Abstract
Paeonol was tested for its anti-inflammatory and analgesic effects in a rat model of carrageenan-evoked thermal hyperalgesia. The possible mechanisms involved in these effects were also investigated.
Pre- and post-treatment with paeonol (30, 50 or 100 mg kg−1, i.p.) dose-dependently inhibited the carrageenan-evoked thermal hyperalgesia.
Treatment with paeonol dose-dependently inhibited tumour necrosis factor-α (TNF-α) and interleukin-lβ (IL-1β) formation, but enhanced IL-10 production in the rat paw exudates both at the early (1.5 h) and late phase (4 h) after carrageenan injection. However, inhibition of IL-6 formation by paeonol was only observed at the late phase.
Paeonol dose-dependently decreased the formation of prostaglandin E2 (PGE2) in rat paw exudates with a greater inhibition at the late phase. However, inhibition of nitrate generation was observed only during the late phase (at 4 h after carrageenan injection), accompanied by an attenuation of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) protein expression in paw tissue.
Elevated myeloperoxidase activity, an indicator of neutrophil infiltration, in carrageenan-injected paws was also dose-dependently reduced in paeonol-treated rats.
Our results suggest that the mechanisms by which paeonol exerts its anti-inflammatory and analgesic effects in this inflammatory model may be associated with decreased production of proinflammatory cytokines, NO and PGE2 and increased production of IL-10, an anti-inflammatory cytokine, in carrageenan-injected rat paws. In addition, attenuation of the elevated iNOS and COX-2 protein expression as well as neutrophil infiltration in carrageenan-injected paws may also be involved in the beneficial effects of paeonol.
Keywords: Paeonol, carrageenan, hyperalgesia, cytokines, nitric oxide, prostaglandin E2
Introduction
The Paeonia species (Paeoniaceae), a traditional Chinese herb, has been used as an analgesic and anti-inflammatory drug and also has a beneficial effect in prevention and treatment of thromboembolic diseases (Zhang et al., 1996). Paeonol (2′-hydroxy-4′-methoxyacetophenone), the main active compound of the radix of Paeonia suffruticosa, has been reported to inhibit platelet aggregation, thromboxane A2 (Hirai et al., 1983) and free radical formation (Zhang et al., 1999). Accordingly, paeonol may be a potential therapeutic agent for treating inflammation-associated conditions, such as inflammatory pain.
Thermal hyperalgesia, evoked by carrageenan, results from the combined effect of proinflammatory cytokines, cyclooxygenase (COX) products and sympathomimetic amines (Nakamura & Ferreira, 1987) and is a model frequently used to study inflammatory pain. Tumour necrosis factor-α (TNF-α) was proposed to play an early and crucial role in the cascade of proinflammatory cytokines and the subsequent inflammatory processes (Cunha et al., 1992). COX catalyses the conversion of arachidonic acid to prostaglandin H2, the precursor of a variety of biological active mediators such as prostaglandin E2 (PGE2), prostacyclin and thromboxane A2 (Smith et al., 1996). It is well known that overproduction of inflammatory prostaglandins by the inducible isoform of COX (COX-2) plays an important pathophysiological role in the development of inflammatory pain (Vane et al., 1994; Portanova et al., 1996). The inhibition of thromboxane A2 formation by paeonol (Hirai et al., 1983) suggests that the beneficial effect of paeonol may be associated with the suppression of COX pathway.
Nitric oxide (NO) synthesized from L-arginine by the enzyme, nitric oxide synthase (NOS), is an important mediator in regulation of cell functions (Moncada et al., 1991). However, overproduction of NO derived from inducible NOS (iNOS) activated by proinflammatory cytokines, free radicals and lipopolysaccharide (LPS) plays an important role in the pathogenesis of inflammatory diseases (Vane et al., 1994; Szabó, 1995). Importantly, it has been demonstrated that NO plays a key role in the development of carrageenan-evoked thermal hyperalgesia (Salvemini et al., 1996). However, there are no reports concerning the analgesic effects and the mechanisms of action of paeonol in the inflammatory pain model.
Thus, in the present study, we first test whether paeonol exerts analgesic effects in this model of inflammatory pain, carrageenan-evoked thermal hyperalgesia in rat paw. Further, the possible mechanisms of the anti-inflammatory effects of paeonol are investigated with special focus on the formation of some important inflammatory mediators such as cytokines, NO and PGE2, as well as neutrophil infiltration in the sites of inflammation.
