Abstract
Mesangial cell proliferation is observed in a number of kidney diseases. The sympathetic cotransmitter ATP is suspected to play a major role in proliferative processes. Therefore, the effects of exogenous ATP on human mesangial cells in culture were studied.
Fresh human kidney cortex was processed to obtain mesangial cells in culture. Effects of nucleotides on [3H]thymidine incorporation, the activation of mitogen-activated protein kinase and the cell number were studied. The involved P2-receptors were characterized pharmacologically. In addition, we searched for mRNA for P2Y- and P2X-receptors by RT–PCR.
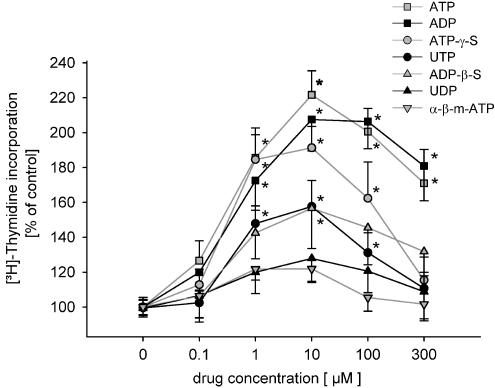
ATP (0.1–300 μM) and related nucleotides induced a significant increase in [3H]thymidine incorporation up to 220% of control. The adenine nucleotides ATP and ADP were about equally effective. Also ATP-γ-S, UTP, ADP-β-S and 2-m-thio-ADP induced a weaker response. UDP and α-β-methylene-ATP failed to induce an effect on [3H]thymidine uptake.
ATP (100μM) induced a fast activation of the MAPK42/44 pathway. The effects of ATP on MAPK42/44 activation and [3H]thymidine incorporation were reduced by the MAPK inhibitor PD 98059. Platelet-derived growth factor (PDGF 5 ng ml−1) increased the cell number to more than 122% of control. ATP (10 μM) on top of PDGF amplified PDGF induced cell proliferation to 136% of control.
RT–PCR products for P2Y1,2,4,6,11,12- and P2X1,2,4,5,6,7-receptor subtypes were detected in human mesangial cells.
ATP has mitogenic effects on human mesangial cells. DNA synthesis is increased by the activation of the MAPK42/44 pathway. ATP amplifies PDGF-induced cell hyperplasia.
Keywords: P2Y-Receptors, ATP, UTP, sympathetic nervous system, human mesangial cells, proliferation, DNA synthesis, MAPK p42/44 activation
Introduction
Mesangial cells form the matrix of the glomerulus, hold the capillary arteries and connect them with the juxtaglomerular apparatus. They can influence the glomerular filtration rate by contracting the mesangium (Haas et al., 1999) and have been shown to release various growth hormones and cytokines; (Lee, 1995; Haas et al., 1999). In healthy individuals glomerular cells have a cell turnover of less than 1% (Pabst & Sterzel, 1983). However, in diabetic nephropathy, Lupusnephritis, IgA nephropathy and other renal diseases marked mesangial cell proliferation and increased extracellular matrix expansion has been observed. Since most of these patients develop end-stage renal failure within years, it is essential to understand the mechanisms involved in mesangial cell proliferation.
It is now well established that inhibition of the renin–angiotensin system and the sympathetic nervous system slows the decline of renal function in chronic renal failure (Rump et al., 2000; Hilgers & Mann, 2002). This is probably due to a reduction in local concentrations of growth promoting hormones and neurotransmitters, such as angiotensin II (Mezzano et al., 2001) and noradrenaline (Aizawa et al., 2001).
Accordingly, the sympatholytic drug moxonidine reduces renal damage independent from blood pressure effects in an experimental model of chronic renal failure (Amann et al., 2000). Since ATP is a cotransmitter of noradrenaline (Starke et al., 1991; Burnstock, 1995) in the kidney (Rump et al., 1996; Vonend et al., 2002), it is our hypothesis that P2-receptor activation by ATP contributes to progression of renal disease. In addition to the neuronal source of ATP, large amounts of ATP are released during cell lysis and blood cell aggregation (Schulze-Lohoff et al., 1996). There is some evidence that ATP increases DNA synthesis (Schulze-Lohoff et al., 1992), cell proliferation and in addition cell death (Schulze-Lohoff et al., 1998; Harada et al., 2000).
ATP can stimulate a variety of receptor subtypes. ATP- (P2) Receptors are divided into two major families, the ion channel coupled P2X and the G-protein coupled P2Y-receptors. Beside their intracellular signal transduction mechanisms there are differences in molecular structures and pharmacological profiles. At least, seven members of the P2X family (P2X1–7) and seven subtypes of the mammalian P2Y receptors (P2Y1,2,4,6,11,12,13) have been cloned (Ralevic & Burnstock, 1998; Communi et al., 2001; Hollopeter et al., 2001).
The aim of the study was to investigate mitogenic effects of extracellular nucleotides in human mesangial cells. Possible receptor subtypes and intracellular signalling pathways were analysed.
Methods
The present study was approved by the local ethics committee. Human kidneys were obtained from patients undergoing nephrectomy for renal cell carcinoma. Only macroscopic intact renal cortex tissue was used for cell preparation. Renal cortical slices were minced, pushed and rinsed through a serial of stainless-steel sieves of different pore sizes of 40, 80 and finally 120 mesh. Glomeruli retained by the 120 mesh sieves were collected in tubes and centrifuged at 1750 × g for 8 min and suspended in RPMI+L-glutamine containing 20% foetal calf serum (FCS) and supplements (nonessential amino acids, Na-Pyruvat, penicillin, streptomycin, HEPES (Biochromchrom, Berlin, Germany), insulin-transferrin-selenite (Boehringer, Mannheim, Germany)). Primary cultures and subcultures were maintained at 37°C and 5% CO2. Plates were left undisturbed for the next 3–6 weeks to facilitate outgrowth of mesangial cells from glomeruli. Then, the cells were trypsinized and passaged. All cells were studied within the first 15 passages.
