Abstract
Squalene synthase is the enzyme that converts farnesyl pyrophosphate to squalene in the cholesterol biosynthesis pathway. We examined the lipid-lowering properties of 1-[[(3R,5S)-1-(3-acetoxy-2,2-dimethylpropyl)-7-chloro-5-(2,3-dimethoxyphenyl)-2-oxo-1,2,3,5-tetrahydro-4,1-benzoxazepin-3-yl]acetyl]piperidine-4-acetic acid (TAK-475), a novel squalene synthase inhibitor.
TAK-475 inhibited hepatic cholesterol biosynthesis in rats (ED50, 2.9 mg kg−1) and showed lipid-lowering effects in beagle dogs, marmosets, cynomolgus monkeys and Wistar fatty rats.
In marmosets, TAK-475 (30, 100 mg kg−1, p.o., for 4 days) lowered both plasma non-high-density lipoprotein (HDL) cholesterol and triglyceride, but did not affect plasma HDL cholesterol. On the other hand, atorvastatin (10, 30 mg kg−1, p.o., for 4 days) lowered the levels of all these lipids. A correlation between decrease in triglyceride and increase in HDL cholesterol was observed, and TAK-475 increased HDL cholesterol with a smaller decrease in triglyceride than did atorvastatin.
TAK-475 (60 mg kg−1, p.o., for 15 days) suppressed the rate of triglyceride secretion from the liver in hypertriglyceridemic Wistar fatty rats, which show an enhanced triglyceride secretion rate from the liver compared with their lean littermates.
In HepG2 cells, TAK-475 and its pharmacologically active metabolite, T-91485, increased the binding of 125I-low-density lipoprotein (LDL) to LDL receptors.
6 These results suggest that TAK-475 has clear hypolipidemic effects in animals via inhibition of hepatic triglyceride secretion and upregulation of LDL receptors, and that TAK-475 might increase HDL cholesterol by decreasing triglyceride. Thus, TAK-475 is expected to be useful for the treatment of dyslipidemia.
Keywords: TAK-475, T-91485, squalene synthase inhibitor, HMG-CoA reductase inhibitor, marmosets, Wistar fatty rats, LDL receptor, HDL cholesterol, hypolipidemic effects
Introduction
Many epidemiological studies, including the Framingham Heart Study, have established an association between dyslipidemia and increased risk of coronary heart disease (CHD) (Gordon et al., 1981; Anderson et al., 1987). An elevated plasma level of low-density lipoprotein (LDL) cholesterol together with an elevated triglyceride level constitutes a significant risk factor for CHD (Austin, 1991). Various lipid-modifying drugs with different mechanisms of action have been developed and used for the treatment of patients with dyslipidemia. Among them, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, known as statins, are the most common lipid-lowering drugs (Hsu et al., 1995; Brown et al., 1998; Olsson, 2001). HMG-CoA reductase inhibitors reduce not only plasma LDL cholesterol but also triglyceride, while they have only a small effect on plasma HDL cholesterol (Bakker-Arkema et al., 1996; Illingworth et al., 2001). The lipid-lowering ability of HMG-CoA reductase inhibitors has been reported to result from upregulation of the LDL receptor and inhibition of triglyceride secretion from the liver (Kasim et al., 1992; Haria & McTavish, 1997). This class of drugs is safe and well tolerated, although adverse effects such as myotoxicity and hepatotoxicity have been reported very occasionally (Ucar et al., 2000; Illingworth et al., 2001). These toxicities of HMG-CoA reductase inhibitors are thought to be the result of deficiencies of isoprenyl metabolites of mevalonic acid, such as ubiquinone, isopentenyl tRNA, dolichols and isoprenylated proteins (Thibault et al., 1996).
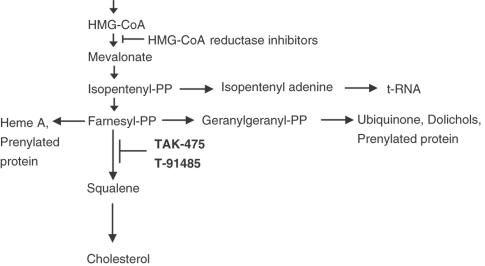
Squalene synthase is the enzyme that converts farnesyl pyrophosphate to squalene in the cholesterol biosynthesis pathway (Figure 1). Since squalene synthase inhibitors inhibit cholesterol biosynthesis without suppression of the synthesis of isoprenyl metabolites, these agents are expected to have hypolipidemic effects without myotoxicity or hepatotoxicity (Flint et al., 1997; Hiyoshi et al., 2000), although the lipid-lowering properties of squalene synthase inhibitors remain to be thoroughly elucidated.
Figure 1.
Cholesterol biosynthesis pathway and its inhibitors.
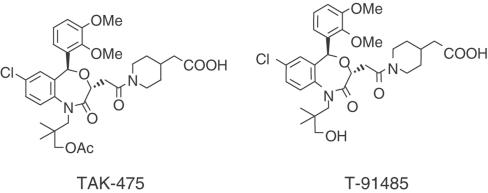
TAK-475, 1-[[(3R,5S)-1-(3-acetoxy-2,2-dimethylpropyl)-7-chloro-5-(2,3-dimethoxyphenyl)-2-oxo-1,2,3,5-tetrahydro-4,1-benzoxazepin-3-yl]acetyl]piperidine-4-acetic acid, is a novel squalene synthase inhibitor (Figure 2, Miki et al., 2002). In the present study, we examined the lipid-lowering properties of TAK-475, comparing its effects with those of HMG-CoA reductase inhibitor atorvastatin in in vivo and in vitro models.
Figure 2.
The chemical structures of the squalene synthase inhibitors TAK-475 and T-91485.
Methods
Materials
TAK-475 and its pharmacologically active metabolite, T-91485, were synthesized in Takeda Chemical Industries, Ltd (Osaka, Japan). Atorvastatin calcium was purchased from Warner-Lambert (Ann Arbor, MI, U.S.A.). Probucol and human lipoprotein-deficient serum (LPDS) were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.). Fetal bovine serum (FBS) was purchased from Life Technologies (Rockville, MD, U.S.A.). Sodium [2-14C] acetate was purchased from Amersham Pharmacia Biotech. (Buckinghamshire, U.K.). Na 125I was purchased from NEN Life Science Products, Inc. (Boston, MA, U.S.A.). Human LDL was purchased from Biomedical Technologies Inc. (Stoughton, MA, U.S.A.). Other chemicals were purchased from Wako Pure Chemical Industries (Osaka, Japan).
