Abstract
Epidemiological and clinical observations suggest the involvement of nicotinic acetylcholine receptors (nAChRs) in depressive illness. Nonetheless, there is no clearcut evidence that nicotine and/or nAChR antagonists produce an antidepressant effect.
In the tail-suspension test (C57/Bl male mice), nicotine (0.8–1.2 mg kg−1 s.c. or i.p.) given 15–60 min before the measurement exerted no effect on immobility.
Given 30 min before the measurement, citalopram (2 mg kg−1) produced a slight decrease in immobility; coadministration of nicotine (0.8 mg kg−1, 15 but not 40 min before the test) to citalopram-treated mice resulted in a robust decrease in immobility. Imipramine (4 mg kg−1) did not affect immobility, but given in combination with 0.8 mg kg−1 of nicotine (15 but not 40 min before the test), a decrease in immobility was observed. Nicotine (0.8 and 1.2 mg kg−1) also produced an enhancement in the anti-immobility effect of imipramine (20 mg kg−1).
We further investigated if nAChR antagonists would influence the antidepressant-like effects of imipramine and citalopram. Unexpectedly, mecamylamine (1–2.5 mg kg−1) and dihydro-β-erythroidine (2 mg kg−1) potentiated the antidepressant-like effect of imipramine (4–20 mg kg−1). Mecamylamine (2.5 mg kg−1) but not dihydro-β-erythroidine also increased the antidepressant-like effect produced by 2 mg kg−1 of citalopram.
The interaction between nAChR antagonists and antidepressants appeared synergistic.
Neither nAChR ligands, antidepressants nor combinations of the two, affected locomotor activity.
The present results demonstrate an unexpected interaction between nAChR ligands and imipramine and citalopram in the tail-suspension test.
Keywords: Depression, antidepressants, nicotinic receptors, nAChR, imipramine, citalopram, mecamylamine, dihydro-β-erythroidine, tail-suspension test, mice
Introduction
Major depression has been treated for more than 40 years with the use of drugs that inhibit the reuptake and/or metabolism of biogenic amines. At present, the most widely prescribed antidepressants are selective reuptake inhibitors that exhibit a good safety profile and are relatively easy to use. Nonetheless, these agents do not exhibit either a faster onset of action or greater efficacy than their predecessors (Skolnick, 1999). Therefore, there are concerted efforts underway to develop antidepressants with advantages over currently used antidepressive drugs (Skolnick et al., 2001).
Converging lines of evidence indicate that nicotinic acetylcholine receptors (nAChRs) are involved in major depression. As pointed by Ferguson et al. (2000), epidemiological findings suggest that smokers more often demonstrate depressive symptoms than nonsmokers, and that depressed patients are less likely to cease smoking. In addition, depressed smokers are being more dependent on cigarettes, and the cessation of smoking is often followed by a depressive episode (Glassman et al., 1990; Lerman et al., 1996; Covey et al., 1998). Finally, smokers with a history of major depressive episode are more likely to relapse than smokers with no history of depression (Fergusson et al., 1996; Kinnunen et al., 1996). Other pharmacological lines of evidence indicate that the antidepressant drug, bupropion, antagonizes neuronal nAChRs (Slemmer et al., 2000), and has also been used as the treatment for smoking cessation (Lief, 1996; Dewey et al., 1999). Furthermore, nicotine patches can improve the mood of depressed patients (Salin-Pascual et al., 1996).
Based on these observations, it has been hypothesized that nAChRs are involved in the major depression. It has been hypothesized that nicotine produces antidepressant effects, and that smokers ‘self-medicate' the underlying depressive illness with nicotine, and/or depressive symptoms produced by nicotine withdrawal (Markou et al., 1998). Another hypothesis that appears at face value, contradictory to the first, is based on findings indicating that most clinically effective antidepressants antagonize nAChRs (Rana et al., 1993; Fryer & Lukas, 1999; Slemmer et al., 2000; Lopez-Valdes & Garcia-Colunga, 2001). This latter hypothesis assumes that the common final pathway for the antidepressant effects is the inhibition of nAChRs (Shytle et al., 2002). Finally, there is a hypothesis that emphasizes genetic factors predisposing to vulnerability to both major depression and smoking (Picciotto et al., 2002).