Methods
Animals
Male Sprague–Dawley rats (aged 8–9 weeks), weighing 200–250 g, purchased from the National Animal Center (Taipei, Taiwan), were used in this study. The present study was approved by the local institutional Animal Care and Use Committee. Animals were housed in a standard environment and maintained on tap water and rodent food ad libitum throughout the investigation.
Induction and assessment of carrageenan-evoked thermal hyperalgesia
To induce a local inflammation, 2 mg of λ-carrageenan (Sigma, St Louis, MO, U.S.A. 100 μl of 2% (wv−1) in saline) was injected subcutaneously into the plantar surface (i.pl.) of the right hind paw at time zero (T=0). Hyperalgesia was assessed by placing the hind paw above a radiant heat source and measuring the paw-withdrawal latency with a non-noxious heat stimulus, using a commercially available device (7370 Plantar Test, UGO Basile, Comerio, Italy) as previously described by Hargreaves et al. (1988). The heat source was set to a low intensity of 20. Paw-withdrawal latencies were determined both in carrageenan-injured and -uninjured (contralateral) left hind paws. The paw-withdrawal latencies were evaluated every 30 min for 240 min, starting 60 min after carrageenan injection and the baseline latencies were assessed at 40 min before carrageenan injection (T=−40). Each test was calculated as a mean of three repeated measurements. Each rat was used once and immediately killed according to the experimental protocol given below.
Drug treatment
Paeonol was purchased from Wako Chemicals (Osaka, Japan) and was dissolved in dimethyl sulphoxide (DMSO). Paeonol (30, 50 or 100 mg kg−1, i.p.), vehicle (DMSO) or ibuprofen (50 mg kg−1, i.p.) (Sigma, St Louis, MO, U.S.A.) delivered in a volume of 0.2 ml (i.p.) was injected at 30 min (T=−30) before or 165 min (T=165) after carrageenan injection. The vehicle (i.p.)-treated and the saline (i.pl.)-injected rats acted as the control group. Each group comprised six rats.
Measurement of cytokines in paw exudates
To obtain paw exudates, rats were stunned by a blow to the head and killed by exsanguination. Then, each hind paw was cut at the level of the calcaneus bone and centrifuged at 400 × g for 15 min at 4°C to collect the exudate (oedema fluid). The levels of TNF-α, IL-10, IL-1β and IL-6 in paw exudates at 1.5 or 4 h after carrageenan injection were measured by using enzyme immunoassay (EIA) kits for the corresponding rat cytokines (Genzyme Corporation, Cambridge, U.S.A.).
Measurement of nitrate and PGE2 concentration in paw exudates
Concentrations of nitrate and PGE2 were determined in paw exudates collected as above, 1.5 or 4 h after carrageenan injection. Nitrate measured in paw exudates is the total of nitrite and nitrate concentrations. In this method, nitrite and nitrate were reduced to NO by adding a reducing agent (0.8% VCl3 in 1 M HCl). Then, the NO was drawn into a Sievers Nitric Oxide Analyzer (Sievers 280 NOA, Sievers, Boulder, CO, U.S.A.), as we have previously described (Chou et al., 2001).
Nitrate concentration was calculated by comparison with a standard solution of sodium nitrate (Sigma, St Louis, MO, U.S.A.). The levels of PGE2 were measured by the rat EIA kit (Genzyme Corporation, Cambridge, U.S.A.).
Western blot analysis of iNOS and COX-2
Soft tissues were removed from individual rat paws and homogenized in a solution containing 10 mM CHAPS, 1 mM phenylmethylsulphonyl fluoride (PMSF), 5 μg ml−1 aprotinin, 1 μM pepstatin and 10 μM leupeptin. The homogenates were centrifuged at 12,000 × g for 20 min, and 50 μg of protein from the supernatants was then separated on 10% sodium dodecylsulphate–polyacrylamide gel and transferred to polyvinylidene difluoride membranes (Millipore, MA, U.S.A.). Following transfer, the membrane was blocked for 2 h at room temperature with 5% skim milk in Tris-buffered saline-Tween (TBST; 20 mM Tris, 500 mM NaCl, pH 7.5, 0.1% Tween 20). The membranes were then incubated with mouse monoclonal anti-iNOS (1 : 1000 dilution, Transduction Lab., Lexington, KY, U.S.A.) or anti-COX-2 (1 : 1000 dilution, Transduction Laboratories, Lexington, KY, U.S.A.) antibody in 5% skim milk in TBST for 2 h at room temperature. The membranes were washed three times with TBST at room temperature and then incubated with a 1 : 2000 dilution of anti-mouse IgG secondary antibody conjugated to horseradish peroxidase (Sigma, St Louis, MO, U.S.A.) in 2.5% skim milk in TBST for 1 h at room temperature. The membranes were washed three times and the immunoreactive proteins were detected by enhanced chemiluminescence (ECL) using hyperfilm and ECL reagent (Amersham International plc., Buckinghamshire, U.K.). The relative optical density of bands was quantified by densitometric scanning of the Western blots using Image-pro plus software.
