Abstract
Adhesion of neutrophils (PMNs) to vascular endothelial cells (EC) is a critical step in recruitment and infiltration of leukocytes into tissues during inflammation. Substance P (SP), a neuropeptide released from sensory nerves, evoked PMN adhesion to EC. The NK receptor subtype(s) and the cell type(s) involved were investigated.
SP was coincubated with human PMNs and EC from the human umbilical vein (HUVEC); adhesion was quantitated by computerised microimaging fluorescence analysis.
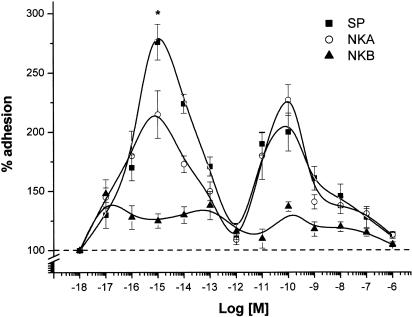
The proadhesive effects of SP (range 10−18–10−6 M) were illustrated in a biphasic dose–response curve, with a maximum at 10−15 M (276±16% adhesion vs control; P<0.01) and another one at 10−10 M (200±18% adhesion vs control; P<0.01). Neurokinin A was less active and neurokinin B was inactive. The adhesion molecules LFA-1 and OKM-1, but not selectins, were involved according to results with selective mAbs.
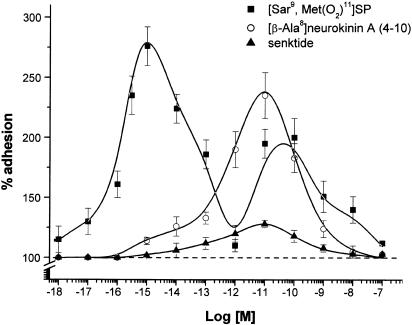
The NK1 agonist [Sar9,Met(O2)11]SP reproduced the effects of SP, whereas the NK2 agonist [βAla8]-neurokininA (4–10) acted at 10−13–10−8 M only. The NK3 agonist, senktide, was ineffective.
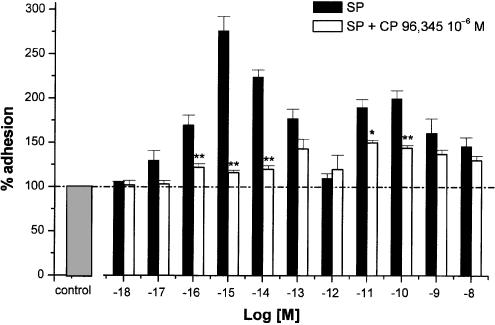
The NK1 antagonists, CP 96,345 and L 703,606 (both 10−6 M), abolished the effect of 10−15 M SP and inhibited that of 10−10 M SP by 56±5% (P<0.01). By comparison, the NK2 antagonist, SR 48,968 (10−7 M), partially antagonised the adhesion evoked by 10−10 M SP (% inhibition: 61±6; P<0.05).
Since preincubation of PMNs and HUVEC with SP gave the same results it is clear that both cell types contributed to its proadhesive effects.
These results indicate that SP induced a proadhesive effect during inflammatory processes, which was mediated by NK1 and NK2 receptors.
Keywords: Substance P; HUVEC; PMNs; neutrophil adhesion; tachykinin; NK1 receptor; NK2 receptor; CP 96,345; SR 48,968
Introduction
The peripheral effects evoked by substance P (SP) and other tachykinins released from unmyelinated sensory nerve endings are collectively referred to as ‘neurogenic inflammation' (Foreman, 1984). SP's effects included arteriolar dilatation, plasma extravasation in postcapillary venules and leukocyte activation and recruitment (Joos & Pawels, 2000; Harrison & Geppetti, 2001). Migration of polymorphonuclear cells (PMNs) into the inflammatory area requires their prior adhesion to the vascular endothelium and extravasion into tissues where they are retained and activated to mediate the inflammatory responses. These, too, involve adhesive interactions (Springer, 1990).
Tachykinins are a family of peptides that share the common C-terminal sequence Phe-X-Gly-Leu-MetNH2. In mammals, they include SP, neurokinin A (NKA) and neurokinin B (NKB), all of which interact with three receptors, namely NK1, NK2 and NK3 (Patacchini & Maggi, 1995). SP is the most selective agonist for NK1, NKA for NK2, and NKB for NK3 receptors (Regoli et al., 1994). An additional receptor, NK4, has been postulated, but has not been characterised (Donaldson et al., 1996; Krause et al., 1997). SP's proinflammatory properties seem to involve NK1 receptor (Walsh et al., 1995). NK1-deficient mice, in fact, display defective neutrophil recruitment into tissues and oedema formation (Cao et al., 2000). Transfection of the human NK1 receptor onto CHO cells shows that it couples to Gq/11, Gαs and Gαo proteins (Roush & Kwatra, 1998), whereas the roles of NK2 and NK3 receptors are not known.
SP's effects in neurogenic inflammation include the triggering of mast cell degranulation (Dianzani & Foreman, 1986) and costimulation of the PMN response to several activation stimuli (Brunelleschi et al., 1991; Sterner-Kock et al., 1999; Dianzani et al., 2001). It also modulates PMN and eosinophil migration by inducing chemotactic activity (Carolan & Casale, 1993). Moreover, it is not clear whether SP targets PMNs or endothelial cells (EC), and the concentrations needed to trigger its effects have not been established. Tominaga et al. (1999) suggested that SP acts by activating protein-kinase C on human umbilical vein endothelial cells (HUVEC) via NK1 receptor in the concentration range 10−10–10−7 M. HUVEC do, in fact, express mRNA for NK1, NK2 and NK3 receptors. Moreover, several types of vascular EC have been reported to predominantly express NK1 receptors. This appears to be partly dependent on cell activation (Greeno et al., 1993; Baluk et al., 1997; Quinlam et al., 1998).
Other authors, however, suggest that SP acts directly on neutrophils at 10−8–10−16 M (De Rose et al., 1994) or 10−10–10−12 M (Zimimerman et al., 1992). Shipp et al. (1991) have shown that 10−7 M SP upmodulates expression of the adhesion molecule LFA-l (CD11/CD18) on human neutrophils, and we have demonstrated that μM doses prime the PMN response to IL-8, probably by acting on NK1 receptors (Dianzani et al., 2001).
The aim of this study was to investigate the effect of tachykinins on PMN adhesion to HUVEC. Their effects on the two cell types are assessed and the role of the NK receptors is determined from their responses to receptor-specific agonists and antagonists. We show that SP promotes PMN adhesion by acting on both PMNs and HUVEC, and that the effect of its low doses is primarily mediated by NK1 receptors, whereas that of high doses also involves NK2 receptors.