Cell identification of mesangial cells
Cell purity was assessed by the peroxidase antiperoxidase (PAP) staining from DAKO (Hamburg, Germany) following the manufacturer's manual. Primary antihuman antibodies used were mouse antifactor VIII-related antigen, mouse anticytokeratin, mouse antivimentin, mouse antidesmin, mouse antismooth muscle actin (all from DAKO) and rabbit anti-WT-1 (Santa Cruz, CA, U.S.A.). Secondary antibodies were mouse anti-rabbit IgG and rabbit anti-mouse IgG (both 1 : 100, DAKO). Mesangial cells were factor VIII and WT-1 negative, showed weak signals for desmin and smooth-muscle actin and strong signals for cytokeratin and vimentin.
Proliferation assays
All experiments were done under growth-arrested conditions. Mesangial cells were plated at a density of 30,000 cells per well in 24-well plates and grown to 80% confluence in RPMI, 20% FCS and supplements before they were placed in growth-arrest medium (RPMI medium supplemented with 0.5% FCS; 5 μg insulin ml−1, Sigma, Deisendorf, Germany; and 100 U penicillin and 100 μg streptomycin ml−1) for 72 h to decrease proliferation.
Determination of DNA synthesis
DNA synthesis was assessed by measuring [3H]thymidine incorporation. All studied substances were added to resting cells (four wells for each concentration) for 24 h. The cells were incubated with [3H]thymidine (1 μCi ml−1) during the last 6 h. The medium was then aspirated, the cells washed three times with phosphate buffer and for DNA precipitation twice with ice-cold 10% trichloroacetic acid (TCA). The fixed cellular material was solubilized in 1 ml 0.5 M NaOH for 2 h mixed with 10 ml scintillation fluid (Ultima-Gold, Canberra Packard, Frankfurt, Germany) to measure the amount of radioactivity present in the TCA insoluble fraction. Data are expressed as the mean ratio of radioactivity present in four wells with identical concentration divided by control (0.5% FCS) values (% of control).
RNA extraction and RT–PCR of P2X and P2Y-receptors
RNA was gained from 2 × 106 cells using the RNAeasy Kit (Qiagen, Hilden, Germany). Following DNA digestion (Rnase free Dnase, Invitrogen, Gibco-BRL, Karlsruhe, Germany) 2 μg of RNA was used in the superscript first-strand system (Gibco-BRL) for synthesis of cDNA. The amplification was performed with specific primer (Table 1) in a volume of 50 μl with 10% of the first-strand cDNA with AmpliTaq Gold (Applied Biosystems, Weiterstadt, Germany) according to the supplier's protocol. After 5 min, 95°C followed by 35–40 cycles consisting of 1 min, 95°C, 1 min 55–65°C, 1 min, 72°C and for termination 8 min 72°C, 10 μl of the reaction products were analysed on a 1.5% agarose gel stained with ethidium bromide.
Table 1.
Sequence, predicted product size and source of primers used for RT–PCR
| Primer | Sequence | Product size | Source* | |
|---|---|---|---|---|
| P2Y1 | Sense: | 5′-TGC CAG CCC TGA TCT TCT ACT ACT-3′ | ||
| Antisense: | 5′-ATA CGT GGC ATA AAC CCT GTC ATT-3′ | 608 bp | NM_002563 | |
| P2Y2 | Sense: | 5′-GCC GGG GCC GTG TGG GTG TT-3′ | ||
| Antisense: | 5′-CGG GTG ACG TGG AAT GGC AGG AA-3′ | 335 bp | NM_176072 | |
| P2Y4 | Sense: | 5′-GGG ATG CAA CGG CCA CCT ACA-3′ | ||
| Antisense: | 5′-GCA CGA AGC AGA CAG CAA AGA CAG-3′ | 579 bp | NM_002565 | |
| P2Y6 | Sense: | 5′-CCC TGC TGG CCT GCT ACT GTC TCC-3′ | ||
| Antisense: | 5′-TTC TCC GCA TGG TTT GGG GTT GGT-3′ | 452 bp | U52464 | |
| P2Y11 | Sense: | 5′-CCC CCG CTG GCC GCC TAC CTC TTA-3′ | ||
| Antisense: | 5′-GCC CAA CCC CGC CAG CAC CAG-3′ | 393 bp | AF030335 | |
| P2Y12 | Sense: | 5′-CTC TGT TGT CAT CTG GGC ATT CAT-3′ | ||
| Antisense: | 5′-GGT TTG GCT CAG GGT GTA AGG A-3′ | 361 bp | AF313449 | |
| P2Y13 | Sense: | 5′-TGT GTC GTT TTT CTT CGG TG-3′ | ||
| Antisense: | 5′-CTG CCA AAA AGA GAG TTG-3′ | 575 bp | NM_176894 | |
| (Communi et al., 2001) | ||||
| P2X1 | Sense: | 5′-CTT TCC ACG CTT CAA GGT CAA CA-3′ | ||
| Antisense: | 5′-GCC ACC CCA AAG ATG CCA AT-3′ | 453 bp | NM_002558 | |
| (Lynch et al., 1999) | ||||
| P2X2a-d | Sense: | 5′-TTT ATC GTG GAG AAG GCT GGG GAG-3′ | ||
| Antisense: | 5′-TTT CGT GGA GAT GCT CCG CTA CTG-3′ | 600–879 bp | AF190822-5 | |
| P2X3 | Sense: | 5′-TCT GTG CTC CGG ACC TGT GAG AT-3′ | ||
| Antisense: | 5′-AAG CGG ATG CCA AAA GCC TTC A-3′ | 491 bp | AB016608 | |
| P2X4 | Sense: | 5′-CCT TCT GCC CCA TAT TCC GTC T-3′ | ||
| Antisense: | 5′-GTT GAT CAT AGT GGG GAT GAT GTC A-3′ | 341 bp | AF191093 | |
| P2X5a/b | Sense: | 5′-GAG TGC TGT CAT CAC CAA AGT CAA-3′ | ||
| Antisense: | 5′-CCA GTC GGA AGA TGG GGC AGT A-3′ | 512/440 bp | U49395 | |
| P2X6 | Sense: | 5′-CCT GTG AGA TCT GGA GTT GGT GC-3′ | ||
| Antisense: | 5′-GTG TCC AGG TCA CAA TCC CAG T-3′ | 319 bp | AF065385 | |
| P2X7 | Sense: | 5′-CGA CTT CCC CGG CCA CAA CTA-3′ | ||
| Antisense: | 5′-TGC CAA AAA CCA GGA TGT CAA AAC-3′ | 383 bp | NM_002562 | |
| β-actin | Sense: | 5′-ACC TTC AAC ACC CCA GCC ATG TAC G-3′ | ||
| Antisense: | 5′-CTG ATC CAC ATC TGC TGG AAG GTG G-3′ | 645 bp | V00481 |
Sources are described by the accession number in GenBank.