Myeloperoxidase activity assay in paw tissue
At 4 h after injection of carrageenan, rats were killed and the tissues of carrageenan-injected paws were removed, using a scalpel. Each sample of paw tissue was washed with sterile normal saline, blotted dry, weighed and homogenized in ice-cold 0.5% hexadecyltrimethylammonium bromide (HTAB) in 50 mM phosphate buffer (pH=6.0, 5 ml HTAB g tissue−1) on ice using a homogenizer (Pro model 200, Monroe, CT, U.S.A.) at a setting of 4 for 10 s. The resulting homogenate was sonicated and then centrifuged at 15,000 × g for 15 min at 4°C. Protein in the resulting supernatant was determined by using dye-binding assay (Bio-Rad) with bovine serum albumin as a standard. For myeloperoxidase (MPO) assay, samples of the supernatant were mixed with assay buffer (1 : 30, vv−1) and read at 460 nm. The assay buffer consisted of 100 mM potassium phosphate, pH 6.0, 0.083 ml H2O2 (Sigma; 30% stock diluted 1 : 1000) and 0.834 ml o-dianisidine hydrochloride (Sigma; 10 mg ml−1). MPO activity was calculated as the change in absorbance (ΔA) at 460 nm over 1 min and expressed as ΔA460 min−1 mg protein−1.
Statistical analysis
Data were expressed as mean±s.e.m. All results were analysed by one-way ANOVA followed by a multiple comparison test (Scheffe test). A P-value of less than 0.05 was considered statistically significant.
Results
Inhibition of the hyperalgesic response to carrageenan by paeonol
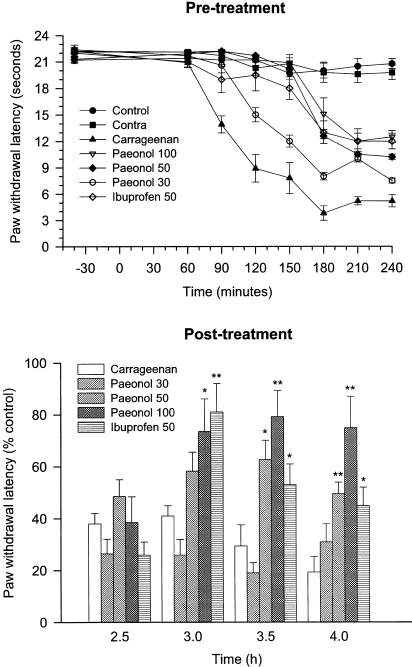
Baseline latencies for all experimental groups before carrageenan injection were similar with a basal withdrawal latency of 21.5±0.4 s. Contralateral left (carrageenan-uninjected) hind paw-withdrawal latencies remained constant at basal levels for the entire experiment. Injection of carrageenan (2 mg paw−1, i.pl.) evoked a thermal hyperalgesia with a significant decrease in withdrawal latencies that began from 60 min, after carrageenan injection, and reached the lowest value of 3.8±0.6 s at 3 h after carrageenan injection. Administration of paeonol (30–100 mg kg−1, i.p.), either 30 min before or 165 min after carrageenan injection, significantly inhibited carrageenan-evoked thermal hyperalgesia (P<0.01 vs vehicle-treated and carrageenan-injected rats) in a dose-dependent manner (Figure 1). Pre- or post-treatment with the same dose (50 mg kg−1, i.p.) of paeonol or ibuprofen, a known nonsteroidal anti-inflammatory drug, had a similar analgesic effect.
Figure 1.
Effect of paeonol on carrageenan-evoked paw thermal hyperalgesia. Paeonol (30, 50 or 100 mg kg−1, i.p.), vehicle (DMSO, i.p.) or ibuprofen (50 mg kg−1, i.p.) was administered 30 min before (a) or 165 min after (b) carrageenan (2 mg paw−1, i.pl.) injection. Paw-withdrawal latencies were assessed for both the carrageenan-injected and uninjected (contralateral, contra) hind paws of each rat at specific time. The vehicle (i.p.)-treated and saline (i.pl.)-injected rats acted as the control group. *P<0.05, **P<0.01 vs vehicle-treated and carrageenan-injected group (n=6 in each group).