Methods
Cell preparation
PMNs were prepared from the citrated blood of healthy male and female volunteers aged 25–45 years by standard dextran sedimentation followed by Histopaque®1077 gradient centrifugation. Residual erythrocytes were removed by hypotonic lysis and PMNs were resuspended in buffered salt solution (BSS) (138 mmol l−1 NaCl, 2.7 mmol l−1 KCl, 8.1 mmol l−1 Na2HPO4, 1.5 mmol l−1 KH2PO4, 1 mmol l−1 MgCl2, 1 mmol l−1 CaCl2, pH 7.4) supplemented with 1 mg ml−1 glucose and 1 mg ml−1 human serum albumin (HSA). Purity of the final cell suspension and cell viability, assessed by the trypan blue-exclusion test, were always >95%. Cell viability was not affected by drug treatment.
HUVEC were isolated from human umbilical veins by trypsin treatment (1%) and cultured in M199 medium with the addition of 20% bovine calf serum (BCS) and 10 ng ml−1 human fibroblast growth factor (FGF). Purity of the HUVEC preparation, evaluated by morphology and immunostaining for factor VIII, was >95%. Contaminant leukocytes were detected by immunostaining for CD45. HUVEC were grown to confluence in flasks and used at the third to fifth passage. Informed consent was obtained from the volunteers.
Adhesion assay: effect of agonists
HUVEC were grown to confluence in 24-well plates, washed, and rested for 1 day in M199 plus 10% BCS without FGF. PMNs (107 cells ml−1) were labelled with fluorescein diacetate (5 μg ml−1) for 30 min at 37°C, washed with BSS, and plated at 106 cells well−1 in a final volume of 0.25 ml BSS on HUVEC treated or not with either SP, or [Sar9,Met(O2)11]SP, or [β-Ala8] neurokinin A-(4–10), or neurokinin A (NKA), or neurokinin B (NKB), or senktide for 10 min. All these agonists were tested in the 10−18–10−6 M range and all concentrations were used concurrently for each stimulus in the same experiment. After incubation, nonadherent PMNs were removed by washing three times with 1 ml BSS. The centre of each well was analysed by fluorescence image analysis. Adherent cells were counted by the Image Pro Plus Software for microimaging (Media Cybernetics, version 4.1 for Windows 98). Single experimental points were assayed in quadruplicate, and standard error mean (s.e.m.) of the four replicates was always lower than 10%. Data are presented as per cent of adhesion, calculate as follows:
where control adhesion was measured in the absence of any treatment of HUVEC, and corresponds to an adhesion of 78±15 cells per microscope fields (n=50).
The direct effect on HUVEC was assessed by preincubating HUVEC with tachykinin for 10 min at 37°C. HUVEC were washed three times to remove tachykinin and used in the adhesion assay with untreated PMNs.
The direct effect on PMNs was assessed in the same way, by preincubating only PMNs with tachykinin for 10 min at 37°C. PMNs were washed and centrifugated three times to remove tachykinins and used in the adhesion assay with untreated HUVEC.
To confirm the data obtained with this method, PMNs were seeded on 24-well plates lacking EC for 10 min at 37°C in the presence of tachykinin. The plates were previously coated with heat-inactivated calf serum for 3 h to reduce spontaneous adhesion to the plastic wells. In the time-course experiments, HUVEC were incubated with SP (10−15 and 10−10 M) and PMNs for 2, 5, 10, 15, 20, 40 min before washing out PMNs.
Adhesion assay: effects of antagonists
HUVEC were pretreated for 15 min (Maggi et al., 1993) with the selective NK1 antagonists (±)CP 96,345 (Sachais et al., 1993) or L-703,606 (Cascieri et al., 1992) (both at 10−6 M), or the NK2 antagonists SR 48,968 (10−7 M), MEN 10,376 (10−6 M), R396 (10−6 M) (Renzetti et al., 1999), and then coincubated with SP (10−18–10−8 M) and PMNs for 10 min.
To antagonise the whole dose-response curve of SP, the antagonists were tested at concentrations that evoked maximum inhibitory effects in other experimental models (Greeno et al., 1993; Warner et al., 1999).
The adhesion inhibition percentage was calculated as follows: [100−(a)/(b)] × 100, where a is the adhesion measured in the presence of tachykinin and NK antagonist minus basal adhesion and b is the adhesion elicited by tachykinin minus basal adhesion.
Adhesion assay: effect of monoclonal antibodies
HUVEC and PMNs were coincubated for 10 min with monoclonal antibodies (mAbs) to LFA-1, OKM-1, L-selectin, P-selectin and E-selectin in the presence of 10−15 M SP. The ability of these mAbs to block adhesion molecules in a selective way has been previously demonstrated (Altieri et al., 1990; Bragardo et al., 1997; Kitayama et al., 1997). UCHL-1 (CD45R0) was tested as a control mAb (Dianzani et al., 1994).
Each mAb was used at the concentration that demonstrated saturation in binding assays or maximal inhibitory effects in adhesion assays (20 μg ml−1) (Zimmerman et al., 1992).
Statistical analysis
Results were expressed as mean±s.e.m.; n indicates the number of experiments. Statistics were obtained by one-way analysis of variance to ascertain whether the differences among the means were significant. The statistical analysis was further implemented by using the Student–Newman–Keuls multiple comparisons post-test to determine significant differences between specific mean pairs. Differences were considered to be statistically significant at a value of P<0.05.
Materials
Dextran T500 was obtained from Pharmacia Biotech (Uppsala, Sweden). Bovine calf serum (BCS, endotoxin tested) was obtained from Hyclone Laboratories Inc. (Logan, UT, U.S.A.). Trypsin was obtained from Difco Laboratories Inc, Detroit, MI, U.S.A. Histopaque®1077, fluorescein diacetate, M199 (endotoxin tested), SP, neurokinin A (NKA) and neurokinin B (NKB) were obtained from Sigma (St Louis, MO, U.S.A.). SP was also purchased from Peninsula (St Helens, Merseyside, U.K.). The selective NK1 agonist [Sar9,Met(O2)11]SP, the selective NK2 agonist [β-ala8] neurokinin A (4–10), the selective NK3 agonist senktide (Suc-Asp-Phe-MePhe-Gly-Leu-Met-NH2), the selective NK1 antagonist L703,606, oxalate salt (cis-2-(diphenylmethyl)-N-[(2-iodophenyl)methyl]-1- azabicyclo[2.2.2]octan-3 amine oxalate) were purchased from RBI/Sigma (Natick, MA, U.S.A.). The selective NK1 antagonist (±)CP 96,345 (2-(diphenylmethyl)-N-[(2- methoxyphenyl)methyl]-1-azabicyclo[2.2.2]octan-3 amine), MEN 10,376 ([Tyr5,D-Trp6,8,9,Lys10] NKA 4-10) and R 396 (Ac-Leu-Asp-Gln-Trp-Phe-Gly-NH2) were kindly supplied by Dr. C.A. Maggi (A. Menarini Pharmaceuticals, Firenze, Italy). The selective NK2 antagonist SR 48,968 [(S)-N-methyl-N-(4-(4-acetylamino-4-phenylpiperidino)-2-(3,4 dichlorophenyl)butyl)benzamide] was a gift from Dr. X. Edmonds-Alt (Sanofi Recherche, Montpellier, France).
