Abstract
Using in vivo intracerebral microdialysis in conscious, freely moving rats, we examined the effect of flibanserin, a potential antidepressant drug with high affinity for human 5-HT1A receptors and four–50-fold lower affinity for 5-HT2A and D4 receptors, on basal extracellular concentrations of serotonin (5-hydroxytryptamine, 5-HT), dopamine (DA) and noradrenaline (NA) in selected regions of the rat brain.
Flibanserin at 3 and 10 mg kg−1 significantly reduced extracellular 5-HT in the prefrontal cortex (by 30 and 45%) and dorsal raphe (35 and 44%), but had no effect on extracellular 5-HT in the ventral hippocampus. The 3 and 10 mg kg−1 doses raised extracellular NA to a similar extent in the prefrontal cortex (47 and 50%). In all, 10 mg kg−1 raised extracellular DA in the prefrontal cortex (63%) whereas 3 mg kg−1 had no significant effect.
Pretreatment with the selective 5-HT1A receptor antagonist WAY100,635 (0.3 mg kg−1) 30 min before 10 mg kg−1 flibanserin completely antagonized the latter's effects on extracellular 5-HT, DA and NA in the prefrontal cortex. WAY100,635 by itself had no effect on cortical extracellular monoamines.
The results show that the stimulation of 5-HT1A receptors plays a major role in the effect of flibanserin on brain extracellular 5-HT, DA and NA.
Keywords: 5-HT1A receptors, 5-HT2A receptors, 5-hydroxytryptamine, dopamine, noradrenaline, antidepressant drugs, prefrontal cortex, dorsal raphe, ventral hippocampus, microdialysis
Introduction
The serotonergic system has long been implicated in depression and in the response to antidepressant drugs. Among the seven main families of 5-hydroxytryptamine (5-HT) receptors known to date (Hoyer et al., 1994; Barnes & Sharp, 1999) attention has focussed particularly on the role of 5-HT1A and 5-HT2 subtypes in the mechanism of action of antidepressant drugs. 5-HT1A receptor agonists from different chemical classes are active in animal models predictive of antidepressant activity (Cervo & Samanin, 1991; De Vry, 1995) and may have antidepressant effects in man (Stahl et al., 1992). The nonselective 5-HT1A receptor antagonists such as pindolol accelerate the antidepressant effect of selective serotonin reuptake inhibitors (SSRI) (Artigas et al., 1994; Perez et al., 1997) and it has been suggested that the antidepressant effect of both types of substances is related to the desensitization of raphe 5-HT1A autoreceptors with repeated administration (Blier & de Montigny, 1994; Invernizzi et al., 1994, 1996; Rutter et al., 1994). Thus, 5-HT1A receptor knockout mice give antidepressant-like responses in the tail-suspension (Heisler et al., 1998) and forced swimming (Ramboz et al., 1998) tests, two models widely used to assess the potential antidepressant effect of drugs (Porsolt et al., 1978; Steru et al., 1985).
Changes in 5-HT2 receptor density are observed in depressed patients (Stanley & Mann, 1983; Yates et al., 1990; Risch & Nemeroff, 1992) and in response to chronic administration of classical antidepressant drugs (Peroutka & Snyder, 1980). In addition, blockade of 5-HT2A receptors might contribute to the antidepressant effect of mirtazapine, mianserin and nefazodone which potently inhibit 5-HT2A receptors (de Boer et al., 1988), and to the antidepressant effect of ritanserin, a 5-HT2A/2C receptor antagonist (Bersani et al., 1991).
Recent strategies aimed at developing new antidepressant drugs have focused on compounds acting as 5-HT1A receptor agonists and 5-HT2A receptor antagonists. This approach stems from electrophysiological studies in rats suggesting a functional opposition between 5-HT2A and 5-HT1A receptors in the cortex (Araneda & Andrade, 1991; Ashby et al., 1994), one of the brain regions where metabolic alterations have been consistently reported in depressed patients (Drevets, 1998).
Flibanserin is a potential antidepressant drug with high affinity for human 5-HT1A receptors (Ki=1 nM) and lower affinity for 5-HT2A (Ki=49 nM) and D4 (Ki=4–24 nM) receptors, but negligible affinity for a variety of other neurotransmitter receptors and ion channels (Borsini et al., 2002). In vitro studies showed that flibanserin reduced forskolin-stimulated cAMP formation in cells and rat tissues and antagonized the accumulation of phosphatidyl inositol turnover induced by 5-HT in the mouse cortex (Borsini et al., 1995). This suggested that the drug may be a 5-HT1A receptor agonist and 5-HT2A receptor antagonist. In vivo, flibanserin displayed some effects compatible with the activation of 5-HT1A receptors. Flibanserin inhibited the firing rate of serotonergic neurons of the dorsal raphe (DR) (Rueter et al., 1998) and this effect was antagonized by the selective 5-HT1A receptor antagonist WAY100,635 (Forster et al., 1995). Similar to the selective 5-HT1A receptor agonists, flibanserin reduced the accumulation of brain 5-hydroxytryptophan induced by the blockade of aromatic amino-acid decarboxylase (ED50=8 mg kg−1; Brambilla et al., 1999). The fact that flibanserin reduced the head-twices (ED50=4.1 mg kg−1) in mice and electrophysiological effects induced by the nonselective 5-HT2A agonist 2,5-dimethoxy-4-iodoamphetamine (DOI) in rats (Rueter & Blier, 1999; Borsini et al., 2002) suggests that this compound may block 5-HT2A receptors in vivo.
In the present study, we employed the microdialysis technique in conscious rats to investigate the effect of 3 and 10 mg kg−1 flibanserin on the extracellular concentration of 5-HT in various brain regions. These doses are in the range of those inhibiting brain 5-HT synthesis and antagonizing the effect of DOI (Brambilla et al., 1999; Borsini et al., 2002). Since 5-HT1A receptor agonists stimulate the activity of noradrenergic cells of the locus coeruleus (Szabo & Blier, 2001) and dopaminergic neurons of the ventrotegmental area (Arborelius et al., 1993a; Prisco et al., 1994), and increase extracellular noradrenaline (NA) and dopamine (DA) in the respective projection regions (Arborelius et al., 1993b; Wedzony et al., 1996), we investigated the effect of flibanserin on extracellular DA and NA in the prefrontal cortex. Finally, to prove the involvement of 5-HT1A receptors, in one experiment we studied the effect of flibanserin on cortical monoamine release in rats pretreated with the selective 5-HT1A receptor antagonist WAY100,635. Part of these results has been published in preliminary form (Borsini et al., 2002).