Animals
Female Wistar fatty rats and their lean littermates were bred in Takeda Chemical Industries, Ltd (Osaka, Japan). Male Wistar rats were purchased from Clea (Osaka, Japan). They were fed a commercial chow diet (CE-2; Clea) and allowed access to water ad libitum. Male beagle dogs (6–7 years old) were purchased from Oriental Yeast, Tokyo, Japan. Dogs were fed a commercial chow diet (Type DS; Oriental Yeast) and allowed access to water ad libitum. Male common marmosets (300–493 g) were purchased from Clea, and fed a commercial chow diet (CMS-1; Clea) and allowed access to water ad libitum. Cynomolgus monkeys (2.60–8.15 kg) were purchased from A.T. VIRI Inc. (Batangas, Philippines). Cynomolgus monkeys were individually provided with 100 g of food per day (Certified Primate Diet No. 5048; PMI Feed, Inc., Richmond, IN, U.S.A.) and allowed access to water ad libitum. Uneaten food was removed at around 15:00. A fresh banana was given to each monkey as a supplementary food item once a day. All animal experiments were carried out according to the Takeda Animal Care Guidelines.
Measurement of hepatic cholesterol biosynthesis in rats
The inhibition of de novo cholesterol biosynthesis in rat livers was determined by measuring the conversion of intravenously injected [2-14C] acetate into cholesterol. TAK-475 (n=10) and atorvastatin (n=8) suspended in 0.5% methylcellulose solution were administered orally to 6-week-old male Wistar rats under fed conditions. At 1 h after drug administration, [2-14C] acetate (50 μCi kg−1) was injected intravenously. The rats were killed 1 h after the injection of [2-14C] acetate. Their livers were quickly isolated, 1 g of liver was saponified with 1 ml of 90% (w v−1) KOH and 2 ml of ethanol at 100°C for 4 h, and lipids were extracted with petroleum ether. The petroleum ether extracts were dried under N2 gas, and dissolved in ethanol–acetone (1 : 1) solution. The sterol fractions were precipitated by digitonin solution. The radioactivities of the precipitates were measured by liquid scintillation counting.
Biochemical analysis of plasma lipids
Beagle dogs study
Before the experiment, a blood sample was collected under fasted conditions. Plasma total cholesterol and triglyceride were measured enzymatically with a biochemical autoanalyzer (Hitachi Auto Analyzer 7070; Hitachi, Tokyo, Japan). High-density lipoprotein (HDL) cholesterol was separated by chemical precipitation of apoB-containing lipoprotein with phosphotungstate and Mg2+ and then the cholesterol was measured enzymatically. Beagle dogs were divided into two groups matched for body weight, plasma non-HDL cholesterol (the value obtained by subtraction of plasma HDL cholesterol from total cholesterol), HDL cholesterol and triglyceride. These two groups were assigned to receive vehicle (0.5% methylcellulose solution, n=4) or TAK-475 (30 mg kg−1, n=4). Drugs were administered orally once a day for 14 days. On the mornings after the seventh and the final administrations, blood samples were collected under fasted conditions and plasma parameters were measured.
Common marmosets study
Before the experiments, blood samples were collected under nonfasted conditions. Plasma total cholesterol, triglyceride and HDL cholesterol were measured as described above. Common marmosets were divided into two groups for experiment 1 (control vs TAK-475, 30 mg kg−1, n=5), two groups for experiment 3 (control vs atorvastatin, 10 mg kg−1, n=6), two groups for experiment 4 (control vs atorvastatin, 30 mg kg−1, n=5 and 6, respectively) and three groups for experiment 5 (control vs TAK-475, 100 mg kg−1, vs atorvastatin, 30 mg kg−1, n=5); groups were matched for body weight, plasma HDL cholesterol, triglyceride and non-HDL cholesterol. One group for experiment 2 (TAK-475, 100 mg kg−1, n=6) was also set up. Each experiment was performed individually. Drugs were suspended in 0.5% methylcellulose solution and administered orally once a day for 4 days. On the morning after the final administration of drugs, blood samples were collected under nonfasted conditions and plasma parameters were measured. (This procedure was adopted, because fasted conditions are too stressful for marmosets. Under our nonfasted conditions, food was eaten on the previous day and appeared to be almost completely digested when blood was collected.)
Cynomolgus monkeys study
On the day before the start of drug administration, blood samples were collected under fasted conditions. Plasma total cholesterol and triglyceride, and aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities were measured enzymatically with a biochemical autoanalyzer (Hitachi Auto Analyzer 911; Hitachi, Tokyo, Japan). Cynomolgus monkeys were divided into three groups matched for body weight, plasma total cholesterol and triglyceride, and AST and ALT activities, and were assigned to receive vehicle (0.5% methylcellulose solution, 5 ml kg−1, n=5), TAK-475 (n=5) or atorvastatin (n=5). Drugs were administered orally once a day at low doses (30 mg kg−1 for TAK-475 and 10 mg kg−1 for atorvastatin) for 7 days and then at high doses (100 mg kg−1 for TAK-475 and 30 mg kg−1 for atorvastatin) for the next 7 days. On the mornings after the seventh and the final administrations, blood samples were collected under fasted conditions and plasma parameters were measured.
Evaluation of hepatic triglyceride secretion rate in hypertriglyceridemic female Wistar fatty rats
Blood samples were collected from 18-week-old Wistar fatty rats after 4 h of fasting. Plasma total cholesterol, triglyceride and HDL cholesterol were measured as described above. Wistar fatty rats were divided into two groups matched for body weight, plasma HDL cholesterol, triglyceride and non-HDL cholesterol. These groups were assigned to receive vehicle (0.5% methylcellulose solution, 5 ml kg−1, n=9) or TAK-475 (60 mg kg−1, n=9). Their lean littermates were used as normal controls (n=9). Drugs were administered orally once a day for 15 days. On the morning after the final administration, Triton WR-1339 (400 mg kg−1, i.v.) was injected under fasted conditions in order to inhibit catabolism of triglyceride. Blood samples were collected before and 60 and 120 min after Triton WR-1339 injection and the plasma parameters were measured as described above. Hepatic triglyceride secretion rates are expressed as increment of plasma triglyceride per hour.