Determination of MAPK42/44 activation
Kinetics of MAPK42/44 phosphorylation were done by stimulation of resting mesangial cells plated on six-well plates with ATP (100 μM) for 0, 2, 5, 10, 15, 20 and 60 min. To determinate concentration dependency, various concentrations of ATP, α-β-methylene-ATP and UTP were applied on the cells for 10 min. For experiments using the mitogen-activated protein (MAP) kinase (MEK) inhibitor PD-98059 (New England BioLabs, Beverly, U.S.A.), cells were incubated for 30 min at 37°C in growth-arresting medium that contained the inhibitor before the addition of agonists. After treatment, media were aspirated, solubilized in 200 μl 2 × Laemmli sample buffer with 200 mM dithiothreitol, and boiled for 5 min. Lysates were sonicated to disrupt DNA, and proteins were separated on 10% SDS–PAGE gels. The proteins were electrophoretically transferred to nitrocellulose in buffer containing 25 mM Tris, 192 mM glycine, 20% methanol and 0.02% SDS. The nitrocellulose was blocked with 5% nonfat dry milk in 20 mM Tris, pH 7.4, 150 mM NaCl, and 0.01% Tween-20. The membranes were probed either with a polyclonal phosphotyrosyl-MAP kinase-specific antibody (New England BioLabs) which recognizes only the tyrosine-phosphorylated (active) form of p44 MAP kinase (ERK1) and p42 MAP kinase (ERK2) or with a MAP kinase-specific antibody (New England BioLabs) which recognizes the total amount of ERK1 and ERK2.
Accordingly specific antibodies raised against phosphorylated and total p38 MAP kinase SAPK/JNK MAP kinase (New England BioLabs) were used. The buffer used for incubation contained 20 mM Tris, pH 7.4, 150 mM NaCl, 3% BSA, and 0.01% Tween-20. The blots were then washed in 20 mM Tris, pH 7.4, 150 mM NaCl and 0.01% Tween-20, and bound antibody was detected by a horseradish peroxidaseconjugated anti-rabbit IgG and enhanced chemiluminescence (Pierce, Rockford, IL, U.S.A). The detection and estimation of the ratio between activated and total protein was performed with the FluorChem (Alpha Innotech Corporation, U.S.A.) Imaging System. The density ratio was used as the quantum of MAPK activation.
Cell number
The number of viable cells was determined by a colorimetric method (CellTiter 96®AQueous, Promega, Mannheim, Germany) first described by Cory and co-workers (Cory et al., 1991). In brief, a MTS tetrazolium compound is bioreduced by cells into a coloured formazan product that can be measured by absorbance at 490 nm in a plate reader.
The cells were plated into a 96-well plate. After reaching 70% confluence in growth medium and another 2 days in growth-arrest medium the cells were stimulated with the tested substances (ATP, PDGF) for 24 h. The cells were incubated with 20 μl of CellTiter substrate at 37°C in 5% CO2. After 1 h the absorbance was read at 490 nm. By using standard curves the actual cell number was estimated.
Statistics
All data are expressed as means±s.e.m. Multiple comparisons with single control were analysed by ANOVA with a post-hoc test by Dunnetts. To determine a rank order of potency on DNA synthesis, concentration–response curves were compared by ANOVA (>indicating significant higher potency). Differences in two groups were tested by Student's t-test. Values of P<0.05 were considered statistically significant. All data were analysed by SPSS 11.0 (Sigma-Plot, U.S.A.).
Results
Nucleotide-induced [3H]thymidine incorporation in human mesangial cells
In control experiments [3H]thymidine incorporation into mesangial cells was measured in growth-arrest medium containing for 24 h 0.5% FCS but no further drugs. The mean value was set to 100% (control, 0.5% FCS). To test the effect of various drugs, different concentrations were added to the growth-arrest medium for 24 h. ATP, ADP, ATP-γ-S, UTP and ADP-β-S concentration dependently increased [3H]thymidine uptake (Figure 1). 2-m-thio ADP had a similar potency to ADP-β-S (data not shown). UDP and α-β-methylene-ATP failed to induce an effect on [3H]thymidine incorporation (Figure 1).
Figure 1.
Dose–response curves of various nucleotides on [3H]thymidine incorporation as a marker for DNA synthesis are shown. Medium containing 0.5% FCS was used as a control and set to 100%. Each concentration represents n=10–85 data points. *Indicates significant difference between 0.5% FCS (control) and stimulation (ANOVA with post-hoc test by Dunnet's).
[3H]thymidine uptake induced by ATP (10 μM) was significantly reduced by the non-subtype selective P2-receptor antagonist suramin (30 μM) from 176±13% (n=31) to 61±4% (n=31) of control (FCS 0.5%) (P<0.05).