Mab LFA-1 (CD11a) was a gift from Prof. U. Dianzani (University of Piemonte Orientale, Novara, Italy). MAb OKM-1 (CD11b) was obtained from American Type Culture Collection (Rockville, MD, U.S.A.); UCHL-1 (CD45R0) was obtained from Dako Igs (Copenhagen, Denmark); anti-human P-selectin, CD62P and anti-human E-selectin, CD62E, were purchased by R&D Systems (Minneapolis, U.S.A.) and anti-human L-selectin, CD62L was purchased by ImmunoKontact (Frankfurt, Germany). All the other reagents and solvents were from Merck (Darmstadt, Germany).
NKB, CP 96,345 and SR 48,968 were dissolved in dimethyl sulphoxide (DMSO); the final concentration of DMSO was not higher than 0.1%. The same amount of DMSO was added to the control samples, and it did not affect the absolute control adhesion. L703,606 were dissolved in methanol; the final concentration of methanol was not higher than 0.1%. The same amount of methanol was added to the control samples, and it did not affect the absolute control adhesion. Stock solutions of tachykinins were prepared daily and diluted in M199 to the appropriate concentrations before each experiment.
Results
Effects of SP on PMN/HUVEC adhesion
The SP effect on PMN adhesion to HUVEC was evaluated by coincubating both cell types for 10 min with SP tested in the 10−18–10−6 M range (Figure 1). The control adhesion was 78±15 cells per microscope field (mean±s.e.m., n=50). The dose–response curve gave two bell-shaped curves: the first in the 10−18–10−12 M range, with the maximum proadhesive effect at 10−15 M (276±16% vs control, mean±s.e.m.), the second in the 10−11–10−8 M range, with the maximum effect at 10−10 M (200±18% vs control, mean±s.e.m.). 10−12 M SP was inactive.
Figure 1.
Effects of SP (▪) compared with those of NKA (○) and NKB (▴) on PMN adhesion to HUVEC. PMNs and HUVEC were incubated with increasing concentrations of tachykinins for 10 min at 37°C. Data are expressed as per cent of adhesion. Statistical analysis established the following significance: P<0.01 vs control for the concentration range of SP 10−16–10−13 and 10−11–10−9 M; P<0.01 vs control for NKA 10−16–10−14 and 10−11–10−10 M; P<0.05 vs control for NKA 10−13 and 10−9 M. Asterisks mark values significantly different from NKA 10−15 M (*P<0.05). Control value corresponds to an absolute adhesion of 78±15 cells/microscope field. Data are expressed as means±s.e.m.; n=10.
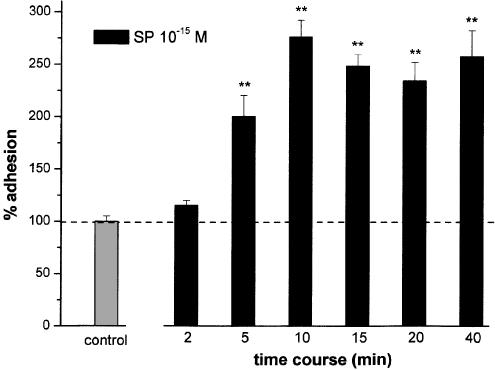
The same response was obtained with SP from two distinct sources (Peninsula and Sigma). Time-course experiments assessing the effect of 10−15 M SP (Figure 2) showed that adhesion induction was clearly detectable after 5 min and reached a plateau at 10 min, which lasted up to 40 min (P<0.01). Prolongation of the kinetics was not possible because of bleaching of the fluorescent dye labelling PMNs. A similar kinetics was obtained using 10−10 M SP (data not shown). The effects evoked by 10 min coincubation of SP were not statistically different from those measured until 40 min. We therefore chose 10 min of incubation for all the experiments.
Figure 2.
Time course- of adhesion effects of SP (10−15 M). PMNs and HUVEC were coincubated for 2–40 min with 10−15 M SP. Data are expressed as means±s.e.m.; n=7. Asterisks mark values significantly different from control (**P<0.01).
Effects of agonists
To detect the receptor(s) involved in SP-induced adhesion, we first compared the effect of SP with that of NKA and NKB, which preferentially bind to different tachykinin receptors.
Figure 1 shows that also NKA, but not NKB, induced PMN adhesion to HUVEC and gave two bell-shaped curves, with maximum effects at 10−15 and 10−10 M, similarly to SP. Quantitative comparison of the adhesion evoked by SP and NKA showed that the effects at 10−15 M were significantly higher for SP (P<0.05 SP vs NKA), whereas there was no significant difference at 10−10 M. Since SP interacts preferentially with NK1 receptors, as does NKA with NK2 receptors, and NKB with NK3 receptors, these data suggested that the effects evoked in the femtomolar range preferentially involved NK1 receptors, while those evoked in the nanomolar range involved both NK1 and NK2 receptors, and NK3 receptors were not involved.
To confirm these data, we assessed the effects of the NK1 receptor agonist [Sar9,Met(O2)11]SP, the NK2 receptor agonist [βAla8]-neurokinin A (4–10) and the NK3 receptor agonist senktide in the 10−18–10−7 M range. Figure 3 shows that [Sar9,Met(O2)11]SP displayed a biphasic dose–response curve, which is never statistically different from that evoked by SP. [βAla8]-neurokinin A (4–10) displayed only one bell-shaped curve, with a maximum effect at 10−11 M. Senktide was always ineffective. These data further confirm those obtained with the natural tachykinins.
Figure 3.
