Abstract
One classical example of how neuropeptides can affect the function of ligand-gated receptors is the modulation of neuronal nicotinic receptors (nAChRs) by substance P. The present review updates current understanding of this action by substance P and compares it with other neuropeptides more recently found to modulate nAChRs in the autonomic nervous system.
Calcitonin gene-related peptide (CGRP) and its N-terminal fragments have been shown to exert complex inhibitory as well facilitatory actions on nAChRs. Fragments such as CGRP1–4, CGRP1–5 and CGRP1–6 rapidly and reversibly enhance agonist sensitivity of nAChRs without directly activating those receptors. Longer fragments or the full-length peptide potently inhibit responses mediated by nAChRs via an apparently competitive-type antagonism. This phenomenon differs from the substance P-induced block, which is agonist use-dependent and preferential towards large nicotinic responses.
It is argued that the full-length peptides CGRP and substance P might play distinct roles in the activity-dependent modulation of cholinergic neurotransmission, by inhibiting background noise in the case of CGRP or by reducing excessive excitation in the case of substance P. Hence, multiple neuropeptide mechanisms may represent a wide array of fine-tuning processes to regulate nicotinic synaptic transmission.
The availability of novel CGRP derivatives with a strong enhancing action on nAChRs may offer new leads for the drug design targeted for potentiation of nAChRs in the autonomic nervous system as well as in the brain, a subject of interest to counteract the deficit of the nAChR function associated with neurodegenerative diseases like Alzheimer's and Parkinson's diseases.
Keywords: Acetylcholine, neuropeptide, allosteric modulator, nicotine, autonomic nervous system
Neuronal nicotinic receptors as prototypes of ionotropic, ligand-gated receptors
Neuronal nicotinic receptors (nAChRs) are integral membrane proteins that mediate central and peripheral effects of endogenously released acetylcholine (ACh) as well as the action of nicotine delivered via tobacco smoking (for a review, see Paterson & Nordberg, 2000). Receptor activation is characterized by an opening of its central aqueous pore through which Na+, Ca2+ and K+ permeate to generate membrane depolarization with a consequent increase in neuronal excitability (Changeux & Edelstein, 1998; Itier & Bertrand, 2001). AChRs are a heterogeneous group with discrete tissue distribution (Gotti et al., 1997). The pleiotropy of nAChRs makes their systematic classification difficult, although a broad subdivision may be based on homomeric α7 receptors and heteromeric non-α7 receptors. Even if the relation between nAChR structure and neuronal function is poorly understood, it seems likely that different subunit compositions may determine amplitude, kinetics or rate of desensitization of nicotinic receptor-mediated responses (Groot-Kormelink et al., 1998; Yu & Role, 1998; Le Novère et al., 2002). Further details concerning nAChR mechanisms may be found in recent review articles (Karlin, 2002; Le Novere et al., 2002; Quick & Lester, 2002; Sine, 2002). Considerable interest in nAChRs stems from the realization that several neuropsychiatric disorders are accompanied by a strong deficit in nAChR function, which is probably responsible for at least part of the clinical symptomatology (Paterson & Nordberg, 2000; Picciotto & Zoli, 2002; Raggenbass & Bertrand, 2002). To obtain amelioration of these conditions, it would be potentially useful to amplify either the extracellular concentration of the endogenous neurotransmitter ACh or the nAChR signalling mechanism. The present review will discuss some novel approaches to the latter issue based on direct actions of neuropeptides, which represent a new and rapidly growing field of neuropharmacology.
Modulation of nAChRs
Receptor modulation may be defined as an up- or downregulation of receptor function associated with direct modification of the receptor protein, often of reversible nature. In principle, it is possible to imagine at least two conditions when receptor modulation may take place. In one case, receptor upmodulation is desirable to boost the faltering activity of nAChRs during the progressive, slow onset of neurological diseases (Pereira et al., 2002; Picciotto & Zoli, 2002). In this instance, the aim is to enhance receptor responsiveness in view of their reduced number or decreased concentration of the endogenous transmitter. This type of modulation may be observed with administration of allosteric modulators that, by themselves, cannot significantly activate receptors, yet they facilitate agonist/receptor interaction (Le Novere et al., 2002; Pereira et al., 2002). As far as the cholinergic system is concerned, drugs like galanthamine or tacrine are thought to be allosteric modulators of nAChRs and are useful for controlling symptoms of Alzheimer's disease (Grutzendler & Morris, 2001; Pereira et al., 2002).
A different case of nAChR modulation may be observed with large or persistently applied concentrations of nicotinic agonists (Buisson & Bertrand, 2001). In this instance, it is likely that strong and/or persistent ligand occupation of a large receptor fraction triggers the activation of intracellular second messengers, in turn modifying nAChR responsiveness, for instance, by phosphorylation of certain nAChR domains (Wonnacott, 1990; Khiroug et al., 1998; Fenster et al., 1999; Harkness & Millar, 2002). More intriguing is the possibility that there are endogenous modulators to fine-tune the activity of nAChRs. Such modulators could be present constitutively to set the background responsiveness of nAChRs, or they could be released during intense activity to reverse receptor desensitization or, conversely, they could inhibit nAChRs to avoid excessive activation. Hence, the ability of nAChRs to mediate ACh-evoked responses might be a state-dependent phenomenon with rapid adaptive changes determined by the extent of neuronal signalling in conjunction with locally released substances. Within this framework, the present report will focus on the potential modulatory role of two endogenous neuropeptides, namely CGRP and substance P, which are often colocalized within the same neurons (Lee et al., 1985; Ma et al., 2001; Lawson et al., 2002). While substance P has long been known to inhibit nAChRs (Clapham & Neher, 1984; Valenta et al., 1993; Stafford et al., 1994), the demonstration of an apparently similar effect by CGRP on nAChRs is a more recent discovery (Giniatullin et al., 1999).
Complex mechanism of action of CGRP or substance P
The most typical action by neuropeptides on target neurons is slow neurotransmission (often referred to as ‘volume transmission') via activation of G-protein coupled receptors to which they bind with very high affinity after having travelled a considerable distance from their site of release (Agnati et al., 1995; Zoli & Agnati, 1996; Jansson et al., 2000). These properties usually confer long latency to peptide-mediated responses that are expressed through changes in membrane conductance and cell excitability (Hokfelt, 1991; Otsuka & Yoshioka, 1993). Peptides can exert a modulatory role on fast transmitter-gated channels through at least two distinct processes: (1) an indirect mechanism mediated by the peptide G-protein-coupled receptors that, through changes in intracellular free Ca2+ concentration ([Ca2+]i) and other intracellular second messengers, control the phosphorylation state of the fast transmitter receptors and their ability to bind receptor agonists (Huganir & Greengard, 1990; Levitan, 1994; Smart, 1997). This phenomenon is just one example of the process of cross talk which often occurs between different transmitter systems (Kotter, 1994; Barbour & Hausser, 1997) and will not be further examined here: (2) An incompletely understood effect that involves direct interaction of the neuropeptide with certain subunits of the fast transmitter receptor with subsequent alteration in receptor signalling (Clapham & Neher, 1984; Stafford et al., 1994; Giniatullin et al., 1999; Di Angelantonio et al., 2002). We consider this property as representative of direct receptor modulation and will discuss it in relation to nAChRs. The aim of the present review is to discuss evidence pertaining to the action of CGRP and substance P on autonomic neurons for the main reason that the direct accessibility of such cells makes high-resolution studies of the molecular mechanisms underlying the observed phenomena possible. It is also useful to consider such studies as a model for expanding and interpreting current data obtained with brain neurons.
Cholinergic transmission is a target for CGRP modulation
Calcitonin gene-related peptide (CGRP), cloned in the early 1980 s from the gene encoding calcitonin (Amara et al., 1982), is a 37 amino-acid peptide, with a characteristic six amino-acid ring made by a disulphide bridge between Cys 2 and Cys 7 at the N-terminal region.
Although at the rodent neuromuscular junction, CGRP is contained in large, dense-core vesicles distinct from ACh-filled ones, these two substances can be coreleased (Matteoli et al., 1988) with the result that CGRP prolongs the open time of muscle nicotinic channels (Lu et al., 1993). Furthermore, on the same receptors, CGRP facilitates desensitization by phoshorylating certain receptor subunits (Mulle et al., 1988; Miles et al., 1989; but see Lu et al., 1993) and, ultimately, it increases receptor biosynthesis (Changeux et al., 1992). Traditionally, the analysis of the modulatory role of CGRP was limited to muscle nicotinic receptors (Eusebi et al., 1988; Mulle et al., 1988; Miles et al., 1989; Lu et al., 1993). A convenient model for investigating the modulatory action of CGRP on nAChRs is the adrenal chromaffin cell because it expresses a large population of nAChRs (predominantly of the α3β4 type; Campos-Caro et al., 1997; Di Angelantonio et al., 2003) normally activated by ACh released from the splanchnic nerve terminals (Douglas & Rubin, 1961; Douglas et al., 1967). In the adrenal medulla, CGRP is present in nerve fibres (Costa et al., 1994; Heym et al., 1995) and in the chromaffin cells themselves (Kuramoto et al., 1987).