Methods
Animals
Male Sprague–Dawley rats (CD-COBS, Charles River, Italy) (250–350 g) were used, housed at constant temperature (21±1°C) and relative humidity (60±5%) under a regular light–dark schedule (light 7:00–19:00 h) with food and water freely available.
Procedures involving animals and their care were conducted in conformity with the institutional guidelines that are in compliance with national (D.L. n. 116, G.U., suppl. 40, 18 Febbraio 1992, Circolare No. 8, G.U., 14 Luglio 1994) and international laws and policies (EEC Council Directive 86/609, OJ L 358,1, 12 December 1987; Guide for the Care and Use of Laboratory Animals, U.S. National Research Council, 1996).
Dialysis procedure
Rats were anaesthetized with 3.5 ml kg−1. Equithesin and placed on a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, U.S.A.). A hole was drilled in the parietal or frontal bone and a small incision was made in the dura with a bent needle tip. The probe was perfused with artificial cerebrospinal fluid (aCSF; see below for composition) and lowered slowly into the rat prefrontal cortex, ventral hippocampus and DR nucleus, then fixed vertically to the skull using two or three stainless-steel anchorage screws and acrylic cement. Stereotaxic coordinates relative to the probe tip were as follows (in mm): prefrontal cortex, AP=12.7, L=±0.6 and V=4.6; ventral hippocampus, AP=4.2, L=±4.8 and V=1.6; DR, AP=1.1, L=±0.6 and V=3.0 with an 8° angle to the sagittal plane. Coordinates were taken from the interaural line according to the Paxinos & Watson (1986) atlas.
The dialysis probes were of the concentric type and were prepared essentially as described by Robinson & Whishaw (1988) except that the dialysis membrane was made of polyacrylonitrile-sodium methallyl sulphonate (AN69, Hospal). Since diffusion of 5-HT through AN69 membrane is markedly delayed (Tao & Hjorth, 1992), we used Cuprophan membranes (216 μm outer diameter, Sorin Biomedica, Italy) for the measurement of 5-HT. The length of the exposed membrane was 4 mm for the prefrontal cortex and ventral hippocampus and 1.5 mm for the DR. In vitro recovery was about 8 and 20% respectively for 1.5 and 4 mm Cuprophan membranes and 22–29% for 4 mm AN69 membranes. Each rat was implanted with a single probe in the DR or ventral hippocampus. Bilateral probes were implanted in the prefrontal cortices to allow the detection of changes in extracellular 5-HT and DA or NA in the same subject.
Rats were allowed to recover from anaesthesia, one per cage with free access to food and water. About 24 h after surgery, each rat was placed in a cage and the inlet cannula was connected by polyethylene tubing to a 2.5 ml syringe containing aCSF (composition in mM: 145 NaCl, 3 KCl, 1.26 CaCl2·2 H2O, 1 MgCl2·6 H2O in distilled water and buffered at pH 7.4 with 2 mM sodium phosphate buffer) containing 1 μM citalopram to improve 5-HT detectability. Each probe was perfused at a constant flow-rate of 1 μl min−1 with a microinfusion pump (CMA 100, CMA/Microdialysis, Stockholm, Sweden). After a 30 min washout period, consecutive 30 min samples of perfusate were collected in minivials. Samples were immediately injected into the high-performance liquid chromatograph with electrochemical detection (HPLC-ED) without prior purification for the determination of monoamines as previously described (Invernizzi et al., 1992a,1992b). 5-HT, NA and DA were determined in separate samples of dialysate. Separation of 5-HT was achieved by a reverse-phase column (Supelcosil LC18-DB 3 μm, 150 × 4.6 mm; Supelchem, Italy) and a mobile phase consisting of (mM) citric acid 9, sodium acetate trihydrate 48, Na2EDTA 0.1, 100 μl l−1 triethylamine and 40 ml l−1 acetonitrile, pumped at 1 ml min−1. Separation of NA was obtained using a reverse-phase column (Hypersil-ODS 5 μm, 125 × 3.1 mm, Bischoff, Italy). The mobile phase, consisting of (mM) citric acid 25, sodium acetate 24, sodium octyl sulphate 1.55 and 80 ml l−1 CH3OH was pumped at 1 ml min−1. DA was separated through a 150 × 4.6 reverse-phase column (Supelcosil LC18-DB 3 μm, 150 × 4.6 mm; Supelchem, Milan, Italy) using a mobile phase containing 0.1 M sodium acetate, 60 ml l−1 CH3OH, pH 4.2 with acetic acid, pumped at 1 ml min−1. 5-HT, NA and DA were measured by a Coulochem II electrochemical detector equipped with a 5011 analytical cell at the following potentials (E1/E2): 5-HT 50/180 mV, NA 200/−250 mV and DA 300/−325 mV. Monoamines were read as the second electrode output signal.
Histological procedure
At the end of the experiments, rats were deeply anaesthetized with chloral hydrate (400 mg kg−1) and killed by decapitation, their brains were immediately removed and the correct placement of the probes was checked by examining the probe tracks. Only rats with correct probe placement were considered in the results.
Drug treatment
Flibanserin (previously BIMT17; 1-[2-[4-(3-trifluoromethylphenyl)piperazin-1-yl] ethyl] benzimidazol-[1 H]-2-one) (Boehringer-Ingelheim, Milan, Italy) was dissolved in a vehicle containing 250 ml l−1 polyethylene glycol-400 and 22.7 ml l−1. 1 M HCl, warmed at about 40°C. On the day of the experiment (24 h after probe implantation), once basal levels of monoamines in the dialysate were stable (no more than 15% difference between three consecutive samples), rats were injected intraperitoneally with vehicle (2 ml kg−1) or 3 and 10 mg kg−1 flibanserin (as base). WAY100,635 (N-[2-[methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl) cyclohexane carboxamide trihydrochloride) (Pharmacia, Nerviano, Italy) was dissolved in saline and injected subcutaneously 30 min before flibanserin or vehicle.