Although these hypotheses are based on the assumption that nicotine, the major (but not only) constituent of cigarettes, may have antidepressant properties, the preclinical data on the antidepressant-like (AD-like) effects of nAChR ligands are ambiguous. For example, in intact, although aged (25–30 months old) rats, Nakamura & Tanaka (2001) reported that nicotine at a dose of 0.1 mg kg−1 i.p., produced an AD-like effect in the forced swimming test. However, this finding could be attributed to a much higher immobility time of control rats in a particular set of comparisons than observed for other controls, suggesting that nicotine produced the same effect as was observed for other controls (Nakamura & Tanaka, 2001). In Ferguson et al. (2000) studies, nicotine appeared not to influence the learned helplessness response, although subtype-selective nAChR agonist produced AD-like effect. Perhaps more suggestive are studies on the Flinders Sensitive Line (FSL) rats, regarded as a ‘genetic animal model of depression'. In these rats, which in drug-free state demonstrate an exaggerated immobility in the forced swimming test, acute or chronic administration of nicotine (0.4 mg kg−1 s.c.) significantly improved the performance in the forced swimming test (Tizabi et al., 1999). This effect was prevented by pretreatment with nAChR antagonist, mecamylamine (MEC), which by itself did not affect forced swimming response (Tizabi et al., 2000). Since FSL rats are selectively bred for their hyper-responsiveness to cholinergic stimulation, these findings cannot be regarded as surprising. The most convincing study demonstrating AD-like effect of nicotine was reported by Semba et al. (1998), who showed that the chronic treatment with nicotine produced AD-like effects in a learned helplessness ‘model of depression'. Thus, it appears that the ‘antidepressant' activity of nicotine is not readily detectable with the use of behavioral despair tests, that is, standard screening procedures involving naïve and standard laboratory animals (Porsolt & Lenegre, 1992). Similarly, we failed to find data supporting the AD-like effects of nAChR antagonists with the exception of one study, in which MEC, at a high dose (10 mg kg−1), decreased immobility in aged (25–30 months old) rats (Nakamura & Tanaka, 2001). It should be mentioned, however, that a recent preliminary clinical report indicates that MEC may produce antidepressive effects (Shytle et al., 2002).
The lack of convincing preclinical data demonstrating that nicotine and/or nAChR antagonists produce AD-like effects in the commonly used screening procedures (such as the forced swimming and tail-suspension tests) is surprising. This might be due to the fact that these screening tests are insensitive to all the potential mechanisms of antidepressant actions, and/or that such experiments have not been yet reported, and/or that nicotinic agents are devoid of AD-like actions.
In the present study, we investigated if nicotine and/or nAChR antagonists, MEC and dihydro-β-erythroidine (DHβE), may produce and/or influence the AD-like effects of imipramine (IMI) and citalopram (CIT) in the tail-suspension test in mice (Steru et al., 1985). This test is often used in screening for potential antidepressants, because it is characterized by a high predictive validity after single-dose treatment (Willner, 1991).
Methods
Subjects
Male C57Bl/6J/Han/IMP mice (IMP, Lodz, Poland) weighing 25–30 g at the start of the experiments were used. Mice were group-housed in the standard laboratory cages and kept in a humidity- and temperature (21±2°C)-controlled colony room with a 12-h light/dark cycle (lights on: 07:00, off: 19:00). Commercial food and tap water were available ad libitum. All mice were used only once.
Apparatus and procedure
The immobility was induced according to the procedure of Steru et al. (1985). Mice were individually suspended 75 cm above the tabletop with an adhesive tape placed ∼1 cm from the tip of the tail. Immobility duration was recorded for 6 min. Mice were considered immobile only when they hung passively and completely motionless. A trained observer, unaware of the treatment conditions, used PC computer and the ‘PORSOLT' program (Infallible Software, Res Tri. Pk., NC, U.S.A.) to measure the duration of immobility. There were 5–13 of mice per treatment group.
Mice were treated with an nAChR ligand (or placebo) and the antidepressant (or placebo). Antidepressants were always given i.p., 30 min before the test. In studies aimed to investigate the effects of nicotine on antidepressant response, nicotine (or placebo) was administered (if not indicated otherwise) s.c., 15 or 40 min before the test. In the studies aimed to investigate the effects of nAChR antagonists on antidepressant response, MEC, DHβE or placebo were given 40 min before the test.
The shorter (15 min) interval between nicotine administration and the test was due to the short half-life of nicotine (Mathieu-Kia et al., 2002). The longer interval (40 min) between nicotine administration and the test was used for comparison with experiments involving nAChR antagonists.
Locomotor activity
Locomotor activity was measured in custom-made metal circular actometers (25 cm in diameter) under low illumination. Two pairs of photocell beams automatically recorded gross movements; due to the construction of apparatus, the minor movements (that would reflect stereotypies) were not recorded. Photocells were located 1.5 cm above the bottom of the apparatus and the beams crossed each other at the center of the apparatus. Mice were transferred for adaptation into the testing room at least 2 h prior to drug administration and placed into the apparatus for 90 min of habituation.
To study the effects of nicotine and antidepressants, to follow the design of injections described for the tail-suspension test, mice were injected i.p. with the antidepressant 30 min, and s.c. with nicotine 15 min, before the first measurement, respectively. The measurements continued for the following 40 min in 10 min epochs.
To study the effects of nAChR antagonists and antidepressants, like in the tail-suspension test, mice were injected s.c. with the nAChR antagonists 40 min, and i.p. with the antidepressants 30 min before the first measurement. The measurements continued for the following 80 min in 30 min and then 10 min epochs. This time of testing maximized chances to detect possible change in locomotion due to the treatments. Each group consisted of 6–10 mice with the exception that placebo+placebo and placebo+IMI groups consisted of 15–17 mice due to the fact that experiments lasted for several days and more controls were needed. There were no differences among the control groups, such that control data were pooled for statistical analysis and graphical presentation.
Data presentation and statistics
Immobility (in s) data were used for statistical analysis (one-way ANOVA followed by Newman–Keul's test). For simplicity, data from locomotor activity experiments were calculated with the use of trapezoidal rule and are presented as the area under the curve (AUC). These data were analyzed with one-way ANOVA. P<0.05 was considered significant. Statistica 5.0 for Windows was used throughout.
