POSH) (Tapon et al., 1998), MEKK1 (Mercurio et al., 1997) and TAK1 (Sakurai et al., 1998), these represent potential targets for inhibition by PDTC, which may act through its iron-chelating properties (Sunderman, 1991; Bowie et al., 1997), its pro- or antioxidant properties (Brennan & O'Neill, 1996), or its ability to increase intracellular copper levels (Iseki et al., 2000). Similarly, TLCK may act directly upon these proteins to inhibit activity or regulate rates of proteolysis. This possibility is currently being examined in our laboratory.
As parallel activation of SAP kinase and NFκB signalling usually occurs in response to LPS and agents such as TNFα, it is reasonable to suggest that PDTC may inhibit the cascade at a site common to both pathways. However, to our surprise, we found that inhibition of the IKK/ IκB / NFκB axis by PDTC resulted in an enhanced activation of JNK Figure (4 and Figure 7) and p38 MAP kinase (data not shown). This effect was also observed in downstream activation of MAPKAP kinase-2 (results not shown). Interestingly, of the cell types tested, the phenomenon was restricted to RASMCs and cardiac myocytes (Wilson, Paul & Plevin, unpublished results). In RAW 264.7 macrophages, only a minor potentiation of LPS-stimulated SAP kinase activity was observed (Paul & Plevin, unpublished results). The effects of LPS in either smooth muscle cells or cardiac myocytes may not involve the interaction with the same TLR as in RAW 264.7 macrophages or endothelial cells, cell-type-specific differences in TLR expression for smooth muscle, endothelial cells and monocytes have been described (Zhang & Ghosh, 2000). Thus, the differing effects of PDTC in each cell type reflects the different intermediates that are linked to TLR subtypes, for example, MyD88 is associated with TLR2, −4 and −9 signalling, the related protein Mal (MyD88-adapter-like) is involved in TLR4 signalling but activates NFκB through a different subset of intermediates (Fitzgerald et al., 2001).
The enhanced activation of JNK following treatment of cells with PDTC has recently been described in several cell types. PDTC was observed to cause a sustained activation of JNK in HUVECs (Liao et al., 2000), in PC12 cells PDTC stimulated ERK and JNK but not p38 MAPK (Chung et al., 2000) and in ROS 17/2.8 osteoblasts PDTC increased p38 MAPK activity and JNK1 phosphotransferase activity (Chae et al., 2001). Effects upon JNK phosphatases such as M3/6 have been implicated as a mechanism of activation (Chen et al., 2001). However, these studies did not examine the interaction between the NFκB and SAP kinase pathways. Since TLCK, a markedly different compound from PDTC, also strongly potentiated the activity of both SAP kinases, this points to an indirect effect of PDTC on SAP kinase signalling in RASMCs rather than a direct effect on JNK or JNK phosphatases. Furthermore, preliminary studies showed that MEK-4 and MEK3/6 activation, assessed using phospho-specific antibodies, was also enhanced with PDTC, suggesting a site of action upstream of these kinases (results not shown).
Rather our findings indicate that the ability of PDTC and TLCK to potentiate LPS-stimulated SAP kinase activity is through their action on the NFκB pathway. This idea was supported by experiments using well-characterised adenoviral constructs expressing wild-type or dominant-negative components of the NFκB pathway. The Ad.IκBα construct has previously been observed to block the expression of NFκB -dependent genes such as pIAP in porcine endothelial cells (Tang et al., 2001), IAP-1 in human and rat aortic smooth muscle cells (Erl et al., 1999) and VCAM-1, ICAM-1, E-selectin, monocyte chemoattractant protein-1 and growth-related activity-α in endothelial cells (Weber et al., 1999). Similarly, the Ad.IKKβ+/− construct has been shown to inhibit NFκB DNA-binding and translocation, and ICAM-1, VCAM-1, E-selectin and IL-8 expression in endothelial cells (Oitzinger et al., 2001).
