Abstract
Human airway smooth muscle cells (HASMC) contribute to airway inflammation in asthma by virtue of their capacity to produce several inflammatory mediators including IL-8, GM-CSF and RANTES. The intracellular signal pathway underlying the production of these cytokines in HASMC is not entirely elucidated.
We examined the role of the mitogen-activated protein kinase (MAPK) c-jun N-terminal kinase (JNK) in TNFα- and IL-1β-induced GM-CSF, RANTES and IL-8 production in HASMC by using a novel specific inhibitor for JNK (SP600125).
Confluent HASMC were treated with TNFα or IL-1β (10 ng ml−1) for 24 h in the presence or absence of SP600125 (1–100 μM). JNK activity was determined by a kinase assay. Phosphorylation of JNK, p38 MAPK and ERK was examined by Western blotting. Culture supernatants were assayed for GM-CSF, RANTES and IL-8 content by ELISA.
Maximum TNFα- or IL-1β-induced phosphorylation of JNK in HASMC occurred after 15 min and returned to baseline levels after 4 h. SP600125 inhibited TNFα- and IL-1β-induced JNK activity in HASMC as shown by the reduced phosphorylation of its substrate c-jun. Furthermore, GM-CSF, RANTES and to a lesser extent IL-8 release from HASMC treated with TNFα and IL-1β was inhibited dosedependently by SP600125.
JNK activation is involved in TNFα- and IL-1β-induced GM-CSF, RANTES and IL-8 production from HASMC. JNK may therefore represent a critical pathway for cytokine production in HASMC.
Keywords: c-jun N-terminal kinase, airway smooth muscle cells, cytokines, asthma, airway inflammation, signal transduction
Introduction
Human airway smooth muscle cells, apart from having contractile and proliferative functions, may contribute directly to airway inflammation through their synthetic properties. Airway smooth muscle cells produce a wide array of cytokines including granulocyte–macrophage colony stimulating factor (GM-CSF), RANTES, eotaxin, interleukin (IL)-6 and IL-8 in response to inflammatory mediators (John et al., 1997; 1998; Saunders et al., 1997; Watson et al., 1998; Chung et al., 1999; Hashimoto et al., 2000; Pascual et al., 2001; Shin et al., 2002) and may be important effector cells in causing lung inflammation. Cytokines and chemokines play an integral role in the coordination and persistence of airway inflammation. GM-CSF, for example, stimulates proliferation, maturation and function of haematopoietic cells and prolongs eosinophil survival (Hallsworth et al., 1998). Chemokines such as RANTES and IL-8 contribute to cell accumulation in inflamed airways by enhancing leukocyte migration through the endothelium and extracellular matrix (Adams & Lloyd, 1997). IL-8 is a C-X-C chemokine that attracts neutrophils, eosinophils, and to a lesser extent, T-lymphocytes (Bacon & Camp, 1990; Shute, 1994). RANTES, a member of the C-C chemokine family, acts mainly on eosinophils, but also affects T-cell and monocyte migration (Schall et al., 1990; Kameyoshi et al., 1992). However, the intracellular events that determine airway smooth muscle production of these mediators are only partially understood.
Mitogen-activated protein kinases (MAPK) are a family of serine/threonine kinases that transduce extracellular signals to the nucleus. In mammalian cells, three major groups of MAPK that differ in their substrate specificity have been characterized: extracellular signal-regulated protein kinase (ERK), c-Jun NH2-terminal kinase (JNK) and p38 MAP kinase. Activation of MAPK requires dual phosphorylation on threonine and tyrosine by upstream kinases and occurs in response to diverse stimuli, such as environmental stress (hyperosmotic shock, heat shock, UV irradiation), endotoxins, mitogenic stimuli and proinflammatory cytokines (IL-1β and TNFα). Once activated, MAPKs phosphorylate selected intracellular proteins including transcription factors. The resulting changes in gene expression affect fundamental cellular processes such as proliferation, differentiation, survival and inflammation (for review see: Herlaar & Brown, 1999; Barr & Bogoyevitch, 2001; Dong et al., 2002).
The c-jun N-terminal (JNK) group of MAPK consists of three isoforms, encoded by three different genes, of which the JNK1 and 2 isoforms are widely distributed, while JNK3 is mainly located in neuronal tissue (Martin et al., 1996). JNKs enhance the transcriptional activity of AP-1 by phosphorylation of the AP-1 component c-Jun on serine 63 and serine 73 of its amino-transactivation domain (Hibi et al., 1993). Binding sites for AP-1 exist in the promoter regions of several genes including those encoding for IL-8, RANTES and GM-CSF (Mukaida et al., 1989; Nelson et al., 1993; Ye et al., 1996), suggesting a role for JNK in regulating the expression of these cytokines.
Recent investigations have led to significant insight into the roles of ERK and p38 MAPK in airway smooth muscle function. These studies have been much facilitated by the development of specific MAPK inhibitors and indicate that the production of several proinflammatory cytokines in airway smooth muscle cells depends on p38 MAPK and/or ERK activation. Hallsworth et al. (2001) showed reduced eotaxin, RANTES and GM-CSF release after ERK inhibition, while suppression of p38 MAPK activity led to the inhibition of eotaxin, but enhancement of GM-CSF production. Moreover, IL-6 and IL-8 production in response to inflammatory mediators required ERK and p38 MAPK activation (Hedges et al., 2000). In contrast, the role of JNK in airway smooth muscle synthetic function is poorly understood due to the lack of a selective JNK inhibitor. Involvement of JNK in cytokine production is conceiveable though, since an inhibitor of the mixed lineage kinase (MLK) family (CP-1347), which is located upstream of JNK, reduced RANTES production in human lung epithelial cells (Kujime et al., 2000).