Drugs
Imipramine HCl (ICN Polfa, Krakow, Poland), citalopram, DHβE, MEC HCl and (−)-nicotine hydrogen bitartrate (Sigma-Aldrich, Poznan, Poland) were dissolved in sterile saline (placebo). When appropriate, pH was adjusted to 7.0 by the addition of 10 N NaOH solution. Drug or placebo solutions were administered in a volume of 10 ml kg−1. Doses of nicotine are expressed in terms of its free base concentrations and for all other compounds were calculated as respective salts.
All experiments were carried out according to the National Institutes of Health Guide for Care and Use of Laboratory Animals (revised 1996) and were approved by the Institute of Pharmacology, Polish Academy of Sciences in Krakow Animal Care and Use Bioethics Commission.
Results
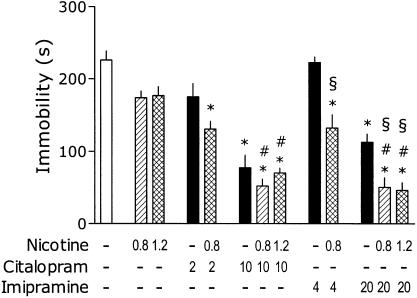
The data presented in Figure 1 summarize the effects of nicotine administration (15 min before the test) on AD-like action of IMI and CIT. One-way ANOVA revealed a significant effect of treatments: F(12,88)=23.01, P<0.001. Nicotine given s.c. 15 min before the test did not significantly influence the immobility of mice. Citalopram (2 mg kg−1) produced insignificant effect on immobility (P=0.08, post hoc Newman–Keul's test). However, the same dose of CIT significantly reduced immobility in mice cotreated with 0.8 mg kg−1 of nicotine. Citalopram (10 mg kg−1) remarkably reduced the immobility and this effect was not further potentiated by nicotine (0.8 or 1.2 mg kg−1). Nicotine (0.8 mg kg−1) appeared to reveal the ‘antidepressant' effect of IMI (4 mg kg−1). At the higher dose of 20 mg kg−1, IMI produced reliable decrease in immobility and this effect was further increased by nicotine (0.8 and 1.2 mg kg−1).
Figure 1.
Effects of nicotine on immobility time in the tail-suspension test and its interaction with CIT and IMI. Male C57/Bl mice were injected i.p., 30 min before the test with the antidepressant and s.c., 15 min before the test with nicotine. Minus signs indicate the absence of a given treatment, the numbers on abscissa represent the doses in mg kg−1. Post hoc analysis demonstrates significant (P<0.05) differences as compared to: ‘*' placebo, ‘#' respective dose of nicotine and ‘§' respective dose of imipramine. Note: for the clarity of figure, the precise P is not shown.
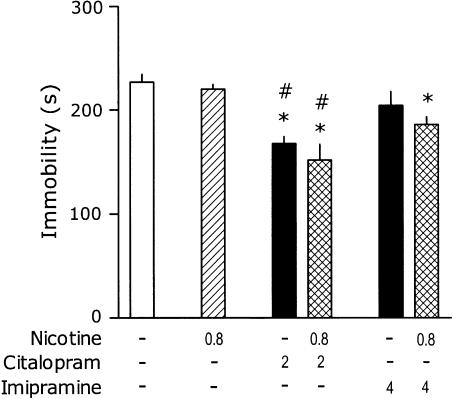
In a repetition of this experiment with selected doses of antidepressants, nicotine (0.8 mg kg−1) was administered 40 min, instead of 15 min before the test. One-way ANOVA revealed a significant effect of treatments: F(5,50)=8.4, P<0.001. Nicotine given s.c. 40 min before the test did not influence immobility. In this experiment, citalopram (2 mg kg−1) produced a significant effect on immobility, but coadministration of 0.8 mg kg−1 of nicotine did not significantly affect CIT effect. Imipramine (4 mg kg−1) did not influence immobility and coadministration of 0.8 mg kg−1 of nicotine did not affect IMI response (Figure 2).
Figure 2.
Effects of nicotine on immobility time in the tail-suspension test and its interaction with CIT and IMI. Mice were injected s.c., 40 min before the test with nicotine and i.p., 30 min before the test with the antidepressant. Minus signs indicate the absence of a given treatment, the numbers on abscissa represent the doses in mg kg−1. Post hoc analysis demonstrates significant (P<0.05) differences as compared to: ‘*' placebo and, ‘#' respective dose of nicotine. Note: for the clarity of figure, the precise P is not shown.
Since s.c. nicotine failed to exert any effects on immobility, in a separate experiment mice were treated i.p. with 1.2 mg kg−1 of nicotine (or placebo) 15 min, and separately, 60 min before the tail suspension test. The immobility scores for mice treated with i.p. nicotine 15 and 60 min before the test were 187±13.5 and 170±8.3 s, respectively, and for mice treated with placebo 15 and 60 min before the test were 179±14.4 and 200±22.6 s, respectively (there were eight mice per treatment in each group). One-way ANOVA demonstrated no effect of treatment: F(3,31)=0.6.
It appeared that in the tail-suspension test, nicotine used at various doses, routes of administration and intervals between the injection and the test did not influence immobility of mice.
