Abstract
The regional haemodynamic effects of the putative nNOS inhibitor, S-methyl-L-thiocitrulline (SMTC), were compared with those of the nonselective NOS inhibitor, NG-nitro-L-arginine methyl ester (L-NAME), in conscious, male Sprague–Dawley rats.
SMTC (0.3 mg kg−1 bolus) produced a significant, short-lived, pressor effect associated with renal, mesenteric and hindquarters vasoconstriction; the same dose of L-NAME did not affect mean blood pressure (BP), although it caused bradycardia and mesenteric vasoconstriction.
At the highest dose tested (10 mg kg−1), L-NAME produced a significantly greater bradycardia and fall in mesenteric vascular conductance than SMTC, although the initial pressor response to SMTC was greater, but less sustained, than that to L-NAME.
Infusion of SMTC or L-NAME (3 mg kg−1 h−1) induced rises in BP and falls in renal, mesenteric and hindquarters vascular conductances, but the effects of L-NAME were greater than those of SMTC, and L-NAME also caused bradycardia.
The renal vasodilator response to acetylcholine was markedly attenuated by infusion of L-NAME, but unaffected by SMTC. The hindquarters vasodilatation induced by salbutamol was attenuated by L-NAME, but not by SMTC. The mesenteric vasodilator response to bradykinin was modestly enhanced by SMTC, but not by L-NAME. The depressor and renal, mesenteric and hindquarters vasodilator responses to sodium nitroprusside were enhanced by L-NAME, whereas SMTC modestly enhanced the hypotensive and renal vasodilator effects of sodium nitroprusside, but attenuated the accompanying tachycardia.
The results are consistent with the cardiovascular effects of low doses of SMTC being attributable to nNOS inhibition.
Keywords: Nitric oxide synthase (NOS) inhibitors, regional haemodynamics, neuronal NOS, endothelial NOS, S-methyl-L-thiocitrulline
Introduction
Nitric oxide (NO) is synthesised as a product of the conversion of its physiological precursor, L-arginine, to L-citrulline, a reaction which is catalysed by a family of enzymes known as the neuronal on synthases (NOS). Three isoforms of NOS have been identified in mammalian tissues, namely, NOS 1 (neuronal NOS, nNOS), NOS 2 (inducible NOS, iNOS) and NOS 3 (endothelial NOS eNOS) (for review see Alderton et al., 2001).
The involvement of NO in cardiovascular regulation was originally described in the context of its production by endothelial cells causing vascular smooth muscle cell relaxation, via activation of soluble guanylyl cyclase and increases in cGMP (Palmer et al., 1988). Subsequent experiments, investigating the possible cardiovascular role(s) for NO in vivo, used nonselective NOS inhibitors such as NG monomethyl-L-arginine (L-NMMA) and NG nitro-L-arginine (L-NAME), and showed NO to be an important signalling molecule in the regulation of regional vascular conductance in conscious animals (Gardiner et al., 1990a, 1990b). As detailed knowledge of different isoforms of NOS was not available at that time, those results were taken to indicate a role for endothelial-derived NO in the control of regional blood flow (Gardiner et al., 1990a, 1990b). However, there is increasing recognition that NO, derived from neuronal sources (under the control of nNOS), may contribute to cardiovascular regulation. Thus, nonadrenergic and noncholinergic, NO-containing, ‘nitroxidergic' perivascular nerves have been identified in many regions, including rat cerebral (Estrada et al., 1993; Toda & Okamura, 1996), mesenteric (Marin & Balfagón 1988; Toda & Okamura, 1992), and hindquarters vasculature (Davisson et al., 1994). NO released from these nerves may act directly on vascular smooth muscle to produce vasodilatation, and/or may modulate the release and/or activity of other neurotransmitters (for review see Esplugues, 2002).
Identifying a role for nNOS-derived NO in cardiovascular regulation in vivo requires the means of effectively manipulating the system, and several pharmacological agents have now been reported to show some (relative) selectivity for nNOS. Thus, S-methyl- L-thiocitrulline (SMTC) has been shown to be 10-fold more potent against nNOS than eNOS, and 17-fold more selective for rat nNOS in neuronal tissue than rat eNOS in vascular endothelium in vitro (Furfine et al., 1994). Pressor effects following acute administration of SMTC have been described in anaesthetised (Narayanan et al., 1995; Komers et al., 2000) and conscious (Gozal et al., 1996) rats, but these findings have been interpreted in different ways – either as indicating a role for nNOS in the control of blood pressure (BP) (Komers et al., 2000), or as an effect of SMTC against eNOS (Narayanan et al., 1995, Gozal et al., 1996). None of the above mentioned studies measured the regional haemodynamic changes associated with the pressor effects of SMTC.
We reasoned that a comparison of the regional haemodynamic responses to a range of doses of SMTC and L-NAME would help to determine whether or not the changes in BP associated with systemic administration of the inhibitors were due to common underlying mechanisms. However, such studies would not allow us to distinguish between SMTC-induced inhibition of eNOS and L-NAME-induced inhibition of nNOS. Therefore, we hypothesised that a comparison of the influence of SMTC and L-NAME on the effects of a range of vasodilators might reveal differences that would help in distinguishing actions due to inhibition of nNOS vs eNOS. Thus, in additional experiments, we assessed the effects of SMTC and L-NAME on responses to acetylcholine, which causes L-NAME-sensitive renal vasodilatation in conscious rats (Gardiner et al., 1991b); bradykinin, which causes mesenteric vasodilatation that is resistant to L-NAME (Randall et al., 1996); salbutamol, which causes L-NAME-sensitive, β2-adrenoceptor-mediated, hindquarters vasodilatation (Gardiner et al., 1991b); and sodium nitroprusside, which causes enhanced mesenteric vasodilatation in the presence of L-NAME, possibly due to upregulation of guanylyl cyclase sensitivity (Gardiner et al., 1998). Some of this work has been presented to the British Pharmacological Society (Wakefield et al., 2002a,2002b,2002c).