In the present study, we investigated the role of JNK in TNFα- and IL-1β-induced GM-CSF, RANTES and IL-8 production in airway smooth muscle cells. A novel specific JNK inhibitor (SP600125) was used for this purpose. SP600125 is a reversible ATP-competitive inhibitor of JNK that inhibits c-jun phosphorylation and the expression of inflammatory genes such as IL-2, IFNγ and TNFα (Bennett et al., 2001).
Methods
Materials
Tissue culture reagents and drugs were obtained from Sigma (Poole, U.K.). Cell culture plasticware was purchased from Falcon Labware (Becton Dickinson, Oxford, U.K.). Recombinant human IL-β and TNFα and matched antibody pairs for RANTES and IL-8 enzyme-linked immunosorbent assays (ELISA) were purchased from R&D Systems (DuoSet, Abingdon, U.K.). Matched antibody pairs for GM-CSF ELISA and substrate solution were purchased from BD Pharmingen (Oxford, U.K.). Protease inhibitor cocktail was obtained from Roche Diagnostic (Lewes, U.K.). All other chemical reagents were obtained from Sigma (Poole, U.K.).
Isolation and culture of human airway smooth muscle cells
Human airway smooth muscle was obtained from lobar or main bronchus from patients undergoing lung resection for carcinoma of the bronchus. The smooth muscle was dissected out under sterile conditions and placed in culture as described previously (Belvisi et al., 1997). Cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% FCS supplemented with sodium pyruvate (1 mM), L-glutamine (2 mM), nonessential amino acids (1 : 100), penicillin (100 U ml−1)/streptomycin (100 μg ml−1) and amphotericin B (1.5 μg ml−1) in a humidified atmosphere at 37°C in air/CO2 (95 : 5% v/v−1). Immunofluorescence techniques for calponin, smooth muscle α-actin and myosin heavy chain revealed that more than 95% of the cells displayed the characteristics of smooth muscle cells in culture. HASMC at passages 3–7 from 9 different donors were used in the studies described below.
Cell stimulation
Human airway smooth muscle cells were plated at an initial seeding density of 1 × 104 cells cm−2 onto 24-well plates for assessment of cytokine protein release, six-well plates for p-JNK Western blotting and 75-cm2 flasks (7.5 × 105 cells per flask) for JNK kinase assay and for Western blotting for p-ERK and p-p38. Prior to experiments, confluent cells were growth-arrested by FCS deprivation for 24 h in DMEM supplemented with sodium pyruvate (1 mM), L-glutamine (2 mM), nonessential amino acids (1 : 100), penicillin (100 U ml−1)/streptomycin (100 μg ml−1), amphotericin B (1.5 μg ml−1), insulin (1 μM), transferrin (5 μg ml−1), ascorbic acid (100 μM) and bovine serum albumin (0.1%). Cells were stimulated in duplicate in a fresh FCS-free medium containing the cytokines IL-1β or TNF-α (10 ng ml−1) for 24 h. To examine the effect of the JNK-inhibitor, SP600125 (1–100 μM), was added 30 min prior to the addition of IL-1β or TNF-α (10 ng ml−1) and cells were studied 24 h later.
Cell viability
The effect of SP600125 on human airway smooth muscle viability was assessed by the mitochondrial-dependent reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to formazan. Cells grown in 24-well plates were treated as indicated above, washed with PBS and 400 μl MTT solution (1 mg ml−1) was added to each well. After 3 h of incubation at 37°C, the MTT solution was removed and the converted dye was solubilized with dimethyl sulphoxide (DMSO). A sample (100 μl) from each well was then transferred in duplicate to a 96-well microplate and the optical density (OD) was measured using a spectrophotometer set to 550 nm.
Cytokine assay
Cell supernatants were harvested 24 h after stimulation and stored at −70°C until assayed for RANTES, GM-CSF and IL-8. Cytokine levels were determined by using specific sandwich ELISA according to the manufacturers' instructions.
Western immunoblot analysis of JNK, p38 and ERK phosphorylation
Confluent HASMC were serum-deprived for 24 h and then stimulated with 10 ng ml−1 IL-β or TNFα in the presence or absence of SP600125 (10 and 50 μM). At the indicated time points (15 min – 4 h), cells were rinsed with ice-cold PBS containing protease inhibitors (200 μM Na3VO4, 2 mM phenylmethylsulphonyl fluoride) and lysed in radioimmunoprecipitation assay (RIPA) buffer (PBS containing 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulphate (SDS), 1% Igepal, and 200 μM Na3VO4, 1 tablet protease inhibitor cocktail 10 ml−1 buffer). Cells were scraped off the flasks and solubilized by sonication followed by centrifugation (10,000 × g, 4°C, 4 min). Protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad Laboratories, Hertfordshire, U.K.). Lysates were boiled for 10 min and total protein extracts (20 μg per lane) were separated by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) on a 4–12% acrylamide precast gel (Novex, Invitrogen, Paisley, U.K.). The separated proteins were transferred electrophoretically onto a nitrocellulose membrane in transfer buffer (Novex) and the membrane was then blocked with 5% nonfat dry milk in TBS containing 0.1% Tween 20 (TBS-T) for at least 1 h at room temperature. Blots were then incubated overnight at 4°C with a phospho-specific SAPK/JNK, p38 or ERK antibody (New England BioLabs, Hertfordshire, U.K.) in TBS-T containing 1% dried nonfat milk and 1% BSA (p-JNK), 5% dried nonfat milk (p-ERK) or 5% BSA (p-p38) at a 1 : 1000 dilution. The next day, the membrane was washed five times with TBS-T and then incubated for 1 h with a 1 : 4000 dilution of goat anti-mouse (p-JNK) or a 1 : 2000 dilution of goat anti-rabbit (p-p38 and p-ERK) HRP-conjugated secondary antibody in TBS-T containing 5% nonfat dry milk. The membrane was then washed as before and visualized by enhanced chemiluminescence (ECL-solution, Amersham, Buckinghamshire, U.K.). Membranes were reprobed with a mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody (1 : 5000, Biogenesis, Poole, U.K.) in order to show the amount of protein loaded. Signals were quantified by scanning densitometry using software from Ultra-Violet Products (UVP) (Cambridge, U.K.). Densitometric data were normalized for GAPDH values.