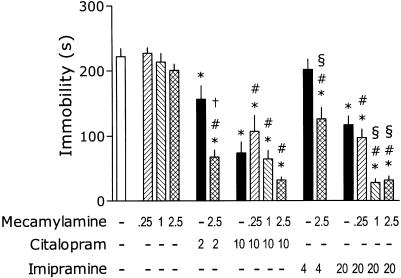
MEC at doses 0.25–2.5 mg kg−1 did not influence the immobility, although one-way ANOVA revealed significant overall effect of treatments: F(15,133)=40.39, P<0.001 (Figure 3). In this set of experiments, CIT (2 mg kg−1) significantly inhibited the immobility and this effect was further increased by MEC (2.5 mg kg−1). CIT (10 mg kg−1) remarkably reduced the immobility, but this effect was not further influenced by MEC (0.25–1 mg kg−1). As was the case with nicotine, MEC (2.5 mg kg−1) appeared to ‘unmask' an AD-like effect of 4 mg kg−1 of IMI. At the higher dose of 20 mg kg−1, IMI produced a reliable decrease in immobility, and this effect was further increased by MEC (1 and 2.5 but not 0.25 mg kg−1).
Figure 3.
Effects of MEC on immobility time in the tail-suspension test and its interaction with CIT and IMI. Mice were injected s.c., 40 min before the test with MEC and i.p., 30 min before the test with the antidepressant. Minus signs indicate the absence of a given treatment; the numbers on abscissa represent the doses in mg kg−1. Post hoc analysis demonstrates significant (P<0.05) differences as compared to: ‘*' placebo, ‘#' respective dose of MEC, ‘§' respective dose of IMI and ‘†' respective dose of CIT. Note: for the clarity of figure, the precise P is not shown.
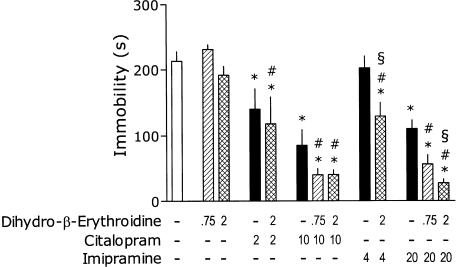
DHβE at doses of 0.75 and 2 mg kg−1 did not affect the immobility (Figure 4). One-way ANOVA revealed a significant overall effect of treatments: F(12,119)=25.66, P<0.001. CIT (2 mg kg−1) significantly inhibited immobility, but this effect was not affected by DHβE (2 mg kg−1). CIT (10 mg kg−1) remarkably reduced the immobility and this effect was not influenced by DHβE (0.75 or 2 mg kg−1). As was the case of nicotine and MEC, DHβE (2 mg kg−1) appeared to reveal the ‘antidepressant' effect of IMI (4 mg kg−1). At the higher dose of 20 mg kg−1, IMI produced a reliable decrease in immobility and this effect was further potentiated by DHβE (2 but not 0.75 mg kg−1).
Figure 4.
Effects of DHβE on immobility time in the tail-suspension test and its interaction with CIT and IMI. Mice were injected s.c., 40 min before the test with DHβE and i.p., 30 min before the test with the antidepressant. Minus signs indicate the absence of a given treatment; the numbers on abscissa represent the doses in mg kg−1. Post hoc analysis demonstrates significant (P<0.05) differences as compared to: ‘*' placebo, ‘#' respective dose of DHβE and ‘§'respective dose of IMI. Note: for the clarity of figure, the precise P is not shown.
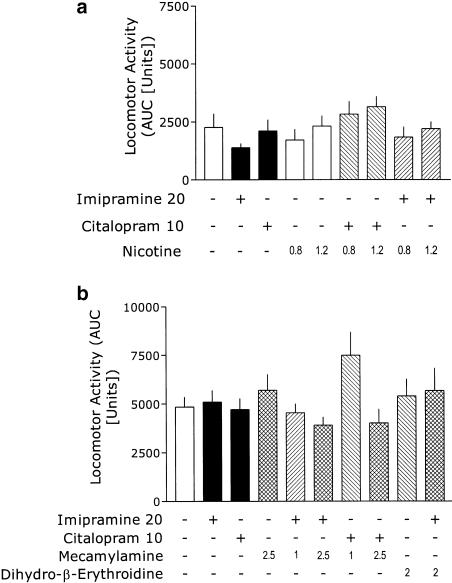
The last experiment was carried out to investigate if the decrease in immobility could be explained by an increase of the locomotor activity of mice.
Neither nicotine, nor the antidepressants itself or in combination with nicotine, produced significant effects on this measure (Figure 5a), since one-way ANOVA demonstrated no significant effects of treatment: F(8,49)=1.5. Similarly, neither MEC, DHβE, nor the antidepressants itself or in combination with nAChR antagonists, significantly affected locomotor activity (Figure 5b), since one-way ANOVA demonstrated no significant effects of treatment: F(9,84)=1.69. The combination of MEC (1 mg kg−1) and CIT (10 mg kg−1) appear to slightly increase the locomotor activity, but this effect did not reach statistical significance.
Figure 5.