Statistical analysis
The effects of flibanserin on extracellular 5-HT, NA and DA were analysed by ANOVA for repeated measures (split-plot) with treatment and time as between and within factors, respectively. Post-hoc comparisons were made by Tukey–Kramer's test. Values missing because of occasional problems in sample collection or analysis were replaced by the mean of the samples immediately before and after. Statistical analysis was done using the StatView 5.0 statistical package for Apple-Macintosh computer (SAS Institute Inc., SAS Campus Drive, Cary, NC, U.S.A.).
Results
Effect of flibanserin on extracellular 5-HT in the prefrontal cortex, ventral hippocampus and dorsal raphe
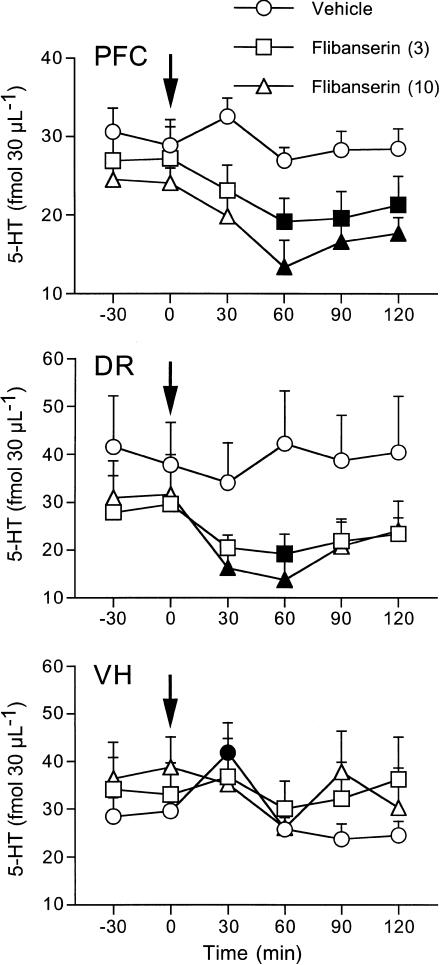
Basal concentrations of extracellular 5-HT (fmol 30 μl−1) measured in the presence of 1 μM citalopram in the perfusion medium were as follows (mean±s.e.m.): prefrontal cortex 27.6±1.3 (n=38), ventral hippocampus 33.6±3.1 (n=19), DR 33.3±4.8 (n=16). The vehicle had no effect on extracellular 5-HT in the prefrontal cortex and DR but it significantly raised extracellular 5-HT (by 33%) in the ventral hippocampus 30 min after injection. Overall, flibanserin significantly reduced extracellular 5-HT in the prefrontal cortex (F2,16=4.4, P<0.03) and DR (F8,64=2.3, P<0.03), but not in the hippocampus (F8,60=1.9, P>0.06). In all, 3 and 10 mg kg−1 flibanserin significantly reduced extracellular 5-HT in the prefrontal cortex, by respectively, 30 and 45% (Figure 1). The reduction was maximal at 60 min and lasted to 120 min after both doses. The effect of flibanserin on extracellular 5-HT in the DR was similar to that on the prefrontal cortex: 3 and 10 mg kg−1 reduced extracellular 5-HT by, respectively, 35 and 44% (Figure 1). However, the effect was short-lasting, being significant 30 and 60 min after 10 mg kg−1 and only 30 min after 3 mg kg−1.
Figure 1.
Extracellular 5-HT in the prefrontal cortex (PFC), dorsal raphe (DR) and ventral hippocampus (VH) of rats given 3 and 10 mg kg−1 flibanserin or vehicle intraperitoneally. Mean±s.e.m. of 5–7 rats. The arrows indicate the time of drug or vehicle injection. Solid symbols indicate P<0.05 vs basal values (Tukey–Kramer's test).
Effect of flibanserin on extracellular DA and NA in the prefrontal cortex
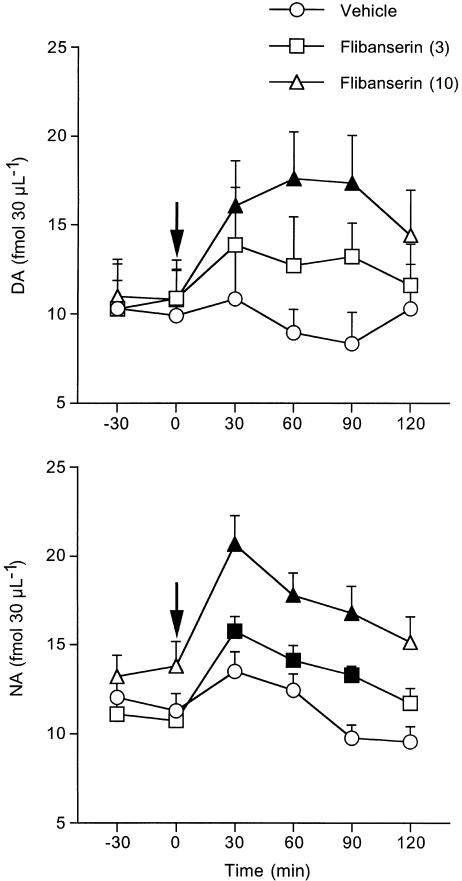
Basal concentrations of extracellular NA and DA (fmol 30 μl−1) in the prefrontal cortex in the presence of 1 μM citalopram in the perfusion medium (mean±s.e.m.) were, respectively, 13.8±0.7 (n=35) and 13.4±0.9 (n=36). This concentration of citalopram had no significant effects on extracellular DA and NA (Pozzi et al., 1999 and unpublished results). The vehicle had no effect on extracellular DA and NA in the prefrontal cortex.
As shown in Figure 2, 10 mg kg−1 flibanserin significantly increased extracellular DA (63%) and NA (50%) in the prefrontal cortex (F values for DA and NA were F8,56=3.2, P<0.005 and F2,13=9.7, P<0.003). The increases of extracellular DA and NA were significant from 30 to 90 min after injection. The lower dose of flibanserin significantly raised extracellular NA, by 47%, but it had no effect on extracellular DA (Figure 2).
Figure 2.
Extracellular DA and NA in the prefrontal cortex (PFC) of rats given 3 and 10 mg kg−1 flibanserin or vehicle intraperitoneally. Mean±s.e.m. of 5–6 rats. The arrows indicate the time of drug or vehicle injection. Solid symbols indicate P<0.05 vs basal values (Tukey–Kramer's test).