Involvement of the MAPK pathway
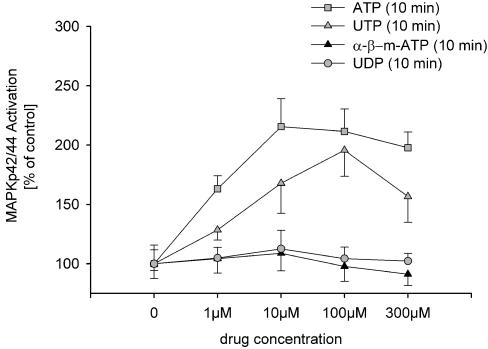
In human mesangial cells ATP (100 μM) induced a fast activation of the MAPK42/44 pathway (Figure 2a and b). The maximal activation of MAPK42/44 was observed 10 min after addition of ATP. A more than two-fold increase was still present 20 min after incubation with ATP (100 μM). ATP and UTP (10 min) concentration dependently increased MAPK42/44 activity (Figure 3). α-β-methylene-ATP and UDP failed to activate MAPK42/44 (Figure 3).
Figure 2.
(a) Representative Western blot of ATP (10 μM) induced activation of MAPK42/44. Antibody recognizing only the phosphorylated form of MAPK42/44 (upper blot) or total MAPK42/44 (lower blot) was used. (b) The ratio phosphorylated to total MAPK42/44 of three individual preparations was used to demonstrate MAPK42/44 activation. The ratio at minute 0 was used as a control and set to 100%. Maximal MAPK42/44 activation is present 10 min after adding ATP (100 μM). The number in the column [n] equals the number of experiments.
Figure 3.
The ratios of phosphorylated to total MAPK42/44 of three individual preparations demonstrate concentration-dependent activation of MAPK42/44 after 10 min ATP or UTP. α-β-Methylene-ATP and UTP failed to stimulate MAPK42/44. The ratio after 10 min without agonists was used as a control and set to 100%.
The selective MAPK42/44 blocker PD 98059 (100 μM) abolished MAPK42/44 activation induced by 2 and 5 min 10 μM ATP (157±10 and 217±28% of control) to 82±17 and 94±16% of control (n=3), respectively (activation of MAPK42/44 at 0 min in cells treated with PD 98059 100 μM was set to 100%). PD 98059 (100 μM) alone decreased MAPK42/44 activity by 84±19%, compared to values of unstimulated, resting cells (n=3).
In parallel, basal and ATP (10 μM) induced [3H]thymidine incorporation was reduced by PD 98059 in a concentration of 10 and 100 μM significantly (Figure 4). Stimulation with ATP (10 μM) did not alter [3H]thymidine uptake in cells treated with 100 μM PD 98059 when compared with cells treated with PD 98059 in the presence of FCS 0.5%. In contrast to that, application of 20% serum did increase [3H]thymidine uptake significantly, suggesting activation of MAPK42/44 independent pathways.
Figure 4.
The selective MAPK42/44 pathway inhibitor PD 98059 reduced basal (FCS 0.5%) and ATP (10 μM) increased [3H]thymidine incorporation in a concentration-dependent manner. FCS 20% but not ATP (10 μM) altered [3H]thymidine uptake in cells treated with 100 μM PD 98059. The number in the column [n] equals the number of experiments. *Indicates significant difference between 0.5% FCS (control) and 10 or 100 μM PD 98059 (ANOVA with post-hoc test by Dunnet's). +Indicates significant difference between 100 μM PD 98059 with and without FCS 20% (Student's t-test).
Besides the effects on the MAPK42/44 pathway, ATP (1–100 μM) failed to activate MAPKjnk or MAPKp38 in human mesangial cells (data not shown).
ATP amplifies growth factor induced cell proliferation
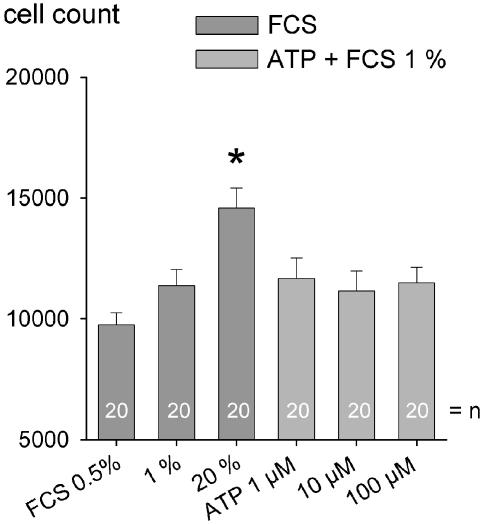
Mesangial cells were plated into a 96-well plate. After reaching 70% confluence, cells were left for 24 h in growth-arrest medium. FCS (1 and 20%), added to the medium, increased cell number in a concentration-dependent manner (Figure 5). ATP (1–100 μM) added in addition to FCS 1% did not further increase the cell number (Figure 5). Platelet-derived growth factor (PDGF; 1 and 5 ng ml−1) also increased the cell number in a concentration-dependent manner (Figure 6). ATP (10 μM) on top of PDGF, amplified PDGF-induced cell proliferation in a synergistic manner (Figure 6). ATP (10 μM) alone had no effect.
Figure 5.
Cell number increased 2 days after incubation with FCS 1% and FCS 20% compared to FCS 0.5%. No additive effects were observed when ATP (1–100 μM) was given on top of FCS 1%. The number in the column [n] equals the number of experiments. *Indicates significant difference between 0.5% FCS (control) and FCS 1% and 20% (ANOVA with post-hoc test by Dunnet's).
Figure 6.