Effects of antidepressants, nAChR ligands, and their combination on locomotor activity of mice. Data are presented as the mean+s.e.m. AUC. Mice were injected with the antidepressant 30 min, and with nicotine 15 min, before the test, respectively; the measurements continued for the next 40 min (a), or with nAChR antagonist 40 min, and with the antidepressant 30 min before the test, respectively; the measurements continued for the next 80 min (b). Plus and minus signs indicate the presence or absence of a given treatment, respectively. The numbers on abscissa represent the doses in mg kg−1. One-way ANOVAs showed no overall effects of treatments.
Discussion
In the present study, nicotine was inactive in the tail-suspension test but increased the antidepressant-like activity of IMI. This facilitating action of nicotine was observable at doses that did not affect locomotor activity. In our separate, unpublished studies, no effect of nicotine (0.125–1 mg kg−1, i.p., 30 min before measurement) was observed in the forced swimming test (Porsolt et al., 1977) in albino Swiss mice (data not shown). Unexpectedly, the nAChR antagonists MEC and DHβE also enhanced the AD-like action of IMI. In addition, MEC, but not DHβE, facilitated AD-like effects of CIT. The effect of nAChR antagonists was unexpected, and may be viewed at face value as counterintuitive. MEC and DHβE themselves failed to affect the immobility time in the tail-suspension test and did not influence the locomotor activity.
Since IMI is structurally dissimilar and may exert its AD-like effect by a different mechanism than CIT, it is likely that the reported effects are not due to a pharmacokinetic interaction between these antidepressants and the nAChR ligands. Also, MEC and DHβE differ from each other in many respects. MEC, currently used clinically in the treatment of hypertension, is a relatively nonspecific noncompetitive nAChR antagonist (Taylor, 1980; Varanda et al., 1985) though it demonstrates a slightly higher selectivity for the α-3- and β-4-containing nAChRs. In contrast, DHβE is a competitive nAChR antagonist that exhibits high affinity and selectivity for the α-4- and β-2-containing receptor subunits (Harvey et al., 1996). Despite these differences, the observation that MEC and DHβE produce similar (but not identical) behavioral effects in the tail-suspension test, indicates that the sites of action of these compounds are nAChRs, and not other receptors (5-HT3, NMDA) sensitive to MEC (Kenny et al., 2000), but not DHβE. In addition, this indicates that the peripheral ‘side effects' (e.g., hypotension) produced by MEC, but not DHβE, were not involved in the facilitation of AD-like action.
The concentrations of 5-HT, NE and DA in the brain are critical for mood and motivational processes, and disturbances in these monoamine systems are considered to be responsible for mood instability and depression (for reviews, see Skolnick (1999) and Vetulani & Nalepa (2000)). nAChRs are located mainly presynaptically and modulate the release of a variety of neurotransmitters including ACh itself, DA, NE and 5-HT (Toth et al., 1992) (for a review, see McGehee & Role (1996)). The antidepressant activity of IMI and CIT is believed to be due to the inhibition of monoamine reuptake and a resulting increase in monoamine's concentration in the synapse (Vetulani & Nalepa, 2000). Thus, combined administration of nicotine and IMI or CIT may result in the significant increase in concentration and/or prolongation of the presence of monoamines in synaptic cleft as a consequence of enhanced monoamine release (nicotine) and inhibition of monoamine reuptake (antidepressants). The behavioral outcome could be demonstrated as a decrease in immobility time in tail-suspension test. This hypothesis implies that the potential AD-like effect of nicotine would not be detected until nicotine administration is accompanied by administration of monoamine reuptake inhibitors. In support, Ribeiro et al. (1993) found in microdialysis studies in anesthetized rats that s.c. injection of nicotine at doses 2–8 mg kg−1 (at least ∼2 times higher than used in the present study) increased 5-HT concentration in the frontal cortex for the first 15 min after nicotine injection; however, in the presence of SSRI antidepressant fluoxetine, even 1.6 mg kg−1 of nicotine increased 5-HT concentration and prolonged its presence. Although this hypothesis may explain a potentiation of the AD-like effect by the combination of nicotine and antidepressants, it fails to explain the same interaction between antidepressants and nAChR antagonists.
Despite the opposite interactions with nAChRs, nicotine and nAChR antagonists have been shown to share some physiological and behavioral effects. For example, MEC at the low (0.5 μM) concentration antagonized nicotine-induced 5-HT release in rat hippocampal slices, but it itself stimulated 5-HT release at concentrations of 1–50 μM (Kenny et al., 2000). In Mihailescu et al. (1998) study, not only nicotine (10–300 μM) but also MEC (1–20 μM) stimulated 5-HT dorsal raphe neurons. At the behavioral level, nicotine and MEC share anxiogenic effects in rats (File et al., 2000).
Other lines of evidence suggest that apart from its agonistic activity, nicotine might act as a functional antagonist at nAChRs due to its ability to produce a desensitization of nAChRs that follows activation phase (Fenster et al., 1997). Continuous exposure to nicotine may ‘lock' nAChRs in a desensitized state that is functionally equivalent to the treatment with an antagonist (Wonnacott, 1990). Such an effect depends on the subunit composition of nAChRs; if this action is manifested in vivo at the nAChRs involved in the AD-like effects of drugs in the tail-suspension test, it may explain why both nicotine and nAChR antagonists facilitate the effects of IMI and CIT.