Effect of WAY100,635 on flibanserin-induced changes of extracellular 5-HT, DA and NA in the prefrontal cortex
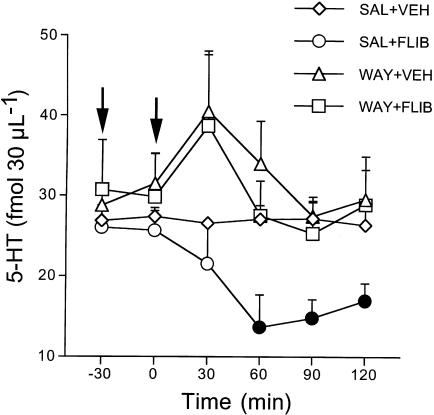
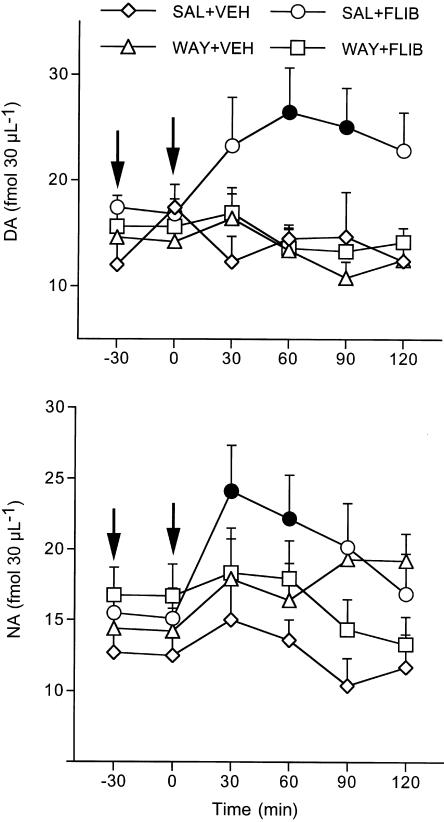
In rats given saline, 10 mg kg−1 flibanserin reduced extracellular 5-HT by 47% at 60 min (Figure 3). Pretreatment with 0.3 mg kg−1 WAY100,635 completely prevented this reduction (F5,50=2.6, P<0.01). As shown in Figure 4, 10 mg kg−1 flibanserin significantly increased extracellular DA (58%) and NA (60%) in the prefrontal cortex of rats pretreated with saline. WAY100,635 given 30 min before flibanserin prevented both these rises (DA, F5,50=4.8, P<0.001, NA, F5,55=3.1, P<0.01) (Figure 4). WAY100,635 by itself had no effects on extracellular 5-HT, DA and NA.
Figure 3.
Effect of flibanserin alone and in combination with the selective 5-HT1A autoreceptor antagonist WAY100,635 on extracellular 5-HT in the prefrontal cortex. Rats were pretreated subcutaneously with saline (SAL) or 0.3 mg kg−1 WAY100,635 (WAY; first arrow). After 30 min, they received 10 mg kg−1 flibanserin (FLIB) or vehicle (VEH) intraperitoneally (second arrow) and extracellular 5-HT was measured for 2 h. Mean±s.e.m. of 3–6 rats. Solid symbols indicate P<0.05 vs basal values (Tukey–Kramer's test).
Figure 4.
Effect of flibanserin alone and in combination with the selective 5-HT1A autoreceptor antagonist WAY100,635 on extracellular DA and NA in the prefrontal cortex. Rats were injected subcutaneously with saline (SAL) or 0.3 mg kg−1 WAY100,635 (WAY; first arrow) and 30 min later received 10 mg kg−1 flibanserin (FLIB) or vehicle (VEH) intraperitoneally (second arrow); extracellular DA and NA were measured for 2 h. Mean±s.e.m. of 2–6 rats. Solid symbols indicate P<0.05 vs basal values (Tukey–Kramer's test).
Discussion
The present study shows that flibanserin lowered extracellular 5-HT in the prefrontal cortex and dorsal raphe and raised extracellular DA and NA in the prefrontal cortex. The fact that the doses of flibanserin that affected extracellular monoamine concentrations are in the range of those active in chronic mild stress and bulbectomized rat models of depression (Borsini et al., 1997; D'Aquila et al., 1997), and in the ultrasonic vocalization model of anxiety (Podhorna & Brown, 2000), suggests that the drug's action on monoamines may contribute to these effects. In line with the short half-life of flibanserin (1–2 h) in rats (Borsini et al., 2002), changes in extracellular 5-HT, DA and NA peaked at 30–60 min and, except for cortical 5-HT, lasted less than 2 h.
The selective 5-HT1A receptor antagonist WAY100,635 (Forster et al., 1995) completely antagonized the effect of flibanserin on extracellular 5-HT in the prefrontal cortex. This finding is consistent with the drug's high affinity for 5-HT1A receptors (Borsini et al., 1995) and suggests that the reduction of cortical extracellular 5-HT by flibanserin depends on the stimulation of 5-HT1A receptors. 5-HT1A autoreceptors on 5-HT neurons of the raphe play a pivotal role in regulating the activity of 5-HT neurons, and in previous studies WAY100,635 antagonized the flibanserin-induced decrease of firing rate of 5-HT neurons of the DR (Rueter et al., 1998). These results suggest that 5-HT1A autoreceptors of the DR are quite likely involved in the drug's effect on extracellular 5-HT in the prefrontal cortex. 5-HT1A receptors are also present on postsynaptic elements in the prefrontal cortex (Pompeiano et al., 1992) and their stimulation may reduce the activity of serotonergic neurons of the DR and the release of 5-HT in the prefrontal cortex (Ceci et al., 1994; Hajos et al., 1999; Celada et al., 2001). Electrophysiological studies have shown that flibanserin inhibits the activity of cortical pyramidal neuron by stimulating 5-HT1A receptors in the prefrontal cortex. The involvement of postsynaptic 5-HT1A receptors is supported by the fact that the effect of flibanserin was anatgonized by WAY100,135 and tertatolol, two 5-HT1A receptor antagonists, but not by the destruction of 5-HT containing neurons with the neurotoxin 5,7-dihydroxytryptamine (Borsini et al., 1995). Although in subsequent studies WAY100,635 failed to antagonize the inhibitory effect of flibanserin on the activity of cortical neurons (Rueter et al., 1998), a role of postsynaptic 5-HT1A receptors in the effect of flibanserin on cortical extracellular 5-HT cannot be excluded.