Cell number increased 2 days after incubation with 1 and 5 ng PDGF ml−1 (light-grey column) as compared to FCS 0.5%. ATP (10 μM) alone had no effects on top of 0.5% FCS, but amplified PDGF-induced cell proliferation. The number in the column [n] equals the number of experiments. *Indicates significant difference between 0.5% FCS (control) and stimulation (ANOVA with post-hoc test by Dunnet's). +Indicates significant difference between PDGF (5 ng ml−1) and ATP (10 μM) in combination with PDGF (5 ng ml−1) (Student's t-test).
RT–PCR analysis of P2Y- and P2X-receptor mRNA
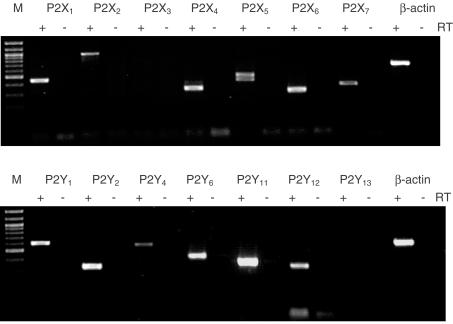
Resting cells were harvested and the RNA extracted. Under these conditions RT–PCR revealed products of the expected lengths for P2Y1,2,4,6,11,12- and P2X1,2,4,5,6,7-receptors in human mesangial cells (Figure 7). No expression of P2X3 and P2Y13 could be detected. The housekeeper β-actin was used as a positive and negative control. Experiments without reverse transcriptase (−) confirmed that the PCR products originated from mRNA but not from genomic DNA.
Figure 7.
P2Y- and P2X-receptor subtype expression in human cultured mesangial cells. PCR with (+) and without reverse transcriptase (−) and specific primers produced amplification products for P2Y1,2,4,6,11,12 and P2X1,2,4,5,6,7 at the expected size. Marker is a 100 bp ladder. PCR without RT (−) showed no amplification products.
Discussion
Overactivity of the sympathetic nervous system is a hallmark of various renal diseases (Converse et al., 1992). Moreover, it has been shown that the sympathetic nervous system plays an important role for progression of glomerulosclerosis in an experimental model of chronic renal failure. In this model, an increased release of noradrenaline from renal cortex has been observed (Amann et al., 2000). Noradrenaline, however, is not the only neurotransmitter of the sympathetic nervous system. For example, in human and rat kidney cortex neuronal release of the sympathetic cotransmitter ATP has been demonstrated (Rump et al., 2000; Vonend et al., 2002). In the present study, the possibility that extracellular ATP mediates human glomerular cell proliferation was tested.
DNA synthesis is the first important step of the cell cycle towards proliferation. It was shown that ATP increases [3H]thymidine incorporation, as a marker for DNA synthesis. ATP was the most potent P2-receptor agonist used increasing DNA synthesis by more than 200% as compared to control. Comparable results were reported by others using rat mesangial cells in culture (Schulze-Lohoff et al., 1992; Huwiler & Pfeilschifter, 1994; Harada et al., 2000).
The next step was to evaluate the effects of ATP on cell number as a marker for hyperplasia. Increasing effects of ATP on the cell number have been shown in experiments on rat mesangial (Schulze-Lohoff et al., 1992; 1995) and smooth muscle cells (Wang et al., 1992; Erlinge et al., 1993). In our experiments on human mesangial cells, ATP given in addition to PDGF increased the cell number whereas no increasing effects were observed in the presence of FCS alone (i.e. without the addition of PDGF). Thus, in some cell types such as the human mesangial cells the growth promoting effect of ATP seems to require the presence of other growth factors. Since ATP by itself did not increase cell number, ATP and PDGF seem to have synergistic effects as previously shown in cultured smooth muscle cells (Crowley et al., 1994; Erlinge, 1998). A possible explanation for the described dissociation of DNA synthesis and increase of cell number by ATP was put forward by Schulze-Lohoff and co-workers. He suggested that ATP stimulates cells to proceed into the S phase but fails to promote further steps essential for cell division (Schulze-Lohoff et al., 1995).
To further prove the role of ATP as an extracellular signalling molecule involved in cell proliferation, we investigated whether ATP activates the MAPK signalling cascades as one of the most important links between receptor activation and cellular responses. Different MAPK pathways are associated with cell proliferation, cell differentiation, cell movement and cell death. These are the MAPK42/44 and the stress-induced MAPKjnk and MAPKp38 cascades, which can be activated by various substances including cytokines, growth factors and neurotransmitters. In the present study, we investigate whether ATP-triggered proliferation involves phosphorylation of MAPK42/44, MAPKjnk and MAPKp38. ATP induced a time- and concentration-dependent activation only of the MAPK42/44 pathway. A coupling to this pathway was confirmed by the blockade of the effects of ATP on MAPK42/44 phosphorylation and in parallel on DNA synthesis by the selective MEK inhibitor PD 98059. Since the MAPK42/44 pathway is shared by a variety of growth factors, blockade by PD 98059 leads to reduction in DNA synthesis even in cells not stimulated by ATP. ATP also activates MAPK42/44 activity in rat mesangial cells (Huwiler & Pfeilschifter, 1994), glioma (Tu et al., 2000), PC12 (Soltoff et al., 1998; Swanson et al., 1998) cell lines and vascular smooth muscle cells (Wilden et al., 1998).
In contrast to observations in rat mesangial cells (Huwiler et al., 1997; 2000) we could not demonstrate activation of MAPKjnk and MAPKp38 in human mesangial cells. This suggests that there are important differences between species in P2-receptor-mediated signalling processes involved in ATP-mediated glomerular cell proliferation.
Interestingly, the dose–response curves of ATP-analogue on DNA synthesis revealed, that after reaching the maximum, a decrease in synthesis can be obtained by using higher concentrations. At present no plausible explanation for this observation can be given. Further studies whether the cytolytic P2X7 receptor might here be involved, activating antiproliferative pathways as postulated for rat mesangial cells (Schulze-Lohoff et al., 1998; Harada et al., 2000) are needed and are currently under investigation in our laboratory.