Methods
Animals and surgical preparation
All experiments were performed on male, Sprague–Dawley rats (350–450 g: Charles River, U.K.). Animals were kept in the Biomedical Services Unit at Nottingham for at least 1 week after delivery, before any procedures were carried out. The procedures were approved by the University of Nottingham Ethical Review Committee and were performed under Home Office Project Licence authority.
All surgeries were performed under general anaesthesia (fentanyl and medetomidine, 300 μg kg−1 of each i.p., reversed with atipamezole, and analgesia provided by nalbuphine, 1 mg kg−1 of each s.c.). Surgery was carried out in two stages; initially, miniaturised pulsed Doppler flow probes were sutured around the left renal and superior mesenteric arteries, and around the distal abdominal aorta (to monitor hindquarters flow). At least 10 days later, under anaesthesia (as above), catheters were implanted in the distal abdominal aorta (via the ventral caudal artery), for monitoring arterial BP and heart rate, and in the right jugular vein for the administration of substances. Following at least 24 h recovery from the procedures for catheterization, when animals were fully conscious and freely moving, the experiments began.
Cardiovascular responses to SMTC or L-NAME
On the day after catheterisation (day 1), animals (n=7) received bolus i.v. injections (0.1 ml) of either saline (vehicle), and 0.3 and 3 mg kg−1 SMTC (n=4), or 0.1, 1 and 10 mg kg−1 SMTC (n=3). On day 3, the dose regimen was switched to ensure that each animal had received all the doses of SMTC. On each day, drugs were given in ascending dose-order, and at least 60 min was allowed between doses. The intervening day (day 2) was allowed for wash-out of any drug effects. This protocol was repeated with L-NAME in a different group of rats (n=8).
Effects of infusion of SMTC or L-NAME on resting cardiovascular variables and on responses to acetylcholine, salbutamol, sodium nitroprusside and bradykinin
On day 1, animals in group 1 (n=8) and group 2 (n=9) received an i.v. infusion (0.4 ml h−1) of saline (vehicle). After 90 min, during continued infusion of the vehicle, animals were given 3 min infusions (0.15 ml min−1) of acetylcholine (10 μg kg−1 min−1), salbutamol (0.6 μg kg−1 min−1), sodium nitroprusside (20 μg kg−1 min−1) and bradykinin (38 μg kg−1 min−1). The order of administration was randomised between animals within the groups, with at least 10 min between each substance to allow return to baseline values. The doses of vasodilators were chosen on the basis of previous experiments (Gardiner et al., 1991b; Randall et al., 1996; Gardiner et al., 1998), which showed that they produced robust, steady-state responses.
On day 3, group 1 received SMTC and group 2 received L-NAME (both at 3 mg kg−1 h−1 i.v.). Starting 90 min later, while the infusions of SMTC or L-NAME were continued, animals received 3 min infusions of acetylcholine, salbutamol, sodium nitroprusside and bradykinin using the same doses and protocol as for day 1.
Data analysis
All data were collected using the Haemodynamics Data Acquisition System designed and built at the University of Maastricht. The system sampled every 2 ms and averaged each cardiac cycle and stored data to disc every 5 s. Offline, data were analysed using software (Datview, Maastricht), which provided electronically averaged values over times selected by the analyst.
Within-group analyses were carried out using a nonparametric equivalent of ANOVA (Friedman's test, Theodorsson-Norheim, 1987) or Wilcoxon's test. Between-group analyses of areas under or over the curve (AUC, AOC) were analysed by the Mann–Whitney U-test. A P-value ⩽0.05 was taken as significant.
Drugs
Fentanyl citrate was obtained from Martindale; medetomidine hydrochloride (Domitor) and atipamezole hydrochloride (Antisedan) were obtained from Pfizer; nalbuphine hydrochloride (Nubain) was obtained from DuPont.
SMTC was obtained from Tocris (Bristol, U.K.); acetylcholine, salbutamol, sodium nitroprusside and L-NAME were obtained from Sigma (Dorset, U.K.); bradykinin was obtained from Bachem (Saffron Walden, U.K.). All substances were dissolved in sterile saline. Bolus injections were given in a volume of 0.1 ml and infusions were at a rate of 0.4 ml h−1 (L-NAME and SMTC) or 0.15 ml min−1 (acetylcholine, sodium nitroprusside, bradykinin and salbutamol).
Results
Cardiovascular responses to SMTC and L-NAME
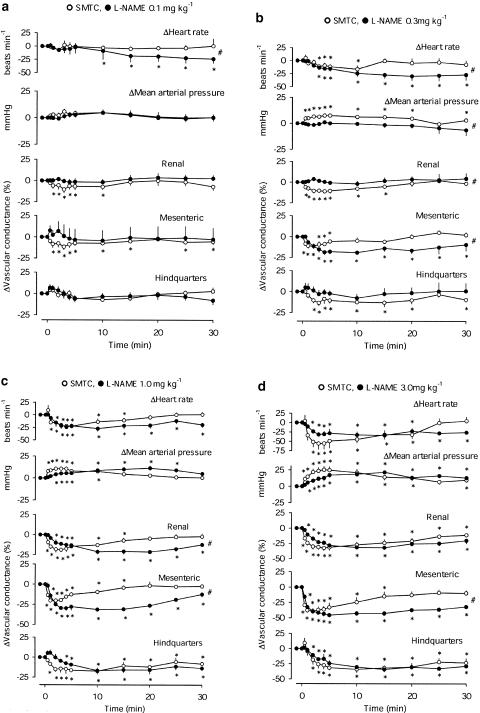
There were no significant effects of vehicle in either group of rats (data not shown). At a dose of 0.1 mg kg−1 (Figure 1a), neither SMTC nor L-NAME affected mean BP or hindquarters vascular conductance. L-NAME caused a delayed fall in heart rate, and SMTC caused a modest, and transient, renal and mesenteric vasoconstriction. The integrated change in heart rate following L-NAME (0.1 mg kg−1) was significantly greater than that of SMTC (Figure 1a).