Slow effects of CGRP on [Ca2+]i and membrane current induced by nicotine
Chromaffin cells provide a clear example (Figure 1) of how CGRP can produce a delayed [Ca2+]i rise (without associated change in membrane current) due to Ca2+ release from internal stores via activation of G-protein coupled receptors (Mazzocchi et al., 1996a,1996b; Ayar et al., 1999). Unlike CGRP, the action of nicotine (applied by brief pressure pulses from a puffer pipette to mimic the synaptic activity of the natural transmitter ACh) is much faster and accompanied by large, rapid [Ca2+]i increases (due to Ca2+ influx) and fast inward currents (see also Vernino et al., 1994; Khiroug et al., 1997). Note, however, that responses induced by nicotine after the application of CGRP are clearly depressed versus control, demonstrating that the neuropeptide had induced a transient downregulation of nAChRs. The question next arises whether such a downregulation is contingent upon elevated [Ca2+]i or independent from it. The CGRP-mediated [Ca2+]i rise is prevented by the CGRP receptor antagonist hCGRP8–37 (Quirion et al., 1992, Giniatullin et al., 1999, Poyner et al., 2002), a pharmacological agent selective against G-protein coupled CGRP receptors (Bell & McDermott, 1996). Conversely, hCGRP8–37 does not change either nicotine-evoked responses or their depression by CGRP. These results confirm that the reduction of nicotine-induced currents by CGRP is not due to persistent [Ca2+]i rise or activation of CGRP G-protein receptors.
Figure 1.
Changes in [Ca2+]i and membrane current induced by CGRP or nicotine. Top: [Ca2+]i fluorescence signals induced by pressure application of nicotine (Nic; 20 ms; 0.1 mM; see arrow-heads) or CGRP (1 min; 1 μM; horizontal filled bar). Note that the [Ca2+]i rise evoked by CGRP is smaller and slower than the one evoked by nicotine, but it induces a lasting depression of nicotine responses evoked at 30 s intervals. Bottom: Membrane currents induced by the same application of nicotine. CGRP did not change baseline current, but depressed subsequent responses to nicotine. Note different time scales between the top and bottom records. All traces are from the same cell (Giniatullin et al., 1999 with permission from the Journal of Neuroscience. Copyright 1999 Society for Neuroscience).
Fast action of CGRP on nicotine-mediated responses
Any depression of nicotinic responses occurring independent from metabotropic receptor activity should prompt studying the basis of this phenomenon in a much faster time domain. In fact, when CGRP is briefly applied for just a few seconds prior to nicotine, even without changing [Ca2+]i, it strongly (and reversibly) depresses the inward current induced by nicotine (Figure 2a). The extent of this block does not intensify during continuous application of CGRP, and is unrelated to [Ca2+]i buffering (Giniatullin et al., 1999). This observation suggests that CGRP interacts directly with nAChRs on a rapid timescale. In support of this notion is the finding that CGRP acts as fast as nicotine itself, because the nicotine current response is decreased even when CGRP and nicotine are briefly coapplied (Giniatullin et al., 1999). Since nAChR desensitization has a time constant of approximately 100 ms (Khiroug et al., 1997, 1998; Quick & Lester, 2002), the inhibitory action in CGRP takes place before receptors can be significantly desensitized. Thus, either CGRP can dramatically speed up desensitization kinetics or it can act via a different mechanism to depress nicotinic responses. It seems unlikely that the first possibility holds true because large responses to nicotine (which should be more prone to desensitization; Valenta et al., 1993; Khiroug et al., 1997, 1998) are actually less affected by the peptide as indicated by the plots in Figure 2b (Giniatullin et al., 1999). Note also that the ability of CGRP to modulate receptors does not extend to ionotropic γ-aminobutyric acid (GABAA) receptors (which belong to the same superfamily comprising nAChRs; Barnard, 1996), indicating nAChR specificity to the blocking action of CGRP.
Figure 2.
Rapid downregulation of nicotine-induced responses by CGRP and its N-terminal fragment CGRP1–7. (a) Current records obtained with 20 ms nicotine (0.1 mM; left), 15 s after starting pressure application of CGRP (1 μM, middle) and 45 s after washout of CGRP. Note the reduction in nicotine current amplitude. (b) Plot of nicotine current amplitudes versus increasing duration of nicotine pressure pulses in control solution, or the presence of CGRP. Ordinate, current amplitude normalized with respect to the response evoked by 10 ms nicotine in control solution for each cell. Abscissa, pulse duration of nicotine (0.1 mM) applications. CGRP (1 μM) was applied for 15 s before each nicotine response (n=6–9 cells). Note the rightward shift of the plot without decrease in maximal response. (c) Current records obtained with 20 ms nicotine (0.1 mM; left), 15 s after starting pressure application of CGRP1–7 (1 μM; middle) and 45 s after washout of CGRP1–7. Note the reduction in nicotine current amplitude. (d) Plot of nicotine current amplitudes versus increasing duration of nicotine pressure pulses in control solution, or the presence of CGRP1–7, constructed as in b (n=8 cells). Note the rightward shift of the plot without decrease in maximal response. Data are based on results by Giniatullin et al. (1999).
Mechanism of CGRP fast block on nAChRs
CGRP does not apparently act as an open channel blocker of nicotinic receptors such as, for instance, local anaesthetics (Neher & Steinbach, 1978) because its block is neither agonist use-dependent nor voltage-dependent (Giniatullin et al., 1999). It is apparent that CGRP preferentially inhibits small responses to nicotine, and that increasing the amount of nicotine delivered to the cell counteracts the inhibitory effect of CGRP (Figure 2b). In fact, plotting the fractional response amplitude versus the amount of nicotine shows a rightward, parallel shift of the dose–response curve in the presence of CGRP, consistent with an apparently competitive antagonism (Figure 2b). Hence, the mode of action of CGRP is similar to the one of the competitive antagonist N,N,N-trimethyl-1-(4-trans-stilbenoxy)-2-propylammonium iodide (F3; Di Angelantonio et al., 2000); indeed, coapplication of CGRP and F3 produces linear summation of antagonism. It is also unlikely that the blocking effect of CGRP is caused by negative allosteric modulation of nAChRs like the one reported for substance P, which elicits a downward shift of the agonist dose–response curve (Akasu et al., 1983; Stafford et al., 1994).
Molecular determinants of the fast blocking action of CGRP
Since hCGRP8–37 is inactive on nicotine-induced currents, it follows that the N-terminal sequence series of seven amino acids is actually the main determinant for the CGRP modulation of nAChRs. Indeed, CGRP1–7 modulates nicotine-induced currents (Figure 2c) like full-length CGRP, with analogous effectiveness and shift in the dose–response curve (Figure 2d). Sustained application of CGRP1–7 fails to raise [Ca2+]i, confirming that the N-terminal sequence of the peptide is responsible for rapid block of nAChRs, while the full-length molecule is necessary for the slow [Ca2+]i increase mediated by metabotropic CGRP receptors (Giniatullin et al., 1999).
Potentiation of nAChRs by CGRP1–6 and other short-length peptide fragments
Structure–activity studies aimed at identifying the minimal CGRP fragments capable of interacting with nAChRs have revealed further, unexpected effects (Di Angelantonio et al., 2002). In fact, deleting Cys7 from CGRP1–7 generates CGRP1–6 which rapidly and reversibly enhances responses mediated by nAChR activation (Figure 3a). CGRP1–6 is not a partial agonist on nAChRs because it evokes no change in baseline current or input conductance. Its potentiating action is not use-dependent and rapidly manifested even with the first response to nicotine in CGRP1–6 solution, suggesting that this peptide fragment can bind nAChRs in the absence of their agonist. The action of CGRP1–6 is voltage- and agonist-independent, as responses to cytisine or nicotine are equally increased at various membrane potentials (Di Angelantonio et al., 2002). Figure 3b shows that the curve relating the fractional response amplitude to the amount of nicotine is displaced to the left (with unchanged maximum response) in the presence of CGRP1–6. The CGRP1–6 concentration threshold is 0.1 μM and its enhancing action levels off at 50 μM (Nistri & Di Angelantonio, 2002). Thus, this peptide increases the sensitivity of nAChRs to their agonist nicotine without changing the agonist efficacy on them. It is, however, clear that CGRP1–6 cannot modulate responses mediated by muscle-type nicotinic receptors or GABAA receptors (Di Angelantonio et al., 2002).
Figure 3.
Effect of CGRP1–6 and shorter CGRP fragments on nicotine-evoked currents. (a) Submaximal currents induced by a short nicotine pulse (20 ms) are potentiated by 1 μM CGRP1–6 fragment. (b) Dose–Response curve (expressed as pulse duration versus response) for nicotine in control and in the presence of CGRP1–6 (1 μM); the plot in the presence of the peptide is shifted to the left in a parallel manner without altering maximal responses (n=5–12). Currents are normalized with respect to response to 20 ms pulse in control solution and fitted with the logistic equation. P refers to significant difference between control and CGRP1–6 data indicated by asterisks. (c) Histograms summarizing the action of different fragments of CGRP (all compounds applied at 1 μM concentration) on nicotine-mediated responses. The efficacy of CGRP1–5 is less than the one observed with 1–6 fragments. CGRP1–4 maintains a slight potentiating effect, whereas CGRP1–3 is inactive. For comparison, data corresponding to the antagonism exerted by CGRP and CGRP1–7 are reported. Responses are expressed as ratios of currents (ICGRP) in the presence of CGRP or its fragment with respect to controls. P-values refer to significant differences with respect to control responses. Data are based on results by Di Angelantonio et al. (2002).