In RASMCs both viruses showed selective inhibition of NFκB signalling in the predicted manner, Ad.IκBα inhibited NFκB DNA-binding and prevented total IκB loss by providing an excess of IκB protein (as described in Weber et al., 1999) but was without effect upon IKK activity, while Ad.IKKβ+/− inhibited IKK activity, IκB loss and NFκB DNA-binding. However, infection with either construct substantially increased JNK activity. This clearly indicates that it is the inhibition of NFκB DNA-binding that results in a potentiation of SAP kinase activity in LPS-stimulated RASMCs. Thus, the effects of PDTC and TLCK on LPS-stimulated SAP kinase activity can be entirely explained by their inhibition of the NFκB pathway and we have found no evidence of a direct crosstalk between IKK and SAP kinases. We suggest that an indirect but functional relation exists, whereby a possible protective effect of NFκB is lost, which in turn causes stress kinase activation through an unidentified mechanism. This conclusion is supported by the recent description of XIAP (Tang et al., 2001) and Gadd45β (De Smaele et al., 2001) as the products of NFκB target genes that can inhibit JNK activity. These studies were on murine embryonic fibroblasts from ‘knockout' mice, not mature differentiated cells; however, it is possible that members of the X chromosome-linked inhibitor of apoptosis (XIAP) and/or Gadd45β protein families may also be involved in the antiapoptotic mechanism in RASMCs.
As both NFκB and SAP kinases have been implicated in the regulation of growth and apoptosis in numerous cell types, it is apparent that the balance between these two pathways is crucial in determining cell survival. Thus, enhanced activation of JNK and p38 MAP kinase, that is a sustained rather than transient response, may explain the observed apoptosis in a number of systems following inhibition of NFκB. In smooth muscle cells, enhanced NFκB activity has been implicated in atherosclerosis. Since the aetiology of this disease involves enhanced smooth muscle remodelling as well as inflammation (Bellas et al., 1995), inhibition of the NFκB cascade in smooth muscle cells is an important target to consider for novel therapies in the future.
Acknowledgments
We thank, Professors Philip Cohen, Ron Hay, Chris Marshall and James Woodgett, and for kind gifts of reagents used in this study. This project was supported by grants from The British Heart Foundation and from the University of Strathclyde.
Abbreviations
- Ad.IKKβ+/−
IKKβ dominant-negative adenoviral construct
- Ad.IκBα
wild-type IκBα adenoviral construct
- DMEM
Dulbecco's modified Eagle's medium
- DTT
dithiothreitol
- EMSA
electrophoretic mobility shift assay
- FCS
foetal calf serum
- GFP
green fluorescent protein
- GSH
glutathione
- GST
glutathione S-transferase
- HRP
horseradish peroxidase
- HUVEC
human umbilical vein endothelial cells
- IκB
inhibitory kappa B
- IKK
inhibitory kappa B kinase
- IL-1
interleukin-1
- iNOS
inducible nitric oxide synthase
- IRAK
IL-1 receptor-associated kinase
- JNK
c-Jun N-terminal kinase
- LBP
LPS-binding protein
- LPS
lipopolysaccharide
- MAP kinase
mitogen-activated protein kinase
- MAPKAP kinase-2
MAP kinase-activated protein kinase-2
- MEK
MAP kinase kinase
- MEKK1
MEK kinase 1
- m.o.i.