Figure 1.
Cardiovascular changes in conscious male Sprague–Dawley rats, following i.v. bolus administration of (a) 0.1 mg kg−1, (b) 0.3 mg kg−1, (c) 1 mg kg−1, (d) 3 mg kg−1 SMTC or L-NAME. Values are means and the vertical bars show s.e.m. *P<0.05 vs baseline (Friedman's test), #P<0.05 for AUC or AOC in the presence of L-NAME and SMTC (Mann–Whitney test).
At a dose of 0.3 mg kg−1 (Figure 1b), SMTC, but not L-NAME, caused a rise in mean BP. The pressor effect of SMTC was accompanied by falls in renal, mesenteric and hindquarters vascular conductance, and there was bradycardia. L-NAME caused bradycardia and mesenteric vasoconstriction only. The integrated pressor, and renal and mesenteric vasoconstrictor effects of SMTC were greater than those of L-NAME, whereas the integrated bradycardic effect of L-NAME was greater than that of SMTC (Figure 1b).
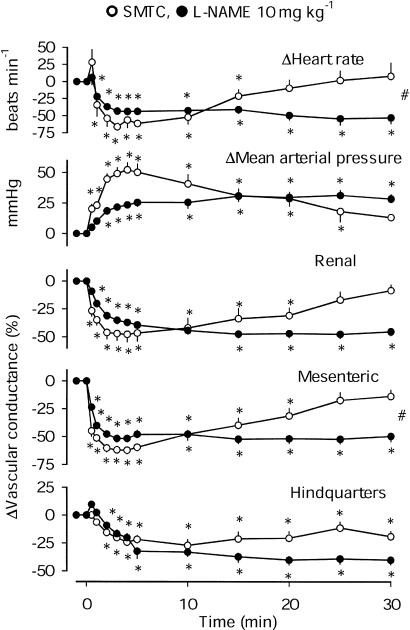
At doses of 1.0, 3.0 and 10 mg kg−1 (Figures 1c, d and Figure 2), L-NAME and SMTC both caused falls in heart rate, rises in BP and vasoconstriction in all three vascular beds. The integrated responses to SMTC and L-NAME were generally similar, with the exception of the mesenteric vasoconstrictor effect of L-NAME, which was consistently greater than that of SMTC. In addition, at a dose of 1 mg kg−1, the integrated renal vasoconstrictor effect of L-NAME was greater than that of SMTC (Figure 1c), and at a dose of 10 mg kg−1, L-NAME caused a greater bradycardia (Figure 2).
Figure 2.
Cardiovascular changes in conscious male Sprague–Dawley rats, following i.v. bolus administration of 10 mg kg−1 SMTC or L-NAME. Values are means and the vertical bars show s.e.m. *P<0.05 vs baseline (Friedman's test), #P<0.05 for AUC or AOC in the presence of L-NAME and SMTC (Mann–Whitney test).
Although the integrated (0–30 min) pressor effects of 10 mg kg−1 L-NAME and SMTC were not significantly different, it was notable that the initial rise in BP was more rapid and marked following SMTC (Figure 2). For example, 4 min after administration, the pressor response to SMTC (+52±7 mmHg) was significantly (Mann–Whitney) greater than the response to L-NAME at that juncture (+24±2 mmHg).
Effects of infusion of SMTC or L-NAME on resting cardiovascular variables and on responses to acetylcholine, salbutamol, sodium nitroprusside and bradykinin
Resting cardiovascular variables
Cardiovascular variables before and after 90 min infusion of vehicle (Groups 1 and 2, day 1), SMTC (Group 1, day 3) or L-NAME (Group 2, day 3) are shown in Table 1 . In both groups of rats, there were no consistent cardiovascular changes associated with infusion of vehicle.
Table 1.
Cardiovascular variables for groups 1 (n=8) and 2 (n=9), before and after 90 min infusion with vehicle (day 1), SMTC (Group 1, day 3) or L-NAME (Group 2; day 3)
| Group 1 | Group 2 | Group 1 | Group 2 | |||||
|---|---|---|---|---|---|---|---|---|
| Day 1 Vehicle | Day 1 Vehicle | Day 3 SMTC | Day 3 L-NAME | |||||
| 0 min | 90 min | 0 min | 90 min | 0 min | 90 min | 0 min | 90 min | |
| Heart rate (beats min−1) | 338±9 | 326±8 | 346±15 | 352±15 | 321±9 | 325±15 | 336±13 | 292±12* |
| Blood pressure (mmHg) | 104±4 | 102±3 | 102±4 | 104±5 | 100±3 | 110±4* | 100±2 | 132±4*# |
| Renal Doppler shift (kHz) | 10±0.7 | 9.9±0.7 | 8.7±0.7 | 9.1±0.6 | 10±0.9 | 9.8±1.0 | 8.2±1.1 | 8.1±1.2# |
| Renal VC (units) | 98±5 | 97±6 | 85±6 | 88±5 | 100±7 | 88±7* | 82±10 | 63±9*# |
| Mesenteric Doppler shift (kHz) | 10±0.7 | 10±0.7 | 10±0.9 | 10±0.9 | 9.5±0.8 | 8.6±0.6* | 9.8±0.9 | 7.3±0.8*# |
| MesentericVC (units) | 106±8 | 100±8 | 107±11 | 100±13 | 96±10 | 80±8* | 99±10 | 55±7*# |
| Hindquarters Doppler shift (kHz) | 4.9±0.8 | 4.2±0.2 | 4.4±0.3 | 4.2±0.3 | 3.9±0.2 | 3.6±0.2 | 3.9±0.4 | 3.0±0.3*# |
| Hindquarters VC (units) | 47±7 | 42±3 | 44±3 | 41±3 | 40±3 | 34±3* | 40±3 | 23±3*# |
Units for vascular conductance (VC) are (kHz mmHg−1)103.
P<0.05 vs baseline (Friedman's test)
P<0.05 vs Group 1 (SMTC) (Mann–Whitney test).