JNK assay
The activitiy of JNK was analysed using commercially available kits (SAPK/JNK kinase assay kit, New England BioLabs) according to the manufacturer's instructions. The kit employs an N-terminal c-Jun fusion protein bound to glutathione sepharose beads to selectively pull down JNK from cell lysates. An in vitro kinase reaction in the presence of unlabeled ATP is followed by measuring c-Jun phosphorylation using an antiphospho-specific c-Jun antibody that specifically detects JNK-induced phosphorylation of c-Jun. Briefly, 250 μg of total cell extracts were incubated overnight at 4°C with the c-Jun fusion protein beads. Precipitates were washed twice in the lysis buffer and then twice in the kinase buffer supplied in the kit. The kinase assay was performed by resuspending the samples in kinase buffer containing 100 μM ATP for 30 min at 30°C. The reaction was stopped by the addition of 4 × SDS–PAGE sample buffer and heating to 90°C for 5 min. Samples were separated by SDS–Page on 4–12% acrylamide precast gel (Novex, Invitrogen, Paisley, U.K.), transferred to membranes, and blotted with antiphospho-specific c-Jun antibody. Following incubation with an HRP conjugated anti-rabbit antibody (1 : 4000), membranes were visualized by enhanced chemiluminescence (LumiGLO; KPL Europe, Guildford, U.K.). Membranes were reprobed with a phosphorylation state-independent JNK antibody in order to determine the amount of JNK loaded. Signals were quantified by scanning densitometry using UVP software. Densitometric data were normalized for total JNK values.
Data and statistical analysis
Data are presented as mean±s.e.m. Data were compared using one-way analysis of variance (ANOVA) followed by Bonferroni's t-test post hoc to determine statistical differences. A P-value <0.05 was considered to be significant. SigmaStat software (Jandel Scientific, Germany) was used for statistical analysis.
Results
Inhibition of JNK activity in airway smooth muscle cells by SP600125
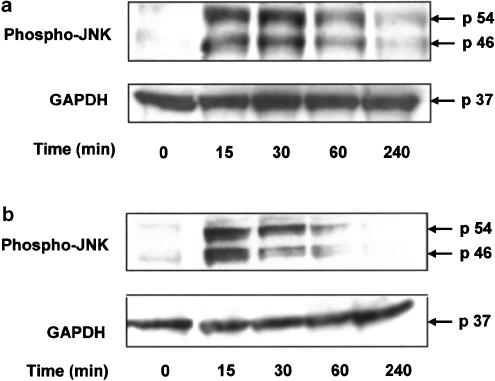
The capacity of TNFα and IL-1β to phosphorylate JNK in airway smooth muscle was first examined. For these experiments, cells were treated with TNFα or IL-1β (10 ng ml−1) followed by measurement of JNK phosphorylation by immunoblotting. After 15 min, TNFα or IL-1β induced marked phosphorylation of JNK in airway smooth muscle cells. Levels of pJNK remained high after 30 min and then returned to baseline levels after 4 h (Figure 1).
Figure 1.
TNFα (10 ng ml−1) (panel a) and IL-1β (10 ng ml−1) (panel b) induce phosphorylation of JNK in human airway smooth muscle cells. Phosphorylation of JNK was detected by Western blotting using a specific antibody to phosphorylated threonine and tyrosine of JNK. A representative example of two identical experiments is shown.
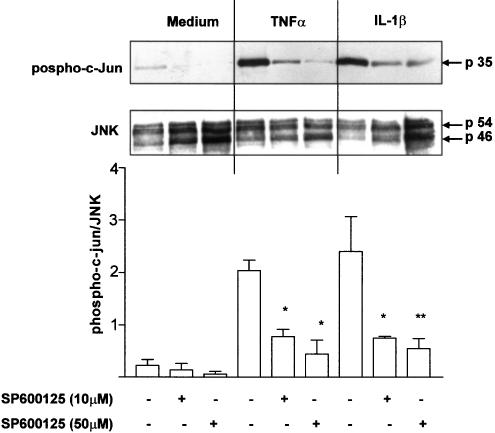
We next examined the effect of the selective JNK inhibitor SP600125 on JNK activity in airway smooth muscle cells. After 30 min pretreatment with SP600125, airway smooth muscle cells were stimulated with TNFα or IL-1β (10 ng ml−1) for another 15 min, when maximum JNK phosphorylation occurs. TNFα and IL-1β enhanced JNK activity as demonstrated by the increased phosphorylation of its substrate c-Jun (Figure 2). Pretreatment with SP600125 (10 and 50 μM) markedly inhibited baseline as well as TNFα- or IL-1β-induced JNK activity in airway smooth muscle cells.