Quantification of LDL receptor activities in HepG2 cells
Human LDL was labeled with Na 125I according to the method of Bilheimer et al. (1972). Cellular LDL receptor activity was determined by detecting the incorporation of labeled LDL. HepG2 cells (American Type Culture Collection, Rockville, MD, U.S.A.) were seeded onto type 1 collagen-coated six-well plates in minimum essential medium (MEM) supplemented with 10% FBS at 37°C under 5% CO2. HepG2 cells under subconfluent conditions were preincubated with MEM supplemented with 10% human LPDS for 16 h. LDL-binding experiments were performed according to the method of Stopeck et al. (1993) with slight modifications. HepG2 cells were incubated in 10% LPDS MEM with or without drugs for 24 h. Cells were then incubated with 4 μg ml−1 125I-LDL (111 cpm ng−1 protein) in the presence or absence of an excess amount of native LDL (300 μg ml−1) at 4°C for 2 h. The medium was removed and cells were washed three times with phosphate-buffered saline (PBS) containing 1% bovine serum albumin (fatty acid free) and twice with PBS. To dissociate 125I-LDL from the LDL receptor, cells were treated with dextran sulfate (4 mg ml−1) and incubated at 4°C for 1 h. Radioactivity (free 125I-LDL) in the medium was counted with a gamma counter (Aloka ARC-1000, Tokyo, Japan). Cells were dissolved in 1 ml of 0.5 N NaOH for the measurement of cell protein concentrations by the method of Lowry. Specific binding was calculated as the difference between dextran-releasable 125I-LDL with and without a 75-fold excess of unlabeled LDL. Binding activities were expressed as radioactivity per milligram cell protein.
Measurement of acceptor-dependent cholesterol efflux from macrophage-derived foam cells
Human THP-1 monocytes (American Type Culture Collection, Rockville, MD, U.S.A.) were induced to differentiate into macrophages by treatment with PMA (400 ng ml−1) in RPMI 1640 medium containing 10% FBS for 3 days at 37°C under 5% CO2, and then foam cells were prepared by exposure to rabbit β-very low-density lipoprotein (β-VLDL) (150 μg cholesterol ml−1), together with [1,2,6,7-3H] cholesterol (1 μCi ml−1) and 167 μM oleate/0.2% bovine serum albumin complex. The THP-1 macrophage-derived foam cells were incubated with or without drugs for 24 h in RPMI 1640 medium at 37°C under 5% CO2, followed by incubation with human apoAI (2 μg ml−1) or HDL (200 μg ml−1) for 24 h. Radioactivities in the culture supernatant were measured by liquid scintillation counting and normalized to cell protein mass (mg of cell protein).
Statistical analysis
Results are represented as mean±s.e.m. Comparisons among groups were performed with Student's t-test, one-tailed Williams' test, Dunnett's test or Tukey's test. Comparisons of pretreatment with post-treatment in each group were performed with a paired t-test.
Results
Effect of TAK-475 and atorvastatin on hepatic cholesterol biosynthesis in Wistar rats
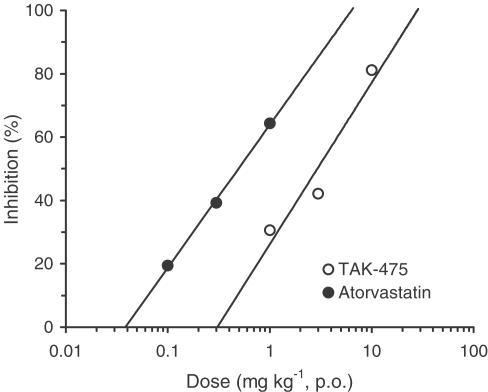
The effect of TAK-475 on hepatic cholesterol biosynthesis in rats was evaluated. TAK-475 (1, 3 and 10 mg kg−1, p.o.) dose-dependently inhibited hepatic cholesterol biosynthesis in Wistar rats with an ED50 value of 2.9 mg kg−1 (Figure 3). Atorvastatin (0.1, 0.3 and 1 mg kg−1, p.o.) also inhibited hepatic cholesterol biosynthesis with an ED50 value of 0.49 mg kg−1.
Figure 3.
Inhibitory effects of TAK-475 and atorvastatin on hepatic cholesterol biosynthesis in Wistar rats. TAK-475 and atorvastatin were administered orally to 6-week-old male Wistar rats. Data are represented as means for eight to 10 animals.ED50 values for TAK-475 and atorvastatin were 2.9 mg kg−1, p.o. (95% confidence interval 2.1–4.0) and 0.49 mg kg−1, p.o. (95% confidence interval 0.30–1.13), respectively.
Lipid-lowering effects of TAK-475 in beagle dogs
TAK-475 (30 mg kg−1, p.o. for 14 days) significantly reduced plasma non-HDL cholesterol by 40±4% (Table 1). TAK-475 tended to reduce plasma triglyceride, but had no effect on plasma HDL cholesterol.
Table 1.
Lipid-lowering effects of TAK-475 in beagle dogs
| Treatment | Non-HDL-C % change | TG % change | HDL-C % change |
|---|---|---|---|
| Control | −6±11 | −14±13 | −7±2 |
| TAK-475 (30 mg kg−1) | −40±4* | −33±6 | −5±2 |
TAK-475 (30 mg kg−1) or vehicle were administered orally once a day to male beagle dogs for 14 days. The initial values of non-HDL cholesterol (Non-HDL-C) were 24±3 and 27±5 mg dl−1, of triglyceride (TG) were 23±2 and 22±3 mg dl−1 and of HDL cholesterol (HDL-C) were 110±10 and 110±9 mg dl−1 for the control and TAK-475-treated groups, respectively. Data are represented as mean±s.e.m. changes in levels compared with initial values (n=4).
P<0.05 vs control values by Student's t-test.