Values are mean±s.e.m.
SMTC and L-NAME both caused increases in mean BP, associated with vasoconstriction in all three vascular beds. The pressor and vasoconstrictor effects of L-NAME were significantly greater than those of SMTC and there was an accompanying bradycardic effect of L-NAME not seen with SMTC (Table 1).
Responses to acetylcholine
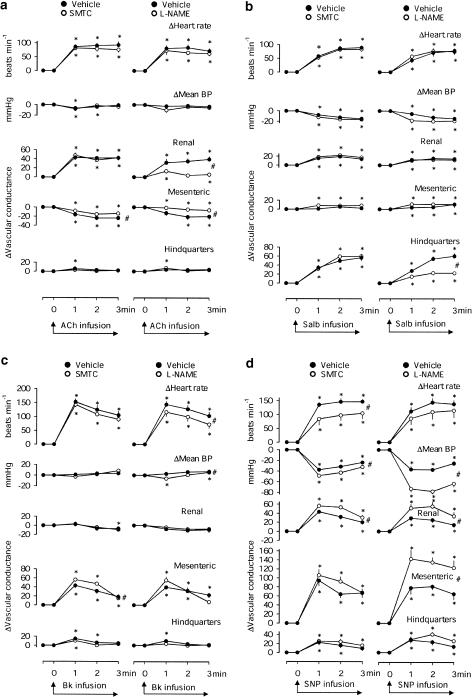
In the presence of vehicle, in both groups of rats, acetylcholine caused consistent tachycardia, renal vasodilatation and mesenteric vasoconstriction. L-NAME, but not SMTC, significantly attenuated the integrated renal vasodilator response to acetylcholine (Figure 3a). L-NAME and SMTC both attenuated the mesenteric vasoconstriction caused by acetylcholine.
Figure 3.
Cardiovascular changes during 3 min infusions of (a) acetylcholine (ACh, 10 μg kg−1 min−1), (b) salbutamol (Salb, 0.6 μg kg−1 min−1), (c) bradykinin (Bk, 38 μg kg−1 min−1) and (d) sodium nitroprusside (SNP, 20 μg kg−1 min−1) in conscious male Sprague–Dawley rats, in the presence of vehicle, SMTC (n=8) or L-NAME (n=9), both at 3 mg−1 kg−1 h−1. Values are means and the bars show s.e.m. *P<0.05 vs baseline (Friedman's test), #P<0.05 for AUC or AOC in the presence of SMTC or L-NAME (Wilcoxon's test).
Responses to salbutamol
In the presence of vehicle, in both groups of rats, salbutamol caused tachycardia, a fall in mean BP, modest renal vasodilatation and marked hindquarters vasodilatation. L-NAME, but not SMTC, significantly attenuated the integrated hindquarters vasodilator response to salbutamol (Figure 3b).
Responses to bradykinin
In the presence of vehicle, in both groups of rats, bradykinin caused tachycardia and mesenteric and transient hindquarters vasodilatation. L-NAME and SMTC both slightly enhanced the integrated mesenteric vasodilator response to bradykinin, but the change was only significant for SMTC (Figure 3c).
Responses to sodium nitroprusside
In the presence of vehicle, in both groups of rats, sodium nitroprusside caused hypotension, tachycardia and vasodilatation in all three vascular beds. L-NAME enhanced the hypotensive and vasodilator effects of sodium nitroprusside. The hypotensive and renal vasodilator effects of sodium nitroprusside were also modestly, but significantly, enhanced by SMTC. However, SMTC attenuated the tachycardic response to sodium nitroprusside (Figure 3d).
Discussion
The major aim of the present studies was to delineate the detailed cardiovascular effects of the putatively selective nNOS inhibitor, SMTC (Furfine et al., 1994), in conscious rats.
The effects of acute inhibition of nNOS on BP and heart rate have been reported previously, but most studies have used the inhibitor, 7-nitroindazole (7-NI) (Moore et al., 1993). Whether or not acute administration of 7-NI raises BP is controversial (e.g., Moore et al., 1993; Zagvazdin et al., 1996; Ollerstam et al., 1997; Vaupel et al., 1997), and the results may depend on experimental conditions, such as the choice of anaesthetic (Zagvazdin et al., 1996). However, 7-NI shows poor selectivity for nNOS and, indeed, it has been suggested that 7-NI should not be described as a selective nNOS inhibitor (Alderton et al., 2001). The selectivity of SMTC for nNOS is better than that of 7-NI, although, in vivo, it may still be less than the ideal pharmacological agent (Alderton et al., 2001).
A pressor response to systemic administration of SMTC has been consistently reported (Narayanan et al., 1995; Gozal et al., 1996; Vaupel et al., 1997; Komers et al., 2000), but whether or not the pressor response is due to a selective effect on nNOS, or due to additional actions on eNOS is unclear. Komers et al. (2000) reported pressor effects of SMTC at doses up to 0.5 mg kg−1, with no influence on depressor responses to acetylcholine. They suggested, therefore, that the effects of SMTC were atttributable to nNOS inhibition. In that study, a higher dose of SMTC (5 mg kg−1) did reduce the effects of acetylcholine, suggesting effects on eNOS at that dose (Komers et al., 2000).
At a dose of 0.3 mg kg−1, we were able to show effects of SMTC on BP and renal vascular conductance that were not seen with L-NAME. Others have suggested that, under certain conditions, nNOS-mediated effects may be particularly important in the kidney (Ichihara et al., 1998; Wilcox et al., 1998; Komers et al., 2000), and our findings may support that claim. However, a recent study using Nω-propyl-L-arginine (which shows 150-fold greater potency against nNOS than eNOS), concluded that nNOS was not an important regulator of renal blood flow (Kakoki et al., 2001). In passing, Kakoki et al. (2001) reported that selective nNOS inhibition decreased, rather than increased, BP. Such a phenomenon has been alluded to previously in rats (Wilcox et al., 1998), and in transgenic mice (Kurihara et al., 1998), and it has been suggested, therefore, that nNOS-derived NO may play a pressor role, possibly through effects on the central nervous system and baroreflexes, although the direct evidence for this is lacking (Kurihara et al., 1998). However, we did not see any tendency for BP to fall following acute SMTC administration, nor did we see any concurrent dilatation and constriction of different vascular beds preventing a change in BP. Thus, any pressor role for nNOS-derived NO may only be apparent under chronic conditions. Alternatively, it is feasible that such effects may only be revealed with the use of a more selective inhibitor of nNOS such as Nω-propyl-L-arginine.