Flibanserin at the two doses tested reduced extracellular 5-HT in the prefrontal cortex and DR of conscious rats but had no effect in the ventral hippocampus. In line with this finding, flibanserin was less effective in reducing 5-HT synthesis in the hippocampus than in the prefrontal cortex (Brambilla et al., 1999). These findings confirm the results of previous studies showing that selective 5-HT1A receptor agonists such as 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) preferentially reduced extracellular 5-HT and 5-HT synthesis in the prefrontal cortex in respect to the hippocampus (Invernizzi et al., 1991, 1994, 1995; Casanovas & Artigas, 1996; Casanovas et al., 1997). It should be considered, however, that the stimulation of 5-HT1A receptors with appropriate doses of the agonist reduced extracellular 5-HT in the ventral hippocampus (Sharp et al., 1989; Kreiss & Lucki, 1994). It is conceivable therefore that similar effects may have been observed had we administered flibanserin at higher doses.
The finding that WAY100,635 completely antagonized the flibanserin-induced increase of extracellular DA and NA in the prefrontal cortex strongly supports the role of 5-HT1A receptors in the drug's effect on cortical catecholamines. Accordingly, microdialysis studies have consistently reported that selective 5-HT1A receptor agonists raise extracellular concentrations of DA and NA in the prefrontal cortex (Arborelius et al., 1993b; Done & Sharp, 1994; Wedzony et al., 1996). 5-HT1A receptor agonists-induced increase of extracellular DA may be secondary to the disinhibition of the activity of mesocortical dopaminergic neurons in the ventrotegmental area (VTA). In support of this hypothesis, such a mechanism has already been shown to account for the disinhibitory effect of 8-OH-DPAT on the firing activity of dopaminergic neurons of the VTA (Prisco et al., 1994). Consistently, depletion of endogenous 5-HT by p-chlorophenylalanine attenuated 8-OH-DPAT-induced increase of extracellular DA in the VTA (Chen & Reith, 1995). On the contrary, 5-HT depletion left unaltered the effect of 8-OH-DPAT on extracellular NA (Chen & Reith, 1995). The involvement of presynaptic mechanism in the effect of 5-HT1A receptor agonists on extracellular DA was not confirmed in a subsequent study showing that the increase of cortical extracellular DA induced by MKC-242, a selective 5-HT1A receptor agonist, was not prevented by the neurotoxic lesion of 5-HT containing neurons with 5,7-DHT (Sakaue et al., 2000). Therefore, the control exerted by pre- and postsynaptic 5-HT1A receptors on DA and NA release is still controversial and further studies are needed to clarify the mechanism by which flibanserin increased extracellular catecholamines in the prefrontal cortex.
Although in vitro studies showing that flibanserin has higher affinity for 5-HT1A receptors than 5-HT2A receptors (Borsini et al., 1995) suggest a major role of 5-HT1A receptors in the effect of flibanserin, in vivo flibanserin binds 5-HT1A and 5-HT2A receptors to a similar extent (Scandroglio et al., 2001). Thus, 5-HT2A receptor blockade may be involved in the effects of flibanserin on cortical monoamines. We did not address this question in the present study, but it is unlikely that blockade of 5-HT2A receptors by itself was responsible for flibanserin's effects on cortical monoamines since the selective blockade of these receptors with M100,907 has no effect on extracellular 5-HT, DA and NA in the prefrontal cortex (Gobert & Millan, 1999; Rollema et al., 2000; Ichikawa et al., 2001). It cannot be excluded, however, that the blockade of 5-HT2A receptors may have contributed to flibanserin-induced increase of dopamine in the prefrontal cortex since microdialysis studies have shown that 5-HT1A receptor stimulation and 5-HT2A receptor blockade act synergistically to increase extracellular DA in the prefrontal cortex (Ichikawa et al., 2001).
5-HT2C receptors exert an important tonic inhibitory control on dopaminergic and noradrenergic neurons innervating the prefrontal cortex and antagonists at these receptors consistently increase extracellular DA and NA in the prefrontal cortex (Gobert et al., 2000; Pozzi et al., 2002). However, because of the low affinity of flibanserin for 5-HT2C receptors (Borsini et al., 2002), these receptors are unlikely involved in the effect of flibanserin on extracellular catecholamines
In view of the high affinity of flibanserin for D4 receptors, the interaction with these sites may contribute to some of the effects observed in the present study. In vitro studies in cloned cells found that flibanserin behaved as an antagonist or, albeit at higher concentrations, as an agonist or partial agonist at D4 receptors (Borsini et al., 2002). Selective antagonists of D4 receptors had no effect on extracellular NA and 5-HT (Broderick & Piercey, 1998; Millan et al., 1998) in the prefrontal cortex and, although there are reports that selective D4 receptor antagonists raise extracellular DA in the prefrontal cortex (Millan et al., 1998; Broderick & Piercey, 1998), it has been argued that this occurs at doses higher than those believed to block D4 receptors selectively (Millan et al., 1998). Taken together, these findings suggest that blockade of D4 receptors is unlikely to have contributed to flibanserin-induced changes in extracellular monoamines in the prefrontal cortex.
In summary, the present results show that the stimulation of 5-HT1A receptors plays a major role in the effect of flibanserin on extracellular 5-HT, DA and NA and suggest that these actions could constitute a basis for interpreting the drug's antidepressant-like effects.