multiplicity of infection
- NFκB
nuclear factor kappa B
- NIK
NFκB-inducing kinase
- PAGE
polyacrylamide gel electrophoresis
- PDTC
pyrrolidine dithiocarbamate
- pIAP
porcine inhibitor of apoptosis protein
- POSH
plenty of SH3
- RASMCs
rat aortic smooth muscle cells
- SAP kinase
stress-activated protein kinase
- TAK1
transforming growth factor-β-activated kinase
- TLCK
Nα-p-tosyl-L-lysine-chloro-methylketone
- TLR
Toll-like receptor
- TRAF2
TNF receptor-associated factor-2
- XIAP
X chromosome-linked inhibitor of apoptosis
References
- BELLAS R.E., LEE J.S., SONENSHEIN G.E. Expression of a constitutive NF-kappa B-like activity is essential for proliferation of cultured bovine vascular smooth muscle cells. J. Clin. Invest. 1995;96:2521–2527. doi: 10.1172/JCI118313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOWIE A.G., MOYNAGH P.N., O'NEILL L.A.J. Lipid peroxidation is involved in the activation of NF-kappaB by tumor necrosis factor but not interleukin-1 in the human endothelial cell line ECV304. Lack of involvement of H2O2 in NF-kappaB activation by either cytokine in both primary and transformed endothelial cells. J. Biol. Chem. 1997;272:25941–25950. doi: 10.1074/jbc.272.41.25941. [DOI] [PubMed] [Google Scholar]
- BOWIE A.G., O'NEILL L.A. Vitamin C inhibits NF-kappa B activation by TNF via the activation of p38 mitogen-activated protein kinase. J Immunol. 2000;165:7180–7188. doi: 10.4049/jimmunol.165.12.7180. [DOI] [PubMed] [Google Scholar]
- BRENNAN P., O'NEILL L.A.J. 2-mercaptoethanol restores the ability of nuclear factor kappa B (NF kappa B) to bind DNA in nuclear extracts from interleukin 1-treated cells incubated with pyrollidine dithiocarbamate (PDTC). Evidence for oxidation of glutathione in the mechanism of inhibition of NF kappa B by PDTC. Biochem. J. 1996;320:975–981. doi: 10.1042/bj3200975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAE H., CHAE S., PARK N., BANG B., CHO S., KIM J., KIM H., LEE Z. Pyrrolidine dithiocarbamate inhibits serum-induced NF-kappaB activation and induces apoptosis in ROS 17/2.8 osteoblasts. Int. Immunopharmacol. 2001;1:255–263. doi: 10.1016/s1567-5769(00)00025-4. [DOI] [PubMed] [Google Scholar]
- CHEN B.-C., LIN W.-W. PKC- and ERK-dependent activation of I kappa B kinase by lipopolysaccharide in macrophages: enhancement by P2Y receptor-mediated CaMK activation. Br. J. Pharm. 2001;134:1055–1065. doi: 10.1038/sj.bjp.0704334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN Y.-R., SHRIVASTAVA A., TAN T.-H. Down-regulation of the c-Jun N-terminal kinase (JNK) phosphatase M3/6 and activation of JNK by hydrogen peroxide and pyrrolidine dithiocarbamate. Oncogene. 2001;20:367–374. doi: 10.1038/sj.onc.1204105. [DOI] [PubMed] [Google Scholar]
- CHUNG K.C., PARK J.H., KIM C.H., LEE H.W., SATO N., UCHIYAMA Y., AHN Y.S. Novel biphasic effect of pyrrolidine dithiocarbamate on neuronal cell viability is mediated by the differential regulation of intracellular zinc and copper ion levels, NF-kappaB, and MAP kinases. J. Neurosci. Res. 2000;59:117–125. [PubMed] [Google Scholar]
- CRAIG R., LARKIN A., MINGO A.M., THUERAUF D.J., ANDREWS C., MCDONOUGH P.M., GLEMBOTSKI C.C. p38 MAPK and NF-kappa B collaborate to induce interleukin-6 gene expression and release. Evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J. Biol. Chem. 2000;31:23814–23824. doi: 10.1074/jbc.M909695199. [DOI] [PubMed] [Google Scholar]