Comparison of data in Figure 2d with those in Figure 3b shows that CGRP1–7 and CGRP1–6 have mirror-like actions on nicotinic currents. This finding suggests that a discrete change in the amino-acid sequence, consisting of a single amino-acid deletion, could transform an antagonist into a potentiating substance. This observation outlines the possibility that these fragments interact with similar sites located at a discrete (although yet unidentified) region of nAChRs. In support of this notion, equimolar concentrations of CGRP1–6 and CGRP1–7, coapplied to the same cell, leave nicotine-induced submaximal currents unchanged, while solutions containing mixtures of these peptides in dissimilar concentration evoke facilitation or depression of nicotinic currents depending on the prevailing fragment concentration (Di Angelantonio et al., 2002).
Further reduction in the amino-acid sequence length has been tested to identify the minimal structure for receptor modulation and to outline some structural characteristics of the peptide molecules, which might be exploited with molecular dynamics studies to unveil analogies or differences in spatial conformation.
Deleting one amino acid from the carboxyl end of the CGRP1–6 sequence yields a compound (CGRP1–5) still endowed with potentiating activity on nAChRs (although with reduced potency). Even the CGRP1–4 fragment retains a slight, yet significant potentiation, absent, however, with CGRP1–3 (Figure 3c). Such a potentiation of nicotinic receptors suggests CGRP1–6 and its derivatives to be prototypes of a new class of molecules capable of enhancing responses mediated by nAChRs.
The mechanism of action of CGRP1–6 differs from the one of a typical allosteric potentiator
One possibility is that CGRP1–6 might act as an allosterically potentiating ligand (APL) on nAChRs (Changeux & Edelstein, 1998). When used at submicromolar concentration APLs, like physostigmine, facilitate nicotine-induced responses even if generated by desensitized receptors, while a 10 times higher dose of the same APL depresses nAChR-mediated responses (Maelicke et al., 1997; Maelicke & Albuquerque, 2000). Coapplication of enhancing concentrations of physostigmine and CGRP1–6 leads to linear summation of the individual effects, while CGRP1–6 can partly reverse the depression induced by a large concentration of physostigmine (Di Angelantonio et al., 2002). Thus, CGRP1–6 and physostigmine have pharmacologically different effects on nicotine-induced currents, suggesting functionally distinct sites of action for CGRP1–6 and physostigmine. These data, however, do not preclude that CGRP1–6 might exert a different type of allosteric enhancement as they simply show an action distinct from that of a typical APL.
Structure/function studies of CGRP fragments
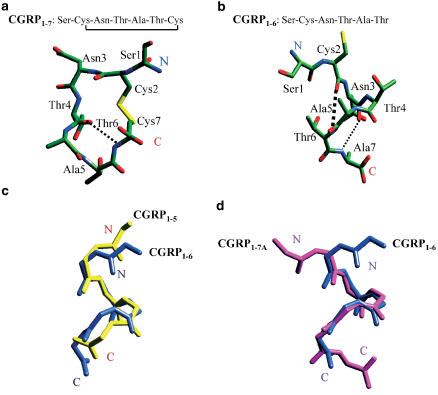
Inspection of the primary structure of the CGRP fragments reveals one major difference between CGRP1–7 and CGRP1–6, namely the presence of a disulphide bridge between Cys2 and Cys7 responsible for the closed ring structure of CGRP1–7. Molecular dynamics simulations indicate that the ring structure of CGRP1–7 (Figure 4a) is stabilized by an inner ring hydrogen bond as confirmed by circular dichroism spectra (Di Angelantonio et al., 2002). This rather rigid structure might be responsible for blocking agonist binding to nAChRs. Conversely, molecular dynamics studies of CGRP1–6 show it to be a flexible molecule with considerable freedom to assume various spatial conformations (Figure 4b). Both CGRP1–6 (blue) and CGRP1–5 (yellow) turn out to preferentially adopt a flexible structure partly with an α-helix conformation (Figure 4c). The helical conformations of CGRP1–6 and CGRP1–5 may be essential for peptide/receptor interaction and are probably responsible for the enhancing action of the agonist on nAChRs, without any direct activity on the agonist binding site. A helical motif has also been shown to be the spatial conformation assumed by the CGRP8–18 fragment (Howitt et al., 2003) responsible for the high-affinity binding of the antagonist CGRP8–37 to the metabotropic CGRP type 1 receptor (Lynch & Kaiser, 1988).
Figure 4.
Molecular dynamics analysis of CGRP fragments. Top: Schematic conformations of CGRP1–7 and CGRP1–6 in an aqueous solution as obtained with molecular dynamics calculations. Hydrogen atoms are not displayed for the sake of clarity. Hydrogen bonds are indicated as dashed lines of different thickness according to the strength of the bond: (a) CGRP1–7; (b) CGRP1–6. Atoms are coloured using the following code: oxygen (red), nitrogen (blue), carbon (green) and sulphide (yellow). Bottom: Spatial alignment of CGRP1–6, 1–5, or 1–7A backbones. (c) Comparison of CGRP1–6 backbone (blue) with the one of CGRP1–5 (yellow). (d) Comparison of CGRP1–6 backbone (blue) with the one of CGRP1–7A (magenta). Data are based on results by Di Angelantonio et al. (2002).
These data raise two possibilities, namely that either the overall amino-acid length or the absence of a disulphide bridge is responsible for the nAChR enhancing activity. This issue can be investigated by testing custom designed peptides with the following characteristics: (1) a six amino-acid peptide CGRP2–7 retaining the rigid ring structure of the disulphide bridge through deletion of Ser1 from CGRP1–7; (2) a seven amino-acid peptide CGRP1–7A analogous to CGRP1–7, except that the terminal Cys7 is replaced by Ala and thus devoid of the disulphide bridge. The conformation of these new compounds assumed after the molecular dynamics simulations is shown in Figure 5a and c. CGRP2–7, as expected, maintains the rigid ring conformation of the CGRP1–7, while the 3D structure of the CGRP1–7A (magenta) is similar to the one of CGRP1–6 (blue; Figure 4d). In particular, they differ only in terms of the conformation assumed by their N-terminal residues that, in the case of CGRP1–6, is constrained to the backbone by the two hydrogen bonds.
Figure 5.
Structure/function studies of CGRP fragments. (a) Schematic conformations of CGRP2–7 in an aqueous solution as obtained with molecular dynamics calculations. Hydrogen atoms are not displayed for the sake of clarity. Hydrogen bonds are indicated as dashed lines of different thicknesses according to the strength of the bond. Atoms are coloured using the following code: oxygen (red), nitrogen (blue), carbon (green) and sulphide (yellow). (b) Current records obtained with 20 ms nicotine (0.1 mM), 15 s after starting pressure application of CGRP2–7 (1 μM) and 45 s after washout of CGRP2–7. Note the reversible reduction in nicotine current amplitude. (c) Schematic conformations of CGRP1–7A in an aqueous solution as obtained with molecular dynamics calculations (for colour legend see a). (d) Example of current (induced by 20 ms nicotine) potentiated by 1 μM CGRP1–7A (preapplied for 15 s). This effect is reversible after peptide washout (right). Data are based on results by Di Angelantonio et al. (2002).
Functional tests confirm the molecular dynamics simulations by indicating that CGRP2–7 behaves like a rapid and reversible blocker of nicotine-evoked responses (Figure 5b), whereas CGRP1–7A behaves like its shorter length counterpart CGRP1–6 in potentiating nAChRs, although with somewhat reduced potency (Figure 5d; Di Angelantonio et al., 2002).
At present, it is difficult to identify endogenous peptides which would share a facilitatory action on nAChRs similar to the one of CGRP1–6. However, the recently discovered Lynx-1 protein that is widely expressed by neurons in the cortex, hippocampus and cerebellum, coexists with α4β2 and α7 receptors and can enhance the activity of nAChRs expressed by Xenopus oocytes (Miwa et al., 1999), although a subsequent report demonstrates that the main action of Lynx-1 is to facilitate nAChR desensitization and recovery from it (Ibanez-Tallon et al., 2002). Thus, the function of Lynx-1 remains to be established.
The results of CGRP derivatives may provide design leads for developing powerful, nonpeptide modulators of nAChRs.
Electrophysiological modulation by substance P of peripheral nAChRs
Most studies dealing with the effects of substance P on nicotinic systems have used adrenal chromaffin and rat pheochromocytoma cells (PC12) cells. In fact, substance P, which belongs to the tachykinin peptide family (Leeman & Ferguson, 2000), has been shown to be colocalized with ACh within the splanchnic nerve terminals in the adrenal gland from which it can be released in response to stress (Livett & Boksa, 1984). The action of substance P on chromaffin cells appears complex. Electrophysiological studies have consistently demonstrated that substance P inhibits membrane currents generated by nAChR activation (Clapham & Neher, 1984; Role, 1984; Boyd, 1987) via a mechanism not mediated by G-protein coupled tachykinin receptors (Stafford et al., 1994). nAChRs containing β4 subunits display the highest sensitivity to substance P-induced block with certain residues present on β subunits responsible for the action of the peptide (Stafford et al., 1998). These data suggest that substance P binds to an allosteric site on the nAChR to generate transient downregulation of nicotinic receptor activity and have prompted a number of investigations into the molecular mechanisms underlying this phenomenon.