Acknowledgments
This work was partially supported by Boehringer Ingelheim (Milan, Italy). We are grateful to Pharmacia for the generous gift of WAY100,635 and to J. Baggott for stylistic editing.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- DA
dopamine
- 5-HT
5-hydroxytryptamine
- NA
noradrenaline
- SSRI
selective serotonin reuptake inhibitors
References
- ARANEDA R., ANDRADE R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- ARBORELIUS L., CHERGUI K., MURASE S., NOMIKOS G.G., HOOK B.B., CHOUVET G., HACKSELL U., SVENSSON T.H. The 5-HT1A receptor selective ligands, (R)-8-OH-DPAT and (S)-UH-301, differentially affect the activity of midbrain dopamine neurons. Naunyn Schmiedebergs Arch. Pharmacol. 1993a;347:353–362. doi: 10.1007/BF00165384. [DOI] [PubMed] [Google Scholar]
- ARBORELIUS L., NOMIKOS G.G., HACKSELL U., SVENSSON T.H. (R)-8-OH-DPAT preferentially increases dopamine release in rat medial prefrontal cortex. Acta. Physiol. Scand. 1993b;148:465–466. doi: 10.1111/j.1748-1716.1993.tb09584.x. [DOI] [PubMed] [Google Scholar]
- ARTIGAS F., PEREZ V., ALVAREZ E. Pindolol induces a rapid improvement of depressed patients treated with serotonin reuptake inhibitors. Arch. Gen. Psychiatry. 1994;51:248–251. doi: 10.1001/archpsyc.1994.03950030084009. [DOI] [PubMed] [Google Scholar]
- ASHBY C.R., JR, EDWARDS E., WANG R.Y. Electrophysiological evidence for a functional interaction between 5-HT1A and 5-HT2A receptors in the rat medial prefrontal cortex: an iontophoretic study. Synapse. 1994;17:173–181. doi: 10.1002/syn.890170306. [DOI] [PubMed] [Google Scholar]
- BARNES N.M., SHARP T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- BERSANI G., POZZI F., MARINI S., GRISPINI A., PASINI A., CIANI N. 5-HT2 receptor antagonism in dysthymic disorder: a double-blind placebo-controlled study with ritanserin. Acta Psychiatr. Scand. 1991;83:244–248. doi: 10.1111/j.1600-0447.1991.tb05533.x. [DOI] [PubMed] [Google Scholar]
- BLIER P., DE MONTIGNY C. Current advances and trends in the treatment of depression. Trends Pharmacol. Sci. 1994;15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- BORSINI F., CESANA R., KELLY J., LEONARD B.E., McNAMARA M., RICHARDS J., SEIDEN L. BIMT 17: a putative antidepressant with a fast onset of action. Psychopharmacology (Berl.) 1997;134:378–386. doi: 10.1007/s002130050474. [DOI] [PubMed] [Google Scholar]
- BORSINI F., EVANS K., JASON K., ROHDE F., ALEXANDER B., POLLENTIER S. Pharmacology of flibanserin. CNS Drug Rev. 2002;8:117–142. doi: 10.1111/j.1527-3458.2002.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORSINI F., GIRALDO E., MONFERINI E., ANTONINI G., PARENTI M., BIETTI G., DONETTI A. BIMT 17, a 5-HT2A receptor antagonist and 5-HT1A receptor full agonist in rat cerebral cortex. Naunyn Schmiedebergs Arch. Pharmacol. 1995;352:276–282. doi: 10.1007/BF00168557. [DOI] [PubMed] [Google Scholar]
- BRAMBILLA A., BASCHIROTTO A., GRIPPA N., BORSINI F. Effect of flibanserin (BIMT 17), fluoxetine, 8-OH-DPAT and buspirone on serotonin synthesis in rat brain. Eur. Neuropsychopharmacol. 1999;10:63–67. doi: 10.1016/s0924-977x(99)00056-5. [DOI] [PubMed] [Google Scholar]
- BRODERICK P.A., PIERCEY M.F. Clozapine, haloperidol, and the D4 antagonist PNU-101387G: in vivo effects on mesocortical, mesolimbic, and nigrostriatal dopamine and serotonin release. J. Neural Transm. 1998;105:749–767. doi: 10.1007/s007020050093. [DOI] [PubMed] [Google Scholar]
- CASANOVAS J.M., ARTIGAS F. Differential effects of ipsapirone on 5-hydroxytryptamine release in the dorsal and median raphe neuronal pathways. J. Neurochem. 1996;67:1945–1952. doi: 10.1046/j.1471-4159.1996.67051945.x. [DOI] [PubMed] [Google Scholar]
- CASANOVAS J.M., LESOURD M., ARTIGAS F. The effect of the selective 5-HT1A agonists alnespirone (S-20499) and 8-OH-DPAT on extracellular 5-hydroxytryptamine in different regions of rat brain. Br. J. Pharmacol. 1997;122:733–741. doi: 10.1038/sj.bjp.0701420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CECI A., BASCHIROTTO A., BORSINI F. The inhibitory effect of 8-OH-DPAT on the firing activity of dorsal raphe serotoninergic neurons in rats is attenuated by lesion of the frontal cortex. Neuropharmacology. 1994;33:709–713. doi: 10.1016/0028-3908(94)90177-5. [DOI] [PubMed] [Google Scholar]
- CELADA P., PUIG M.V., CASANOVAS J.M., GUILLAZO G., ARTIGAS F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: involvement of serotonin-1A, GABA(A), and glutamate receptors. J. Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CERVO L., SAMANIN R. Effect of chronic treatment with 8-OH-DPAT in the forced swimming test requires the integrity of presynaptic serotonergic mechanisms. Psychopharmacology. 1991;103:524–528. doi: 10.1007/BF02244253. [DOI] [PubMed] [Google Scholar]
- CHEN N.H., REITH M.E. Monoamine interactions measured by microdialysis in the ventral tegmental area of rats treated systemically with (+/−)-8-hydroxy-2-(di-n-propylamino)tetralin. J. Neurochem. 1995;64:1585–1597. doi: 10.1046/j.1471-4159.1995.64041585.x. [DOI] [PubMed] [Google Scholar]
- D'AQUILA P., MONLEON S., BORSINI F., BRAIN P., WILLNER P. Anti-anhedonic actions of the novel serotonergic agent flibanserin, a potential rapidly-acting antidepressant. Eur. J. Pharmacol. 1997;340:121–132. doi: 10.1016/s0014-2999(97)01412-x. [DOI] [PubMed] [Google Scholar]