At doses above 0.3 mg kg−1, L-NAME and SMTC both caused falls in heart rate, rises in BP and vasoconstriction in all three vascular beds. This suggests that SMTC inhibits eNOS as well as nNOS at these doses, and this would be consistent with the conclusion of Komers et al. (2000). However, although the integrated (0–30 min) pressor effects of 10 mg kg−1 L-NAME and SMTC were similar, it was notable that the profiles of BP change were different. Thus, following SMTC, BP initially rose higher, but then declined more rapidly, than following L-NAME. This phenomenon has been observed previously (Gozal et al., 1996), and may reflect the time required for the demethylation of L-NAME to its active product, L-nitroarginine (Pfeiffer et al., 1996), and a rapidly resolving inhibition of eNOS by SMTC (Narayanan et al., 1995; Gozal et al., 1996). An alternative, more provocative, interpretation would be that nNOS has a greater influence than eNOS on basal tone, but evidence from the use of more selective nNOS inhibitors would be required to support this.
A specific involvement of nNOS in heart rate control has been reported (Jumrussirikul et al., 1998; Choate et al., 2001), and those studies indicate a bradycardic role for nNOS-derived NO. However, in our study, we did not observe any tachycardic response to SMTC, and its bradycardic effects tended to be temporally associated with the pressor response, and were, therefore, likely to be baroreflex mediated. In contrast, in the study of Gozal et al. (1996) the duration of the bradycardic effect of SMTC (>5 h) was far greater than that of the pressor response (20 min).
In our study, unlike SMTC, L-NAME caused bradycardic effects independent of BP changes. We have previously shown that the heart rate response to L-NAME administration is predominantly autonomically mediated, although a residual, modest bradycardic response to L-NAME does occur during combined antagonism of muscarinic and β-adrenoceptors (Widdop et al., 1992). Whether or not the BP-independent effects of L-NAME on heart rate were autonomically mediated is not known, but since they were not seen with SMTC, it would suggest they were not due to inhibition of nNOS.
In the second part of the study, we chose to infuse the NOS inhibitors, and compare their effects on a range of vasodilators, some of the responses to which we have previously shown to be influenced by L-NAME (Gardiner et al., 1991b; 1992; 1998).
Responses to acetylcholine
Acetylcholine produced a selective, hyperaemic, renal vasodilatation that was attenuated by L-NAME, but not by SMTC. The effect of L-NAME on acetylcholine-induced renal vasodilatation in vivo has been reported by us previously (Gardiner et al., 1991b), and is consistent with in vitro observations (Moore et al., 1990; Rees et al., 1990) indicating that at least part of the vasodilator response to acetylcholine is mediated through the release of endothelial-derived NO. The lack of effect of SMTC on the renal vasodilator response to acetylcholine would indicate that, at the dose used for the infusion studies, SMTC was not acting to inhibit eNOS. Thus, the modest baseline cardiovascular effects observed during SMTC infusion could be attributed to nNOS inhibition.
Although L-NAME attenuated the integrated response to acetylcholine, it was notable that, in its presence, there was still a small, transient renal vasodilator response to acetylcholine. There are several ways in which acetylcholine could cause vasodilatation independently of eNOS-derived NO (Vanhoutte & Levy, 1980; Parkington et al., 1993), one of which is that the initial renal hyperaemic vasodilatation produced by acetylcholine in the presence of L-NAME is due to the release of NO or nitrosyl factors from preformed pools (Aisaka et al., 1989; Davisson et al., 1996). Thus, Aisaka et al. (1989) found that NOS inhibition did not affect the initial hypotensive response to acetylcholine, but substantially reduced the duration of effect, and they proposed the existence of preformed pools of NO or a nitroso-compound, possibly in acid-containing vesicles in the endothelium. Later, Davisson et al. (1994); (1996) also produced evidence for ‘use-dependent' loss of an NO-mediated response, which they suggested could be explained by depletion of nitrosyl factors from preformed pools.
As observed previously in Sprague–Dawley rats (Gardiner et al., 1998), acetylcholine caused mesenteric vasoconstriction. The mechanism for this is unknown but, interestingly, it was slightly, but significantly, attenuated in the presence of either SMTC or L-NAME, suggesting a modulatory role of nNOS-derived NO in the process. Others have suggested that nNOS-derived NO may play an excitatory role in the regulation of baroreceptor-mediated vasomotor tone (for a review see Esplugues, 2002), thus, one possibility is that the mesenteric vasoconstriction seen during acetylcholine administration was a baroreceptor-mediated reflex response.
Responses to salbutamol
Salbutamol, under our experimental conditions, produced hindquarters vasodilatation, which was attenuated in the presence of L-NAME, consistent with previous findings suggesting that β2-adrenoceptor-mediated hindlimb vasodilatation is due, at least in part, to an endothelium-dependent mechanism (Rubanyi & Vanhoutte, 1985; Gray & Marshall, 1992; Gardiner et al., 1991b). SMTC did not influence the hindquarters vasodilator response to salbutamol, reinforcing the suggestion that SMTC was not acting as a nonspecific NOS inhibitor. There is some evidence to suggest that presynaptic β-adrenoceptors may increase neuronal NO release in some vascular beds (Ferrer & Balfagón, 2001); hence, it might be hypothesised that a component of the response to salbutamol would be sensitive to nNOS inhibition. However, our results indicate that salbutamol-induced vasodilatation does not involve nNOS-mediated processes.