In vitro studies demonstrated that a number of antidepressants may inhibit the function of nAChRs (Rana et al., 1993; Fryer & Lukas, 1999; Slemmer et al., 2000; Lopez-Valdes & Garcia-Colunga, 2001). The blockade occurs at the external side of the nAChR channel when receptor is inactive, and within the channel when the receptor is activated (Lopez-Valdes & Garcia-Colunga, 2001). Fluoxetine, the antidepressant with similar mechanism of action to CIT, not only blocks the activated nAChRs but also increases the rate of desensitization of nAChRs in Xenopus oocytes (Garcia-Colunga et al., 1997).
Although the present results and methodology cannot precisely explain the interaction between antidepressants and nAChR ligands at the nAChRs, it is worth noting that this interaction appeared supra-additive (synergistic). This is because none of the nAChR ligands produced an AD-like effect, but some potentiated the antidepressant-like effects of IMI and CIT. Thus, it is likely that if antidepressants decreased the immobility by inhibiting the function of nAChRs, then the site or nature of interaction with nAChRs must be different from that involved in the action of nAChR ligands, since synergistic interactions are more likely when compounds act via different mechanisms (Tallarida et al., 1997).
The present studies indicate that the interaction between various nAChR ligands and antidepressants is not identical. While MEC pretreatment enhanced the AD-like effect of IMI and CIT, pretreatment with nicotine produced only a modest effect on CIT action and DHβE had no effect on CIT AD-like action at all. Since all the three nAChR ligands potentiated the AD-like action of IMI, it appears that the different nature of interaction between antidepressants and nAChR ligands is due to a different mechanism of AD-like action of IMI and CIT, different mechanism of action of MEC and DHβE, or both mechanisms. It is known that NE and 5-HT neurons contain nAChRs of different subunit composition (Lena et al., 1999; Miller et al., 2002a, b) and it is possible that either α-4-containing nAChRs (which DHβE preferentially binds to Harvey et al. (1996)) are not abundant at 5-HT neurons, or that they play a minor role in the effect of antidepressants. It is also likely that different antidepressants act with different selectivity at nAChRs that differ in subunit composition and/or brain localization as discussed in detail by Miller et al. (2002b). Studies with more selective nAChR agonists could also aid in elucidating the nAChRs subtypes contributing to these phenomena.
Acknowledgments
The study was supported by statutory activity of IF PAN, Krakow, Poland. We gratefully thank Dr P. Skolnick for his helpful comments and the linguistic correction of the manuscript.
Abbreviations
- ACh
acetylcholine
- CIT
citalopram
- DA
dopamine
- DHβE
dihydro-β-erythroidine
- GABA
gamma amino butyric acid
- 5-HT
serotonin
- IMI
imipramine
- i.p.
intraperitoneally
- MEC
mecamylamine
- nAChR
acetylcholine nicotinic receptor
- NMDA
N-methyl-D-aspartate
- NE
norepinephrine
- s.c.
subcutaneously
- SSRI
selective serotonin reuptake inhibitor
References
- COVEY L.S., GLASSMAN A.H., STETNER F. Cigarette smoking and major depression. J. Addict. Dis. 1998;17:35–46. doi: 10.1300/J069v17n01_04. [DOI] [PubMed] [Google Scholar]
- DEWEY S.L., BRODIE J.D., GERASIMOV M., HORAN B., GARDNER E.L., ASHBY C.R., JR A pharmacologic strategy for the treatment of nicotine addiction. Synapse. 1999;31:76–86. doi: 10.1002/(SICI)1098-2396(199901)31:1<76::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- FENSTER C.P., RAINS M.F., NOERAGER B., QUICK M.W., LESTER R.A. Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. J. Neurosci. 1997;17:5747–5759. doi: 10.1523/JNEUROSCI.17-15-05747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERGUSON S.M., BRODKIN J.D., LLOYD G.K., MENZAGHI F. Antidepressant-like effects of the subtype-selective nicotinic acetylcholine receptor agonist, SIB–1508Y, in the learned helplessness rat model of depression. Psychopharmacology (Berl.) 2000;152:295–303. doi: 10.1007/s002130000531. [DOI] [PubMed] [Google Scholar]
- FERGUSSON D.M., LYNSKEY M.T., HORWOOD L.J. Comorbidity between depressive disorders and nicotine dependence in a cohort of 16-year-olds. Arch. Gen. Psychiatry. 1996;53:1043–1047. doi: 10.1001/archpsyc.1996.01830110081010. [DOI] [PubMed] [Google Scholar]