Lipid-lowering effects of TAK-475 in common marmosets
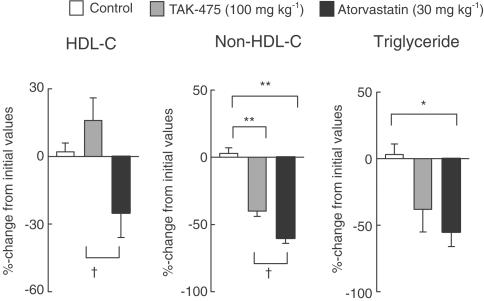
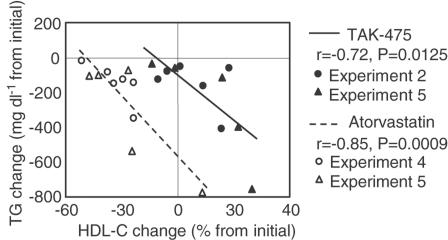
The four individual experiments are summarized in Table 2. TAK-475 at 30 mg kg−1 significantly lowered plasma non-HDL cholesterol and at 100 mg kg−1 lowered both plasma non-HDL cholesterol and triglyceride. TAK-475 did not cause significant changes in plasma HDL cholesterol, although TAK-475 at 100 mg kg−1 tended to increase the level of this lipoprotein. Atorvastatin (10 and 30 mg kg−1, p.o.) significantly lowered plasma non-HDL cholesterol and triglyceride and also significantly lowered plasma HDL cholesterol (Table 2). In experiment 5, which was the comparative study between TAK-475 (100 mg kg−1) and atorvastatin (30 mg kg−1), plasma HDL cholesterol in the TAK-475-treated group was significantly higher than that in the atorvastatin-treated group (Figure 4). Since TAK-475 did not cause the same reduction in plasma non-HDL cholesterol as did atorvastatin, it was difficult to ascertain the relative effects of TAK-475 and atorvastatin on plasma HDL cholesterol from the results of experiment 5. Thus, to elucidate in detail the difference in effect between TAK-475 and atorvastatin on plasma HDL cholesterol, the relation between changes from pretreatment values of plasma triglyceride and HDL cholesterol was plotted for the TAK-475 (100 mg kg−1)- and atorvastatin (30 mg kg−1)-treated groups (Figure 5). A significant negative correlation was observed between the changes in plasma HDL cholesterol (percentage change from initial levels) and in plasma triglyceride (mg dl−1 change from initial levels). Figure 5 clearly indicates that, compared with atorvastatin, TAK-475 increased plasma HDL cholesterol with a smaller decrease in plasma triglyceride.
Table 2.
Effects of TAK-475 and atorvastatin on plasma lipid levels in marmosets
| Treatment | Non-HDL-C % change | TG % change | HDL-C % change |
|---|---|---|---|
| Experiment 1 (n=5) | |||
| Control | −8±5 (107±9) | 59±83 (320±130) | −12±9 (76±16) |
| TAK-475, 30 mg kg−1 | −23±7† (106±13) | −41±17 (211±81) | 0±9 (74±7) |
| Experiment 2 (n=6) | |||
| TAK-475, 100 mg kg–1 | −43±8†† (116±12) | −33±8†† (206±99) | 8±6 (70±11) |
| Experiment 3 (n=6) | |||
| Control | 7±4 (103±15) | 4±13 (115±26) | −11±3†† (94±14) |
| Atorvastatin, 10 mg kg–1 | −30±4**††(99±4) | −39±7*††(115±37) | −34±5**††(82±8) |
| Experiment 4 (n=5–6) | |||
| Control | 1±13 (101±7) | −4±15 (168±89) | −6±5 (76±10) |
| Atorvastatin, 30 mg kg–1 | −50±2*††(109±17) | −46±15† (137±33) | −33±4**††(78±14) |
TAK-475 (30 and 100 mg kg−1), atorvastatin (10 and 30 mg kg−1) or vehicle were administered orally once a day to male common marmosets for 4 days. Initial values (mg dl−1) are shown in parentheses. Non-HDL-C, plasma non-HDL cholesterol; TG, plasma triglyceride; HDL-C, plasma HDL cholesterol. Data are represented as mean ± s.e.m. changes in levels compared with initial values (n=5 or 6).
P<0.05 vs initial values by paired t-test.
P<0.01 vs initial values by paired t-test.
P<0.05 vs control values by Student's t-test.
P<0.01 vs control values by Student's t-test.
Figure 4.
Comparative effects of TAK-475 and atorvastatin on plasma lipid profile in common marmosets (experiment 5). TAK-475 (100 mg kg−1), atorvastatin (30 mg kg−1) or vehicle were administered orally once a day to male common marmosets for 4 days. Initial values of plasma non-HDL cholesterol (non-HDL-C) were 116±18, 104±13 and 111±10 mg dl−1, of plasma HDL cholesterol (HDL-C) were 69±9, 71±9 and 70±8mg dl−1, and of plasma triglyceride (TG) were 246±103, 296±139 and 296±132 mg dl−1 for the control group, TAK-475- and atorvastatin-treated groups, respectively. Data are represented as mean±s.e.m. percentage changes from initial values (n=5). *P<0.05 and **P<0.01 vs control group by Tukey's test. †P<0.05 vs TAK-475-treated group by Tukey's test.
Figure 5.
Correlation between changes in plasma HDL cholesterol (percentage change from initial levels) and plasma triglyceride (mg dl−1 change from initial levels) in TAK-475 (100 mg kg−1)- and atorvastatin (30 mg kg−1)-treated common marmosets. Data from experiments 2, 4 and 5 (shown in Table 2 and Figure 4) are plotted. Solid symbols indicated TAK-475-treated marmosets (n=11). Clear symbols indicate atorvastatin-treated marmosets (n=11).