It is well known that many cell types express more than one P2-receptor (Ralevic & Burnstock, 1998). Accordingly, in the present study, the RT–PCR analysis demonstrated P2Y1,2,4,6,11,12 and P2X1,2,4,5,6,7 mRNA in human mesangial cells. Therefore, the question arises which P2-receptor subtype mediates the observed mitogenic effects of ATP. Generally, the rank order of potency of nucleotides on DNA synthesis was ATP≈ADP>ATP-γ-S>UTP≈ADP-β-S≈2-m-thio-ADP>UDP≈α-β-methylene-ATP.
This profile and the observed antagonism of suramin excludes an involvement of the α-β-methylene-ATP-sensitive P2X1- and P2X3- and suramin-insensitive P2X4-, P2X6- and P2X7-receptor subtypes in the effects induced by ATP (Khakh et al., 2001; Lambrecht et al., 2002). Since our RT–PCR data suggest the presence of α-β-methylene-ATP-insensitive, suramin-sensitive P2X2 and P2X5 receptors in human mesangial cells, an involvement of these receptor subtypes in DNA synthesis cannot be ruled out. However, ADP and UTP, which both do not activate P2X2 and P2X5 receptors, significantly increased [3H]thymidine uptake. Therefore, it seems to be convincing that P2Y-receptors play a major role as a comitogen in human mesangial cells. This is in contrast to observations on PC12- (Swanson et al., 1998) or MG-63-cells (Nakamura et al., 2000), where ATP but not ADP or UTP induced MAPK activation and DNA synthesis.
The effectiveness of ATP, ADP, ATP-γ-S, ADP-β-S and 2-methylthio-ADP is in agreement with an involvement of P2Y1-, P2Y11-, P2Y12- and P2Y13- receptors (Ralevic & Burnstock, 1998; von Kugelgen & Wetter, 2000; Communi et al., 2001). The P2Y13-receptor does not seem to play a major role in human mesangial cells since RT–PCR could not detect mRNA expression. Suramin (30 μM) reduced the effect on DNA synthesis. Because the P2Y12-receptor subtype is only weakly antagonized by suramin (Unterberger et al., 2002), a major involvement of this receptor is also less likely. In contrast to that suramin, given at a relatively low concentration of 30 μM, can antagonize P2Y1- and P2Y11-receptors (von Kugelgen & Wetter, 2000), suggesting their involvement in modulating proliferative effects in human mesangial cells. However, in haematological cells transfected with human P2Y11 receptors, α-β-methylene-ATP was able to activate this receptor subtype (van der Weyden et al., 2000). As no response to α-β-methylene-ATP could be observed in our study, one could argue against major involvement of this receptor subtype. The effect of UTP indicates the additional presence of an UTP-sensitive P2Y2- or P2Y4-receptor (Ralevic & Burnstock, 1998; von Kugelgen & Wetter, 2000). P2Y6-receptors does not seem to have an effects on MAPK42/44 activation and DNA synthesis as shown by the lack of efficacy of the P2Y6-agonist UDP (Ralevic & Burnstock, 1998; von Kugelgen & Wetter, 2000). The mitogenic effect of UTP is in clear contrast to observations in another human renal cell line (Vonend et al., 2002). Although ATP had a strong effect on DNA synthesis in human podocytes comparable to that in human mesangial cells, UTP was without an effect (Vonend et al., 2002). This underlines functional diversities of P2Y-receptors even within one organ and species.
Subtype-selective ligands are still lacking for most of the human P2-receptor subtypes. However, the presented data suggest a role of the adenine nucleotide sensitive P2Y1-receptors on the one hand and of the uracil nucleotide sensitive P2Y2- or P2Y4-receptors on the other hand on cell proliferation in human mesangial cells. Nevertheless, a supplementary role of other subtypes, like P2X2- or P2X5-receptors cannot be excluded when the endogenous agonist ATP is present.
In conclusion, we demonstrated the ability of the extracellular signal molecule ATP to trigger proliferation in cultured human mesangial cells. P2Y-receptor stimulation activates the Ras, Raf, MAPK42/44 signal transduction pathway, increases DNA synthesis and leads to amplification of growth factor induced cell proliferation. Therefore, ATP release by cell stress or sympathetic overactivity has the potential to play a major role in the progression of chronic renal failure by accumulating extracellular matrix. Further studies with subtype selective antagonists or P2-receptor subtype overexpression are needed for further classification of P2-receptors in human mesangial cells. Experiments with subtype selective antagonists will also facilitate the understanding of the role of ATP in maladaptive changes observed in a variety of renal diseases.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (RU 401/5-6). We thank the Department of Urology of the Univeristy of Freiburg (Professor H. Sommerkamp) and the Marienhospital Herne, Ruhr-University Bochum (Professor T. Senge) for supplying the human kidney cortex used for primary culture. The expert technical assistance of Petra Stunz and Bettina Priesch and the statistical advice of Dr Holland-Letz are greatly acknowledged.