- FILE S.E., KENNY P.J., CHEETA S. The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacol. Biochem. Behav. 2000;66:65–72. doi: 10.1016/s0091-3057(00)00198-2. [DOI] [PubMed] [Google Scholar]
- FRYER J.D., LUKAS R.J. Antidepressants noncompetitively inhibit nicotinic acetylcholine receptor function. J. Neurochem. 1999;72:1117–1124. doi: 10.1046/j.1471-4159.1999.0721117.x. [DOI] [PubMed] [Google Scholar]
- GARCIA-COLUNGA J., AWAD J.N., MILEDI R. Blockage of muscle and neuronal nicotinic acetylcholine receptors by fluoxetine (Prozac) Proc. Natl. Acad. Sci. U.S.A. 1997;94:2041–2044. doi: 10.1073/pnas.94.5.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLASSMAN A.H., HELZER J.E., COVEY L.S., COTTLER L.B., STETNER F., TIPP J.E., JOHNSON J. Smoking, smoking cessation, and major depression. JAMA. 1990;264:1546–1549. [PubMed] [Google Scholar]
- HARVEY S.C., MADDOX F.N., LUETJE C.W. Multiple determinants of dihydro-beta-erythroidine sensitivity on rat neuronal nicotinic receptor alpha subunits. J. Neurochem. 1996;67:1953–1959. doi: 10.1046/j.1471-4159.1996.67051953.x. [DOI] [PubMed] [Google Scholar]
- KENNY P.J., FILE S.E., NEAL M.J. Evidence for a complex influence of nicotinic acetylcholine receptors on hippocampal serotonin release. J. Neurochem. 2000;75:2409–2414. doi: 10.1046/j.1471-4159.2000.0752409.x. [DOI] [PubMed] [Google Scholar]
- KINNUNEN T., DOHERTY K., MILITELLO F.S., GARVEY A.J. Depression and smoking cessation: characteristics of depressed smokers and effects of nicotine replacement. J. Consult. Clin. Psychol. 1996;64:791–798. doi: 10.1037//0022-006x.64.4.791. [DOI] [PubMed] [Google Scholar]
- LENA C., DE KERCHOVE D.'E., CORDERO-ERAUSQUIN M., LE NOVERE N., MAR ARROYO-JIMENEZ M., CHANGEUX J.P. Diversity and distribution of nicotinic acetylcholine receptors in the locus ceruleus neurons. Proc. Natl. Acad. Sci. U.S.A. 1999;96:12126–12131. doi: 10.1073/pnas.96.21.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LERMAN C., AUDRAIN J., ORLEANS C.T., BOYD R., GOLD K., MAIN D., CAPORASO N. Investigation of mechanisms linking depressed mood to nicotine dependence. Addict. Behav. 1996;21:9–19. doi: 10.1016/0306-4603(95)00032-1. [DOI] [PubMed] [Google Scholar]
- LIEF H.I. Bupropion treatment of depression to assist smoking cessation. Am. J. Psychiatry. 1996;153:442. doi: 10.1176/ajp.153.3.442a. [DOI] [PubMed] [Google Scholar]
- LOPEZ-VALDES H.E., GARCIA-COLUNGA J. Antagonism of nicotinic acetylcholine receptors by inhibitors of monoamine uptake. Mol. Psychiatry. 2001;6:511–519. doi: 10.1038/sj.mp.4000885. [DOI] [PubMed] [Google Scholar]
- MARKOU A., KOSTEN T.R., KOOB G.F. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- MATHIEU-KIA A.M., KELLOGG S.H., BUTELMAN E.R., KREEK M.J. Nicotine addiction: insights from recent animal studies. Psychopharmacology (Berl.) 2002;162:102–118. doi: 10.1007/s00213-002-1096-0. [DOI] [PubMed] [Google Scholar]
- MCGEHEE D.S., ROLE L.W. Presynaptic ionotropic receptors. Curr. Opin. Neurobiol. 1996;6:342–349. doi: 10.1016/s0959-4388(96)80118-8. [DOI] [PubMed] [Google Scholar]
- MIHAILESCU S., PALOMERO-RIVERO M., MEADE-HUERTA P., MAZA-FLORES A., DRUCKER-COLIN R. Effects of nicotine and mecamylamine on rat dorsal raphe neurons. Eur. J. Pharmacol. 1998;360:31–36. doi: 10.1016/s0014-2999(98)00658-x. [DOI] [PubMed] [Google Scholar]
- MILLER D.K., SUMITHRAN S.P., DWOSKIN L.P. Bupropion inhibits nicotine-evoked [3H]overflow from rat striatal slices preloaded with [3H]dopamine and from rat hippocampal slices preloaded with [3H]norepinephrine. J. Pharmacol. Exp. Ther. 2002a;302:1113–1122. doi: 10.1124/jpet.102.033852. [DOI] [PubMed] [Google Scholar]
- MILLER D.K., WONG E.H., CHESNUT M.D., DWOSKIN L.P. Reboxetine: functional inhibition of monoamine transporters and nicotinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 2002b;302:687–695. doi: 10.1124/jpet.302.2.687. [DOI] [PubMed] [Google Scholar]
- NAKAMURA K., TANAKA Y. Antidepressant-like effects of aniracetam in aged rats and its mode of action. Psychopharmacology (Berl.) 2001;158:205–212. doi: 10.1007/s002130100849. [DOI] [PubMed] [Google Scholar]