Effects of the three selective NK1, NK2 and NK3 receptor agonists, respectively [Sar9,Met(O2)11]SP (▪), [β-Ala8]neurokinin A (○) and senktide (▴) on PMN adhesion to HUVEC. Incubation time and methods were identical to those selected for SP. Data are expressed as means±s.e.m.; n=7. Statistical analysis established the following significance: P<0.01 vs control for the concentration range of [Sar9,Met(O2)11]SP 10−16–10−13 and 10−11–10−9 M; P<0.01 vs control for [β-Ala8]neurokinin A 10−12–10−10 M.
Effects of antagonists
Involvement of NK1 and NK2 receptors was further evaluated by assessing the effects displayed on SP-induced PMN/HUVEC adhesion by the nonpeptide antagonists CP 96,345 and SR 48,968. These are specific for human NK1 and NK2 receptors, respectively.
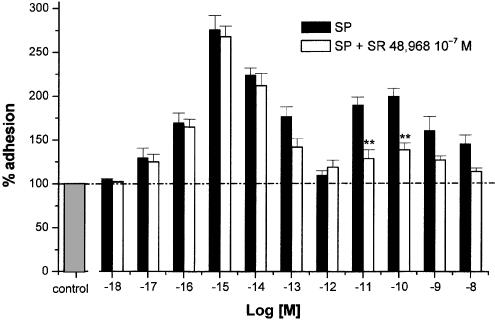
Figure 4 shows that adhesion induced by 10−15 M SP is completely inhibited by CP 96,345 (10−6 M), whereas that evoked by 10−10 M SP is only partially inhibited (% inhibition: 56±5%; P<0.01). When L703,606, another potent nonpeptide human NK1 receptor antagonist, was evaluated at this concentration, the qualitative and quantitative effects of the two antagonists were so close that their respective inhibitory effects could not be differentiated (data not shown). By contrast, SR 48,968(10−7 M) did not inhibit the maximum response induced by 10−15 M SP, but almost halved that induced by 10−10 M SP (% inhibition: 61±6%; P<0.01, Figure 5). Further experiments were performed by incubating PMNs and HUVEC with SP (10−10 M) in the contemporaneous presence of CP 96,345 (10−6 M) and SR 48,968 (10−7 M). The two antagonists caused complete inhibition and thus displayed an additive effect.
Figure 4.
Effect of the NK1 receptor antagonist CP 96,345 (open columns) on the adhesion effects evoked by SP (closed columns). HUVEC were incubated 15 min with the antagonist (10−6 M), then exposed to PMNs and increasing concentrations of SP (10−18–10−8 M). Data are expressed as means±s.e.m.; n=7. Asterisks mark statistically significant inhibition values (*P<0.05 and **P<0.01).
Figure 5.
Effects of the NK2 receptor antagonist SR 48,968 (open columns) on the adhesion effects evoked by SP (closed columns). HUVEC were incubated 15 min with the antagonist (10−7 M), then exposed to PMNs and to increasing concentrations of SP (10−18–10−8 M). Data are expressed as means±s.e.m.; n=7. Asterisks mark statistically significant inhibition values (**P<0.01).
The biphasic effects evoked by [Sar9, Met(O2)11]SP were completely inhibited by the NK1 receptor antagonists (data not shown).
To confirm the involvement of NK2 receptors, the effects of the NK2 receptor antagonists SR 48,968 (10−7 M), MEN 10,376 (10−6 M), and R 396 (10−6 M) on adhesion induced by NKA (10−10 M) and [[βAla8]-neurokinin A (4–10) (10−11 M) were assessed. Results (not shown) demonstrated that all three antagonists inhibited the adhesion in a rank order similar to that observed by Warner et al. (1999) in a different model: SR 48,968>MEN 10,376>R 396. None of the antagonists affected the basal adhesion of resting PMNs.
Effects of monoclonal antibodies
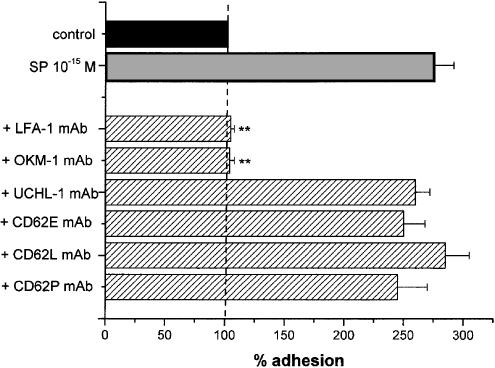
Figure 6 summarises the effects of mAbs that bind selectively different adhesion molecules involved in the interaction between PMNs and ECs (Albelda et al., 1994). The adhesion evoked by 10−15 M SP was significantly inhibited by mAbs against the integrin adhesion molecules, LFA-1 and OKM-1, that have been demonstrated on PMNs (% inhibition: 97±2 and 95±3%; both P<0.01). UCHL-1, which is a mAb directed against a lymphocyte molecule (CD45R0), was used as a control and did not exert any effect in our experimental model, thus supporting the specificity of the observed inhibition. In comparison, no significant inhibitions were noted with mAbs directed against the selectins CD62E, CD62L, CD62P. None of the mAbs affected the basal adhesion of resting PMNs.
Figure 6.
Effect of mAbs (striped columns) on the adhesion effects evoked by SP (grey column). LFA-1 mAb and OKM-1 mAb were selectively directed against neutrophil adhesion molecules, CD62E mAb, CD62L mAb and CD62P mAb against the selectins, UCHL-1 mAb was used as a control. PMNs and HUVEC were coincubated for 10 min with 10−15 M SP and 20 μg ml−1 mAbs. Data are expressed as means±s.e.m.; n=7. Asterisks mark values significantly different from 10−15 M SP (**P<0.01).
Similar results were obtained using 10−10 M SP (data not shown).
Individual treatment of PMNs or HUVEC
To evaluate whether SP acted on PMNs or HUVEC, we evaluated the effect of SP, [Sar9,Met(O2)11]SP, [βA1a8]-neurokinin A (4–10) and senktide in the three experimental systems, previously described. The SP incubation time with only PMNs or HUVEC was always 10 min, in accordance with the coincubation time-course results that demonstrated no statistical differences between 10 and 40 min.
Table 1 shows that all the adhesion patterns are similar to those found in the standard PMN/HUVEC adhesion assay, with no statistical differences. The four basal adhesion values, too, were always similar. To simplify, only the adhesion peaks are included in Table 1 because there were no statistical differences at any point along the 10−18–10−7 M range. It is evident, therefore, that both PMNs and HUVEC contributed to the proadhesive effects of the agonists.
Table 1.