Figure 2.
SP600125 inhibits JNK activity induced by TNFα (10 ng ml−1) or IL-1β (10 ng ml−1) in human airway smooth muscle cells. Phosphorylation of c-Jun was detected by Western blotting using an antiphospho-specific c-Jun antibody. The blot shown in the upper panel was stripped and reprobed using a phosphorylation state-independent JNK antibody to show the amount of JNK loaded. A representative example of three identical experiments is shown. In the lower panel, densitometric analysis of phospho-c-jun expression, normalized by total JNK expression, is shown. Data are expressed as mean±s.e. of the mean (s.e.m.). *P<0.05 compared to cells treated with TNFα or IL-1β in the absence of SP600125.
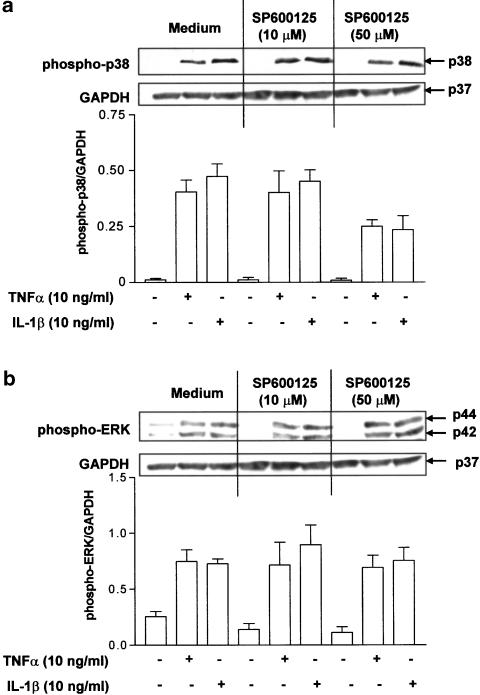
Higher concentrations of SP600125 (50 μM) have previously been reported to inhibit p38 MAPK phosphorylation in a T-cell line (Bennett et al., 2001). Therefore, to exclude the possibility that SP600125 may affect the activity of other related MAPKs in airway smooth muscle cells, the effect of SP600125 on p38 MAPK and ERK phosphorylation was examined. Levels of phosphorylated ERK in airway smooth muscle cell cultures treated with TNFα and IL-1β were not affected by 10 or 50 μM SP600125. Partial inhibition of p38 MAPK phosphorylation by SP600125 in airway smooth muscle cells stimulated with TNFα and IL-1β was observed at 50 μM but not at 10 μM, indicating some effect on p38 MAPK phosphorylation at the high dose of SP600125 (Figure 3).
Figure 3.
Effect of SP600125 on p38 MAPK (panel a) and ERK (panel b) phosphorylation induced by TNFα (10 ng ml−1) or IL-1β (10 ng ml−1). Phosphorylation of p-38 MAPK was detected by Western blotting using a specific antibody to phosphorylated p-38 MAPK. The membrane was stripped and reprobed using a specific antibody to phosphorylated ERK and for GAPDH. A representative example of three identical experiments is shown. The graphs in panels (a) and (b) show the densitometric analysis of phospho-p38 and phospho-pERK expression, normalized by GAPDH expression. Data are expressed as mean±s.e.m.
Effect of SP600125 on RANTES, GM-CSF and IL-8 production from airway smooth muscle cells
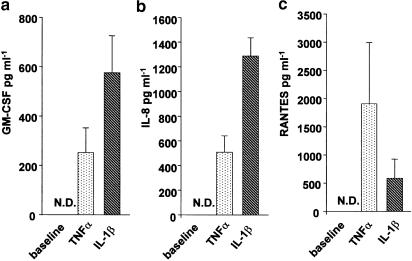
The possible involvement of JNK activation in TNFα- and IL-1β-induced RANTES, GM-CSF and IL-8 release from airway smooth muscle cells was determined by examining the effect of SP600125. After 24 h, release of RANTES, IL-8 and GM-CSF from unstimulated airway smooth muscle cells was not detectable by ELISA. Stimulation with TNFα or IL-1β induced substantial amounts of RANTES, IL-8 and GM-CSF in airway smooth muscle cultures (Figure 4). GM-CSF release in response to TNFα stimulation was below the detection limit of the ELISA in some cell donors. Therefore, only cultures where significant GM-CSF release was detected were used for data analysis. Preincubation for 30 min with SP600125 (1–100 μM) reduced TNFα- or IL-1β-induced GM-CSF, RANTES and IL-8 levels in cell culture supernatants in a dose-dependent manner (Figure 5). Significant inhibition of TNFα-mediated cytokine production occurred at a concentration of 1 μM for GM-CSF, 5 μM for RANTES and at 50 μM for IL-8 release. IL-1β-induced RANTES release from airway smooth muscle cells was significantly reduced by 1 μM SP600125 pretreatment. The production of GM-CSF and IL-8 in response to IL-1β was inhibited by 10 μM SP600125. The maximum inhibition of all cytokines was seen at the highest SP600125 concentration (100 μM). SP600125 was more effective in reducing RANTES and GM-CSF than IL-8.