- PICCIOTTO M.R., BRUNZELL D.H., CALDARONE B.J. Effect of nicotine and nicotinic receptors on anxiety and depression. NeuroReport. 2002;13:1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- PORSOLT R.D., BERTIN A., JALFRE M. Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- PORSOLT R.D., LENEGRE A.Behavioral models of depression Experimental Approaches to Anxiety and Depression 1992London: John Wiley & Sons; 73–85.ed. Elliott, J.M., Heal, D.J. & Marsden, C.A, pp [Google Scholar]
- RANA B., MCMORN S.O., REEVE H.L., WYATT C.N., VAUGHAN P.F, PEERS C. Inhibition of neuronal nicotinic acetylcholine receptors by imipramine and desipramine. Eur. J. Pharmacol. 1993;250:247–251. doi: 10.1016/0014-2999(93)90388-x. [DOI] [PubMed] [Google Scholar]
- RIBEIRO E.B., BETTIKER R.L., BOGDANOV M., WURTMAN R.J. Effects of systemic nicotine on serotonin release in rat brain. Brain Res. 1993;621:311–318. doi: 10.1016/0006-8993(93)90121-3. [DOI] [PubMed] [Google Scholar]
- SALIN-PASCUAL R.J., ROSAS M., JIMENEZ-GENCHI A., RIVERA-MEZA B.L., DELGADO-PARRA V. Antidepressant effect of transdermal nicotine patches in nonsmoking patients with major depression. J. Clin. Psychiatry. 1996;57:387–389. [PubMed] [Google Scholar]
- SEMBA J., MATAKI C., YAMADA S., NANKAI M., TORU M. Antidepressantlike effects of chronic nicotine on learned helplessness paradigm in rats. Biol. Psychiatry. 1998;43:389–391. doi: 10.1016/s0006-3223(97)00477-0. [DOI] [PubMed] [Google Scholar]
- SHYTLE R.D., SILVER A.A., LUKAS R.J., NEWMAN M.B., SHEEHAN D.V., SANBERG P.R. Nicotinic acetylcholine receptors as targets for antidepressants. Mol. Psychiatry. 2002;7:525–535. doi: 10.1038/sj.mp.4001035. [DOI] [PubMed] [Google Scholar]
- SKOLNICK P. Antidepressants for the new millenium. Eur. J. Pharmacol. 1999;375:31–40. doi: 10.1016/s0014-2999(99)00330-1. [DOI] [PubMed] [Google Scholar]
- SKOLNICK P., LEGUTKO B., LI X., BYMASTER F.P. Current perspectives on the development of non-biogenic amine-based antidepressants. Pharmacol. Res. 2001;43:411–423. doi: 10.1006/phrs.2000.0806. [DOI] [PubMed] [Google Scholar]
- SLEMMER J.E., MARTIN B.R., DAMAJ M.I. Bupropion is a nicotinic antagonist. J. Pharmacol. Exp. Ther. 2000;295:321–327. [PubMed] [Google Scholar]
- STERU L., CHERMAT R., THIERRY B., SIMON P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- TALLARIDA R.J., STONE D.J., JR, RAFFA R.B. Efficient designs for studying synergistic drug combinations. Life Sci. 1997;61:417–425. doi: 10.1016/s0024-3205(97)01030-8. [DOI] [PubMed] [Google Scholar]
- TAYLOR P.Ganglionic stimulating and blocking agents The Pharmacological Basis of Therapeutics 1980New York: Macmillan Publishing Company; 211–219.6th edn. Ed. Gilman, A.G., Goodman, L.S & Gillman, A., pp [Google Scholar]
- TIZABI Y., OVERSTREET D.H., REZVANI A.H., LOUIS V.A., CLARK E., JR, JANOWSKY D.S., KLING M.A. Antidepressant effects of nicotine in an animal model of depression. Psychopharmacology. 1999;142:193–199. doi: 10.1007/s002130050879. [DOI] [PubMed] [Google Scholar]
- TIZABI Y., REZVANI A.M., RUSSELL L.T., TYLER K.Y., OVERSTREET D.H. Depressive characteristics of FSL rats: involvement of central nicotinic receptors. Pharmacol. Biochem. Behav. 2000;66:73–77. doi: 10.1016/s0091-3057(00)00236-7. [DOI] [PubMed] [Google Scholar]
- TOTH E., SERSHEN H., HASHIM A., VIZI E.S., LAJTHA A. Effect of nicotine on extracellular levels of neurotransmitters assessed by microdialysis in various brain regions: role of glutamic acid. Neurochem. Res. 1992;17:265–271. doi: 10.1007/BF00966669. [DOI] [PubMed] [Google Scholar]
- VARANDA W.A., ARACAVA Y., SHERBY S.M., VANMETER W.G., ELDEFRAWI M.E., ALBUQUERQUE E.X. The acetylcholine receptor of the neuromuscular junction recognizes mecamylamine as a noncompetitive antagonist. Mol. Pharmacol. 1985;28:128–137. [PubMed] [Google Scholar]
- VETULANI J., NALEPA I. Antidepressants: past, present and future. Eur. J. Pharmacol. 2000;405:351–363. doi: 10.1016/s0014-2999(00)00565-3. [DOI] [PubMed] [Google Scholar]
- WILLNER P.Animal models of depression Behavioural Models in Psychopharmacology: Theoretical, Industrial and Clinical Perspectives 1991Cambridge: Cambridge University Press; 91–124.ed. Willner, P., pp [Google Scholar]
- WONNACOTT S. The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Pharmacol. Sci. 1990;11:216–219. doi: 10.1016/0165-6147(90)90242-z. [DOI] [PubMed] [Google Scholar]