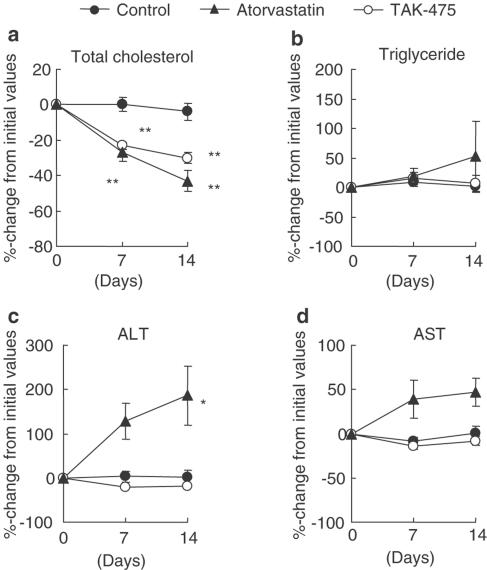
Lipid-lowering effects and hepatotoxicity in cynomolgus monkeys
TAK-475 (30 mg kg−1) and atorvastatin (10 mg kg−1) showed comparable effects in lowering plasma total cholesterol in cynomolgus monkeys (Figure 6). The plasma triglyceride level was not changed by treatment with TAK-475 or atorvastatin. Even at a dose of 100 mg kg−1, TAK-475 did not affect plasma AST and ALT activities. Atorvastatin at 10 mg kg−1, on the other hand, tended to increase plasma ALT and AST activities, and at 30 mg kg−1 significantly increased plasma ALT activity.
Figure 6.
Effects of TAK-475 and atorvastatin on plasma lipid levels and transaminase activities in male cynomolgus monkeys. Drugs were administered orally once a day at low doses (30 mg kg−1 for TAK-475 and 10 mg kg−1 for atorvastatin) for 7 days and at high doses (100 mg kg−1 for TAK-475 and 30 mg kg−1 for atorvastatin) for the next 7 days. Initial values of plasma total cholesterol were 137±9, 136±12 and 137±18 mg dl−1 and of plasma triglyceride were 29±3, 38±4 and 39±7 mg dl−1 for the control groups, TAK-475- and atorvastatin-treated groups, respectively. Initial values of AST activity were 23±1, 26±2 and 26±1 IU l−1 and of ALT activity were 49±6, 46±5 and 42±11 IU l−1 for the control group, TAK-475- and atorvastatin-treated groups, respectively. Data are represented as mean±s.e.m. percentage changes from initial values (n=5). *P<0.05 and **P <0.01 vs control by Dunnett's test.
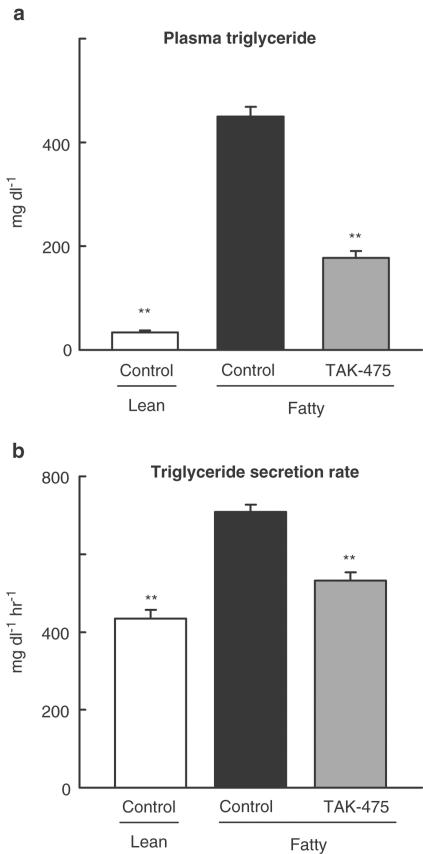
Effect of TAK-475 on hepatic triglyceride secretion rate in hypertriglyceridemic Wistar fatty rats
TAK-475 (60 mg kg−1, p.o.) significantly lowered plasma triglyceride by 61±3% (Figure 7a). To investigate the mechanism of the plasma triglyceride-lowering effect of TAK-475, we evaluated hepatic triglyceride secretion after blocking clearance with Triton WR-1339. At the age of 20 weeks, the secretion of triglyceride from the liver in female Wistar fatty rats was faster than that in Wistar lean rats (Figure 7b). TAK-475 significantly inhibited this enhanced secretion rate of triglyceride from the liver in female Wistar fatty rats (Figure 7b).
Figure 7.
Effects of TAK-475 on plasma triglyceride (a) and hepatic triglyceride secretion rate (b) in hypertriglyceridemic female Wistar fatty rats. TAK-475 (60 mg kg−1) or vehicle were administered orally to female Wistar fatty rats and their lean littermates for 15 days. Hepatic triglyceride secretion rates are represented as the increment of plasma triglyceride per hour after the injection of Triton WR-1339 (400 mg kg−1, i.v.). Data are represented as mean ±s.e.m. (n=9). **P<0.01 vs the fatty control by Student's t-test.
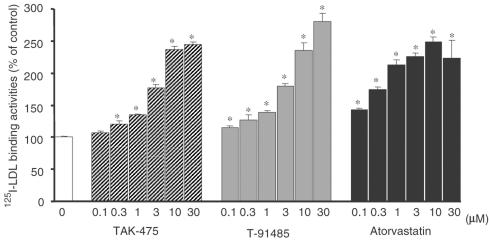
Effects on the binding of 125I-LDL to LDL receptors in HepG2 cells
After oral administration to rats, TAK-475 is absorbed and rapidly hydrolyzed into its pharmacologically active metabolite T-91485. We mainly detected T-91485, but not TAK-475, in the blood and liver of rats (data not shown). T-91485, TAK-475 and atorvastatin inhibited cholesterol synthesis in HepG2 cells with IC50 values of 152, 150 and 26 nM, respectively (Tozawa et al., unpublished observations). As shown in Figure 8, both T-91485 and TAK-475 enhanced the binding of 125I-LDL to the LDL receptor in a concentration-dependent manner, suggesting that these drugs increased LDL receptor expression. Atorvastatin also enhanced the binding. At a concentration of 10 μM, T-91485, TAK-475 and atorvastatin showed almost the same effects on LDL binding.
Figure 8.
Effects of TAK-475, T-91485 and atorvastatin on the binding of 125I-LDL to LDL receptors in HepG2 cells. Binding activities are expressed as radioactivity per milligram cell protein. Control value of binding activity was 32.9±0.5 ng LDL per milligram cell protein. Data are represented as mean±s.e.m. (n=3). *P<0.025 vs control values by one-tailed Williams' test.