Mechanism of substance P action on nAChRs
While CGRP as well as substance P rapidly downregulate nAChR function, it is clear that the depressant action by substance P is phenomenologically different from that of CGRP as shown by an example of rat chromaffin cell nAChRs (see filled squares for control in Figure 6a; details in Khiroug et al., 1998). Repeated test applications of a low dose of nicotine generate stable peak responses, whereas a large dose of the same agonist depresses the response to subsequent test applications of low-dose nicotine (see also Katz & Thesleff, 1957). Subsequent test pulses are used to monitor the time course of nAChR recovery from desensitization. When the same protocol is applied in the presence of 10 μM CGRP (Figure 6a; open triangles), the peak amplitude of currents is depressed by the peptide (cf. Figure 2a and c), yet the extent and time course of the recovery from desensitization is not different from control. Conversely, in the presence of 10 μM substance P (Figure 6a; grey circles), not only is the peak current value more strongly depressed than with CGRP, but also recovery from desensitization is largely delayed. This result illustrates, on the same cell, the clear separation between the effects produced by CGRP or substance P on nicotine-evoked currents.
Figure 6.
Allosteric modulation of substance P on nAChRs. (a) Plot of nicotine-induced peak currents obtained with a protocol consisting of repeated test applications of nicotine before the conditioning (desensitizing; 2 s) dose. After the conditioning pulse, test pulses are resumed at the same rate to monitor the time course of nAChR recovery from desensitization (filled squares). The same protocol is applied in the presence of 10 μM CGRP (open triangles) or 10 μM substance P (grey circles). Reponses to test doses of nicotine are more strongly reduced by CGRP than substance P, while the desensitized current is more intensively depressed by substance P rather than CGRP. The time course of the recovery from desensitization is left unchanged by CGRP and largely prolonged by substance P (R. Giniatullin and E. Sokolova, unpublished). (b) Substance P is not trapped inside nicotinic channels. Control current evoked by nicotine pulses is reduced at steady state by substance P; combination of membrane depolarization to +30 mV (horizontal line) with nicotine pulse does not change the extent of the block tested with repeated application of nicotine (C. Matteoni, unpublished). (c) Trapping of the nicotinic antagonist mecamylamine inside nicotinic channels is revealed by rapid relief of block. Control current evoked by nicotine pulses is reduced at steady state by mecamylamine. After combining depolarization to +30 mV (horizontal line) with nicotine pulse, the subsequent application of nicotine transiently generates a strong inward current, which is then blocked again. Data are based on results by Giniatullin et al. (2000).
Unlike CGRP, substance P binds to the lumen of nicotinic channels and blocks them in a use-dependent fashion (Boyd & Leeman, 1987; Arias, 1998). Such observations suggest that substance P may modulate nAChR desensitization which is a strong characteristic of adrenal chromaffin cell receptors (Marley, 1988; Ochoa et al., 1989). However, another possibility is that substance P induces nicotinic channel block. In fact, in the presence of substance P, there is a large reduction in ACh-evoked current due to increased channel interburst intervals plus decreased channel burst duration and number of channel openings per burst, while single-channel activation, conductance and ion permeation are not changed by substance P (Clapham & Neher, 1984). Thus, the peptide either allosterically binds to the nicotinic receptor and stabilizes it in its desensitized state or induces channel block, which indirectly enhances desensitization (Clapham & Neher, 1984). It is interesting that the action of substance P does not apparently require nAChR activation, thus implying that the peptide can bind agonist-free receptors and convert them into a nonresponsive state (Valenta et al., 1993).
Since Blanton et al. (1994) have shown photo-incorporation of a radioiodinated analogue of substance P into the δ subunit that lines up the channel of Torpedo california AChRs, it is possible that this peptide might work as a ‘trapped blocker' inside the channel from which it could be released during subsequent channel opening coincident with inverted ion flow (Lingle, 1983; Gurney & Rang, 1984). The prototypic agent producing such a transient channel inhibition is the antagonist mecamylamine (Giniatullin et al., 2000), the blocking action of which is temporarily removed whenever the membrane potential is shifted to positive values (so as to generate an outward current) together with nicotine application. One example of this phenomenon is depicted in Figure 6c. When an analogous protocol is applied to the block of nAChRs by substance P, it is apparent that membrane depolarization plus nicotine application cannot transiently reverse the block by substance P (Figure 6b). These data suggest that the mechanism responsible for such a block is different from the typical channel block by mecamylamine. Together with the observation that the blocking action by substance P is voltage independent (Cuevas & Adams, 2000), these findings concur to make it unlikely that the mechanism of action of substance P on nAChRs is to block their open channels. Similar conclusions have also been reached for amphibian ganglion neurons (Akasu et al., 1983).
An indirect component of the action by substance P?
A number of investigations have also considered the possibility that the blocking action by substance P involves intracellular second messengers. In particular, while recording from cell-attached patches of avian ganglion cells, Simmons et al. (1990) have confirmed the facilitation of nAChR desensitization by bath-applied substance P, but have also reported that it must have acted via an intracellular second messenger system, probably requiring the activation of protein kinase C, as the peptide was not applied to the region under the recording pipette (see also Downing & Role, 1987, for the role of protein kinase C in desensitization). This phenomenon has not been corroborated with studies of PC12 cells on which substance P directly facilitates desensitization (Andoh et al., 2001). On rat intracardiac ganglion cells, Cuevas & Adams (2000) have shown that substance P inhibits ACh-activated unitary currents even in the outside-out membrane patches, thus ruling out the contribution by intracellular messengers. Since substance P does not affect binding of ACh to nAChRs (Lukas & Eisenhour, 1996; Weiland et al., 1987), it seems likely that its inhibitory effects are due to a decrease in the number of functional nicotinic channels in accordance with the proposal of facilitation of receptor desensitization.
Biochemical studies of the interaction of substance P with nAChRs
Electrophysiological studies have predominantly addressed the issue of the action of substance P within a relatively short time domain. Biochemical techniques have conversely examined how substance P may influence the release of catecholamines from chromaffin cells either directly or in response to cholinergic agonists. Substance P per se cannot stimulate catecholamine release (Zhou & Livett, 1990; Valenta et al., 1993). Low (nM) concentrations of substance P are reported to enhance the stimulatory action of nicotine on catecholamine release during the first few min and subsequently to inhibit it (Zhou et al., 1991). Conversely, μM concentrations of substance P strongly and consistently inhibit the action of nicotine, although rebound facilitation of release is observed after substance P washout (Zhou et al., 1991). Note that substance P does not change the releasing property of muscarinic agonists, indicating peptide specificity towards nAChR-mediated responses (Zhou et al., 1991; Valenta et al. 1993). When catecholamine release is evoked by electrical field stimulation, substance P is reported to act at various sites: presynaptically to facilitate release of endogenous ACh, and postsynaptically to modulate nAChR desensitization and to protect them from this process over a longer time scale (Zhou & Livett, 1990). These complex data apparently stand against electrophysiological results.
To resolve this discrepancy, Lyford et al. (1990) have used on-line electrochemical detection of catecholamine release to investigate the action of substance P with a more rapid time resolution. They have confirmed that, at short latency after substance P application, there is accelerated desensitization of the release process induced by nicotinic agents. However, over a longer time and after substance P washout, desensitization to nicotinic agonists is much less intense, thus implying that the peptide had ‘protected' receptors from their more profound desensitization (Lyford et al., 1990). This complicated phenomenon probably involves coactivation of two distinct mechanisms, namely tachykinin receptors responsible for protecting nAChRs from desensitization (presumably via intracellular second messengers) and direct interaction of substance P with nAChRs to enhance their desensitization (Khalil et al., 1988). It is noteworthy that studies of 22Na+ influx into PC12 cells have also indicated two phases of nAChR desensitization (Boyd & Leeman, 1987). The first one is a fast process always inhibited by substance P (Stallcup & Patrick, 1980) and possibly corresponds to the phenomenon usually investigated electrophysiologically. The second phase is much slower and actually inhibited by substance P (Boyd & Leeman, 1987). While electrophysiological and imaging studies have indicated that, on rat chromaffin cells, nAChR desensitization does not depend on intracellular second messengers, recovery from desensitization does depend on intracellular protein kinase/phosphatase activity (Khiroug et al., 1998). Hence, it is likely that the recovery from desensitization is the target through which substance P indirectly modulates desensitization of nAChRs responsible for catecholamine release. This process may be species-specific as, unlike mammalian tissues, on chick sympathetic neurons, substance P consistently blocks catecholamine release induced by nicotinic agonists (Valenta et al., 1993).
In conclusion, reconciling biochemical and electrophysiological data is difficult because the two different approaches employ diverse techniques in terms of single versus population responses, fast or slow data sampling, animal species differences, measurements directly related to nAChR occupancy or nonlinearly related to receptor activation (like catecholamine release), etc. Biochemical studies have extended the current understanding of the action by substance P to encompass integrated tissue responses over longer times not usually accessible to high-resolution, yet time constrained, electrophysiological records.
Examples of other endogenous peptides modulating nAChRs
In recent years, several endogenously occurring neuropeptides have been shown to inhibit nAChRs. For the sake of brevity, the present review will not discuss the nAChR blocking action exerted by these substances (listed in Table 1). Evidence in support of their direct effect was obtained with heterologous expression of nAChRs in the absence of native peptide receptors (Herrero et al., 2002; Lioudyno et al., 2002; Grassi et al., 2003), recording from excised membrane patches (Pettit et al., 2001), or insensitivity to G-protein blockers (Oka et al., 1998). Future studies are required to fully understand the molecular mechanism of action of such substances and disclose any structural analogies among them.
Table 1.