- DE SMAELE E., ZAZZERONI F., PAPA S., NGUYEN D.U., JIN R., JONES J., CONG R., FRANZOSO G. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- DELHASE M., HAYAKAWA M., CHEN Y., KARIN M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- ERL W., HANSSON G.K., DE MARTIN R., DRAUDE G., WEBER K.S., WEBER C. Nuclear factor-kappa B regulates induction of apoptosis and inhibitor of apoptosis protein-1 expression in vascular smooth muscle cells. Circ. Res. 1999;84:668–677. doi: 10.1161/01.res.84.6.668. [DOI] [PubMed] [Google Scholar]
- FITZGERALD K.A., PALSSON-MCDERMOTT E.M., BOWIE A.G., JEFFERIES C.A., MANSELL A.S., BRADY G., BRINT E., DUNNE A., GRAY P., HARTE M.T., MCMURRAY D., SMITH D.E., SIMS J.E., BIRD T.A., O'NEILL L.A.J. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- FLODSTROM M., WELSH N., EIZIRIK D.L. Cytokines activate the nuclear factor kappa B (NF-kappa B) and induce nitric oxide production in human pancreatic islets. FEBS Lett. 1996;385:4–6. doi: 10.1016/0014-5793(96)00337-7. [DOI] [PubMed] [Google Scholar]
- HENKEL T., MACHLEIDT T., ALKALAY I., KRONKE M., BEN-NERIAH Y., BAEUERLE P.A. Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappa B. Nature (London) 1993;365:182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- HU Y., BAUD V., DELHASE M., ZHANG P., DEERINCK T., ELLISMAN M., JOHNSON R., KARIN M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- ISEKI A., KAMBE F., OKUMURA K., NIWATA S., YAMAMOTO R., HAYAKAWA T., SEO H. Pyrrolidine dithiocarbamate inhibits TNF-alpha- dependent activation of NF-kappaB by increasing intracellular copper level in human aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 2000;276:88–92. doi: 10.1006/bbrc.2000.3452. [DOI] [PubMed] [Google Scholar]
- LI Q., VAN ANTWERP D., MERCURIO F., LEE K.-F., VERMA I.M. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- LI Z.-W., CHU W., HU Y., DELHASE M., DEERINCK T., ELLISMAN M., JOHNSON R., KARIN M. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. Science. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIAO H.-L., ZHU Y., WANG N., VERNA L., STEMERMAN M.B. Selective activation of endothelial cells by the antioxidant pyrrolidine dithiocarbamate: involvement of C-jun N-terminal kinase and AP-1 activation. Endothelium. 1999;7:121–133. doi: 10.3109/10623320009072207. [DOI] [PubMed] [Google Scholar]
- LIU Z-G., HSU H., GOEDDEL D.V., KARIN M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- LU X., NEMOTO S., LIN A. Identification of c-Jun NH2-teminal protein kinase (JNK)-activating kinase 2 as an activator of JNK but not p38. J. Biol. Chem. 1997;272:24751–24754. doi: 10.1074/jbc.272.40.24751. [DOI] [PubMed] [Google Scholar]
- MALININ N.L., BOLDIN M.P., KOVALENKO A.V., WALLACH D. MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature (London) 1997;386:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- MECHTCHERIAKOVA D., SCHABBAUER G., LUCERNA M., CLAUSS M., DE MARTIN R., BINDER B.R., HOFER E. Specificity, diversity, and convergence in VEGF and TNF-alpha signaling events leading to tissue factor up-regulation via EGR-1 in endothelial cells. FASEB J. 2001;15:230–242. doi: 10.1096/fj.00-0247com. [DOI] [PubMed] [Google Scholar]