Responses to bradykinin
Bradykinin produced tachycardia and mesenteric vasodilatation, but only transient hindquarters vasodilatation. We have previously shown a sustained hindquarters vasodilator response to a 3 min infusion of bradykinin which, we concluded, was mediated by adrenaline acting on β2 adrenoceptors (Gardiner et al., 1992), and this effect, like that of salbutamol, would be expected to be inhibited by L-NAME. However, in the present study, the hindquarters vasodilator response to bradykinin was modest, not sustained during the infusion, and not inhibited by L-NAME. The most obvious difference between these different studies is the strain of rat used; previously, we used Long Evans rats, whereas here we studied Sprague–Dawley rats. Interestingly, in another series of experiments, using a different sub strain of Sprague–Dawley rats (Gardiner et al., 1998), we also measured small hindquarters vasodilator responses to bradykinin that were not influenced by L-NAME. Hence, it is feasible that a strain-dependent difference in adrenomedullary function could explain these disparate findings.
The mesenteric vasodilator response to bradykinin is substantial, but is not sensitive to L-NAME or indomethacin, indicating that it may involve a component due to EDHF (Randall et al., 1996). The present results showed a small augmentation of the mesenteric vasodilator effect of bradykinin by both inhibitors, although the effect was only statistically significant for SMTC. Whether or not this augmentation was due to the underlying vasoconstriction caused by the inhibitors, or signals a change in EDHF-mediated events, remains to be investigated.
Responses to sodium nitroprusside
We, and others, have previously reported increased responses to nitrovasodilators in the presence of nonselective NOS inhibition (Moncada et al., 1991; Gardiner et al., 1993) and this has been attributed to a supersensitivity at the level of soluble guanylyl cyclase in the absence of endogenous NO (Moncada et al., 1991). Some augmentation of response might also be expected due to the change in baseline haemodynamic status caused by the NOS inhibitor. Therefore, a combination of these influences probably explains the augmentation, by L-NAME, of the hypotensive and vasodilator effects of sodium nitroprusside. The smaller influence of SMTC on sodium nitroprusside-induced vascular effects is probably explained by its smaller effect on baseline variables.
The inhibitory effect of SMTC on the heart rate response to sodium nitroprusside was interesting in light of the suggestion that nNOS-derived NO might play a role in the vagal control of heart rate (Jumrussirikul et al., 1998, Choate et al., 2001). The tachycardic response to sodium nitroprusside would be expected to include a component which was due to baroreflex-mediated vagal withdrawal. Thus, if SMTC had inhibited the facilitating effects of nNOS-derived NO on vagal cardiac mechanisms (Jumrussirikul et al., 1998), then this could explain the smaller tachycardic response to sodium nitroprusside that we observed. The lack of effect of L-NAME on baroreflex-mediated changes in heart rate is consistent with our previous findings (Gardiner et al., 1991a).
In conclusion, we have shown that at low bolus doses and modest infusion levels, SMTC can exert pressor and vasoconstrictor effects that do not appear to be due to inhibition of eNOS and, therefore, are likely due to nNOS inhibition. Moreover, the differences in the effects of SMTC and L-NAME on responses to a range of vasodilators support this proposal.
Acknowledgments
We thank Astra-Zeneca for financial support.
Abbreviations
- cGMP
cyclic guanosine monophosphate
- EDHF
endothelium-derived hyperpolarising factor
- eNOS
endothelial nitric oxide synthase
- L-NAME
NG-nitro-L-arginine methyl ester
- L-NMMA
NG-monomethyl-L-arginine
- 7-NI
7-nitroindazole
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NOS
nitric oxide synthase
- SMTC
S-methyl-L-thiocitrulline
References
- AISAKA K., GROSS S.S., GRIFFITH O.W., LEVI R. L-arginine availability determines the duration of acetylcholine-induced systemic vasodilatation in vivo. Biochem. Biophys. Res. Commun. 1989;163:710–717. doi: 10.1016/0006-291x(89)92281-x. [DOI] [PubMed] [Google Scholar]
- ALDERTON W.K., COOPER C.E., KNOWLES R.G. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOATE J.K., DANSON E.J.F., MORRIS J.F., PATERSON D.J. Peripheral vagal control of heart rate is impaired in neuronal NOS knockout mice. Am. J. Physiol. 2001;281:H2310–H2317. doi: 10.1152/ajpheart.2001.281.6.H2310. [DOI] [PubMed] [Google Scholar]
- DAVISSON R.L., JOHNSON A.K., LEWIS S.J. In-vivo evidence that preformed stores of nitrosyl factors mediate active sympathetic neurogenic hindlimb vasodilation in the rat. Hypertension. 1994;24:393–393. [Google Scholar]
- DAVISSON R.L., SHAFFER R.A., JOHNSON A.K., LEWIS S.J. Stimulation of lumber sympathetic nerves may produce hindlimb vasodilatation via the release of pre-formed stores of nitrosyl factors. Neuroscience. 1996;72:881–887. doi: 10.1016/0306-4522(96)00090-5. [DOI] [PubMed] [Google Scholar]
- ESPLUGUES J.V. NO as a signalling molecule in the nervous system. Br. J. Pharmacol. 2002;135:1079–1095. doi: 10.1038/sj.bjp.0704569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESTRADA C., MENGUAL E., GONZALEZ C. Local NADPH diaphorase neurons innervate pial-arteries and lie close or project to intracerebral blood-vessels – a possible role for nitric-oxide in the regulation of cerebral bloodflow. J. Cereb. Blood Flow Metab. 1993;13:978–984. doi: 10.1038/jcbfm.1993.122. [DOI] [PubMed] [Google Scholar]