List of endogenous peptides with direct inhibitory action on nAChRs
| Peptide | Amino-acid length | Cell type | Type of inhibition | References |
|---|---|---|---|---|
| β-amyloid peptide and peptide A12-28 | 42 | Hippocampal interneurons, α7 nAChRs expressed in oocytes | Noncompetitive | Pettit et al. (2001) |
| 17 | Grassi et al. (2003) | |||
| Catestatin | 15 | Chromaffin cells, nAChRs expressed in oocytes | Noncompetitive, voltage- and use-dependent | Herrero et al. (2002) |
| Proadrenomedullin | 20 | PC12 cells | Noncompetitive | Mahata et al. (1998) |
| Dynorphin A | 17 | PC12 cells | Noncompetitive | Oka et al. (1998) |
| Dynorphin A fragments | PC12 cells | Direct, noncompetitive, voltage-independent | Itoh et al. (2000) | |
| (1–13) | 13 | |||
| (2–13) | 12 | |||
| (1–8) | 8 | |||
| Endomorphin-1 and Dynorphin B | 17 | Hair cells and α9/α10 nAChRs expressed in oocytes | Direct, noncompetitive, voltage-independent | Lioudyno et al. (2002) |
| Thymopentin | 5 | Chromaffin cells | Noncompetitive | Afar et al. (1993) |
Functional implications
The chromaffin cell synaptic organization consists of a single axon innervating clusters of postsynaptic cells (Iijima et al., 1992) from which multiple peaks of miniature currents can be recorded indicating heterogeneity of release sites (Kajiwara et al., 1997) with a potential for transmitter spillover to adjacent sites. The observation of consistent subpopulations of nAChRs at perisynaptic or extrasynaptic areas on cardiac (Wilson Horch & Sargent, 1996) and ciliary (Horch & Sargent, 1995) ganglion cells provides an example of likely membrane targets for such a transmitter spillover at autonomic ganglia, even though the demonstration of analogous sites on chromaffin cells is currently lacking. Electrical stimulation of single afferent axons induces variable postsynaptic currents which are not always associated with action potential generation (Holman et al., 1994). Thus, it seems likely that there are spontaneous fluctuations in the extracellular concentration of ACh, especially because presynaptic cells can fire phasically or tonically (Cassell et al., 1986) with presumably different degrees of activation of synaptic and extrasynaptic receptors. These properties suggest that the strength of synaptic transmission between splanchnic nerve fibre and chromaffin cell is potentially susceptible to pharmacological up- or downregulation. We suspect that activation of nAChRs by ACh released from splanchnic nerve terminals could be modulated differentially by CGRP or substance P, thus leading to significant changes in synaptic efficacy.
Figure 7 is an idealized scheme to summarize the complex actions by CGRP and substance P on chromaffin cells taken as representative of autonomic neurons. In the first instance, CGRP (possibly via endogenous release from chromaffin cells and non-neuronal cells) may block postsynaptic responses to relatively low, ambient concentrations of ACh. In this way, CGRP would help to filter out unnecessary signalling. Like most endogenous peptides, CGRP is expected to be broken down by peptidases. It is, however, postulated that, perhaps under certain conditions of intense and persistent CGRP release, short fragments like CGRP1–6 endowed with nAChR enhancing activity might be generated to counteract any decline in cholinergic neurotransmission. At the same time, CGRP could activate its own metabotropic receptors, leading to [Ca2+]i rise and possibly release of catecholamines and modulation of other cell functions.
Figure 7.
Idealized scheme of neuropeptide modulatory sites at synapses between splanchnic nerve terminals and chromaffin cells. Left: Modulatory action by CGRP and its derivative CGRP1–6. CGRP (purple) can be released by the chromaffin cell itself and/or other nearby cells (not shown). CGRP binds to G-protein coupled receptors (CGRP-R; magenta) to increase intracellular free Ca2+. Furthermore, CGRP directly blocks nAChRs (red; decrease in nAChR function is indicated by a negative sign) activated by release of ACh from presynaptic vesicles (orange). Since CGRP preferentially inhibits small rather than large responses mediated by nAChRs, we postulate that this effect can improve the signal-to-noise ratio at this synapse. We also assume that CGRP might be broken down into shorter peptides like CGRP1–6 with facilitatory action on nAChRs (this phenomenon is indicated by a positive sign). The main consequence of chromaffin cell activation is the release of catecholamines (CA; blue) into the bloodstream. Right: Modulatory action by substance P (SP; green) released by presynaptic terminals. Substance P activates G-protein coupled neurokinin receptors (NKR), which exert a positive modulatory role on nAChRs via intracellular messengers. In addition, substance P directly binds to an allosteric site on nAChRs to enhance desensitization so that ACh-mediated responses are depressed (negative signs). Since large responses are preferentially reduced by this action of substance P, this phenomenon is assumed to be useful in preventing excessive depolarization of the chromaffin cell membrane. Other abbreviations and symbols as in left panel.
The action of substance P might be complementary to (yet distinct from) that of CGRP on cholinergic transmission. It could block nAChRs activated by low ACh concentrations, yet protecting them from desensitization (via an indirect pathway) so as to make them fully available to rapid signalling and catecholamine release. However, larger release of substance P may protect cells from excessive activation of nAChRs via facilitation of their desensitization. The extent of nAChR desensitization in chromaffin cells during physiological activity in vivo is currently unknown, and its role remains, therefore, elusive. Assuming that desensitization in vivo can be as strong as the one demonstrated in vitro, the phenomenon induced by substance P might even be paradigmatic for peptide neuroprotection against Ca2+ overloading (Ochoa et al., 1989), especially in view of the fact that nAChRs are highly permeable to Ca2+ (Vernino et al., 1994; McGehee & Role, 1995) and activation of mutant forms of highly Ca2+ permeable α7 receptors is strongly neurotoxic (Lukas et al., 2001).
Acknowledgments
This work was supported by grants from MIUR (co-fin 2000 and 2002), Regione FVG and INFM.
Abbreviations
- ACh
acetylcholine
- APL
allosterically potentiating ligand
- CGRP
calcitonin gene-related peptide, CGRP1−x, 1 to x N-terminal fragment of calcitonin gene-related peptide, where x is 3, 4, 5, 6, or 7
- CGRP1–7A
N-terminal fragment of calcitonin gene-related peptide in which Cys7 is replaced by Ala
- CGRP2–7
N-terminal fragment of calcitonin gene-related peptide missing Ser1
- F3
N,N,N-trimethyl-1-(4-trans-stilbenoxy)-2-propylammonium iodide
- [Ca2+]i
intracellular free Ca2+ concentration
- GABA
γ-aminobutyric acid
- nAChRs
neuronal nicotinic receptors
- hCGRP8–37
8–37 C-terminal fragment of human calcitonin gene-related peptide
- PC12
rat pheochromocytoma cells
References
- AFAR R., TRIFARO J.M., QUIK M. Modulation of nicotinic acetylcholine receptor function in bovine adrenal chromaffin cells by thymopentin. Brain Res. 1993;606:346–650. doi: 10.1016/0006-8993(93)91006-e. [DOI] [PubMed] [Google Scholar]
- AGNATI L.F., ZOLI M., STROMBERG I., FUXE K. Intercellular communication in the brain: wiring versus volume transmission. Neuroscience. 1995;69:711–726. doi: 10.1016/0306-4522(95)00308-6. [DOI] [PubMed] [Google Scholar]
- AKASU T., KOJIMA M., KOKETSU K. Substance P modulates the sensitivity of the nicotinic receptor in amphibian cholinergic transmission. Br. J. Pharmacol. 1983;80:123–131. doi: 10.1111/j.1476-5381.1983.tb11057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMARA S.G., JONAS V., ROSENFELD M.G., ONG E.S., EVANS R.M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298:240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- ANDOH T., ITOH H., WATANABE I., SASAKI T., HIGASHI T. Mechanisms of modulation of neuronal nicotinic receptors by substance P and OAG. Am. J. Physiol. Cell Physiol. 2001;281:C1871–C1880. doi: 10.1152/ajpcell.2001.281.6.C1871. [DOI] [PubMed] [Google Scholar]
- ARIAS H.R. Noncompetitive inhibition of nicotinic acetylcholine receptors by endogenous molecules. J. Neurosci. Res. 1998;52:369–379. doi: 10.1002/(SICI)1097-4547(19980515)52:4<369::AID-JNR1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- AYAR A., STORER C., TATHAM E.L., SCOTT R.H. The effects of changing intracellular Ca2+ buffering on the excitability of cultured dorsal root ganglion neurones. Neurosci. Lett. 1999;271:171–174. doi: 10.1016/s0304-3940(99)00538-8. [DOI] [PubMed] [Google Scholar]