Proadhesive effects of SP and NK-agonists: comparison between different incubation methods
| Coincubation (n=10) | Pretreated HUVEC (n=5) | Pretreated PMNs (n=5) | PMN adhesion to serum-coated plates (n=5) | |
|---|---|---|---|---|
| % adhesion | ||||
| SP 10−15 M | 276±15 | 312±42 | 246±20 | 313±29 |
| [Sar9,Met(O2)11]SP 10−15 M | 260±18 | 260±31 | 225±18 | 290±35 |
| [βAla8]-neurokinin A (4–10) 10–15 M | 110±3 | 120±5 | 108±2 | 112±5 |
| SP 10−10 M | 200±18 | 230±34 | 180±9 | 188±13 |
| [Sar9,Met(O2)11]SP 10−10 M | 200±16 | 217±20 | 176±6 | 216±23 |
| [βAla8]-neurokinin A (4–10) 10–11 M | 235±19 | 280±35 | 205±12 | 270±26 |
Values are expressed as percent adhesion. Data represent the means±s.e.m.
Discussion
The aim of this study was to investigate the effect of tachykinins on PMN adhesion to HUVEC and identify the receptor(s) involved. Dose–response experiments showed that the SP-induced PMN adhesion to HUVEC resulted in a biphasic response, with maxima at femtomolar and at nanomolar concentrations. The magnitude of the adhesion induced was comparable to that we found for other proadhesive agents in the same experimental model (Avanzi et al., 1998). For example, exposure of PMNs and HUVEC to PAF or IL-1β resulted in a two- to three-fold increase over control adhesion, linked to that observed with 10−10 and 10−15 M SP. The proadhesive effect of nanomolar SP concentrations was described by Zimmerman et al. (1992). They did not test the femtomolar concentrations that proved active in our experiments. Femtomolar concentrations stimulated EC differentiation into capillary-like structures in an in vitro model of angiogenesis (Wiedermann et al., 1996). De Rose et al. (1994) showed that 10−16–10−10 M SP increased PMN adhesion to bovine bronchial epithelial cells, with significant effects already detectable at 10−16–10−14 M, and femtomolar concentrations induced the release of neutrophil chemotactic activity in the same cell model (Von Essen et al., 1992). A bell-shaped dose–response curve for SP was previously described by De Rose et al. (1994) and also by Vishwanath & Mukherjee (1996), whose curve was similar to ours, with a maximum effect at 10−10 M and no response at 10−12 M, when the adhesion of EL4 T cell hybridoma, primary T cells and splenocytes to EC was measured.
A biphasic curve at SP concentrations between 10−10 and 10−4 M was reported by Noveral & Grunstein (1995) for airway smooth muscle cell proliferation. These authors used selective receptor agonists and antagonists to demonstrate that this effect was mediated by the NK1 receptor. The response obtained at 10−4 M, however, may involve direct G-protein activation rather than a receptor-mediated effect.
The receptor types involved in SP's proadhesive effects were investigated in our study by using selective NK1, NK2 and NK3 receptor agonists and antagonists. The selective NK3 receptor agonist, senktide, was never effective, in agreement with the negative data obtained with NKB. The selective NK1 receptor agonist [Sar9,Met(O2)11]SP reproduced the entire dose–response curve of SP, displayed the same biphasic pattern and achieved quite close maximum responses. CP 96,345 and L-703,606 antagonised the responses induced by [Sar9,Met(O2)11]SP and by SP, although with a notable difference. Inhibition was complete at both femtomolar and nanomolar concentrations of the selective agonist, but only complete at femtomolar SP concentrations. Addition of the selective NK2 receptor antagonist SR 48,968 caused a full inhibition of the effect of nanomolar SP. Involvement of NK2 receptor was evident from the fact that its selective NK2 receptor agonist [βA1a8]-neurokinin A (4–10) reproduced the response evoked by SP, though not at femtomolar concentrations, unlike [Sar9,Met(O2)11]SP. The selective antagonist SR 48,968 was obviously active in the presence of [βA1a8]-neurokinin A (4–10). In the nanomolar range, [Sar9,Met(O2)11]SP and [βA1a8]-neurokinin A (4–10) achieved effects similar to SP when evaluated individually. Activation of only one receptor type is needed to evoke the same response as SP, which can bind both types. Taken as a whole, these findings indicate that when SP stimulates PMN adhesion to HUVEC it interacts with NK1 receptors only at femtomolar and with both NK1 and NK2 receptors at nanomolar concentrations. The data with NKA agree with this conclusion and the statistical difference at 10−15 M underlie the higher affinity of SP for NK1 receptors.
There is no evident reason why SP induces such a dose–response curve. Comparison with other demonstrations of its proadhesive effects (Vishwanath & Mukherjee, 1996) confirms that 10−12 M SP is a noneffective dose. SP's effects in our study resulted in two bell-shaped dose–response curves. The first (in the femtomolar range) reflects interaction of SP with a NK1 receptor population displaying very high affinity for the ligand and giving the maximum response at 10−15 M. At higher concentrations, the signal and response decline to the 10−12 M no-response level. The second (in the nanomolar range) starts at concentrations higher than 10−12 M. The proadhesive effects in this dose range are apparently attributable to a NK1 receptor population with a lower affinity for SP, together with a NK2 K2 receptors population labelled by selective ligands. SP at 10−10 M was the optimal concentration for evoking PMN adhesion. Higher concentrations induced a decreasing response. Different NK1 receptor isoforms may have been marked by the ligands, but effects on the mechanism of signal transduction cannot be ruled out a priori. NK1 receptors undergo rapid internalisation and desensitisation (Bowden et al., 1994) and interact with different G-proteins (Roush and Kwatra, 1998). In the absence of direct experimental evidence, no conclusions can be reached with regard to this suggestion.
The specific contribution of PMNs and HUVEC on SP-evoked adhesion could not be deduced from these findings, since SP was coincubated with both cell types at the same time. SP was, therefore, incubated with PMNs or HUVEC alone. A 10 min incubation was selected because the response at 10 min was unchanged for up to 40 min in the coincubation model. Individual treatment of both HUVEC and PMNs with SP gave responses whose extent was quite close to that in the co-incubation model. It can thus be assumed that a challenge of either HUVEC or PMNs with SP is sufficient to evoke the full response without additive effects, and the contribution of any effect on the two cell types is the same throughout the time course. The possibility that PMNs are a direct target of the proadhesive effects mediated by tachykinins was strongly supported by the observation that both the femtomolar and the nanomolar concentrations of SP induced proadhesive effects in a HUVEC-free PMN adhesion assay, namely that assessing PMN adhesion to serum-coated wells. Moreover, similar results were obtained in the experiments where PMN were preincubated with SP or with the selective agonists before their seeding on HUVEC.