Inhibition of cytokine formation by paeonol
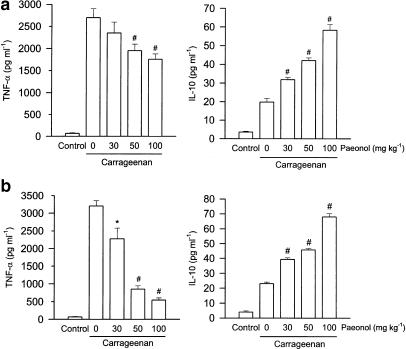
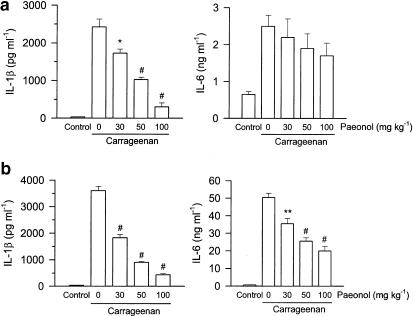
Elevated levels of TNF-α and IL-10 in paw exudates were observed both at the early (1.5 h) and late phase (4 h) after injection of carrageenan. Administration of paeonol 30 min prior to carrageenan injection dose-dependently inhibited the increased level of TNF-α, but further enhanced that of IL-10, both at the early and late phase compared with that of vehicle-treated and carrageenan-injected rats (Figure 2). Concentrations of IL-1β and IL-6 in paw exudates were increased at 1.5 h, and then further increased at 4 h after injection of carrageenan. Administration of paeonol dose-dependently attenuated the level of IL-1β both at the early and late phases, but only inhibited levels of IL-6 at the late phase after carrageenan injection (Figure 3).
Figure 2.
Effect of paeonol on TNF-α and IL-10 formation in carrageenan-injected paws. Paeonol (30, 50 or 100 mg kg−1, i.p.) was administered 30 min before carrageenan injection. At 1.5 h (a) or 4 h (b) after the carrageenan injection, the paw exudates were collected for TNF-α and IL-10 measurement, using EIA kits. The vehicle (i.p.)-treated and saline (i.pl.)-injected rats acted as the control group. *P<0.05, **P<0.01, #P<0.001 vs vehicle-treated and carrageenan-injected group (n=6 in each group).
Figure 3.
Inhibition by paeonol of IL-6 and IL-1β formation in carrageenan-injected paws. Paeonol (30, 50 or 100 mg kg−1, i.p.) was administered 30 min before carrageenan injection and 1.5 h (a) or 4 h (b) later, the paw exudates were collected for IL-6 and IL-1β measurement using EIA kits. The vehicle (i.p.)-treated and saline (i.pl.)-injected rats acted as the control group. *P<0.05, **P<0.01, #P<0.001 vs vehicle-treated and carrageenan-injected group (n=6 in each group).
Inhibition of nitrate and PGE2 formation by paeonol
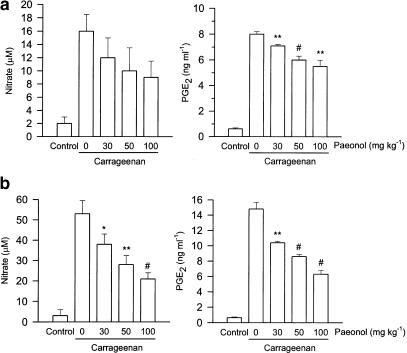
The intraplantar injection of carrageenan in rats led to a time-dependent, increased production of nitrate and PGE2 in the paw exudates. Administration of paeonol 30 min before carrageenan injection dose-dependently inhibited PGE2 production both at early and late phases after carrageenan injection, but inhibition of nitrate levels in paw exudate was observed only at the late phase (Figure 4).
Figure 4.
Inhibition by paeonol of nitrate and PGE2 formation in carrageenan-injected paws. Paeonol (30, 50 or 100 mg kg−1, i.p.) was administered 30 min before carrageenan injection and 1.5 h (a) or 4 h (b) later, the paw exudates were collected for nitrate and PGE2 measurement. The vehicle (i.p.)-treated and saline (i.pl.)-injected rats acted as the control group. *P<0.05, **P<0.01, #P<0.001 vs vehicle-treated and carrageenan-injected group (n=6 in each group).