- DE BOER T.H., MAURA G., RAITERI M., DE VOS C.J., WIERINGA J., PINDER R.M. Neurochemical and autonomic pharmacological profiles of the 6-aza-analogue of mianserin, Org 3770 and its enantiomers. Neuropharmacology. 1988;27:399–408. doi: 10.1016/0028-3908(88)90149-9. [DOI] [PubMed] [Google Scholar]
- DE VRY J. 5-HT1A receptor agonists: recent developments and controversial issues. Psychopharmacology (Berl.) 1995;121:1–26. doi: 10.1007/BF02245588. [DOI] [PubMed] [Google Scholar]
- DONE C.J., SHARP T. Biochemical evidence for the regulation of central noradrenergic activity by 5-HT1A and 5-HT2 receptors: microdialysis studies in the awake and anaesthetized rat. Neuropharmacology. 1994;33:411–421. doi: 10.1016/0028-3908(94)90071-x. [DOI] [PubMed] [Google Scholar]
- DREVETS W.C. Functional neuroimaging studies of depression: the anatomy of melancholia. Annu. Rev. Med. 1998;49:341–361. doi: 10.1146/annurev.med.49.1.341. [DOI] [PubMed] [Google Scholar]
- FORSTER E.A., CLIFFE I.A., BILL D.J., DOVER G.M., JONES D., REILLY Y., FLETCHER A. A pharmacological profile of the selective silent 5-HT1A receptor antagonist, WAY-100635. Eur. J. Pharmacol. 1995;281:81–88. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- GOBERT A., MILLAN M.J. Serotonin (5-HT)2A receptor activation enhances dialysate levels of dopamine and noradrenaline, but not 5-HT, in the frontal cortex of freely moving rats. Neuropharmacology. 1999;38:315–317. doi: 10.1016/s0028-3908(98)00188-9. [DOI] [PubMed] [Google Scholar]
- GOBERT A., RIVET JM., LEJEUNE F., NEWMAN-TANCREDI A., ADHUMEAU-AUCLAIR A., NICOLAS J.P., CISTARELLI L., MELON C., MILLAN M.J. Serotonin(2C) receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic, pathways: a combined dialysis and electrophysiological analysis in the rat. Synapse. 2000;36:205–221. doi: 10.1002/(SICI)1098-2396(20000601)36:3<205::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- HAJOS M., HAJOS-KORCSOK E., SHARP T. Role of the medial prefrontal cortex in 5-HT1A receptor-induced inhibition of 5-HT neuronal activity in the rat. Br. J. Pharmacol. 1999;126:1741–1750. doi: 10.1038/sj.bjp.0702510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEISLER L.K., CHU H.M., BRENNAN T.J., DANAO J.A., BAJWA P., PARSONS L.H., TECOTT L.H. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- ICHIKAWA J., ISHII H., BONACCORSO S., FOWLER W.L., O'LAUGHLIN I.A., MELTZER H.Y. 5-HT(2A) AND D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J. Neurochem. 2001;76:1521–1531. doi: 10.1046/j.1471-4159.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- INVERNIZZI R., BELLI S., SAMANIN R. Citalopram's ability to increase the extracellular concentrations of serotonin in the dorsal raphe prevents the drug's effect in the frontal cortex. Brain Res. 1992a;584:322–324. doi: 10.1016/0006-8993(92)90914-u. [DOI] [PubMed] [Google Scholar]
- INVERNIZZI R., BRAMANTE M., SAMANIN R. Chronic treatment with citalopram facilitates the effect of a challenge dose on cortical serotonin output: role of presynaptic 5-HT1A receptors. Eur. J. Pharmacol. 1994;260:243–246. doi: 10.1016/0014-2999(94)90344-1. [DOI] [PubMed] [Google Scholar]
- INVERNIZZI R., BRAMANTE M., SAMANIN R. Extracellular concentrations of serotonin in the dorsal hippocampus after acute and chronic treatment with citalopram. Brain Res. 1995;696:62–66. doi: 10.1016/0006-8993(95)00730-e. [DOI] [PubMed] [Google Scholar]
- INVERNIZZI R., BRAMANTE M., SAMANIN R. Role of 5-HT1A receptors in the effects of acute chronic fluoxetine on extracellular serotonin in the frontal cortex. Pharmacol. Biochem. Behav. 1996;54:143–147. doi: 10.1016/0091-3057(95)02159-0. [DOI] [PubMed] [Google Scholar]
- INVERNIZZI R., CARLI M., DI CLEMENTE A., SAMANIN R. Administration of 8-hydroxy-2-(Di-n-propylamino)tetralin in raphe nuclei dorsalis and medianus reduces serotonin synthesis in the rat brain: differences in potency and regional sensitivity. J. Neurochem. 1991;56:243–247. doi: 10.1111/j.1471-4159.1991.tb02587.x. [DOI] [PubMed] [Google Scholar]
- INVERNIZZI R., POZZI L., VALLEBUONA F., BONINI I., SACCHETTI G., SAMANIN R. Effect of amineptine on regional extracellular concentrations of dopamine and noradrenaline in the rat brain. J. Pharmacol. Exp. Ther. 1992b;262:769–774. [PubMed] [Google Scholar]
- KREISS D.S., LUCKI I. Differential regulation of serotonin (5-HT) release in the striatum and hippocampus by 5-HT1A autoreceptors of the dorsal and median raphe nuclei. J. Pharmacol. Exp. Ther. 1994;269:1268–1279. [PubMed] [Google Scholar]
- MILLAN M.J., NEWMAN-TANCREDI A., BROCCO M., GOBERT A., LEJEUNE F., AUDINOT V., RIVET J.M., SCHREIBER R., DEKEYNE A., SPEDDING M., NICOLAS J.P., PEGLION J.L. S 18126 ([2-[4-(2,3-dihydrobenzo[1,4]dioxin-6-yl)piperazin-1-yl methyl]indan-2-yl]), a potent, selective and competitive antagonist at dopamine D4 receptors: an in vitro and in vivo comparison with L 745,870 (3-(4-[4-chlorophenyl]piperazin-1-yl)methyl-1H-pyrrolo[2, 3b]pyridine) and raclopride. J. Pharmacol. Exp. Ther. 1998;287:167–186. [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic Press; 1986. [Google Scholar]
- PEREZ V., GILABERTE I., FARIES D., ALVAREZ E., ARTIGAS F. Randomised, double-blind, placebo-controlled trial of pindolol in combination with fluoxetine antidepressant treatment. Lancet. 1997;349:1594–1597. doi: 10.1016/S0140-6736(96)08007-5. [DOI] [PubMed] [Google Scholar]
- PEROUTKA S.J., SNYDER S.H. Long-term antidepressant treatment decreases spiroperidol-labeled serotonin receptor binding. Science. 1980;210:88–90. doi: 10.1126/science.6251550. [DOI] [PubMed] [Google Scholar]