Figure 4.
TNFα and IL-1β induce the release of GM-CSF (panel a), IL-8 (panel b) and RANTES (panel c) from airway smooth muscle cells. HASMC were left untreated or stimulated with TNFα or IL-1β (10 ng ml−1) for 24 h. Cells from 5–7 donors were used for the experiments. N.D.=not detectable. Data are shown as mean±s.e.m.
Figure 5.
SP600125 inhibits TNFα (panel a) or IL-1β (panel b) induced cytokine release from airway smooth muscle cells. HASMC were pretreated with medium or different concentrations of SP600125 for 30 min and then stimulated with TNFα or IL-1β (10 ng ml−1) for 24 h. Data show the percentage of cytokine levels from cells treated with cytokines in the absence of SP600125 (mean±s.e.m.). Cells from 5–7 donors were used for the experiments. *P<0.05 compared to cytokine levels from cells stimulated with TNFα or IL-1β in the absence of the SP600125.
Cell viability as measured by MTT assays was not affected up to the concentration of SP600125 of 100 μM both in the absence or presence of TNFα or IL-1β (Figure 6).
Figure 6.
Effect of SP600125 on human airway smooth muscle cell viability at baseline (panel a) and after stimulation with TNFα (panel b) or IL-1β (panel c) (10 ng ml−1). Cell viability was assessed after 24 h of treatment by the mitochondrial-dependent reduction of MTT to formazan. Data are shown as optical density (OD) measured at a wavelength of 550 nm (mean±s.e.m.). Cells from two donors were used for the experiment.
Discussion
In the present study, we used a novel selective JNK inhibitor (SP600125) to investigate the role of JNK in signalling proinflammatory mediator-induced cytokine production in human airway smooth muscle. The data indicate that SP600125 is effective in inhibiting JNK activity in these cells and that JNK acitvation is an essential event in IL-1β- and TNFα-mediated GM-CSF, RANTES and, to a lesser extent, IL-8 release from human airway smooth muscle cells.
GM-CSF, RANTES and IL-8 are important mediators of leukocyte migration, proliferation and activation in inflammatory lung diseases. Apart from several inflammatory cells such as macrophages, lymphocytes and eosinophils, airway smooth muscle cells have been identified as important sources of these cytokines (John et al., 1997; Saunders et al., 1997; Watson et al., 1998). In asthma, airway smooth muscle cells represent an important component of the airway wall (Ebina et al., 1993). Therefore, it is of importance to understand the intracellular signal pathway underlying the production of proinflammatory mediators in these cells.
Several lines of evidence suggest that JNK activation may be important in inflammatory and immune responses. JNK polarizes the differentiation of CD4+ T cells to a Th1-type immune response (Dong et al., 1998) by a transcriptional mechanism involving the transcription factor NFATc (Chow et al., 2000). Impaired production of IL-6, IL-12 and IFNγ has been demonstrated in double-negative JNK2 fibroblasts, indicating a potential role for JNK in cytokine production (Chu et al., 1999). Furthermore, an inhibitor of the mixed lineage kinase (MLK) family (CP-1347), which are located upstream of JNK, reduced RANTES production in bronchial epithelial cells (Kujime et al., 2000). The JNK inhibitor (SP600125) we used in our study is an ATP competitive inhibitor of the JNK-1, -2 and -3 isoforms with a selectivity of at least 10-fold for the JNK pathway compared to several related MAP kinases such as ERK and p38 (Bennett et al., 2001). SP600125 reduces AP-1-mediated gene transcription by blocking c-jun phosphorylation. However, c-jun mRNA accumulation and AP-1 DNA binding activity is also affected by SP600125 and contributes to its inhibitory effects on AP-1 function (Han et al., 2001; Shin et al., 2002). In our study, cell pretreatment with 10 μM SP600125 inhibited induced JNK activity in airway smooth muscle cells, as shown by the reduced phosphorylation of its substrate c-jun. Further reduction of TNFα- and IL-1β-induced JNK activity was seen at a concentration of 50 μM.
Inhibition of JNK activity by SP600125 was associated with a dose-dependent decline of IL-1β- and TNFα-induced GM-CSF, RANTES and IL-8 production from airway smooth muscle cells. Significant suppression of this cytokine production was observed at a concentration of 10 μM or less except for the effect on TNFα-induced IL-8 release. This concentration was shown to inhibit JNK activity. As suggested before, the inhibitory effect of SP600125 on cytokine production may not only depend on transcriptional mechanisms but may also involve post-transcriptional modifications, since it was demonstrated that SP600125 decreased mRNA stability (Bennett et al., 2001). In our study, inhibition of IL-1β and TNFα induced GM-CSF and RANTES release by SP600125 was greater than that of IL-8 release. This is in line with previous data (Bennett et al., 2001), showing a weak effect of SP600125 on IL-8 production in monocytes. Although a role of c-jun and AP-1 in IL-8 expression has been demonstrated (Mukaida et al., 1989; Holtmann et al., 1999), these results indicate that JNK is not the major pathway in the induction of IL-8 production from airway smooth muscle. Using inhibitors of the p38 MAPK and ERK pathway, a recent study showed the involvement of these two MAPKs in IL-8 production from airway smooth muscle in response to a cytokine cocktail of IFNγ, TNFα and IL-1β (Hedges et al., 2000). Therefore, ERK and p38 MAPK, and not JNK, may be the predominant pathways in IL-8 production from airway smooth muscle cells.