Abbreviations
- ANOVA
analysis of variance
- BSA
bovine serum albumin
- ERK
extracellular signal-regulated kinase
- FCS
foetal calf serum
- HEPES
4-(2-ydroxyethyl)-1-piperazineethanesulphonic acid
- JNK
Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- mRNA
messenger ribonucleic acid
- PBS
phosphate buffer saline
- PDGF
plateled derived growth factor
- RT
reverse transcription
- SDS–PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
References
- AIZAWA T., ISHIZAKA N., KUROKAWA K., NAGAI R., NAKAJIMA H., TAGUCHI J., OHNO M. Different effects of angiotensin II and catecholamine on renal cell apoptosis and proliferation in rats. Kidney Int. 2001;59:645–653. doi: 10.1046/j.1523-1755.2001.059002645.x. [DOI] [PubMed] [Google Scholar]
- AMANN K., RUMP L.C., SIMONAVICIENE A., OBERHAUSER V., WESSELS S., ORTH S.R., GROSS M.L., KOCH A., BIELENBERG G.W., VAN KATS J.P., EHMKE H., MALL G., RITZ E. Effects of low dose sympathetic inhibition on glomerulosclerosis and albuminuria in subtotally nephrectomized rats. J. Am. Soc. Nephrol. 2000;11:1469–1478. doi: 10.1681/ASN.V1181469. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Noradrenaline and ATP: cotransmitters and neuromodulators. J. Physiol. Pharmacol. 1995;46:365–384. [PubMed] [Google Scholar]
- COMMUNI D., GONZALEZ N.S., DETHEUX M., BREZILLON S., LANNOY V., PARMENTIER M., BOEYNAEMS J.M. Identification of a novel human ADP receptor coupled to G(i) J. Biol. Chem. 2001;276:41479–41485. doi: 10.1074/jbc.M105912200. [DOI] [PubMed] [Google Scholar]
- CONVERSE R.L., JR, JACOBSEN T.N., TOTO R.D., JOST C.M., COSENTINO F., FOUAD-TARAZI F., VICTOR R.G. Sympathetic overactivity in patients with chronic renal failure. N. Engl. J. Med. 1992;327:1912–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- CORY A.H., OWEN T.C., BARLTROP J.A., CORY J.G. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207–212. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- CROWLEY S.T., DEMPSEY E.C., HORWITZ K.B., HORWITZ L.D. Platelet-induced vascular smooth muscle cell proliferation is modulated by the growth amplification factors serotonin and adenosine diphosphate. Circulation. 1994;90:1908–1918. doi: 10.1161/01.cir.90.4.1908. [DOI] [PubMed] [Google Scholar]
- ERLINGE D. Extracellular ATP: a growth factor for vascular smooth muscle cells. Gen. Pharmacol. 1998;31:1–8. doi: 10.1016/s0306-3623(97)00420-5. [DOI] [PubMed] [Google Scholar]
- ERLINGE D., YOO H., EDVINSSON L., REIS D.J., WAHLESTEDT C. Mitogenic effects of ATP on vascular smooth muscle cells vs other growth factors and sympathetic cotransmitters. Am. J. Physiol. 1993;265:H1089–H1097. doi: 10.1152/ajpheart.1993.265.4.H1089. [DOI] [PubMed] [Google Scholar]
- HAAS C.S., SCHOCKLMANN H.O., LANG S., KRALEWSKI M., STERZEL R.B. Regulatory mechanism in glomerular mesangial cell proliferation. J. Nephrol. 1999;12:405–415. [PubMed] [Google Scholar]
- HARADA H., CHAN C.M., LOESCH A., UNWIN R., BURNSTOCK G. Induction of proliferation and apoptotic cell death via P2Y and P2X receptors, respectively, in rat glomerular mesangial cells. Kidney Int. 2000;57:949–958. doi: 10.1046/j.1523-1755.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- HILGERS K.F., MANN J.F. ACE inhibitors versus AT(1) receptor antagonists in patients with chronic renal disease. J. Am. Soc. Nephrol. 2002;13:1100–1108. doi: 10.1681/ASN.V1341100. [DOI] [PubMed] [Google Scholar]
- HOLLOPETER G., JANTZEN H.M., VINCENT D., LI G., ENGLAND L., RAMAKRISHNAN V., YANG R.B., NURDEN P., NURDEN A., JULIUS D., CONLEY P.B. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- HUWILER A., PFEILSCHIFTER J. Stimulation by extracellular ATP and UTP of the mitogen-activated protein kinase cascade and proliferation of rat renal mesangial cells. Br. J. Pharmacol. 1994;113:1455–1463. doi: 10.1111/j.1476-5381.1994.tb17160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUWILER A., VAN ROSSUM G., WARTMANN M., PFEILSCHIFTER J. Stimulation by extracellular ATP and UTP of the stress-activated protein kinase cascade in rat renal mesangial cells. Br. J. Pharmacol. 1997;120:807–812. doi: 10.1038/sj.bjp.0700979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUWILER A., WARTMANN M., VAN DEN BOSCH H., PFEILSCHIFTER J. Extracellular nucleotides activate the p38-stress-activated protein kinase cascade in glomerular mesangial cells. Br. J. Pharmacol. 2000;129:612–618. doi: 10.1038/sj.bjp.0703077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHAKH B.S., BURNSTOCK G., KENNEDY C., KING B.F., NORTH R.A., SEGUELA P., VOIGT M., HUMPHREY P.P. International Union of Pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol. Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- LAMBRECHT G., BRAUN K., DAMER M., GANSO M., HILDEBRANDT C., ULLMANN H., KASSACK M.U., NICKEL P. Structure–activity relationships of suramin and pyridoxal-5′-phosphate derivatives as P2 receptor antagonists. Curr. Pharm. Des. 2002;8:2371–2399. doi: 10.2174/1381612023392973. [DOI] [PubMed] [Google Scholar]
- LEE G.S. Mesangial cell culture: its role in the understanding of the pathogenesis of glomerular disease. Ann. Acad. Med. Singapore. 1995;24:851–855. [PubMed] [Google Scholar]