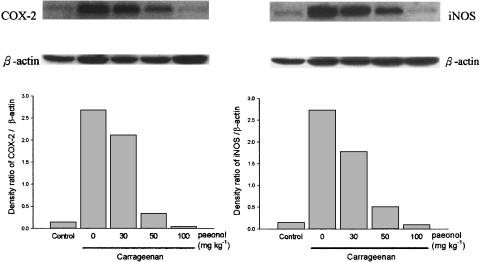
Inhibition of iNOS and COX-2 protein expression in paw tissue by paeonol
In control rat paws, iNOS and COX-2 protein were barely detectable, but 4 h after carrageenan, the expression of iNOS and COX-2 protein in paw tissue was markedly increased. When paeonol was administered, expression of iNOS and COX-2 protein in carrageenan-injected paws was dose-dependently attenuated (Figure 5).
Figure 5.
Effect of paeonol on COX-2 and iNOS protein expression in carrageenan-injected paws. Paeonol (30, 50 or 100 mg kg−1, i.p.) was administered 30 min before carrageenan injection. At 4 h after injection of carrageenan, paws were removed. COX-2 and iNOS protein expression in paw tissue was detected by Western blot analysis, using β-actin as the internal control. One of four experiments, with similar results, is shown (top). In the lower half, the results of densitometric analyses, normalized with respect to β-actin, using a bioimaging analyzer are presented. The vehicle (i.p.)-treated and saline (i.pl.)-injected rats acted as control group.
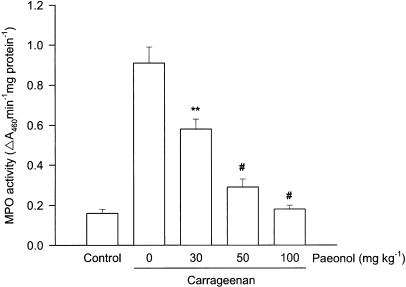
Inhibition of MPO activity in paw tissue by paeonol
The MPO activity of paw tissue was significantly increased at 4 h after injection of carrageenan. This effect was dose-dependently depressed in rats pretreated with paeonol (Figure 6).
Figure 6.
Effect of paeonol on MPO activity in carrageenan-injected paws. Paeonol (30, 50 or 100 mg kg−1, i.p.) was administered 30 min before carrageenan injection. Paw tissue was removed for determination of MPO activity, 4 h after injection of carrageenan. **P<0.01, #P<0.001 vs vehicle-treated and carrageenan-injected group (n=6 in each group).
Discussion
Injection of the inflammatory agent, carrageenan, in the rat hind paws is a model frequently employed to study inflammation and evaluate the anti-inflammatory activity of various compounds. Previous studies in this model have led to the proposal of the following sequence of events. First, carrageenan stimulates the release of TNF-α, which, in turn, (1) induces IL-1β and IL-6, thus stimulating the production of COX products, and (2) induces another cytokine, IL-8, thus stimulating the local production of sympathetic amines (Nakamura & Ferreira, 1987; Ferreira et al., 1993). Thus, a cascade of cytokine release preceded the release of COX products and sympathomimetic amines. An early and crucial role, in this cascade of proinflammatory cytokines, was proposed for TNF-α. This suggestion was confirmed by the fact that injection of antiserum neutralizing endogenous TNF-α markedly decreased the response to carrageenan (Cunha et al., 1992). In contrast to the proinflammatory cytokines, some ‘antagonist cytokines' such as IL-10 produced by murine Th2 lymphocytes and monocytes, have been reported to have an inhibitory effect on the production of proinflammatory cytokines produced by murine Th1 lymphocytes (Fiorentino et al., 1989; Oswald et al., 1992). In addition, IL-10 can also induce the formation of IL-1 receptor antagonist (IL-1ra), another anti-inflammatory cytokine (Howard & O'Garra, 1992; Jenkins et al., 1994). Injection of a monoclonal antibody to mouse IL-10 potentiated carrageenan- and TNF-α-evoked inflammatory pain (Poole et al., 1995), suggesting that IL-10 may have a therapeutic potential in acute and chronic inflammatory diseases.
In the present study, we first demonstrate that pre- or post-treatment with paeonol significantly inhibits carrageenan-evoked thermal hyperalgesia, indicating that paeonol is a potent preventive and therapeutic agent for inflammatory pain. In addition, paeonol time- and dose-dependently inhibits the formation of proinflammatory cytokines, including TNF-α, IL-1β and IL-6, but enhances the production of IL-10 in carrageenan-injected paws, thus resulting in an overall attenuation of the proinflammatory/anti-inflammatory cytokine ratio, which may contribute to its anti-inflammatory and analgesic effects.