For induction of cytokine synthesis, we chose the proinflammatory cytokines IL-1β and TNFα since their stimulatory effect on mediator production and JNK activation in airway smooth muscle cells has been previously documented (John et al., 1997; Chung et al., 1999; Hallsworth et al., 2001; McFarlane et al., 2001). Increased JNK activity has also been reported following serum withdrawal in A549 cells and Schwann cells (Huang et al., 2000; Cheng et al., 2001). However, we and others (Hallsworth et al., 2001) detected only a small degree of JNK phosphorylation and activity in airway smooth muscle cells after serum withdrawal at 24 and 96 h, respectively. Therefore, the stress of serum withdrawal did not activate JNK at the time when the airway smooth muscle cells were studied at 24 h.
To test whether SP600125 interfered with the activity of other MAPK, we investigated the effect of this compound on p38 MAPK and ERK phosphorylation. In a previous study, partial inhibition of phospho-p38 MAPK at concentrations of SP600125 greater than 25 μM was shown in a T-cell line (Bennett et al., 2001) and was postulated to be due to the inhibition of MAPK kinase (MKK) 3 and MKK6. In our experiments, SP600125 did not affect IL-1β- and TNFα-induced ERK phosphorylation at a concentration of 10 and 50 μM, while the amount of phosphorylated p38 MAPK was slightly reduced at 50 μM. This indicates that the effects of SP600125 on airway smooth muscle function at a concentration of 50 μM and higher may be the result of a combined inhibition of p38 MAPK and JNK activities. As mentioned earlier, the p38 MAPK pathway contributes to cytokine-induced IL-8 production from airway smooth muscle cells (Hedges et al., 2000). However, the involvement of ERK, but not p38 MAPK, in IL-1β-induced GM-CSF and RANTES release from airway smooth muscle cells was found by Hallsworth et al. (2001). Another study (Maruoka et al., 2000) showed that p38 MAPK and ERK activation is associated with PAF-induced, but not TNFα-induced RANTES production in airway smooth muscle cells, indicating that the involvement of MAPKs in cytokine production depends on the stimulating signal. Therefore, reduced phosphorylation of p38 MAPK may add to the inhibitory effect of SP600125 on IL-8 release at high concentrations (50 μM), but is unlikely to affect IL-1β-induced GM-CSF as well as IL-1β- and TNFα-induced RANTES production from airway smooth muscle cells. Taken together, these studies and our results also indicate that ERK and JNK are the predominant pathways for GM-CSF and RANTES production, whereas all three MAPK contribute to IL-8 release from airway smooth muscle cells.
In summary, we have shown that JNK is an important signalling pathway for GM-CSF, RANTES and IL-8 production from airway smooth muscle cells. In conjunction with recent studies, our results indicate that the production of these cytokines in airway smooth muscle cells depends not only on the activation of one intracellular signalling pathway but also on the combined action of several kinases. Inhibition of MAPK activity may therefore be a potential approach to reduce the burden of inflammatory mediator release from airway smooth muscle cells in inflammatory lung diseases.
Acknowledgments
This work was supported by a Wellcome Trust (U.K.) grant and by a European Respiratory Society Fellowship to Dr. U. Oltmanns. SP600125 was kindly provided by Celgene Inc., San Diego, CA, U.S.A.
Abbreviations
- ANOVA
analysis of variance
- AP-1
activator protein-1
- ATP
adenosine tri-phosphate
- BSA
bovine serum albumin
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethyl sulphoxide
- ECL
enhanced chemiluminescence
- ELISA
enzyme-linked immunosorbent assay
- ERK
extracellular signal-regulated protein kinase
- FCS
fetal calf serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GM-CSF
granulocyte–macrophage colony stimulation factor
- HASMC
human airway smooth muscle cells
- HRP
horseradish peroxidase
- IFNγ
interferon-γ
- IL
interleukin
- JNK
c-jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MKK
MAPK kinase
- MLK
mixed lineage kinase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- OD
optical density
- PAF
platelet acitvated factor
- PBS
phosphate-buffered saline
- RANTES
regulated upon activation, normal T cell expressed and secreted
- RIPA
radioimmunoprecipitation assay
- SDS
sodium dodecyl sulphate
- TBS
Tris-buffered saline
- TNF
tumour necrosis factor
References
- ADAMS D.H., LLOYD A.R. Chemokines: leucocyte recruitment and activation cytokines. Lancet. 1997;349:490–495. doi: 10.1016/s0140-6736(96)07524-1. [DOI] [PubMed] [Google Scholar]