- BARBOUR B., HAUSSER M. Intersynaptic diffusion of neurotransmitter. Trends Neurosci. 1997;20:377–384. doi: 10.1016/s0166-2236(96)20050-5. [DOI] [PubMed] [Google Scholar]
- BARNARD E.A. The transmitter-gated channels: a range of receptor types and structures. Trends Pharmacol. Sci. 1996;17:305–309. [PubMed] [Google Scholar]
- BELL D., MCDERMOTT B.J. Calcitonin gene-related peptide in the cardiovascular system: characterization of receptor populations and their (patho)physiological significance. Pharmacol. Rev. 1996;48:253–288. [PubMed] [Google Scholar]
- BLANTON M.P., LI Y.M., STIMSON E.R., MAGGIO J.E., COHEN J.B. Agonist-induced photoincorporation of a p-benzoylphenylalanine derivative of substance P into membrane-spanning region 2 of the Torpedo nicotinic acetylcholine receptor delta subunit. Mol. Pharmacol. 1994;46:1048–1055. [PubMed] [Google Scholar]
- BOYD N.D. Two distinct kinetic phases of desensitization of acetylcholine receptors of clonal rat PC12 cells. J. Physiol. 1987;389:45–67. doi: 10.1113/jphysiol.1987.sp016646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD N.D., LEEMAN S.E. Multiple actions of substance P that regulate the functional properties of acetylcholine receptors of clonal rat PC12 cells. J. Physiol. 1987;389:69–97. doi: 10.1113/jphysiol.1987.sp016647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUISSON B., BERTRAND D. Chronic exposure to nicotine upregulates the human α4β2 nicotinic acetylcholine receptor function. J. Neurosci. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPOS-CARO A., SMILLIE F.I., DOMINGUEZ D.T., ROVIRA J.C., VICENTE-AGULLO F., CHAPULI J., JUIZ J.M., SALA S., SALA F., BALLESTA J.J., CRIADO M. Neuronal nicotinic acetylcholine receptors on bovine chromaffin cells: cloning, expression, and genomic organization of receptor subunits. J. Neurochem. 1997;68:488–497. doi: 10.1046/j.1471-4159.1997.68020488.x. [DOI] [PubMed] [Google Scholar]
- CASSELL J.F., CLARK A.L., McLACHLAN E.M. Characteristics of phasic and tonic sympathetic ganglion cells of the guinea-pig. J. Physiol. 1986;372:457–483. doi: 10.1113/jphysiol.1986.sp016020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANGEUX J.P., DUCLERT A., SEKINE S. Calcitonin gene-related peptides and neuromuscular interactions. Ann. N.Y. Acad. Sci. 1992;657:361–378. doi: 10.1111/j.1749-6632.1992.tb22783.x. [DOI] [PubMed] [Google Scholar]
- CHANGEUX J.P., EDELSTEIN S.J. Allosteric receptors after 30 years. Neuron. 1998;21:959–980. doi: 10.1016/s0896-6273(00)80616-9. [DOI] [PubMed] [Google Scholar]
- CLAPHAM D.E., NEHER E. Substance P reduces acetylcholine-induced currents in isolated bovine chromaffin cells. J. Physiol. 1984;347:255–277. doi: 10.1113/jphysiol.1984.sp015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSTA J.J., AVERILL S., CHING Y.P., PRIESTLEY J.V. Immunocytochemical localization of a growth-associated protein (GAP-43) in rat adrenal gland. Cell Tissue Res. 1994;275:555–566. doi: 10.1007/BF00318824. [DOI] [PubMed] [Google Scholar]
- CUEVAS J., ADAMS D.J. Substance P preferentially inhibits large conductance nicotinic ACh receptor channels in rat intracardiac ganglion neurons. J. Neurophysiol. 2000;84:1961–1970. doi: 10.1152/jn.2000.84.4.1961. [DOI] [PubMed] [Google Scholar]
- DI ANGELANTONIO S., COSTA V., CARLONI P., MESSORI L., NISTRI A. A novel class of peptides with facilitating action on neuronal nicotinic receptors of rat chromaffin cells in vitro: functional and molecular dynamics studies. Mol. Pharmacol. 2002;61:43–54. doi: 10.1124/mol.61.1.43. [DOI] [PubMed] [Google Scholar]
- DI ANGELANTONIO S., MATTEONI C., FABBRETTI E., NISTRI A.Molecular biology and electrophysiology of neuronal nicotinic receptors of rat chromaffin cells Eur. J. Neurosci. 2003(in press) [DOI] [PubMed]
- DI ANGELANTONIO S., NISTRI A., MORETTI M., CLEMENTI F., GOTTI C. Antagonism of nicotinic receptors of rat chromaffin cells by N,N,N-trimethyl-1-(4-trans-stilbenoxy)-2-propylammonium iodide: a patch clamp and ligand binding study. Br. J. Pharmacol. 2000;129:1771–1779. doi: 10.1038/sj.bjp.0703264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W.W., KANNO T., SAMPSON S.R. Effects of acetylcholine and other medullary secretagogues and antagonists on the membrane potential of adrenal chromaffin cells: an analysis employing techniques of tissue culture. J. Physiol. 1967;188:107–120. doi: 10.1113/jphysiol.1967.sp008127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W.W., RUBIN R.P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J. Physiol. 1961;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOWNING J.E., ROLE L.W. Activators of protein kinase C enhance acetylcholine receptor desensitization in sympathetic ganglion neurons. Proc. Natl. Acad. Sci. U.S.A. 1987;84:7739–7743. doi: 10.1073/pnas.84.21.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUSEBI F., FARINI D., GRASSI F., MONACO L., RUZZIER F. Effects of calcitonin gene-related peptide on synaptic acetylcholine receptor-channels in rat muscle fibres. Proc. Roy. Soc. London B Biol. Sci. 1988;234:333–342. doi: 10.1098/rspb.1988.0052. [DOI] [PubMed] [Google Scholar]
- FENSTER C.P., WHITWORTH T.L., SHEFFIELD E.B., QUICK M.W., LESTER R.A. Upregulation of surface α4β2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. J. Neurosci. 1999;19:4804–4814. doi: 10.1523/JNEUROSCI.19-12-04804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINIATULLIN R., DI ANGELANTONIO S., MARCHETTI C., SOKOLOVA E., KHIROUG L., NISTRI A. Calcitonin gene-related peptide rapidly downregulates nicotinic receptor function and slowly raises intracellular Ca2+ in rat chromaffin cells in vitro. J. Neurosci. 1999;19:2945–2953. doi: 10.1523/JNEUROSCI.19-08-02945.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINIATULLIN R.A., SOKOLOVA E.M., DI ANGELANTONIO S., SKORINKIN A., TALANTOVA M.V., NISTRI A. Rapid relief of block by mecamylamine of neuronal nicotinic acetylcholine receptors of rat chromaffin cells in vitro: an electrophysiological and modeling study. Mol. Pharmacol. 2000;58:778–787. doi: 10.1124/mol.58.4.778. [DOI] [PubMed] [Google Scholar]
- GOTTI C., FORNASARI D., CLEMENTI F. Human neuronal nicotinic receptors. Prog. Neurobiol. 1997;53:199–237. doi: 10.1016/s0301-0082(97)00034-8. [DOI] [PubMed] [Google Scholar]
- GRASSI F., PALMA E., TONINI R., AMICI M., BALLIVET M., EUSEBI F. Amyloid beta1-42 peptide alters the gating of human and mouse alpha-bungarotoxin-sensitive nicotinic receptors. J. Physiol. 2003;547:147–157. doi: 10.1113/jphysiol.2002.035436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROOT-KORMELINK P.J., LUYTEN W.H., COLQUHOUN D., SIVILOTTI L.G. A reporter mutation approach shows incorporation of the ‘orphan' subunit β3 into a functional nicotinic receptor. J. Biol. Chem. 1998;273:15317–15320. doi: 10.1074/jbc.273.25.15317. [DOI] [PubMed] [Google Scholar]
- GRUTZENDLER J., MORRIS JC. Cholinesterase inhibitors for Alzheimer's disease. Drugs. 2001;61:41–52. doi: 10.2165/00003495-200161010-00005. [DOI] [PubMed] [Google Scholar]
- GURNEY A.M., RANG H.P. The channel-blocking action of methonium compounds on rat submandibular ganglion cells. Br. J. Pharmacol. 1984;82:623–642. doi: 10.1111/j.1476-5381.1984.tb10801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARKNESS P.C., MILLAR N.S. Changes in conformation and subcellular distribution of α4β2 nicotinic acetylcholine receptors revealed by chronic nicotine treatment and expression of subunit chimeras. J. Neurosci. 2002;22:10172–10181. doi: 10.1523/JNEUROSCI.22-23-10172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERRERO CJ, ALES E., PINTADO A.J., LOPEZ M.G., GARCIA-PALOMERO E., MAHATA S.K., O'CONNOR D.T., GARCIA A.G., MONTIEL C. Modulatory mechanism of the endogenous peptide catestatin on neuronal nicotinic acetylcholine receptors and exocytosis. J. Neurosci. 2002;22:377–388. doi: 10.1523/JNEUROSCI.22-02-00377.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEYM C., BRAUN B., KLIMASCHEWSKI L., KUMMER W. Chemical codes of sensory neurons innervating the guinea-pig adrenal gland. Cell Tissue Res. 1995;279:169–181. doi: 10.1007/BF00300702. [DOI] [PubMed] [Google Scholar]
- HOKFELT T. Neuropeptides in perspective: the last ten years. Neuron. 1991;7:867–879. doi: 10.1016/0896-6273(91)90333-u. [DOI] [PubMed] [Google Scholar]