- FERRER M., BALFAGÓN G. Aging alters neuronal oxide release from rat mesenteric arteries: role of presynaptic β-adrenoceptors. Clin. Sci. 2001;101:321–328. [PubMed] [Google Scholar]
- FURFINE E.S., HARMON M.F., PAITH J.E., KNOWLES R.G., SALTER M., KIFF R.J., DUFFY C., HAZELWOOD R., OPLINGER J.A., GARVEY E.P. Potent and selective inhibition of human nitric oxide synthases. Selective inhibition of neuronal nitric oxide synthase by S-methyl-L-thiocitrulline and S-ethyl-L-thiocitrulline. J. Biol. Chem. 1994;269:26677–26683. [PubMed] [Google Scholar]
- GARDINER S.M., COMPTON A.M., BENNETT T., PALMER R.M.J., MONCADA S. Control of regional blood flow by endothelium-derived nitric oxide. Hypertension. 1990a;15:486–492. doi: 10.1161/01.hyp.15.5.486. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., COMPTON A.M., KEMP P.A., BENNETT T. Regional and cardiac haemodynamic-effects of NG-nitro-L-arginine methyl ester in conscious, Long Evans rats. Br. J. Pharmacol. 1990b;101:625–631. doi: 10.1111/j.1476-5381.1990.tb14131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., COMPTON A.M., KEMP P.A., BENNETT T. Effects of NG-nitro-L-arginine methyl ester or indomethacin on differential regional and cardiac haemodynamic actions of arginine vasopressin and lysine vasopressin in conscious rats. Br. J. Pharmacol. 1991a;102:65–72. doi: 10.1111/j.1476-5381.1991.tb12133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., BENNETT T. Effects of NG-nitro-L-arginine methyl ester on vasodilator responses to acetylcholine, 5′-N-ethylcarboxyamidoadenosine or salbutamol in conscious rats. Br. J. Pharmacol. 1991b;103:1725–1732. doi: 10.1111/j.1476-5381.1991.tb09854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., BENNETT T. Effects of chronic treatment with nitric oxide synthase inhibitors on regional haemodynamic responses to vasodilators in conscious Brattleboro rats. Br. J. Pharmacol. 1993;109:222–228. doi: 10.1111/j.1476-5381.1993.tb13557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., BENNETT T., BOSE C., FOULKES R., HUGHES B. Involvement of β2-adrenoceptors in the regional haemodynamic responses to bradykinin in conscious rats. Br. J. Pharmacol. 1992;105:839–848. doi: 10.1111/j.1476-5381.1992.tb09066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., MARCH J.E., KEMP P.A., BENNETT T. The contribution of nitric oxide to cardiovascular status and responses to vasodilators in conscious, hypertensive, transgenic ((mRen-2) 27) rats. Br. J. Pharmacol. 1998;124:299–306. doi: 10.1038/sj.bjp.0701838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOZAL D., TORRES J.E., GOZAL Y.M., LITTWIN S.M. Effect of nitric oxide synthase inhibition on cardiorespiratory responses in the conscious rat. J. Appl. Physiol. 1996;81:2068–2077. doi: 10.1152/jappl.1996.81.5.2068. [DOI] [PubMed] [Google Scholar]
- GRAY D.W., MARSHALL I. Novel signal transduction pathway mediating endothelium-dependent beta-adrenoceptor vasorelaxation in rat thoracic aorta. Br. J. Pharmacol. 1992;107:684–690. doi: 10.1111/j.1476-5381.1992.tb14507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICHIHARA A., INSCHO E.W., IMIG J.D., NAVAR L.G. Neuronal nitric oxide synthase modulates rat renal microvascular function. Am. J. Physiol. 1998;274:F516–F524. doi: 10.1152/ajprenal.1998.274.3.F516. [DOI] [PubMed] [Google Scholar]
- JUMRUSSIRIKUL P., DINERMAN J., DAWSON T.M., DAWSON V.L., EKELUND U., GEORGAKOPOULOS D., SCHRAMM L.P., CALKINS H., SNYDER S.H., HARE J.M., BERGER R.D. Interaction between neuronal nitric oxide synthase and inhibitory G protein activity in heart rate regulation in conscious mice. J. Clin. Invest. 1998;102:1279–1285. doi: 10.1172/JCI2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAKOKI M., ZOU A-P., MATTSON D.L. The influence of nitric oxide synthase 1 on blood flow and interstitial nitric oxide in the kidney. Am. J. Physiol. 2001;281:R91–R97. doi: 10.1152/ajpregu.2001.281.1.R91. [DOI] [PubMed] [Google Scholar]
- KOMERS R., OYAMA T.T., CHAPMAN J.G., ALLISON K.M., ANDERSON S. Effects of systemic inhibition of neuronal nitric oxide synthase in diabetic rats. Hypertension. 2000;35:655–661. doi: 10.1161/01.hyp.35.2.655. [DOI] [PubMed] [Google Scholar]
- KURIHARA N., ALFIE M.E., SIGMON D.H., RHALEB N-R., SHESELY E.G., CARRETERO O.A. Role of nNOS on blood pressure regulation in eNOS null mutant mice. Hypertension. 1998;32:856–861. doi: 10.1161/01.hyp.32.5.856. [DOI] [PubMed] [Google Scholar]
- MARIN J., BALFAGÓN G. Effect of clenbuterol on non-endothelial nitric oxide release in rat mesenteric arteries and the involvement of beta-adrenoceptors. Br. J. Pharmacol. 1998;124:473–478. doi: 10.1038/sj.bjp.0701856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONCADA S., REES D.D., SCHULZ R., PALMER R.M.J. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc. Natl. Acad. Sci. U.S.A. 1991;88:2166–2170. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE P.K., ALSWAYEH O.A., CHONG N.W.S., EVANS R.A., GIBSON A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br. J. Pharmacol. 1990;99:408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE P.K., WALLACE P., GAFFEN Z., HART S.L., BABBEDGE R.C. Characteristics of the novel nitric oxide synthase inhibitor 7-nitro indazole and related indazoles: antinociceptive and cardiovascular effects. Br. J. Pharmacol. 1993;110:219–224. doi: 10.1111/j.1476-5381.1993.tb13795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAYANAN K., SPACK L., MCMILLAN K., KILBOURN R.G., HAYWARD M.A., MASTERS B.S.S., GRIFFITH O.W. S-alkyl-L-thiocitrullines. Potent stereoselective inhibitors of nitric oxide synthase with strong pressor activity in vivo. J. Biol. Chem. 1995;270:11103–11110. doi: 10.1074/jbc.270.19.11103. [DOI] [PubMed] [Google Scholar]