Effects on acceptor-dependent cholesterol efflux from macrophage-derived foam cells
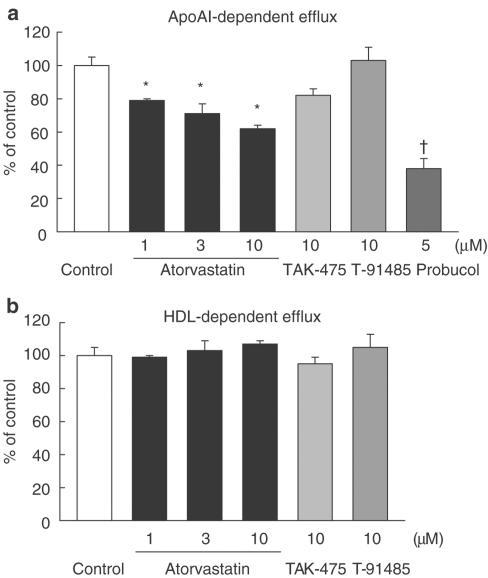
Since some HMG-CoA reductase inhibitors decrease HDL cholesterol in patients with a high baseline HDL cholesterol level, the effects of T-91485, TAK-475 and atorvastatin on cholesterol efflux from THP-1 macrophage-derived foam cells were examined. Atorvastatin (1–10 μM) concentration-dependently and significantly inhibited apoAI-dependent cholesterol efflux from the foam cells, and probucol, which is clinically known to decrease HDL cholesterol, also significantly inhibited apoAI-dependent cholesterol efflux (Figure 9a). T-91485 and TAK-475 had no effect on apoAI-dependent cholesterol efflux. None of the drugs affected HDL-dependent cholesterol efflux (Figure 9b).
Figure 9.
Effects of TAK-475, T-91485 and atorvastatin on apoAI-mediated (a) and HDL-mediated (b) cholesterol efflux from cholesterol-enriched THP-1 differentiated macrophages. Data are represented as mean±s.e.m percentage values of control (n=3). *P<0.025 and †P<0.05 vs control values (apoAI, 41,900±2254 dpm per milligram cell protein; HDL, 133,340±7362 dpm per milligram cell protein) by one-tailed Williams' test and Dunnett's test, respectively.
Discussion
In this study, we examined the lipid-lowering properties of TAK-475, comparing its effects with those of atorvastatin, an HMG-CoA reductase inhibitor. TAK-475 showed lipid-lowering effects in beagle dogs, marmosets, cynomolgus monkeys and hypertriglyceridemic Wistar fatty rats. Many studies have demonstrated that the cholesterol-lowering effects of HMG-CoA reductase inhibitors are due to the induction of LDL receptors. TAK-475 and its pharmacologically active metabolite, T-91485, also increased LDL receptor levels in HepG2 cells. Wistar fatty rats, established by transferring the obesity-inducing fa gene from Zucker fatty rats to Wistar Kyoto rats with blunted insulin sensitivity (Ikeda et al., 1981), have the characteristics of obesity, hyperlipidemia, hyperinsulinemia and peripheral and hepatic insulin resistance. In the present study, we demonstrated that female Wistar fatty rats have higher non-HDL cholesterol and triglyceride levels than their littermate lean controls, with an enhanced hepatic triglyceride secretion rate. TAK-475 potently reduced plasma triglyceride and cholesterol and decreased the hepatic triglyceride secretion rate in these animals. The results obtained from HepG2 cells and Wistar fatty rats indicate that the lipid-lowering effects of TAK-475 are caused by an increase in LDL receptors and suppression of the secretion rate of triglyceride from the liver (Figures 7 and 8).
Common marmosets are useful for the evaluation of lipid-lowering agents, because the lipoprotein profile of this species is similar to that of humans (Miyazaki & Koga, 1998). In common marmosets, both TAK-475 and atorvastatin potently lowered plasma non-HDL cholesterol and triglyceride. TAK-475 did not affect plasma HDL cholesterol, but atorvastatin significantly reduced it (Table 2, Figure 4). Interestingly, a correlation was observed between the decrease in triglyceride level and the increase in plasma HDL cholesterol (Figure 5), suggesting that the increases in plasma HDL cholesterol caused by both TAK-475 and atorvastatin are dependent on the decrease in plasma triglyceride. Compared with atorvastatin, TAK-475 increased HDL cholesterol with a smaller decrease in triglyceride. It has been reported that pravastatin lowered both plasma LDL cholesterol and HDL cholesterol in marmosets, but that the squalene synthase inhibitors, squalestatin 1 and RPR107393, selectively lowered plasma LDL cholesterol without affecting plasma HDL cholesterol (Baxter et al., 1992; Amin et al., 1997; Miyazaki & Koga, 1998). Our observations coincide with these results. Atorvastatin has also reported to lower plasma HDL cholesterol in beagle dogs (Walsh et al., 1996). In contrast, TAK-475 did not affect plasma HDL cholesterol in beagle dogs (Table 1). In studies using patients with a relatively high HDL cholesterol level, HMG-CoA reductase inhibitors have been reported as reducing or tending to reduce HDL cholesterol, although in patients with a low HDL cholesterol level some HMG-CoA reductase inhibitors increase the level of this lipoprotein (Miserez et al., 1994; Narita et al., 1997).
Recent studies show that HDL cholesterol is regulated by apoAI-mediated cholesterol efflux from peripheral tissues and liver (Young & Fielding, 1999; Tsujita et al., 2000). In the present study, the effects of TAK-475, T-91485 and atorvastatin on lipid-free apoAI-dependent and HDL-dependent cholesterol efflux were examined in cholesterol-enriched macrophages. Atorvastatin inhibited apoAI-dependent cholesterol efflux from foam cells (Figure 9), whereas TAK-475 and T-91485 had no effects on this process. These results indicate that atorvastatin, but not TAK-475, might inhibit HDL generation by partially inhibiting apoAI-mediated cholesterol efflux from peripheral cells. This may be one explanation for the difference in the effects of TAK-475 and atorvastatin on plasma HDL cholesterol in marmosets.
In cynomolgus monkeys, TAK-475 reduced plasma cholesterol without affecting plasma hepatic transaminase activities. Atorvastatin, on the other hand, caused marked increases in plasma AST and ALT activities. These results are consistent with the report of Hiyoshi et al. (2000), demonstrating that atorvastatin increased ALT activity in rhesus monkeys but that ER-27856, a squalene synthase inhibitor, did not affect this parameter. In rabbits, lovastatin-induced elevations of AST and ALT activities were completely prevented by coadministration of mevalonate, but not by supplementation with cholesterol (Kornbrust et al., 1989). These observations suggest that the elevations of plasma AST and ALT activities by HMG-CoA reductase inhibitors are mechanism-based adverse effects. Consistent with these preclinical observations, HMG-CoA reductase inhibitors have been clinically reported to cause elevation of plasma AST and ALT activities (Illingworth et al., 2001).