- BACON K.B., CAMP R.D. Interleukin (IL)-8-induced in vitro human lymphocyte migration is inhibited by cholera and pertussis toxins and inhibitors of protein kinase C. Biochem. Biophys. Res. Commun. 1990;169:1099–1104. doi: 10.1016/0006-291x(90)92008-n. [DOI] [PubMed] [Google Scholar]
- BARR R.K., BOGOYEVITCH M.A. The c-Jun N-terminal protein kinase family of mitogen-activated protein kinases (JNK MAPKs) Int. J. Biochem. Cell Biol. 2001;33:1047–1063. doi: 10.1016/s1357-2725(01)00093-0. [DOI] [PubMed] [Google Scholar]
- BELVISI M.G., SAUNDERS M.A., HADDAD E., HIRST S.J., YACOUB M.H., BARNES P.J., MITCHELL J.A. Induction of cyclooxygenase-2 by cytokines in human cultured airway smooth muscle cells: novel inflammatory role of this cell type. Br. J. Pharmacol. 1997;120:910–916. doi: 10.1038/sj.bjp.0700963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENNETT B.L., SASAKI D.T., MURRAY B.W., O'LEARY E.C., SAKATA S.T., XU W., LEISTEN J.C., MOTIWALA A., PIERCE S., SATOH Y., BHAGWAT S.S., MANNING A.M., ANDERSON D.W. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHENG H.L., STEINWAY M.L., XIN X., FELDMAN E.L. Insulin-like growth factor-I and Bcl-X(L) inhibit c-jun N-terminal kinase activation and rescue Schwann cells from apoptosis. J. Neurochem. 2001;76:935–943. doi: 10.1046/j.1471-4159.2001.00110.x. [DOI] [PubMed] [Google Scholar]
- CHOW C.W., DONG C., FLAVELL R.A., DAVIS R.J. c-Jun NH(2)-terminal kinase inhibits targeting of the protein phosphatase calcineurin to NFATc1. Mol. Cell Biol. 2000;20:5227–5234. doi: 10.1128/mcb.20.14.5227-5234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU W.M., OSTERTAG D., LI Z.W., CHANG L., CHEN Y., HU Y., WILLIAMS B., PERRAULT J., KARIN M. JNK2 and IKKbeta are required for activating the innate response to viral infection. Immunity. 1999;11:721–731. doi: 10.1016/s1074-7613(00)80146-6. [DOI] [PubMed] [Google Scholar]
- CHUNG K.F., PATEL H.J., FADLON E.J., ROUSELL J., HADDAD E.B., JOSE P.J., MITCHELL J., BELVISI M. Induction of eotaxin expression and release from human airway smooth muscle cells by IL-1beta and TNFalpha: effects of IL-10 and corticosteroids. Br. J. Pharmacol. 1999;127:1145–1150. doi: 10.1038/sj.bjp.0702660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONG C., DAVIS R.J., FLAVELL R.A. MAP kinases in the immune response. Annu. Rev. Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- DONG C., YANG D.D., WYSK M., WHITMARSH A.J., DAVIS R.J., FLAVELL R.A. Defective T cell differentiation in the absence of Jnk1. Science. 1998;282:2092–2095. doi: 10.1126/science.282.5396.2092. [DOI] [PubMed] [Google Scholar]
- EBINA M., TAKAHASHI T., CHIBA T., MOTOMIYA M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma. A 3-D morphometric study. Am. Rev. Respir. Dis. 1993;148:720–726. doi: 10.1164/ajrccm/148.3.720. [DOI] [PubMed] [Google Scholar]
- HALLSWORTH M.P., MOIR L.M., LAI D., HIRST S.J. Inhibitors of mitogen-activated protein kinases differentially regulate eosinophil-activating cytokine release from human airway smooth muscle. Am. J. Respir. Crit. Care Med. 2001;164:688–697. doi: 10.1164/ajrccm.164.4.2011004. [DOI] [PubMed] [Google Scholar]
- HALLSWORTH M.P., SOH C.P., TWORT C.H., LEE T.H., HIRST S.J. Cultured human airway smooth muscle cells stimulated by interleukin-1beta enhance eosinophil survival. Am. J. Respir. Cell Mol. Biol. 1998;19:910–919. doi: 10.1165/ajrcmb.19.6.3275. [DOI] [PubMed] [Google Scholar]
- HAN Z., BOYLE D.L., CHANG L., BENNETT B., KARIN M., YANG L., MANNING A.M., FIRESTEIN G.S. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J. Clin. Invest. 2001;108:73–81. doi: 10.1172/JCI12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO S., MATSUMOTO K., GON Y., MARUOKA S., KUJIME K., HAYASHI S., TAKESHITA I., HORIE T. p38 MAP kinase regulates TNF alpha-, IL-1 alpha- and PAF-induced RANTES and GM-CSF production by human bronchial epithelial cells. Clin. Exp. Allergy. 2000;30:48–55. doi: 10.1046/j.1365-2222.2000.00641.x. [DOI] [PubMed] [Google Scholar]
- HEDGES J.C., SINGER C.A., GERTHOFFER W.T. Mitogen-activated protein kinases regulate cytokine gene expression in human airway myocytes. Am. J. Respir. Cell Mol. Biol. 2000;23:86–94. doi: 10.1165/ajrcmb.23.1.4014. [DOI] [PubMed] [Google Scholar]
- HERLAAR E., BROWN Z. p38 MAPK signalling cascades in inflammatory disease. Mol. Med. Today. 1999;5:439–447. doi: 10.1016/s1357-4310(99)01544-0. [DOI] [PubMed] [Google Scholar]