Many studies have demonstrated that overproduction of NO and inflammatory prostaglandins such as PGE2, mainly via iNOS and COX-2, respectively, plays an important pathophysiological role in the development of inflammatory hyperalgesia and oedema induced by carrageenan (Ialenti et al., 1992; Meller et al., 1994; Dirig et al., 1998; Nantel et al., 1999). The overproduction of PGE2 induced by bradykinin or cytokines may sensitize pain fibres, which in turn causes a peripherally mediated hyperalgesia (Cunha et al., 1992; Ferreira et al., 1993). Systemic administration of conventional COX-2 inhibitors or PGE2 monoclonal antibodies significantly attenuates the carrageenan-induced inflammatory hyperalgesia (Masferrer et al., 1994; Portanova et al., 1996; Zhang et al., 1997; Dirig et al., 1998), further indicating that overproduction of PGE2 derived from COX-2 plays a critical role in the generation and maintenance of inflammatory pain. Our results showed the inhibition of PGE2 overproduction and of elevated COX-2 protein expression by paeonol in carrageenan-injected paws and these effects may also be involved in the anti-inflammatory action of paeonol.
It has been suggested that another important mediator, NO, produced by constitutive NOS (cNOS) is involved in the development of inflammation at the early phase and NO produced by iNOS is involved in the maintenance of the carrageenan-evoked inflammatory response at the later phase (Salvemini et al., 1996). Peripheral or central administration of NOS inhibitors inhibited carrageenan-induced hyperalgesia in rats (Babbedge et al., 1993; Salvemini et al., 1996). Our results demonstrate that paeonol has an inhibitory effect on nitrate production at the late phase (4 h) (when iNOS activity is activated) but not that at the early phase (1.5 h) in carrageenan-treated paw exudates. This observation is in agreement with inhibition of the expression of iNOS protein in carrageenan-injected paws, indicating that paeonol may be an iNOS inhibitor. Furthermore, NO stimulates the COX pathway, resulting in an enhanced production of proinflammatory prostaglandins (Salvemini et al., 1993; Sautebin et al., 1995). Simultaneously, NO reacting with superoxide anion to form peroxynitrite (ONOO−), a potent oxidizing molecule capable of eliciting lipid peroxidation, may cause serious cellular damage (Beckman et al., 1990). These findings suggest that during inflammatory responses, overproduction of NO may exert multiple cytotoxic effects. The dual inhibition of paeonol on NO and PGE2 overproduction accompanied by inhibition of elevated iNOS and COX-2 protein expression is possibly due to paeonol's initial attenuation of the formation of proinflammatory cytokine.
Activated neutrophils infiltrating sites of inflammation are an important source of oxygen-derived free radicals and many proinflammatory mediators, which in turn causes inflammatory reactions (Fantone & Ward, 1982; Salvemini et al., 1996). In our experiments, paeonol significantly decreased the elevated MPO activity (an indicator of neutrophil infiltration) (Bradley et al., 1982) in inflamed paws, suggesting that inhibition of neutrophil infiltration may be another mechanism by which paeonol achieves its anti-inflammatory effect. It has been reported that cytokine production at an inflammatory site may lead to the induction of particular enzymes and receptors for inflammatory mediators including eicosanoids, which may subsequently act to cooperate with other mediators to enhance inflammation (Oh-ishi et al., 1986; Movat et al., 1987). Simultaneously, prostaglandins also modulate cytokine production and phosphoinositide turnover via different prostaglandin E (EP) receptors including EP1, EP2, EP3 and EP4 receptors (Coleman et al., 1987; Ushikubi et al., 1995). Furthermore, it has been reported that PGE2 regulates production of proinflammatory and anti-inflammatory cytokines predominately through EP2 and EP4 receptors in inflammatory macrophages (Shinomiya et al., 2001). However, we do not know if the beneficial effects of paeonol are mediated via the EP receptors and this possibility needs further investigation.
In summary, our results first demonstrated that paeonol exhibited potent analgesic effects on carrageenan-evoked thermal hyperalgesia. Furthermore, we propose that the possible mechanisms by which paeonol exerts its anti-inflammatory and analgesic effects include the inhibition of the cascade of proinflammatory cytokines and of the overproduction of NO and PGE2. Other possibilities are an increase in the anti-inflammatory cytokine, IL-10, and inhibition of neutrophil infiltration and of the elevated expression of iNOS and COX-2 protein in carrageenan-injected paws.
Acknowledgments
We thank Ms Ting-Hui Chu for technical assistance. This study was partly supported by a research grant from the National Science Council of Taiwan (NSC 88-2314-B016-072), and a grant from the Tri-Service General Hospital (TSGH-C91-15), Republic of China.