Although many squalene synthase inhibitors have been reported to date (Rosenberg, 1998; Hiyoshi et al., 2000; Ugawa et al., 2000), no such drugs have been launched on the market. One of the reasons seems to be toxic acidosis due to overexcretion of farnesol-derived dicarboxylic acids (Rosenberg, 1998). One squalene synthase inhibitor, zaragozic acid A, caused the excretion of unexpectedly high levels of urinary dicarboxylic acids in rats and dogs (Bostedor et al., 1997). In our preliminary results, although rats treated with TAK-475 tended to have increased urinary levels of dicarboxylic acids, accumulation after treatment with TAK-475 was smaller than that with zaragozic acid A. Accumulation of urinary dicarboxylic acids by TAK-475 did not result in toxic acidosis in rats (data not shown).
In conclusion, TAK-475 is a potent and orally effective squalene synthase inhibitor that markedly lowers plasma lipid levels in various animal species. These lipid-lowering effects are due to induction of LDL receptors and inhibition of triglyceride secretion from the liver. TAK-475 may be useful for treatment of dyslipidemia.
Acknowledgments
We thank Drs Zen-ichi Terashita, Hiroshi Mabuchi, Masakuni Kori, Takeo Wada, Masahira Nakamura and Takashi Miki for their continuing advice.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CHD
coronary heart disease
- FBS
fetal bovine serum
- HDL
high-density lipoprotein
- HMG-CoA
3-hydroxy-3-methylglutaryl coenzyme A
- LDL
low-density lipoprotein
- LPDS
lipoprotein-deficient serum
- non-HDL
non-high-density lipoprotein
References
- AMIN D., RUTLEDGE R.Z., NEEDLE S.N., GALCZENSKI H.F., NEUENSCHWANDER K., SCOTESE A.C., MAGUIRE M.P., BUSH R.C., HELE D.J., BILDER G.E., PERRONE M.H. RPR 107393, a potent squalene synthase inhibitor and orally effective cholesterol-lowering agent:comparison with inhibitors of HMG-CoA reductase. J. Pharmacol. Exp. Ther. 1997;281:746–752. [PubMed] [Google Scholar]
- ANDERSON K.M., CASTELLI W.P., LEVY D. Cholesterol and mortality. 30 years of follow-up from the Framingham Study. J. Am. Med. Assoc. 1987;257:2176–2180. doi: 10.1001/jama.257.16.2176. [DOI] [PubMed] [Google Scholar]
- AUSTIN M.A. Plasma triglyceride and coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 1991;11:2–14. doi: 10.1161/01.atv.11.1.2. [DOI] [PubMed] [Google Scholar]
- BAKKER-ARKEMA R.G., DAVIDSON M.H., GOLDSTEIN R.J., DAVIGNON J., ISAACSOHN J.L., WEISS S.R., KEILSON L.M., BROWN W.V., MILLER V.T., SHURZINSKE L.J., BLACK D.M. Efficacy and safety of a new HMG-CoA reductase inhibitor, atorvastatin, in patients with hypertriglyceridemia. J. Am. Med. Assoc. 1996;275:128–133. [PubMed] [Google Scholar]
- BAXTER A., FITZGERALD B.J., HUTSON J.L., MCCARTHY A.D., MOTTERAM J.M., ROSS B.C., SAPRA M., SNOWDEN M.A., WATSON N.S., WILLIAMS R.J., WRIGHT C. Squalestatin 1, a potent inhibitor of squalene synthase, which lowers serum cholesterol in vivo. J. Biol. Chem. 1992;267:11705–11708. [PubMed] [Google Scholar]
- BILHEIMER D.W., EISENBERG S., LEVY R.I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim. Biophys. Acta. 1972;260:212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- BOSTEDOR R.G., KARKAS J.D., ARISON B.H., BANSAI V.S., VAIDYA S., GERMERSHAUSEN J.I., KURTZ M.M., BERGSTROM J.D. Farnesol-derived dicarboxylic acids in the urine of animals treated with zaragozic acid A or with farnesol. J. Biol. Chem. 1997;272:9197–9203. doi: 10.1074/jbc.272.14.9197. [DOI] [PubMed] [Google Scholar]
- BROWN A.S., BAKKER-ARKEMA R.G., YELLEN L., HENLEY R.W., JR., GUTHRIE R., CAMPBELL C.F., KOREN M., WOO W., MCLAIN R., BLACK D.M. Treating patients with documented atherosclerosis to National Cholesterol Education Program-recommended low-density-lipoprotein cholesterol goals with atorvastatin, fluvastatin, lovastatin and simvastatin. J. Am. Coll. Cardiol. 1998;32:665–672. doi: 10.1016/s0735-1097(98)00300-3. [DOI] [PubMed] [Google Scholar]
- FLINT O.P., MASTERS B.A., GREGG R.E., DURHAM S.K. Inhibition of cholesterol synthesis by squalene synthase inhibitors does not induce myotoxicity in vitro. Toxicol. Appl. Pharmacol. 1997;145:91–98. doi: 10.1006/taap.1997.8131. [DOI] [PubMed] [Google Scholar]
- GORDON T., KANNEL W.B., CASTELLI W.P., DAWBER T.R. Lipoproteins, cardiovascular disease, and death: the Framingham study. Arch. Intern. Med. 1981;141:1128–1131. [PubMed] [Google Scholar]
- HARIA M., MCTAVISH D. Pravastatin. Drugs. 1997;53:299–336. doi: 10.2165/00003495-199753020-00008. [DOI] [PubMed] [Google Scholar]
- HIYOSHI H., YANAGIMACHI M., ITO M., OHTSUKA I., YOSHIDA I., SAEKI T., TANAKA H. Effect of ER-27856, a novel squalene synthase inhibitor, on plasma cholesterol in rhesus monkeys: comparison with 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors. J. Lipid Res. 2000;41:1136–1144. [PubMed] [Google Scholar]