- MELLITS K.H., HAY R.T., GOODBOURN S. Proteolytic degradation of MAD3 (I kappa B alpha) and enhanced processing of the NF-kappa B precursor p105 are obligatory steps in the activation of NF-kappa B. Nucleic Acids Res. 1993;21:5059–5066. doi: 10.1093/nar/21.22.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERCURIO F., ZHU H., MURRAY B.W., SHEVCHENKO A., BENNETT B.L., WU LI J., YOUNG D.B., BARBOSA M., MANN M., MANNING A., RAO A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- MULSCH A., SCHRAY-UTZ B., MORDVINTEEV P.I., HAUSCHILDT S., BUSSE R. Diethyldithiocarbamate inhibits induction of macrophage NO synthase. FEBS Lett. 1993;321:215–218. doi: 10.1016/0014-5793(93)80111-7. [DOI] [PubMed] [Google Scholar]
- NATOLI G., COSTANZO A., MORETTI F., FULCO M., BALSANO C., LEVRERO M. Tumor necrosis factor (TNF) receptor 1 signaling downstream of TNF receptor-associated factor 2. Nuclear factor kappaB (NFkappaB)-inducing kinase requirement for activation of activating protein 1 and NFkappaB but not of c-Jun N-terminal kinase/stress-activated protein kinase. J. Biol. Chem. 1997;272:26079–26082. doi: 10.1074/jbc.272.42.26079. [DOI] [PubMed] [Google Scholar]
- NICKLIN S.A., BAKER A.H. Methods in Molecular Medicine: Vascular Disease: Molecular Biology and Gene Therapy Protocols 1999The Humana Press, Totowa, NJ; 271–283.ed. Baker A.H [Google Scholar]
- OITZINGER W., HOFER-WARBINEK R., SCHMID J.A., KOSHELNICK Y., BINDER B.R., DE MARTIN R. Adenovirus-mediated expression of a mutant IkappaB kinase 2 inhibits the response of endothelial cells to inflammatory stimuli. Blood. 2001;97:1611–1617. doi: 10.1182/blood.v97.6.1611. [DOI] [PubMed] [Google Scholar]
- PAUL A., BRYANT C., LAWSON M.F., CHILVERS E.R., PLEVIN R. Dissociation of lipopolysaccharide-mediated induction of nitric oxide synthase and inhibition of DNA synthesis in RAW 264.7 macrophages and rat aortic smooth muscle cells. Br. J. Pharmacol. 1997;120:1439–1444. doi: 10.1038/sj.bjp.0701070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLEVIN R., SCOTT P.H., ROBINSON C.J.M., GOULD G.W. Efficacy of agonist-stimulated MEK activation determines the susceptibility of mitogen-activated protein (MAP) kinase to inhibition in rat aortic smooth muscle cells. Biochem. J. 1996;318:657–663. doi: 10.1042/bj3180657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHWARF D.M., KARIN M. The NF-kappa B activation pathway: a paradigm in information transfer from membrane to nucleus. SciSTKE. 1999;1999 5:RE1. doi: 10.1126/stke.1999.5.re1. [DOI] [PubMed] [Google Scholar]
- SCHREIBER E., MATTHIAS P., MULLER M.M., SCHAFFNER W. Rapid detection of octamer binding proteins with ‘mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKURAI H., SHIGEMORI N., HASEGAWA K., SUGITA T. TGF-beta-activated kinase 1 stimulates NF-kappa B activation by an NF-kappa B-inducing kinase-independent mechanism. Biochem. Biophys. Res. Commun. 1998;243:545–549. doi: 10.1006/bbrc.1998.8124. [DOI] [PubMed] [Google Scholar]
- SEYDEL U., OIKAWA M., FUKASE K., KUSUMOTO S., BRANDENBURG K. Intrinsic conformation of lipid A is responsible for agonistic and antagonistic activity. Eur. J. Biochem. 2000;267:3032–3039. doi: 10.1046/j.1432-1033.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- SHERMAN M.P., AEBERHARD E.E., WONG V.Z., GRISCAVAGE J.M., IGNARRO L.J. Pyrrolidine dithiocarbamate inhibits induction of nitric oxide synthase activity in rat alveolar macrophages. Biochem. Biophys. Res. Commun. 1993;191:1301–1308. doi: 10.1006/bbrc.1993.1359. [DOI] [PubMed] [Google Scholar]