- HOLMAN M.E., COLEMAN H.A., TONTA M.A., PARKINGTON H.C. Synaptic transmission from splanchnic nerves to the adrenal medulla of guinea-pigs. J. Physiol. 1994;478:115–124. doi: 10.1113/jphysiol.1994.sp020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORCH H.L., SARGENT P.B. Perisynaptic surface distribution of multiple classes of nicotinic acetylcholine receptors on neurons in the chicken ciliary ganglion. J. Neurosci. 1995;15:7778–7795. doi: 10.1523/JNEUROSCI.15-12-07778.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWITT S.G., KILK K., WANG Y., SMITH D.M., LANGEL U., POYNER D.R. The role of the 8–18 helix of CGRP8–37 in mediating high affinity binding to CGRP receptors; coulombic and steric interactions. Br. J. Pharmacol. 2003;138:325–332. doi: 10.1038/sj.bjp.0705040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGANIR R.L., GREENGARD P. Regulation of neurotransmitter receptor desensitization by protein phosphorylation. Neuron. 1990;5:555–567. doi: 10.1016/0896-6273(90)90211-w. [DOI] [PubMed] [Google Scholar]
- IBANEZ-TALLON I., MIWA J.M., WANG H.L., ADAMS N.C., CRABTREE G.W., SINE S.M., HEINTZ N. Novel modulation of neuronal nicotinic acetylcholine receptors by association with the endogenous prototoxin lynx1. Neuron. 2002;33:893–903. doi: 10.1016/s0896-6273(02)00632-3. [DOI] [PubMed] [Google Scholar]
- IIJIMA T., MATSUMOTO G., KIDOKORO Y. Synaptic activation of rat adrenal medulla examined with a large photodiode array in combination with a voltage-sensitive dye. Neuroscience. 1992;51:211–219. doi: 10.1016/0306-4522(92)90486-l. [DOI] [PubMed] [Google Scholar]
- ITIER V., BERTRAND D. Neuronal nicotinic receptors: from protein structure to function. FEBS Lett. 2001;504:118–125. doi: 10.1016/s0014-5793(01)02702-8. [DOI] [PubMed] [Google Scholar]
- ITOH H., ANDOH T., WATANABE I., SASAKI T., KAMIYA Y., OKUMURA F. Dynorphins directly inhibit neuronal nicotinic acetylcholine receptors in PC12 cells. Eur. J. Neurosci. 2000;12:1253–1262. doi: 10.1046/j.1460-9568.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- JANSSON A., LIPPOLDT A., MAZEL T., BARTFAI T., OGREN S.O., SYKOVA E., AGNATI L.F., FUXE K. Long distance signalling in volume transmission. Focus on clearance mechanisms. Prog. Brain Res. 2000;125:399–413. doi: 10.1016/S0079-6123(00)25028-0. [DOI] [PubMed] [Google Scholar]
- KAJIWARA R., SAND O., KIDOKORO Y., BARISH M.E., IIJIMA T. Functional organization of chromaffin cells and cholinergic synaptic transmission in rat adrenal medulla. Jpn. J. Physiol. 1997;47:449–464. doi: 10.2170/jjphysiol.47.449. [DOI] [PubMed] [Google Scholar]
- KARLIN A. Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 2002;3:102–114. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the ‘desensitization' produced by acetylcholine at the motor end-plate. J. Physiol. 1957;138:63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHALIL Z., MARLEY P.D., LIVETT B.G. Effect of substance P on nicotine-induced desensitization of cultured bovine adrenal chromaffin cells: possible receptor subtypes. Brain Res. 1988;459:282–288. doi: 10.1016/0006-8993(88)90644-0. [DOI] [PubMed] [Google Scholar]
- KHIROUG L., GINIATULLIN R., SOKOLOVA E., TALANTOVA M., NISTRI A. Imaging of intracellular calcium during desensitization of nicotinic acetylcholine receptors of rat chromaffin cells. Br. J. Pharmacol. 1997;122:1323–1332. doi: 10.1038/sj.bjp.0701518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHIROUG L., SOKOLOVA E., GINIATULLIN R., AFZALOV R., NISTRI A. Recovery from desensitization of neuronal nicotinic acetylcholine receptors of rat chromaffin cells is modulated by intracellular calcium through distinct second messengers. J. Neurosci. 1998;18:2458–2466. doi: 10.1523/JNEUROSCI.18-07-02458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOTTER R. Postsynaptic integration of glutamatergic and dopaminergic signals in the striatum. Prog. Neurobiol. 1994;44:163–196. doi: 10.1016/0301-0082(94)90037-x. [DOI] [PubMed] [Google Scholar]
- KURAMOTO H., KONDO H., FUJITA T. Calcitonin gene-related peptide (CGRP)-like immunoreactivity in scattered chromaffin cells and nerve fibers in the adrenal gland of rats. Cell Tissue Res. 1987;247:309–315. doi: 10.1007/BF00218312. [DOI] [PubMed] [Google Scholar]
- LAWSON S.N., CREPPS B., PERL E.R. Calcitonin gene-related peptide immunoreactivity and afferent receptive properties of dorsal root ganglion neurones in guinea-pigs. J.Physiol. 2002;540:989–1002. doi: 10.1113/jphysiol.2001.013086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LE NOVERE N., CORRINGER P.J., CHANGEUX J.P. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J. Neurobiol. 2002;53:447–456. doi: 10.1002/neu.10153. [DOI] [PubMed] [Google Scholar]
- LEE Y., TAKAMI K., KAWAI Y., GIRGIS S., HILLYARD C.J., MACINTYRE I., EMSON P.C., TOHYAMA M. Distribution of calcitonin gene-related peptide in the rat peripheral nervous system with reference to its coexistence with substance P. Neuroscience. 1985;15:1227–1237. doi: 10.1016/0306-4522(85)90265-9. [DOI] [PubMed] [Google Scholar]
- LEEMAN S.E., FERGUSON S.L. Substance P: an historical perspective. Neuropeptides. 2000;34:249–254. doi: 10.1054/npep.2000.0826. [DOI] [PubMed] [Google Scholar]
- LEVITAN I.B. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu. Rev. Physiol. 1994;56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- LINGLE C. Different types of blockade of crustacean acetylcholine-induced currents. J. Physiol. 1983;339:419–437. doi: 10.1113/jphysiol.1983.sp014724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIOUDYNO M.I., VERBITSKY M., GLOWATZKI E., HOLT J.C., BOULTER J., ZADINA J.E., ELGOYHEN A.B., GUTH P.S. The alpha9/alpha10-containing nicotinic ACh receptor is directly modulated by opioid peptides, endomorphin-1, and dynorphin B, proposed efferent cotransmitters in the inner ear. Mol. Cell. Neurosci. 2002;20:695–711. doi: 10.1006/mcne.2002.1150. [DOI] [PubMed] [Google Scholar]
- LIVETT B.G., BOKSA P. Receptors and receptor modulation in cultured chromaffin cells. Can. J. Physiol. Pharmacol. 1984;62:467–476. doi: 10.1139/y84-076. [DOI] [PubMed] [Google Scholar]
- LU B., FU W.M., GREENGARD P., POO M.M. Calcitonin gene-related peptide potentiates synaptic responses at developing neuromuscular junction. Nature. 1993;363:76–79. doi: 10.1038/363076a0. [DOI] [PubMed] [Google Scholar]
- LUKAS R.J., EISENHOUR C.M. Interactions between tachykinins and diverse, human nicotinic acetylcholine receptor subtypes. Neurochem. Res. 1996;21:1245–1257. doi: 10.1007/BF02532402. [DOI] [PubMed] [Google Scholar]
- LUKAS R.J., LUCERO L., BUISSON B., GALZI J.L., PUCHACZ E., FRYER J.D., CHANGEUX J.P., BERTRAND D. Neurotoxicity of channel mutations in heterologously expressed alpha7-nicotinic acetylcholine receptors. Eur. J. Neurosci. 2001;13:1849–1860. doi: 10.1046/j.0953-816x.2001.01560.x. [DOI] [PubMed] [Google Scholar]
- LYFORD L.K., KENT-BRAUN J.A., WESTHEAD E.W. Substance P enhances desensitization of the nicotinic response in bovine chromaffin cells but enhances secretion upon removal. J. Neurochem. 1990;55:1960–1965. doi: 10.1111/j.1471-4159.1990.tb05782.x. [DOI] [PubMed] [Google Scholar]
- LYNCH B., KAISER E.T. Biological properties of two models of calcitonin gene related peptide with idealized amphiphilic alpha-helices of different lengths. Biochemistry. 1988;27:7600–7607. doi: 10.1021/bi00420a005. [DOI] [PubMed] [Google Scholar]
- MA Q.P., HILL R., SIRINATHSINGHJI D. Colocalization of CGRP with 5-HT1B/1D receptors and substance P in trigeminal ganglion neurons in rats. Eur. J. Neurosci. 2001;13:2099–2104. doi: 10.1046/j.0953-816x.2001.01586.x. [DOI] [PubMed] [Google Scholar]
- MAELICKE A., ALBUQUERQUE E.X. Allosteric modulation of nicotinic acetylcholine receptors as a treatment strategy for Alzheimer's disease. Eur. J. Pharmacol. 2000;393:165–170. doi: 10.1016/s0014-2999(00)00093-5. [DOI] [PubMed] [Google Scholar]
- MAELICKE A., COBAN T., STORCH A., SCHRATTENHOLZ A., PEREIRA E.F., ALBUQUERQUE E.X. Allosteric modulation of Torpedo nicotinic acetylcholine receptor ion channel activity by noncompetitive agonists. J. Receptor Signal Transduction Res. 1997;17:11–28. doi: 10.3109/10799899709036592. [DOI] [PubMed] [Google Scholar]
- MAHATA M., MAHATA S.K., PARMER R.J., O'CONNOR D.T. Proadrenomedullin N-terminal 20 peptide: minimal active region to regulate nicotinic receptors. Hypertension. 1998;32:907–916. doi: 10.1161/01.hyp.32.5.907. [DOI] [PubMed] [Google Scholar]