The observation that HUVEC pretreatment with SP or the agonists induced the same biphasic effect suggested that tachykinins also acted on EC. This would agree with the reports that these cells express NK1 receptor (Greeno et al., 1993). However, another possibility is that tachykinins may have bound to ‘non specific' sites exposed on the HUVEC surface during pretreatment and were not removed by washing. These HUVEC-bound tachykinins could have exerted their effects on PMNs when they were seeded on the HUVEC. This possibility is in line with that reported for chemokines and other chemoattractants whose binding to membrane-bound proteoglycans may present them on the surface of EC to circulating leukocytes (Tanaka et al., 1998; Vaday & Lider, 2000; Fernandez-Botran et al., 2002). A similar mechanism has also been demonstrated for TNF-α, which binds to fibronectin, laminin and collagen in the extracellular matrix, and augmented the integrin-mediated adhesion of leukocytes to fibronectin (Alon et al., 1994). The two bell-shaped dose–response curve of SP may thus be ascribable not only to the recruitment of different tachykinin receptors on PMNs, but also to the involvement of different EC ‘binding' sites. The model would also help to explain the effect of femtomolar SP concentrations, since a high affinity binding of SP to HUVEC binding sites might increase its effectiveness on PMNs.
A prevalent effect on PMNs may explain the rapid kinetics of the SP-induced adhesion already detectable after 5 min. A similar time course in both in vivo and in vitro experiments has been reported by other authors (De Rose et al., 1994; Mancuso et al., 1995; Mantyh et al., 1995; Baluk et al., 1995). This rapid kinetics can be attributed to the triggering of inside–out signalling on PMN that increases the avidity of their β2-integrins for the ligands. A similar effect on PMNs displayed by chemokines and other chemoattractants is inhibited by PKC inhibitors and agents able to increase cAMP levels. This effect may involve (1) induction of conformational changes that increase the intrinsic integrin affinity for ligands and (2) induction of integrin aggregation that increases the number of integrin/ligand interactions at the cell–cell interface. According to this model, we performed experiments showing that the SP-induced PMN/HUVEC adhesion was completely blocked by mAbs to the PMN β2-integrins LFA-1 and OKM-1.
Other authors have detected a direct effect of SP on EC and shown that it upregulates their expression of the β2-integrin ligands ICAM-1 and VCAM-1, in a time-dependent manner, probably by acting on the NK1 receptor (Nakagawa et al., 1993,1995; Quinlam et al., 2000). However, this effect was relatively late since it required de novo synthesis of these ligands. It was presumably undetectable in our experiments that only assessed the earlier effects of tachykinins. As Zimmerman et al. (1992) have stated, SP probably does not increase adhesion molecule expression on PMNs or HUVEC in a few minutes. In our system, early modulation (within 5–10 min) of HUVEC adhesion could theoretically be mediated by upmodulation of the expression of P-selectin, which is stored, preformed, inside EC and translocated to the plasma membrane upon activation. This accounts for PMN rolling on vascular EC.
However, involvement of selectins in our system was unlikely, since no substantial inhibition of SP-induced PMN/HUVEC adhesion was found in experiments with anti-selectin mAbs, probably because we used a static and not a dynamic adhesion assay.
In conclusion, this work provides experimental evidence that SP induces an early proadhesive response of PMNs and allows their adhesion to HUVEC. This effect was mainly mediated by NK1 receptors, but NK2 receptors were also recruited when higher SP doses were used. The nerve fibres that innervate lymphoid organs are an important in vivo source of SP (Payan, 1989), but it seems that it can also be secreted by activated EC in a later phase of the inflammatory response (Vishwanath & Mukherjee, 1996; Quinlam et al., 2000). Therefore, SP may be released upon antigenic, mechanical, thermal or chemical stimuli and modulate leukocyte recruitment in both the early and late phase of the inflammation.
Abbreviations
- HUVEC
human umbilical vein endothelial cells
- PMNs
polymorphonuclear neutrophils
References
- ALBELDA S.M., SMITH C.W., WARD P.A. Adhesion molecules and inflammatory injury. FASEB J. 1994;8:504–512. [PubMed] [Google Scholar]
- ALON R., CAHALON L., HERSHKOVIZ R., ELBAZ D., REIZIS B., WALLACH D., AKIYAMA S.K., YAMADA K.M., LIDER O. TNF-alpha binds to the N-terminal domain of fibronectin and augments the beta1-integrin-mediated adhesion of CD4+ T lymphocytes to the glycoprotein. J. Immunol. 1994;152:1304–1313. [PubMed] [Google Scholar]
- ALTIERI D.C., AGBANYO F.R., PLESCIA J., GINSBERG M.H., EDGINGTON T.S., PLOW E.F. A unique recognition site mediates the interaction of fibrinogen with the leukocyte integrin Mac-1 (CD11b/CD18) J. Biol. Chem. 1990;265:12119–12122. [PubMed] [Google Scholar]
- AVANZI G.C., GALLICCHIO M., BOTTAREL F., GAMMAITONI L., CAVALLLONI G., BUONFIGLIO D., BRAGARDO M., BELLOMO G., ALBANO E., FANTOZZI R., GARBARINO G., VARNUM B., AGLIETTA M., SAGLIO G., DIANZANI U., DIANZANI C. GAS6 inhibits granulocyte adhesion to endothelial cells. Blood. 1998;91:2334–2340. [PubMed] [Google Scholar]
- BALUK P., BERTRAND C., GEPPETTI P., MCDONALD D.M., NADEL J.A. NK1 receptors mediate leukocyte adhesion in neurogenic inflammation in the rat trachea. Am. J. Physiol. 1995;268:L263–L269. doi: 10.1152/ajplung.1995.268.2.L263. [DOI] [PubMed] [Google Scholar]