Abbreviations
- COX
cyclooxygenase
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- IL-10
interleukin-10
- iNOS
inducible nitric oxide synthase
- NO
nitric oxide
- PGE2
prostaglandin E2
- TNF-α
tumour necrosis factor-α
References
- BABBEDGE R.C., HART S.L., MOORE P.K. Anti-nociceptive activity of nitric oxide synthase inhibitors in the mouse: dissociation between the effect of L-NAME and L-NMMA. J. Pharm. Pharmacol. 1993;45:77–79. doi: 10.1111/j.2042-7158.1993.tb03686.x. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., BECKMAN T.W., CHEN T.W., MARSHALL P.A., FREEMAN B.A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADLEY P.P., PRIEBAT D.A., CHRISTENSEN R.D., ROTHSTEIN G. Measurement of cutaneous inflammation. Estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- CHOU T.C., LI C.Y., WU T.M., TANG S.T., LEE A.R., DING Y.A. Beneficial effect of HCL-31D in murine models of endotoxaemia. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;364:213–219. doi: 10.1007/s002100100438. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., KENNEDY I., SHELDRICK R.L.G. New evidence with selective agonists and antagonists for the subclassification of PGE2-sensitive (EP) receptors. Adv. Prostaglandin Thromboxane Leukotr. Res. 1987;17:467–470. [PubMed] [Google Scholar]
- CUNHA F.Q., POOLE S., LORENZETTI B.B., FERREIRA S.H. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br. J. Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIRIG D.M., ISAKSON P.C., YAKSH T.L. Effect of COX-1 and COX-2 inhibition on induction and maintenance of carrageenan-evoked thermal hyperalgesia in rats. J. Pharmacol. Exp. Ther. 1998;285:1031–1038. [PubMed] [Google Scholar]
- FANTONE J.C., WARD P.A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am. J. Pathol. 1982;107:395–418. [PMC free article] [PubMed] [Google Scholar]
- FERREIRA S.H., LORENZETTI B.B., POLLLE S. Bradykinin initiates cytokine mediated inflammatory hyperalgesia. Br. J. Pharmacol. 1993;110:1227–1231. doi: 10.1111/j.1476-5381.1993.tb13946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIORENTINO D.F., BOND M.W., MOSMANN T.R. Two types of mouse helper T cell IV Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARGREAVES K., DUBNER R., BROWN F., FLORES C., JORIS J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- HIRAI A., TERANO T., HAMAZAKI T., SAJIKI J., SAITO H., TAHARA K., TAMURA Y., KUMAGAI A. Studies on the mechanism of antiaggregatory effect of Moutan Cortex. Thromb. Res. 1983;31:29–40. doi: 10.1016/0049-3848(83)90005-1. [DOI] [PubMed] [Google Scholar]
- HOWARD M., O'GARRA A. Biological properties of IL-10. Immunol. Today. 1992;13:198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- IALENTI A., IANARO A., MONCADA S., ROSA M.D. Modulation of acute inflammation by endogenous nitric oxide. Eur. J. Pharmacol. 1992;211:177–182. doi: 10.1016/0014-2999(92)90526-a. [DOI] [PubMed] [Google Scholar]
- JENKINS J.K., MALYAK M., AREND W.P. The effects of interleukin-10 on interleukin-1 receptor and antagonist and interleukin-1 production in human monocytes and neurtrophils. Lymphokine Cytokine Res. 1994;13:47–54. [PubMed] [Google Scholar]
- MASFERRER J.L., ZWEIFEL B.S., MANNING P.T., HAUSER S.D., LEAHY K.M., SMITH W.G., ISAKSON P.C., SEIBERT K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELLER S.T., CUMMINGS C.P., TRAUB R.J., GEBHART G.F. The role of nitric oxide in the development and maintenance of hyperalgesia produced by intraplantar injection of carrageenan in the rat. Neuroscience. 1994;60:367–374. doi: 10.1016/0306-4522(94)90250-x. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- MOVAT H.Z., CYBULSKY M.I., COLDITZ I.G., CHAN M.K., DINARELLO C.A. Acute inflammation in gram-negative infection: endotoxin, interleukin 1, tumor necrosis factor, and neutrophils. Fed. Proc. 1987;46:97–104. [PubMed] [Google Scholar]
