Long QT syndrome (LQTS) is a cardiac disease resulting from impaired repolarisation of the ventricular action potential. Patients with LQTS are at increased risk of the dangerous Torsades de Pointes ventricular tachyarrhythmias that can cause short episodes of loss of consciousness (syncope) or sudden cardiac death (Keating & Sanguinetti, 2001). The inherited form of the disease results from mutations in ion channel subunits or in the adapter protein ankyrin B that coordinates cellular organisation of key proteins involved in normal calcium signalling in cardiac myocytes (Mohler et al., 2003). However, a far more common reason for LQTS is pharmacological inhibition of the rapid component of the delayed rectifier potassium current (IKr). This current is carried by channels encoded by the human ether-a-go-go related gene (HERG). A spectrum of therapeutically and structurally unrelated drugs have been linked to LQTS and have been shown to inhibit IKr and HERG channels with high potency (Keating & Sanguinetti, 2001). The problem of medication-induced LQTS has been a major issue for the pharmaceutical industry and drug-regulatory bodies. Strategies to evaluate the potential of drugs to cause Torsade de Pointes have improved in recent years, but a detailed mechanistic understanding of where and how drugs block HERG channels would be helpful for reducing the cardiotoxic risk of future drugs.
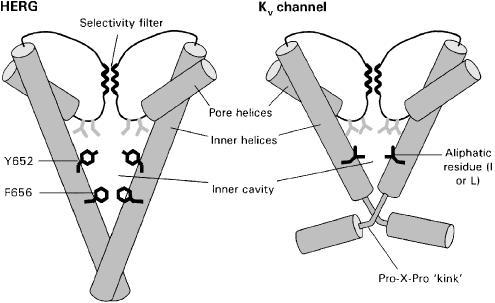
Most LQTS-associated drugs show open-channel block of HERG channels and slow recovery from block upon washing the compounds off. These drugs are likely to bind to sites within the inner cavity of the channel, behind the activation gate. Thus, drugs only get access to their receptor site when the channel opens, and recovery from block is slow because they become trapped by closure of the activation gate upon membrane potential repolarisation (Carmeliet, 1993; Mitcheson et al., 2000b). Drug trapping and structure–function studies suggest that the inner cavity of HERG is larger than other voltage-gated potassium channels (Kv) and is therefore able to accommodate diverse chemical structures. HERG lacks a highly conserved proline-X-proline motif found on the inner helices of most Kv channels (Figure 1). The prolines are proposed to ‘kink' the inner helices and consequently reduce the space within the inner cavity (del Camino et al., 2000). Mutagenesis studies also show that whereas the inner helices of most voltage-gated K channels are lined by aliphatic isoleucine or valine residues, the inner helices of HERG contain two aromatic residues (Y652 and F656; see Figure 1) that are important structural determinants of binding for all drugs tested to date (Lees-Miller et al., 2000; Mitcheson et al., 2000a; Kamiya et al., 2001; Sanchez-Chapula et al., 2002). In addition to hydrophobic interactions, the pi-electrons on the face of aromatic residues may enable polar and cation–pi interactions with drug molecules. Y652 and F656 face into the inner cavity and are accessible to drugs entering from the cytoplasm when the channels open. Channel inactivation may provide additional conformational changes within the inner cavity that maximise interactions with some drugs (Chen et al., 2002). Other residues that line the inner cavity and may be important for drug binding are polar residues (T623 and S624) located on the bottom loop of the pore helices and G648 on the inner helices (Mitcheson et al., 2000a).
Figure 1.
The structures of two of the four subunits that form the pore and inner cavity of HERG and Kv channels are shown. The inner helices and loops extending from the pore helices to the selectivity filter form the inner cavity and drug-binding site of HERG. Several structural features that help explain the nonspecific drug-binding properties of HERG are illustrated. The inner cavity of HERG is long, creating a relatively large space for trapping drugs and for channel–drug interactions. Aromatic residues (black) not found in Kv channels are critical sites for interaction for most compounds, but not for fluvoxamine. Other sites for drug interaction are polar residues (grey) located close to the selectivity filter. Kv channels have a proline-X-proline motif that is proposed to insert a ‘kink' in the inner helices, resulting in a relatively small inner cavity. The inner cavity is lined by aliphatic rather than aromatic residues.
For all drugs investigated to date, one or both of the aromatic residues in the inner cavity were key molecular determinants of drug binding. In particular, mutation of F656 dramatically reduced the potency of each of the tested drugs. However, in this issue, Milnes et al. have for the first time identified a drug, the selective seratonin reuptake inhibitor fluvoxamine, with HERG channel block properties that are relatively insensitive to mutation of F656 and Y652.