- STEHLIK C., DE MARTIN R., BINDER B.R., LIPP J. Cytokine induced expression of porcine inhibitor of apoptosis protein (iap) family member is regulated by NF-kappa B. Biochem. Biophys. Res. Commun. 1998;243:827–832. doi: 10.1006/bbrc.1998.8185. [DOI] [PubMed] [Google Scholar]
- SU Y.C., HAN J., XU S., COBB M., SKOLNIK E.Y. NIK is a new Ste20-related kinase that binds NCK and MEKK1 and activates the SAPK/JNK cascade via a conserved regulatory domain. EMBO J. 1997;16:1279–1290. doi: 10.1093/emboj/16.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUNDERMAN F.W. Therapeutic properties of sodium diethyldithiocarbamate: its role as an inhibitor in the progression of AIDS. Ann. Clin. Lab. Sci. 1991;21:70–81. [PubMed] [Google Scholar]
- TAKEDA K., TAKEUCHI O., TSUJIMURA T., ITAMI S., ADACHI O., KAWAI T., SANJO H., YOSHIKAWA K., TERADA N., AKIRA S. Limb and skin abnormalities in mice lacking IKKalpha. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI O., AKIRA S. Toll-like receptors; their physiological role and signal transduction system. Int. Immunopharmacol. 2001;1:625–635. doi: 10.1016/s1567-5769(01)00010-8. [DOI] [PubMed] [Google Scholar]
- TANAKA M., FUENTES M.E., YAMAGUCHI K., DURNIN M.H., DALRYMPLE S.A., HARDY K.L., GOEDDEL D.V. Embryonic lethality, liver degeneration, and impaired NF-kappa B activation in IKK-beta-deficient mice. Immunity. 1999;10:421–429. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- TANG G., MINEMOTO Y., DIBLING B., PURCELL N.H., LI Z., KARIN M., LIN A. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- TAPON N., NAGATA K.-I., LAMARCHE N., HALL A. A new rac target POSH is an SH3-containing scaffold protein involved in the JNK and NF-kappaB signalling pathways. EMBO. 1998;17:1395–1404. doi: 10.1093/emboj/17.5.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODISCO A., RAMAMOORTHY S., PAUSAWASDI N., TACEY K. Carbachol activates IkappaB kinase in isolated canine gastric parietal cells. Biochem. Biophys. Res. Commun. 1999;261:877–884. doi: 10.1006/bbrc.1999.1141. [DOI] [PubMed] [Google Scholar]
- TORRIE L.J., MACKENZIE C.J., PAUL A., PLEVIN R.P. Hydrogen peroxide-mediated inhibition of lipopolysaccharide-stimulated inhibitory kappa B kinase activity in rat aortic smooth muscle cells. Br. J. Pharmacol. 2001;134:393–401. doi: 10.1038/sj.bjp.0704259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER K.S., DRAUDE G., ERL W., DE MARTIN R., WEBER C. Monocyte arrest and transmigration on inflamed endothelium in shear flow is inhibited by adenovirus-mediated gene transfer of IkappaB-alpha. Blood. 1999;93:3685–3693. [PubMed] [Google Scholar]
- WRIGHTON C.J., HOFER-WARBINEK R., MOLL T., EYTNER R., BACH F.H., DE MARTIN R. Inhibition of endothelial cell activation by adenovirus-mediated expression of I kappa B alpha, an inhibitor of the transcription factor NF-kappa B. J. Exp. Med. 1996;183:1013–1022. doi: 10.1084/jem.183.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIE Q.-W., KASHIWABARA Y., NATHAN C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- ZHANG G., GHOSH S. Molecular mechanisms of NF-kappaB activation induced by bacterial lipopolysaccharide through Toll-like receptors. J. Endotoxin Res. 2000;6:453–457. doi: 10.1179/096805100101532414. [DOI] [PubMed] [Google Scholar]