- BALUK P., BOWDEN J.J., LEFEVRE P.M., MCDONALD D.M. Upregulation of substance P receptors in angiogenesis associated with chronic airway inflammation in rats. Am. J. Physiol. 1997;273:L565–L571. doi: 10.1152/ajplung.1997.273.3.L565. [DOI] [PubMed] [Google Scholar]
- BOWDEN J.J., GARLAND A.M., BALUK P., LEFEVRE P., GRADY E.F., VIGNA S.R., BUNNETT N.W., MCDONALD D.M. Direct observation of substance P-induced internalization of neurokinin 1 (NK1) receptors at sites of inflammation. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8964–8968. doi: 10.1073/pnas.91.19.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAGARDO M., BUONFIGLIO D., FEITO M.J., BONISSONI S., REDOGLIA V., ROJO J.M., BALLESTER S., PORTOLES P., GARBARINO G., MALAVASI F., DIANZANI U. Modulation of lymphocyte interaction with endothelium and homing by HIV-1 gp120. J. Immunol. 1997;159:1619–1627. [PubMed] [Google Scholar]
- BRUNELLESCHI S., TARLI S., GIOTTI A., FANTOZZI R. Priming effects of mammalian tachykinins on human neutrophils. Life Sci. 1991;48:PL1–PL5. doi: 10.1016/0024-3205(91)90416-9. [DOI] [PubMed] [Google Scholar]
- CAO T., PINTER E., AL-RASHED S., GERARD N., HOULT J.R., BRAIN S.D. Neurokinin-1 receptor agonists are involved in mediating neutrophil accumulation in the inflamed, but not normal, cutaneous microvasculature: an in vivo study using neurokinin-1 receptor knockout mice. J. Immunol. 2000;164:5424–5429. doi: 10.4049/jimmunol.164.10.5424. [DOI] [PubMed] [Google Scholar]
- CAROLAN E.J., CASALE T.B. Effects of neuropeptides on neutrophil migration through noncellular and endothelial barriers. J. Allergy Clin. Immunol. 1993;92:589–598. doi: 10.1016/0091-6749(93)90083-r. [DOI] [PubMed] [Google Scholar]
- CASCIERI M.A., BER E., FONG T.M., SADOWSKI S., BANSAL A., SWAIN C., SEWARD E., FRANCES B., BURNS D., STRADER C.D. Characterization of the binding of a potent, selective, radioidinated antagonist to the human neurokinin-1 receptor. Mol. Pharmacol. 1992;42:458–463. [PubMed] [Google Scholar]
- DE ROSE V., ROBBINS R.A., SNIDER R.M., SPURZEM J.R., THIELE G.M., RENNARD S.I., RUBINSTEIN I. Substance P increases neutrophil adhesion to bronchial epithelial cells. J. Immunol. 1994;152:1339–1346. [PubMed] [Google Scholar]
- DIANZANI C., FOREMAN J.C. Desensitization of rat peritoneal mast cells to substance P. Agents Actions. 1986;23:214–216. doi: 10.1007/BF02142544. [DOI] [PubMed] [Google Scholar]
- DIANZANI C., LOMBARDI G., COLLINO M., FERRARA C., CASSONE M.C., FANTOZZI R. Priming effects of substance P on calcium changed evoked by interleukin-8 in human neutrophils. J. Leuk. Biol. 2001;69:1013–1018. [PubMed] [Google Scholar]
- DIANZANI U., FUNARO A., DIFRANCO D., GARBARINO G., BRAGARDO M., REDOGLIA V., BUONFIGLIO D., DE MONTE LB., PILERI A., MALAVASI F. Interaction between endothelium and CD4+CD45RA+ lymphocytes. Role of the human CD38 molecule. J. Immunol. 1994;153:952–959. [PubMed] [Google Scholar]
- DONALDSON L.F., HASKELL C.A., HANLEY M.R. Functional characterisation by heterologous expression of a novel cloned tachykinin peptide receptor. Biochem. J. 1996;320:1–5. doi: 10.1042/bj3200001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERNANDEZ-BOTRAN R., GORANTLA V., SUN X.C., REN X.P., PEREZ-ABADIA G., CRESPO F.A., OLIVER R., ORHUN H.I., QUAN E.E., MALDONADO C., RAY M., BARKER J.H. Targeting of glycosaminoglycan–cytokine interactions as a novel therapeutic approach in allotransplantation. Transplantation. 2002;74:623–629. doi: 10.1097/00007890-200209150-00007. [DOI] [PubMed] [Google Scholar]
- FOREMAN C. Neurogenic inflammation. Trends Pharmacol. Sci. 1984;5:116–119. [Google Scholar]
- GREENO E.-W., MANTYH P., VERCELLOTTI G.M., MOLDOW C.F. Functional neurokinin 1 receptors for substance P are expressed by human vascular endothelium. J. Exp. Med. 1993;177:1269–1276. doi: 10.1084/jem.177.5.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON S., GEPPETTI P. Substance P. Int. J. Biochem. Cell Biol. 2001;33:555–576. doi: 10.1016/s1357-2725(01)00031-0. [DOI] [PubMed] [Google Scholar]
- JOOS G.F., PAWELS R.A. Pro-inflammatory effects of substance P: new perspectives for the treatment of airway diseases. Trends Pharmacol. Sci. 2000;21:131–133. doi: 10.1016/s0165-6147(00)01458-9. [DOI] [PubMed] [Google Scholar]
- KITAYAMA J., FUHLBRIGGE R.C., PURI K.D., SPRINGER T.A. P-selectin, L-selectin, and alpha 4 integrin have distinct roles in eosinophil tethering and arrest on vascular endothelial cell under physiological flow conditions. J. Immunol. 1997;159:3929–3939. [PubMed] [Google Scholar]
- KRAUSE J.E., STAVETEIG P.T., MENTZER J.N., SCHMIDT S.K., TUCKER J.B., BRODBECK R.M., BU J.-Y., KARPITSKIY V.V. Functional expression of a novel human neurokinin-3 receptor homolog that binds [3H]senktide and [125I-MePhe7]neurokinin B, and is responsive to tachykinin peptide agonists. Proc. Natl. Acad. Sci. U.S.A. 1997;94:310–315. doi: 10.1073/pnas.94.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGGI C.A., PATACCHINI R., GIULIANI S., GIACHETTI A. In vivo and in vitro pharmacology of SR 48,968, a non-peptide tachykinin NK-2 receptor antagonist. Eur. J. Pharmacol. 1993;234:83–90. doi: 10.1016/0014-2999(93)90709-q. [DOI] [PubMed] [Google Scholar]
- MANCUSO F., FLOWER R.J., PERRETTI M. Leukocyte transmigration, but not rolling or adhesion, is selectively inhibited by dexamethasone in the hamster post-capillary venule. J. lmmunol. 1995;155:377–386. [PubMed] [Google Scholar]