Fluvoxamine is a relatively low-potency blocker of HERG channels with an IC50 of 3.8 μM. In a careful and thorough study, Milnes et al. show that the kinetics and apparent state dependence of block of HERG is quite different from most HERG channel blockers. The onset of block is very rapid, occurring within 10 ms of depolarisation, suggesting that fluvoxamine exhibits either closed-state block or very rapid open-channel block. As the authors point out, it is extremely difficult to distinguish between these possibilities. Certainly, the time course of block is far more rapid than the more potent methanesulphonanilides as well as lower potency blockers such as vesnarinone and propafenone. Channel inactivation is also not a prerequisite of block by fluvoxamine. The F656/Y652-independent block and unusual pharmacological properties of fluvoxamine raise several questions. Is fluvoxamine binding outside the inner cavity? If so, where is this additional binding site and do many other compounds also bind there? It has been suggested that the molecular determinants of low-affinity HERG channel blockers may be different from high-affinity blockers. So far, the evidence does not support this hypothesis. The low-potency blockers chloroquine and vesnarinone both bind within the inner cavity (Kamiya et al., 2001; Sanchez-Chapula et al., 2002). Mutating F656 and/or Y652 dramatically attenuates channel block. Fluvoxamine is the only blocker identified that does not appear to bind at these residues. The rapid onset of block is consistent with binding to an extracellular site, possibly at the outer mouth of the channel where erg-specific peptide toxins bind (Pardo-Lopez et al., 2002). However, a distinct site within the inner cavity that is not dependent on aromatic residues (perhaps located higher up within the cavity) cannot be ruled out at this stage. Further experiments are needed to determine if fluvoxamine binds from the cytoplasmic or extracellular side of the channel and if mutation of other residues within the inner cavity reduces the potency of fluvoxamine.
The observation that fluvoxamine does not bind to either Y652 or F656 is highly significant and suggests that there are alternative mechanisms of action for LQTS-associated compounds. It is likely that a number of additional nontoxin compounds will be identified that do not bind within the inner cavity. For example, the large size of macrolide antibiotics such as erythromycin suggests that they are unlikely to fit within the inner cavity and must bind elsewhere. Questions remain about whether alternative mechanisms of block are associated with different risks of inducing arrhythmias. To fully understand and prevent unwanted HERG channel block, it is important that potential sites of drug action are identified and characterised.
Acknowledgments
The work of this author is supported by funding from the Medical Research Council, British Heart Foundation and a collaborative grant from Pfizer Global Research and Development.
Abbreviations
- HERG
human ether-a-go-go related gene
- IKr
current through rapid delayed rectifier potassium channels
- LQTS
long QT syndrome
References
- CARMELIET E. Use-dependent block and use-dependent unblock of the delayed rectifier K+ current by almokalant in rabbit ventricular myocytes. Circ. Res. 1993;73:857–868. doi: 10.1161/01.res.73.5.857. [DOI] [PubMed] [Google Scholar]
- CHEN J., SEEBOHM G., SANGUINETTI M.C. Position of aromatic residues in the S6 domain, not inactivation, dictates cisapride sensitivity of HERG and eag potassium channels. Proc. Natl. Acad. Sci. U.S.A. 2002;99:12461–12466. doi: 10.1073/pnas.192367299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CAMINO D., HOLMGREN M., LIU Y., YELLEN G. Blocker protection in the pore of a voltage-gated K+ channel and its structural implications. Nature. 2000;403:321–325. doi: 10.1038/35002099. [DOI] [PubMed] [Google Scholar]
- KAMIYA K., MITCHESON J.S., YASUI K., KODAMA I., SANGUINETTI M.C. Open channel block of HERG K+ channels by vesnarinone. Mol. Pharmacol. 2001;60:244–253. doi: 10.1124/mol.60.2.244. [DOI] [PubMed] [Google Scholar]
- KEATING M.T., SANGUINETTI M.C. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104:569–580. doi: 10.1016/s0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- LEES-MILLER J.P., DUAN Y., TENG G.Q., DUFF H.J. Molecular determinant of high-affinity dofetilide binding to HERG1 expressed in Xenopus oocytes: involvement of S6 sites. Mol. Pharmacol. 2000;57:367–374. [PubMed] [Google Scholar]
- MITCHESON J.S., CHEN J., LIN M., CULBERSON C., SANGUINETTI M.C. A structural basis for drug-induced long QT syndrome. Proc. Natl. Acad. Sci. U.S.A. 2000a;97:12329–12333. doi: 10.1073/pnas.210244497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHESON J.S., CHEN J., SANGUINETTI M.C. Trapping of a methanesulfonanilide by closure of the HERG potassium channel activation gate. J. Gen. Physiol. 2000b;115:229–240. doi: 10.1085/jgp.115.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOHLER P.J., SCHOTT J.J., GRAMOLINI A.O., DILLY K.W., GUATIMOSIM S., DUBELL W.H., SONG L.S., HAUROGNE K., KYNDT F., ALL M.E., ROGERS T.B., LEDERER W.J., ESCANDE D., LE MAREC H., BENNETT V. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–639. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- PARDO-LOPEZ L., GARCIA-VALDES J., GURROLA G.B., ROBERTSON G.A., POSSANI L.D. Mapping the receptor site for ergtoxin, a specific blocker of ERG channels. FEBS Lett. 2002;510:45–49. doi: 10.1016/s0014-5793(01)03218-5. [DOI] [PubMed] [Google Scholar]
- SANCHEZ-CHAPULA J.A., NAVARRO-POLANCO R.A., CULBERSON C., CHEN J., SANGUINETTI M.C. Molecular determinants of voltage-dependent human ether-a-go-go related gene (HERG) K+ channel block. J. Biol. Chem. 2002;277:23587–23595. doi: 10.1074/jbc.M200448200. [DOI] [PubMed] [Google Scholar]