- MARLEY P.D. Desensitization of the nicotinic secretory response of adrenal chromaffin cells. Trends Pharmacol. Sci. 1988;9:102–107. doi: 10.1016/0165-6147(88)90177-0. [DOI] [PubMed] [Google Scholar]
- MATTEOLI M., HAIMANN C., TORRI-TARELLI F., POLAK J.M., CECCARELLI B., DE CAMILLI P. Differential effect of alpha-latrotoxin on exocytosis from small synaptic vesicles and from large dense-core vesicles containing calcitonin gene-related peptide at the frog neuromuscular junction. Proc. Natl. Acad. Sci. U.S.A. 1988;85:7366–7370. doi: 10.1073/pnas.85.19.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZZOCCHI G., MUSAJO F., NERI G., GOTTARDO G., NUSSDORFER G.G. Adrenomedullin stimulates steroid secretion by the isolated perfused rat adrenal gland in situ: comparison with calcitonin gene-related peptide effects. Peptides. 1996a;17:853–857. doi: 10.1016/0196-9781(96)00109-x. [DOI] [PubMed] [Google Scholar]
- MAZZOCCHI G., REBUFFAT P., GOTTARDO G., NUSSDORFER G.G. Adrenomedullin and calcitonin gene-related peptide inhibit aldosterone secretion in rats, acting via a common receptor. Life Sci. 1996b;58:839–844. doi: 10.1016/0024-3205(96)00017-3. [DOI] [PubMed] [Google Scholar]
- MCGEHEE D.S., ROLE L.W. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu. Rev. Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- MILES K., GREENGARD P., HUGANIR R.L. Calcitonin gene-related peptide regulates phosphorylation of the nicotinic acetylcholine receptor in rat myotubes. Neuron. 1989;2:1517–1524. doi: 10.1016/0896-6273(89)90198-0. [DOI] [PubMed] [Google Scholar]
- MIWA J.M., IBANEZ-TALLON I., CRABTREE G.W., SANCHEZ R., SALI A., ROLE L.W., HEINTZ N. Lynx1, an endogenous toxin-like modulator of nicotinic acetylcholine receptors in the mammalian CNS. Neuron. 1999;23:105–114. doi: 10.1016/s0896-6273(00)80757-6. [DOI] [PubMed] [Google Scholar]
- MULLE C., BENOIT P., PINSET C., ROA M., CHANGEUX J.P. Calcitonin gene-related peptide enhances the rate of desensitization of the nicotinic acetylcholine receptor in cultured mouse muscle cells. Proc. Natl. Acad. Sci. U.S.A. 1988;85:5728–5732. doi: 10.1073/pnas.85.15.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEHER E., STEINBACH J.H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J. Physiol. 1978;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISTRI A., DI ANGELANTONIO S. Enhancement of neuronal nicotinic receptor activity of rat chromaffin cells by a novel class of peptides. Ann. N.Y. Acad. Sci. 2002;971:100–107. doi: 10.1111/j.1749-6632.2002.tb04443.x. [DOI] [PubMed] [Google Scholar]
- OCHOA E.L., CHATTOPADHYAY A., MCNAMEE M.G. Desensitization of the nicotinic acetylcholine receptor: molecular mechanisms and effect of modulators. Cell. Mol. Neurobiol. 1989;9:141–178. doi: 10.1007/BF00713026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKA K., ANDOH T., WATANABE I., KAMIYA Y., ITO H. Inhibition of the neuronal nicotinic receptor-mediated current by kappa opioid receptor agonists in PC12 cells. Pflugers Arch. 1998;436:887–893. doi: 10.1007/s004240050719. [DOI] [PubMed] [Google Scholar]
- OTSUKA M., YOSHIOKA K. Neurotransmitter functions of mammalian tachykinins. Physiol. Rev. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- PATERSON D., NORDBERG A. Neuronal nicotinic receptors in the human brain. Prog. Neurobiol. 2000;61:75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- PEREIRA E.F., HILMAS C., SANTOS M.D., ALKONDON M., MAELICKE A., ALBUQUERQUE E.X. Unconventional ligands and modulators of nicotinic receptors. J. Neurobiol. 2002;53:479–500. doi: 10.1002/neu.10146. [DOI] [PubMed] [Google Scholar]
- PETTIT D.L., SHAO Z., YAKEL J.L. Beta-amyloid(1-42) peptide directly modulates nicotinic receptors in the rat hippocampal slice. J. Neurosci. 2001;21:RC120. doi: 10.1523/JNEUROSCI.21-01-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICCIOTTO M.R., ZOLI M. Nicotinic receptors in aging and dementia. J. Neurobiol. 2002;53:641–655. doi: 10.1002/neu.10102. [DOI] [PubMed] [Google Scholar]
- POYNER D.R., SEXTON P.M., MARSHALL I., SMITH D.M., QUIRION R., BORN W., MUFF R., FISCHER J.A., FOORD S.M. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol. Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- QUICK M.W., LESTER R.A. Desensitization of neuronal nicotinic receptors. J. Neurobiol. 2002;53:457–478. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- QUIRION R., VAN ROSSUM D., DUMONT Y., ST-PIERRE S., FOURNIER A. Characterization of CGRP1 and CGRP2 receptor subtypes. Ann. N.Y. Acad. Sci. 1992;657:88–105. doi: 10.1111/j.1749-6632.1992.tb22759.x. [DOI] [PubMed] [Google Scholar]
- RAGGENBASS M., BERTRAND D. Nicotinic receptors in circuit excitability and epilepsy. J. Neurobiol. 2002;53:580–589. doi: 10.1002/neu.10152. [DOI] [PubMed] [Google Scholar]
- ROLE L.W. Substance P modulation of acetylcholine-induced currents in embryonic chicken sympathetic and ciliary ganglion neurons. Proc. Natl. Acad. Sci. U.S.A. 1984;81:2924–2928. doi: 10.1073/pnas.81.9.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMMONS L.K., SCHUETZE S.M., ROLE L.W. Substance P modulates single-channel properties of neuronal nicotinic acetylcholine receptors. Neuron. 1990;4:393–403. doi: 10.1016/0896-6273(90)90051-g. [DOI] [PubMed] [Google Scholar]
- SINE S.M. The nicotinic receptor ligand binding domain. J. Neurobiol. 2002;53:431–446. doi: 10.1002/neu.10139. [DOI] [PubMed] [Google Scholar]
- SMART T.G. Regulation of excitatory and inhibitory neurotransmitter-gated ion channels by protein phosphorylation. Curr. Opin. Neurobiol. 1997;7:358–367. doi: 10.1016/s0959-4388(97)80063-3. [DOI] [PubMed] [Google Scholar]
- STAFFORD G.A., OSWALD R.E., FIGL A., COHEN B.N., WEILAND G.A. Two domains of the beta subunit of neuronal nicotinic acetylcholine receptors contribute to the affinity of substance P. J. Pharmacol. Exp. Ther. 1998;286:619–626. [PubMed] [Google Scholar]
- STAFFORD G.A., OSWALD R.E., WEILAND G.A. The beta subunit of neuronal nicotinic acetylcholine receptors is a determinant of the affinity for substance P inhibition. Mol. Pharmacol. 1994;45:758–762. [PubMed] [Google Scholar]
- STALLCUP W.B., PATRICK J. Substance P enhances cholinergic receptor desensitization in a clonal nerve cell line. Proc. Natl. Acad. Sci. U.S.A. 1980;77:634–638. doi: 10.1073/pnas.77.1.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALENTA D.C., DOWNING J.E., ROLE L.W. Peptide modulation of ACh receptor desensitization controls neurotransmitter release from chicken sympathetic neurons. J. Neurophysiol. 1993;69:928–942. doi: 10.1152/jn.1993.69.3.928. [DOI] [PubMed] [Google Scholar]
- VERNINO S., ROGERS M., RADCLIFFE K.A., DANI J.A. Quantitative measurement of calcium flux through muscle and neuronal nicotinic acetylcholine receptors. J. Neurosci. 1994;14:5514–5524. doi: 10.1523/JNEUROSCI.14-09-05514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEILAND G.A., DURKIN J.A., HENLEY J.M., SIMASKO S.M. Effects of substance P on the binding of ligands to nicotinic acetylcholine receptors. Mol. Pharmacol. 1987;32:625–632. [PubMed] [Google Scholar]
- WILSON HORCH H.L., SARGENT P.B. Synaptic and extrasynaptic distribution of two distinct populations of nicotinic acetylcholine receptor clusters in the frog cardiac ganglion. J. Neurocytol. 1996;25:67–77. doi: 10.1007/BF02284786. [DOI] [PubMed] [Google Scholar]
- WONNACOTT S. The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Pharmacol. Sci. 1990;11:216–219. doi: 10.1016/0165-6147(90)90242-z. [DOI] [PubMed] [Google Scholar]
- YU C.R., ROLE L.W. Functional contribution of the α5 subunit to neuronal nicotinic channels expressed by chick sympathetic ganglion neurones. J. Physiol. 1998;509:667–681. doi: 10.1111/j.1469-7793.1998.667bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU X.F., LIVETT B.G. Substance P increases catecholamine secretion from perfused rat adrenal glands evoked by prolonged field stimulation. J. Physiol. 1990;425:321–334. doi: 10.1113/jphysiol.1990.sp018105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU X.F., MARLEY P.D., LIVETT B.G. Substance P modulates the time course of nicotinic but not muscarinic catecholamine secretion from perfused adrenal glands of rat. Br. J. Pharmacol. 1991;104:159–165. doi: 10.1111/j.1476-5381.1991.tb12401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOLI M., AGNATI L.F. Wiring and volume transmission in the central nervous system: the concept of closed and open synapses. Prog. Neurobiol. 1996;49:363–380. doi: 10.1016/0301-0082(96)00020-2. [DOI] [PubMed] [Google Scholar]