- LYNCH K.J., TOUMA E., NIFORATOS W., KAGE K.L., BURGARD E.C., VAN BIESEN T., KOWALUK E.A., JARVIS M.F. Molecular and functional characterization of human P2X(2) receptors. Mol. Pharmacol. 1999;56:1171–1181. doi: 10.1124/mol.56.6.1171. [DOI] [PubMed] [Google Scholar]
- MEZZANO S.A., RUIZ-ORTEGA M., EGIDO J. Angiotensin II and renal fibrosis. Hypertension. 2001;38:635–638. doi: 10.1161/hy09t1.094234. [DOI] [PubMed] [Google Scholar]
- NAKAMURA E., UEZONO Y., NARUSAWA K., SHIBUYA I., OISHI Y., TANAKA M., YANAGIHARA N., NAKAMURA T., IZUMI F. ATP activates DNA synthesis by acting on P2X receptors in human osteoblast-like MG-63 cells. Am. J. Physiol. Cell. Physiol. 2000;279:C510–C519. doi: 10.1152/ajpcell.2000.279.2.C510. [DOI] [PubMed] [Google Scholar]
- PABST R., STERZEL R.B. Cell renewal of glomerular cell types in normal rats. An autoradiographic analysis. Kidney Int. 1983;24:626–631. doi: 10.1038/ki.1983.203. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- RUMP L.C., AMANN K., ORTH S., RITZ E. Sympathetic overactivity in renal disease: a window to understand progression and cardiovascular complications of uraemia. Nephrol. Dial. Transplant. 2000;15:1735–1738. doi: 10.1093/ndt/15.11.1735. [DOI] [PubMed] [Google Scholar]
- RUMP L.C., BOHMANN C., SCHWERTFEGER E., KRUMME B., VON KUGELGEN I., SCHOLLMEYER P. Extracellular ATP in the human kidney: mode of release and vascular effects. J. Auton. Pharmacol. 1996;16:371–375. doi: 10.1111/j.1474-8673.1996.tb00056.x. [DOI] [PubMed] [Google Scholar]
- SCHULZE-LOHOFF E., HUGO C., ROST S., ARNOLD S., GRUBER A., BRUNE B., STERZEL R.B. Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am. J. Physiol. 1998;275:F962–F971. doi: 10.1152/ajprenal.1998.275.6.F962. [DOI] [PubMed] [Google Scholar]
- SCHULZE-LOHOFF E., OGILVIE A., STERZEL R.B. Extracellular nucleotides as signalling molecules for renal mesangial cells. J. Auton. Pharmacol. 1996;16:381–384. doi: 10.1111/j.1474-8673.1996.tb00058.x. [DOI] [PubMed] [Google Scholar]
- SCHULZE-LOHOFF E., ZANNER S., OGILVIE A., STERZEL R.B. Extracellular ATP stimulates proliferation of cultured mesangial cells via P2-purinergic receptors. Am. J. Physiol. 1992;263:F374–F383. doi: 10.1152/ajprenal.1992.263.3.F374. [DOI] [PubMed] [Google Scholar]
- SCHULZE-LOHOFF E., ZANNER S., OGILVIE A., STERZEL R.B. Vasoactive diadenosine polyphosphates promote growth of cultured renal mesangial cells. Hypertension. 1995;26:899–904. doi: 10.1161/01.hyp.26.6.899. [DOI] [PubMed] [Google Scholar]
- SOLTOFF S.P., AVRAHAM H., AVRAHAM S., CANTLEY L.C. Activation of P2Y2 receptors by UTP and ATP stimulates mitogen-activated kinase activity through a pathway that involves related adhesion focal tyrosine kinase and protein kinase C. J. Biol. Chem. 1998;273:2653–2660. doi: 10.1074/jbc.273.5.2653. [DOI] [PubMed] [Google Scholar]
- STARKE K., VON KUGELGEN I., BULLOCH J.M., ILLES P. Nucleotides as cotransmitters in vascular sympathetic neuroeffector transmission. Blood Vessels. 1991;28:19–26. doi: 10.1159/000158839. [DOI] [PubMed] [Google Scholar]
- SWANSON K.D., REIGH C., LANDRETH G.E. ATP-stimulated activation of the mitogen-activated protein kinases through ionotrophic P2X2 purinoreceptors in PC12 cells. Difference in purinoreceptor sensitivity in two PC12 cell lines. J. Biol. Chem. 1998;273:19965–19971. doi: 10.1074/jbc.273.32.19965. [DOI] [PubMed] [Google Scholar]
- TU M.T., LUO S.F., WANG C.C., CHIEN C.S., CHIU C.T., LIN C.C., YANG C.M. P2Y(2) receptor-mediated proliferation of C(6) glioma cells via activation of Ras/Raf/MEK/MAPK pathway. Br. J. Pharmacol. 2000;129:1481–1489. doi: 10.1038/sj.bjp.0703182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNTERBERGER U., MOSKVINA E., SCHOLZE T., FREISSMUTH M., BOEHM S. Inhibition of adenylyl cyclase by neuronal P2Y receptors. Br. J. Pharmacol. 2002;135:673–684. doi: 10.1038/sj.bjp.0704514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER WEYDEN L., ADAMS D.J., LUTTRELL B.M., CONIGRAVE A.D., MORRIS M.B. Pharmacological characterisation of the P2Y11 receptor in stably transfected haematological cell lines. Mol. Cell. Biochem. 2000;213:75–81. doi: 10.1023/a:1007168215748. [DOI] [PubMed] [Google Scholar]
- VON KUGELGEN I., WETTER A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- VONEND O., OBERHAUSER V., VON KUGELGEN I., APEL T.W., AMANN K., RITZ E., RUMP L.C. ATP release in human kidney cortex and its mitogenic effects in visceral glomerular epithelial cells. Kidney Int. 2002;61:1617–1626. doi: 10.1046/j.1523-1755.2002.00315.x. [DOI] [PubMed] [Google Scholar]
- WANG D.J., HUANG N.N., HEPPEL L.A. Extracellular ATP and ADP stimulate proliferation of porcine aortic smooth muscle cells. J. Cell. Physiol. 1992;153:221–233. doi: 10.1002/jcp.1041530202. [DOI] [PubMed] [Google Scholar]
- WILDEN P.A., AGAZIE Y.M., KAUFMAN R., HALENDA S.P. ATP-stimulated smooth muscle cell proliferation requires independent ERK and PI3K signaling pathways. Am. J. Physiol. 1998;275:H1209–H1215. doi: 10.1152/ajpheart.1998.275.4.H1209. [DOI] [PubMed] [Google Scholar]