- HSU I., SPRINLER S.A., JOHNSON N.E. Comparative evaluation of the safety and efficacy of HMG-CoA reductase inhibitor monotherapy in the treatment of primary hypercholesterolemia. Ann. Pharmacother. 1995;29:743–759. doi: 10.1177/106002809502907-818. [DOI] [PubMed] [Google Scholar]
- IKEDA H., SHINO A., MATSUO T., IWATSUKA H., SUZUOKI Z. A new genetically obese-hyperglycemic rat (Wistar fatty) Diabetes. 1981;30:1045–1050. doi: 10.2337/diab.30.12.1045. [DOI] [PubMed] [Google Scholar]
- ILLINGWORTH D.R., CROUSE J.R., III, HUNNINGHAKE D.B., DAVIDSON M.H., ESCOBAR I.D., STALENHOEF A.F.H., PARAGH G., MA P.T.S., LIU M., MELINO M.R., O'GRADY L., MERCURI M., MITCHEL Y.B., FOR THE SIMVASTATIN ATORVASTATIN HDL STUDY GROUP A comparison of simvastatin and atorvastatin up to maximal recommended doses in a large multicenter randomized clinical trial. Curr. Med. Res. Opin. 2001;17:43–50. [PubMed] [Google Scholar]
- KASIM S.E., LEBOEUF R.C., KHILNANI S., TALLAPAKA L., DAYANANDA D., JEN K.-L.C. Mechanisms of triglyceride-lowering effect of an HMG-CoA reductase inhibitor in a hypertriglyceridemic animal model, the Zucker obese rat. J. Lipid Res. 1992;33:1–7. [PubMed] [Google Scholar]
- KORNBRUST D.J., MACDONALD J.S., PETER C.P., DUCHAI D.M., STUBBS R.J., GERMERSHAUSEN J.I., ALBERTS A.W. Toxicity of the HMG-coenzyme A reductase inhibitor, lovastatin, to rabbits. J. Pharmacol. Exp. Ther. 1989;248:498–505. [PubMed] [Google Scholar]
- MIKI T., KORI M., MABUCHI H., TOZAWA R., NISHIMOTO T., SUGIYAMA Y., TESHIMA K., YUKIMASA H. Synthesis of novel 4,1-benzoxazepine derivatives as squalene synthase inhibitors and their inhibition of cholesterol synthesis. J. Med. Chem. 2002;45:4571–4580. doi: 10.1021/jm020234o. [DOI] [PubMed] [Google Scholar]
- MISEREZ A.R., ROSSI F.A., KELLER U. Prediction of the therapeutic response to simvastatin by pretreatment lipid concentrations in 2082 subjects. Eur. J. Clin. Pharmacol. 1994;46:107–114. doi: 10.1007/BF00199871. [DOI] [PubMed] [Google Scholar]
- MIYAZAKI A., KOGA T. Lipid lowering effects of pravastatin in common marmosets. Arzneim.-Forsch./Drug Res. 1998;48:154–160. [PubMed] [Google Scholar]
- NARITA Y., KITAZOE Y., KURIHARA Y., OKUHARA Y., TAKAMATSU K., SAITO N., DOI Y. Increase or decrease of HDL-cholesterol concentrations during pravastatin treatment depending on the pre-treatment HDL cholesterol levels. Eur. J. Clin. Pharmacol. 1997;52:461–463. doi: 10.1007/s002280050319. [DOI] [PubMed] [Google Scholar]
- OLSSON A.G. Statin therapy and reductions in low-density lipoprotein cholesterol: initial clinical data on the potent new statin rosuvastatin. Am. J. Cardiol. 2001;87:33B–36B. doi: 10.1016/s0002-9149(01)01455-2. [DOI] [PubMed] [Google Scholar]
- ROSENBERG S.H. Squalene synthase inhibitors 1998. Exp. Opin. Ther. Patents. 1998;8:521–530. [Google Scholar]
- STOPECK A.T., NICHOLSON A.C., MANCINI F.P., HAJJAR D.P. Cytokine regulation of low density lipoprotein receptor gene transcription in HepG2 cells. J. Biol. Chem. 1993;268:17489–17494. [PubMed] [Google Scholar]
- THIBAULT A., SAMID D., TOMPKINS A.C., FIGG W.D., COOPER M.R., HOHL R.J., TREPEL J., LIANG B., PATRONAS N., VENZON D.J., REED E., MYERS C.E. Phase I study of lovastatin, an inhibitor of the mevalonate pathway, in patients with cancer. Clin. Cancer Res. 1996;2:483–491. [PubMed] [Google Scholar]
- TSUJITA M., TOMIMOTO S., OKUMURA-NOJI K., OKAZAKI M., YOKOYAMA S. Apolipoprotein-mediated cellular cholesterol/phospholipid efflux and plasma high density lipoprotein level in mice. Biochim. Biophys. Acta. 2000;1485:199–213. doi: 10.1016/s1388-1981(00)00061-5. [DOI] [PubMed] [Google Scholar]
- UCAR M., MJÖRNDAL T., DAHLQVIST R. HMG-CoA reductase inhibitors and myotoxicity. Drug Saf. 2000;22:441–457. doi: 10.2165/00002018-200022060-00003. [DOI] [PubMed] [Google Scholar]
- UGAWA T., KAKUTA H., MORITANI H., MATSUDA K., ISHIHARA T., YAMAGUCHI M., NAGANUMA S., IIZUMI Y., SHIKAMA H. YM-53601, a novel squalene synthase inhibitor, reduces plasma cholesterol and triglyceride levels in several animal species. Br. J. Pharmacol. 2000;131:63–70. doi: 10.1038/sj.bjp.0703545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG S.G., FIELDING C.J. The ABCs of cholesterol efflux. Nat. Genet. 1999;22:316–318. doi: 10.1038/11878. [DOI] [PubMed] [Google Scholar]
- WALSH K.M., ALBASSAM M.A., CLARKE D.E. Subchronic toxicity of atorvastatin, a hydroxymethylglutaryl-coenzyme A reductase inhibitor, in beagle dogs. Toxicol. Pathol. 1996;24:468–476. doi: 10.1177/019262339602400409. [DOI] [PubMed] [Google Scholar]