- OLLERSTAM A., PITTNER J., PERSSON A.E.G., THORUP C. Increased blood pressure in rats after long-term inhibition of the neuronal isoform of nitric oxide synthase. J. Clin. Invest. 1997;99:2212–2218. doi: 10.1172/JCI119394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALMER R.M.J., REES D.D., ASHTON D.S., MONCADA S. L-arginine is the physiological precursor for the formation of nitric-oxide in endothelium-dependent relaxation. Biochem. Biophys. Res. Commun. 1988;153:1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- PARKINGTON H.C., TARE M., TONTA M.A., COLEMAN H.A. Stretch revealed 3 components in the hyperpolarization of guinea-pig coronary-artery in response to acetylcholine. J. Physiol. 1993;465:459–476. doi: 10.1113/jphysiol.1993.sp019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEIFFER S., LEOPOLD E., SCHMIDT K., BRUNNER F., MAYER B. Inhibition of nitric oxide synthesis by N-G-nitro-L-arginine methyl ester (L-NAME): Requirement for bioactivation to the free acid, N-G-nitro-L-arginine. Br. J. Pharmacol. 1996;118:1433–1440. doi: 10.1111/j.1476-5381.1996.tb15557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDALL M.D., ALEXANDER S.P.H., BENNETT T., BOYD E.A., FRY J.R., GARDINER S.M., KEMP P.A., MCCULLOCH A.I., KENDALL D.A. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem. Biophys. Res. Commun. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- REES D.D., PALMER R.M.J., SCHULZ R., HODSON H.F., MONCADA S. Characterization of 3 inhibitors of endothelial nitric-oxide synthase in vitro and in vivo. Br. J. Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBANYI G., VANHOUTTE P.M. Endothelium removal decreases relaxations of canine coronary arteries caused by beta-adrenergic agonists and adenosine. J. Cardiovasc. Pharmacol. 1985;7:139–144. doi: 10.1097/00005344-198501000-00023. [DOI] [PubMed] [Google Scholar]
- THEODORSSON-NORHEIM E. Friedman and Quade tests: BASIC computer program to perform nonparametric two-way analysis of variance and multiple comparisons on ranks of several related samples. Comput. Biol. Med. 1987;17:85–99. doi: 10.1016/0010-4825(87)90003-5. [DOI] [PubMed] [Google Scholar]
- TODA N., OKAMURA T. Mechanism of neurally induced monkey mesenteric-artery relaxation and contraction. Hypertension. 1992;19:161–166. doi: 10.1161/01.hyp.19.2.161. [DOI] [PubMed] [Google Scholar]
- TODA N., OKAMURA T. Nitroxidergic nerve: regulation of vascular tone and blood flow in the brain. J. Hypertens. 1996;14:423–434. [PubMed] [Google Scholar]
- VANHOUTTE P.M., LEVY M.N. Prejunctional cholinergic modulation of adrenergic neurotransmission in the cardiovascular system. Am. J. Physiol. 1980;238:H275–H281. doi: 10.1152/ajpheart.1980.238.3.H275. [DOI] [PubMed] [Google Scholar]
- VAUPEL D.B., KIMES A.S., LONDON E.D. Further in vivo studies on attenuating morphine withdrawal: isoform-selective nitric oxide synthase inhibitors differ in efficacy. Eur. J. Pharmacol. 1997;324:11–20. doi: 10.1016/s0014-2999(97)00061-7. [DOI] [PubMed] [Google Scholar]
- WAKEFIELD I.D., GARDINER S.M., VALENTIN J-P., BENNETT T. Regional haemodynamic effects of the nitric oxide synthase inhibitor S-methyl-L-thiocitrulline in conscious Sprague Dawley rats. Br. J. Pharmacol. 2002a;135:136P. doi: 10.1038/sj.bjp.0705351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKEFIELD I.D., GARDINER S.M., VALENTIN J-P., BENNETT T. Effects of the nitric oxide synthase inhibitor, S-methyl-L-thiocitrulline (SMTC), on the regional haemodynamic responses to acetylcholine in conscious Sprague Dawley rats. Br. J. Pharmacol. 2002b;135:296P. doi: 10.1038/sj.bjp.0705351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKEFIELD I.D., GARDINER S.M., VALENTIN J-P., BENNETT T. Comparative effects of S-methyl-L-thiocitrulline (SMTC) and NG-nitro-L-arginine methyl ester (L-NAME) on salbutamol-induced hindquarters vasodilatation in conscious rats. Br. J. Pharmacol. 2002c;137:58P. [Google Scholar]
- WIDDOP R.E., GARDINER S.M., KEMP P.A., BENNETT T. The influence of atropine and atenolol on the cardiac haemodynamic effects of NG-nitro-L-arginine methyl ester in conscious Long Evans rats. Br. J. Pharmacol. 1992;105:653–656. doi: 10.1111/j.1476-5381.1992.tb09034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILCOX C.S., DENG X., WELCH W.J. NO generation and action during changes in salt intake: roles of nNOS and macula densa. Am. J. Physiol. 1998;274:R1588–R1593. doi: 10.1152/ajpregu.1998.274.6.R1588. [DOI] [PubMed] [Google Scholar]
- ZAGVAZDIN Y., SANCESARIO G., WANG Y-X., SHARE L., FITZGERALD M.E.C., REINER A. Evidence from its cardiovascular effects that 7-nitroindazole may inhibit endothelial nitric oxide synthase in vivo. Eur. J. Pharmacol. 1996;303:61–69. doi: 10.1016/0014-2999(96)00106-9. [DOI] [PubMed] [Google Scholar]