- MANTYH P.W., ALLEN C.J., GHILARDI J.R., ROGERS S.D., MANTYH C.R., LIU H., BASBAUM A.I., VIGNA S.R., MAGGIO J.E. Rapid endocytosis of a G protein-coupled receptor: substance P evoked internalization of its receptor in the rat striatum in vivo. Proc. Natl. Acad. Sci. U.S.A. 1995;92:2622–2626. doi: 10.1073/pnas.92.7.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAGAWA N., IWAMOTO I., YOSHIDA S. Effect of substance P on the expression of an adhesion molecule ICAM-1 in human vascular endothelial cells. Regul. Pept. 1993;46:223–224. doi: 10.1016/0167-0115(93)90040-f. [DOI] [PubMed] [Google Scholar]
- NAKAGAWA N., SANO H., IWAMOTO I. Substance P induces the expression of intercellular adhesion molecule-1 on vascular endothelial cells and enhances neutrophil transendothelial migration. Peptides. 1995;16:721–725. doi: 10.1016/0196-9781(95)00037-k. [DOI] [PubMed] [Google Scholar]
- NOVERAL J.P., GRUNSTEIN M.M. Tachykinin regulation of airway smooth muscle cell proliferation. Am. J. Physiol. 1995;269:L339–L343. doi: 10.1152/ajplung.1995.269.3.L339. [DOI] [PubMed] [Google Scholar]
- PATACCHINI R., MAGGI C.A. Tachykinin receptors and receptor subtypes. Arch. Int. Pharmacodyn. 1995;329:161–184. [PubMed] [Google Scholar]
- PAYAN D.G. Neuropeptides and inflammation: the role of substance P. Annu. Rev. Med. 1989;40:341–352. doi: 10.1146/annurev.me.40.020189.002013. [DOI] [PubMed] [Google Scholar]
- QUINLAM K.L., CAUGHMAN S.W., OLERUD J.E., BUNNETT N.W, ARMSTRONG C.A., ANSEL J.C. Neural regulation of endothelial cell-mediated inflammation. J. Invest. Dermatol. Symp. Proc. 2000;5:74–78. doi: 10.1046/j.1087-0024.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- QUINLAM K.L., SONG I.-S., BUNNETT N.W., LETRAN E., STEINHOFF M., HARTEN B., OLERUD J.E., ARMSTRONG C.A., CAUGHMAN S.W., ANSEL J.C. Neuropeptide regulation of human dermal microvascular endothelial cell ICAM-1 expression and function. Am. J. Physiol. 1998;275:C1580–C1590. doi: 10.1152/ajpcell.1998.275.6.C1580. [DOI] [PubMed] [Google Scholar]
- REGOLI D., BOUDON A., FAUCHERE J.-L. Receptors and antagonists for substance P and related peptides. Pharmacol. Rev. 1994;46:551–599. [PubMed] [Google Scholar]
- RENZETTI A.R., CATALIOTO R.-M., CRISCUOLI M., CUCCHI P., FERRER C., GIOLITTI A., GUELFI M., ROTONDARO L., WARKER F.J., MAGGI C.A. Relevance of aromatic residues in transmembrane segments V to VII for binding of peptide and nonpeptide antagonists to the human tachykinin NK-2 receptor. J. Pharmacol. Exp. Ther. 1999;290:487–495. [PubMed] [Google Scholar]
- ROUSH E.D., KWATRA M.M. Human Substance P receptor expressed in Chinese hamster ovary cells directly activates Gαq/11, Gαs, and Gαo. FEBS Lett. 1998;428:291–294. doi: 10.1016/s0014-5793(98)00553-5. [DOI] [PubMed] [Google Scholar]
- SACHAIS B.S., SNIDER R.M., LOWE J.A., III, KRAUSE J.E. Molecular basis for the species selectivity of the substance P antagonist CP-96,345. J. Biol. Chem. 1993;268:2319–2323. [PubMed] [Google Scholar]
- SHIPP M.A., STEFANO G.B., SWITZER S.N., GRIFFIN J.D., REINHERZ E.L. CD10 (CALLA)/Neutral peptidase 24.11 modulates inflammatory peptide-induced changes in neutrophil morphology, migration, and adhesion proteins and is itself regulated by neutrophil activation. Blood. 1991;78:1834–1841. [PubMed] [Google Scholar]
- SPRINGER T.A. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- STERNER-KOCK A., BRAUN R., VAN DERVILIET A., SCHRENZEL M., MC DONALD R., KABBUR M., VULLIET R., HYDE D. Substance P primes the formation of hydrogen peroxide and nitric oxide in human neutrophils. J. Leukoc. Biol. 1999;65:834–840. doi: 10.1002/jlb.65.6.834. [DOI] [PubMed] [Google Scholar]
- TANAKA Y., KIMATA K., ADAMS D.H., ETO S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-l beta. Nature. 1998;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- TOMINAGA K., HONDA K., AKAHOSHI A., MAKINO Y., KAWARABAYASHI T., TAKANO Y., KAMIYA H. Substance P causes adhesion of neutrophils to endothelial cells via protein kinase C. Biol. Pharm. Bull. 1999;22:1242–1245. doi: 10.1248/bpb.22.1242. [DOI] [PubMed] [Google Scholar]
- VADAY G.G., LIDER O. Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behaviour and inflammation. J. Leuk. Biol. 2000;67:149–159. doi: 10.1002/jlb.67.2.149. [DOI] [PubMed] [Google Scholar]
- VISHWANATH R., MUKHERJEE R. Substance P promotes lymphocyte–endothelial cell adhesion preferentially via LFA-l/ICAM-1 interactions. J. Neuroimmunol. 1996;71:163–171. doi: 10.1016/s0165-5728(96)00143-9. [DOI] [PubMed] [Google Scholar]
- VON ESSEN S.G., RENNARD S.I., O'NEILL D., ERTL R.F., ROBBINS R.A., KOYAMA S., RUBINSTEIN I. Bronchial epithelial cells release neutrophil chemotactic activity in response to tachykinins. Am. J. Physiol. 1992;263:L226–L231. doi: 10.1152/ajplung.1992.263.2.L226. [DOI] [PubMed] [Google Scholar]
- WALSH D.T., WEG V.B., WILLIAMS T.J., NOURSHARGH S. Substance P- induced inflammatory response in guinea-pig skin: the effect of specific NK-1 receptor antagonists and the role of endogenous mediators. Br. J. Pharmacol. 1995;114:1343–1350. doi: 10.1111/j.1476-5381.1995.tb13354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARNER F.J., COMIS A., MILLER R.C., BURCHER E. Characterization of the [125I] neurokinin A binding site in the circular muscle of human colon. Br. J. Pharmacol. 1999;127:1105–1110. doi: 10.1038/sj.bjp.0702648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIEDERMANN C.J., AUER B., SITTE B., REINISCH N., SCHRATZBERGER P., KAHLER C.M. Induction of endothelial cell differentiation into capillary-like structures by substance P. Eur. J. Pharmacol. 1996;298:335–338. doi: 10.1016/0014-2999(95)00818-7. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN B.J., ANDERSON D.C., GRANGER D.N. Neuropeptides promote neutrophil adherence to endothelial cell monolayers. Am. J. Physiol. 1992;263:G678–G682. doi: 10.1152/ajpgi.1992.263.5.G678. [DOI] [PubMed] [Google Scholar]