- PODHORNA J., BROWN R.E. Flibanserin has anxiolytic effects without locomotor side effects in the infant rat ultrasonic vocalization model of anxiety. Br. J. Pharmacol. 2000;130:739–746. doi: 10.1038/sj.bjp.0703364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POMPEIANO M., PALACIOS J.M., MENGOD G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J. Neurosci. 1992;12:440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORSOLT R.D., ANTON G., BLAVET N., JALFRE M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- POZZI L., ACCONCIA S., CEGLIA I., INVERNIZZI R.W., SAMANIN R. Stimulation of 5-hydroxytryptamine 5-HT2C receptors in the ventrotegmental area inhibits stress-induced but not basal dopamine release in the rat prefrontal cortex. J. Neurochem. 2002;82:93–100. doi: 10.1046/j.1471-4159.2002.00947.x. [DOI] [PubMed] [Google Scholar]
- POZZI L., INVERNIZZI R., GARAVAGLIA C., SAMANIN R. Fluoxetine increases extracellular dopamine in the prefrontal cortex by a mechanism not dependent on serotonin: a comparison with citalopram. J. Neurochem. 1999;73:1051–1057. doi: 10.1046/j.1471-4159.1999.0731051.x. [DOI] [PubMed] [Google Scholar]
- PRISCO S., PAGANNONE S., ESPOSITO E. Serotonin–dopamine interaction in the rat ventral tegmental area: an electrophysiological study in vivo. J. Pharmacol. Exp.Ther. 1994;271:83–90. [PubMed] [Google Scholar]
- RAMBOZ S., OOSTING R., AMARA D.A., KUNG H.F., BLIER P., MENDELSOHN M., MANN J.J., BRUNNER D., HEN R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc. Natl. Acad. Sci. U.S.A. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RISCH S.C., NEMEROFF C.B. Neurochemical alterations of serotonergic neuronal systems in depression. J. Clin. Psychiatry. 1992;53 Suppl:3–7. [PubMed] [Google Scholar]
- ROBINSON T.E., WHISHAW I.Q. Normalization of extracellular dopamine in striatum following recovery from a partial unilateral 6-OHDA lesion of the substantia nigra: a microdialysis study in freely moving rats. Brain Res. 1988;450:209–224. doi: 10.1016/0006-8993(88)91560-0. [DOI] [PubMed] [Google Scholar]
- ROLLEMA H., LU Y., SCHMIDT A.W., SPROUSE J.S., ZORN S.H. 5-HT1A receptor activation contributes to ziprasidone-induced dopamine release in the rat prefrontal cortex. Biol. Psychiatry. 2000;48:229–237. doi: 10.1016/s0006-3223(00)00850-7. [DOI] [PubMed] [Google Scholar]
- RUETER L.E., DE MONTIGNY C., BLIER P. In vivo electrophysiological assessment of the agonistic properties of flibanserin at pre- and postsynaptic 5-HT1A receptors in the rat brain. Synapse. 1998;29:392–405. doi: 10.1002/(SICI)1098-2396(199808)29:4<392::AID-SYN11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- RUETER L.E., BLIER P. Electrophysiological examination of the effects of sustained flibanserin administration on serotonin receptors in rat brain. Br. J. Pharmacol. 1999;126:627–638. doi: 10.1038/sj.bjp.0702344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUTTER J.J., GUNDLAH C., AUERBACH S.B. Increase in extracellular serotonin produced by uptake inhibitors is enhanced after chronic treatment with fluoxetine. Neurosci. Lett. 1994;171:183–186. doi: 10.1016/0304-3940(94)90635-1. [DOI] [PubMed] [Google Scholar]
- SAKAUE M., SOMBOONTHUM P., NISHIHARA B., KOYAMA Y., HASHIMOTO H., BABA A., MATSUDA T. Postsynaptic 5-hydroxytryptamine(1A) receptor activation increases in vivo dopamine release in rat prefrontal cortex. Br. J. Pharmacol. 2000;129:1028–1034. doi: 10.1038/sj.bjp.0703139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCANDROGLIO A., MONFERINI E., BORSINI F. Ex vivo binding of flibanserin to serotonin 5-HT1A and 5-HT2A receptors. Pharmacol. Res. 2001;43:179–183. doi: 10.1006/phrs.2000.0762. [DOI] [PubMed] [Google Scholar]
- SHARP T., BRAMWELL S.R., GRAHAME-SMITH D.G. 5-HT1 agonists reduce 5-hydroxytryptamine release in rat hippocampus in vivo as determined by brain microdialysis. Br. J. Pharmacol. 1989;96:283–290. doi: 10.1111/j.1476-5381.1989.tb11815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAHL S., GASTPAR M., HESSELINK J.K., TRABER J. Serotonin1A Receptors in Depression and Anxiety. New York: Raven Press; 1992. [Google Scholar]
- STANLEY M., MANN J.J. Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet. 1983;1:214–216. doi: 10.1016/s0140-6736(83)92590-4. [DOI] [PubMed] [Google Scholar]
- STERU L., CHERMAT R., THIERRY B., SIMON P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- SZABO S.T., BLIER P. Serotonin (1A) receptor ligands act on norepinephrine neuron firing through excitatory amino acid and GABA(A) receptors: a microiontophoretic study in the rat locus coeruleus. Synapse. 2001;42:203–212. doi: 10.1002/syn.10009. [DOI] [PubMed] [Google Scholar]
- TAO R., HJORTH S. Differences in the in vitro and in vivo 5-hydroxytryptamine extraction performance among three common microdialysis membranes. J. Neurochem. 1992;59:1778–1785. doi: 10.1111/j.1471-4159.1992.tb11010.x. [DOI] [PubMed] [Google Scholar]
- WEDZONY K., MACKOWIAK M., FIJAL K., GOLEMBIOWSKA K. Ipsapirone enhances the dopamine outflow via 5-HT1A receptors in the rat prefrontal cortex. Eur. J. Pharmacol. 1996;305:73–78. doi: 10.1016/0014-2999(96)00150-1. [DOI] [PubMed] [Google Scholar]
- YATES M., LEAKE A., CANDY J.M., FAIRBAIRN A.F., McKEITH I.G., FERRIER I.N. 5HT2 receptor changes in major depression. Biol. Psychiatry. 1990;27:489–496. doi: 10.1016/0006-3223(90)90440-d. [DOI] [PubMed] [Google Scholar]