- NAKAMURA M., FERREIRA S.H. A peripheral sympathetic component in inflammatory hyperalgesia. Eur. J. Pharmacol. 1987;135:145–153. doi: 10.1016/0014-2999(87)90606-6. [DOI] [PubMed] [Google Scholar]
- NANTEL E., DENIS D., GORDON R., NORTHEY A., CIRINO M., METTERS K.M., CHAN C.C. Distribution and regulation of cyclooxygenase-2 in carrageenan-induced inflammation. Br. J. Pharmacol. 1999;128:853–859. doi: 10.1038/sj.bjp.0702866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OH-ISHI S., HAYASHI M., YAMAKI K. Inflammatory effects of acetylglycerylether phosphorycholine; vascular permeability increase and induction of pleurisy in rats. Proc. Leukotr. Med. 1986;22:21–33. doi: 10.1016/0262-1746(86)90019-3. [DOI] [PubMed] [Google Scholar]
- OSWALD I.P., WYNN T.A., SHER A., JAMES S.L. Interleukin-10 inhibits macrophage microbicidal activity by blocking the endogenous production of tumor necrosis factor α required as costimulatory factor for interferon-γ-induced activation. Proc. Natl. Acad. Sci. U.S.A. 1992;89:8676–8680. doi: 10.1073/pnas.89.18.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POOLE S., CUNHA F.Q., SELKIRK S., LORENZETTI B.B., FERREIRA S.H. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-10. Br. J. Pharmacol. 1995;115:684–688. doi: 10.1111/j.1476-5381.1995.tb14987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTANOVA J.P., ZHANG Y., ANDERSON G.D., HAUSER S.D., MASFERRER J.L., SEIBERT K., GREGORY S.A., ISAKSON P.C. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J. Exp. Med. 1996;184:883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., MISKO T.P., SEIBERT K., MASFERRER J.L., CURRIE M.G., NEEDLEMAN P. Nitric oxide activates cyclooxygenase enzymes. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., WYATT P.S., BOURDON D.M., MARRING M.H., MANNING P.T., CURRIE M.G. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br. J. Pharmacol. 1996;118:829–838. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAUTEBIN I., IALENTI A., IANARO A., DIROSA M. Modulation by nitric oxide of prostaglandin biosynthesis in the rat. Br. J. Pharmacol. 1995;114:323–328. doi: 10.1111/j.1476-5381.1995.tb13230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHINOMIYA S., NARABA H., UENO A., UTSUNOMIYA I., MARUYAMA T., OHUCHIDA S., USHIKUBI F., YUKI K., NARUMIYA S., SUGIMOTO Y., ICHIKAWA A., OH-ISHI S. Regulation of TNF-α and interleukin-10 production by prostaglandins I2 and E2: studies with prostaglandin E-receptor subtype-selective synthetic agonists. Biochem. Pharmacol. 2001;61:1153–1160. doi: 10.1016/s0006-2952(01)00586-x. [DOI] [PubMed] [Google Scholar]
- SMITH W.L., GARAVITO R.M., DEWITT D.L. Prostaglandin endoperoxide H synthase (cyclooxygenase)-1 and -2. J. Biol. Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- SZABÓ C. Alterations in nitric oxide production in various forms of circulatory shock. New Horizons. 1995;3:2–32. [PubMed] [Google Scholar]
- USHIKUBI F., HIRATA M., NARUMIYA S. Molecular biology of prostanoid receptors: an overview. J. Lipid Med. Cell Sign. 1995;12:343–359. doi: 10.1016/0929-7855(95)00022-i. [DOI] [PubMed] [Google Scholar]
- VANE J.R., MITCHELL J.A., APPLETON I., TOMLINSON A., BISHOP-BAILEY D., CROXTALL J., WILLOUGHBY D.A. Inducible isoforms of cyclooxygenase and nitric oxide synthase in inflammation. Proc. Natl. Acad. Sci. U.S.A. 1994;91:2046–2050. doi: 10.1073/pnas.91.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG H.Y., GE N., ZHUNG Z.Y. Theoretical elucidation of activity differences of five phenolic antioxidants. Chung-Kuo Yao Li Hsueh Pao-Acta Pharmacol. Sin. 1999;20:363–366. [PubMed] [Google Scholar]
- ZHANG L.H., XIAO P.G., HUNG Y. Recent progresses in pharmacological and clinical studies of paeonol. Chung-Kuo Chung His I Chieh Ho Tsa Chin. 1996;16:187–190. [PubMed] [Google Scholar]
- ZHANG Y., SHAFFERM A., PORTANOVA J., SEIBERT K., ISAKSON P.C. Inhibition of cyclooxygenase-2 rapidly reverses inflammatory hyperalgesia and prostaglandin E2 production. J. Pharmacol. Exp. Ther. 1997;283:1069–1075. [PubMed] [Google Scholar]