- HIBI M., LIN A., SMEAL T., MINDEN A., KARIN M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- HOLTMANN H., WINZEN R., HOLLAND P., EICKEMEIER S., HOFFMANN E., WALLACH D., MALININ N.L., COOPER J.A., RESCH K., KRACHT M. Induction of interleukin-8 synthesis integrates effects on transcription and mRNA degradation from at least three different cytokine- or stress-activated signal transduction pathways. Mol. Cell Biol. 1999;19:6742–6753. doi: 10.1128/mcb.19.10.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG Y., HUTTER D., LIU Y., WANG X., SHEIKH M.S., CHAN A.M., HOLBROOK N.J. Transforming growth factor-beta 1 suppresses serum deprivation-induced death of A549 cells through differential effects on c-Jun and JNK activities. J. Biol. Chem. 2000;275:18234–18242. doi: 10.1074/jbc.M909431199. [DOI] [PubMed] [Google Scholar]
- JOHN M., AU B.T., JOSE P.J., LIM S., SAUNDERS M., BARNES P.J., MITCHELL J.A., BELVISI M.G., CHUNG K.F. Expression and release of interleukin-8 by human airway smooth muscle cells: inhibition by Th-2 cytokines and corticosteroids. Am. J. Respir. Cell Mol. Biol. 1998;18:84–90. doi: 10.1165/ajrcmb.18.1.2813. [DOI] [PubMed] [Google Scholar]
- JOHN M., HIRST S.J., JOSE P.J., ROBICHAUD A., BERKMAN N., WITT C., TWORT C.H., BARNES P.J., CHUNG K.F. Human airway smooth muscle cells express and release RANTES in response to T helper 1 cytokines: regulation by T helper 2 cytokines and corticosteroids. J. Immunol. 1997;158:1841–1847. [PubMed] [Google Scholar]
- KAMEYOSHI Y., DORSCHNER A., MALLET A.I., CHRISTOPHERS E., SCHRODER J.M. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J. Exp. Med. 1992;176:587–592. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUJIME K., HASHIMOTO S., GON Y., SHIMIZU K., HORIE T. p38 mitogen-activated protein kinase and c-jun-NH2-terminal kinase regulate RANTES production by influenza virus-infected human bronchial epithelial cells. J. Immunol. 2000;164:3222–3228. doi: 10.4049/jimmunol.164.6.3222. [DOI] [PubMed] [Google Scholar]
- MARTIN J.H., MOHIT A.A., MILLER C.A. Developmental expression in the mouse nervous system of the p493F12 SAP kinase. Brain Res. Mol. Brain Res. 1996;35:47–57. doi: 10.1016/0169-328x(95)00181-q. [DOI] [PubMed] [Google Scholar]
- MARUOKA S., HASHIMOTO S., GON Y., TAKESHITA I., HORIE T. PAF-induced RANTES production by human airway smooth muscle cells requires both p38 MAP kinase and Erk. Am. J. Respir. Crit. Care Med. 2000;161:922–929. doi: 10.1164/ajrccm.161.3.9906059. [DOI] [PubMed] [Google Scholar]
- MCFARLANE S.M., JUPP O.J., COBBAN H.J., HUNTER I., ANDERSON H.M., VANDENABEELE P., NIXON G.F., MACEWAN D.J. Stimulation of stress-activated but not mitogen-activated protein kinases by tumour necrosis factor receptor subtypes in airway smooth muscle. Biochem. Pharmacol. 2001;61:749–759. doi: 10.1016/s0006-2952(01)00530-5. [DOI] [PubMed] [Google Scholar]
- MUKAIDA N., SHIROO M., MATSUSHIMA K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J. Immunol. 1989;143:1366–1371. [PubMed] [Google Scholar]
- NELSON P.J., KIM H.T., MANNING W.C., GORALSKI T.J., KRENSKY A.M. Genomic organization and transcriptional regulation of the RANTES chemokine gene. J. Immunol. 1993;151:2601–2612. [PubMed] [Google Scholar]
- PASCUAL R.M., BILLINGTON C.K., HALL I.P., PANETTIERI R.A., JR, FISH J.E., PETERS S.P., PENN R.B. Mechanisms of cytokine effects on G protein-coupled receptor-mediated signaling in airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;281:L1425–L1435. doi: 10.1152/ajplung.2001.281.6.L1425. [DOI] [PubMed] [Google Scholar]
- SAUNDERS M.A., MITCHELL J.A., SELDON P.M., YACOUB M.H., BARNES P.J., GIEMBYCZ M.A., BELVISI M.G. Release of granulocyte–macrophage colony stimulating factor by human cultured airway smooth muscle cells: suppression by dexamethasone. Br. J. Pharmacol. 1997;120:545–546. doi: 10.1038/sj.bjp.0700998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHALL T.J., BACON K., TOY K.J., GOEDDEL D.V. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- SHIN M., YAN C., BOYD D. An inhibitor of c-jun aminoterminal kinase (SP600125) represses c-Jun activation, DNA-binding and PMA-inducible 92-kDa type IV collagenase expression. Biochim. Biophys. Acta. 2002;1589:311–316. doi: 10.1016/s0167-4889(02)00195-7. [DOI] [PubMed] [Google Scholar]
- SHUTE J. Interleukin-8 is a potent eosinophil chemo-attractant. Clin. Exp. Allergy. 1994;24:203–206. doi: 10.1111/j.1365-2222.1994.tb00220.x. [DOI] [PubMed] [Google Scholar]
- WATSON M.L., GRIX S.P., JORDAN N.J., PLACE G.A., DODD S., LEITHEAD J., POLL C.T., YOSHIMURA T., WESTWICK J. Interleukin 8 and monocyte chemoattractant protein 1 production by cultured human airway smooth muscle cells. Cytokine. 1998;10:346–352. doi: 10.1006/cyto.1997.0350. [DOI] [PubMed] [Google Scholar]
- YE J., ZHANG X., DONG Z. Characterization of the human granulocyte–macrophage colony-stimulating factor gene promoter: an AP1 complex and an Sp1-related complex transactivate the promoter activity that is suppressed by a YY1 complex. Mol. Cell Biol. 1996;16:157–167. doi: 10.1128/mcb.16.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]